ABSTRACT
Cefiderocol (CFDC), a novel siderophore cephalosporin, demonstrates strong activity against multidrug-resistant (MDR) Acinetobacter baumannii. Limited studies have evaluated CFDC alone and in combination with other Gram-negative antibiotics against MDR A. baumannii isolates. Susceptibility testing revealed lower CFDC MIC values (87% of MICs ≤ 4mg/liter) than the comparator Gram-negative agents. Six isolates, with elevated CFDC MICs (16 to 32 mg/liter) were selected for further experiments. Time-kill analyses presented with synergistic activity and beta-lactamase inhibitors increased CFDC susceptibility in each of the isolates.
KEYWORDS: A. baumannii, Gram-negative, cefiderocol, multidrug-resistant infection
INTRODUCTION
Multidrug-resistant (MDR) Acinetobacter baumannii remains one of the most challenging public health threats due to its ability to escape the activity of the majority of our antibiotic armamentarium (1–3). Carbapenems, as well as colistin (COL) and tigecycline (TGC), have been routinely utilized as appropriate therapy in the treatment of MDR A. baumannii in the clinical setting; however, the continued increase in A. baumannii resistance has led to the decreased efficacy of these agents (4–9), further attesting to the critical need for antibiotics with novel mechanisms of action for evading Gram-negative resistance (10).
Cefiderocol (CFDC) is a novel siderophore-cephalosporin conjugated with an iron-chelating catechol moiety at the 3-position side chain (11). The catechol moiety sequesters free iron to facilitate CFDC’s entry across the outer cell membrane of Gram-negative bacteria in a “Trojan horse”-like approach, via the bacteria’s iron-transport system (11, 12). CFDC has demonstrated strong in vitro activity against MDR A. baumannii isolates, including those that were identified as carbapenem resistant (13, 14). Although these studies lay groundwork to support the potential role for CFDC in mitigating MDR A. baumannii infections, important gaps in knowledge remain.
The objective of this study was to evaluate the antibacterial activity of CFDC against a diverse collection of 150 MDR A. baumannii isolates (including carbapenem-resistant and COL-resistant phenotypes) compared to and in combination with other commercially available Gram-negative antibiotics via broth microdilution (BMD) MIC testing and 24-h time-kill analyses (TKA).
The MIC values of meropenem (MEM), COL, TGC, minocycline (MIN), amikacin (AMK), ceftazidime (CAZ), and ampicillin-sulbactam (SAM) (all purchased through Sigma Chemical Company, St. Louis) were determined in duplicate by the broth microdilution (BMD) method in a 96-well plate, in standard Mueller-Hinton broth (MHB) (Difco, Detroit, MI) supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium, with an inoculum of approximately 106 CFU/ml, per the CLSI guidelines. CFDC MICs were determined in duplicate by BMD using TREK panels supplied by International Health Management Associates, Inc. (IHMA, Inc.). For reference, the ATCC strain 25922 (Escherichia coli) and ATCC 27853 (Pseudomonas aeruginosa) were used in the completion MIC testing (MIC range 0.06 to 0.5 mg/liter). Cefiderocol at a concentration of ≤4 mg/liter inhibited the growth of 87% (130/150) of the A. baumannii isolates evaluated in the study. Of the 32 COL-resistant (COL-R) isolates evaluated, 94% (30/32) of the isolates were susceptible to CFDC. The MIC50 and MIC90 values reflected that CFDC presented with significantly lower MIC values compared to the other commonly used Gram-negative agents and, more specifically, when tested against carbapenem-resistant and COL-R A. baumannii isolates (P < 0.001). The MIC ranges for all of the agents are listed in Table 1.
TABLE 1.
MIC range, MIC50, and MIC90 against 118 carbapenem-resistant, COL-nonresistant isolates (mg/liter)
| Antimicrobial agenta | MIC range 118 COL-non-R isolates | MIC range 32 COL-resistant isolates | MIC50 118 COL-non-R isolates | MIC50 32 COL-resistant isolates | MIC90 118 COL-non-R isolates | MIC90 32 COL-resistant isolates |
|---|---|---|---|---|---|---|
| CFDC | 0.125–32 | 0.06–32 | 1 | 1 | 1 | 4 |
| MEM | 8–128 | 8–128 | 16 | 32 | 32 | 64 |
| COL | 0.25–2 | 4–256 | 0.5 | 16 | 1 | 32 |
| TGC | 2–32 | 4–32 | 4 | 8 | 8 | 8 |
| AMK | 8–>256 | 8–>256 | 256 | 256 | >256 | >256 |
| CAZ | 32–>256 | 32–>256 | 256 | 256 | >256 | >256 |
| MIN | 0.063–32 | 0.125–32 | 1 | 4 | 4 | 16 |
| SAM | 4/2–128/64 | 16/8–128/64 | 32/16 | 64/32 | 64/32 | 64/32 |
CFDC, cefiderocol; MEM, meropenem; COL, colistin; TGC, tigecycline; AMK, amikacin; CAZ, ceftazidime; MIN, minocycline; SAM, ampicillin/sulbactam.
Six isolates presented with elevated MICs (16 to 32 mg/liter) to CFDC. These isolates were submitted to whole-genome sequencing (WGS) and multilocus sequence typing (https://pubmlst.org/abaumannii/) for the identification and analysis of genes related to β-lactam resistance. The sequence types (ST) of these isolates were compared to A. baumannii-calcoaceticus species complex from the SENTRY Antimicrobial Surveillance Program. Isolates with the highest genetic similarity were susceptibility tested against CFDC and used as a control for the genetic analysis. Briefly, WGS was performed with total genomic DNA as input for a DNA library prepared using Nextera XT library construction protocol and index kit (Illumina, San Diego, CA, USA). Sequencing was conducted in a MiSeq using reagent kit v3 (600 cycle; Illumina).
FASTQ format files for each sample set were assembled independently using the de novo assembler SPAdes v3.11.1. An in-house-designed software used the target assembled sequences as queries to align against numerous β-lactam resistance determinants as part of a curated database (https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/). Other resistance genes we searched by reference-guided assembly. Whole-genome sequencing revealed extended-spectrum AmpC (ADC variants) and OXA-51-like-encoding genes, and the acquired OXA-23 and TEM-1 that were present in all six and two isolates, respectively. In addition, AdeB mutations are listed and were noted in comparison to CFDC-susceptible isolates from the same sequence type (ST). The sequencing results of the six isolates are shown in Table 2.
TABLE 2.
Cefiderocol (CFDC) project isolates with CFDC MICs of 16 to 32 mg/litera
| Isolate no. | R no. | Species | Geographical location | Cefiderocol MIC (mg/liter) | CFDC+AVI MIC (mg/liter) | CFDC+SUL MIC (mg/liter) | CFDC+TAZ MIC (mg/liter) | CFDC+CLAV MIC (mg/liter) |
|---|---|---|---|---|---|---|---|---|
| 1 | 11248 | A. baumannii | Thailand | 32 | 0.5 | 2 | 8 | 1 |
| 2 | 10141 | A. baumannii | Thailand | 32 | 1 | 32 | 32 | 32 |
| 3 | 9755 | A. baumannii | Israel | 32 | 1 | 0.5 | 4 | 1 |
| 4 | 11357 | A. baumannii | Israel | 16 | 1 | 1 | 1 | 1 |
| 5 | 11189 | A. baumannii | Thailand | 32 | 1 | 1 | 4 | 0.25 |
| 6 | 10053 | A. baumannii | United States | 32 | 8 | 4 | 32 | 4 |
AVI, avibactam; SUL, sulbactam; TAZ, tazobactam; CLAV, clavulanic acid.
To assess the impact of the addition of beta-lactamase inhibitors (BLIs) on decreasing elevated CFDC MICs, we supplemented tazobactam (TAZ), sulbactam (SUL), avibactam (AVI), and clavulanic acid (CLAV) to CFDC and completed BMD susceptibility testing. CLAV, TAZ, and SUL were purchased through Sigma Chemical Company (St. Louis, MO) and AVI was purchased through Fisher Scientific (Pittsburgh, PA). The BLIs were supplemented in the following ratios to CFDC: 8:1 (TAZ) (15), 2:1 (SUL) (16), 4:1 (AVI) (17), and 4:1 (CLAV) (18). All in vitro testing for CFDC was completed with the use of iron-depleted, cation-adjusted Mueller-Hinton broth (ID-CAMHB; iron concentration <0.1 mg/liter) to ensure the induction of bacterial iron transporters per manufacturer standards (19).We observed a decline in the MIC values with the addition of the BLIs for each of the isolates. AVI produced the most frequent reduction in MIC values for all of the tested isolates, with an average 28-fold reduction in MIC values observed. The CFDC MICs of each of the isolates with the addition of the beta-lactamase inhibitors are provided in Table 3.
TABLE 3.
Beta-lactam resistance mechanisms among the A. baumannii isolates with elevated CFDC MICs
| R no. | MLSTa | CFDCa MIC (mg/liter) | Efflux pump mutations | Acquired beta-lactamases |
|---|---|---|---|---|
| 11248 | 2 | 32 | AdeB (T674S) | ADC-33, OXA-82, OXA-23 |
| 10141 | 823 | 32 | AdeB (Q177R) | ADC-73, OXA-66, OXA-23, TEM-1 |
| 11189 | 2 | 32 | AdeB (E90K; also observed in ST3 CFDC-susceptible isolates) | ADC-73, OXA-66, OXA-23, TEM-1 |
| 10053 | 2 | 32 | AdeB (T674S) | ADC-172, OXA-82, OXA-23 |
| 9755 | 3 | 32 | None detected | ADC-79, OXA-71, OXA-23 |
| 11357 | 3 | 16 | None detected | ADC-6-like, OXA-71, OXA-23 |
MLST, multilocus sequence type; CFDC, cefiderocol.
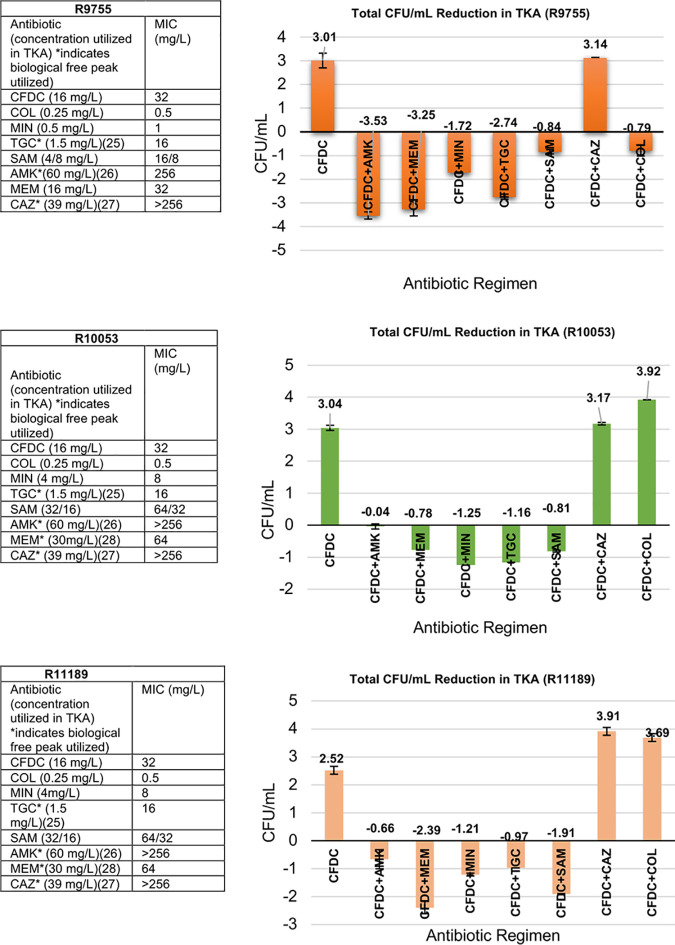
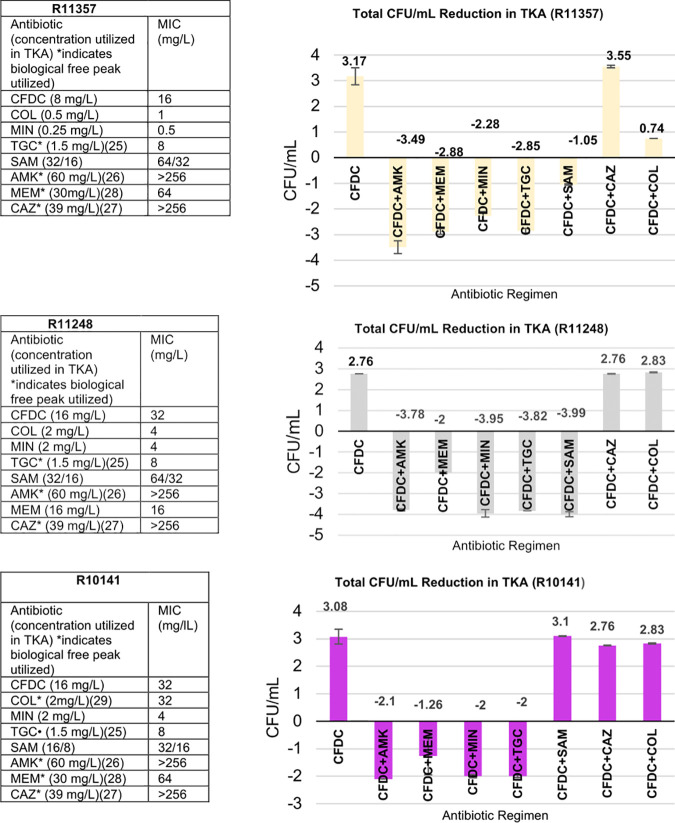
The six isolates with elevated MICs were further evaluated in TKA, with 24-well tissue culture plates utilizing ID-CAMHB as growth medium (supplied by IHMA, Inc.) to assess the potential for enhanced activity when combined with additional Gram-negative agents. All antimicrobials were tested at 0.5× MIC or the maximum concentration of free drug in serum (fCmax), whichever was lower. Synergistic activity was defined as a ≥2-log10 CFU/ml from the most active single agent; bactericidal activity was defined as a ≥3-log10 CFU/ml reduction from the starting inoculum; and bacterial growth of ≥1-log CFU/ml in either combination compared to single agent(s) was considered antagonistic activity. The TKA were conducted in duplicate to ensure reproducibility. Of the isolates that were evaluated, 5/6 had a CFDC MIC of 32 mg/liter, and the single remaining strain had a MIC of 16 mg/liter. We observed synergistic activity in 100% (6/6) of the isolates with the CFDC+MEM, CFDC+AMK, CFDC+TGC, CFDC+MIN, and CFDC+SAM combinations, and bactericidal activity was observed in several of these isolates with the CFDC+MIN, CFDC+TGC, CFDC+MEM, and CFDC+AMK combinations. CFDC+CAZ did not present with improved activity in any of the evaluated isolates, and, in the COL-R strains, the CFDC+COL combination did not reveal enhanced activity.
Of note, in 4/6 of the TKAs, the combinations of CFDC+AMK and CFDC+MEM resulted in the highest reductions in bacterial counts (log10 CFU/ml), despite the increased resistance to either agent (P < 0.001). The six TKAs are pictured in Fig. 1.
FIG 1.
Six isolates with elevated CFDC MICs tested alone and in combination with other Gram-negative agents in TKA.
Acinetobacter baumannii-mediated infections are a leading cause of nosocomial infections, with MDR isolates increasing in prevalence. With respect to this, CFDC retains activity against a large percentage of highly resistant A. baumannii isolates. Nevertheless, the propensity for A. baumannii to develop mechanisms of resistance, and the potential for the emergence of CFDC resistance, calls for the exploration of alternative mitigating strategies.
Overall, the addition of each of the BLIs decreased the elevated MICs of the six evaluated isolates. This is likely attributed to the fact that multiple classes of beta-lactamases (class A, class C, and class D) and efflux genes associated with decreased cephalosporin activity (adeB) were simultaneously present in the isolates (20). Thus, to some extent, the addition of each of the BLIs to CFDC improved its activity. Further, upon the genetic analysis completed in our study, we observed that each of the six A. baumannii isolates had extended-spectrum AmpC beta-lactamases present (ADC variants), which have the potential to severely impact extended-spectrum cephalosporin activity (21, 22). Nevertheless, studies have shown that AVI has activity against ADC variants in Gram-negative organisms, which could possibly attribute to the increased susceptibility of CFDC in the A. baumannii isolates observed with AVI use (23).
Additionally, we observed synergy with the other Gram-negative agents in 100% of the evaluated isolates. Of note, synergy, as well as bactericidal activity, was most often displayed with CFDC+AMK and CFDC+MEM dual therapies, although all isolates evaluated were resistant to MEM and AMK. This may potentially be attributed to increased CFDC permeability induced by an AMK-mediated disruption of the A. baumannii outer membrane, and the complementary binding of PBP2 by MEM and PBP3 binding by CFDC, with the CFDC+AMK and CFDC+MEM combinations, respectively. Notably, synergy was not observed in any of the six resistant isolates when tested in the TKA against the CFDC+CAZ combination. The structure of CFDC is closely related to that of CAZ, with each antimicrobial binding to PBP1a, PBP1b, PBP2, and PBP3 (11, 24). Therefore, it is possible that the saturation of target binding sites eliminates the ability of CAZ to bind to the PBPs to potentiate action and ultimately synergize with CFDC.
Although our study did present with positive findings for CFDC, both MIC testing as well as TKA are short in duration and use static concentrations, thus presenting a limitation when considering clinical applicability. Also, all 150 of the isolates included were not investigated for detailed phenotypes and genotypes regarding specific mechanisms of resistance, which may also limit the application of these findings in clinical settings.
Overall, CFDC presented with high susceptibility compared to other commonly utilized Gram-negative agents against MDR, including COL-R A. baumannii isolates. This study also investigated and revealed that CFDC is capable of producing synergy with other agents, despite increased MICs to either drug. The characteristics of CFDC indicate that it may be a promising agent, given either as a monotherapy or in combination with other commonly utilized antimicrobials. Further research is warranted to solidify the positioning of CFDC as an antibiotic for use against MDR A. baumannii.
Data availability.
Whole-genome sequence data have been deposited in NCBI under BioProject no. PRJNA735707.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Doi Y, Bonomo RA, Hooper DC, Kaye KS, Johnson JR, Clancy CJ, Thaden JT, Stryjewski ME, van Duin D, Gram-Negative Committee of the Antibacterial Resistance Leadership Group (ARLG). 2017. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the antibacterial resistance leadership group. Clin Infect Dis 64:S30–S35. 10.1093/cid/ciw829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzler E, Goff DA, Humphries R, Goldstein EJC. 2017. Anticipating the unpredictable: a review of antimicrobial stewardship and Acinetobacter infections. Infect Dis Ther 6:149–172. 10.1007/s40121-017-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Queenan AM, Pillar CM, Deane J, Sahm DF, Lynch AS, Flamm RK, Peterson J, Davies TA. 2012. Multidrug resistance among Acinetobacter spp. in the USA and activity profile of key agents: results from CAPITAL Surveillance 2010. Diagn Microbiol Infect Dis 73:267–270. 10.1016/j.diagmicrobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.McCann E, Srinivasan A, DeRyke CA, Ye G, DePestel DD, Murray J, Gupta V. 2018. Carbapenem-nonsusceptible Gram-negative pathogens in ICU and non-ICU settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis 5:ofy241. 10.1093/ofid/ofy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pogue JM, Mann T, Barber KE, Kaye KS. 2013. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev Anti Infect Ther 11:383–393. 10.1586/eri.13.14. [DOI] [PubMed] [Google Scholar]
- 6.Garnacho-Montero J, Amaya-Villar R. 2010. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis 23:332–339. 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 7.Dizbay M, Tozlu DK, Cirak MY, Isik Y, Ozdemir K, Arman D. 2010. In vitro synergistic activity of tigecycline and colistin against XDR-Acinetobacter baumannii. J Antibiot (Tokyo) 63:51–53. 10.1038/ja.2009.117. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Chen Y, Fang Y, Wang X, Chen Y, Qi Q, Huang F, Xiao X. 2015. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci Rep 5:17091. 10.1038/srep17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. 2015. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 45:8–18. 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi ZA, Hittle LE, O'Hara JA, Rivera JI, Syed A, Shields RK, Pasculle AW, Ernst RK, Doi Y. 2015. Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 60:1295–1303. 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, Idowu T, Domalaon R, Schweizer F, Zhanel MA, Lagace-Wiens PRS, Walkty AJ, Noreddin A, Lynch Iii JP, Karlowsky JA. 2019. Cefiderocol: a siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 79:271–289. 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 12.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother 61:e00093-17. 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2018. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 62:e01968-17. 10.1128/AAC.01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagel S, Fiedler S, Hohn A, Brinkmann A, Frey OR, Hoyer H, Schlattmann P, Kiehntopf M, Roberts JA, Pletz MW, Group TS, TARGET Study Group. 2019. Therapeutic drug monitoring-based dose optimisation of piperacillin/tazobactam to improve outcome in patients with sepsis (TARGET): a prospective, multi-centre, randomised controlled trial. Trials 20:330. 10.1186/s13063-019-3437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anonymous. 1987. Ampicillin/sulbactam (Unasyn). Med Lett Drugs Ther 29:79–81. [PubMed] [Google Scholar]
- 17.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, LynchJP, 3rd, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/beta-lactamase inhibitor combination. Drugs 73:159–177. 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 18.Sedivy J, Petkov V, Jirkovska A, Stambergova A, Ulbrichova Z, Lupinkova J, Fejfarova V, Bem R. 2004. Optimization of amoxicillin/clavulanate therapy based on pharmacokinetic/pharmacodynamic parameters in patients with diabetic foot infection (in Czech). Klin Mikrobiol Infekc Lek 10:167–175. [PubMed] [Google Scholar]
- 19.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller-Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Wang M, Xie Y, Li X, Dong Z, Liu Y, Wang L, Yang M, Song H, Cao H, Cao W. 2017. Active efflux pump adeB is involved in multidrug resistance of Acinetobacter baumannii induced by antibacterial agents. Exp Ther Med 13:1538–1546. 10.3892/etm.2017.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian G-B, Adams-Haduch JM, Taracila M, Bonomo RA, Wang H-N, Doi Y. 2011. Extended-spectrum AmpC cephalosporinase in Acinetobacter baumannii: ADC-56 confers resistance to cefepime. Antimicrob Agents Chemother 55:4922–4925. 10.1128/AAC.00704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai A, McElheny CL, Iovleva A, Kline EG, Sluis-Cremer N, Shields RK, Doi Y. 2020. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother 64:e00198-20. 10.1128/AAC.00198-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlowsky JA, Biedenbach DJ, Kazmierczak KM, Stone GG, Sahm DF. 2016. Activity of ceftazidime-avibactam against extended-spectrum- and AmpC beta-lactamase-producing Enterobacteriaceae collected in the INFORM Global Surveillance Study from 2012 to 2014. Antimicrob Agents Chemother 60:2849–2857. 10.1128/AAC.02286-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2018. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Download AAC.02646-20-s0001.pdf, PDF file, 0.2 MB (201.7KB, pdf)
Data Availability Statement
Whole-genome sequence data have been deposited in NCBI under BioProject no. PRJNA735707.