Abstract
Purpose of Review
Low anterior resection syndrome is a highly prevalent condition that can develop after anal sphincter-sparing surgery for rectal cancer and impair quality of life. In this review, we summarize the major features and pathophysiology of this syndrome and discuss treatment approaches.
Recent Findings
Quality of life correlates significantly with severity of low anterior resection syndrome. Prompt assessment and initiation of therapy are essential to rehabilitating damaged mechanical and neural structures. Anorectal manometry demonstrates a global decrease in sphincteric function postoperatively, though in many patients, function does recover. Transanal irrigation, pelvic floor rehabilitation, and biofeedback are the mainstays of the treatment of major LARS. Definitive stoma can be considered in therapy refractory LARS > 2 years.
Summary
The development of low anterior resection syndrome likely involves an interplay between mechanical and neural pathways. Clinically, patients present at varying levels of severity, and scoring systems are available to help assess patient symptoms and guide therapy. Treatment approaches range from conservative therapies to biofeedback and sacral nerve stimulation. Future randomized controlled trials aimed at risk stratification of patients and development of severity-based treatment algorithms are warranted.
Keywords: Low anterior resection syndrome, Internal anal sphincter, Fecal incontinence, Urgency, Rectal cancer
Introduction
Colorectal cancer is the third most commonly diagnosed cancer worldwide, and rectal cancer accounts for over one-third of cases, with an age-standardized incidence rate of 7.7 per 100,000 [1]. Low anterior resection of the rectum with total mesorectal excision for the treatment of rectal cancer allows patients to avoid the permanent colostomy that is associated with an abdominoperineal resection [2]. However, a potential consequence of this surgery is low anterior resection syndrome (LARS). The prevalence of LARS is high, with approximately 80–90% of individuals who undergo sphincter-preserving surgery experiencing varying degrees of severity [3].
LARS is difficult to fully define but consists of any altered defecation status occurring after an anal sphincter-preservation operation for rectal cancer. Symptoms include fecal incontinence, urgency, and incomplete evacuation. Short-term symptoms (resolution within 6–12 months after anal sphincter-sparing surgery) are usually due to short-lived neorectal irritability in the postoperative period. Long-term symptoms of LARS (extending more than 12 months after surgery) are more likely due to permanent changes [4]. Around 46–49% of patients who undergo anal sphincter-sparing surgery still experience symptoms of LARS at a follow-up period of 11.1–14.6 years [5–7].
Clinical Manifestations
There are two categories of LARS. The first consists of fecal urgency, incontinence, and increased frequency. The second category involves constipation, feelings of incomplete evacuation, and bowel-emptying difficulties. Some patients report features of both categories, either alternating between the two patterns or experiencing both simultaneously.
Quality of Life
Types of bowel dysfunction that have been found to majorly impact quality of life include fecal incontinence, increased bowel frequency, clustering (i.e., a bowel movement within 1 h of the last bowel movement), and urgency [4, 8]. For this reason, the LARS score places heavier weights on clustering and frequency. A multicenter European study of 1061 patients showed a correlation between decreasing quality of life and higher LARS score, especially if diarrhea-predominant LARS was reported [9]. However, even though patients with minor LARS (as defined by the LARS score detailed in a later section) were found to have a significantly worse quality of life than those with no LARS, the differences were too small to be considered clinically relevant. Patients with major LARS were found to have a clinically and statistically significant worsened quality of life compared with those with minor or no LARS, so the former is a group that should be targeted and followed closely for management.
Risk Factors
Neoadjuvant and adjuvant radiotherapy have consistently been shown to be risk factors for LARS, especially major LARS, even in those with a larger remnant rectum [10–12]. Radiation is thought to lead to poorer rectal compliance and thus increased frequency and urgency, with a study showing that radiation combined with total mesenteric excision was associated with a higher frequency of defecation and significantly reduced rectal compliance at 4 and 12 months postoperatively compared with total mesenteric excision alone [13].
Low tumor height and thus low anastomotic height are also associated with postoperative development of LARS, with a 46% risk of major LARS in those with < 4 cm of remnant rectum compared with 10% risk in those with ≥ 4 cm of remnant rectum [10, 11]. A total mesenteric excision at the time of the low anterior resection is an independent risk factor for LARS (2-fold increased odds), although this may be related to low anastomotic height [14, 15]. Lastly, a complication of the anastomosis, such as an anastomotic leak, has been found to be associated with 3.5-fold increased odds of major LARS [14, 16].
There has been conflicting evidence with regard to age and the risk of major LARS. One study found that having anal sphincter-sparing surgery at the age of 70 was associated with increased odds of major LARS [5], while other studies did not find any association between age and risk of major LARS [10, 16]. There is no known association between gender and development of LARS [10].
Etiology
Normal Physiology
The anal sphincter is extremely important for fecal continence, and the internal anal sphincter (IAS) is responsible for 55– 75% of resting anal tone [17, 18]. The conjoint longitudinal muscle, an extension of the rectal longitudinal muscle, penetrates into the external sphincter and fixes the anorectum to the pelvis [19–21]. During defecation, the conjoint longitudinal muscle and the rectococcygeus muscle, a strong smooth muscle on the dorsal side of the rectum attached to the coccyx, contract to shorten the anal canal and rectum and assist in fecal evacuation [22]. The efferent and afferent visceral nerve supply to the rectum and the IAS comes from the inferior hypogastric and, subsequently, the rectal plexus. The nerve branch to the rectal wall runs below the peritoneal reflection, and the nerve branch to the IAS runs along the levator ani muscle and enters at the dentate line [23].
During the basal phase, the rectum is mostly empty and may contain a small amount of feces without conscious awareness. Defecation is thought to be initiated by a colonic contraction that delivers fecal material into the rectum, causing an urge to defecate [24]. When the intrarectal pressure increases due to the accumulation of feces, the IAS relaxes an automatic process that is termed the rectoanal inhibitory reflex. If it is not a suitable time to defecate, the external anal sphincter and pelvic floor muscles contract voluntarily, and the rectum performs periodic retrograde motor propagation in order to decrease intrarectal pressure and delay defecation [25, 26].
Pathophysiology
The exact etiology of LARS remains unclear. Fecal incontinence may result from direct structural damage to the IAS during intersphincteric resection or secondary damage by insertion of an anastomotic device via the anus during the low anterior resection [27]. Damage to the function of the IAS may also be caused by damage to its nerve supply, especially if the surgical approach reaches the posterolateral side of the prostate (in men) where both the sympathetic and parasympathetic nerve fibers enter the rectal wall [23, 28]. A cohort study of 21 patients undergoing a low anterior resection measured resting IAS pressures before and after surgery and found that those with lower postoperative resting pressures more frequently had LARS, supporting the notion that fecal incontinence related to IAS damage may be associated with the etiology of LARS [29].
The conjoint longitudinal muscle can also be damaged during the surgical dissection of the intersphincteric space during a low anterior resection. Additionally, the rectococcygeus muscle is often divided to obtain a sufficient horizontal margin anally, thus damaging the function of the rectococcygeus muscle [23]. Both can impair the functional role of shortening the rectum during fecal evacuation. Furthermore, curative resection of rectal cancer requires removal of most of the rectum, which reduces the fecal continence that the rectum provides [23].
Poor compliance due to loss of rectal volume may lead to an increased, false urge to defecate and a reduction in maximum tolerable rectal volume after low anterior resection. In the aforementioned cohort study of 21 patients, there was a correlation between the length of the remaining rectum and the ratio of postoperative to preoperative maximum resting pressure, suggesting that the lower the resection, the more incontinence a patient would exhibit [29]. Another study of 232 patients found that a worsened ability to differentiate flatus from feces was associated with decreased anal verge-anastomotic distance [30]. One study of 32 patients found improved outcomes in those patients with ≥ 4 cm of remaining rectum, including retention of the rectoanal inhibitory reflex and increased rectal capacity, compared with those with < 4 cm of remaining rectum [31]. Finally, a study of 30 patients performed rectal volumetry and symptom scoring prior to and 12 months after surgery and found that patients with a low anastomosis had a lower maximum tolerable volume and reported more problems with complete bowel emptying compared with those with a high anastomosis [13]. However, the physical form of the neorectum has not been strongly associated with LARS, as efforts to reduce symptoms by creating a colonic J-pouch or side-to-end anastomosis have not consistently shown benefit (Fig. 1) [32]. Longer term data are needed to help support the creation of a J-pouch, which is often employed in clinical practice.
Fig. 1.
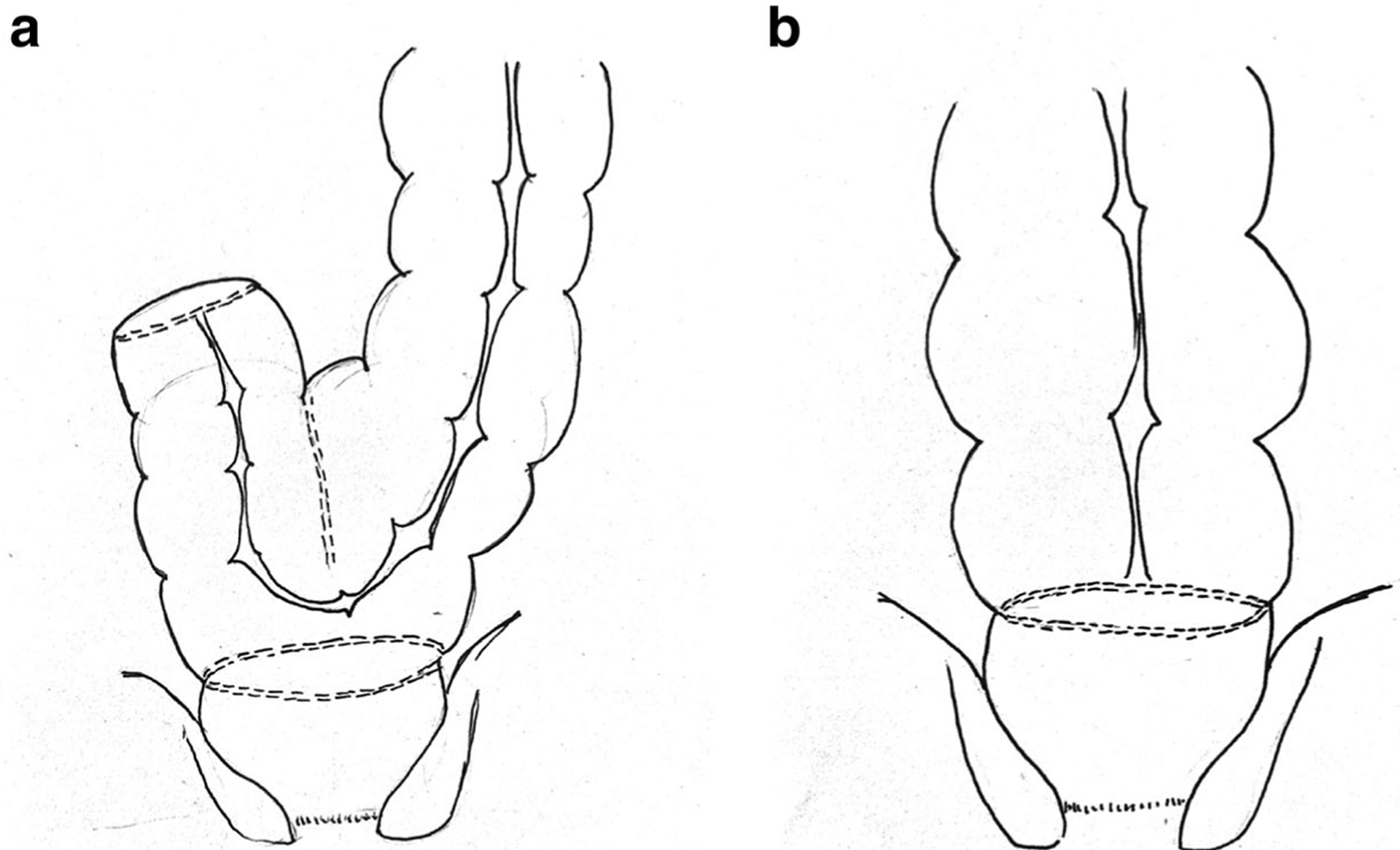
a Colonic J-pouch fashioned from the sigmoid colon to form the proximal portion of the coloanal anastomosis. b End-to-end anastomosis between the sigmoid colon and the anal canal following a very low anterior resection of the rectum
The rectoanal inhibitory reflex is mediated by the extrinsic nerves from the spinal cord, which can also be damaged during a low anterior resection, leading to bowel dysfunction [33, 34]. A study of 18 patients found that loss of the recto-anal inhibitory reflex was an independent predictor of poor function at 12 months after low anterior resection [35]. Additionally, during the total mesorectal excision of a low anterior resection, the nerve supply to the rectum and IAS are at high risk of damage [36]. One study of 23 patients divided those who underwent a low anterior resection into those with and without postoperative incontinence and found a decrease in sensitivity in the lower anal canal at the dentate line in those with incontinence, which may be related to nerve damage [37]. Spastic hypermotility of the neorectum, which may be associated with extrinsic denervation [38, 39], has been correlated with degree of defecatory urgency [40]. Additionally, major LARS has been associated with a significant increase in postprandial pressure in the neorectum, suggesting that LARS may be caused by changes from neural damage [41].
Management
Testing
Two validated patient questionnaires can be used to evaluate for LARS. The Memorial Sloan Kettering Cancer Center Bowel Function Instrument (MSKCC-BFI) is an 18-item validated scoring instrument created in 2004 that can be used to evaluate bowel function after sphincter-preserving surgery [42]. The instrument surveys factors surrounding diet; number, form, quality, and timing of bowel movements; sensation of flatus; anti-diarrheal medication usage; and fecal incontinence in the previous 4 weeks. Scores range from 18 to 90, with higher scores indicating better levels of bowel function.
A second scoring system, the LARS score, is a 5-item validated questionnaire created in 2012 by Emmertsen et al. in a Danish population that assesses for bowel function after sphincter-preserving surgery for rectal cancer (Table 1) [8, 43, 44]. The LARS score covers incontinence of flatus, incontinence of liquid stools, frequency of bowel movements, clustering of stools, and urgency. The score ranges from 0 to 42, with 0–20 signifying no LARS, 21–29 signifying minor LARS, and 30–42 signifying major LARS. Those with a score ≥ 30 (major or severe LARS) were found to have a major impact on their quality of life. The LARS score has since been validated in several languages, including English, Chinese, Lithuanian, Swedish, Spanish, and German [43–48]. This scoring system can be used to stratify patients based on the severity of their symptoms in order to guide therapy. The MSKCC-BFI allows for a more comprehensive and thorough evaluation of LARS but may be of less practical use in a clinical setting compared with the LARS score due to the length of the questions and the tedious nature of the MSKCC-BFI.
Table 1.
The low anterior resection syndrome bowel function questionnaire and scoring system
| Do you ever have occasions when you cannot control your flatus (wind?) | |
| •No, never | 0 points |
| •Yes, less than once per week | 4 points |
| •Yes, at least once per week | 7 points |
| Do you ever have any accidental leakage of liquid stool? | |
| •No, never | 0 points |
| •Yes, less than once per week | 3 points |
| •Yes, at least once per week | 3 points |
| How often do you open your bowels? | |
| •More than 7 times per day (24 h) | 4 points |
| •4–7 times per day (24 h) | 2 points |
| •1–3 times per day (24 h) | 0 points |
| •Less than once per day (24 h) | 5 points |
| Do you ever have to open your bowels again within 1 h of the last bowel opening? | |
| •No, never | 0 points |
| •Yes, less than once per week | 9 points |
| •Yes, at least once per week | 11 points |
| Do you ever have such a strong urge to open your bowels that you have to rush to the toilet? | |
| •No, never | 0 points |
| •Yes, less than once per week | 11 points |
| •Yes, at least once per week | 16 points |
| Total Score: | |
| Interpretation: | |
| 0–20, No LARS | |
| 21–29, Minor LARS | |
| 30–42, Major LARS |
Anorectal manometry evaluates anal sphincter function and rectal capacity objectively and uses a balloon catheter and pressure sensor to record resting pressure, maximum squeezing pressure, rectoanal inhibitory reflex, rectal capacity, and compliance. Anorectal manometry is not required for the diagnosis of LARS but can be used to guide and monitor response to therapy [49]. The results of anorectal manometry have been found to be related to severity of symptoms based on the LARS score [50, 51]. A cohort study of 30 patients who underwent sphincter-sparing surgery for rectal cancer found that the anal resting pressure and maximum tolerated rectal volume were lower after low anterior resection as compared with baseline values, with an absent rectoanal inhibitory reflex in 80% of patients at 1 month after surgery that correlated with severity of symptoms [51]. At 6 months postoperatively, the severity of symptoms as well as manometric parameters tended to recover. In another cohort of 83 patients, resting pressure, rectal sensitivity, and compliance were significantly lower in patients with major LARS compared with patients with minor or no LARS, confirming that manometry parameters correlate with severity of symptoms [50]. A study by Kakodkar et al. found that preoperative anorectal manometry results could not adequately predict postoperative occurrence of LARS, though absence of RAIR was associated with unsatisfactory outcome in multivariate analysis [35].
Endoscopic rectal ultrasound can be used to assess the structure of the sphincter complex and pelvic floor. A study of 20 patients who underwent transanal total mesenteric excision for rectal cancer performed endoscopic ultrasound six to 36 months after surgery to evaluate the sphincter muscles [52]. Four patients (20%) were found to have a partial laceration of the internal anal sphincter, and five had increased or decreased puborectal angulation in the evacuation movement. However, numbers were too small to predict major LARS, as only 10% reported major symptoms 1 year after surgery.
Fecoflowmetry is a useful tool for assessing postoperative anorectal motor function by studying the fecal flow rate (the product of rectal detrusor action against anorectal outlet resistance) through recorded curves representing the changes that occur in flow against time [53]. While monitoring anorectal pressure, 1000 mL of normal saline enema (simulating diarrheic stool) is administered to an individuals in the left lateral position. Additional saline is then given until no longer tolerable. The individual is then asked to hold the enema as long as possible. When no longer possible, the individual is placed on a fecoflowmeter commode and asked to defecate. The defecated volume, flow time, mean and maximum flow rates, and time to maximum flow are then reported in order to obtain a defecation flow curve. Fecoflowmetry has been found to be comparable with symptom severity scores and anorectal manometry in evaluating anorectal motor function in postoperative patients [54].
Therapy
First-line therapy for LARS includes dietary modifications, fiber, and constipating agents (Fig. 2). It is recommended to avoid foods that may soften stools, such as caffeine, citrus, spicy foods, and alcohol, and increase dietary fiber (e.g., methylcellulose) [55–57]. In the Nurses’ Health Study of 58,330 women (7056 incident cases of fecal incontinence), women in the highest quintile of fiber intake (25 g/day) had an 18% decrease in risk of fecal incontinence compared with those in the lowest quintile of fiber intake (13.5 g/day; adjusted hazard ratio, 0.82; 95% confidence interval, 0.76–0.89) [58]. The impact of fiber supplementation was greatest with liquid stool fecal incontinence, in which women with the highest intake of fiber had 31% lower risk of fecal incontinence compared with women with the lowest fiber intake. Medication therapy is mostly comprised of those that prevent excess motility of the colon. Antidiarrheals, such as loperamide and atropine, may be used to reduce colonic motility and possibly increase IAS tone. A 5-HT3 antagonist, such as ramosetron, may reduce bowel frequency and urge fecal incontinence and strong postprandial contractions of the neorectum [59].
Fig. 2.
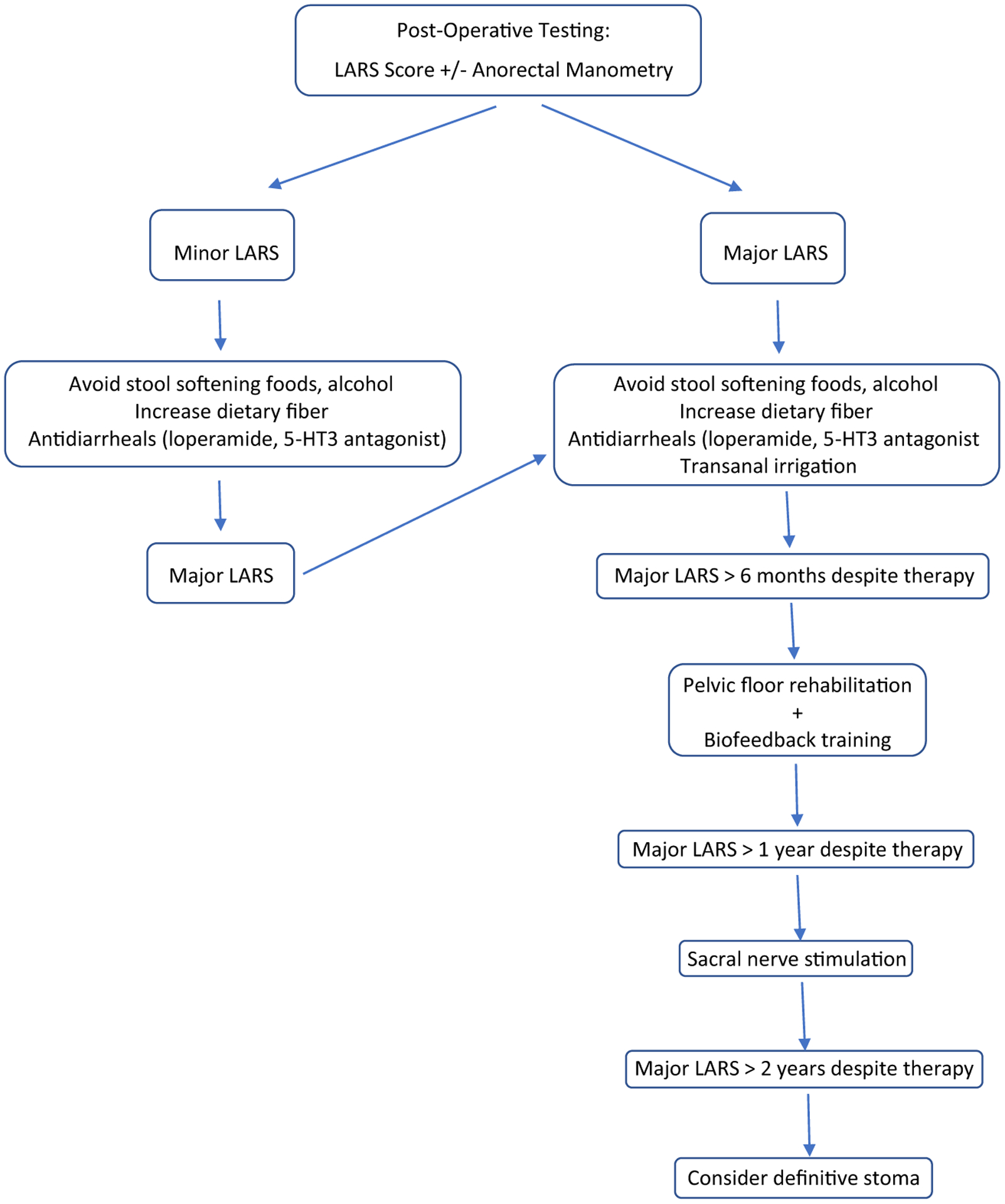
Treatment algorithm for low anterior resection syndrome
Large volume enemas are also recommended as first-line therapy for LARS, especially if major LARS is reported. Transanal irrigation has been found to improve the LARS score, fecal incontinence, defecatory urgency, and reduce bowel frequency [3, 60]. This effect is partly due to a simple mechanical wash-out effect that allows time before the patient has further need to evacuate. In addition, the regular management of bowel function may rehabilitate colonic motility, as enemas above 250 mL have been found to generate colonic mass movements and other colonic functional responses [61, 62]. A randomized controlled trial of 23 patients found that transanal irrigation significantly improved major LARS in 80% of patients, while pretibial nerve stimulation improved LARS in only 38% [63].
If one continues to have major LARS despite the above therapy for > 6 months, the second-line approach is pelvic floor rehabilitation (Kegel exercise training and anal sphincter exercise training) with biofeedback training. Pelvic floor rehabilitation has been reported to be effective in improving fecal incontinence, stool frequency, and quality of life [64, 65]. In a retrospective analysis of 61 patients with LARS, biofeedback therapy was associated with significant improvements in incontinence scale scores, number of daily bowel movements, and anorectal manometry data (maximum resting pressure, maximum squeeze pressure, and rectal capacity) [66]. A single-center randomized controlled trial in China found that biofeedback training with pelvic floor muscle exercises had higher MSKCC-BFI scores 3 months postoperatively compared with pelvic floor muscle exercises alone and controls [67].
If a patient fails first- and second-line therapy for major LARS after 1 year, sacral nerve stimulation is recommended [3]. The mechanism of sacral nerve stimulation is thought to be via the afferent nerves from the anorectum and central levels, decreasing antegrade colonic motility and increasing retrograde activity [68, 69]. FDA approved for use in refractory fecal incontinence, it may help improve the function of the remaining bowel in this patient population. Implantation is a two-stage procedure, with a diagnostic phase and permanent implantation phase [70]. In the diagnostic phase, a quadripolar electrode is implanted after finding a very satisfactory S3 response with corresponding toe twitch. After subcutaneous tunneling of the electrode to the gluteal pocket site of the neurostimulator, an external stimulator is connected, and stimulation is set at a sensory threshold. Patients then complete a bowel diary over a 2-week test stimulation period, and permanent implantation is considered for patients with > 50% improvement in fecal incontinence. Croese et al. demonstrated at least a 75% improvement in fecal incontinence, even in patients previously unresponsive to biofeedback.
Patients with more persistent or intractable symptoms and/or impaired quality of life from major LARS beyond 2 years of therapy may require a definitive stoma, typically permanent colostomy [3, 71]. Previous studies demonstrate that creation of a permanent stoma for bowel dysfunction occurs in 1.8–3.2% who undergo anal sphincter-sparing surgery [72, 73].
Conclusions
LARS is a syndrome of altered bowel function, commonly including fecal or gas incontinence, urgency, clustering, and frequency, that occurs after anal sphincter-sparing surgery for the treatment of rectal cancer. The prevalence of LARS after low anterior resection is high at 80–90%, and of those that develop major LARS, nearly half continue to report major symptoms at follow-up over 11 years later. Given the high correlation between major LARS and poor quality of life, these patients should be followed closely. Treatment algorithms have been suggested and include conservative therapies, biofeedback, and sacral nerve stimulation. However, randomized controlled and longer trials are needed to better target the needs of these patients.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interests.
References
- 1.Observatory TGC. Rectum. In: Sheet RF, editor. Globocan 2018. International Agency for Research on Cancer; 2019. [Google Scholar]
- 2.Monson J, Weiser M, Buie W, et al. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum. 2013;56:535–50. [DOI] [PubMed] [Google Scholar]
- 3.Martellucci J Low anterior resection syndrome: a treatment algorithm. Dis Colon Rectum. 2016;59:79–82. [DOI] [PubMed] [Google Scholar]
- 4.Emmertsen K, Laurberg S, Group RCFS. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013;100:1377–87. [DOI] [PubMed] [Google Scholar]
- 5.Sturiale A, Martellucci J, Zurli L, Vaccaro C, Brusciano L, Limongelli P, et al. Long-term functional follow-up after anterior rectal resection for cancer. Int J Color Dis. 2017;32:83–8. [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Wiltink L, Nout R, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–14. [DOI] [PubMed] [Google Scholar]
- 7.Pieniowski E, Palmer G, Juul T, et al. Low anterior resection syndrome and quality of life after sphincter-sparing rectal cancer surgery: a long-term longitudinal follow-up. Dis Colon Rectum. 2019;62:14–20. [DOI] [PubMed] [Google Scholar]
- 8.Emmertsen K, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–8. [DOI] [PubMed] [Google Scholar]
- 9.Juul T, Ahlberg M, Biondo S, Espin E, Jimenez LM, Matzel KE, et al. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum. 2014;57:585–91. [DOI] [PubMed] [Google Scholar]
- 10.Croese A, Lonie J, Trollope A, et al. A meta-analysis of the prevalence of low anterior resection syndrome and systematic review of risk factors. Int J Surg. 2018;2018:234–41. [DOI] [PubMed] [Google Scholar]
- 11.Bondeven P, Emmertsen K, Laurberg S, et al. Neoadjuvant therapy abolishes the functional benefits of a larger rectal remnant, as measured by magnetic resonance imaging after restorative rectal cancer surgery. Eur J Surg Oncol. 2015;41:1493–9. [DOI] [PubMed] [Google Scholar]
- 12.Qin Q, Huang B, Cao W, Zhou J, Ma T, Zhou Z, et al. Bowel dysfunction after low anterior resection with neoadjuvant chemoradiotherapy or chemotherapy alone for rectal cancer: a cross-sectional study from China. Dis Colon Rectum. 2017;60:697–705. [DOI] [PubMed] [Google Scholar]
- 13.Nesbakken A, Nygaard K, Lunde O. Mesorectal excision for rectal cancer: functional outcome after low anterior resection and colorectal anastomosis without a reservoir. Color Dis. 2002;4:172–6. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Gomez L, Espin-Basany E, Trenti L, et al. Factors associated with low anterior resection syndrome after surgical treatment of rectal cancer. Colorectal Dis. 2018;20(3):195–200. [DOI] [PubMed] [Google Scholar]
- 15.Ekkarat P, Boonpipattanapong T, Tantiphlachiva K, Sangkhathat S. Factors determining low anterior resection syndrome after rectal cancer resection: a study in Thai patients. Asian J Surg. 2016;39: 225–31. [DOI] [PubMed] [Google Scholar]
- 16.Hain E, Manceau G, Maggiori L, Mongin C, Prost à la Denise J, Panis Y. Bowel dysfunction after anastomotic leakage in laparoscopic sphincter-saving operative intervention for rectal cancer: a case-matched study in 46 patients using the low anterior resection score. Surgery. 2017;161:1028–39. [DOI] [PubMed] [Google Scholar]
- 17.Lestar B, Penninckx F, Kerremans R. The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Color Dis. 1989;4:118–22. [DOI] [PubMed] [Google Scholar]
- 18.Schweiger M Method for determining individual contributions of voluntary and involuntary anal sphincters to resting tone. Dis Colon Rectum. 1979;22:415–6. [DOI] [PubMed] [Google Scholar]
- 19.Courtney H Anatomy of the pelvic diaphragm and anorectal musculature as related to sphincter preservation in anorectal surgery. Am J Surg. 1950;79:155–73. [DOI] [PubMed] [Google Scholar]
- 20.Muro S, Yamaguchi K, Nakajima Y, Watanabe K, Harada M, Nimura A, et al. Dynamic intersection of the longitudinal muscle and external anal sphincter in the layered structure of the anal canal posterior wall. Surg Radiol Anat. 2014;36:551–9. [DOI] [PubMed] [Google Scholar]
- 21.Yang H Anal Anatomy. In: Yang H, ed. Hemorrhoids. Berlin: Springer, 2014:5–13. [Google Scholar]
- 22.Nout Y, Leedy G, Beattie M, et al. Alterations in eliminative and sexual reflexes after spinal cord injury: defecatory function and development of spasticity in pelvic floor musculature. Prog Brain Res. 2006;152:359–72. [DOI] [PubMed] [Google Scholar]
- 23.Koda K, Yamazaki M, Shuto K, Kosugi C, Mori M, Narushima K, et al. Etiology and management of low anterior resection syndrome based on the normal defecation mechanism. Surg Today. 2019;49: 803–8. [DOI] [PubMed] [Google Scholar]
- 24.Halls J Bowel content shift during normal defecation. Proc R Soc Med. 1965;58:859–60. [PMC free article] [PubMed] [Google Scholar]
- 25.Palit S, Lunniss P, Scott S. The physiology of human defecation. Dig Dis Sci. 2012;57:1445–64. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Welcher K. Periodic rectal motor activity: the intrinsic colonic gatekeeper? Am J Gastroenterol. 1996;91:890–7. [PubMed] [Google Scholar]
- 27.Farouk R, Duthie G, Lee P, et al. Endosonographic evidence of injury to the internal anal sphincter after low anterior resection: long-term follow-up. Dis Colon Rectum. 1998;41:888–91. [DOI] [PubMed] [Google Scholar]
- 28.Ishiyama G, Hinata N, Kinugasa Y, Murakami G, Fujimiya M. Nerves supplying the internal anal sphincter: an immunohistochemical study using donated elderly cadavers. Surg Radiol Anat. 2014;36:1033–42. [DOI] [PubMed] [Google Scholar]
- 29.Williamson M, Lewis W, Holdsworth P, et al. Decrease in the anorectal pressure gradient after low anterior resection of the rectum. A study using continuous ambulatory manometry. Dis Colon Rectum. 1994;37:1228–31. [DOI] [PubMed] [Google Scholar]
- 30.Karanijia N, Schache D, Heald R. Function of the distal rectum after low anterior resection for carcinoma. Br J Surg. 1992;79:114–6. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Park Y. Serial evaluation of anorectal function following low anterior resection of the rectum. Int J Color Dis. 1998;13:241–6. [DOI] [PubMed] [Google Scholar]
- 32.Marti W, Curti G, Wehrli H, et al. Clinical outcome after rectal replacement with side-to-end, colon-j-pouch, or straight colorectal anastomosis following total mesorectal excision: a Swiss prospective, randomized, multicenter trial (SAKK 40/04). Ann Surg. 2019;269:827–35. [DOI] [PubMed] [Google Scholar]
- 33.Meunier P, Mollard P. Control of the internal anal sphincter (manometric study with human subjects). Pflugers Arch. 1977;370: 233–9. [DOI] [PubMed] [Google Scholar]
- 34.Remes-Troche J, De-Ocampo S, Valestin J, et al. Rectoanal reflexes and sensorimotor response in rectal hyposensitivity. Dis Colon Rectum. 2010;53:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakodkar R, Gupta S, Nundy S. Low anterior resection with total mesorectal excision for rectal cancer: functional assessment and factors affecting outcome. Color Dis. 2006;8:650–6. [DOI] [PubMed] [Google Scholar]
- 36.Bharucha A, Blandon R. Anatomy and physiology of continence. In: Ratto C, Doglietto G, editors. Fecal incontinence. Springer; 2007. p. 3–12. [Google Scholar]
- 37.Tomita R, Igarashi S, Fujisaki S. Studies on anal canal sensitivity in patients with or without soiling after low anterior resection for lower rectal cancer. Hepatogastroenterology. 2008;55:1311–4. [PubMed] [Google Scholar]
- 38.Koda K, Saito N, Seike K, Shimizu K, Kosugi C, Miyazaki M. Denervation of the neorectum as a potential cause of defecatory disorder following low anterior resection for rectal cancer. Dis Colon Rectum. 2005;48:210–7. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu K, Koda K, Kase Y, Satoh K, Seike K, Nishimura M, et al. Induction and recovery of colonic motility/defecatory disorders after extrinsic denervation of the colon and rectum in rats. Surgery. 2006;139:395–406. [DOI] [PubMed] [Google Scholar]
- 40.Iizuka I, Koda K, Seike K, Shimizu K, Takami Y, Fukuda H, et al. Defecatory malfunction caused by motility disorder of the neorectum after anterior resection for rectal cancer. Am J Surg. 2004;188:176–80. [DOI] [PubMed] [Google Scholar]
- 41.Emmertsen K, Brehendahl S, Fassov J, et al. A hyperactive postprandial response in the neorectum-the clue to low anterior resection syndrome after total mesorectal excision surgery? Color Dis. 2013;15:e599–606. [DOI] [PubMed] [Google Scholar]
- 42.Temple LK, Bacik J, Savatta S, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48: 1353–65. [DOI] [PubMed] [Google Scholar]
- 43.Juul T, Battersby N, Christensen P, et al. Validation of the English translation of the low anterior resection syndrome score. Color Dis. 2015;17:908–16. [DOI] [PubMed] [Google Scholar]
- 44.Juul T, Ahlberg M, Biondo S, et al. Internal validation of the low anterior resection syndrome score. Ann Surg. 2014;259:728–34. [DOI] [PubMed] [Google Scholar]
- 45.Hou X, Pang D, Lu Q, Yang P, Jin SL, Zhou YJ, et al. Validation of the Chinese version of the low anterior resection syndrome score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients. Eur J Oncol Nurs. 2015;19:495–501. [DOI] [PubMed] [Google Scholar]
- 46.Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109: 822–7. [DOI] [PubMed] [Google Scholar]
- 47.Samalavicius N, Dulskas A, Lasinskas M, et al. Validity and reliability of a Lithuanian version of low anterior resection syndrome score. Tech Coloproctol. 2016;20:215–20. [DOI] [PubMed] [Google Scholar]
- 48.Hupkens B, Breukink S, Olde Reuver of Briel C, et al. Dutch validation of the low anterior resection syndrome score. Color Dis. 2018;20:881–7. [DOI] [PubMed] [Google Scholar]
- 49.Pales CGC, An S, Cruz JP, et al. Postoperative bowel function after anal sphincter-preserving rectal cancer surgery: risk factors, diagnostic modalities, and management. Ann Coloproctol. 2019;35: 160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihnat P, Vavra P, Prokop J, et al. Functional outcome of low rectal resection evaluated by anorectal manometry. ANZ J Surg. 2018;88: E512–6. [DOI] [PubMed] [Google Scholar]
- 51.Efthimiadis C, Basdanis G, Zatagias A, Tzeveleki I, Kosmidis C, Karamanlis E, et al. Manometric and clinical evaluation of patients after low anterior resection for rectal cancer. Tech Coloproctol. 2004;8:s205–7. [DOI] [PubMed] [Google Scholar]
- 52.Leao P, Santos C, Goulart A, et al. TaTME: analysis of the evacuatory outcomes and EUS anal sphincter. Minim Invasive Ther Allied Technol. 2019;28:332–7. [DOI] [PubMed] [Google Scholar]
- 53.Shafik A, Abdel-Moneim K. Fecoflowmetry: a new parameter assessing rectal function in normal and constipated subjects. Dis Colon Rectum. 1993;36:35–42. [DOI] [PubMed] [Google Scholar]
- 54.Ryu Y, Akagi Y, Yagi M, Sasatomi T, Kinugasa T, Yamaguchi K, et al. Fecoflowmetric analysis of anorectal motor function in postoperative anal-preserving surgery patients with low rectal cancer comparison with the wexner score and anorectal manometry. Int Surg. 2015;100:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao S. Current and emerging treatment options for fecal incontinence. J Clin Gastroenterol. 2014;48:752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bliss D, Savik K, Jung H, et al. Dietary fiber supplementation for fecal incontinence: a randomized clinical trial. Res Nurs Health. 2014;37:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paquette I, Varma M, Kaiser A, et al. The American Society of Colon and Rectal Surgeons’ clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623–36. [DOI] [PubMed] [Google Scholar]
- 58.Staller K, Song M, Grodstein F, Whitehead WE, Matthews CA, Kuo B, et al. Increased long-term dietary fiber intake is associated with a decreased risk of fecal incontinence in older women. Gastroenterology. 2018;155:661–7. [DOI] [PubMed] [Google Scholar]
- 59.Itagaki R, Koda K, Yamazaki M, Shuto K, Kosugi C, Hirano A, et al. Serotonin (5-HT3) receptor antagonists for the reduction of symptoms of low anterior resection syndrome. Clin Exp Gastroenterol. 2014;7:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCutchan G, Hughes D, Davies Z, et al. Acceptability and benefit of rectal irrigation in patients with low anterior resection syndrome: a qualitative study. Colorectal Dis. 2018;20(3):O76–O84. [DOI] [PubMed] [Google Scholar]
- 61.Rosen H, Robert-Yap J, Tentschert G, Lechner M, Roche B. Transanal irrigation improves quality of life in patients with low anterior resection syndrome. Color Dis. 2011;13:e335–8. [DOI] [PubMed] [Google Scholar]
- 62.Koch S, Rietveld M, Govaert B, et al. Retrograde colonic irrigation for faecal incontinence after low anterior resection. Int J Color Dis. 2009;24:1019–22. [DOI] [PubMed] [Google Scholar]
- 63.Enriquez-Navascues JM, Labaka-Arteaga I, Aguirre-Allende I, et al. A randomized trial comparing transanal irrigation and percutaneous tibial nerve stimulation in the management of low anterior resection syndrome. Colorectal Dis. 2020;22(3):303–9. [DOI] [PubMed] [Google Scholar]
- 64.Visser W, Te Riele W, Boerma D, et al. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol. 2014;30:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin K, Granger C, Denehy L, et al. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review. Neurourol Urodyn. 2015;34:703–12. [DOI] [PubMed] [Google Scholar]
- 66.Liang Z, Ding W, Chen W, Wang Z, du P, Cui L. Therapeutic evaluation of biofeedback therapy in the treatment of anterior resection syndrome after sphincter-saving surgery for rectal cancer. Clin Colorectal Cancer. 2016;15:e101–7. [DOI] [PubMed] [Google Scholar]
- 67.Wu XD, Fu CF, Chen YL, Kong LH, Pan ZZ, Zheng MC. Intervention effect of biofeedback combined with pelvic floor muscle exercise on low anterior resection syndrome in patients with low anus-preserving rectal cancer. Zhonghua Yi Xue Za Zhi. 2019;99: 2337–43. [DOI] [PubMed] [Google Scholar]
- 68.Ramage L, Qiu S, Kontovounisios C, Tekkis P, Rasheed S, Tan E. A systematic review of sacral nerve stimulation for low anterior resection syndrome. Color Dis. 2015;17:762–71. [DOI] [PubMed] [Google Scholar]
- 69.Mege D, Meurette G, Vitton V, Leroi AM, Bridoux V, Zerbib P, et al. Sacral nerve stimulation can alleviate symptoms of bowel dysfunction after colorectal resections. Color Dis. 2017;19:756–63. [DOI] [PubMed] [Google Scholar]
- 70.Croese AD, Whiting S, Vangaveti VN, Ho YH. Using sacral nerve modulation to improve continence and quality of life in patients suffering from low anterior resection syndrome. ANZ J Surg. 2018;88:E787–91. [DOI] [PubMed] [Google Scholar]
- 71.Sarcher T, Dupont B, Alves A, Menahem B. Anterior resection syndrome: what should we tell practitioners and patients in 2018? J Visc Surg. 2018;155:383–91. [DOI] [PubMed] [Google Scholar]
- 72.Keane C, Park J, Oberg S, et al. Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br J Surg. 2019;106:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinnewitzer A, Jager T, Nawara C, et al. Cumulative incidence of permanent stoma after sphincter preserving low anterior resection of mid and low rectal cancer. Dis Colon Rectum. 2013;56:1134–42. [DOI] [PubMed] [Google Scholar]


