Abstract
Background and Aims:
Persons with opioid use disorder (OUD) and co-occurring alcohol use disorder (AUD) are understudied. We identified whether co-occurring AUD was associated with OUD treatment type, compared associations between treatment type and six-month treatment retention and determined whether co-occurring AUD moderated these relationships.
Methods:
We used an observational cohort study design to analyze insurance claims data from 2011–2016 from persons aged 12–64 with an opioid abuse or opioid dependence diagnosis and OUD treatment claim. Our unit of analysis was the treatment episode; we used logistic regression for analyses.
Results:
Of 211,047 treatment episodes analyzed, 14% had co-occurring alcohol abuse or dependence diagnoses. Among persons with opioid dependence, persons with co-occurring alcohol dependence were 25% less likely to receive medication treatment relative to those without AUD. Further, alcohol dependence was associated with decreased likelihood of treatment with buprenorphine (AOR 0.47, 95% CI 0.44–0.49) or methadone (AOR 0.31, 95% CI 0.28–0.35) and increased likelihood of treatment with extended-release (AOR 1.36, 95% CI 1.21–1.54) or oral (AOR 1.73, 95% CI 1.57–1.90) naltrexone relative to psychosocial treatment. Buprenorphine and methadone were associated with highest retention prevalence regardless of OUD or AUD severity. Co-occurring alcohol abuse or dependence did not meaningfully change retention prevalence associated with buprenorphine or methadone. Co-occurring AUD was not associated with improved retention among persons receiving either formulation of naltrexone.
Conclusions:
Buprenorphine and methadone are associated with high likelihood of treatment retention among persons opioid and alcohol dependence, but are disproportionately under-prescribed.
Keywords: opioid use disorder, alcohol use disorder, buprenorphine, methadone, naltrexone, treatment retention
1. Introduction:
Opioid use disorder (OUD) and alcohol use disorder (AUD) increase mortality risk (Kendler et al., 2016; Olfson et al., 2018): Approximately 47,000 Americans died of an opioid-related overdose in 2018 (Wilson et al., 2020), and more than 72,000 Americans died from alcohol-related causes in 2017 (White et al., 2020). Not surprisingly, OUD is associated with increased likelihood of AUD (Hartzler et al., 2010; Hser et al., 2017; Saha et al., 2016; Soyka, 2015), and co-occurring diagnoses portend worse outcomes than having either diagnosis alone, with elevated risk of relapse to either substance (Friedmann et al., 2018; Witkiewitz et al., 2018). Further, as central nervous system depressants, opioid and alcohol co-use increases risk of respiratory depression, overdose, and death (Bogdanowicz et al., 2015; Darke and Zador, 1996; Morgan et al., 2019; Webster et al., 2011). Thus, persons with co-occurring OUD and AUD represent a particularly vulnerable population for whom effective treatment is critical.
Of the three medications with FDA approval for treatment of moderate or severe OUD, two are opioid agonists: buprenorphine, a partial agonist; and methadone, a full agonist. The effectiveness of opioid agonist therapy (OAT) for OUD is clear: randomized controlled trials and observational studies have shown treatment with either buprenorphine or methadone is associated with increased treatment retention and decreased risk of relapse, with consistent results across differing study populations (Dole and Nyswander, 1965; Hser et al., 2016; Johnson et al., 1995; Kakko et al., 2003; Mattick et al., 2009, 2014; Mintz et al., 2020). Both buprenorphine and methadone cause physiologic dependence and abrupt discontinuation from either medication may lead to withdrawal (Substance Abuse and Mental Health Services Administration, 2020), which may partially contribute to the improved treatment retention associated with these medications. The protective benefits of OAT, however, are clear: multiple observational studies have demonstrated that OAT is associated with decreased risk of overdose (Morgan et al., 2019; Wakeman et al., 2020; Williams et al., 2020) and mortality (Larochelle et al., 2018; Sordo et al., 2017) among persons with OUD.
Naltrexone, an opioid antagonist that comes in both extended-release (XR) depot and oral formulations, uniquely carries FDA approval for OUD and for AUD. XR Naltrexone has been shown to be effective for relapse prevention among persons with OUD who have been abstinent from opioids for several days (Lee et al., 2018; Tanum et al., 2017), however, studies to date—though limited—have not demonstrated reduction in overdose risk (Morgan et al., 2019). Oral naltrexone has been shown to be largely ineffective for OUD treatment (Minozzi et al., 2011) and may increase risk of opioid-related overdose (Morgan et al., 2019). Naltrexone in either formulation has been shown to be moderately effective for persons with moderate to severe AUD, with multiple studies demonstrating naltrexone’s association with decreased risk of relapse and decreased number of heavy drinking days among persons with AUD (Anton et al., 2006; Jonas et al., 2014; Kranzler and Soyka, 2018).
Despite the prevalence of data on medication treatment effectiveness in OUD and AUD populations separately, data on treatment effectiveness for persons with co-occurring OUD and AUD are lacking (Witkiewitz and Vowles, 2018). For example, it has been suggested that naltrexone may confer particular benefit for persons with OUD and AUD given its indications for each diagnosis separately (Volkow and Blanco, 2020; Witkiewitz and Vowles, 2018), although to our knowledge this hypothesis has not been formally tested. Further, whether naltrexone’s dual indications makes providers more likely to prescribe naltrexone for persons with both OUD and AUD compared to those with OUD alone is an important related question.
Finally, whether co-occurring AUD moderates the protective effect of OAT has not been formally examined, yet has clinical implications for practitioners treating patients with these disorders. Studies examining associations between OAT and treatment outcomes in persons with OUD and AUD are few and interpretation of results is complicated by differing findings, study designs, inclusion criteria and outcome measures. For example, in a 2007 systemic review examining associations between methadone maintenance treatment and alcohol consumption among persons with OUD found that among 15 studies reviewed, three concluded alcohol use increased with methadone treatment, three found that alcohol use decreased, and nine studies found no association between methadone treatment and alcohol use (Srivastava et al., 2008). Of these 15 studies, only three were randomized control trials, and follow-up times varied from four weeks to several years. A 2008 open-label Italian study found that among 218 persons with heroin use disorder and alcohol dependence, treatment with either buprenorphine or methadone decreased subsequent alcohol intake (Nava et al., 2008); however, interpretation of these results is limited by small sample size and lack of control group.
Treatment retention is often used as a measure of treatment effectiveness for substance use disorders (Clark et al., 2015; Hadland et al., 2018; Mintz et al., 2020) as it can be relatively easily measured and is clinically relevant. In addition, there is evidence that six-month retention is correlated with overdose risk in persons with OUD (Wakeman et al., 2020), further increasing the clinical importance of this outcome. Administrative claims data and observational study designs allow for inclusion of large sample sizes to examine real-world outcomes such as treatment retention.
When assessing treatment effectiveness of pharmacotherapy for OUD and AUD, disease severity warrants attention. Substance use disorder diagnoses and their severities- classified as “mild,” “moderate” or “severe”- are currently defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th Edition, which has been in clinical use since 2013. The previous DSM-IV classification categorized substance use disorder severity as either “abuse” or “dependence,” and these terms are still used for International Classification of Disease (ICD) diagnostic codes. In general, it is assumed that “abuse” is synonymous with mild severity while “dependence” connotes moderate or severe illness. As pharmacotherapy is only FDA-approved for opioid and alcohol dependence, treatment outcomes in these populations is of particular interest.
In this study, we used IBM® MarketScan® Commercial and Multi-State Medicaid databases to examine the following among persons with an opioid abuse or dependence diagnosis: 1) whether co-occurring alcohol abuse or dependence was associated with type of OUD treatment received, 2) the relative effectiveness of buprenorphine, methadone, XR naltrexone, and oral naltrexone in persons with co-occurring alcohol abuse or dependence, and 3) whether co-occurring alcohol abuse or dependence moderated the association between treatment type and six-month retention.
2. Methods:
2.1. Data Source
MarketScan Commercial and Multi-State Medicaid databases provided de-identified claims data for diagnoses, inpatient and outpatient health care services, and prescription drugs for persons aged 12–64. As many persons transition to Medicare insurance coverage at age 65, coverage plans in persons above age 64 were considered incomplete. The commercial database included claims for employees, spouses, and dependents from several large employers and health plans from a nationally-representative sample; the Medicaid database included claims from states selected by IBM Watson Health. Because XR-naltrexone was not approved for OUD until October 2010, we limited analyses from 2011 to 2016 (most recent year for which data were available). This study was exempted from human subjects review by the institutional review board at Washington University School of Medicine.
2.2. Sample Selection
Our sample was composed of persons with an OUD diagnosis as defined by an ICD-9 or -10 code (Supplementary Table 1) and a corresponding treatment claim for a medication for opioid use disorder and/or multiple psychosocial treatment claims (Supplementary Table 2). We classified our sample further into (1) an “opioid abuse” category, composed of persons with ICD-9 or 10 codes corresponding to diagnoses of opioid abuse or unspecified opioid use and (2) the more severe “opioid dependence” category, which was composed of persons with ICD-9 or -10 codes corresponding to a diagnosis of opioid dependence. There were a small number of persons with both “dependence” and “abuse” diagnoses (n=15,641); as we could not be certain which severity was predominant during a treatment episode, we excluded these persons from analyses. Treatment services were characterized using Current Procedural Terminology codes, the Healthcare Common Procedure Coding System (HCPCS), and ICD-9 and -10 codes. Treatment with buprenorphine (with or without naloxone) or with oral naltrexone was identified from pharmacy claims using appropriate National Drug Codes (NDCs). XR naltrexone treatment was identified using NDCs and HCPCS code J2315. Methadone treatment was identified using HCPCS code H0020. Psychosocial treatment codes were identified using HCPCS, CPT and ICD-9/10 codes. As psychosocial treatment claims do not specify for which SUD a person is receiving treatment, to maximize the likelihood we captured psychosocial treatment for OUD, we only counted psychosocial treatment services that linked to persons with a primary diagnosis of OUD at the time of the claim. The list of CPT, HCPCS, NDCs and ICD codes used to define treatment services is detailed in Supplementary Table 2.
Our unit of analysis was the treatment episode. As in our previous work (Mintz et al., 2020), we defined treatment episodes as consecutive treatment days without a 45-day gap in medication or psychosocial treatment claims. Claims for buprenorphine and for oral naltrexone included the day supply of medication dispensed by a pharmacy; each day for which a patient was presumed to have a prescribed medication supply was considered a day of treatment with this medication. The 28 days following an XR naltrexone injection were counted as days of XR naltrexone treatment since the medication is presumed to be active during this period.
Episodes that included only psychosocial services were categorized as psychosocial treatment only. Episodes that included medication with or without psychosocial treatment were categorized by the type of medication received. The small number of treatment episodes that included more than one type of medication treatment were excluded from analyses. Individuals could undergo multiple treatment episodes within the data set. Detailed inclusion and exclusion criteria have been described previously (Mintz et al., 2020) and are outlined in Supplementary Figure 1.
AUD was defined by ICD-9 and 10 codes (Supplementary Table 1). As with OUD diagnoses, we classified AUD diagnoses by severity: persons with an ICD-9 or -10 code corresponding to alcohol abuse or intoxication was placed in the less severe “alcohol abuse” category and those with an ICD code corresponding to alcohol dependence and more severe “alcohol dependence” category. Those with codes for both alcohol abuse and dependence (n=6,868) were excluded from analyses due to inability to determine from claims data which severity of illness predominated during the treatment episode of interest.
Our final sample, then included six subgroups: 1) opioid abuse without co-occurring AUD, 2) opioid abuse and co-occurring alcohol abuse, 3) opioid abuse and alcohol dependence, 4) opioid dependence without co-occurring AUD, 5) opioid dependence and co-occurring alcohol abuse and 6) opioid dependence and alcohol dependence.
2.3. Variables of Interest
The primary outcome for our first objective was OUD treatment type: buprenorphine, methadone, XR naltrexone, oral naltrexone, or psychosocial only. The primary outcome for our second and third objectives was six-month treatment retention.
Covariates included age, sex, insurance type, year, medical comorbidities, comorbid SUD illness, comorbid non-SUD psychiatric illness, and OUD diagnosis prior to current treatment episode. Age was treated as categorical variable divided into five groups: 12–17, 18–25, 26–34, 35–49, and 50–64. Because contributing health plans may change over time, binary indicator variables were assigned to each insurance cohort for each year included in the study period. More specifically, we created variables for the product of insurance status (Medicaid or commercial) and year and estimated a separate effect for each insurance-year cohort.
The Charlson Comorbidity Index was used as an indicator of overall health status, with higher scores indicating a higher severity of comorbid illness. The following SUD diagnoses were included as covariates: cannabis, cocaine, sedative, stimulant and other (composed of hallucinogen, inhalant, and “other” substance use disorder diagnostic codes). The following non-SUD psychiatric diagnoses were included as covariates: mood disorder (including depressive and bipolar disorders), anxiety disorder, psychotic disorder and personality disorder. ICD-9 and 10 codes used to identify comorbidities are included in Supplementary Table 1. SUD and non-SUD psychiatric disorders were included as covariates if there was a relevant diagnostic code in the six months prior to the start of the treatment episode or first day of treatment episode. Almost half of the episodes included a diagnosis of OUD without any treatment receipt that predated the current treatment episode; thus, previous OUD diagnosis was included as covariate.
Race/ethnicity was available only in Medicaid sample, so was not included as a covariate, but was included in descriptive analyses.
2.4. Analyses
Analyses were conducted with SAS® version 9.4. We used chi square and ANOVA tests to evaluate for demographic differences between groups. To examine the relationship between co-occurring AUD and type of OUD treatment received, we first calculated the unadjusted prevalence of a) any medication treatment and b) specific treatment type within each of the six clinical groups of interest. We then stratified the sample by opioid abuse or dependence status and used logistic regression to estimate adjusted effect sizes conferred by co-occurring alcohol abuse and co-occurring alcohol dependence relative to no AUD on likelihood of receiving medication treatment relative to psychosocial treatment alone.
To examine the associations between AUD and treatment type on six-month treatment retention, we used logistic regression to model the prevalence of retention as a function of co-occurring AUD status (no AUD, alcohol abuse, or alcohol dependence), treatment type, and the interaction between AUD status and treatment type while adjusting for covariates. We modeled retention for the opioid abuse and opioid dependence subgroups separately. We used the “lsmeans” option within SAS to produce adjusted prevalence estimates for six-month retention, which were equivalent to population margins based on covariates as observed in the sample.
For all analyses, we used the “survey” procedures within SAS to account for clustering of treatment episodes within individuals.
3. Results:
3.1. Demographics and Clinical Characteristics of the Sample
Demographic information and clinical characteristics of the sample are shown in Table 1. Of the 211,047 treatment episodes included in analyses, 26.8% (n=56,545) were included in the opioid abuse category and 73.2% (n=154,502) were included in the opioid dependence category. Within the opioid abuse group, 5.8% (n =3,282) met criteria for alcohol abuse and 10.3% (n=5,851) met criteria for alcohol dependence. Within the opioid dependence group, 4.8% (n=7,348) met criteria for alcohol abuse and 7.8% (n=12,078) met criteria for alcohol dependence.
Table 1.
Demographic and clinical characteristics of study sample.
| Opioid Abuse (n=56,545) | Opioid Dependence (n=154,502) | ||||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | ||||||
| No AUD | Alcohol Abuse | Alcohol Dependence | No AUD | Alcohol Abuse | Alcohol Dependence | ||
| n=47, 412 | n=3,282 | n=5,851 | n=135,076 | n=7,348 | n=12,078 | ||
| Characteristic | |||||||
| Prevalence within total sample | 22.5 (22.3, 22.7) | 1.6 (1.5, 1.6) | 2.8 (2.7, 2.9) | 64.0 (63.8, 64.2) | 3.5 (3.4, 3.6) | 5.7 (5.6, 5.8) | |
| Comorbid SUD□ | |||||||
| Amphetamine-type | 3.6 (3.5, 3.8) | 9.8 (8.7, 10.9) | 9.2 (8.4, 10.0) | 3.1 (3.0, 3.2) | 7.2 (6.6, 7.8) | 8.7 (8.1, 9.2) | |
| Cannabis | 8.9 (8.6, 9.2) | 37.8 (36.0, 39.6) | 27.5 (26.3, 28.8) | 10.4 (10.3, 10.6) | 32.8 (31.6, 33.9) | 29.5 (28.6, 30.4) | |
| Cocaine | 4.3 (4.1, 4.6) | 14.8 (13.6, 16.1) | 17.4 (16.3, 18.5) | 6.1 (5.9, 6.2) | 19.2 (18.2, 20.2) | 20.9 (20.1, 21.7) | |
| Sedative | 4.5 (4.3, 4.7) | 13.2 (12.0, 14.4) | 15.5 (14.5, 16.5) | 6.0 (5.8, 6.1) | 15.3 (14.4, 16.2) | 19.3 (18.5, 20.0) | |
| Other | 15.8 (15.4, 16.1) | 30.4 (28.8, 32.1) | 23.2 (22.1, 24.3) | 17.9 (17.7, 18.1) | 33.5 (32.4, 34.6) | 32.3 (31.4, 33.2) | |
| Previous OUD diagnosis | 21.5 (21.1, 21.9) | 33.9 (32.3, 35.6) | 32.1 (30.9, 33.4) | 56.1 (55.9, 56.4) | 61.9 (60.7, 63.0) | 65.2 (64.3, 66.0) | |
| Comorbid psychiatric illness□□ | |||||||
| Anxiety | 30.8 (30.3, 31.3) | 47.8 (46.0, 49.6) | 41.7 (40.4, 43.1) | 28.2 (28.0, 28.5) | 43.7 (42.5, 44.9) | 38.4 (37.5, 39.3) | |
| Mood | 38.9 (38.4, 39.4) | 64.6 (62.9, 66.4) | 57.9 (56.6, 59.3) | 33.4 (33.1, 33.7) | 53.9 (52.7, 55.1) | 48.4 (47.4, 49.4) | |
| Personality | 2.7 (2.6, 2.9) | 9.0 (7.9, 10.1) | 5.3 (4.7, 5.9) | 1.7 (1.6, 1.8) | 5.3 (4.7, 5.9) | 3.9 (3.5, 4.2) | |
| Psychotic | 3.2 (3.0, 3.4) | 10.8 (9.7, 11.9) | 5.8 (5.2, 6.5) | 1.9 (1.8, 2.0) | 5.6 (5.1, 6.2) | 4.0 (3.7, 4.4) | |
| Charlson comorbidity indexa | 0.0 (1.0–10.0) | 0.0 (0.0–11.0) | 0.0 (1.0–10.0) | 0.0 (1.0–12.0) | 0.0 (1.0–10.0) | 0.0 (1.0–13.0) | |
| Insurance | |||||||
| Commercial | 60.0 (59.5, 60.5) | 42.1 (40.3, 43.9) | 58.9 (57.5, 60.4) | 40.8 (40.4, 41.1) | 36.6 (35.3, 37.8) | 54.9 (53.9, 55.9) | |
| Medicaid | 40.0 (39.5, 40.5) | 57.9 (56.0, 59.7) | 41.1 (39.6, 42.5) | 59.2 (58.9, 59.6) | 63.4 (62.2, 64.7) | 45.1 (44.1, 46.1) | |
| Sex | |||||||
| Female | 51.1 (50.6, 51.7) | 46.1 (44.2, 48.0) | 45.0 (43.5, 46.4) | 54.1 (53.8, 54.4) | 49.8 (48.5, 51.1) | 42.1 (41.1, 43.1) | |
| Male | 48.9 (48.3, 49.4) | 53.9 (52.0, 55.8) | 55.0 (53.7, 56.5) | 45.9 (45.6, 46.2) | 50.2 (48.9, 51.5) | 57.9 (56.9, 58.9) | |
| Age | |||||||
| 12–17 | 2.4 (2.3, 2.6) | 11.5 (10.3, 12.6) | 2.6 (2.2, 3.0) | 1.0 (0.9, 1.0) | 2.7 (2.3, 3.1) | 1.0 (0.8, 1.2) | |
| 18–25 | 25.2 (24.7, 25.7) | 26.7 (25.1, 28.3) | 21.5 (20.4, 22.7) | 29.1 (28.8, 29.5) | 32.6 (31.4, 33.8) | 34.8 (33.8, 35.8) | |
| 26–34 | 27.4 (26.9, 27.8) | 21.3 (19.7, 22.8) | 19.2 (18.1, 20.3) | 33.6 (33.3, 34.0) | 30.2 (29.1, 31.4) | 24.0 (23.1, 24.8) | |
| 35–49 | 29.4 (28.9, 29.9) | 25.7 (24.1, 27.4) | 33.2 (31.9, 34.6) | 26.5 (26.2, 26.8) | 24.1 (23.0, 25.2) | 27.0 (26.1, 27.9) | |
| 50–64 | 15.6 (15.2, 16.0) | 14.8 (13.5, 16.2) | 23.4 (22.2, 24.7) | 9.7 (9.5, 9.9) | 10.3 (9.6, 11.1) | 13.2 (12.6, 13.9) | |
| Race/ethnicity (Medicaid only) | n=18,642 | n=1,861 | n=2,347 | n=78,161 | n=4,529 | n=5,248 | |
| Black | 7.0 (6.6, 7.4) | 10.8 (9.2, 12.4) | 12.4 (10.6, 14.1) | 6.7 (6.5, 7.0) | 7.7 (6.8, 8.5) | 8.9 (8.1, 9.8) | |
| White | 82.4 (81.8, 83.1) | 77.3 (75.2, 79.4) | 75.6 (73.6, 77.7) | 84.2 (83.9, 84.5) | 82.4 (81.2, 83.6) | 79.4 (78.2, 80.7) | |
| Hispanic | 1.1 (0.9, 1.2) | 1.3 (0.8, 1.9) | 1.4 (0.9, 2.0) | 1.1 (1.0, 1.2) | 1.2 (0.9, 1.6) | 1.1 (0.8, 1.4) | |
| Other | 9.5 (9.0, 10.0) | 10.6 (9.1, 12.0) | 10.6 (9.2, 11.9) | 7.9 (7.7, 8.1) | 8.7 (7.9, 9.6) | 10.5 (9.6, 11.4) | |
OUD= Opioid use disorder; AUD=alcohol use disorder; SUD= substance use disorder; CI= confidence interval.
Note: Variables with mutually exclusive response choices may not always sum to 100.0 due to rounding.
Each comorbid SUD is binary (yes/no) variable.
Each comorbid non-SUD psychiatric disease is binary (yes/no) variable.
Median (range) presented.
All comparisons are significant at p<.0001 level.
Most notably, those with co-occurring alcohol abuse or dependence had a higher overall disease burden compared to those without AUD: comorbid SUDs, comorbid non-SUD psychiatric illness, and a previous OUD diagnosis were all more common among persons with both OUD and AUD (Table 1).
3.2. Association between co-occurring AUD severity and OUD treatment type
Table 2 shows the prevalence of OUD treatment type by co-occurring AUD status among persons with opioid abuse and with opioid dependence. Surprisingly, persons with opioid dependence without co-occurring AUD were 33% less likely to receive any OUD medication compared to their opioid abuse counterparts (Table 2). Similar overall patterns of medication receipt were observed in the opioid dependence group as for the opioid abuse group. Persons with co-occurring alcohol abuse or dependence were less likely to receive any medication treatment than their OUD-only counterparts. Further, persons with co-occurring alcohol abuse or dependence were less likely to receive opioid agonist treatment and were more likely to receive either formulation of naltrexone compared to persons without co-occurring AUD.
Table 2.
Prevalence of OUD treatment type by OUD and AUD disease severity among OUD treatment episodes from 2011–2016.
| Opioid Abuse (n=56,545) | Opioid Dependence (n=154,502) | ||||||
|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | ||||||
| No AUD (n=47,412) | Alcohol Abuse (n=3,282) | Alcohol Dependence (n=5,851) | No AUD (n=135,076) | Alcohol Abuse (n=7,348) | Alcohol Dependence (n=12,078) | ||
| Treatment type | |||||||
| Any medication | 74.5 (74.0, 75.0) | 36.0 (34.3, 37.8) | 44.3 (42.9, 45.7) | 49.7 (49.4, 50.0) | 34.2 (33.1, 35.3) | 37.1 (36.2, 38.0) | |
| Buprenorphine | 66.1 (65.6, 66.7) | 20.6 (19.2, 22.1) | 16.3 (15.3, 17.3) | 37.8 (37.5, 38.1) | 25.0 (23.9, 26.0) | 24.5 (23.7, 25.3) | |
| Methadone | 2.9 (2.8, 3.1) | 0.9 (0.6, 1.3) | 0.9 (0.7, 1.2) | 8.8 (8.6, 8.9) | 4.4 (3.9, 4.9) | 2.5 (2.2, 2.8) | |
| XR naltrexone | 0.8 (0.7, 0.9) | 1.1 (0.7, 1.5) | 3.3 (2.7, 3.9) | 1.5 (1.4, 1.5) | 1.8 (1.5, 2.1) | 3.5 (3.2, 3.9) | |
| Oral naltrexone | 4.6 (4.4, 4.8) | 13.3 (12.1, 14.6) | 23.8 (22.5, 25.0) | 1.7 (1.7, 1.8) | 3.1 (2.7, 3.5) | 6.6 (6.1, 7.0) | |
| Psychosocial only | 25.5 (25.0, 26.0) | 64.0 (62.2, 65.7) | 55.7 (54.3, 57.2) | 50.3 (50.0, 50.6) | 65.8 (64.7, 66.9) | 62.9 (62.0, 63.8) | |
OUD= Opioid use disorder; AUD= alcohol use disorder; CI=confidence interval; XR= extended-release.
Table 3 shows adjusted odds ratios reflecting the association between AUD status and severity and likelihood of OUD medication treatment type relative to psychosocial treatment. For both the opioid abuse the opioid dependence groups, co-occurring alcohol abuse or dependence was associated with decreased likelihood of OAT and increased likelihood of treatment with oral naltrexone compared to psychosocial treatment only. Alcohol dependence was associated with increased likelihood of treatment with XR naltrexone compared to psychosocial treatment alone regardless of OUD severity. Within both opioid abuse and opioid dependence groups, persons with co-occurring alcohol dependence had lowest odds of receiving OAT and highest odds of receiving naltrexone treatment in either formulation.
Table 3.
Associations between co-occurring AUD, AUD severity and treatment type among OUD treatment episodes from 2011–2016.
| Buprenorphine vs psychosocial only | Methadone vs psychosocial only | XR Naltrexone vs psychosocial only | Oral Naltrexone vs psychosocial only | |
|---|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Opioid Abuse (n=56,545) | ||||
| Alcohol dependence | 0.14 (0.13, 0.16)**** | 0.21 (0.15, 0.28)**** | 1.69 (1.36, 2.10)**** | 2.18 (1.98, 2.41)**** |
| Alcohol abuse | 0.29 (0.25, 0.32)**** | 0.25 (0.17, 0.36)**** | 0.78 (0.54, 1.13) | 1.48 (1.29, 1.69)**** |
| No AUD | Ref | Ref | Ref | Ref |
| Opioid Dependence (n=154,502) | ||||
| Alcohol dependence | 0.47 (0.44, 0.49)**** | 0.31 (0.28, 0.35)**** | 1.36 (1.21, 1.54)**** | 1.73 (1.57, 1.90)**** |
| Alcohol abuse | 0.65 (0.61, 0.69)**** | 0.57 (0.51, 0.64)**** | 0.95 (0.79, 1.15) | 1.22 (1.05, 1.42)* |
| No AUD | Ref | Ref | Ref | Ref |
AUD= alcohol use disorder; OUD= opioid use disorder; XR= extended-release; AOR=adjusted odds ratio; CI= confidence interval; SUD= substance use disorder.
Models adjusted for co-occurring substance use disorder, previous OUD diagnosis, psychiatric comorbidity, medical comorbidity as measured by Charlson comorbidity index, sex, age, and insurance-year cohort.
p<.0001.
3.3. Associations between co-occurring AUD severity, OUD treatment type, and six-month retention
We modeled six-month retention as a function of co-occurring AUD severity, treatment type, and the interaction between co-occurring AUD and treatment type while adjusting for covariates. Separate models were conducted for the opioid abuse and opioid dependence groups; results are shown in Table 4. For both opioid abuse and opioid dependence models, there were statistically significant main effects for alcohol dependence but not alcohol abuse, such that alcohol dependence was associated with decreased likelihood of retention. There were also significant main effects of treatment type such that buprenorphine, methadone and XR naltrexone were associated with higher likelihood of retention compared to psychosocial treatment alone in both the opioid abuse and the opioid dependence models.
Table 4.
Associations between OUD and AUD disease severity and six-month treatment retention among OUD treatment episodes from 2011–2016.
| Opioid Abuse | Opioid Dependence | ||
|---|---|---|---|
| n=56,545 | n=154,502 | ||
| AOR (95% CI) | AOR (95% CI) | ||
| Co-occurring AUD | |||
| Alcohol dependence | 0.8 (0.7, 0.9)*** | 0.8 (0.8, 0.9)**** | |
| Alcohol abuse | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.0) | |
| No AUD | Ref | Ref | |
| Treatment type | |||
| Buprenorphine | 4.9 (4.6, 5.2)**** | 4.5 (4.4, 4.6)**** | |
| Methadone | 4.6 (4.0, 5.2)**** | 7.6 (7.3, 7.9)**** | |
| XR Naltrexone | 2.0 (1.5, 2.5)**** | 2.7 (2.4, 2.9)**** | |
| Oral naltrexone | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | |
| Psychosocial | Ref | Ref | |
| Treatment type*AUD status | |||
| Buprenorphine*Alcohol dependence | 1.0 (0.8, 1.2) | 1.2 (1.1, 1.3)** | |
| Buprenorphine*Alcohol abuse | 0.8 (0.7, 1.0)* | 1.0 (0.9, 1.1) | |
| Buprenorphine*No AUD | Ref | Ref | |
| Methadone*Alcohol dependence | 5.5 (2.9, 10.4)*** | 1.3 (1.0, 1.6) | |
| Methadone*Alcohol abuse | 0.7 (0.3, 1.5) | 1.0 (0.8, 1.2) | |
| Methadone*No AUD | Ref | Ref | |
| XR Naltrexone*Alcohol dependence | 1.0 (0.7, 1.6) | 0.9 (0.7, 1.1) | |
| XR Naltrexone*Alcohol abuse | 0.9 (0.4, 2.2) | 1.2 (0.8, 1.7) | |
| XR Naltrexone*No AUD | Ref | Ref | |
| Oral naltrexone* Alcohol dependence | 1.4 (1.1, 1.7)** | 1.2 (1.0, 1.5) | |
| Oral naltrexone*Alcohol abuse | 1.0 (0.8, 1.4) | 1.3 (0.9, 1.9) | |
| Oral naltrexone*No AUD | Ref | Ref | |
| Psychosocial only*Alcohol dependence | Ref | Ref | |
| Psychosocial only*Alcohol abuse | Ref | Ref | |
| Psychosocial*No AUD | Ref | Ref |
AUD= alcohol use disorder; OUD= opioid use disorder; AOR=adjusted odds ratio; CI= confidence interval; XR= extended-release.
Models adjusted for co-occurring substance use disorders, previous OUD diagnosis, psychiatric comorbidity, medical comorbidity as measured by Charlson comorbidity index, sex, age, and insurance-year cohort.
p<.05;
p<.01,
p<.001,
p<.0001.
Relatively few significant interaction effects between treatment type and AUD severity were observed. Among persons with opioid abuse, buprenorphine was statistically less effective in persons with co-occurring alcohol abuse relative to those without AUD, and methadone was more effective in persons with co-occurring alcohol dependence relative to those without AUD. Among those with opioid dependence, buprenorphine appeared to be more effective in those with co-occurring alcohol dependence relative to those without AUD. AUD severity did not appear to alter the effectiveness of XR naltrexone in either OUD group; among persons with opioid abuse, oral naltrexone was more effective among persons with co-occurring alcohol dependence relative to those without AUD.
As statistical significance does not always connote clinical significance, we illustrated modeled prevalence of six-month treatment retention adjusted for these and covariate effects to evaluate for clinically meaningful differences between groups. Retention patterns for those with opioid abuse are illustrated in Figure 1a and patterns for those with opioid dependence are illustrated in Figure 1b. Similar rank-ordering of retention by treatment type was observed regardless of OUD or AUD severity: OAT with either buprenorphine or methadone was associated highest retention prevalence, XR naltrexone was associated with lower retention compared to OAT but higher retention compared to oral naltrexone or psychosocial treatment.
Figure 1a.
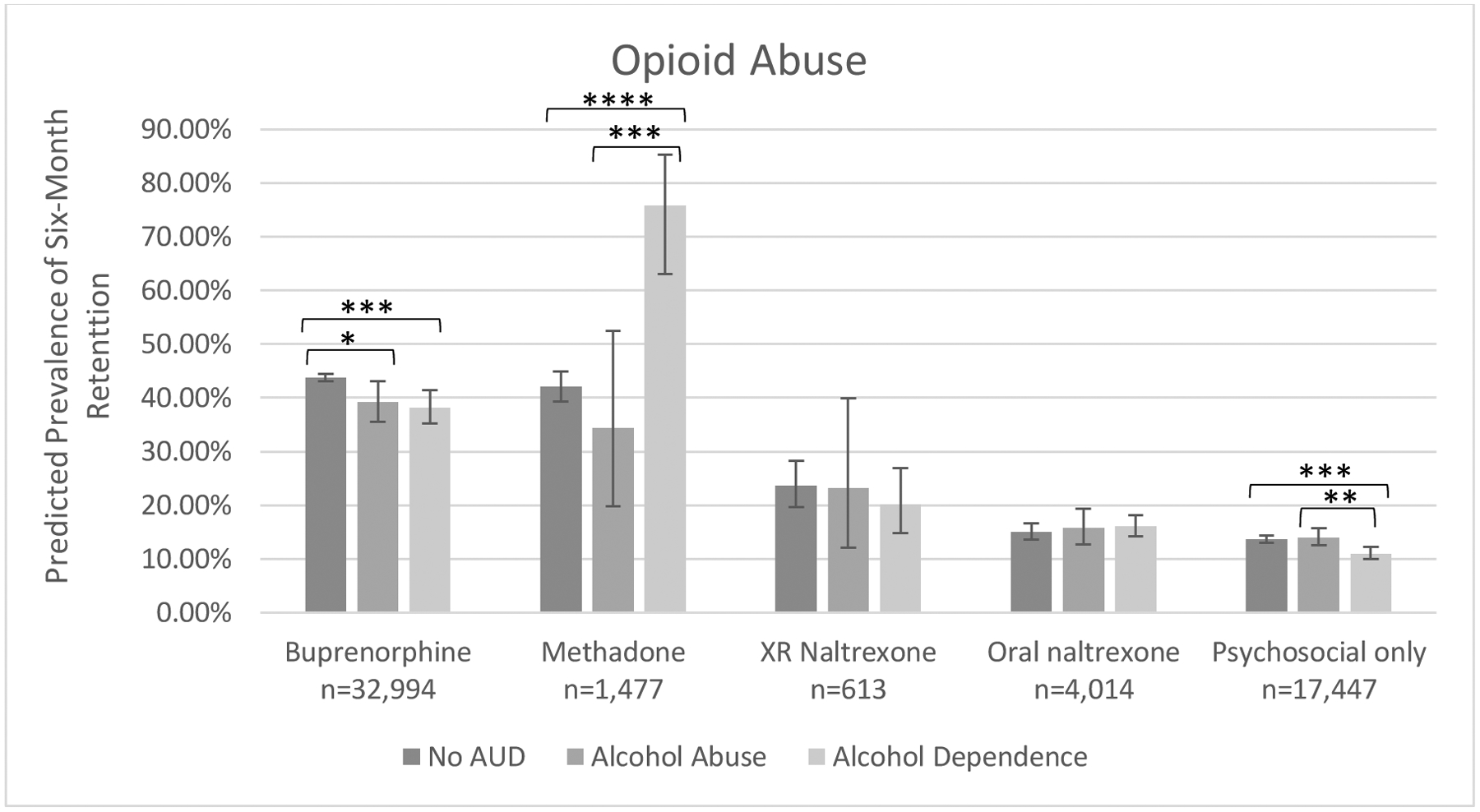
Predicted prevalence of six-month treatment retention by among persons with opioid abuse by treatment type and co-occurring alcohol use disorder severity.
Model adjusted for alcohol use disorder disease severity, opioid use disorder treatment type, disease severity*treatment type, sex, age, insurance-year cohort, previous opioid use disorder diagnosis, comorbid sedative, cocaine, stimulant, cannabis and other substance use disorder(s), comorbid mood, anxiety, psychotic and personality disorder(s), and medical comorbidities.
*p<.05, ***p<.001, ****p<.0001.
Figure 1b.
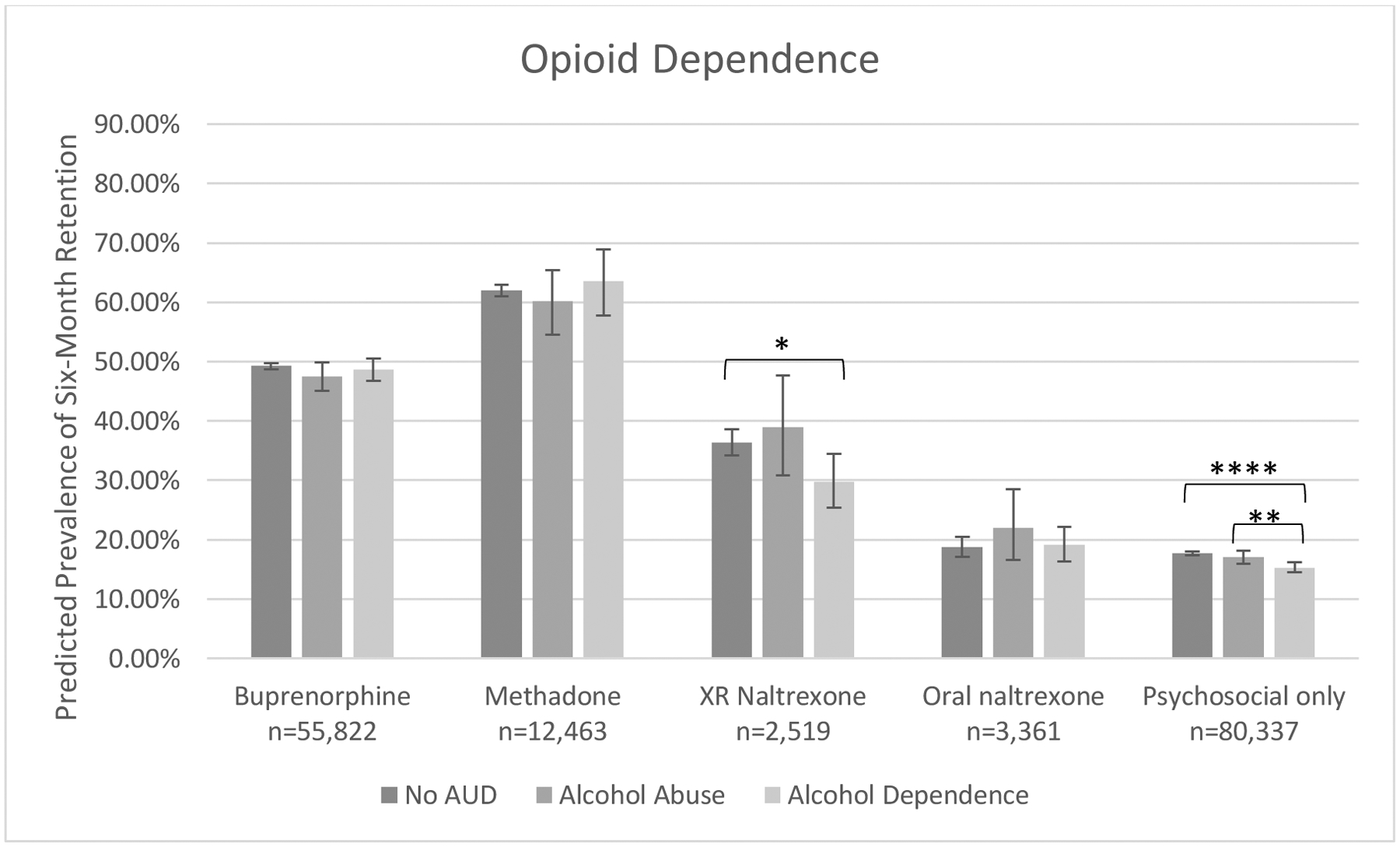
Predicted prevalence of six-month treatment retention by among persons with opioid dependence by treatment type and co-occurring alcohol use disorder severity.
Model adjusted for alcohol use disorder disease severity, opioid use disorder treatment type, disease severity*treatment type, sex, age, insurance-year cohort, previous opioid use disorder diagnosis, comorbid sedative, cocaine, stimulant, cannabis and other substance use disorder(s), comorbid mood, anxiety, psychotic and personality disorder(s), and medical comorbidities.
**p<.01, ***p<.001, ****p<.0001.
Co-occurring alcohol abuse or dependence did not decrease the effectiveness of buprenorphine among those with opioid dependence; within the opioid abuse group, co-occurring alcohol abuse or dependence was associated with slightly lower retention compared to absence of AUD. Co-occurring alcohol abuse or dependence was not associated with decreased effectiveness of methadone among either opioid severity group, although wide confidence intervals for retention prevalence estimates within the opioid abuse subgroup limited interpretation of findings. Co-occurring alcohol abuse or dependence was not associated with increased effectiveness of XR naltrexone in the opioid abuse subgroup, although wide confidence intervals were noted. Among those with opioid dependence, co-occurring alcohol dependence was associated with decreased effectiveness of XR naltrexone relative to those with alcohol abuse or no AUD. Co-occurring alcohol dependence was associated with decreased effectiveness of psychosocial treatment alone in both opioid severity groups, but the absolute differences in prevalence estimates compared to those with alcohol abuse or without AUD were fewer than 4%.
4. Discussion:
In this large, nationally representative cohort of persons with OUD, we found that for those with co-occurring alcohol abuse or dependence, treatment with buprenorphine or methadone was associated with greatest likelihood of six-month treatment retention, but were disproportionately under-prescribed.
To our knowledge, this is the first study to compare associations between OUD treatments and an indicator of treatment outcome in persons with OUD and co-occurring AUD. As FDA indications for OUD medications are specified for opioid dependence, we focus our interpretation of results on this subgroup. However, it is worth noting that in our database, persons with opioid abuse without co-occurring AUD were 40% more likely to receive treatment with buprenorphine than their opioid dependent counterparts. The finding that persons with more severe disease were substantially less likely to receive a highly effective evidence-based treatment was surprising, and warrants additional investigation in future studies.
In persons with the most severe forms of OUD and AUD—that is, those with opioid and alcohol dependence—OAT was superior to other treatments: buprenorphine was associated with 49% retention prevalence at six months and methadone was associated with 64% retention, compared to 30% for XR naltrexone, 19% for oral naltrexone and 15% for psychosocial treatment alone. Importantly, retention rates for OAT were similar in those with both opioid dependence and alcohol dependence to those with opioid dependence without co-occurring AUD, indicating that co-occurring AUD does not dramatically decrease the effectiveness of treatment with either buprenorphine or methadone among persons with opioid dependence.
Despite its superiority for treatment retention, we found that persons with opioid and alcohol dependence were significantly less likely to receive OAT compared to their counterparts without AUD: persons with opioid and alcohol dependence were approximately 35% less likely to receive buprenorphine and 70% less likely to receive methadone compared to those with opioid dependence without co-occurring AUD. Further, they were also significantly less likely to receive any pharmacologic treatment relative to their opioid dependence-only counterparts—more than 60% of persons with concurrent opioid and alcohol dependence received no medication treatment. These findings are especially concerning given the high disease burden associated with the this group: persons with both opioid and alcohol dependence were more likely to have other SUD, psychiatric, and other medical comorbidities relative those without a comorbid AUD diagnosis. In a previous study, we found a similar phenomenon- adolescents with OUD had higher psychiatric comorbidities compared to their adult counterparts, yet were significantly less likely to receive pharmacologic treatment despite medication treatment being more strongly associated with treatment retention compared to psychosocial treatment alone (Mintz et al., 2020). These results illuminate a significant treatment implementation gap and highlight the need to improve the provision of care for the most severely ill populations.
The low rate of OAT prescribed to persons with OUD and co-occurring AUD is likely in part be due to the relatively higher rates of naltrexone prescriptions we observed in that group. Among persons with opioid dependence, persons with co-occurring alcohol dependence were almost twice as likely to receive XR naltrexone and almost four times as likely to receive oral naltrexone relative to those without AUD. Though the nature of administrative claims data does not allow us to know for which substance use disorder a particular medication is being prescribed, our findings regarding naltrexone prevalence among persons with co-occurring AUD likely reflect that naltrexone carries FDA approval for both disorders and perhaps a subsequent belief among practitioners that naltrexone may therefore be particularly effective among persons with OUD and AUD. Our results indicate, however, that naltrexone does not confer a greater protective effect on treatment retention for persons with opioid and alcohol dependence, and XR naltrexone may be less effective in persons with both opioid and alcohol dependence relative to those with opioid dependence alone. Even for the subgroup for whom AUD was more severe than OUD- that is, those with opioid abuse and alcohol dependence- retention prevalence with either formulation of naltrexone were not superior to those without AUD.
The higher prevalence of naltrexone treatment among persons with opioid and alcohol dependence relative to persons with opioid dependence only may also reflect provider concern regarding overdose risk. As both OAT and alcohol can act as central nervous system depressants if consumed in excess, it is possible providers may hesitate to prescribe OAT in persons with OUD and AUD due to fears of increasing overdose risk. Our group recently showed, however, that buprenorphine is associated decreased risk of alcohol-related acute events as measured by emergency room or hospital visits in persons with OUD and AUD relative to no treatment (Xu et al., 2021), providing further evidence that OAT is safe and effective in this population.
Thus, our finding that co-occurring AUD was most strongly associated with decreased likelihood of receipt of the most effective medications, as well as receipt of any medication treatment highlights a significant implementation treatment gap in the high-risk population of persons with OUD and AUD.
4.1. Limitations
The use of administrative data offers the opportunity to capture a large sample size in a real-world setting and increase our knowledge of an understudied population, but is not without limitations. Covariates were limited to those provided in the data set; there may be important confounding variables for which we could not adjust. For example, race/ethnicity was not provided for the commercial insurance database and thus was not included as a covariate in our analyses. As our main sample was an OUD population, we focused our treatment types on medications approved for OUD, and thus we did not examine the effectiveness of other pharmacologic treatment approved for AUD such as disulfiram or acamprosate. Whether co-administration of these medications with OUD medication treatment in persons with OUD and AUD is affects treatment outcomes an important area of future research. It is important to note our sample was restricted to those with an OUD diagnosis and treatment claim who had received an AUD diagnosis prior to or within the first week of the OUD treatment episode that was analyzed. As only a minority of persons with substance use disorders receive treatment (Substance Abuse and Mental Health Services Administration, 2019) our results may not generalize to all persons with OUD and AUD. Finally, treatment retention does not necessarily indicate sobriety; however, given the growing data indicating treatment retention is correlated with increased likelihood of sobriety (Kakko et al., 2003; Mattick et al., 2014) and that six-month treatment retention in persons taking OAT in particular may be correlated with decreased risk of overdose (Wakeman et al., 2020), treatment retention remains an important proxy for treatment success.
5. Conclusions
Pharmacologic treatment, especially buprenorphine and methadone, is associated with improved six-month treatment retention in persons with opioid and alcohol dependence. Despite their effectiveness, persons with opioid and alcohol dependence are less likely to receive buprenorphine or methadone compared to persons with opioid dependence alone, highlighting a need to improve implementation of evidence-based treatment in this particularly high-risk population.
Supplementary Material
Highlights:
Opioid agonist therapy improves treatment retention in persons with OUD and AUD.
Co-occurring AUD does not decrease effectiveness of opioid agonist therapy.
Opioid agonist therapy is under-prescribed to persons with OUD and AUD.
Role of Funding
This work was supported by the following: K12DA041449-03 (CMM, LJB); NIH R25 MH112473-01 (KYX); NIH R21 DA04744 (RAG); Saint Louis University Research Institute (RAG); NIAAA R21 AA024888 (SMH), NIAAA R01 AA029308 (SMH), SAMHSA H79TI082566 (CMM, SMH); Center for Administrative Data Research at Washington University (JMS). The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ). Research for this publication was also supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsible of the authors and does not necessarily represent the official view of the NIH.
IBM Watson Health and MarketScan are trademarks of IBM Corporation in the United States, other countries or both.
Abbreviations:
- OUD
opioid use disorder
- AUD
alcohol use disorder
- OAT
opioid agonist therapy
- ICD
International Classification of Diseases
- HCPCS
Healthcare Common Procedure Coding System
- CPT
Current Procedural Terminology
- NDC
National Drug Codes
- SUD
substance use disorder
Footnotes
Conflict of Interest
Dr. Bierut is listed as an inventor on Issued U.S. Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction; Dr. Bierut is a Speaker Bureau member for Imedex. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR, 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295(17), 2003–2017. [DOI] [PubMed] [Google Scholar]
- Bogdanowicz KM, Stewart R, Broadbent M, Hatch SL, Hotopf M, Strang J, Hayes RD, 2015. Double trouble: Psychiatric comorbidity and opioid addiction-all-cause and cause-specific mortality. Drug Alcohol Depend 148, 85–92. [DOI] [PubMed] [Google Scholar]
- Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA, 2015. Risk Factors for Relapse and Higher Costs Among Medicaid Members with Opioid Dependence or Abuse: Opioid Agonists, Comorbidities, and Treatment History. J Subst Abuse Treat 57, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Zador D, 1996. Fatal heroin ‘overdose’: a review. Addiction 91(12), 1765–1772. [DOI] [PubMed] [Google Scholar]
- Dole VP, Nyswander M, 1965. A Medical Treatment for Diacetylmorphine (Heroin) Addiction. A Clinical Trial with Methadone Hydrochloride. JAMA 193, 646–650. [DOI] [PubMed] [Google Scholar]
- Friedmann PD, Wilson D, Nunes EV, Hoskinson R Jr., Lee JD, Gordon M, Murphy SM, Bonnie RJ, Chen DT, Boney TY, O’Brien CP, 2018. Do patient characteristics moderate the effect of extended-release naltrexone (XR-NTX) for opioid use disorder? J Subst Abuse Treat 85, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Bagley SM, Rodean J, Silverstein M, Levy S, Larochelle MR, Samet JH, Zima BT, 2018. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. JAMA Pediatr 172(11), 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Donovan DM, Huang Z, 2010. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat 39(2), 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG, Hatch-Maillette M, Jelstrom E, Wiest K, McLaughlin P, Ling W, 2016. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111(4), 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D, 2017. Chronic pain among patients with opioid use disorder: Results from electronic health records data. J Subst Abuse Treat 77, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE, 1995. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend 40(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC, 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311(18), 1889–1900. [DOI] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M, 2003. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet 361(9358), 662–668. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist J, Sundquist K, 2016. Alcohol Use Disorder and Mortality Across the Lifespan: A Longitudinal Cohort and Co-relative Analysis. JAMA Psychiatry 73(6), 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M, 2018. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320(8), 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med 169(3), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, Rotrosen J, 2018. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391(10118), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2009. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev(3), CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev(2), CD002207. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A, 2011. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev(4), CD001333. [DOI] [PubMed] [Google Scholar]
- Mintz CM, Presnall NJ, Sahrmann JM, Borodovsky JT, Glaser PEA, Bierut LJ, Grucza RA, 2020. Age disparities in six-month treatment retention for opioid use disorder. Drug Alcohol Depend 213, 108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP, 2019. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend 200, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Manzato E, Leonardi C, Lucchini A, 2008. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry 32(8), 1867–1872. [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Wall M, Wang S, Liu SM, Blanco C, 2018. Causes of Death After Nonfatal Opioid Overdose. JAMA Psychiatry 75(8), 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, Grant BF, 2016. Nonmedical Prescription Opioid Use and DSM-5 Nonmedical Prescription Opioid Use Disorder in the United States. J Clin Psychiatry 77(6), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 357, j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, 2015. Alcohol use disorders in opioid maintenance therapy: prevalence, clinical correlates and treatment. Eur Addict Res 21(2), 78–87. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Kahan M, Ross S, 2008. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat 34(2), 215–223. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020. Medications for Opioid Use Disorder. Treatment Improvement Protocol (TIP) 63. Publication No. PEP20-02-01-006.Rockville, MD: Substance Abuse and Mental Health Services Administration, 2020. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K, Krajci P, Kunoe N, 2017. Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry 74(12), 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Blanco C, 2020. Medications for opioid use disorders: clinical and pharmacological considerations. J Clin Invest 130(1), 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, Sanghavi DM, 2020. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open 3(2), e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, Grey T, Johnson EM, Lee LK, Passik SD, Peppin J, Porucznik CA, Ray A, Schnoll SH, Stieg RL, Wakeland W, 2011. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med 12Suppl 2, S26–35. [DOI] [PubMed] [Google Scholar]
- White AM, Castle IP, Hingson RW, Powell PA, 2020. Using Death Certificates to Explore Changes in Alcohol-Related Mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res 44(1), 178–187. [DOI] [PubMed] [Google Scholar]
- Williams AR, Samples H, Crystal S, Olfson M, 2020. Acute Care, Prescription Opioid Use, and Overdose Following Discontinuation of Long-Term Buprenorphine Treatment for Opioid Use Disorder. Am J Psychiatry 177(2), 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kariisa M, Seth P, Smith H.t., Davis NL, 2020. Drug and Opioid-Involved Overdose Deaths - United States, 2017–2018. MMWR Morb Mortal Wkly Rep 69(11), 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Votaw VR, Vowles KE, Kranzler HR, 2018. Opioid Misuse as a Predictor of Alcohol Treatment Outcomes in the COMBINE Study: Mediation by Medication Adherence. Alcohol Clin Exp Res 42(7), 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, 2018. Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcohol Clin Exp Res 42(3), 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KY, Presnall N, Mintz CM, Borodovsky JT, Bhat NR, Bierut LJ, Grucza RA, 2021. Association of Opioid Use Disorder Treatment With Alcohol-Related Acute Events. JAMA Netw Open 4(2), e210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


