ABSTRACT
Antifungal activity of anidulafungin, voriconazole, isavuconazole, and fluconazole in the treatment of Candida auris was determined in vitro and in vivo. MICs for anidulafungin, voriconazole, isavuconazole, fluconazole, and amphotericin B were 0.5, 1, >64, 0.25, and 4 μg/ml, respectively. Significant in vivo efficacy was observed in the anidulafungin- and voriconazole-treated groups in survival and reduction in kidney tissue fungal burden compared to that in the untreated group (P values of <0.001 and 0.044, respectively). Our data showed that anidulafungin and voriconazole had comparable efficacies against C. auris, whereas isavuconazole did not show significant in vivo activity.
KEYWORDS: voriconazole, isavuconazole, fluconazole, anidulafungin, Candida auris, disseminated candidiasis, murine model, in vitro, in vivo, multidrug resistance
INTRODUCTION
Candida auris is an emerging infection that was first described in 2009, followed by multiple reports from countries around the world, including the United States (1–3). C. auris poses a challenge in both identification and effective treatment caused by its unique characteristics and the rather complex identification tools necessary, making it a global concern (4–8). C. auris is a multidrug-resistant (MDR) organism, with emergence of pan-resistant strains, characterized by reduced susceptibility to the three main antifungal groups (azoles, polyenes, and echinocandins), thereby making C. auris one of the most difficult pathogens to treat of all clinically relevant Candida species (9–11). This increases the need to identify drugs that are efficacious against this pathogen. In the current study, we evaluated the in vitro activity and efficacy of a number of antifungal agents, including anidulafungin (ANID), voriconazole (VOR), isavuconazole (ISA), and fluconazole (FLU), in the treatment of C. auris using an immunocompromised murine model of disseminated candidiasis.
A clinical isolate of C. auris (MRL 35368) known to be infective (12–14) was used in this study. Susceptibility testing was performed according to Clinical and Laboratory Standards Institute (CLSI) document M27 (15). After 24 h of incubation, the MICs for ANID, FLU, ISA, amphotericin B (AMB), and VOR were 0.5, >64, 0.25, 4, and 1 μg/ml, respectively. Using tentative C. auris breakpoints suggested by the CDC (16), this clinical isolate is resistant to AMB and FLU.
In vivo testing was performed using a previously described disseminated C. auris infection model (12–14). All procedures were performed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (17) and with approval of the Case Western Reserve University Institutional Animal Care and Use Committee (IACUC). Female BALB/c mice (n = 15) (weighing ∼20 g; Charles River Laboratories, Wilmington, MA) were used in the study. Treatment was initiated 2 h postchallenge. Treatment groups consisted of twice a day dosing of (i) ANID at 12 mg/kg of body weight intraperitoneally (i.p.), (ii) VOR at 12 mg/kg i.p., (iii) ISA at 20 mg/kg per os (p.o.), or (iv) FLU at 20 mg/kg p.o. Untreated control animals were also included; 10 animals were used to assess survival, while 5 animals were used to assess the effect on tissue fungal burden per group. Efficacy endpoints used were animal survival and kidney and brain fungal load.
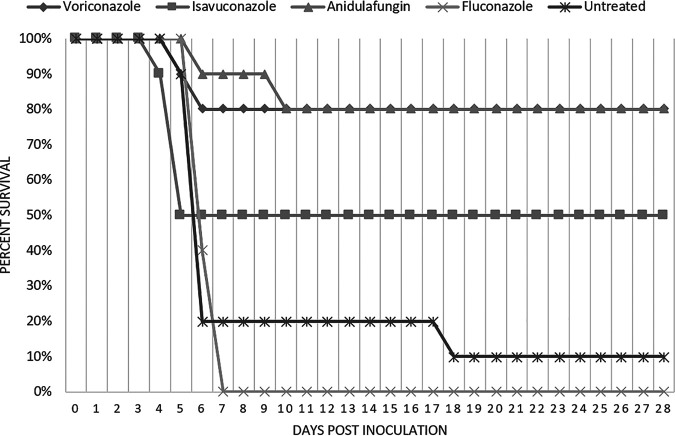
Survival was monitored for 28 days postinoculation (Fig. 1). The animals treated with VOR and ANID at 12 mg/kg showed the highest survival rates (80% and 90%, respectively) at day 7 postinoculation and showed a survival rate of 80% for both drugs at day 28 postinoculation, which is significantly prolonged compared to that of the untreated control group, for which only 10% survival was observed at day 28 (P values of 0.009 and 0.005, respectively). Additionally, the VOR- and ANID-treated groups demonstrated significantly better survival rates than the FLU-treated group (P value of <0.001). The group treated with ISA at 20 mg/kg showed an average survival rate of 50%, while the group treated with FLU at 20 mg/kg showed the lowest percent survival (0% by day 7 postinoculation).
FIG 1.
Effects of various antifungals compared to untreated controls on the survival of mice infected with C. auris.
A subgroup of animals (n = 5) were euthanized on day 8 postinfection. Kidneys were removed aseptically, weighed, homogenized in 1 ml of phosphate-buffered saline (PBS), serially diluted, and then plated onto potato dextrose agar (Becton, Dickinson and Company, Sparks, MD) and cultured at 37°C for 48 h to determine the fungal tissue burden, expressed as CFU per gram of tissue. Efficacy of antifungal agents was evaluated as a reduction in log10 CFU compared with those of control and other tested groups.
Table 1 shows the tissue fungal burden in the kidneys and the brains of mice challenged with C. auris (expressed as average log CFU per gram ± standard deviation [SD]). As expected, the mice in the untreated control group showed the highest tissue fungal burden (8.75 ± 0.6 and 5.92 ± 0.5 average log CFU/g for kidneys and brain, respectively). The ANID- and VOR-treated groups showed significant reductions in the kidney tissue fungal burden (2.90 ± 0.5 and 5.36 ± 3.0 average log CFU/g, respectively) compared to that of the untreated group (P value < 0.001 and P value = 0.044, respectively). Moreover, there was no significant difference between ANID and VOR (P value = 0.296). Additionally, the ANID-treated group demonstrated a significant reduction in kidney fungal burden compared to those of the FLU-treated group and ISA-treated group (6.76 ± 1.8 and 7.25 ± 0.3 average log CFU/g ± SD, respectively; P values = 0.009 and 0.016, respectively). On the other hand, FLU and ISA did not exhibit a significant reduction in tissue fungal burden in the kidneys compared to that in the untreated group (P values of 0.73 and 1.00, respectively)
TABLE 1.
Effects of antifungals on kidney and brain tissue fungal burdens compared to those in the untreated control
| Group | Kidney |
Brain |
||
|---|---|---|---|---|
| Avg log CFU ± SD | P value vs untreated | Avg log CFU ± SD | P value vs untreated | |
| Untreated control | 8.75 ± 0.6 | 5.92 ± 0.5 | ||
| Anidulafungin | 2.90 ± 0.5 | <0.001 | 4.62 ± 0.3 | 0.421 |
| Voriconazole | 5.36 ± 3.0 | 0.044 | 4.26 ± 1.3 | 0.118 |
| Fluconazole | 6.76 ± 1.8 | 0.73 | 5.14 ± 1.5 | 1.000 |
| Isavuconazole | 7.25 ± 0.3 | 1.000 | 5.36 ± 0.2 | 1.000 |
Finally, none of the of the tested compounds demonstrated significant reduction in brain tissue fungal burden compared to that in untreated controls (P values = 0.421, 0.118, 1.00, and 1.00 for ANID, VOR, FLU, and ISA, respectively). This suggests poor central nervous system (CNS) penetration of the tested agents.
Evaluation of the in vitro activity of echinocandins against the MDR C. auris in different studies, which included larger numbers of strains, showed that these agents are active against most C. auris strains (18, 19). However, some strains were found to show resistance even to this class of antifungals, especially those with an S639F mutation in FKS1 hot spot region 1 (18, 19). In our study, we confirmed that ANID showed the greatest effect in reduction of tissue fungal burden as well as potent in vitro activity against a highly infective clinical isolate of C. auris. In the present study, ANID showed a significant effect through reduction of kidney fungal tissue burden. However, unlike APX001A/APX001 (fosmanogepix), which significantly reduced brain tissue fungal burden (13), ANID failed to do so, suggesting that it may have poor CNS penetration compared to that of fosmanogepix. However, since different C. auris strains were used in these studies, evaluating the efficacies of different agents in a head-to-head comparison is not possible. Recently, echinocandin-resistant C. auris has been reported in a number of cases (20, 21); however, some promising newly developed antifungals have shown activity against these strains (19, 22–24).
Interestingly, VOR showed significant reduction of tissue fungal burden in kidneys, which was not the case for ISA, as ISA demonstrated the highest in vitro activity of the three azoles tested but failed to exhibit significant reduction of tissue fungal burden in vivo compared to that in the untreated group. This may be explained by the fact that ISA is highly bound to plasma proteins and achieves a lower maximum plasma concentration and therefore less tissue penetration in animal models than VOR (25, 26). However, the relationship between tissue concentration and efficacy of VOR was reported as variable in various studies (27, 28), which may be caused by distribution of the drug to the wrong subcompartment, lack of bioavailability, or accumulation (in tissue) at a concentration below the threshold required for activity (29). Additionally, ISA failed to demonstrate noninferiority relative to caspofungin (CAS) in the treatment of candidemia and invasive Candida infections (30).
In conclusion, ANID showed potent in vitro and in vivo activities against a clinical isolate of C. auris (MRL 35368) which demonstrate resistance to FLU, as well as AMB. VOR has activity against this isolate at levels comparable to that of ANID. Although ANID and VOR were effective in reducing tissue fungal burden in vivo in kidneys, they were less active in clearing brain infection. Further work is needed to determine factors that play a role in the ability of an antifungal to penetrate various tissues, including the CNS, and effectively eliminate C. auris. These studies may lead to the development of drugs that can act effectively in various tissues. Additionally, further work is necessary to evaluate the variation observed among different azoles used against C. auris. Finally, confirmation of these studies using additional clinical isolates of C. auris is recommended.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (grant number R21 AI143305-01).
In compliance with the ICMJE uniform disclosure form, Mahmoud Ghannoum declares that he received funding from Pfizer, Scynexis, Inc., Cidara Therapeutics, and Amplyx Pharmaceuticals. All other authors have declared that there are no other relationships or activities that could appear to have influenced this work.
REFERENCES
- 1.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 2.Oh BJ, Shin JH, Kim MN, Sung H, Lee K, Joo MY, Shin MG, Suh SP, Ryang DW. 2011. Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Med Mycol 49:98–102. doi: 10.3109/13693786.2010.493563. [DOI] [PubMed] [Google Scholar]
- 3.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. 2017. Multidrug-resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis 23:195–203. doi: 10.3201/eid2302.161486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Gaitan A, Moret AM, Tasias-Pitarch M, Aleixandre-Lopez AI, Martinez-Morel H, Calabuig E, Salavert-Lleti M, Ramirez P, Lopez-Hontangas JL, Hagen F, Meis JF, Mollar-Maseres J, Peman J. 2018. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 6.CDC. 2020. General information about Candida auris. https://www.cdc.gov/fungal/candida-auris/candida-auris-qanda.html.
- 7.Kordalewska M, Perlin DS. 2019. Molecular diagnostics in the times of surveillance for Candida auris. J Fungi (Basel) 5:77. doi: 10.3390/jof5030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, Litvintseva AP. 2017. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 55:2996–3005. doi: 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup MC, Patterson TF. 2017. Multidrug-resistant Candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S445–S451. doi: 10.1093/infdis/jix131. [DOI] [PubMed] [Google Scholar]
- 10.Navalkele BD, Revankar S, Chandrasekar P. 2017. Candida auris: a worrisome, globally emerging pathogen. Expert Rev Anti Infect Ther 15:819–827. doi: 10.1080/14787210.2017.1364992. [DOI] [PubMed] [Google Scholar]
- 11.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, Group CIW, C. auris Investigation Work Group. 2020. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. doi: 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hager CL, Larkin EL, Long LA, Ghannoum MA. 2018. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 73:2085–2088. doi: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrada J, Gamal A, Long L, Sanchez SP, McCormick TS, Ghannoum MA. 2021. In vitro and in vivo antifungal activity of AmBisome compared to conventional amphotericin B and fluconazole against Candida auris. Antimicrob Agents Chemother 65:e00306-21. doi: 10.1128/AAC.00306-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed. CLSI document M27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.CDC. 2020. Antifungal susceptibility testing and interpretation. https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html.
- 17.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 18.Kordalewska M, Lee A, Park S, Berrio I, Chowdhary A, Zhao Y, Perlin DS. 2018. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrob Agents Chemother 62:e00238-18. doi: 10.1128/AAC.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendrup MC, Jørgensen KM, Hare RK, Chowdhary A. 2020. In vitro activity of ibrexafungerp (SCY-078) against Candida auris isolates as determined by EUCAST methodology and comparison with activity against C. albicans and C. glabrata and with the activities of six comparator agents. Antimicrob Agents Chemother 64:e02136-19. doi: 10.1128/AAC.02136-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2017. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. Am J Transplant 17:296–299. doi: 10.1111/ajt.14121. [DOI] [PubMed] [Google Scholar]
- 21.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghannoum M, Arendrup MC, Chaturvedi VP, Lockhart SR, McCormick TS, Chaturvedi S, Berkow EL, Juneja D, Tarai B, Azie N, Angulo D, Walsh TJ. 2020. Ibrexafungerp: a novel oral triterpenoid antifungal in development for the treatment of Candida auris infections. Antibiotics (Basel) 9:539. doi: 10.3390/antibiotics9090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamal A, Chu S, McCormick TS, Borroto-Esoda K, Angulo D, Ghannoum MA. 2021. Ibrexafungerp, a novel oral triterpenoid antifungal in development: overview of antifungal activity against Candida glabrata. Front Cell Infect Microbiol 11:126. doi: 10.3389/fcimb.2021.642358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghannoum M, Isham N, Angulo D, Borroto-Esoda K, Barat S, Long L. 2020. Efficacy of ibrexafungerp (SCY-078) against Candida auris in an in vivo guinea pig cutaneous infection model. Antimicrob Agents Chemother 64:e00854-20. doi: 10.1128/AAC.00854-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rybak JM, Marx KR, Nishimoto AT, Rogers PD. 2015. Isavuconazole: pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy 35:1037–1051. doi: 10.1002/phar.1652. [DOI] [PubMed] [Google Scholar]
- 26.Bellmann R, Smuszkiewicz P. 2017. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection 45:737–779. doi: 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutsar I, Hodges MR, Tomaszewski K, Troke PF, Wood ND. 2003. Safety of voriconazole and dose individualization. Clin Infect Dis 36:1087–1088. doi: 10.1086/374248. [DOI] [PubMed] [Google Scholar]
- 28.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. 2006. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 50:1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felton T, Troke PF, Hope WW. 2014. Tissue penetration of antifungal agents. Clin Microbiol Rev 27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullberg BJ, Viscoli C, Pappas PG, Vazquez J, Ostrosky-Zeichner L, Rotstein C, Sobel JD, Herbrecht R, Rahav G, Jaruratanasirikul S, Chetchotisakd P, Van Wijngaerden E, De Waele J, Lademacher C, Engelhardt M, Kovanda L, Croos-Dabrera R, Fredericks C, Thompson GR. 2019. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: the ACTIVE trial. Clin Infect Dis 68:1981–1989. doi: 10.1093/cid/ciy827. [DOI] [PubMed] [Google Scholar]