ABSTRACT
A series of (Z)-2-(nitroheteroarylmethylene)-3(2H)-benzofuranones possessing nitroheteroaryl groups of nitroimidazole, nitrofuran, and nitrothiophene moieties was screened for antiplasmodium activity against a drug-sensitive strain (3D7 strain) and a multidrug-resistant (chloroquine [CQ] and pyrimethamine) strain (K1 strain) of Plasmodium falciparum. 5-Nitroimidazole and 4-nitroimidazole analogs were highly selective and active against resistant parasites, while 5-nitrofuran and 5-nitrothiophene derivatives were more potent against the 3D7 strain than against the K1 strain. Among the synthetic analogues, (Z)-6-chloro-2-(1-methyl-5-nitroimidazol-2-ylmethylene)-3(2H)-benzofuranone (compound 5h) exhibited the highest activity (50% inhibitory concentration [IC50], 0.654 nM) against the K1 strain and (Z)-7-methoxy-2-(5-nitrothiophen-2-ylmethylene)-3(2H)-benzofuranone (10g) showed the highest activity (IC50, 0.28 μM) against the 3D7 strain in comparison with the activities of CQ (IC50s of 3.13 and 206.3 nM against 3D7 and K1 strains, respectively). The more active compounds, with IC50s lower than 5 μg/ml (∼20 μM), were further studied for their cytotoxicity responses using KB cells. From these studies, 5-nitroimidazole, 4-nitroimidazole, and 5-nitrofuran analogues were shown to be cytotoxic against KB cells, while 5-nitrothiophene analogues were shown to have the least cytotoxic effects. To gain some insight into their potential contributing mechanisms of action, three derivatives, 10e, 10g, and 10h (from the nitrothiophene subgroup, possessing 6-methoxy, 7-methoxy, and 6,7-dimethoxy substituents, respectively, on their benzofuranone moieties), showing the least toxicity and highest selectivity indices were assessed for their β-hematin formation inhibition activity. Compound 10g demonstrated the highest inhibition activity (IC50, 10.78 μM) in comparison with that of CQ (IC50, 2.63 μM) as the reference drug. Finally, these three analogues (10e, 10g, and 10h) were further evaluated for their in vivo activities against the Plasmodium berghei/albino mouse model (Peter’s test). The tested analogues were shown to be active, reducing the percentages of erythrocytes that contained parasites by 53.4, 48.8, and 32.4%, respectively.
KEYWORDS: antimalarial activity, antiplasmodial activity, β-hematin formation, in vivo, benzofuranone, nitrofuran, nitroimidazole, nitrothiophene
TEXT
Malaria is a lethal parasitic disease that was responsible for 229 million acute illnesses and 409,000 deaths worldwide in 2019 (1). Improvements in vector control over the past 2 decades have significantly decreased the incidence of malaria across the tropical regions. However, malaria-associated morbidity is still high (2, 3). The fact that 67% (274,000) of all malaria deaths worldwide in 2019 were of children under the age of 5 years, who are the most vulnerable group affected by malaria, represents the severity of this disease (1).
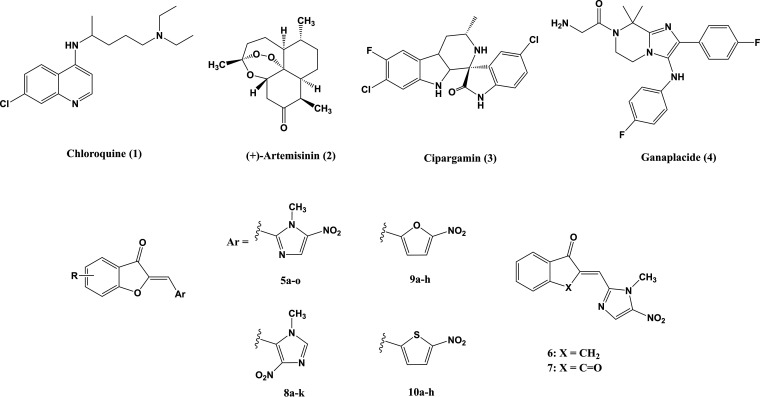
Despite the recent development of many effective antimalarials, the extent of malaria infections is increasing, mainly as a result of the rapid spread of drug-resistant malaria parasite strains in many parts of the world. Currently used antimalarials belong to four main families: quinolines, or aryl aminoalcohols (e.g., chloroquine [CQ] [Fig. 1, compound 1] and mefloquine), antifolates (sulfadoxine and pyrimethamine), artemisinin derivatives (Fig. 1, compound 2), and antimicrobials (tetracycline and doxycycline) (4).
FIG 1.
Representative examples of antimalarial drugs currently in use (1, 2) or in development (3, 4) and chemicals used in this study (5 to 10).
Currently, the first- and second-line World Health Organization (WHO)-recommended treatments for uncomplicated Plasmodium falciparum malaria are artemisinin-based combination therapies (ACT), while for the treatment of uncomplicated Plasmodium vivax malaria, the WHO recommends either CQ or an ACT in areas with CQ-resistant P. vivax (5). Most recently, resistance to artemisinin-based combination therapies has emerged (6).
Emerging drug resistance necessitates the urgent development of novel and affordable antimalarials, particularly against resistant strains of P. falciparum (3, 7).
In the past decade, some promising candidates from known chemical classes but new to malaria chemotherapy, such as cipargamin (KAE609) (Fig. 1, compound 3) (8), ganaplacide (KAF156) (Fig. 1, compound 4) (9), and DSM265, have successfully progressed to clinical trials (10). Some studies have focused on modifying existing compounds to increase their efficacy on resistant strains, and others have targeted new molecules or pathways in the parasite.
Quinoline-based antimalarials, the drugs of choice for the treatment of malaria caused by P. falciparum, act through the inhibition of hemozoin formation from the heme released by the digestion of hemoglobin (Hb) (11). The malaria parasite requires the hemoglobin of its host erythrocytes to obtain amino acids. As a result of hemoglobin digestion, which occurs inside the digestive vacuole of the parasite, a large amount of toxic heme is released. The free heme is autoxidized to potentially toxic hematin, which lyses membranes, inhibits a variety of enzymes, and leads to parasite death. Plasmodia have a unique mechanism for heme detoxification; hematin is converted to a highly insoluble substance via a biocrystallization process (polymerization). In this process, heme is sequestered into large insoluble crystals called hemozoin (the malarial pigment), which are chemically similar to β-hematin (12). CQ resistance is due to a decreased accumulation of CQ in the food vacuole.
On the other hand, studies on a series of sulfonamide chalcone and phenylurenyl chalcone derivatives have demonstrated that they exert their antimalarial activity through inhibition of β-hematin (hemozoin) formation (13, 14).
As part of our ongoing program in developing new antimicrobial agents, we had reported the synthesis of a new class of (Z)-2-(nitroimidazolylmethylene)-3(2H)-benzofuranone derivatives and demonstrated the strong antibacterial activity of some of its subclasses against various bacterial strains, in particular methicillin-resistant Staphylococcus aureus (MRSA) strains (15). Moreover, some analogues were also highly active in reducing Leishmania donovani promastigotes and amastigotes and had high selectivity indices (SIs) (cytotoxicity studied on rat L6 myoblast cell line) (L. Navidpour, M. L. Lopes, R. Milne, S. Wyllie, N. Hadj-Esfandiari, M. I. Choudhary, S. Khan, and S. Yousuf, submitted for publication), which made them interesting candidates for further investigations.
These compounds are hybrids of a nitroheteroaryl moiety and a benzofuranone core. Nitroaryl compounds are known to disrupt DNA molecules of pathogens via the formation of active radical anions. Interestingly, we found that the benzofuranone moiety is also essential for the observed high potency, as substitution of this moiety with indanone and indandione diminished the effectiveness against Leishmania major (Navidpour et al., submitted for publication). Furthermore, their similarity to the chalcone scaffold makes benzofuranones suitable candidates for inhibition of β-hematin formation.
As reported in this paper, we screened our previously synthesized (Z)-2-(nitroheteroarylmethylene)-3(2H)-benzofuranone compounds against a drug-sensitive strain (3D7 strain) and, more importantly, a multidrug-resistant strain (K1 strain) of P. falciparum. The cytotoxicities of the active compounds were also evaluated to select promising candidates for investigation of the mechanism, i.e., assessing the inhibitory activity toward β-hematin formation. Finally, the efficacies of selected derivatives were evaluated for their in vivo activities against the P. berghei/albino mouse model (Peter’s test).
RESULTS AND DISCUSSION
Synthesis.
The synthetic reactions to produce the title compounds (compound series 5 to 10) (Fig. 1) have been described previously (15).
In vitro activities of compounds against 3D7 and resistant K1 strains of Plasmodium falciparum.
The antiplasmodium properties of the 3(2H)-benzofuranone derivatives condensed with 1-methyl-5-nitroimidazole-2-carbaldehyde (compounds 5a to 5n), 1-methyl-4-nitroimidazole-5-carbaldehyde (8a to 8k), 5-nitrofuran-2-carbaldehyde (9a to 9h), and 5-nitrothiophene-2-carbaldehyde (10a to 10h) (Fig. 1) against the drug-sensitive 3D7 clone of isolate NF54 (unknown origin) and the chloroquine (CQ)- and pyrimethamine-resistant K1 strain (Thailand) of P. falciparum were assessed in vitro using the [3H]hypoxanthine incorporation assay (16, 17).
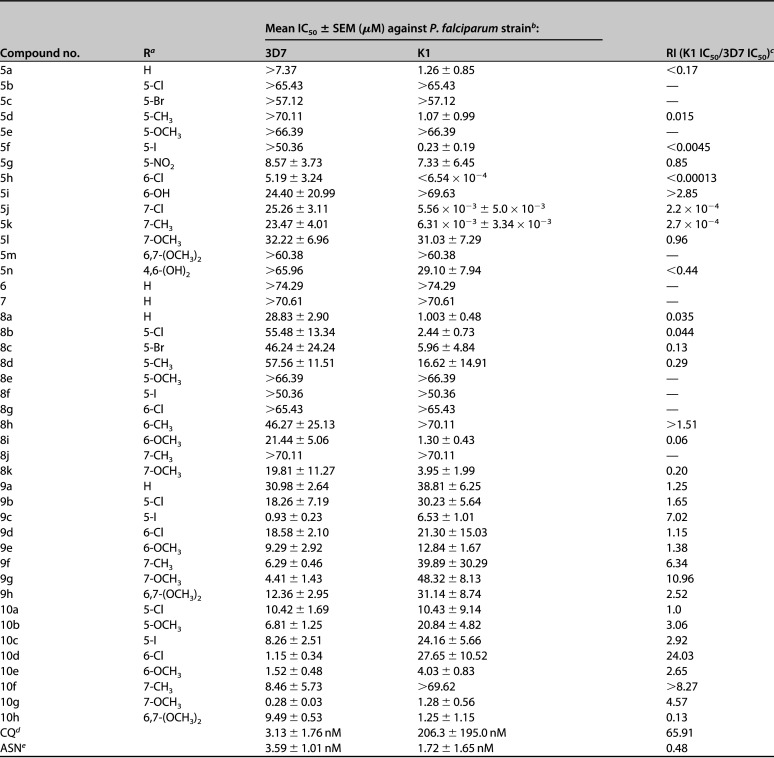
The 50% inhibitory concentration (IC50s) of each compound (μM) was calculated and compared with those of chloroquine diphosphate and asparagine as standard drugs. The IC50s and the resistance indices (RIs) are displayed in Table 1.
TABLE 1.
In vitro antiplasmodium activities against P. falciparum in the 48-h [3H]hypoxanthine incorporation assay
R represents the substituent(s) on benzofuranone moiety.
The IC50s ± SEMs were calculated from triplicate determinations for each concentration.
RI, resistance index; —, RI = 0.
CQ, chloroquine.
ASN, asparagine.
In general, 5-nitroimidazole and 4-nitroimidazole derivatives were more active against the K1 strain than against the 3D7 strain, while 5-nitrofuran and 5-nitrothiophene derivatives were found to be less active against the K1 strain relative to their activities against the 3D7 strain. This is expressed as the RIs for these compounds in Table 1.
Within the 5-nitroimidazole series, some compounds were found to be significantly more active (5h, 5j, and 5k, with IC50s of <0.654, 5.56, and 6.31 nM, respectively) and some less active than CQ (IC50, 206.3 nM) against the K1 strain. All compounds in this subgroup (5a to 5n) were less active than CQ against the 3D7 strain.
Substitution of the benzofuranone moiety with indanone (6) and indandione (7) moieties resulted in less active compounds. This observation suggests the importance of the oxygen atom of the benzofuranone moiety in antiplasmodium activity.
When 5-nitroimidazole was substituted with 4-nitroimidazole, all the resulting compounds (8a to 8k) were shown to be less active than CQ against both the K1 and 3D7 strains. Among these analogues, the unsubstituted (8a) and 6-methoxy-substituted (8i) benzofuranone showed poor (IC50s of 28.83 and 21.44 μM, respectively, against the 3D7 strain) and good (1.0 and 1.30 μM against the K1 strain) antiplasmodium activities against the different strains.
Substitution of nitroimidazoles with 5-nitrofuran (9a to 9h) and 5-nitrothiophene (10a to 10h) scaffolds resulted in greater activities against the 3D7 strain and comparable activities against the K1 strain. Among 5-nitrofuran analogues (9a to 9h), 5-iodo-substituted (9c) benzofuranone showed the highest antiplasmodium activities against the 3D7 (IC50, 0.93 μM) and K1 (IC50, 6.53 μM) strains. In the 5-nitrothiophene subgroup (10a to 10h), 6-chloro- (10d), 6-methoxy- (10e), and 7-methoxy-substituted (10g) analogues of benzofuranone were more active against the 3D7 strain (with IC50s of 1.15, 1.52, and 0.28 μM, respectively) than CQ, while being less active than CQ against the K1 strain.
Finally, among the synthesized analogues, (Z)-7-methoxy-2-(5-nitrothiophene-2-ylmethylene)-3(2H)-benzofuranone (10g) showed the highest activity (IC50, 0.28 μM) against the 3D7 strain while (Z)-6-chloro-2-(1-methyl-5-nitroimidazole-2-ylmethylene)-3(2H)-benzofuranone (5h) exhibited the highest activity (IC50, 0.654 nM) against the K1 strain in comparison with CQ, which showed IC50s of 3.13 and 206.3 nM against the 3D7 and K1 strains, respectively.
Toxicity testing: in vitro test using KB cells.
Compounds showing promising antiplasmodium activities with IC50s of less than 5 μg/ml on 3D7 and K1 strains were further studied for their cytotoxicity responses using KB cells (18). As shown by the results in Table 2, 5-nitroimidazole and 5-nitrofuran analogues were cytotoxic against KB cells, with low selectivity indices (SIs) for antiplasmodium activity over cytotoxicity on KB cells. On the other hand, 5-nitrothiophene analogues had the lowest cytotoxicities with the highest SIs.
TABLE 2.
The in vitro antiplasmodium and cytotoxic activities of the compounds with IC50s of <5 μg/ml
| Compound no. | Ra | Mean IC50 ± SEM (μM) for growth of P. falciparum strainb: |
Mean CC50 (μM) on KB cellsc | SI (CC50/IC50) on KB cells of straind: |
||
|---|---|---|---|---|---|---|
| 3D7 | K1 | 3D7 | K1 | |||
| 5a | H | >7.37 | 1.26 ± 0.85 | 0.169 | <0.023 | 0.13 |
| 5g | 5-NO2 | 8.57 ± 3.73 | 7.33 ± 6.45 | 1.53 | 0.18 | 0.21 |
| 5h | 6-Cl | 5.19 ± 3.24 | <6.54 × 10−4 | 0.291 | 0.056 | >444.9 |
| 8k | 7-OCH3 | 19.81 ± 11.27 | 3.95 ± 1.99 | 13.95 | 0.70 | 3.53 |
| 9c | 5-I | 0.93 ± 0.23 | 6.53 ± 1.01 | 0.522 | 0.56 | 0.08 |
| 9e | 6-OCH3 | 9.29 ± 2.92 | 12.84 ± 1.67 | 0.79 | 0.08 | 0.06 |
| 10a | 5-Cl | 10.42 ± 1.69 | 10.43 ± 9.14 | 7.17 | 0.69 | 0.69 |
| 10e | 6-OCH3 | 1.52 ± 0.48 | 4.03 ± 0.83 | 280.94 | 184.82 | 69.71 |
| 10g | 7-OCH3 | 0.28 ± 0.03 | 1.28 ± 0.56 | 228.82 | 817.21 | 178.76 |
| 10h | 6,7-(OCH3)2 | 9.49 ± 0.53 | 1.25 ± 1.15 | 185.79 | 19.58 | 148.63 |
| CQe | 3.13 ± 1.76 nM | 206.3 ± 195.0 nM | >312.62 | >99,865 | >1,515 | |
| PODf | <0.024 | |||||
R represents the substituent(s) on benzofuranone moiety.
The IC50s ± SEMs were calculated from triplicate determinations for each concentration.
The CC50s ± SEMs were calculated from duplicate determinations for each concentration.
SI, selectivity index.
CQ, chloroquine.
POD, podophyllotoxin.
Cell-free β-hematin formation assay.
In order to gain insight into the possible contributing mechanisms of antiplasmodium killing of the analogues evaluated and in view of the fact that related chalcone derivatives have been shown to exhibit antiplasmodium activity through their inhibitory activity on β-hematin formation (13, 14), a cell-free β-hematin formation assay (19, 20) was performed in the presence of the most promising compounds with the highest selectivity indices (10e, 10g, and 10h).
As shown by the results in Table 3, the compounds presented moderate to prominent activities, with IC50s of 53.20, 10.78, and 14.67 μM for 10e, 10g, and 10h, respectively, in comparison with CQ, with an IC50 of 2.63 μM.
TABLE 3.
β-Hematin formation inhibition and in vivo antiplasmodium assays for compounds with SIs of >20
| Compound no. | Ra | Mean IC50 ± SEM for β-hematin formation (μM)b | Value for in vivo antiplasmodium assay |
|
|---|---|---|---|---|
| Dose (mg/kg) | % parasite growth inhibition | |||
| 10e | 6-OCH3 | 53.20 ± 1.04 | 10 | 53.4 |
| 10g | 7-OCH3 | 10.78 ± 1.17 | 10 | 48.9 |
| 10h | 6,7-(OCH3)2 | 14.67 ± 1.03 | 10 | 32.4 |
| CQc | 2.63 ± 0.66 | 25 | 100 | |
R represents the substituent(s) on benzofuranone moiety.
The IC50s ± SEMs were calculated from triplicate determinations for each concentration.
CQ, chloroquine.
The lack of correlation between the IC50s of the compounds in the β-hematin formation assay and their whole-cell antiplasmodium activities is noteworthy and may be explained on the basis of three factors. First, this may suggest the involvement of other mechanisms also contributing to the observed antiplasmodium activity. Second, there is the possibility that the compounds do not possess appropriate physicochemical and/or transport properties to cross the membranes of infected host red blood cells, as well as the parasite cell membrane, to reach the target site of action, the parasite acidic food vacuole where hemozoin formation occurs. Third, the β-hematin formation assay used is cell free, which implies that the assay does not factor in compound permeation across the infected host red blood cell and parasite membranes to reach the site of action. In contrast, the antiplasmodium activities are determined in a cellular (parasite) environment.
In vivo antimalarial assay against P. berghei in mice.
The rodent parasite Plasmodium berghei has proven to be very convenient for the detection of antimalarial activity, and it is generally considered that infection with this parasite in the mouse is a valid model for the primary screening of drugs for eventual use against human malaria (21, 22). Based on their IC50s against the CQ-sensitive 3D7 strain and CQ-resistant K1 strain and their SI values, the 5-nitrothiophene series having 6-methoxy, 7-methoxy, and 6,7-dimethoxy substituent(s) on the benzofuranone moiety, 10e, 10g, and 10h, were further evaluated for in vivo activity. The compounds were administered intraperitoneally (i.p.) at 10 mg/kg of body weight against the P. berghei/albino mouse model (Peter’s test) in comparison with chloroquine (25 mg/kg) as the reference compound.
The BALB/c mice were infected with 107 parasitized donor erythrocytes i.p. Two hours following sporozoite inoculation, treatment started for 4 consecutive days through the i.p. route with 10e, 10g, 10h, and chloroquine. Parasites were counted in Giemsa-stained thin films made from tail blood on the fourth day (D + 4) after the day of infection (D 0), and the percentages of erythrocytes that contained parasites (erythrocytes infection rate) in tail blood samples from treated groups were compared with the percentages for untreated negative controls on D + 4. As shown by the results in Table 3, significant reductions in parasite-containing erythrocytes were observed for compounds 10e, 10g, and 10h, while the percentages of parasite growth inhibition were 53.4%, 48.9%, and 32.4%, respectively.
In conclusion, a series of compounds possessing a 3(2H)-benzofuranone core condensed with nitroheteroarylcarbaldehydes that included 5-nitroimidazole, 4-nitroimidazole, 5-nitrofuran, and 5-nitrothiophene moieties were screened for their antiplasmodium activities against drug-sensitive (3D7) and multidrug-resistant (K1) strains of Plasmodium falciparum. The nitroimidazole analogs demonstrated strong inhibition activities against resistant strains, while the nitrofuran and nitrothiophene analogs showed significant inhibition activities against sensitive strains. Further studies on cytotoxic activities showed that, among the different nitroheteroaryl derivatives, methoxy-substituted nitrothiophenes demonstrated the lowest cytotoxicities and the highest selectivity indices. To obtain some insight into their mechanism of antiplasmodium activity, the compounds with the highest selectivity indices were assessed for their β-hematin formation inhibition activities, where they demonstrated moderate to prominent inhibitory effects. Finally, these nitrothiophene derivatives (10e, 10g, and 10h) were found to be active in vivo against the P. berghei/albino mouse model (Peter’s test). Therefore, (Z)-2-(nitroheteroarylmethylene)-3(2H)-benzofuranones could serve as a potential novel scaffold for further developing new antimalarial drug candidates.
MATERIALS AND METHODS
Reagents and drug synthesis.
The chemical characterization of the synthesized compounds has been presented previously (Navidpour et al., submitted for publication).
In vitro activities of compounds against 3D7 and K1 strains of Plasmodium falciparum.
Two strains of P. falciparum were used in this study, (i) the drug-sensitive 3D7 clone of isolate NF54 (unknown origin) and (ii) the chloroquine- and pyrimethamine-resistant K1 strain (Thailand), which is sensitive to mefloquine. The cultures were naturally asynchronous (65 to 75% ring stage) and maintained in continuous log-phase growth in RPMI 1640 medium supplemented with 5% washed human A+ erythrocytes, 25 mM HEPES, 32 mM NaHCO3, and liquid-rich bovine serum albumin (complete medium). All cultures and assays were conducted at 37°C under 5% CO2 and 5% O2, with a balance of N2. In vitro parasite growth inhibition was assessed based on the method used by Desjardins et al. (16) by the incorporation of [3H]hypoxanthine.
[3H]hypoxanthine incorporation assay.
Stock compound solutions were dissolved in 100% dimethyl sulfoxide and further diluted to 10-fold dilution series (20.0, 2.00, 0.20, 0.02, 0.002, and 0.0002 μg/ml) in assay medium (RPMI 1640 supplemented with 0.5% Albumax II [Invitrogen], 0.2% wt/vol glucose, 0.03% l-glutamine, and 5 μM hypoxanthine). Assays were performed in sterile 96-well microtiter plates in triplicate. Each plate contained 100 μl parasite culture (0.5% parasitemia and 2.5% hematocrit) with or without 10 μl compound, and the parasite growth was compared to the growth in medium with CQ as the reference drug and in blank (uninfected erythrocytes) wells. After 24 h of incubation at 37°C, 0.5 μCi of [3H]hypoxanthine was added to each well. Cultures were incubated for a further 24 h before they were harvested onto glass fiber filter mats. The radioactivity was counted using a Wallac Microbeta 1450 scintillation counter. The results were recorded as counts per minute (cpm) per well for blank and reference wells and for each compound concentration. The percentage of inhibition was calculated from comparison of blank and control wells, and IC50s were calculated using the Microsoft Excel line-fitting add-in XLFit (IDBS, London, UK).
Toxicity testing: in vitro test using KB cells.
The alamarBlue method was used to assess the cytotoxicity of the compounds on KB cells as previously described (18). Briefly, microtiter plates were seeded at a density of 4 × 104 KB cells/ml in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (HIFCS) (complete medium) and l-glutamine (KB cell medium). Amounts of 100 μl were added to wells in 96-well plates (4 × 103 cells/well). Plates were incubated at 37°C under a 5% CO2, 95% air mixture for 24 h to allow the cells to adhere. Test compounds and podophyllotoxin (reference drug) were prepared in 100% dimethyl sulfoxide (DMSO) and diluted down to a starting concentration of 200 μg/ml (2× top concentration, resulting in a final start concentration of 100 μg/ml) with KB cell medium. A 10-fold serial dilution was performed for all test compounds, and 100 μl of each compound concentration was transferred to the KB cell plates. The plates were incubated for a further 72 h at 37°C under a 5% CO2, 95% air mixture. Each well was assessed by microscopic observation. Twenty microliters of alamarBlue was then added to each well, and the plates were incubated for a further 2 to 4 h. Fluorescence emission at 580 nm was measured in a SpectraMax Gemini plate reader after excitation at 530 nm (with cutoff at 550 nm). The 50% cytotoxic concentrations (CC50s) were calculated using sigmoidal regression analysis (XLfit).
Cell-free β-hematin formation assay.
The antimalarial activities of the compounds were evaluated by slight modifications to the in vitro cell-free β-hematin formation assay described by Afshar et al. (19, 20). Initially, various concentrations of the compounds (0.2 to 2 mg/ml in DMSO) were prepared. Subsequently, the samples were incubated with hematin (100 μl, 3 mM), oleic acid (10 μl, 10 mM), and HCl (10 μl, 1 M). The final volume was adjusted to 1,000 μl by adding sodium acetate buffer (pH 5). Each assay was set up in triplicate and incubated overnight at 37°C with regular shaking of the reaction mixtures. The reactions were stopped by centrifugation (14,000 rpm for 10 min at 25°C), and pellets were repeatedly resuspended in SDS (2.5% [wt/vol] in phosphate-buffered saline) and then centrifuged (14,000 rpm for 10 min at 25°C) to purify the collected hemozoin (β-hematin) pellets (usually 3 to 8 washes). This process was followed by a final wash in sodium bicarbonate (0.1 M) until the supernatant was clear. Ultimately, the supernatant was removed and the pure pellets were resuspended in NaOH (1 ml, 0.1 M). For quantification of the hemozoin content, the absorbance was measured at 400 nm by UV spectrophotometer. The inhibition of heme crystallization was expressed as percent inhibition (I%) using the following equation: I% = [(AB − AA)/AB] × 100, where AB is the absorbance of the blank and AA is the absorbance of the test sample. The data assessment was accomplished with GraphPad Prism 6.0 software, and the IC50s ± standard errors of the means (SEMs) of at least triplicate determinations were calculated from the nonlinear regression analysis. Chloroquine diphosphate, known to be an antimalarial drug, was employed as the reference drug in this assay.
In vivo antimalarial assay.
Adult male albino mice weighing 30 ± 3 g (Pasteur Institute of Iran) were used throughout this study. The animals were housed in a temperature-controlled room (24 ± 1°C) on a 12-h light/dark cycle with free access to food and water. All procedures were carried out in accordance with institutional guidelines for animal care and use.
The animals were inoculated with approximately 107 parasitized donor erythrocytes intraperitoneally (i.p.), the volume of the inoculums being 0.2 ml. The day of infection was called D 0, and subsequent days D + 1, + 2 etc. All test mice were infected in random order before being divided into groups of five. Test compounds were dissolved in Tween 20/DMSO 2.5% (20 mg/ml) and administered intraperitoneally (i.p.) at 10 mg/kg daily on D 0 (2 h following sporozoite inoculation), D + 1, D + 2, and D + 3. Two control groups were used in this experiment, with one being treated with CQ (25 mg/kg) as a reference compound (positive control) and the other kept untreated as a negative control. Parasites were counted in Giemsa-stained thin films made from tail blood on D + 4. The percentages of erythrocytes that contained parasites (erythrocyte infection rate) in tail blood samples from treated groups were compared with the results for untreated negative controls on D + 4 (20, 21).
ACKNOWLEDGMENTS
We are grateful to Zahra Tavakoli for her kind assistance.
The University of Cape Town, South African Medical Research Council, and South African Research Chairs Initiative of the Department of Science and Innovation, administered through the South African National Research Foundation, are gratefully acknowledged for support (K.C.).
REFERENCES
- 1.WHO. 2019. The “World malaria report 2019” at a glance. World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019.
- 2.Ferguson NM. 2018. Challenges and opportunities in controlling mosquito-borne infections. Nature 559:490–497. doi: 10.1038/s41586-018-0318-5. [DOI] [PubMed] [Google Scholar]
- 3.Kapesa A, Kweka EJ, Atieli H, Afrane YA, Kamugisha E, Lee MC, Zhou G, Githeko AK, Yan G. 2018. The current malaria morbidity and mortality in different transmission settings in Western Kenya. PLoS One 13:e0202031. doi: 10.1371/journal.pone.0202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra M, Mishra VK, Kashaw V, Iyer AK, Kashaw SK. 2017. Comprehensive review on various strategies for antimalarial drug discovery. Eur J Med Chem 125:1300–1320. doi: 10.1016/j.ejmech.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 5.WHO. 2020. Tackling antimalarial drug resistance. Launch of the WHO Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance. (2010–2019). World Health Organization, Geneva, Switzerland. https://www.who.int/docs/default-source/malaria/drug-resistance/who-ucn-gmp-2020-07-eng.pdf?sfvrsn=a2c11533_2&download=true.
- 6.Nsanzabana C. 2019. Resistance to artemisinin combination therapies (ACTs): do not forget the partner drug! Trop Med Infect Dis 4:26. doi: 10.3390/tropicalmed4010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tse EG, Korsik M, Todd MH. 2019. The past, present and future of anti-malarial medicines. Malar J 18:93. doi: 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouwman SAM, Zoleko-Manego R, Renner KC, Schmitt EK, Mombo-Ngoma G, Grobusch MP. 2020. The early preclinical and clinical development of cipragamin (KAE609), a novel antimalarial compound. Travel Med Infect Dis 36:101765. doi: 10.1016/j.tmaid.2020.101765. [DOI] [PubMed] [Google Scholar]
- 9.Koller R, Mombo-Ngoma G, Grobusch MP. 2018. The early preclinical and clinical development of ganaplacide (KAF156), a novel antimalarial compound. Expert Opin Invest Drugs 27:803–810. doi: 10.1080/13543784.2018.1524871. [DOI] [PubMed] [Google Scholar]
- 10.Held J, Jeyaraj S, Kreidenweiss A. 2015. Antimalarial compounds in phase II clinical development. Expert Opin Invest Drugs 24:363–382. doi: 10.1517/13543784.2015.1000483. [DOI] [PubMed] [Google Scholar]
- 11.Pandey AV, Singh N, Tekwani BL, Puri SK, Chauhan VS. 1999. Assay of β-hematin formation by malaria parasite. J Pharm Biomed Anal 20:203–207. doi: 10.1016/S0731-7085(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 12.M S, Koringa K, Dave U, Gatne D. 2015. A modified precise analytical method for anti-malarial screening: heme polymerization assay. Mol Biochem Parasitol 201:112–115. doi: 10.1016/j.molbiopara.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez JN, León C, Rodrigues J, Gamboa de Domínguez N, Gut J, Rosenthal PJ. 2005. Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Farmaco 60:307–311. doi: 10.1016/j.farmac.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Domínguez JN, León C, Rodrigues J, Gamboa de Domínguez N, Gut J, Rosenthal PJ. 2005. Synthesis and evaluation of new antimalarial phenylurenyl chalcone derivatives. J Med Chem 48:3654–3658. doi: 10.1021/jm058208o. [DOI] [PubMed] [Google Scholar]
- 15.Hadj-esfandiari N, Navidpour L, Shadnia H, Amini M, Samadi N, Faramarzi MA, Shafiee A. 2007. Synthesis, antibacterial activity, and quantitative structure-activity relationships of new (Z)-2-(nitroimidazolylmethylene)-3(2H)-benzofuranone derivatives. Bioorg Med Chem Lett 17:6354–6363. doi: 10.1016/j.bmcl.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718. doi: 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobarfard F, Yardley V, Little S, Daryaee F, Chibale K. 2012. Synthesis of aminoquinoline-based aminoalcohols and oxazolidinones and their antiplasmodial activity. Chem Biol Drug Des 79:326–331. doi: 10.1111/j.1747-0285.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- 18.Cameron A, Read J, Tranter R, Winter VJ, Sessions RB, Brady RL, Vivas L, Easton A, Kendrick H, Croft SL, Barros D, Lavandera JL, Martin JJ, Risco F, Garcia-Ochoa S, Gamo FJ, Sanz L, Leon L, Ruiz R, Gabarro JR, Mallo A, de las Heras FG. 2004. Identification and activity of a series of azole-based compounds with lactate dehydrogenase-directed anti-malarial activity. J Biol Chem 279:31429–31439. doi: 10.1074/jbc.M402433200. [DOI] [PubMed] [Google Scholar]
- 19.Afshar FH, Delazar A, Asnaashari S, Vaez H, Zolali E, Asgharian P. 2018. Screening of anti-malarial activity of different extracts obtained from three species of Scrophularia growing in Iran. Iran J Pharm Res 17:668–676. doi: 10.22037/IJPR.2018.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herraiz T, Guillén H, González-Peña D, Arán VJ. 2019. Antimalarial quinoline drugs inhibit β-hematin and increase free hemin catalyzing peroxidative reactions and inhibition of cysteine proteases. Sci Rep 9:15398. doi: 10.1038/s41598-019-51604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters W. 1967. Rational methods in the search for antimalarial drugs. Trans R Soc Trop Med Hyg 61:400–410. doi: 10.1016/0035-9203(67)90015-6. [DOI] [Google Scholar]
- 22.Peters W, Portus JH, Robinson BL. 1975. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P berghei in screening for blood schizonticidal activity. Ann Trop Med Parasitol 69:155–171. doi: 10.1080/00034983.1975.11686997. [DOI] [PubMed] [Google Scholar]