ABSTRACT
Antibiotic combinations, including ceftazidime/avibactam (CAZ/AVI), are frequently employed to combat KPC-producing Klebsiella pneumoniae (KPC-Kp), though such combinations have not been rationally optimized. Clinical KPC-Kp isolates with common genes encoding aminoglycoside-modifying enzymes (AMEs), aac(6′)-Ib′ or aac(6′)-Ib, were used in static time-kill assays (n = 4 isolates) and the hollow-fiber infection model (HFIM; n = 2 isolates) to evaluate the activity of gentamicin, amikacin, and CAZ/AVI alone and in combinations. A short course, one-time aminoglycoside dose was also evaluated. Gentamicin plus CAZ/AVI was then tested in a mouse pneumonia model. Synergy with CAZ/AVI was more common with amikacin for aac(6′)-Ib′-containing KPC-Kp but more common with gentamicin for aac(6′)-Ib-containing isolates in time-kill assays. In the HFIM, although the isolates were aminoglycoside-susceptible at baseline, aminoglycoside monotherapies displayed variable initial killing, followed by regrowth and resistance emergence. CAZ/AVI combined with amikacin or gentamicin resulted in undetectable counts 50 h sooner than CAZ/AVI monotherapy against KPC-Kp with aac(6′)-Ib′. CAZ/AVI monotherapy failed to eradicate KPC-Kp with aac(6′)-Ib and a combination with gentamicin led to undetectable counts 70 h sooner than with amikacin. A one-time aminoglycoside dose with CAZ/AVI provided similar killing to aminoglycosides dosed for 7 days. In the mouse pneumonia model (n = 1 isolate), gentamicin and CAZ/AVI achieved a 6.0-log10 CFU/lung reduction at 24 h, which was significantly greater than either monotherapy (P < 0.005). Aminoglycosides in combination with CAZ/AVI were promising for KPC-Kp infections; this was true even for a one-time aminoglycoside dose. Selecting aminoglycosides based on AME genes or susceptibilities can improve the pharmacodynamic activity of the combination.
KEYWORDS: KPC, Klebsiella, aminoglycoside-modifying enzymes, aminoglycosides, antimicrobial combinations, carbapenemase
TEXT
Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp) remains an urgent health threat worldwide and can cause severe infections with high mortality rates (1, 2). The β-lactam/β-lactamase inhibitor combination ceftazidime/avibactam (CAZ/AVI) has become an important therapeutic option for KPC-Kp infections with susceptibility rates for this pathogen exceeding 90% (3–5). Despite susceptibility to CAZ/AVI, treatment failure still occurs in 28 to 45% of patients with KPC-Kp infections (6–8). Aminoglycosides are frequently used in combination with CAZ/AVI for KPC-Kp infections and hold promise as an adjuvant given their high susceptibility rates among KPC-Kp (8–10). Furthermore, synergy between β-lactams and aminoglycosides has been previously observed (11). However, aminoglycoside susceptibility varies between KPC-Kp isolates since they often harbor multiple aminoglycoside-modifying enzymes (AMEs), which are the primary mechanism of aminoglycoside resistance in K. pneumoniae. The variation in susceptibility is due to the specificity of each AME for a subset of the aminoglycosides (12, 13). For example, about 90% of KPC-Kp harbor the AME AAC(6′)-Ib, which partially inactivates gentamicin and fully inactivates amikacin, while some KPC-Kp harbor AAC(6′)-Ib′, which fully inactivates gentamicin but not amikacin (12–14). Selection of an aminoglycoside based on AME genes present may ultimately facilitate more rapid optimization of therapy.
Several studies have detected synergy between CAZ/AVI and a secondary agent against KPC-Kp isolates using checkerboard or Etest synergy assays (3, 15, 16). However, very limited data exist for combinations with CAZ/AVI and aminoglycosides for KPC-Kp. Mikhail et al. found that CAZ/AVI in combination with amikacin was synergistic against one KPC-Kp isolate in a static time-kill assay (17). However, there are no published studies that evaluate combinations with CAZ/AVI against KPC-Kp isolates in dynamic in vitro models. We sought here to evaluate combinations with CAZ/AVI and an aminoglycoside using static time-kill assays, the hollow-fiber infection model (HFIM), and a neutropenic mouse pneumonia model to identify optimal combinations for KPC-Kp with clinically relevant AMEs, aac(6′)-Ib or aac(6′)-Ib′.
RESULTS
Antibiotic susceptibility testing and time-kill assays.
The KPC-Kp NU-CRE61, -CRE78, -CRE85, and -CRE213 CAZ/AVI MICs were 0.25/4, 0.5/4, 0.5/4, and 0.03/4 mg/liter, respectively; the gentamicin MICs were 4, 4, 0.25, and 0.25 mg/liter, respectively; and the amikacin MICs were 2, 2, 8, and 8 mg/liter, respectively (Table 1). Each isolate was susceptible to all tested drugs according to CLSI (18).
TABLE 1.
MICs and aminoglycoside acetyltransferase gene allele for blaKPC-3-harboring K. pneumoniae isolatesa
| Isolateb | MIC (mg/liter) |
Aminoglycoside acetyltransferase gene allele | ||
|---|---|---|---|---|
| CAZ/AVI | GEN | AMK | ||
| NU-CRE61* | 0.25/4 | 4 | 2 | aac(6′)-Ib′ |
| NU-CRE78* | 0.5/4 | 4 | 2 | aac(6′)-Ib′ |
| NU-CRE85* | 0.5/4 | 0.25 | 8 | aac(6′)-Ib |
| NU-CRE213† | 0.03/4 | 0.25 | 8 | aac(6′)-Ib |
CAZ/AVI, ceftazidime/avibactam; GEN, gentamicin; AMK, amikacin. The CLSI susceptible breakpoints for GEN and AMK are ≤4 and ≤16 mg/liter, respectively. The CLSI resistance breakpoints for GEN and AMK are ≥16 and ≥64 mg/liter, respectively.
*, Isolate that harbors other AME genes: ant(3″)-Ia, aph(6)-Ia, and aph(6)-Id; †, isolate that harbors other AME genes: ant(3″)-Ia and aph(3)-Ia.
In time-kill assays, CAZ/AVI concentrations of 20/3.75 mg/liter achieved mean bacterial killing of 3.10 log10 CFU/ml at 24 h (Fig. 1 and 2). For aac(6′)-Ib-containing KPC-Kp isolates, gentamicin concentrations of ≥2.5 mg/liter were bactericidal (Fig. 1, C3 to C5 and D3 to D5), whereas no gentamicin concentration was bactericidal for aac(6′)-Ib′-containing isolates (Fig. 1, A1 to A5 and B1 to B5). Amikacin monotherapy displayed 2.73 log10 CFU/ml greater mean bacterial killing against aac(6′)-Ib′-containing isolates than it did against aac(6′)-Ib-containing isolates at 15 mg/liter. For aac(6′)-Ib′-containing isolates, CAZ/AVI at 80/15 mg/liter combined with amikacin at ≥7.5 mg/liter was synergistic (Fig. 2, A2 to A5 and B2 to B5; Fig. 3, A2 and B2), whereas synergy was only achieved with amikacin against one isolate with aac(6′)-Ib at 60 mg/liter (Fig. 2, D5; Fig. 3, D2). Synergy with CAZ/AVI was more common with amikacin for aac(6′)-Ib′-containing isolates, whereas it was more common with gentamicin for aac(6′)-Ib-containing isolates (Fig. 3). Higher concentrations of gentamicin did not achieve synergy with CAZ/AVI against aac(6′)-Ib-containing isolates since monotherapies were highly active.
FIG 1.
Activity of gentamicin (GEN) combined with CAZ/AVI against KPC-Kp isolates NU-CRE61 and -CRE78 containing aac(6′)-Ib′ (A1 to A5, B1 to B5) and NU-CRE85 and -CRE213 containing aac(6′)-Ib (C1 to C5, D1 to D5) in time-kill assays over 24 h. For each isolate, the growth control and monotherapy lines are duplicated in multiple panels to enable easier comparisons with combinations. The LLQ for bacterial density was 2.4 log10 (CFU/ml).
FIG 2.
Activity of amikacin (AMK) combined with CAZ/AVI against KPC-Kp isolates NU-CRE61 and -CRE78 containing aac(6′)-Ib′ (A1 to A5, B1 to B5) and NU-CRE85 and -CRE213 containing aac(6′)-Ib (C1 to C5, D1 to D5) in time-kill experiments over 24 h. For each isolate, the growth control and monotherapy lines are duplicated in multiple panels to enable easier comparisons with combinations. The LLQ for bacterial density was 2.4 log10 (CFU/ml).
FIG 3.
Heat map depicting interactions between gentamicin or amikacin combined with CAZ/AVI against KPC-Kp isolates NU-CRE61 and -CRE78 containing aac(6′)-Ib′ (A and B, E and F) and NU-CRE85 and -CRE213 containing aac(6′)-Ib (C and D, G and H) at 24 h in time-kill assays. Green and white boxes represent synergy and no synergy, respectively.
HFIM and LC-MS/MS analysis.
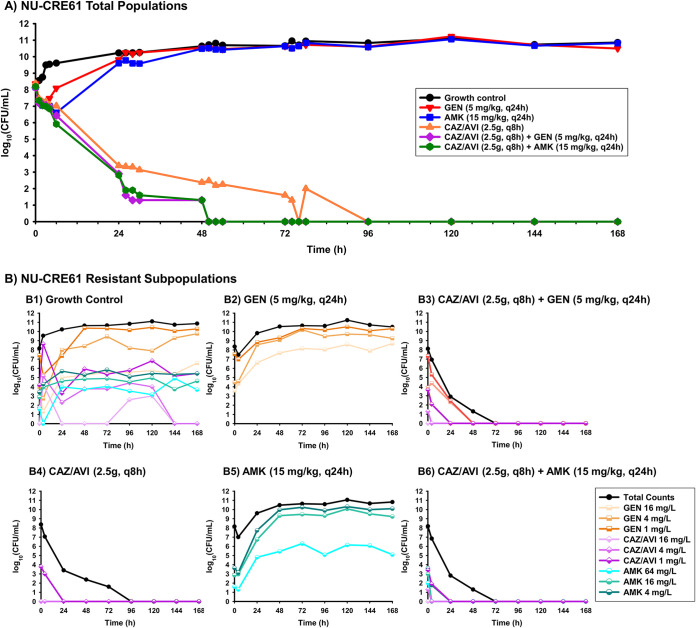
In the HFIM, there was good agreement between the detected and predicted pharmacokinetic profiles for ceftazidime (R2 = 0.986, slope = 1.011, and intercept = −0.086), avibactam (R2 = 0.979, slope = 1.276, and intercept = 0.374), and amikacin (R2 = 0.980, slope = 1.015, and intercept = −0.207) (see Fig. S1 in the supplemental material). For aac(6′)-Ib′-containing KPC-Kp isolate NU-CRE61, CAZ/AVI monotherapy caused 1.36- and 5.90-log10 CFU/ml reductions at 6 and 50 h, respectively (Fig. 4). Bacterial counts were undetectable at 96 h of exposure to CAZ/AVI, and no growth was observed after that time. Amikacin monotherapy caused 1.56-log10 CFU/ml reduction at 6 h but regrew to 10.52 log10 CFU/ml by 50 h. Gentamicin monotherapy caused 1.16-log10 CFU/ml reduction at 2 h but regrew to 10.51 log10 CFU/ml by 50 h. CAZ/AVI in combination with amikacin caused bacterial reductions of 2.27 and 8.19 log10 CFU/ml at 6 and 50 h, respectively. The combination of CAZ/AVI and amikacin caused 10.55-log10 CFU/ml greater reduction at 50 h than did amikacin monotherapy. The combination of CAZ/AVI and gentamicin achieved killing similar to the combination of CAZ/AVI and amikacin. Undetectable counts occurred ∼46 h earlier (∼22 h earlier if ignoring counts below the lower limit of quantification [LLQ]) with both combinations than with CAZ/AVI monotherapy.
FIG 4.
(A) HFIM total bacterial population counts over 168 h for KPC-Kp NU-CRE61 exposed to monotherapies (CAZ/AVI, 2.5 g q8h; gentamicin [GEN], 5 mg/kg q24h; or amikacin [AMK], 15 mg/kg q24h) and combination regimens with a starting inoculum of ∼108 CFU/ml. (B) Population analysis profiles for KPC-Kp NU-CRE61 were prepared to quantify CAZ/AVI-, GEN-, and AMK-resistant subpopulations, which are fractions of the respective total population (black lines). B1 represents resistant subpopulations detected without antibiotic treatment (growth control), while B2, B4, and B5 represent resistant subpopulations detected during exposure to monotherapies, and B3 and B6 represent resistant subpopulations detected during exposure to combinations in the HFIM. The LLQ for bacterial density was 2.4 log10 (CFU/ml).
For aac(6′)-Ib-containing KPC-Kp isolate NU-CRE85, CAZ/AVI monotherapy resulted in bacterial reductions of 1.75 and 3.06 log10 CFU/ml at 6 and 50 h, respectively, though 2.75 log10 CFU/ml of NU-CRE85 remained at 7 days (Fig. 5). Gentamicin monotherapy caused undetectable bacterial counts at 6 h but rapidly regrew to 10.06 log10 CFU/ml by 50 h, whereas amikacin monotherapy only resulted in 0.14-log10 CFU/ml reduction at 2 h and regrew to 10.88 log10 CFU/ml at 76 h. CAZ/AVI in combination with gentamicin resulted in undetectable NU-CRE85 counts at 6 h, and no growth was observed after that time. At 6 h, this combination demonstrated bacterial killing that was 6.56 log10 CFU/ml greater than that of either monotherapy. CAZ/AVI in combination with amikacin resulted in bacterial reductions of 2.03 log10 CFU/ml at 6 h and undetectable viable bacterial counts by 76 h, which was a 4.17-log10 CFU/ml greater reduction than that of either monotherapy at 76 h.
FIG 5.
(A) HFIM total bacterial population counts in log10 CFU/ml over 168 h for KPC-Kp NU-CRE85 versus monotherapies (CAZ/AVI, 2.5 g q8h; gentamicin [GEN], 5 mg/kg q24h; and amikacin [AMK], 15 mg/kg q24h) and combination regimens with a starting inoculum of ∼108 CFU/ml. (B) Population analysis profiles for KPC-Kp NU-CRE85 were performed to quantify CAZ/AVI (1, 4, and 16 mg/liter)-, GEN (1, 4, and 16 mg/liter)-, and AMK (4, 16, and 64 mg/liter)-resistant subpopulations, which are fractions of the respective total population (black lines). B1 represents nonantibiotic treatment growth control, B2, B4, and B5 represent monotherapies, and B3 and B6 represent combinations in the HFIM. The LLQ for bacterial density was 2.4 log10 (CFU/ml).
Regrowth after exposure to either aminoglycoside monotherapy corresponded to amplification of aminoglycoside-resistant subpopulations through 168 h, even on the highest concentrations of gentamicin (16 mg/liter)- or amikacin (64 mg/liter)-containing plates (Fig. 4, B2 and B5; Fig. 5, B2 and B5). CAZ/AVI monotherapy did not lead to amplification of CAZ/AVI-resistant subpopulations (Fig. 4, B4; Fig. 5, B4). When gentamicin or amikacin was combined with CAZ/AVI, the proliferation of gentamicin- or amikacin-resistant subpopulations was suppressed within 96 h (Fig. 4, B3 and B6; Fig. 5, B3 and B6). Amikacin combined with CAZ/AVI killed the aminoglycoside-resistant subpopulation 44 h faster than gentamicin combined with CAZ/AVI against aac(6′)-Ib′-containing NU-CRE061 (Fig. 4, B3 and B6). In contrast, the combination of gentamicin and CAZ/AVI killed the aminoglycoside-resistant subpopulation 94 h faster than amikacin combined with CAZ/AVI against aac(6′)-Ib-containing NU-CRE085 (Fig. 5, B3 and B6).
Reducing the duration of aminoglycoside treatment to a one-time dose administered at 0 h resulted in similar killing compared to seven aminoglycoside doses administered every 24 h (Fig. 6). For example, CAZ/AVI plus a one-time dose of amikacin led to undetectable bacterial counts of NU-CRE61 [aac(6′)-Ib′] by 48 h (Fig. 6, A1), which was similar to the time required for CAZ/AVI plus a multiple dose regimen of amikacin to achieve undetectable counts (50 h) (Fig. 4A). Similarly, against NU-CRE85, CAZ/AVI in combination with gentamicin administered as a one-time dose or once-daily dose achieved undetectable bacterial counts at 24 and 6 h, respectively (Fig. 6, A2; Fig. 5A). Similar suppression of antibiotic-resistant subpopulations was achieved for aminoglycosides administered one-time (Fig. 4, B6; Fig. 5, B3; Fig. 6, B1 and B2).
FIG 6.
HFIM total bacterial population counts and population analysis profiles in log10 CFU/ml over 168 h for KPC-Kp NU-CRE61 versus CAZ/AVI (2.5g q8h) + one dose of amikacin (AMK, 15 mg/kg q24h) (A1) and NU-CRE85 versus CAZ/AVI (2.5g q8h) + one dose of gentamicin (GEN, 5 mg/kg q24h) (A2) with a starting inoculum of ∼108 CFU/ml. Population analysis profiles (B1, B2) were performed to quantify CAZ/AVI (1, 4, and 16 mg/liter)-, GEN (1, 4, and 16 mg/liter)-, or AMK (4, 16, and 64 mg/liter)-resistant subpopulations, which are fractions of the respective total population (black lines). The LLQ for bacterial density was 2.4 log10 (CFU/ml).
Neutropenic mouse pneumonia model.
The neutropenic mouse pneumonia model was used as a proof-of-concept to evaluate the efficacy of CAZ/AVI and aminoglycoside combinations in vivo (Fig. 7). At 24 h, mean bacterial lung concentrations (± the standard deviation) for the untreated control group, gentamicin, CAZ/AVI, and a combination of gentamicin and CAZ/AVI were 8.97 ± 0.09, 4.64 ± 0.34, 5.95 ± 0.25, and 2.95 ± 1.07 log10 CFU/lung, respectively. Compared to the untreated control group at 24 h, gentamicin and CAZ/AVI monotherapies decreased the bacterial lung concentrations of NU-CRE85 by 4.33 and 3.02 log10 CFU/lung (PAdj < 0.0001), respectively. The combination of gentamicin and CAZ/AVI showed significantly enhanced bacterial killing compared to the most potent monotherapy (gentamicin) (6.02- versus 4.33-log10 CFU/lung reductions, PAdj < 0.005).
FIG 7.

Efficacy of gentamicin combined with CAZ/AVI against KPC-Kp isolate NU-CRE85 in murine pneumonia model. The black bars represent mean values.
DISCUSSION
In this study, we investigated combinations between CAZ/AVI and aminoglycosides against KPC-Kp isolates with clinically relevant AME genes, aac(6′)-Ib′ or aac(6′)-Ib. We found that this combination yielded rapid, extensive, and synergistic killing and suppressed resistance, although the benefit of adding an aminoglycoside to CAZ/AVI may be somewhat reduced for isolates that are highly susceptible to CAZ/AVI (e.g., NU-CRE61). Remarkably, we were able to achieve similar pharmacodynamic activity by the combination even when aminoglycoside treatment duration was reduced from seven daily doses to a one-time dose. As expected, the degree of bacterial killing by the aminoglycosides was dependent on the AME the isolate harbored. Thus, selecting the appropriate aminoglycoside based on the AME or the aminoglycoside MIC may improve the success of this combination.
In the present study, gentamicin in combination with CAZ/AVI generally performed better against KPC-Kp with aac(6′)-Ib, while amikacin in combination with CAZ/AVI displayed better activity against KPC-Kp with aac(6′)-Ib′. The variation in antibacterial activity between aminoglycosides occurred despite all KPC-Kp isolates being considered susceptible to gentamicin and amikacin according to CLSI [per EUCAST, all isolates were susceptible to amikacin but the two isolates with aac(6′)-Ib′ were resistant to gentamicin]. The selectivity of the AMEs for certain aminoglycosides can largely explain differences in the pharmacodynamic activity we observed between KPC-Kp isolates; AAC(6′)-Ib inactivates amikacin and only some of the gentamicin congeners (not gentamicin C1), while AAC(6′)-Ib′ primarily inactivates gentamicin but not amikacin (13, 19, 20). Our findings are in agreement with previous time-kill assay results, which showed that AAC(6′)-Ib conferred intermediate-level amikacin resistance in KPC-Kp (21). This is also consistent with our previous time-kill and one-compartment model studies which demonstrated that gentamicin may be preferred over amikacin or tobramycin for KPC-Kp with aac(6′)-Ib (22).
To our knowledge, we are the first to investigate combinations with aminoglycosides and CAZ/AVI against KPC-Kp in a dynamic pharmacokinetic/pharmacodynamic model or an animal infection model. Remarkably, selection of an aminoglycoside specific to the KPC-Kp isolate’s AMEs and aminoglycoside MICs enhanced the pharmacodynamic activity of the combination with CAZ/AVI against NU-CRE85 in the HFIM. Against the other isolate (NU-CRE61) where CAZ/AVI displayed better pharmacodynamic activity, combinations with either aminoglycoside displayed similar effectiveness. This suggests selection of a specific aminoglycoside is more important when CAZ/AVI is less active against a KPC-Kp isolate. Although the combination of an aminoglycoside and CAZ/AVI has not been rigorously optimized in vitro, the combination is being utilized clinically. A small study by Shields et al. reported a survival rate of 75% (6/8) among patients receiving CAZ/AVI alone for carbapenem-resistant K. pneumoniae bacteremia and 100% (5/5) when CAZ/AVI was given in combination with gentamicin (7). A larger retrospective study recently investigated outcomes for 577 patients treated with CAZ/AVI for KPC-Kp infections, in which 15% of patients concurrently received an aminoglycoside (8). Although all-cause mortality did not significantly vary between patients receiving monotherapy and those receiving any combination (26.1% versus 25.0%; P = 0.79), outcomes for the subset of patients receiving aminoglycosides was not provided. Together, our findings suggest that detection of AME genes in a patient’s KPC-Kp isolates, through advances in rapid diagnostics, could replace aminoglycoside MIC testing to enable more rapid selection of the optimal aminoglycoside for use in combination with CAZ/AVI.
There are multiple potential mechanistic explanations for the synergy and rapid pharmacodynamic activity we observed with the combinations. First, the combinations of CAZ/AVI with aminoglycosides displayed enhanced killing of antibiotic-resistant subpopulations compared to monotherapies, suggesting synergy achieved against the resistant subpopulations may in part explain the effectiveness of the combination (23, 24). Second, aminoglycoside-induced aberrant protein synthesis may enhance the activity of CAZ/AVI by decreasing carbapenemase expression (25). Aberrant proteins incorporated into the cell membrane may also increase β-lactam entry. Third, direct aminoglycoside-mediated cell membrane permeabilization has also been shown to improve carbapenem target site penetration (11, 26). Through this mechanism, it may also be possible that the aminoglycosides directly enhance CAZ/AVI penetration into the periplasm. Fourth, it is also possible that the aminoglycosides block the replication of bacterial resistance plasmids that carry carbapenemases and other types of antibiotic resistance genes, as has been shown with kasugamycin and apramycin (27, 28). Blocking replication of these plasmids may sensitize these isolates to other antibiotics such as CAZ/AVI. Future studies, such as leveraging a new antibiotic penetration assay platform (29), are required to elucidate the specific mechanism(s) responsible for the remarkable synergy we observed between CAZ/AVI and aminoglycosides.
Although the aminoglycosides are one of the most potent classes of antimicrobials, their clinical use can be restricted by their nephrotoxicity. Aminoglycoside-induced nephrotoxicity results from accumulation of the drug in the renal cortex (30). Nephrotoxicity in patients receiving aminoglycosides has consistently been associated with extended drug treatment durations (31–33). Therefore, in the present study a one-time dose of an aminoglycoside combined with CAZ/AVI was explored in the HFIM in an effort to minimize the duration of aminoglycoside therapy. Promisingly, the pharmacodynamic activity of the combination with a one-time aminoglycoside dose was comparable to the combination’s activity with seven aminoglycoside doses. Single-dose aminoglycoside monotherapy has previously been shown to be an efficacious and safe dosing strategy for urinary tract infections with only 0.5% of patients experiencing any adverse effect (nephrotoxicity, vestibular toxicity, injection site reactions, or hearing loss) (34). To our knowledge, this is the first study to evaluate combinations that include a one-time aminoglycoside dose for KPC-Kp. This promising combination strategy may reduce the potential for aminoglycosides to cause toxicities while maintaining maximal bacterial killing.
There are a few limitations to the present study to note. First, each HFIM experiment was only conducted once. Although this is standard practice for HFIM experiments in many laboratories, biologic variability cannot be assessed for these experiments in the present study. Second, the dosing regimens were not humanized in the neutropenic mouse pneumonia model. Additional experiments are required to verify that the combination demonstrates significantly better killing than monotherapy in vivo when dosing is humanized. Third, the experiments were conducted with a limited number of clinical isolates; future studies with additional isolates are required to confirm our findings.
In conclusion, combinations between an aminoglycoside and CAZ/AVI against KPC-Kp are synergistic and demonstrate rapid bacterial killing at human simulated antibiotic exposures. Precisely selecting an aminoglycoside to combine with CAZ/AVI based on genotypic resistance mechanisms or phenotypic MIC may be critical to optimizing therapy for all isolates. Gentamicin may be preferred in combination with CAZ/AVI against KPC-Kp that harbor aac(6′)-Ib, whereas amikacin performed better against isolates with aac(6′)-Ib′. A one-time aminoglycoside dose in combination with CAZ/AVI demonstrated remarkable pharmacodynamic activity and provides a potentially promising approach to treatment of KPC-Kp infections. These findings should be validated against additional isolates, especially those with different MICs to CAZ/AVI and aminoglycosides, prior to translation to the clinical setting.
MATERIALS AND METHODS
Bacterial isolates and antibiotic susceptibility testing.
Four clinical KPC-Kp isolates that previously underwent whole-genome sequencing and were determined to harbor blaKPC-3 genes were used for static time-kill assays; two coharbored aac(6′)-Ib′ (NU-CRE61 and -CRE78), while two coharbored aac(6′)-Ib (NU-CRE85 and -CRE213) (22). One isolate that harbored aac(6′)-Ib′ (NU-CRE61) and one isolate that harbored aac(6′)-Ib (NU-CRE85) were evaluated in the hollow-fiber infection model (HFIM). One isolate (NU-CRE85) was evaluated in the neutropenic mouse pneumonia model as a proof of concept. The NCBI assembly accession numbers for NU-CRE61, NU-CRE78, NU-CRE85, and NU-CRE213 are GCA_016802965.1, GCA_016802925.1, GCA_012029975.2, and GCA_012030545.2, respectively. Gentamicin (lot SLBT5354), amikacin (lot SLBT0718), ceftazidime (lot 117M4826V), and avibactam (lot 14605) were purchased from Sigma-Aldrich (St. Louis, MO). MICs for each isolate were determined in triplicate using broth microdilution according to CLSI specifications (35).
Static time-kill assays.
Static concentration time-kill assays were conducted to initially evaluate the pharmacodynamic activity of gentamicin or amikacin alone and combined with CAZ/AVI against KPC-Kp isolates at a starting inoculum of ∼108 CFU/ml, as previously described (36, 37). This starting inoculum was selected to simulate severe infections such as ventilator associated pneumonia, where bacterial burdens often exceed 107 CFU/ml (38). Evaluating antibiotic combinations against the high end of clinically relevant bacterial burdens can also identify concentrations required for mutation prevention (39). A 4:1 ratio of ceftazidime to avibactam (5/0.94, 20/3.75, and 80/15 mg/liter) was selected to mimic the approximate concentration ratio observed in patients and based on the ratio of the drugs in the commercially available formulation (40). CAZ/AVI was tested alone and in combination with gentamicin (0.625, 1.25, 2.5, 5, and 10 mg/liter) or amikacin (3.75, 7.5, 15, 30, and 60 mg/liter). Viable colony counts were performed by obtaining samples at 0, 1, 2, 4, 6, 8, and 24 h of antibiotic exposure, streaking these samples onto CAMHA plates, and incubating the plates for 24 h at 37°C prior to enumeration. Synergy for in vitro experiments was defined as a ≥2-log10 CFU/ml decrease by the combination compared to the most active agent alone (17). Bactericidal activity for in vitro experiments was defined as a ≥3-log10 CFU/ml reduction in viable bacterial count at 24 h compared to the initial inoculum. The LLQ for bacterial colony counts was 250 CFU/ml (2.4 log10 CFU/ml) in both time-kill assays and the HFIM. Data below the LLQ are included to provide the greatest amount of information but should be interpreted with caution.
HFIM and LC-MS/MS analysis.
The HFIM was conducted to quantify the pharmacodynamic effects of CAZ/AVI alone or in combination with an aminoglycoside during exposure to antibiotic profiles that mimic those observed in patients. KPC-Kp isolates NU-CRE61 [(aac(6′)-Ib′] and NU-CRE85 [aac(6′)-Ib] were used in the HFIM at a starting inoculum of ∼108 CFU/ml. HFIM experiments were conducted over 168 h (7 days) as previously described (41, 42), with a growth control, monotherapies (CAZ/AVI at 2.5 g every 8 h [q8h], infusion over 2 h, gentamicin at 5 mg/kg q24h, infusion over 0.5 h, or amikacin at 15 mg/kg q24h, infusion over 0.5 h), and combination regimens (CAZ/AVI plus gentamicin or CAZ/AVI plus amikacin). Bacterial density was enumerated between 0 and 168 h. In addition, population analysis profiles (PAPs) were determined to quantify CAZ/AVI-, gentamicin-, and amikacin-resistant subpopulations at 0, 4, 24, 48, 72, 96, 120, 144, and 168 h. Specifically, PAPs employed drug-containing agar plates (CAZ/AVI at 1, 4, and 16 mg/liter; gentamicin at 1, 4, and 16 mg/liter; and amikacin at 4, 16, and 64 mg/liter), and resistant colonies were quantified after 48 h of incubation. To determine whether the aminoglycoside treatment duration could be reduced, CAZ/AVI at 2.5g q8h plus a one-time dose of gentamicin at 5 mg/kg and CAZ/AVI at 2.5 g q8h plus a one-time dose of amikacin at 15 mg/kg were tested against NU-CRE85 [aac(6′)-Ib] and NU-CRE61 [aac(6′)-Ib′], respectively. Human pharmacokinetics were used to simulate drug regimens as follows: (i) gentamicin at 5 mg/kg, t1/2 2 h, fCmax 15.1 mg/liter, and fCmin 0.01 mg/liter (43); (ii) amikacin at 15 mg/kg, t1/2 2 h, fCmax 50 mg/liter, and fCmin 0.02 mg/liter (44); and (iii) CAZ/AVI at 2.5 g, t1/2 2 h, CAZ fCmax 84.2 mg/liter, CAZ fCmin 10.8 mg/liter, AVI fCmax 13.2 mg/liter, and AVI fCmin 1.7 mg/liter (45).
The β-lactam and aminoglycoside concentrations from representative experiments were determined using an Acquity I-Class UPLC system (Waters, Milford, MA) equipped with a Triple Quad 6500+ mass spectrometry (AB Sciex, Framingham, MA). The UPLC separation of CAZ, AVI, and the internal standard (diclofenac) was performed using a Kinetex Polar C18 column (100 × 2.1 mm, 2.6 μm; Phenomenex, Inc., Torrance, CA) at 40°C with a run time of 4.0 min. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B) at a flow rate of 0.4 ml/min. The gradient profile was 1% phase B from 0 to 0.7 min, 1 to 70% phase B from 0.7 to 1.2 min, 70 to 90% phase B from 1.2 to 2.5 min, 90 to 95% phase B from 2.5 to 2.9 min, 95 to 1% phase B from 2.9 to 3.1 min, and 1% phase B from 3.1 to 4.0 min.
The UPLC separation of amikacin and the internal standard (diclofenac) was performed using an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm; Waters, Milford, MA) at 40°C with a run time of 3.5 min. The mobile phase consisted of 5 mM heptafluorobutyric anhydride (HFBA) in water (A) and 0.1% (wt/vol) formic acid in methanol (B) at a flow rate of 0.4 ml/min. The gradient profile was 5% phase B from 0 to 0.3 min, 5 to 90% phase B from 0.3 to 2 min, 90% phase B from 2 to 2.6 min, 90 to 5% phase B from 2.6 to 2.7 min, and 5% phase B from 2.7 to 3.5 min. Gentamicin concentrations were not evaluated due to the unavailability of purified gentamicin components to use as controls (e.g., C1, C1a, C2, and C2a).
An injection volume of 3 μl was used for all sample analyses. The Triple Quad 6500+ MS/MS system was operated in both positive-ion mode (CAZ) and negative-ion mode (AVI) using the turbo spray IonDrive. The source and gas parameters were as follows: curtain gas (CUR), 30 lb/in2; collision gas (CAD), 9 lb/in2; ion-spray voltage (IS), ±5,500 V; temperature (TEM), 550°C; ion source gas 1 (GS1), 50 lb/in2; and ion source gas 2 (GS2), 50 lb/in2. The optimized multiple reaction monitoring (MRM) conditions were m/z 547.1 to 468.0 for CAZ, 264.1 to 96.8 for AVI, 586.3 to 425.2 for AMK, and 296.0 to 215.1 for diclofenac in positive mode and 294.0 to 214.1 for diclofenac in negative mode. The dwell time was 30 ms for all compounds, except diclofenac and AVI, which had a dwell time of 50 ms. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) peak integration and data analysis were performed in the Analyst software package (AB Sciex, Framingham, MA).
Samples were 10-fold diluted and quantified against a calibration curve generated from the batch calibrators and assay performance was ensured by batch acceptance criteria. The precisions were 5.2, 5.4, 4.7, and 4.8%, and the accuracies were 0.2, −3.2, −1.3, and 1.5% at 0.02 (LLQ), 0.1, 3, and 30 mg/liter of CAZ, respectively. The precisions were 8.8, 7.5, 4.3, and 1.3%, and the accuracies were 7.5, −2.3, 8.7, and −6.8% at 0.1 (LLQ), 0.3, 3, and 30 mg/liter of AVI, respectively. The precisions were 0.5, 6.8, 4.0, and 4.6%, and the accuracies were 3.5, 0.2, −0.3, and 8.7% at 0.01 (LLQ), 0.1, 3, and 30 mg/liter of AMK, respectively.
Neutropenic mouse pneumonia model.
Animal experiments were approved by the Monash University Animal Ethics Committee. Female Swiss mice (8 weeks old; 25 to 30 g) were obtained from Monash Animal Services (Clayton, Victoria, Australia) and handled, fed, and housed according to the criteria of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Food and water were available ad libitum. A neutropenic mouse pneumonia model was employed as previously described (46, 47). Briefly, mice were made neutropenic by intraperitoneal injection of cyclophosphamide and anesthetized via placement into the isoflurane induction chamber. Anesthetized mice were placed on a Perspex support in a vertical upright position, which allowed mice to be temporarily immobilized. A MicroSprayer (model IA-1C; Penn-Century, Philadelphia, PA) was used to deliver 50 μl of a suspension of NU-CRE85 into each lung (∼1.4 × 106 bacterial cells per lung). After inoculation, each mouse was kept in an upright position for approximately 1 to 2 min and then placed onto a warm pad for recovery. Five groups of mice were examined: (i) untreated control at 0 h, (ii) untreated control at 24 h, (iii) gentamicin monotherapy at 24 h (10 mg/kg q8h, subcutaneously), (iv) CAZ/AVI monotherapy at 24 h (24/6 mg/kg q8h, subcutaneously), and (v) combination of gentamicin and CAZ/AVI at 24 h. At the corresponding time point, the lungs were aseptically removed and homogenized for quantitative culture (data are reported in log10 CFU/lung). To compare the efficacy (change in log10 bacterial counts per lung) of the combination to monotherapies, each group was compared using an analysis of variance with a Tukey’s post hoc test. A P value of ≤0.05 was considered statistically significant (48).
ACKNOWLEDGMENTS
The project was funded in part by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (to Z.P.B. and A.R.H.). This project has also been funded in part by the National Institutes of Health (NIH), under Grant KL2TR002002 (to Z.P.B.). The bioanalysis of this study was supported by R01AI136803 (to J.B.B.). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. J.L. is an Australian National Health and Medical Research Council Principal Research Fellow.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.CDC. 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. www.cdc.gov/DrugResistance/Biggest-Threats.html. [Google Scholar]
- 2.WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Papalini C, Sabbatini S, Monari C, Mencacci A, Francisci D, Perito S, Pasticci MB. 2020. In vitro antibacterial activity of ceftazidime/avibactam in combination against planktonic and biofilm carbapenemase-producing Klebsiella pneumoniae isolated from blood. J Glob Antimicrob Resist 23:4–8. 10.1016/j.jgar.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Cui X, Shan B, Zhang X, Qu F, Jia W, Huang B, Yu H, Tang YW, Chen L, Du H. 2020. Reduced ceftazidime-avibactam susceptibility in KPC-producing Klebsiella pneumoniae from patients without ceftazidime-avibactam use history: a multicenter study in China. Front Microbiol 11:1365. 10.3389/fmicb.2020.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satlin MJ, Chen L, Patel G, Gomez-Simmonds A, Weston G, Kim AC, Seo SK, Rosenthal ME, Sperber SJ, Jenkins SG, Hamula CL, Uhlemann AC, Levi MH, Fries BC, Tang YW, Juretschko S, Rojtman AD, Hong T, Mathema B, Jacobs MR, Walsh TJ, Bonomo RA, Kreiswirth BN. 2017. Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant Enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrob Agents Chemother 61. 10.1128/AAC.02349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, De Gasperi A, Mularoni A, Tascini C, Parruti G, Pallotto C, Sica S, Concia E, Cultrera R, De Pascale G, Capone A, Antinori S, Corcione S, Righi E, Losito AR, Digaetano M, Amadori F, Giacobbe DR, Ceccarelli G, Mazza E, Raffaelli F, Spanu T, Cauda R, Viale P. 2019. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 68:355–364. 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 7.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61. 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, Venditti M, De Rosa FG, Sarmati L, Bassetti M, Brindicci G, Rossi M, Luzzati R, Grossi PA, Corona A, Capone A, Falcone M, Mussini C, Trecarichi EM, Cascio A, Guffanti E, Russo A, De Pascale G, Tascini C, Gentile I, Losito AR, Bussini L, Conti G, Ceccarelli G, Corcione S, Compagno M, Giacobbe DR, Saracino A, Fantoni M, Antinori S, Peghin M, Bonfanti P, Oliva A, De Gasperi A, Tiseo G, Rovelli C, Meschiari M, Shbaklo N, Spanu T, Cauda R, Viale P. 2021. Ceftazidime-avibactam use for KPC-Kp infections: a retrospective observational multicenter study. Clin Infect Dis 10.1093/cid/ciab176. [DOI] [PubMed] [Google Scholar]
- 9.Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Souli M, Study Collaborators. 2019. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, With regard to plazomicin and aminoglycoside resistance. BMC Infect Dis 19:167. 10.1186/s12879-019-3801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Kashikar A, Bush K. 2017. In vitro activity of plazomicin against beta-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J Antimicrob Chemother 72:2792–2795. 10.1093/jac/dkx261. [DOI] [PubMed] [Google Scholar]
- 11.Yadav R, Bulitta JB, Schneider EK, Shin BS, Velkov T, Nation RL, Landersdorfer CB. 2017. Aminoglycoside concentrations required for synergy with carbapenems against Pseudomonas aeruginosa determined via mechanistic studies and modeling. Antimicrob Agents Chemother 61:e00722-17. 10.1128/AAC.00722-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haidar G, Alkroud A, Cheng S, Churilla TM, Churilla BM, Shields RK, Doi Y, Clancy CJ, Nguyen MH. 2016. Association between the presence of aminoglycoside-modifying enzymes and in vitro activity of gentamicin, tobramycin, amikacin, and plazomicin against Klebsiella pneumoniae carbapenemase- and extended-spectrum-beta-lactamase-producing Enterobacter species. Antimicrob Agents Chemother 60:5208–5214. 10.1128/AAC.00869-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez MS, Tolmasky ME. 2010. Aminoglycoside modifying enzymes. Drug Resist Updat 13:151–171. 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanelli F, De Robertis A, Carone G, Dalfino L, Stufano M, Del Prete R, Mosca A. 2020. In vitro activity of ceftazidime/avibactam alone and in combination with fosfomycin and carbapenems against KPC-producing Klebsiella pneumoniae. New Microbiol 43:136–138. [PubMed] [Google Scholar]
- 16.Ojdana D, Gutowska A, Sacha P, Majewski P, Wieczorek P, Tryniszewska E. 2019. Activity of ceftazidime-avibactam alone and in combination with ertapenem, fosfomycin, and tigecycline against carbapenemase-producing Klebsiella pneumoniae. Microb Drug Resist 25:1357–1364. 10.1089/mdr.2018.0234. [DOI] [PubMed] [Google Scholar]
- 17.Mikhail S, Singh NB, Kebriaei R, Rice SA, Stamper KC, Castanheira M, Rybak MJ. 2019. Evaluation of the synergy of ceftazidime-avibactam in combination with meropenem, amikacin, aztreonam, colistin, or fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 63:e00779-19. 10.1128/AAC.00779-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CLSI. 2019. Performance standards for antimicrobial susceptibility testing, document M100-S29. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Ramirez MS, Nikolaidis N, Tolmasky ME. 2013. Rise and dissemination of aminoglycoside resistance: the aac(6′)-Ib paradigm. Front Microbiol 4:121. 10.3389/fmicb.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulman ZP, Cirz R, Hildebrandt D, Kane T, Rosario Z, Wlasichuk K, Park M, Andrews LD. 2020. Unraveling the gentamicin drug product complexity reveals variation in microbiological activities and nephrotoxicity. Antimicrob Agents Chemother 64:e00533-20. 10.1128/AAC.00533-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bremmer DN, Clancy CJ, Press EG, Almaghrabi R, Chen L, Doi Y, Nguyen MH, Shields RK. 2014. KPC-producing Klebsiella pneumoniae strains that harbor AAC(6′)-Ib exhibit intermediate resistance to amikacin. Antimicrob Agents Chemother 58:7597–7600. 10.1128/AAC.03831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler DA, Rana AP, Krapp F, Patel SR, Huang Y, Ozer EA, Hauser AR, Bulman ZP. 2020. Optimizing aminoglycoside selection for KPC-producing Klebsiella pneumoniae with the aminoglycoside-modifying enzyme (AME) gene aac(6′)-Ib. J Antimicrob Chemother 76:671–679. 10.1093/jac/dkaa480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landersdorfer CB, Ly NS, Xu H, Tsuji BT, Bulitta JB. 2013. Quantifying subpopulation synergy for antibiotic combinations via mechanism-based modeling and a sequential dosing design. Antimicrob Agents Chemother 57:2343–2351. 10.1128/AAC.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zavascki AP, Klee BO, Bulitta JB. 2017. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15:519–526. 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- 25.Krause KM, Serio AW, Kane TR, Connolly LE. 2016. Aminoglycosides: an overview. Cold Spring Harb Perspect Med 6:a027029. 10.1101/cshperspect.a027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock RE, Farmer SW, Li ZS, Poole K. 1991. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother 35:1309–1314. 10.1128/AAC.35.7.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zulauf KE, Kirby JE. 2020. Discovery of small-molecule inhibitors of multidrug-resistance plasmid maintenance using a high-throughput screening approach. Proc Natl Acad Sci U S A 117:29839–29850. 10.1073/pnas.2005948117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denap JC, Thomas JR, Musk DJ, Hergenrother PJ. 2004. Combating drug-resistant bacteria: small molecule mimics of plasmid incompatibility as antiplasmid compounds. J Am Chem Soc 126:15402–15404. 10.1021/ja044207u. [DOI] [PubMed] [Google Scholar]
- 29.Lang Y, Shah NR, Tao X, Reeve SM, Zhou J, Moya B, Sayed ARM, Dharuman S, Oyer JL, Copik AJ, Fleischer BA, Shin E, Werkman C, Basso KB, Deveson Lucas D, Sutaria DS, Mégroz M, Kim TH, Loudon-Hossler V, Wright A, Jimenez-Nieves RH, Wallace MJ, Cadet KC, Jiao Y, Boyce JD, LoVullo ED, Schweizer HP, Bonomo RA, Bharatham N, Tsuji BT, Landersdorfer CB, Norris MH, Soo Shin B, Louie A, Balasubramanian V, Lee RE, Drusano GL, Bulitta JB. 2021. Combating multidrug-resistant bacteria by integrating a novel target site penetration and receptor binding assay platform into translational modeling. Clin Pharmacol Ther 109:1000–1020. 10.1002/cpt.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahi-Birjand M, Yaghoubi S, Abdollahpour-Alitappeh M, Keshtkaran Z, Bagheri N, Pirouzi A, Khatami M, Sineh Sepehr K, Peymani P, Karimzadeh I. 2020. Protective effects of pharmacological agents against aminoglycoside-induced nephrotoxicity: a systematic review. Expert Opin Drug Saf 19:167–186. 10.1080/14740338.2020.1712357. [DOI] [PubMed] [Google Scholar]
- 31.Streetman DS, Nafziger AN, Destache CJ, BertinoAS, Jr.. 2001. Individualized pharmacokinetic monitoring results in less aminoglycoside-associated nephrotoxicity and fewer associated costs. Pharmacotherapy 21:443–451. 10.1592/phco.21.5.443.34490. [DOI] [PubMed] [Google Scholar]
- 32.Paquette F, Bernier-Jean A, Brunette V, Ammann H, Lavergne V, Pichette V, Troyanov S, Bouchard J. 2015. Acute kidney injury and renal recovery with the use of aminoglycosides: a large retrospective study. Nephron 131:153–160. 10.1159/000440867. [DOI] [PubMed] [Google Scholar]
- 33.BertinoJS, Jr, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN. 1993. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 167:173–179. 10.1093/infdis/167.1.173. [DOI] [PubMed] [Google Scholar]
- 34.Goodlet KJ, Benhalima FZ, Nailor MD. 2019. A systematic review of single-dose aminoglycoside therapy for urinary tract infection: is it time to resurrect an old strategy? Antimicrob Agents Chemother 63:e02165-18. 10.1128/AAC.02165-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9, vol 32,no 2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Bulman ZP, Ly NS, Lenhard JR, Holden PN, Bulitta JB, Tsuji BT. 2017. Influence of rhlR and lasR on polymyxin pharmacodynamics in Pseudomonas aeruginosa and implications for quorum sensing inhibition with azithromycin. Antimicrob Agents Chemother 61:e00096-16. 10.1128/AAC.00096-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulman ZP, Chen L, Walsh TJ, Satlin MJ, Qian Y, Bulitta JB, Peloquin CA, Holden PN, Nation RL, Li J, Kreiswirth BN, Tsuji BT. 2017. Polymyxin combinations combat Escherichia coli harboring mcr-1 and blaNDM-5: preparation for a postantibiotic era. mBio 8:e00540-17. 10.1128/mBio.00540-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drusano GL, Liu W, Fikes S, Cirz R, Robbins N, Kurhanewicz S, Rodriquez J, Brown D, Baluya D, Louie A. 2014. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis 210:1319–1324. 10.1093/infdis/jiu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. 10.1128/AAC.02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Lovern M, Green ML, Chiu J, Zhou D, Comisar C, Xiong Y, Hing J, MacPherson M, Wright JG, Riccobene T, Carrothers TJ, Das S. 2019. Ceftazidime-avibactam population pharmacokinetic modeling and pharmacodynamic target attainment across adult indications and patient subgroups. Clin Transl Sci 12:151–163. 10.1111/cts.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenhard JR, Rana AP, Wenzler E, Huang Y, Kreiswirth BN, Chen L, Bulman ZP. 2020. A coup d’état by NDM-producing Klebsiella pneumoniae overthrows the major bacterial population during KPC-directed therapy. Diagn Microbiol Infect Dis 98:115080. 10.1016/j.diagmicrobio.2020.115080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onufrak NJ, Smith NM, Satlin MJ, Bulitta JB, Tan X, Holden PN, Nation RL, Li J, Forrest A, Tsuji BT, Bulman ZP. 2020. In pursuit of the triple crown: mechanism-based pharmacodynamic modelling for the optimization of three-drug combinations against KPC-producing Klebsiella pneumoniae. Clin Microbiol Infect 26:1256.e1–1256.e8. 10.1016/j.cmi.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodiamont CJ, Janssen JM, de Jong MD, Mathot RA, Juffermans NP, van Hest RM. 2017. Therapeutic drug monitoring of gentamicin peak concentrations in critically ill patients. Ther Drug Monit 39:522–530. 10.1097/FTD.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 44.Maller R, Ahrne H, Holmen C, Lausen I, Nilsson LE, Smedjegard J. 1993. Once- versus twice-daily amikacin regimen: efficacy and safety in systemic gram-negative infections. Scandinavian Amikacin Once Daily Study Group. J Antimicrob Chemother 31:939–948. 10.1093/jac/31.6.939. [DOI] [PubMed] [Google Scholar]
- 45.Shields RK. 2020. Ceftazidime-avibactam plasma levels in critically ill patients, including those receiving continuous renal replacement therapy. ClinicalTrials.gov, NCT04358991. https://clinicaltrials.gov/ProvidedDocs/91/NCT04358991/Prot_SAP_000.pdf.
- 46.Lin YW, Zhou QT, Cheah SE, Zhao J, Chen K, Wang J, Chan HK, Li J. 2017. Pharmacokinetics/pharmacodynamics of pulmonary delivery of colistin against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother 61:e02025-16. 10.1128/AAC.02025-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 48.Aye SM, Galani I, Yu H, Wang J, Chen K, Wickremasinghe H, Karaiskos I, Bergen PJ, Zhao J, Velkov T, Giamarellou H, Lin YW, Tsuji BT, Li J. 2020. Polymyxin triple combinations against polymyxin-resistant, multidrug-resistant, KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 64. 10.1128/AAC.00246-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download AAC.00692-21-s0001.pdf, PDF file, 0.1 MB (122.5KB, pdf)