ABSTRACT
Mycobacterium tuberculosis, the causative agent of human tuberculosis, harbors a branched electron transport chain, preventing the bactericidal action of cytochrome bc1 inhibitors (e.g., TB47). Here, we investigated, using luminescent mycobacterial strains, the in vitro combination activity of cytochrome bc1 inhibitors and nitric oxide (NO) donors including pretomanid (PMD) and explored the mechanisms of combination activity. The TB47 and PMD combination quickly abolished the light emission of luminescent bacilli, as was the case for the combination of TB47 and aurachin D, a putative cytochrome bd inhibitor. The TB47 and PMD combination inhibited M. tuberculosis oxygen consumption, decreased ATP levels, and had a delayed bactericidal effect. The NO scavenger carboxy-PTIO prevented the bactericidal activity of the drug combination, suggesting the requirement for NO. In addition, cytochrome bc1 inhibitors were largely bactericidal when administered with DETA NONOate, another NO donor. Proteomic analysis revealed that the cotreated bacilli had a compromised expression of the dormancy regulon proteins, PE/PPE proteins, and proteins required for the biosynthesis of several cofactors, including mycofactocin. Some of these proteomic changes, e.g., the impaired dormancy regulon induction, were attributed to PMD. In conclusion, combination of cytochrome bc1 inhibitors with PMD inhibited M. tuberculosis respiration and killed the bacilli. The activity of cytochrome bc1 inhibitors can be greatly enhanced by NO donors. Monitoring of luminescence may be further exploited to screen cytochrome bd inhibitors.
KEYWORDS: Mycobacterium tuberculosis, electron transport chain, cytochrome bc1 inhibitor, cytochrome bd oxidase, pretomanid (PA-824), nitric oxide, LuxAB luciferase, luminescence
INTRODUCTION
The disease tuberculosis (TB), primarily caused by Mycobacterium tuberculosis, represents a top infectious disease worldwide, with >10 million new TB patients in 2018 (1). Chemotherapy with the combination of rifampin, isoniazid, pyrazinamide, and ethambutol is key to treating drug-susceptible TB. However, this treatment option not only requires a long period but also may be associated with severe side effects, such as liver damage (2). Furthermore, the emergence of drug-resistant TB further complicates the situation. Hence, studies of novel anti-TB drugs to identify effective combinations should be explored further.
The M. tuberculosis electron transport chain (ETC) has emerged as a target for new anti-TB agents, leading to the discovery of FoF1 ATP synthase inhibitors (3) and of cytochrome bc1 inhibitors, including Telacebec (previously known as Q203) (4), TB47 (5), and lansoprazole (6). These ETC-interfering agents compromise M. tuberculosis energetics essential for maintaining the bacillary viability/growth (7, 8). However, the M. tuberculosis ETC is branched, as demonstrated by its expression of a cytochrome bd oxidase as a compensatory electron transport route when the cytochrome bc1 is inhibited (9, 10). This plasticity could delay and even prevent the bactericidal action of ETC-interfering agents (9, 11). Indeed, interruption of cytochrome bd oxidase sensitized M. tuberculosis to bedaquiline and Telacebec (12–14).
Although cytochrome bd oxidase prevents the bactericidal action of cytochrome bc1 inhibitors (5, 9), a recent study seemed to support a bactericidal effect of Telacebec in human trials (15). It is likely that during infection the bacilli are confronted with adverse factors that could inhibit the cytochrome bd oxidase. During infection, the bacilli reside predominantly in lung macrophages (16), which could produce nitric oxide (NO) as part of innate immune responses (17, 18). Interestingly, NO was reported in other microorganisms to inhibit both the heme-copper oxidase (e.g., cytochrome bo3 for E. coli) and cytochrome bd oxidase (19, 20). Compared to the heme-copper oxidase, the cytochrome bd oxidase is more resistant to NO inhibition due to its higher NO dissociation rate (21).
The killing effect of pretomanid (PMD; previously known as PA-824), a bicyclic nitroimidazole recently approved for the treatment of drug-resistant TB, involves the production of NO (22). PMD exhibits good safety profiles and has passed phase III clinical trials (1, 23). PMD-treated M. tuberculosis exhibited an upregulation of cytochrome bd oxidase gene expression (24). Furthermore, the drug also affected the redox state of menaquinone, an essential electron-shuttling respiratory carrier in M. tuberculosis ETC (24). Thus, PMD appears to have multiple interference effects on the mycobacterial ETC.
Synergy between M. tuberculosis ETC-targeting drugs has been well documented (10, 25, 26). Given the potential effect of PMD on the M. tuberculosis ETC, we speculated that the drug enhanced the activity of cytochrome bc1 inhibitors. In addition, cytochrome bc1 inhibitors enhanced metabolic flux through the pentose phosphate pathway required for PMD activation (27, 28), leading us to reason that cytochrome bc1 inhibitors could augment the activity of PMD. Hence, we investigated the combination effect of PMD and cytochrome bc1 inhibitors.
RESULTS
The TB47 and PMD combination rapidly inhibits the light emission of luminescent mycobacteria.
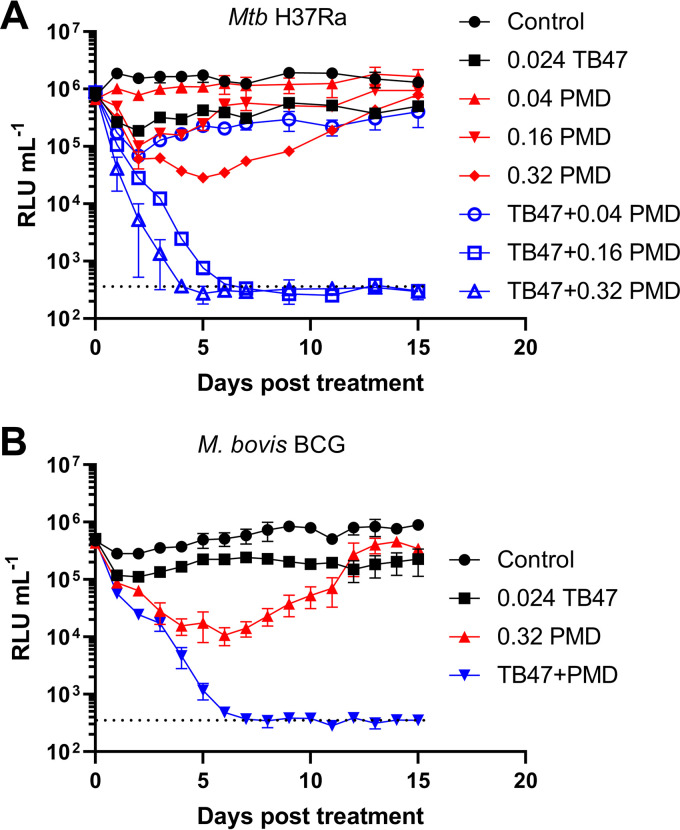
The MICs of TB47 and PMD were determined to be 0.003 μg/ml and 0.04 μg/ml, respectively (see Fig. S1 in the supplemental material), in the same range of those reported for another cytochrome bc1 inhibitor and PMD (4, 29). Next, a fixed concentration of TB47 (i.e., 8-fold MIC) was combined with increasing concentrations of PMD to treat luminescent M. tuberculosis (Fig. 1A). Interestingly, we observed a continuous decrease in light emission following TB47 and PMD cotreatment, which was dependent on the concentration of PMD, as TB47 combined with 0.04 μg/ml PMD failed to inhibit light production (Fig. 1A). Similar results were observed in M. bovis BCG (Fig. 1B). In contrast and as expected, treatment of luminescent M. abscessus (30) with TB47 and PMD had no effect on light emission (Fig. S2). M. abscessus is not susceptible to TB47 (30) or to PMD (31).
FIG 1.
Light production of luminescent M. tuberculosis H37Ra (A) and M. bovis BCG (B) treated with different compounds. (A) Luminescent M. tuberculosis H37Ra was treated with increasing concentrations (μg/ml) of PMD (±0.024 μg/ml TB47), followed by luminescence measurement for 15 days. (B) Luminescent M. bovis BCG was treated with different compounds. The dotted lines indicate the lower limit of detection for this assay. These experiments were performed at least 3 independent times (each in triplicate). Representative data are shown.
The TB47 and PMD combination has a delayed bactericidal effect and prevents the emergence of resistant colonies.
The inhibition of light production (Fig. 1) could be due to cell viability loss. Hence, we measured number of CFU as a read out for cell viability. Surprisingly, TB47 was found to largely protect the bacilli from PMD-mediated killing after 1 and 2 days of treatment (Fig. 2A). Similar protection was observed for bedaquiline (Fig. S3), the FoF1 ATP synthase inhibitor (11). These results suggest that these ETC-targeting agents inhibited the early bactericidal activity of PMD, as was recently reported for isoniazid (32).
FIG 2.
Viability of M. tuberculosis H37Ra treated with different drugs. (A) Exponential M. tuberculosis H37Ra cultures were measured for number of CFU and dispensed to different groups, followed by treatment for 1 and 2 days with 0.024 μg/ml TB47 and/or PMD at a concentration of 0.2 or 0.4 μg/ml. The dotted line indicates the mean viability prior to drug treatment. *, P < 0.05; **, P < 0.01 (by unpaired t test with Welch’s correction). (B) Viability was measured after exposure to 0.024 μg/ml TB47 and/or 0.32 μg/ml PMD for 1, 5, 10, and 21 days. **, P < 0.01 (by unpaired t test with Welch’s correction). (C and D) Cells treated with PMD or the TB47 and PMD combination for 21 days, as shown in panel B, were plated on plain or 0.32 μg/ml PMD-containing 7H11 agar plates. Representative images are shown in panel C. Left, 10 μl of 10−3 dilution of PMD-treated culture was plated on PMD-containing plates; right, 10 μl of the undiluted cotreated culture was plated on PMD-containing plates. (D) CFU data obtained on plain or PMD-containing 7H11 plates. ***, P < 0.001 (by unpaired t test with Welch’s correction). The dotted line indicates the lower limit of detection in this assay. ND, not detectable. These experiments were performed at least 3 times. Representative data are shown.
To determine the effect of the drug combination in more details, the bacilli were treated for 21 days (Fig. 2B). As expected, TB47 only elicited a bacteriostatic activity against M. tuberculosis, as is the case for other cytochrome bc1 inhibitors (33). As was observed in the short-term assay, the TB47- and PMD-cotreated culture had a much larger number of CFU than that treated with PMD alone during the initial 5 days. Strikingly, the viability of the cotreated culture gradually declined thereafter, reaching at day 21 a level that was >2 logs lower than the initial CFU number (Fig. 2B). In some of our repeated experiments, the viability of the cotreated culture declined at day 21 to <1,000 CFU/ml (data not shown), demonstrating a delayed but continuous bactericidal activity for the combination. The viability of the PMD-treated culture increased after 5 days, reflecting the emergence of PMD-resistant colonies. Indeed, at day 21, colonies were recovered from the PMD-treated culture on agar plates containing 0.32 μg/ml PMD (Fig. 2C and D). Interestingly, no PMD-resistant colonies were observed for the cotreated culture at day 21 (Fig. 2C and D, with a limit of detection of 100 for this experiment and of 10 for another repeated experiment), suggesting that the majority of the surviving bacteria in the cotreated culture was PMD sensitive.
To further confirm that the TB47 and PMD combination inhibited the emergence of drug-resistant mutants, we plated approximately 5 × 107 CFU bacilli on 7H11 agar plates containing different drugs (Fig. S4). On plates containing 2.5 μg/ml PMD, 600 to 800 colonies appeared, with a mean frequency of resistant colonies of 1.3 × 10−5. In contrast, no colonies were seen on plates containing both TB47 and PMD. Thus, the drug combination prevented the emergence of drug resistance.
The TB47 and PMD combination inhibits respiration and depletes ATP production.
In mycobacteria, inhibition of respiration kills the bacilli (9). We hypothesized that the TB47 and PMD combination inhibited M. tuberculosis respiration. To test this, methylene blue was used to monitor oxygen consumption (32). As expected, the TB47-treated culture could eventually consume the residual oxygen, as indicated by the decolorization of the oxygen dye (Fig. 3A). Likewise, the PMD culture was also decolorized (Fig. 3A), indicating that PMD could not fully inhibit M. tuberculosis respiration despite its ability to generate NO (22). Importantly, the culture cotreated with TB47 and PMD failed to decolorize methylene blue, even after 28 days of treatment (Fig. 3A and Fig. S5). Similar results were obtained for the combination of Telacebec and PMD (Fig. S6). In contrast, the culture cotreated with TB47/Telacebec and isoniazid could eventually decolorize methylene blue (Fig. S7 and data not shown for Telacebec and isoniazid combination).
FIG 3.
Oxygen consumption of M. tuberculosis H37Ra treated with different compounds. (A) Exponential M. tuberculosis H37Ra cultures were dispensed to glass tubes and treated with 0.024 μg/ml TB47 and/or 0.2 μg/ml PMD. Oxygen consumption was indicated by decolorization of methylene blue (6 μg/ml). (B) M. tuberculosis H37Ra was treated with 0.024 μg/ml TB47 and/or 0.2 μg/ml PMD, followed by fluorescence detection at the indicated time points. The increase in fluorescence reflects a decrease in extracellular oxygen content. Proper controls, e.g., medium with probe, were included in this experiment. The probe indicates the phosphorescent oxygen probe provided in the Cayman oxygen consumption rate assay kit. *, P < 0.05; **, P < 0.01; determined by unpaired t test (relative to PMD-treated cells at corresponding points). (C) Increase of fluorescence signal at 72 h relative to hour 0 (i.e., comparison between the initial and end points in panel B). **, P < 0.01 by unpaired t test (relative to untreated control). These experiments were performed 3 times (each in triplicate). Representative data are shown.
We also performed a fluorescence-based assay to detect oxygen consumption. As shown in Fig. 3B, the fluorescence for control- or single drug-treated cultures gradually increased, reflecting oxygen consumption. In contrast, the fluorescence increase for the cotreated culture was relatively marginal (Fig. 3C). Altogether, these results demonstrate that the combination of cytochrome bc1 inhibitors with PMD inhibited M. tuberculosis respiration.
Measurement of ATP for 11 days showed that the TB47- and PMD-cotreated bacilli had much lower ATP levels (Fig. 4A and B). As a control, the combination of PMD with bedaquiline caused no further reduction in ATP levels compared to the bedaquiline-treated cells (Fig. S8). Hence, in addition to the inhibition of oxygen consumption, the combination of PMD, a NO donor (22), with TB47 lowered mycobacterial ATP levels.
FIG 4.
ATP and viability of M. tuberculosis H37Ra and M. bovis BCG treated with different compounds. (A) ATP kinetics of M. tuberculosis H37Ra culture after treatment with 0.024 μg/ml TB47 and/or 0.32 μg/ml PMD for 11 days. (B) ATP and CFU numbers were determined after 1 day treatment, as for panel A. The ATP data were normalized by dividing by respective viability data. **, P < 0.01; ***, P < 0.001; by unpaired t test. (C) ATP of M. tuberculosis H37Ra treated with various compounds for 2 days was measured. The concentrations of carboxy-PTIO (C-PTIO) were 60 μM, 100 μM, and 158 μM. P values were <0.01 (**), <0.001 (***), and <0.0001 (#), respectively, compared to the TB47 and PMD-cotreated cells. (D) Viability of M. bovis BCG was determined after exposure to compounds for 20 days. The concentration of C-PTIO was 40 μM. ND, not detectable. The dotted line indicates the lower detection limit for this assay.
Protective effects of a NO scavenger.
The inhibited oxygen consumption, together with the lower ATP levels, strongly demonstrates the inhibition of ETC by the TB47 and PMD combination. To investigate whether the lethal effect of the combination was dependent on NO, we investigated the effect of carboxy-PTIO, a validated NO scavenger in mycobacteria (34, 35). We found that the NO scavenger increased the ATP levels in the bacilli cotreated with TB47 and PMD in a concentration-dependent manner (Fig. 4C). Unexpectedly, the NO scavenger did not increase ATP levels for the PMD-treated bacilli (Fig. 4C). Under the tested condition, the NO scavenger did not protect M. tuberculosis from the killing effect of the TB47 and PMD combination (Fig. S9). However, the scavenger was found to protect M. bovis BCG from the lethal effect of the drug combination (Fig. 4D), suggesting that NO contributes to the lethal effect of the drug combination.
We observed that the inhibition of light production by the TB47 and PMD combination was not prevented by the NO scavenger (Fig. S10), suggesting either that the protective effect of the scavenger is incomplete or that other potentially affected pathways, in addition to ETC inhibition, contribute to the inhibition of light production by the drug combination. To understand this better, we assessed whether the combination of TB47 and aurachin D, a putative cytochrome bd oxidase inhibitor (13), could interfere with light production. The combination quickly abrogated the light emission even before bactericidal activity was seen (Fig. S11), as was the case for the TB47 and PMD combination. Hence, the abrogation of light production by the TB47 and PMD combination (Fig. 1) may be explained by inhibition of the ETC. The LuxAB luciferase-mediated light production necessitates, among others, ATP-dependent recycling of long-chain aldehydes (36). The addition of decanal, a 10-carbon aldehyde, partly restored the light production of the TB47- and PMD-cotreated bacilli (Fig. S12). Hence, the severely decreased ATP levels following TB47 and PMD cotreatment may contribute to the observed light abrogation through compromising aldehyde recycling.
Lethal effect of the combination of cytochrome bc1 inhibitors and a NO donor.
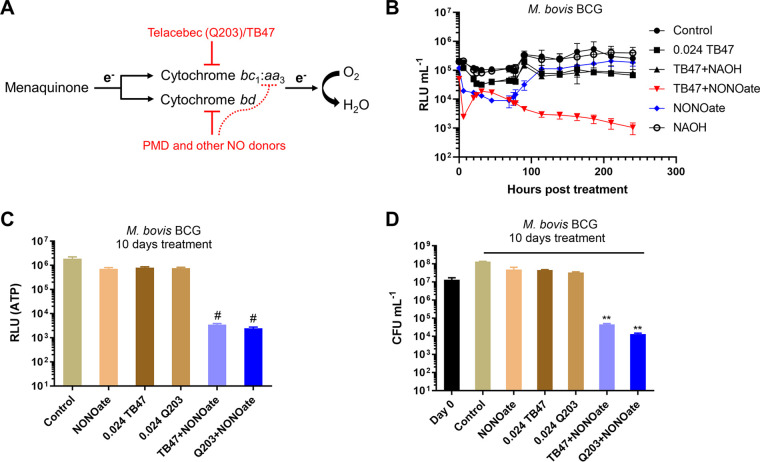
The dependence of NO for the lethal effect of the TB47 and PMD combination led us to further investigate the combination of cytochrome bc1 inhibitors and other sources of NO (Fig. 5A). We used DETA NONOate, a slow releaser of NO with an approximate half-life of 20 h (37). Using the luminescent M. bovis BCG, we observed that the combination of TB47 with DETA NONOate provoked two waves of decrease in light emission, the first one occurring almost instantly after the challenge and the other after 17 to 45 h of treatment (depending on experiments). Notably, following the onset of the second decrease, the light production of the cotreated culture declined gradually to the detection limit (Fig. 5B). In stark contrast, the light production of the NO donor-treated culture eventually reached control levels (Fig. 5B). Similar results were observed for the combination of Telacebec and DETA NONOate (data not shown). The cytochrome bc1 inhibitors and NO donor combination was also found to decrease ATP levels in a time-dependent fashion (Fig. 5C and Fig. S13), implying good bactericidal activity. Indeed, the combination was highly bactericidal against the bacilli (Fig. 5D and Fig. S13). The light abrogation and the strong bactericidal effect of TB47 and the NO donor combination were similarly observed in M. tuberculosis (Fig. S14).
FIG 5.
Cytochrome bc1 inhibitors were largely bactericidal when administered with a NO donor. (A) Schematic representation of mycobacterial ETC inhibition by the combination of cytochrome bc1 inhibitors (e.g., TB47) and PMD (or other sources of NO). The dotted line indicates a potential inhibition of the cytochrome bc1:aa3 branch by NO, as reported in many other microorganisms. However, a recent publication on mycobacteria also suggests that the cytochrome bc1:aa3 branch of the bacilli is less susceptible to NO inhibition than expected (54). (B) Light intensity of luminescent M. bovis BCG was monitored following treatment with 0.024 μg/ml TB47 and/or 0.5 mM DETA NONOate. DETA NONOate was prepared in 0.01 M NAOH (pH 12). Proper controls with NAOH were included in this assay. ATP (C) and viability (D) were determined after 10 days of treatment with different compounds. The concentration of DETA NONOate was 0.5 mM. **, P < 0.01; #, P < 0.0001; relative to the other groups. These experiments were performed 3 independent times (each in triplicate). Representative data are shown.
Proteomic signature of the TB47- and PMD-cotreated cells.
To provide insights into M. tuberculosis adaptation during TB47 and PMD cotreatment, proteomic analysis was performed. The bacilli were treated for 2 days. At this point, the combination markedly decreased ATP levels (Fig. 4) without significantly reducing CFU numbers (Fig. 2).
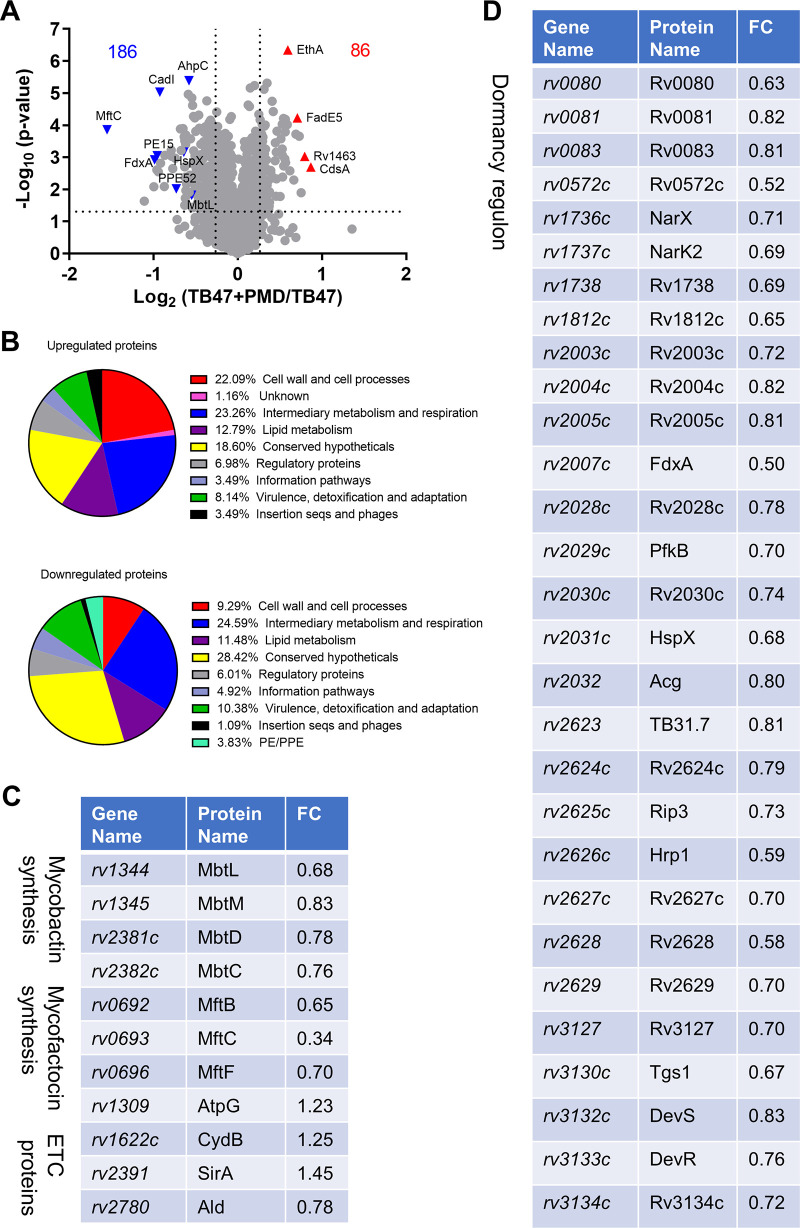
We first compared the cotreated cells with the TB47-treated cells. The complete proteomic data can be found in Data Set S1. In total, 86 and 186 proteins were upregulated and downregulated (with a fold change of >1.2 and a P value of <0.05), respectively, in the cotreated bacilli relative to the TB47-treated cells (Fig. 6A). Further functional category analysis revealed that proteins participating or classified as the intermediary metabolism and respiration, conserved hypotheticals, and lipid metabolism were affected most abundantly. Proteins involved in the cell wall and cell processes were present more predominantly in the upregulated proteins. Many of the upregulated “cell wall and cell processes” proteins are membrane-bound transporters (e.g., ChaA, GlnH, Rv0412c, Rv1140, Rv1634, and Rv2041c) involved in translocating substrates such as ions, amino acids, and drugs (some of these proteins, e.g., GlnH, Rv0412c, and Rv1140, were also increased in the PMD-treated bacilli, suggesting an effect for PMD; Data Set S2). In contrast, many of the downregulated “cell wall and cell processes” proteins participate in the assembly of cell wall components (e.g., Rv0225 for lipopolysaccharide synthesis, LdtB and DacB2 for peptidoglycan synthesis) or are ESAT-6-like proteins (e.g., EsxC and EsxL) (LdtB and EsxC were also decreased in the PMD-treated bacilli; Data Set S2). Proteins involved in mycolic acid synthesis or cell wall mycoloylation (e.g., UmaA, Pks13, AccD6, and FbpB) were downregulated in the cotreated bacilli (UmaA and FbpB were also decreased in the PMD-treated bacilli; Data Set S2).
FIG 6.
Proteomic profiling of M. tuberculosis H37Ra after 2 days of treatment with 0.024 μg/ml TB47 and 0.32 μg/ml PMD. (A) Volcano plot showing downregulated (upper left) and upregulated (upper right) proteins in the cotreated bacilli compared to the TB47-treated cells. Names of particular proteins are given. The dotted line parallel to the x axis indicates a y value of 1.301 (i.e., −log10 0.05). The two dotted lines parallel to the y axis indicate x values of −0.263 (i.e., log2 0.833) and 0.263 (i.e., log2 1.2), respectively. (B) Functional category analysis of upregulated and downregulated proteins was performed based on Mycobrowser (https://mycobrowser.epfl.ch/). (C and D) Differential expression of proteins involved in the synthesis of mycobactin and mycofactocin and in ETC functions, as well as of proteins expressed in the dormancy regulon, was observed in the cotreated bacilli. FC, fold change relative to the TB47-treated cells.
In addition, PE/PPE proteins were only seen in the downregulated proteins (Fig. 6B), including PE15, PPE51, and PPE62. PE/PPE proteins, known for the conserved proline (P) and glutamic acid (E) residues in the N-terminal regions, play multiple roles, including host immunity regulation (e.g., PE15 [38]), uptake of nutrition (e.g., PPE51 [39, 40]), and acquisition of the iron-containing heme (e.g., PPE62 [41]). Proteins involved in the biosynthesis of high-affinity iron-chelating siderophores, i.e., mycobactins (42), were also downregulated (Fig. 6C), suggesting a disrupted iron homeostasis in the cotreated bacilli.
We observed in the cotreated bacilli an altered expression of ETC proteins, including upregulated cytochrome bd component CydB and FoF1 ATP synthase subunit AtpG (Fig. 6C). The enhanced expression of CydB and AtpG could reflect a rescuing mechanism. CydB and AtpG, both expressed in operons, were also upregulated in M. tuberculosis treated with bedaquiline (11). Among the identified proteins involved in ETC function were the nitrate reductase NarX and the nitrate/H+ symporter protein NarK2. The two proteins, involved in nitrate respiration (43), were significantly downregulated in the cotreated bacilli (Fig. 6D).
Expression of narX and narK2 is controlled by the DevR/DevS/DosT two-component system (44). The hypoxia or NO-triggered DevR regulon (also known as the dormancy regulon) comprises ∼50 genes (34, 44). Of note, expression levels of 29 proteins in this regulon decreased (Fig. 6A and D), strongly demonstrating an impaired induction of the dormancy regulon in the cotreated bacilli. Previously reported transcriptomics demonstrated an induction of the dormancy regulon after 30 min to 180 min of treatment with bedaquiline, which also decreased cellular ATP level significantly (11), in contrast to the impaired dormancy regulon induction observed after 2 days of TB47 and PMD cotreatment. The dormancy regulon also expresses the glycolytic protein 6-phosphofructokinase. The decreased expression of this protein (Fig. 6D) may further exacerbate the energetic depletion in the cotreated bacilli. In addition, the dormancy regulon expresses FdxA, a ferredoxin participating in alternative electron transport pathways (Fig. 6D). Ferredoxins are cognate redox partners for cytochrome P450 enzymes involved in lipid and steroid metabolism (45). Interestingly, 4 of the M. tuberculosis 20 cytochrome P450 enzymes were differentially expressed in the cotreated bacilli (Data Set S1). The dormancy regulon also expresses multiple universal stress proteins to enhance bacterial survival under stresses. Some of these proteins, e.g., Rv2005, Rv2028, TB31.7, and Rv2624, were downregulated in the cotreated bacilli (Fig. 6D).
Interestingly, proteins participating in the biosynthesis of mycofactocin were downregulated in the cotreated bacilli (Fig. 6C). Mycofactocins are redox cofactors required for the oxidation of primary alcohols to aldehydes (46, 47). As aldehydes are necessary for the LuxAB-catalyzed light production (48), the observed abrogation of light production following TB47 and PMD cotreatment (Fig. 1) could be due to the decreased expression of mycofactocin-synthesizing proteins.
In addition to these changes, we observed in the cotreated bacilli an altered expression of proteins involved in lipid metabolism (e.g., upregulated methylcitrate dehydratase PrpD and the methylcitrate cycle regulator PrpR [49]). Proteins of the toxin/antitoxin family, proteins required for Fe-S cluster biogenesis (e.g., upregulated cysteine desulfurase Csd), and those with antioxidant (e.g., upregulation of KatG and downregulation of AhpCD) or chaperone (e.g., downregulation of GroEL2, GroES, ClpC1, ClpX, and a transcriptional regulator, ClgR [50]) function were also differentially expressed in the cotreated bacilli. Additionally, proteins involved in the synthesis of several cofactors, such as thiamine (e.g., ThiO and ThiC), biotin (e.g., BioB and BioF2), and cobalamin (e.g., CobQ1), decreased in the cotreated bacilli (Data Set S1). Thiamine is required for the activities of enzymes involved in metabolic processes such as glycolysis, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway (51). Biotin is necessary for the function of a wide range of carboxylases and decarboxylases, e.g., pyruvate carboxylase (52). Cobalamin, also known as vitamin B12, is required for metabolic pathways, including methionine synthesis, methylmalonyl-coenzyme A (CoA) pathway assimilating propionyl-CoA, and biosynthesis of deoxyribonucleotides (53). Interestingly, the cotreated bacilli also exhibited a differential expression of proteins implicated in methionine (e.g., downregulated MetA and MetC) and deoxyribonucleotide biosynthesis (e.g., downregulated NrdF2) (Data Set S1).
Proteomic signature of the PMD-treated bacilli.
We also compared the proteomics of the PMD-treated cells with untreated cells to better understand the mode of action of the drug (Data Set S2). Proteins participating in the methylcitrate cycle (e.g., PrpR, PrpD, and PrpC) were upregulated in the PMD-treated bacilli, as in the cotreated bacilli (Data Set S1). Proteins involved in glycolysis (e.g., Gap and Pgk), in the pentose phosphate pathway (e.g., Rpe and DevB), and in the tricarboxylic acid cycle (e.g., Icd1) were all upregulated in PMD-treated cells, showing some adaptations for the central carbon metabolism. The PMD-treated cells downregulated expression of proteins participating in the electron transport chain (i.e., MenA, NarJ, CydC, and Ndh), suggesting an effect for PMD on respiratory function. The PMD-treated bacilli also showed an altered expression of proteins involved in cell wall/mycolic acid metabolism (e.g., upregulated IniBAC proteins, upregulated FabD, AcpM, and KasAB, downregulated FbpABD, and downregulated MmaA2/MmaA3). Many of these changes, e.g., upregulation of IniBAC, were also noted by transcriptomics previously (24). Proteins required for synthesizing other important cell wall components, e.g., phthiocerol dimycocerosate/phenolic glycolipid (e.g., upregulated FadD26 and downregulated FadD22), arabinogalactan (e.g., downregulated WbbL2 and Rfe), and peptidoglycan (e.g., downregulated LdtB), were also observed in the PMD-treated bacilli.
Interestingly, proteins involved in mycofactocin (e.g., MftC, MftE, and MftF) and mycobactin (e.g., MbtC, MbtD, MbtG, and MbtM) synthesis were downregulated in the PMD-treated cells, suggesting that PMD was responsible for the observed downregulation in the cotreated bacilli of mycofactocin- or mycobactin-synthesizing proteins (Fig. 6). Relative to PMD-treated cells, the cotreated cells expressed significantly less mycobactin-synthesizing proteins (including MbtC, MbtD, MbtG, and MbtM; S. Zeng, J. Zhang, M. Sun, X. Zhang, and T. Zhang, unpublished data), again suggesting disrupted iron homeostasis in the cotreated bacilli. Proteins required for synthesizing several other cofactors, such as thiamine (e.g., ThiO and ThiC) and biotin (e.g., BioB and BioF2), were decreased, and those involved in folate (i.e., FolP2, FolB, FolP1 and FolE) and pantothenate/CoA (i.e., CoaA, PanB, PanC and PanD) metabolism increased in the PMD-treated bacilli relative to untreated cells.
Importantly, 39 proteins expressed in the dormancy regulon (e.g., DevS, DevR, Tgs1, HspX, PfkB, NarX, and NarK2) were found to be downregulated in the PMD-treated bacilli, suggesting that the drug compromised dormancy regulon expression. We further compared proteomics of the cotreated cells with that of the PMD-treated cells (Zeng et al., unpublished). Only 3 proteins expressed in the regulon (i.e., Rv0081, Rv0082, and BfrB) were decreased in the cotreated cells relative to PMD-treated cells. Thus, the impaired dormancy regulon induction in the cotreated cells (Fig. 6) was mainly attributed to PMD.
DISCUSSION
In the present study, we investigated the in vitro antitubercular activity of the combination of cytochrome bc1 inhibitors and PMD, a NO donor (22). The combination was shown to largely decrease cellular ATP levels, inhibited oxygen consumption, and had a delayed bactericidal activity. Furthermore, combination of cytochrome bc1 inhibitors with another NO donor also showed strong bactericidal activity. These combinations also abrogated quickly the light emission of luminescent mycobacterial strains expressing the LuxAB luciferase.
The LuxAB luciferase-mediated light production necessitates, among others, molecular oxygen, reduced flavin mononucleotide, and ATP-dependent recycling of long-chain aldehydes (36). As the oxygen content for the culture cotreated with the cytochrome bc1 inhibitor and PMD should be sufficient for the reaction producing light (the combination inhibited ETC-mediated oxygen consumption), the level of oxygen did not seem to be responsible for the inhibited light emission. Interestingly, the combination of cytochrome bc1 inhibitors with aurachin D, a putative cytochrome bd oxidase inhibitor (13), also quickly abrogated the light emission of luminescent mycobacteria. As the combination of cytochrome bc1 inhibitors and PMD decreased cellular ATP levels significantly (likely also the combination of cytochrome bc1 inhibitors and aurachin D), it is possible that the severely decreased ATP levels following treatment with these combinations contribute to the observed light abrogation, potentially by interfering with aldehyde recycling. Supporting this notion was the finding that decanal addition partly restored the light production of the bacilli cotreated with TB47 and PMD. Despite this, further metabolomics are required to confirm the likely deficiency of aldehyde substrates required for the luciferase-mediated light emission. In addition, the quickly abrogated light emission by TB47 and aurachin D also suggest that the LuxCDABE-expressing luminescent strains are suitable for screening cytochrome bd inhibitors.
Similar to PMD, DETA NONOate, another NO donor (37), also boosted the activity of cytochrome bc1 inhibitors. As treatment with this NO donor alone had no bactericidal activity (Fig. 5), we propose that the NO donor could not fully block the electron transport chain of the bacilli (Fig. 5A). Previously, NO was reported to inhibit respiratory oxidases in other microorganisms (19, 20), with the cytochrome bd oxidase being more resistant to the inhibition (21). However, it was recently reported that mycobacterial cytochrome bc1-aa3 supercomplex has NO-metabolizing activities and, thus, could confer some levels of NO resistance (54). This previously unrecognized role for the cytochrome bc1-aa3 may partly explain our finding that the NO donor becomes highly bactericidal when administered in combination with cytochrome bc1 inhibitors. Another possible interpretation of our data could be that the TB47- or Q203-induced inhibition of the cytochrome bc1-aa3 pathway sensitized the bacilli to the toxic effects of NO-releasing compounds: even a slight (but continuous) inhibition of the cytochrome bd oxidase by NO could lead to blockade of the electron transport chain and result in bactericidal effects. The quick abrogation of light emission following cytochrome bc1 inhibitor and DETA NONOate cotreatment, similarly observed for coadministration with cytochrome bc1 inhibitor and PMD/aurachin D, also supports that the electron transport chain may be blocked by the cytochrome bc1 inhibitor and NO donor combination (Fig. 5A). Based on the results of the present work, we propose a novel combination strategy, using NO donors, for boosting the activity of cytochrome bc1 inhibitors. Given the generation of NO by human immune cells following M. tuberculosis infection (55), it is also possible that the activity of cytochrome bc1 inhibitors could be enhanced when administered in human, as suggested by a recent clinical trial (15).
Another interesting finding of the study is that PMD significantly suppressed the induction of the dormancy regulon, possibly accounting for the impaired dormancy regulon induction observed for the cotreated bacilli (Fig. 6). Although not observed previously, by transcriptomic analysis, for the PMD-treated bacilli (24), a recent paper showed decreased expression of dormancy regulon genes in cells treated with delamanid, another bicyclic nitroimidazole drug (56). Nitric oxide was shown to be one of the stimuli that could trigger the induction of the dormancy regulon (34). The impairment of the dormancy regulon induction following treatment with PMD, a NO donor (22), suggests that the level of NO released during PMD activation is not optimal (and may even be detrimental) for dormancy regulon induction. As the dormancy regulon is essential for mycobacterial survival under, e.g., the hypoxia-induced nonreplicating state (57), bicyclic nitroimidazole drugs such as PMD may kill the nonreplicating bacilli by inhibiting the expression of this dormancy regulon.
Under the tested aerobic condition, the NO scavenger increased the ATP levels of cells cotreated with TB47 and PMD but not those of the PMD-treated cells (Fig. 4C). This finding suggests a different metabolic state of the cotreated cells, allowing for the observed effect of carboxy-PTIO. Although the NO scavenger protected M. bovis BCG from the lethal effect by the TB47 and PMD combination, no similar protections were seen in M. tuberculosis. The reasons for this inconsistency are not known and may reflect the differences between the two species.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and compounds.
M. tuberculosis H37Ra and M. bovis BCG Tice were used as attenuated strains for the pathogenic M. tuberculosis H37Rv or M. bovis in the study. M. tuberculosis H37Ra, M. bovis BCG, and the relevant luminescent strains expressing the luxCDABE operon for light production (58) were grown in 7H9 medium containing 0.2% glycerol, 0.05% Tween 80, and 10% oleic acid-albumin-dextrose-catalase (OADC; Becton, Dickinson). Luminescent M. abscessus (30) was grown in the same medium. TB47 was synthesized in a batch and supplied by Guangzhou Eggbio Co. Ltd. (5, 27). Telacebec and bedaquiline were purchased from MedChemExpress. PMD was kindly provided by TB Alliance (https://www.tballiance.org). Isoniazid was from Tokyo Chemical Industry. Aurachin D was from Aobious. 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO) was from Shanghai Yuanye Bio-Technology, and (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino]diazen-1-ium-1,2-diolate (diethylenetriamine NONOate, DETA NONOate) was from Cayman Chemical.
MIC determination.
MICs of TB47, Telacebec, and PMD were measured using a luminescence-based method (5). Briefly, the MIC was the lowest drug concentration reducing >90% relative light units (RLUs) compared to that of the solvent control. The assay was performed with 200 μl diluted mycobacterial culture (∼106 CFU/ml) containing a series of 2-fold-diluted drugs, and luminescence was measured daily for 4 or 5 days.
ATP measurement.
ATP was measured using a BacTiter-Glo microbial cell viability assay kit (Promega) (32). Briefly, 20 μl mycobacterial culture was mixed with an equal volume of BacTiter-Glo reagent, followed by incubation for 5 min. Luminescence was recorded using a GloMax 20/20 luminometer (Promega).
Monitoring of oxygen consumption.
To assess oxygen consumption, exponential M. tuberculosis cultures were diluted to an optical density at 600 nm (OD600) of 0.2 and dispensed into 2-ml glass tubes with screw caps (1.9 ml culture per tube). Methylene blue, an oxygen indicator (32), was added to a final concentration of 6 μg/ml, followed by the addition of drugs. The tubes were then tightly capped and incubated at 37°C.
Alternatively, oxygen consumption was monitored using an oxygen consumption rate assay kit (Cayman Chemical). M. tuberculosis H37Ra cultures were diluted to an OD600 of 0.5 and treated with drugs. One hundred fifty microliters of the treated culture was dispensed to each well of the 96-well plate, followed by the addition of 10 μl phosphorescent oxygen probe. The surface was overlaid with 2 drops of HS mineral oil to block air exchange. Fluorescence (380 nm for excitation and 650 nm for emission) was recorded using a Mithras2 LB 943 multimode microplate reader (Berthold).
Time-kill assay.
Exponential-phase mycobacterial cultures were diluted to an OD600 of 0.1 and treated with drugs for 21 days. The number of CFU was measured by plating dilutions on 7H11 agar supplemented with 10% OADC and counting after incubation at 37°C for 3 to 4 weeks.
Proteomic analysis.
M. tuberculosis H37Ra was treated for 2 days, in triplicate, with 0.024 μg/ml TB47, 0.32 μg/ml PMD, or 0.024 μg/ml TB47 plus 0.32 μg/ml PMD. The untreated cells were diluted on the same day the compounds were added to reach a comparable cell density (between untreated, TB47-treated, and cotreated cells) before sample collection. The cells were resuspended in a lysis buffer (4% SDS, 100 mM Tris-HCl, 1 mM 1,4-dithiothreitol, pH 7.6) and boiled for 20 min. The extracted proteins were quantified with a bicinchoninic acid protein assay kit (Bio-Rad, USA). Trypsin digestion was performed with a filter-aided sample preparation method (59), followed by desaltination on C18 cartridges (Empore SPE cartridges; Sigma), concentration through vacuum centrifugation, and reconstitution in 40 μl 0.1% (vol/vol) formic acid. One hundred micrograms of peptide mixture was labeled with TMT reagent (Thermo Scientific). Using a high pH reversed-phase peptide fractionation kit (Thermo Scientific), the labeled peptides were fractionated.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was performed with a Q Exactive mass spectrometer (Thermo Scientific). The peptides were loaded to a reverse-phase trap column (Thermo Scientific) connected to a C18-reversed phase analytical column (Thermo Scientific) in buffer A (0.1% formic acid) and separated with buffer B (i.e., 84% acetonitrile and 0.1% formic acid) at a flow rate of 300 nl/min. The mass spectrometer was operated in positive ion mode. MS data were acquired using a data-dependent top 10 method dynamically choosing the most abundant precursor ions from the survey scan (300 to 1,800 m/z). Automatic gain control target was set to 1e6, maximum inject time to 50 ms, and dynamic exclusion duration to 60 s. Survey scans were acquired at a resolution of 70,000 at m/z 200, and resolution for higher energy collisional dissociation spectra was set to 17,500 at m/z 200 with an isolation window of 2 m/z.
MS raw data were searched using Mascot 2.2 engine (Matrix Science) coupled to Proteome Discoverer 1.4 for protein identification and quantitation and a UniProt M. tuberculosis database (https://www.uniprot.org/). Only proteins with a fold change (FC) of >1.2 and a P value of <0.05 (determined by t test) were further analyzed. Functional category analysis was conducted according to Mycobrowser (https://mycobrowser.epfl.ch/). Protein functional annotation was performed with DAVID (https://david.ncifcrf.gov/summary.jsp).
Statistical analysis.
Statistical analysis was performed with GraphPad Prism 9. Unpaired t test (with Welch’s correction, if necessary) was applied to determine statistical significance. A P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
Sheng Zeng was supported by an Overseas Young Postdoctoral Program from Guangdong Province, China. The study was mainly supported by a project from China Postdoctoral Science Foundation (2020M672855) and by research funds from the State Key Laboratory of Respiratory Disease (SKLRD-Z-202016). The study was also supported by the National Mega-project of China for Innovative Drugs (2019ZX09721001-003-003), by the National Natural Science Foundation of China (81973372, 21920102003, and 82061138019), by the Health Research Council of New Zealand-NSFC Biomedical Collaboration Fund (20/1211 and 8206112800), by the Chinese Academy of Sciences Grants (154144KYSB20190005 and YJKYYQ20170036), and by the Department of Science and Technology of Guangdong Province (2016TX03R095; Science and Technology Innovation Leader of Guangdong Province to T.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to TB Alliance (https://www.tballiance.org) for generously providing PMD. TB Alliance received support from Australia's Department of Foreign Affairs and Trade, the Bill & Melinda Gates Foundation, Germany's Federal Ministry of Education and Research through KfW, Irish Aid, Netherlands Ministry of Foreign Affairs, United Kingdom Department of Health, United Kingdom Foreign, Commonwealth and Development Office, and the United States Agency for International Development.
TB47 was synthesized in a batch and supplied by Guangzhou Eggbio Co. Ltd. GIR Medicine Co. Ltd. has been developing TB47 as a therapeutic agent against tuberculosis and other diseases. Neither entity had any role in study design, data collection and analysis, and decision to publish the manuscript. Guangzhou Institutes of Biomedicine and Health (GIBH), Chinese Academy of Sciences (CAS), has filed two Chinese patent applications (filing number 2015104607516 [authorized] and number 201810106538.9) and two PCT applications (filing number CN2015/086852 [authorized in the United States under number US10155756B2] and number CN2018/077992), entitled “Pyrazolo [1,5-a] pyridines and their applications” and “New use of a set of pyridine compounds,” respectively, listing T.Z. as one of the inventors.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2019. Global tuberculosis report 2019. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Shin HJ, Kwon YS. 2015. Treatment of drug susceptible pulmonary tuberculosis. Tuberc Respir Dis 78:161–167. 10.4046/trd.2015.78.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 4.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han SJ, No Z, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Gao Y, Liu J, Tan Y, Liu Z, Chhotaray C, Jiang H, Lu Z, Chiwala G, Wang S, Makafe G, Islam MM, Hameed HMA, Cai X, Wang C, Li X, Tan S, Zhang T. 2019. The compound TB47 is highly bactericidal against Mycobacterium ulcerans in a Buruli ulcer mouse model. Nat Commun 10:524. 10.1038/s41467-019-08464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rybniker J, Vocat A, Sala C, Busso P, Pojer F, Benjak A, Cole ST. 2015. Lansoprazole is an antituberculous prodrug targeting cytochrome bc1. Nat Commun 6:7659. 10.1038/ncomms8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Gardete S, Jansen RS, Shetty A, Dick T, Rhee KY, Dartois V. 2018. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob Agents Chemother 62:e02107-17. 10.1128/AAC.02107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao SP, Alonso S, Rand L, Dick T, Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 105:11945–11950. 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalia NP, Hasenoehrl EJ, Ab Rahman NB, Koh VH, Ang MLT, Sajorda DR, Hards K, Gruber G, Alonso S, Cook GM, Berney M, Pethe K. 2017. Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc Natl Acad Sci U S A 114:7426–7431. 10.1073/pnas.1706139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamprecht DA, Finin PM, Rahman MA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJ. 2016. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7:12393. 10.1038/ncomms12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koul A, Vranckx L, Dhar N, Gohlmann HW, Ozdemir E, Neefs JM, Schulz M, Lu P, Mortz E, McKinney JD, Andries K, Bald D. 2014. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat Commun 5:3369. 10.1038/ncomms4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moosa A, Lamprecht DA, Arora K, BarryCE, III, Boshoff HIM, Ioerger TR, Steyn AJC, Mizrahi V, Warner DF. 2017. Susceptibility of Mycobacterium tuberculosis Cytochrome bd Oxidase Mutants to Compounds Targeting the Terminal Respiratory Oxidase, Cytochrome c. Antimicrob Agents Chemother 61:e01338-17. 10.1128/AAC.01338-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu P, Asseri AH, Kremer M, Maaskant J, Ummels R, Lill H, Bald D. 2018. The anti-mycobacterial activity of the cytochrome bcc inhibitor Q203 can be enhanced by small-molecule inhibition of cytochrome bd. Sci Rep 8:2625. 10.1038/s41598-018-20989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu P, Heineke MH, Koul A, Andries K, Cook GM, Lill H, van Spanning R, Bald D. 2015. The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci Rep 5:10333. 10.1038/srep10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jager VR, Dawson R, van Niekerk C, Hutchings J, Kim J, Vanker N, van der Merwe L, Choi J, Nam K, Diacon AH. 2020. Telacebec (Q203), a new antituberculosis agent. N Engl J Med 382:1280–1281. 10.1056/NEJMc1913327. [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Singh VK, Hunter RL, Jagannath C. 2019. Macrophage heterogeneity and plasticity in tuberculosis. J Leukoc Biol 106:275–282. 10.1002/JLB.MR0318-095RR. [DOI] [PubMed] [Google Scholar]
- 17.MacMicking J, Xie QW, Nathan C. 1997. Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 18.Bogdan C. 2015. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 36:161–178. 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Borisov VB, Forte E, Konstantinov AA, Poole RK, Sarti P, Giuffre A. 2004. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett 576:201–204. 10.1016/j.febslet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Giuffre A, Borisov VB, Mastronicola D, Sarti P, Forte E. 2012. Cytochrome bd oxidase and nitric oxide: from reaction mechanisms to bacterial physiology. FEBS Lett 586:622–629. 10.1016/j.febslet.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Mason MG, Shepherd M, Nicholls P, Dobbin PS, Dodsworth KS, Poole RK, Cooper CE. 2009. Cytochrome bd confers nitric oxide resistance to Escherichia coli. Nat Chem Biol 5:94–96. 10.1038/nchembio.135. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, BarryCE, III.. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395. 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. 2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother 53:3720–3725. 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manjunatha U, Boshoff HI, Barry CE. 2009. The mechanism of action of PA-824: novel insights from transcriptional profiling. Commun Integr Biol 2:215–218. 10.4161/cib.2.3.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berube BJ, Parish T. 2018. Combinations of respiratory chain inhibitors have enhanced bactericidal activity against Mycobacterium tuberculosis. Antimicrob Agents Chemother 62:e01677-17. 10.1128/AAC.01677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berube BJ, Russell D, Castro L, Choi SR, Narayanasamy P, Parish T. 2019. Novel MenA inhibitors are bactericidal against Mycobacterium tuberculosis and synergize with electron transport chain inhibitors. Antimicrob Agents Chemother 63:e02661-18. 10.1128/AAC.02661-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X, Williams Z, Hards K, Tang J, Cheung CY, Aung HL, Wang B, Liu Z, Hu X, Lenaerts A, Woolhiser L, Hastings C, Zhang X, Wang Z, Rhee K, Ding K, Zhang T, Cook GM. 2019. Pyrazolo[1,5- a]pyridine inhibitor of the respiratory cytochrome bcc complex for the treatment of drug-resistant tuberculosis. ACS Infect Dis 5:239–249. 10.1021/acsinfecdis.8b00225. [DOI] [PubMed] [Google Scholar]
- 28.Baptista R, Fazakerley DM, Beckmann M, Baillie L, Mur LAJ. 2018. Untargeted metabolomics reveals a new mode of action of pretomanid (PA-824). Sci Rep 8:5084. 10.1038/s41598-018-23110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bigelow KM, Tasneen R, Chang YS, Dooley KE, Nuermberger EL. 2020. Preserved efficacy and reduced toxicity with intermittent linezolid dosing in combination with bedaquiline and pretomanid in a murine tuberculosis model. Antimicrob Agents Chemother 64:e01178-20. 10.1128/AAC.01178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Tan Y, Islam MM, Cao Y, Lu X, Zeng S, Hameed HMA, Zhou P, Cai X, Wang S, Mugweru JN, Zhang G, Yin H, Liu J, Nuermberger E, Zhang T. 2020. Assessment of clofazimine and TB47 combination activity against Mycobacterium abscessus using a bioluminescent approach. Antimicrob Agents Chemother 64:e01881-19. 10.1128/AAC.01881-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Li S, Wen S, Zhang T, Shang Y, Huo F, Xue Y, Li L, Pang Y. 2020. Comparison of in vitro susceptibility of mycobacteria against PA-824 to identify key residues of Ddn, the deazoflavin-dependent nitroreductase from Mycobacterium tuberculosis. Infect Drug Resist 13:815–822. 10.2147/IDR.S240716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng S, Soetaert K, Ravon F, Vandeput M, Bald D, Kauffmann JM, Mathys V, Wattiez R, Fontaine V. 2019. Isoniazid bactericidal activity involves electron transport chain perturbation. Antimicrob Agents Chemother 63:e01841-18. 10.1128/AAC.01841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupien A, Foo CS, Savina S, Vocat A, Piton J, Monakhova N, Benjak A, Lamprecht DA, Steyn AJC, Pethe K, Makarov VA, Cole ST. 2020. New 2-ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLoS Pathog 16:e1008270. 10.1371/journal.ppat.1008270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198:705–713. 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmins GS, Master S, Rusnak F, Deretic V. 2004. Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis. Antimicrob Agents Chemother 48:3006–3009. 10.1128/AAC.48.8.3006-3009.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodl E, Winkler A, Macheroux P. 2018. Molecular mechanisms of bacterial bioluminescence. Comput Struct Biotechnol J 16:551–564. 10.1016/j.csbj.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purwantini E, Mukhopadhyay B. 2009. Conversion of NO2 to NO by reduced coenzyme F420 protects mycobacteria from nitrosative damage. Proc Natl Acad Sci U S A 106:6333–6338. 10.1073/pnas.0812883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari BM, Kannan N, Vemu L, Raghunand TR. 2012. The Mycobacterium tuberculosis PE proteins Rv0285 and Rv1386 modulate innate immunity and mediate bacillary survival in macrophages. PLoS One 7:e51686. 10.1371/journal.pone.0051686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korycka-Machała M, Pawełczyk J, Borówka P, Dziadek B, Brzostek A, Kawka M, Bekier A, Rykowski S, Olejniczak AB, Strapagiel D, Witczak Z, Dziadek J. 2020. PPE51 is involved in the uptake of disaccharides by Mycobacterium tuberculosis. Cells 9:603. 10.3390/cells9030603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Boshoff HIM, Harrison JR, Ray PC, Green SR, Wyatt PG, BarryCE, III.. 2020. PE/PPE proteins mediate nutrient transport across the outer membrane of Mycobacterium tuberculosis. Science 367:1147–1151. 10.1126/science.aav5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitra A, Ko YH, Cingolani G, Niederweis M. 2019. Heme and hemoglobin utilization by Mycobacterium tuberculosis. Nat Commun 10:4260. 10.1038/s41467-019-12109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Hendrickson RC, Meikle V, Lefkowitz EJ, Ioerger TR, Niederweis M. 2020. Comprehensive analysis of iron utilization by Mycobacterium tuberculosis. PLoS Pathog 16:e1008337. 10.1371/journal.ppat.1008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Q, Abdalla AE, Xie J. 2015. Phylogenomics of mycobacterium nitrate reductase operon. Curr Microbiol 71:121–128. 10.1007/s00284-015-0838-2. [DOI] [PubMed] [Google Scholar]
- 44.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48:833–843. 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega Ugalde S, Boot M, Commandeur JNM, Jennings P, Bitter W, Vos JC. 2019. Function, essentiality, and expression of cytochrome P450 enzymes and their cognate redox partners in Mycobacterium tuberculosis: are they drug targets? Appl Microbiol Biotechnol 103:3597–3614. 10.1007/s00253-019-09697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayikpoe R, Govindarajan V, Latham JA. 2019. Occurrence, function, and biosynthesis of mycofactocin. Appl Microbiol Biotechnol 103:2903–2912. 10.1007/s00253-019-09684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnamoorthy G, Kaiser P, Lozza L, Hahnke K, Mollenkopf HJ, Kaufmann SHE. 2019. Mycofactocin is associated with ethanol metabolism in mycobacteria. mBio 10:e00190-19. 10.1128/mBio.00190-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welham PA, Stekel DJ. 2009. Mathematical model of the Lux luminescence system in the terrestrial bacterium Photorhabdus luminescens. Mol Biosyst 5:68–76. 10.1039/b812094c. [DOI] [PubMed] [Google Scholar]
- 49.Masiewicz P, Brzostek A, Wolański M, Dziadek J, Zakrzewska-Czerwińska J. 2012. A novel role of the PrpR as a transcription factor involved in the regulation of methylcitrate pathway in Mycobacterium tuberculosis. PLoS One 7:e43651. 10.1371/journal.pone.0043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estorninho M, Smith H, Thole J, Harders-Westerveen J, Kierzek A, Butler RE, Neyrolles O, Stewart GR. 2010. ClgR regulation of chaperone and protease systems is essential for Mycobacterium tuberculosis parasitism of the macrophage. Microbiology 156:3445–3455. 10.1099/mic.0.042275-0. [DOI] [PubMed] [Google Scholar]
- 51.Khare G, Kar R, Tyagi AK. 2011. Identification of inhibitors against Mycobacterium tuberculosis thiamin phosphate synthase, an important target for the development of anti-TB drugs. PLoS One 6:e22441. 10.1371/journal.pone.0022441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazar N, Fay A, Nandakumar M, Boyle KE, Xavier J, Rhee K, Glickman MS. 2017. Control of biotin biosynthesis in mycobacteria by a pyruvate carboxylase dependent metabolic signal. Mol Microbiol 106:1018–1031. 10.1111/mmi.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young DB, Comas I, de Carvalho LP. 2015. Phylogenetic analysis of vitamin B12-related metabolism in Mycobacterium tuberculosis. Front Mol Biosci 2:6. 10.3389/fmolb.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forte E, Giuffre A, Huang LS, Berry EA, Borisov VB. 2020. Nitric oxide does not inhibit but is metabolized by the cytochrome bcc-aa3 supercomplex. Int J Mol Sci 21:8521. 10.3390/ijms21228521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamaati H, Mortaz E, Pajouhi Z, Folkerts G, Movassaghi M, Moloudizargari M, Adcock IM, Garssen J. 2017. Nitric oxide in the pathogenesis and treatment of tuberculosis. Front Microbiol 8:2008. 10.3389/fmicb.2017.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Bossche A, Varet H, Sury A, Sismeiro O, Legendre R, Coppee JY, Mathys V, Ceyssens PJ. 2019. Transcriptional profiling of a laboratory and clinical Mycobacterium tuberculosis strain suggests respiratory poisoning upon exposure to delamanid. Tuberculosis 117:18–23. 10.1016/j.tube.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Boon C, Dick T. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J Bacteriol 184:6760–6767. 10.1128/JB.184.24.6760-6767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang F, Njire MM, Liu J, Wu T, Wang B, Liu T, Cao Y, Liu Z, Wan J, Tu Z, Tan Y, Tan S, Zhang T. 2015. Engineering more stable, selectable marker-free autoluminescent mycobacteria by one step. PLoS One 10:e0119341. 10.1371/journal.pone.0119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. 2009. Universal sample preparation method for proteome analysis. Nat Methods 6:359–362. 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and legends. Download AAC.00956-21-s0001.pdf, PDF file, 0.8 MB (845.6KB, pdf)
Data Set S1. Download AAC.00956-21-s0002.xlsx, XLSX file, 0.5 MB (469.1KB, xlsx)
Data Set S2. Download AAC.00956-21-s0003.xlsx, XLSX file, 0.4 MB (450.5KB, xlsx)