ABSTRACT
The rate of eradication of periprosthetic joint infection (PJI) caused by methicillin-resistant Staphylococcus aureus (MRSA) is still not satisfactory with systemic vancomycin administration after one-stage revision arthroplasty. This study aimed to explore the effectiveness and safety of intraarticular (IA) injection of vancomycin in the control of MRSA PJI after one-stage revision surgery in a rat model. Two weeks of intraperitoneal (IP) and/or IA injection of vancomycin was used to control the infection after one-stage revision surgery. The MRSA PJI rats treated with IA injection of vancomycin showed better outcomes in skin temperature, bacterial counts, biofilm on the prosthesis, serum α1-acid glycoprotein levels, residual bone volume, and inflammatory reaction in the joint tissue, compared with those treated with IP vancomycin, while the rats treated with IP and IA administration showed the best outcomes. However, only the IP and IA administration of vancomycin could eradicate MRSA. Minimal changes in renal pathology were observed in the IP and IP plus IA groups but not in the IA group, while no obvious changes were observed in the liver or in levels of serum markers, including creatinine, alanine aminotransferase, and aspartate aminotransferase. Therefore, IA use of vancomycin is effective and safe in the MRSA PJI rat model and is better than systemic administration, while IA and systemic vancomycin treatment could eradicate the infection with a 2-week treatment course.
KEYWORDS: periprosthetic joint infection, one-stage revision, methicillin-resistant Staphylococcus aureus, intraarticular injection, vancomycin
INTRODUCTION
Periprosthetic joint infection (PJI) is one of the catastrophic complications after artificial joint replacement and occurs in 1.2% to 2.2% of primary arthroplasty cases (1). Staphylococcus aureus is one of the common pathogens (2), and approximately 47% of S. aureus clinical isolates in the United States are methicillin resistant (3), with reported rates as high as 50% to 74% in some regions (4, 5). Studies reported that, after revision of methicillin-resistant S. aureus (MRSA) or methicillin-resistant Staphylococcus epidermidis (MRSE) infection, the risk of failure or reinfection was higher (2), with recurrence rates of infection of up to 28.6% (6–8).
One-stage revision arthroplasty is an important PJI treatment strategies; it has advantages such as fewer operations, faster recovery, and lower surgical mortality rates and has been praised highly by a growing number of scholars in recent years. However, the infection control rates varied greatly, ranging between 75% and 95% (9). The main reason for the difficulty of PJI eradication and recurrence is the formation of bacterial biofilms; the tolerance of mature biofilms to most antimicrobial agents is often 103 times greater than that of their planktonic counterparts (10). In addition to complete debridement during surgery, postoperative antibiotic management is crucial. The levels of vancomycin in synovial fluid were about one-third of those in serum with intravenous (IV) injection of vancomycin after revision arthroplasty (11), which generally could reach the MICs of S. aureus or S. epidermidis strains, even MRSA strains. In addition, the minimum biofilm eradication concentration (MBEC) was several orders of magnitude (about 100 to 1,000 times) above the MIC, which could be a better indicator of the antibiofilm activity than the MIC of planktonic bacteria (12). Regrettably, the MBEC at the site of the joint infection is not achievable with traditional IV injection administration, and increased doses or concentrations of IV injections may result in serious adverse effects. Therefore, intraarticular (IA) injection of vancomycin may be one of the most effective ways to improve the success rate of one-stage revision arthroplasty.
IA injection of vancomycin has been applied to control MRSA PJI following single-stage revision arthroplasty. Although inspiring results were obtained, the grade of the evidence was low, because the data were from retrospective studies or even case reports and experience sharing. Thus, this study intends to explore the effectiveness and safety of IA injection of vancomycin to control PJI caused by MRSA after one-stage revision surgery in a rat model, to provide the experimental basis for the clinical development of a postoperative antibiotic management plan.
RESULTS
Establishment and evaluation of rat MRSA PJI model.
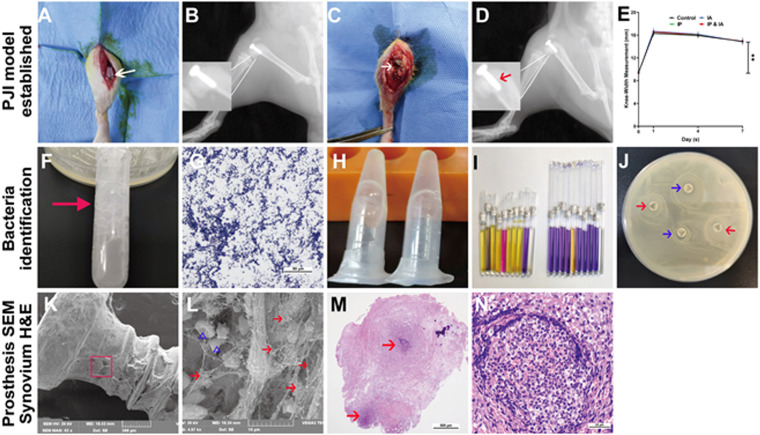
On day 7 after bacteria were inoculated, the surgical knee joint was swollen, and the maximal mediolateral width of the knee was increased (Fig. 1E). Subcutaneous and IA abscess or ulcers (Fig. 1C) and suspicious osteolysis and prosthesis loosening (Fig. 1D) clearly indicated soft tissue and bone infection. Bacteria from the prosthesis and ulcerated tissue were cultured and further confirmed as MRSA by catalase testing, Gram staining, rapid agglutination testing of rabbit plasma coagulase, Staphylococcus identification kits, and cefoxitin susceptibility test discs (Fig. 1F to J). Scanning electron microscopy (SEM) showed that a large number of MRSA organisms grew on the prosthesis, accompanied by a small amount of biofilm formation (Fig. 1K and L). Hematoxylin and eosin (H&E) staining of the synovial tissue also showed infection (Fig. 1M and N). The vancomycin MIC of the strain used in this study (MRSA BAA-1026) was determined to be 2 μg/ml.
FIG 1.
Establishment and evaluation of the PJI model after knee prosthesis implantation in rats. (A) Artificial prosthesis implanted in the knee joint. (B) Lateral X-ray after surgery. (C) Subcutaneous macroscopic examination of the knee joint on postsurgical day 7. The white arrow indicates subcutaneous and IA abscess or ulcer. (D) Lateral X-ray of the knee on postsurgical day 7. The red arrow indicates prosthesis loosening and suspicious osteolysis around the prosthesis. (E) Changes of the surgical knee width on postsurgical days 1, 4, and 7. (F) Microbial catalase testing (many bubbles appear after the microorganisms contact hydrogen peroxide). (G) Microbial Gram staining (Gram-positive bacteria appear purple blue, indicating positive results). (H) Rapid agglutination testing of fresh rabbit plasma (plasma coagulates in a gelatinous form). (I) Staphylococcus identification kit testing (the corresponding indicator reagent tube changes color, indicating that the microorganism is Staphylococcus aureus). (J) Cefoxitin susceptibility disc diffusion testing in LB agar plates with cultured microorganisms (the antibiotic susceptibility test discs [cefoxitin and norfloxacin] have no inhibition zone, indicating that the bacteria are resistant to cefoxitin and norfloxacin; the red arrows indicate cefoxitin sensitivity test discs, and the blue arrows indicate norfloxacin sensitivity test discs). (K and L) Prosthesis taken from the knee for SEM observations at low magnification (×60) (K) and high magnification (×5,000) (L). The red arrows indicate S. aureus, and the blue triangles indicate red blood cells. (M and N) H&E staining of the surgical knee synovium, at low magnification (×40) (M) and high magnification (×400) (N). The red arrows indicate abscess areas. **, P < 0.01. Significance was evaluated using a two-way ANOVA for the comparison of rat knee widths between different treatment groups.
Changes in general status and inflammatory markers.
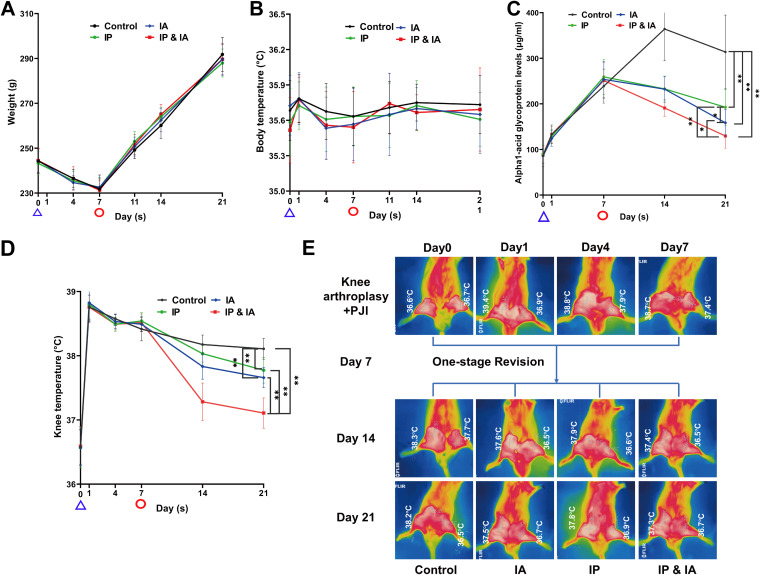
There were no significant differences among the four treatment groups in body weight or body temperature during the whole experiment (P > 0.05) (Fig. 3A and B). Skin temperatures of the surgical knee in the IA, intraperitoneal (IP), and IP plus IA groups were significantly lower than that in the control group; there was no significant difference between the IA and IP groups. The serum α1-acid glycoprotein (α1-AGP) level was lower in the IA group than in the IP and control groups at day 21, while the IP plus IA group showed the lowest skin temperature and serum α1-AGP level (P < 0.05) (Fig. 3C to E).
FIG 3.
Changes in general conditions of the body after PJI rat model establishment, one-stage revision, and corresponding drug treatment in each group. (A) Body weight change curves (n = 12). (B) Body temperature change curves (n = 12). (C) Changes in serum α1-AGP levels during the whole experiment (for the tests on days 0, 7, and 14, n = 6; for the test on day 21, n = 12). (D) Local right knee skin temperature change curves (n = 12). (E) Typical infrared thermal imaging after PJI rat model establishment, revision, and vancomycin treatment of rats in each group (n = 6). Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP plus IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed. The time indicated by the blue triangles is the knee arthroplasty operation and IA inoculation of MRSA in rats, while the time indicated by the red circles is the operation for debridement and implantation of a new elongated revision prosthesis (one-stage revision) in rats. *, P < 0.05; **, P < 0.01. Significance was evaluated using a two-way ANOVA for the comparison of general animal status (weight, body temperature, and local skin temperature of the knee) in different treatment groups.
Radiological evaluation.
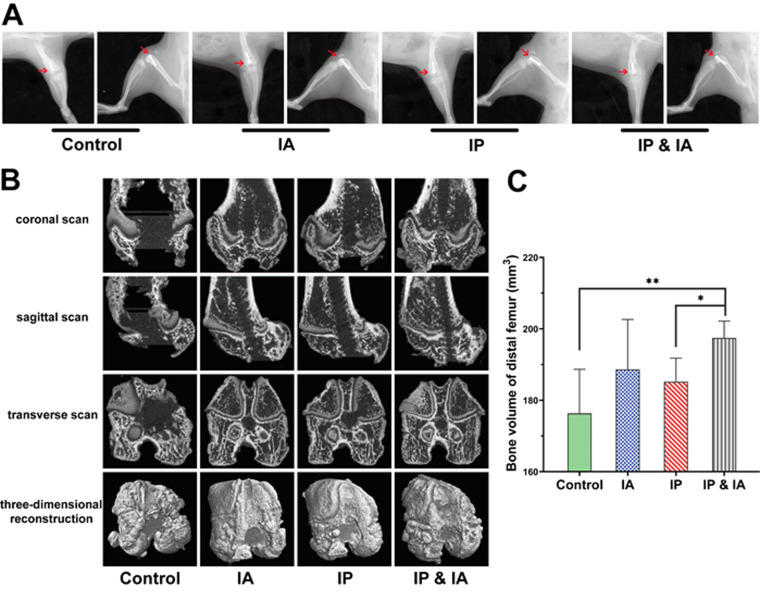
On day 21, prostheses were still in the femoral metaphysis, but all of them were accompanied by signs of bone destruction around the prosthesis, with that in the control group being the most serious (Fig. 4A). There were different degrees of alleviation in the IA, IP, and IP plus IA groups, of which that in the IP plus IA group was the best (Fig. 4A and B). The bone volumes of the distal femur in the control group and the IP group were less than that in the IP plus IA group, and the difference was significant; there was no significant difference among the control, IA, and IP groups (Fig. 4C).
FIG 4.
Radiological evaluation of the knee joint in rats with PJI after debridement and treatment with vancomycin. (A) X-ray of each group on postrevision day 14. (B) Three-dimensional CT scans and bone reconstruction of the distal femur on day 21 (postrevision day 14). (C) Bone volume analysis of the distal femur. Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP plus IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed. *, P < 0.05; **, P < 0.01 (n = 6). The red arrows indicate the position of the prosthesis and the destruction of the distal femur in each group. Significance was evaluated using an unpaired one-tailed Mann-Whitney test for the comparison of residual bone volumes in different treatment groups.
Histopathological evaluation.
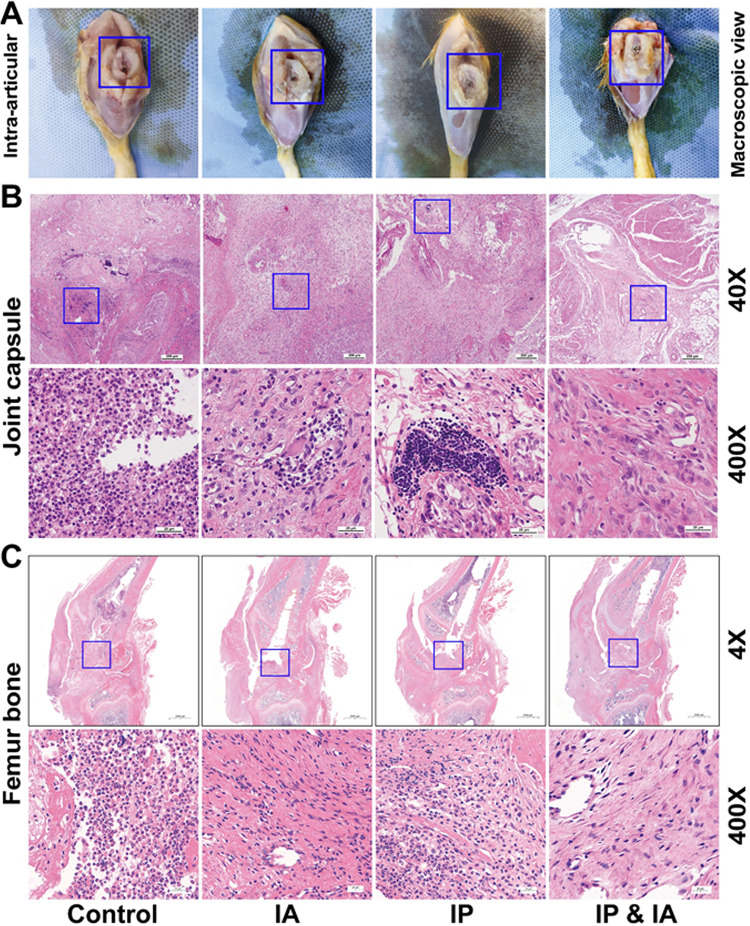
Animals were euthanized on day 21, and then a macroscopic examination of the intraarticular bone and prosthesis was performed. The femoral condylar cartilage was damaged to different degrees in each treatment group. The control group had the most severe cartilage defects, accompanied by prosthesis displacement, while the IP plus IA group had the best cartilage preservation and the prosthesis was stably in place (Fig. 5A). As shown in Fig. 5B, a large number of inflammatory cells were observed in the control group, accompanied by abscess formation. All vancomycin treatment groups (IP, IA, or IP plus IA) showed different degrees of inflammation reduction; that in the IP plus IA group was the most obvious. Osteomyelitis changes, such as intramedullary abscesses, necrotic bone formation, trabecular bone structure changes, and inflammatory cell aggregation, were observed in the control animals (Fig. 5C), while all of these changes were attenuated after vancomycin treatment, especially in the IA and IP plus IA groups (Fig. 5C). No obvious inflammatory bacterial infiltration was observed in the IP plus IA group, while the entrance of the bone tunnel at the femoral trochlea was filled with fibrous connective tissue.
FIG 5.
Macroscopic examination and histopathological assessment of the surrounding knee joint after one-stage revision and treatment with vancomycin in rats with PJI. (A) Macroscopic examination of intraarticular bone and the prosthesis. (B) Pathological H&E staining of joint capsules in each group on day 21. (C) Pathological H&E staining of the knee joint (femur and tibia) in each group on day 21. Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP and IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed. The blue boxes in panels A show the position of the prosthesis and the destruction of the cartilage around the prosthesis, the blue boxes in panels B show the area where inflammation is concentrated, and the blue boxes in panels C represent the entrance of the bone tunnel at the femoral trochlea.
Microbiology.
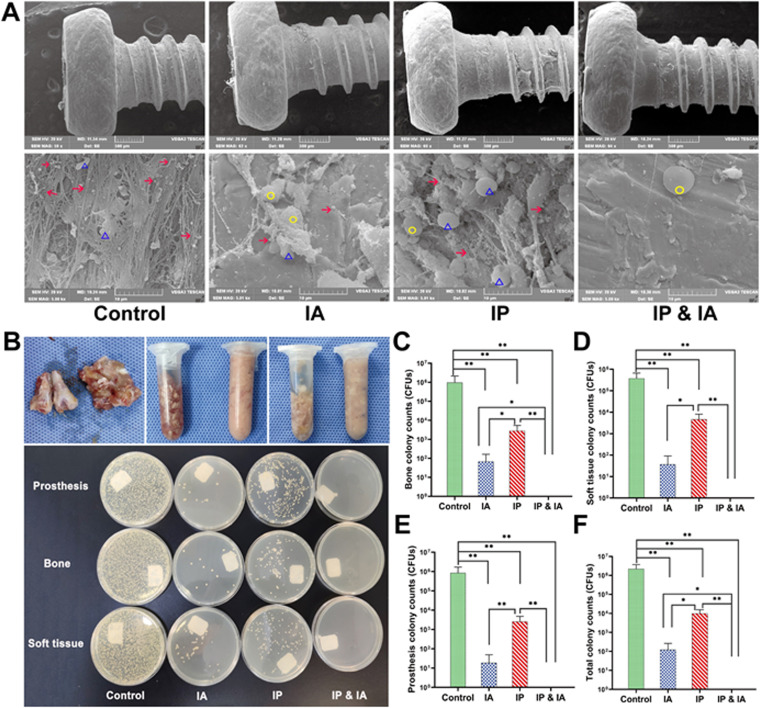
A MRSA biofilm, surrounded by a large number of host leukocytes, was observed in the control group. No other microbial contamination was found in any field of view. A small amount of MRSA was observed on the prosthesis in the IP group, while only several MRSA residues were observed in the IA group. The bacteria in the IP group were slightly more numerous than those in the IA group, while the IP plus IA group showed no microbial growth, and the bacteria on the surface of the prosthesis were completely eliminated (Fig. 6A). All tissues from the PJI knee in the control group were cultured positively on Luria-Bertani (LB) agar plates, while all tissues from the IP plus IA group were free of bacteria (Fig. 6B). The average numbers of bacterial colonies in the control group (prosthesis, 8.57 × 105 ± 8.36 × 105 CFU; bone, 9.78 × 105 ± 11.79 × 105 CFU; soft tissue, 3.78 × 105 ± 2.86 × 105 CFU; whole animals, 2.21 × 106 ± 1.50 × 106 CFU) were significantly greater than those in the IA group (prosthesis, 18 ± 30 CFU; bone, 68 ± 94 CFU; soft tissue, 37 ± 53 CFU; whole animals, 124 ± 140 CFU), the IP group (prosthesis, 2,575 ± 2,220 CFU; bone, 2,815 ± 2,569 CFU; soft tissue, 4,690 ± 3,433 CFU; whole animals, 10,080 ± 5,499 CFU), and the IP plus IA group (no bacterial colonies in any tissue culture) (Fig. 6C to F). The average numbers of colonies for each specimen in the IP group were significantly greater than those in the IA group or the IP plus IA group; only in the IP plus IA group were no bacteria detected (P < 0.05) (Fig. 6C to F).
FIG 6.
Microorganisms in joint tissues of rats in each group after one-stage revision and treatment with vancomycin. (A) On day 21, the microbes on the surface of the prosthesis in each group were observed by SEM, at low magnification (×60) (upper) and high magnification (×5,000) (lower). (B) Before and after homogenization (70 HZ, 10 min) of the knee joint bone and all soft tissues around the knee (upper), images of LB agar plates of microbial cultures (37°C, 24 h) of the prosthesis, bone, and soft tissue in each group of animals (lower) were obtained. (C) Analysis of microbial culture counts for knee joint bones of animals in each group. (D) Analysis of microbial culture counts for all soft tissues around the knee joints of animals in each group. (E) Analysis of microbial culture counts for the prostheses of animals in each group. (F) Analysis of microbial culture counts for the whole animals in each group. Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP and IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed. *, P < 0.05; **, P < 0.01 (n = 6). The red arrows indicate MRSA, the yellow circles indicate leukocytes, and the blue triangles indicate red blood cells. Significance was evaluated using an unpaired one-tailed Mann-Whitney test for the comparison of bacterial counts between different treatment groups.
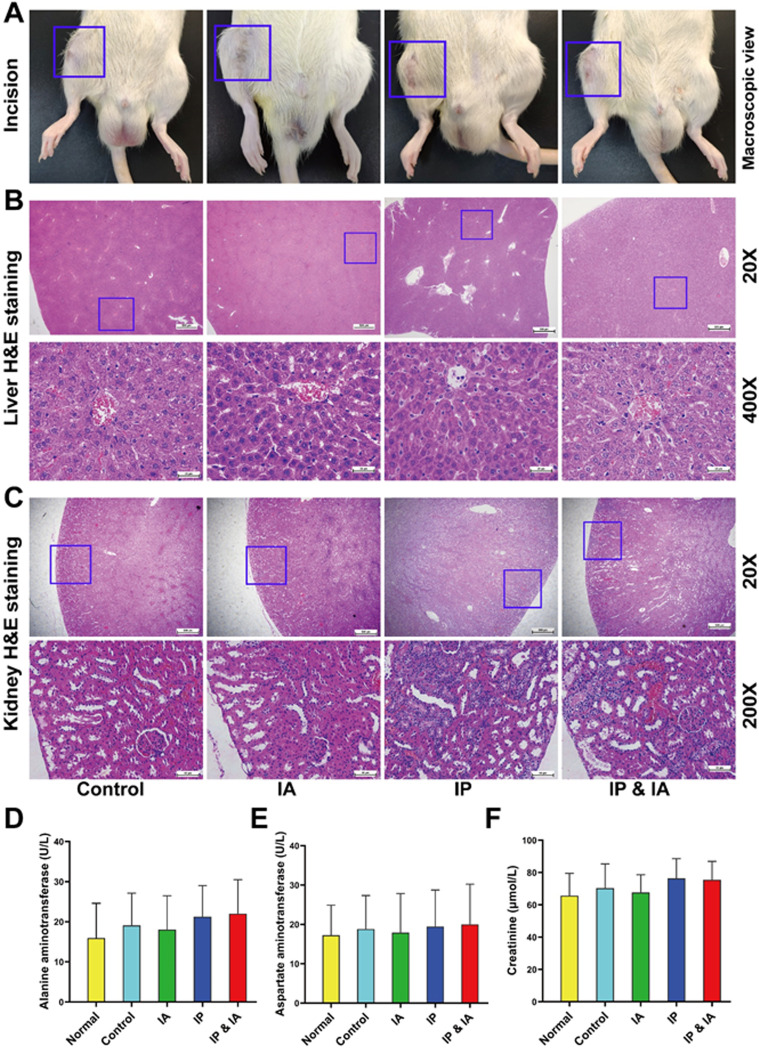
Drug safety assessments.
Macroscopically, the incisions in each group were healed without wound rupture or exudation (Fig. 7A). No obvious pathological changes, such as degeneration and necrosis, were observed in the livers of any of the animals (Fig. 7B). The serum alanine aminotransferase (ALT) results showed that the mean values for the control, IA, IP, and IP plus IA groups were 19.13 ± 8.01 U/liter, 16.76 ± 9.06 U/liter, 21.28 ± 7.73 U/liter, and 22.05 ± 8.45 U/liter, respectively, all within the normal range; there was no significant difference between the four treatment groups and the normal serum ALT result (15.96 ± 8.65 U/liter) (P > 0.05) (Fig. 7D). Aspartate aminotransferase (AST) results showed that the mean values for the control, IA, IP, and IP plus IA groups were 18.85 ± 8.48 U/liter, 17.90 ± 9.95 U/liter, 19.48 ± 9.26 U/liter, and 20.04 ± 10.19 U/liter, all within the normal range (Fig. 7E). No obvious pathological changes, such as degeneration and necrosis of the kidneys, were observed in the control and IA groups. However, inflammatory cell infiltration and mild cortical tubular dilation in a few areas of renal pathology were observed in the IP and IP plus IA groups, of grade 1 (Fig. 7C). The results of serum creatinine (Cr) measurements showed that the mean values for the control, IA, IP, and IP plus IA groups were 70.27 ± 15.05 μmol/liter, 67.62 ± 10.95 μmol/liter, 74.87 ± 12.14 μmol/liter, and 75.49 ± 11.42 μmol/liter, all within the normal range; there was no significant difference between the four treatment groups and normal serum Cr levels (65.70 ± 13.80 μmol/liter) (P > 0.05) (Fig. 7F). The serum levels of vancomycin in all treatment groups were less than 15 μg/ml at 0.5, 2, and 4 h after vancomycin injection on day 21, and the values for both the IA and IP groups were below the limit of detection at 4 h after injection. In addition, serum vancomycin concentrations in all groups were below the limit of detection before injection (Table 1).
FIG 7.
Photographs of wound healing, pathological H&E staining of the liver and kidney, and liver and kidney biochemical indicator test results for rats with PJI in each group after corresponding treatment. (A) Incision healing of rats. (B) Pathological H&E staining of the liver. (C) Pathological H&E staining of the kidney. (D) Serum ALT levels. (E) Serum AST levels. (F) Serum Cr levels. Normal indicates normal serum biochemical values before surgery. Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP and IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed. *, P < 0.05; **, P < 0.01 (n = 12). The blue boxes in panels A show the incision healing in each treatment group, the blue boxes in panels B show the liver lobular structure of the areas of interest, and the blue boxes in panels C represent the renal cortical region of the areas of interest. Significance was evaluated using a two-way ANOVA for the comparison of serum ALT, AST, and Cr levels in different treatment groups.
TABLE 1.
Serum vancomycin levels in each treatment group
| Groupa | Vancomycin level (μg/ml)b |
|||
|---|---|---|---|---|
| Preinjection | 0.5 h | 2 h | 4 h | |
| Control | 0 | 0 | 0 | 0 |
| IA | c | 5.64 ± 0.82 | 3.55 ± 0.26 | c |
| IP | c | 9.39 ± 1.35 | 4.04 ± 0.35 | c |
| IP plus IA | c | 14.31 ± 1.35 | 9.75 ± 1.07 | 1.59 ± 0.40 |
Control (no antibiotics), IP injection of vancomycin (88 mg/kg, q12h), IA injection of vancomycin (44 mg/kg, qd), and IP and IA injection of vancomycin (combined IP treatment at 88 mg/kg, q12h, and IA treatment at 44 mg/kg, qd) were assessed (n = 6).
Serum vancomycin levels were detected before the last injection and 0.5, 2, and 4 h after vancomycin injection in each treatment group on day 21.
Below the limit of detection (1 μg/ml).
DISCUSSION
Although the high bacterial load (106 CFU) of MRSA used in this experiment does not exactly mimic clinical conditions in elective surgery, this bacterial load was based on previous animal studies that indicated a reliable acute infection site contamination model (13, 14). We considered that lower concentrations of bacteria could fail to develop high infection rates in surgically treated rats because of high-level immunity against bacteria in rats (15–17). Therefore, the use of high bacterial concentrations was necessary to have good reproducibility in the rat model.
Vancomycin is currently the preferred IV antibiotic after PJI revision arthroplasty, especially for MRSA or MRSE, because it inhibits bacterial cell wall synthesis (18). Previous clinical studies suggested that both IA injection and systemic vancomycin treatment could achieve therapeutic synovial concentrations, but the peak concentrations in synovial fluid with IA use of vancomycin were many orders of magnitude higher than those with systemic vancomycin treatment and were accompanied by therapeutic serum and synovial fluid levels of vancomycin for about 24 h (11, 19). Regrettably, although our results indicated that IA injection of vancomycin alone was better than IP injection, it could not eliminate the bacteria in 2 weeks. In addition, the International Consensus Meeting suggested that cost and resistance rates are lower when the duration of antibiotics is decreased (20). Thus, IA injection combined with systemic vancomycin treatment might be ideal for treatment, which was confirmed in our current study showing that all bacteria were eliminated in the IA plus IP group. Our data indicated that IA injection combined with systemic vancomycin treatment might be an effective and quick way to eliminate MRSA PJI following one-stage revision arthroplasty.
Our current experiment evaluated one strain of MRSA. Due to the low treatment success rate and high recurrence after MRSA infection, MRSA PJI was even considered by some surgeons to be a contraindication for one-stage revision. The data in the present study suggested that, in the MRSA PJI rat model after knee prosthesis implantation, IA injection of vancomycin could significantly reduce the levels of infecting bacteria and was more effective than IP injection. IP plus IA injection of vancomycin for 2 weeks could eliminate the bacteria in all tissues. In this study, no adverse reactions, such as sinus tract and secondary infections through the injection channel, caused by IA injection were observed; these data corroborate prior clinical IA delivery of vancomycin (11, 19, 21–24). There were no significant changes in liver pathology and serum biochemical markers. However, mild pathological changes in the kidney were observed in both the IP and IP plus IA groups, which were possibly considered early nephrotoxicity caused by systemic vancomycin treatment. This phenomenon was consistent with previous experimental studies on the renal toxicity of vancomycin reported in the literature (25–30). Clinical cases of nephrotoxicity with therapeutic vancomycin levels have been reported, with nephrotoxicity incidence rates ranging from 0% to 17% (31–33), but this dose was routine and relatively safe in clinical application. Further observation and summary in future clinical applications are needed.
However, our approach does have limitations. Firstly, there is some disagreement between the International Consensus Group (ICG) and the Infectious Diseases Society of America (IDSA) about the optimal duration of antibiotic treatment after one-stage revision arthroplasty. The ICG strongly recommends 2 to 6 weeks. The IDSA recommends that the duration of antimicrobial therapy be no more than 6 weeks, including 2 to 6 weeks of IV antibiotic treatment (20). Although we chose 2 weeks as the research endpoint referring to the minimum duration of IV therapy (34), it may be too early to eradicate the bacteria with a single treatment such as IV or IA injection. Because local administration may not cause any systemic adverse effects, the duration could be extended to 3 weeks or even longer, which might lead to better outcomes. Secondly, although available clinical studies and our current experiments have not been reported iatrogenic infections caused by IA injection or infusion of vancomycin, the potential adverse effect exists because of the invasive treatment approach. Thirdly, our experiment mainly discussed the differences in the efficacy of different injection approaches for vancomycin, without adjuvant oral antibiotics such as rifampin or levofloxacin. We plan to address this limitation by subsequently comparing oral rifampin plus systemic vancomycin treatment with oral rifampin plus IA vancomycin treatment. Fourthly, in our study, the serum Cr and vancomycin concentrations were in the normal range. Although renal pathology showed mild changes, it is not a routine way to observe nephrotoxicity. There was no denying that IA injection combined with systemic vancomycin treatment was effective and safe in the treatment of PJI; further experimental studies are required in the future. Fifthly, since synovial fluid from the knee cavity in rats was too sparse to be obtained, we could not measure the concentration and pharmacodynamics of vancomycin in the synovial fluid. We plan to address this limitation in subsequent additional preclinical studies with larger animals. Lastly, the data in our experiment were based on an uncemented prosthesis. Another type of prosthesis, with antibiotic-cemented fixation, is also used; however, antibiotics in the cement should be limited to 1 to 2 g per 40 g cement powder or the mechanical properties of the cement would be significantly affected (35). In addition, recent in vitro studies suggested that less than 5% of total antibiotics were eventually released if more than 1 g of antibiotics was added to 40 g cement powder (36, 37). Further studies are needed to explore the efficacy of different injection approaches for vancomycin by using an antibiotic-cemented prosthesis.
In conclusion, in the current MRSA PJI rat model, IA injection of vancomycin is effective and safe in controlling the infection, while systemic vancomycin treatment combined with IA injection of vancomycin can completely eradicate the infection. Our experimental data support the potential application of IA injection of vancomycin in MRSA PJI. More related research is needed to further evaluate the safety and effectiveness of IA injection of vancomycin.
MATERIALS AND METHODS
Animals and main reagents.
Wistar rats of specific-pathogen-free grade (male, weighing 239 ± 5 g) were obtained from the Center for Disease Control and Prevention (Hubei, China). The protocol for all animal experiments was approved by the Committee on the Ethics of Animal Experiments of the School of Medicine, Wuhan University. All animal experimental procedures were performed following the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee. Clinical-grade vancomycin hydrochloride for injection was obtained from Lilly (Japan). Rat serum α1-AGP enzyme-linked immunosorbent assay (ELISA) kits, serum Cr kits, and serum ALT and AST kits were purchased from Cusabio (China).
Bacterial preparation.
A MRSA strain (ATCC BAA-1026) was streaked on LB agar plates and incubated overnight at 37°C for about 20 to 24 h. Individual colonies were cultured in LB broth overnight at 37°C with shaking (about 20 to 24 h, at 220 rpm). A 1:50 dilution of the overnight culture was subcultured for 2 h at 37°C with shaking. Bacteria were washed in phosphate-buffered saline (PBS), reconstituted to an inoculum of 1.58 × 108 CFU in 10 ml of PBS, as determined by absorbance at 600 nm, and confirmed by overnight culture on LB agar plates after dilution. Vancomycin was added to 96-well plate with serial dilutions, and the initial BAA-2016 in each well was adjusted to a concentration of 105 CFU/ml and incubated at 37°C for 24 h. The vancomycin MIC was defined as the lowest concentration that inhibited visible growth.
Study design.
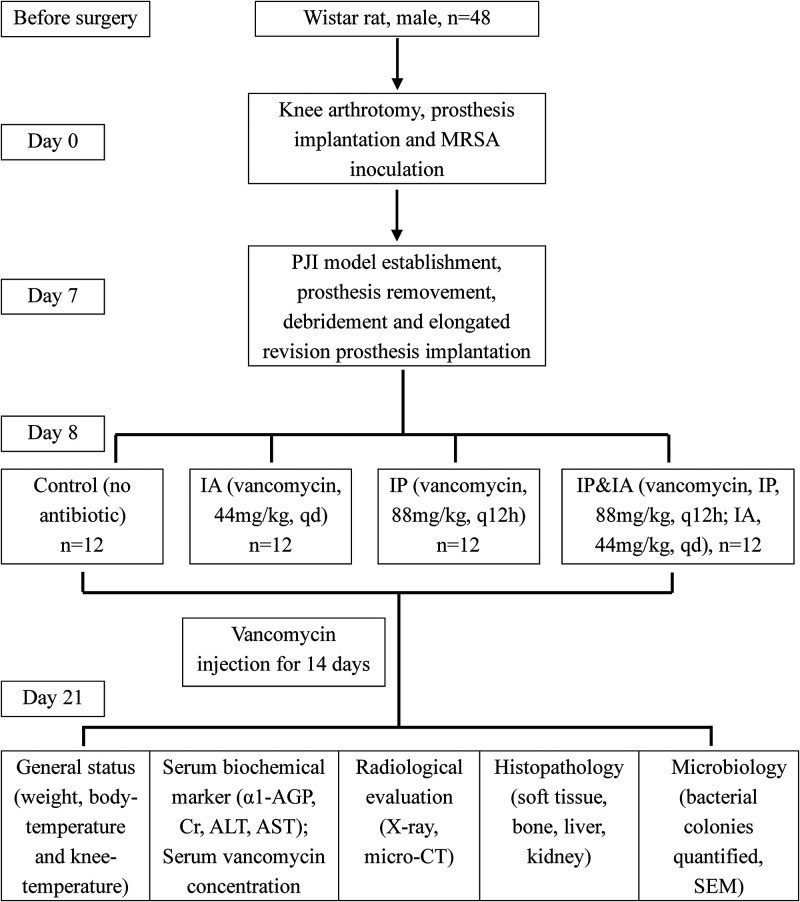
On the basis of previously described murine PJI models (38–42), we chose orthopedic-grade titanium alloy screws to mimic clinical knee arthroplasty and prosthesis implantation in the femoral bone marrow. Forty-eight male Wistar rats were randomly divided into four groups after revision surgery, as follows: (i) control (no antibiotics), (ii) IP injection of vancomycin (88 mg/kg, every 12 h [q12h]; equivalent to 1 g in a 70-kg human, q12h), (iii) IA injection of vancomycin (44 mg/kg, once a day [qd]; equivalent to 0.5 g in a 70-kg human, qd), or (iv) IP plus IA injection of vancomycin (combining IP treatment at 44 mg/kg, q12h, and IA treatment at 44 mg/kg, qd). Doses for the weight-based IP and IA administration of vancomycin were based on routine therapeutic antibiotic doses used for orthopedic infections in humans (19, 43–46) and corresponded to doses of vancomycin used in prior rat models (25, 26, 47).
Surgical procedures and postoperative management.
All operations were performed within a sterile surgical field, using aseptic techniques and sterile instruments and materials. The rats were anesthetized using 2.5% inhalational isoflurane delivered via nose cone and were given preoperative analgesics consisting of subcutaneous buprenorphine (0.1 mg/kg) and parecoxib (2 mg/kg). The maximal medial-lateral width of the knee joint was measured manually with a digital caliper after the knee hair was shaved. After sterile draping of the surgical site, a 2-cm midline longitudinal skin incision was made over the right knee. After knee joint exposure, a 1.2-mm drill bit was used to access the femoral canal just anterior to the Blumensaat line, and the prosthesis (diameter, 1.4 mm; length, 5 mm) was manually placed through retrograde insertion with a screwdriver, with the 1-mm screw cap protruding into the joint (Fig. 1A). The patella was relocated, the capsule was sutured, and the joint was injected with 50 μl of 1.58 × 108 CFU/ml BAA-1026 using a 29-gauge needle. Postoperative radiographs were obtained to verify the placement of the prosthesis (Fig. 1B). Pain was controlled with buprenorphine (0.1 mg/kg) after surgery. The surgical knee was swollen, compared with the preoperative state, on day 7 (Fig. 1E), the subcutaneous and intraarticular tissues of the knee were purulent and ulcerated (Fig. 1C), and X-rays indicated that the prosthesis was loosened (Fig. 1D). The prosthesis was removed aseptically by surgery, and microbiological cultures and related identification tests (catalase testing, Gram staining, rapid agglutination test of rabbit plasma coagulase, Staphylococcus identification kits, and cefoxitin susceptibility test discs) were carried out to confirm that the MRSA PJI model was successfully established (Fig. 1F to J). After complete debridement, a new elongated revision prosthesis (diameter, 1.5 mm; length, 10 mm) was implanted. Animals were treated in groups for 14 consecutive days from the first day after revision surgery (on day 8). All animals were euthanized on day 21 for blood collection and tissue harvesting in accordance with the Institutional Animal Care and Use Committee-approved protocol (Fig. 2).
FIG 2.
Graph illustrating the treatment scheme for the animals in this experiment.
General status and serological marker analyses.
General status, including body weight, body temperature, and local temperature of the knee joint, were measured during the whole experimental study process. The body weights of the rats were recorded before surgery and on days 1, 4, 7, 11, 14, and 21. An electronic thermometer for animals and an infrared thermometer were used to measure the temperature of the anus and rectum of the rats. The body temperature was recorded before surgery and on days 1, 4, 7, 11, 14, and 21. An infrared thermometer and an infrared thermal imager (FLIR, USA) were used to measure the local skin temperature of the surgical knee of rats. The local knee temperature data and images were recorded before surgery and on days 1, 4, 7, 14, and 21. Because α1-AGP is a characteristic serum marker of acute infection in rats that was reported to be better than C-reactive protein (CRP), blood leukocytes, and interleukins (39, 48–50), we choose it as the marker for the prognosis of infection, revision, and drug treatment. We measured serum α1-AGP levels before surgery and on days 1, 7, 14, and 21. The serum Cr, ALT, and AST levels were measured before surgery and on day 21. Because synovial fluid from the rat knee cavity was too sparse to be obtained, we could not measure the concentration and pharmacodynamics of vancomycin in the synovial fluid. We measured the serum concentrations of vancomycin by high-performance liquid chromatography-mass spectrometry (Thermo Fisher Scientific, USA) (preinjection and 0.5, 2, and 4 h after vancomycin injection on day 21).
X-ray imaging.
Anteroposterior and lateral X-ray images of the right hind limbs were taken after anesthesia on day 21 to confirm prosthesis positioning and bone destruction (Bruker, Germany).
Micro-computed tomographic imaging and data analysis.
To evaluate infective bone reaction in bone resorption and osteomyelitis, micro-computed tomographic analysis was performed on the surgical bone with a SkyScan 1276 system (Bruker, Germany). Scan images were reconstructed and bone parameters surrounding the prosthesis were assessed using CT Viewer (Materialise, Belgium). We analyzed the three-dimensional reconstructed images with CTAn v1.15.4 software (Bruker). After scan calibration, we created one-box volumes of interest (size, 1,327.446 mm3 [x, 13.55 mm; y, 13.55 mm; z, 9.03 mm) for the femoral metaphysis. The bone volume (in cubic millimeters) within the volume of interest was quantitatively measured and reported as the residual bone volume.
SEM.
Prostheses were carefully removed, and their surfaces were examined with SEM (Zeiss, Germany) by a single, experienced observer who was blinded to the treatment. S. aureus was identified as spherical structures with the following features: no surface deformities, organized in pairs or clusters, and approximately 0.8 μm to 1 μm in diameter (41).
Histopathology.
Histological analyses (knee joint bone and capsule) were carried out to assess the tissue morphology, with particular attention to signs of inflammation, bone necrosis, and osteomyelitis, as well as to verify the presence or absence of degeneration and necrosis of liver and kidney tissues. Samples were processed, and paraffin-embedded sections (4 μm) were stained with H&E. The field of interest was selected for observation. The histological extent of renal injury was assessed using a six-tier grading system, as follows: grade 0, normal; grade 1, cloudy swelling of the proximal tubular epithelium without necrosis; grade 2, <25% necrosis of the cortical area; grade 3, 25 to 50% necrosis of the cortical area; grade 4, 50 to 75% necrosis of the cortical area; grade 5, >75% necrosis of the cortical area (51).
Microbiology and data analyses.
After euthanasia on day 21, the surgical skin incision was reopened under sterile conditions. Sterile surgical instruments were used to harvest wound tissue, including all of the muscles and soft tissue around the knee joint, the knee joint (including the distal femur, patella, and proximal tibia), and the prosthesis. The tissues were combined with the same amount (10 ml) of sterile PBS and homogenized with a fast tissue grinder. Then, 100 μl of supernatant was inoculated onto LB agar plates and grown for 24 to 48 h at 37°C. The retrieved prosthesis was placed in 5 ml of sterile PBS containing 0.3% Tween 20 and was sonicated to stimulate the release of bacterial biofilm from the prosthesis. A 100-μl aliquot of prosthesis supernatant was plated in the same manner as the other tissue supernatants (14, 52, 53). Bacterial colonies were quantified using the plate count method.
Statistical analysis.
Data were analyzed using the SPSS program (version 22.0; SPSS Inc., Chicago, IL, USA) and are presented as the mean and standard error of the mean. Data were compared by two-way analysis of variance (ANOVA) or unpaired one-tailed Mann-Whitney test. P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
This research was financially supported by grants from the National Natural Science Foundation of China (grants 81603214, 81673490, 81673524, and 81972036) and the Key Research and Development Project of Hubei Province (grant 2020BCA071).
We thank Qi Yin from the microbiology laboratory of Zhongnan Hospital of Wuhan University (Wuhan, China) for providing the MRSA strain ATCC BAA-1026.
REFERENCES
- 1.Miller RJ, Thompson JM, Zheng J, Marchitto MC, Archer NK, Pinsker BL, Ortines RV, Jiang X, Martin RA, Brown ID, Wang Y, Sterling RS, Mao HQ, Miller LS. 2019. In vivo bioluminescence imaging in a rabbit model of orthopaedic implant-associated infection to monitor efficacy of an antibiotic-releasing coating. J Bone Joint Surg 101:e12. 10.2106/JBJS.18.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hischebeth GT, Randau TM, Ploeger MM, Friedrich MJ, Kaup E, Jacobs C, Molitor E, Hoerauf A, Gravius S, Wimmer MD. 2019. Staphylococcus aureus versus Staphylococcus epidermidis in periprosthetic joint infection: outcome analysis of methicillin-resistant versus methicillin-susceptible strains. Diagn Microbiol Infect Dis 93:125–130. 10.1016/j.diagmicrobio.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Garvin KL, Hinrichs SH, Urban JA. 1999. Emerging antibiotic-resistant bacteria: their treatment in total joint arthroplasty. Clin Orthop Relat Res 369:110–123. 10.1097/00003086-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355:666–674. 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J, Ghanem E, Azzam K, Davis E, Jaberi F, Hozack W. 2008. Periprosthetic infection: are current treatment strategies adequate? Acta Orthop Belg 74:793–800. [PubMed] [Google Scholar]
- 6.Petis SM, Abdel MP, Perry KI, Mabry TM, Hanssen AD, Berry DJ. 2019. Long-term results of a 2-stage exchange protocol for periprosthetic joint infection following total hip arthroplasty in 164 hips. J Bone Joint Surg 101:74–84. 10.2106/JBJS.17.01103. [DOI] [PubMed] [Google Scholar]
- 7.Cochran AR, Ong KL, Lau E, Mont MA, Malkani AL. 2016. Risk of reinfection after treatment of infected total knee arthroplasty. J Arthroplasty 31:156–161. 10.1016/j.arth.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Santoso A, Yoon TR, Park KS, Anwar IB, Utomo P, Soetjahjo B, Sibarani T. 2020. The results of two-stage revision for methicillin-resistant periprosthetic joint infection (PJI) of the hip. Malays Orthop J 14:18–23. 10.5704/MOJ.2003.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bialecki J, Bucsi L, Fernando N, Foguet P, Guo S, Haddad F, Hansen E, Janvari K, Jones S, Keogh P, McHale S, Molloy R, Mont MA, Morgan-Jones R, Ohlmeier M, Saldana A, Sodhi N, Toms A, Walker R, Zahar A. 2019. Hip and knee section, treatment, one stage exchange: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34(Suppl):S421–S426. 10.1016/j.arth.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnsholt T, Ciofu O, Molin S, Givskov M, Hoiby N. 2013. Applying insights from biofilm biology to drug development: can a new approach be developed? Nat Rev Drug Discov 12:791–808. 10.1038/nrd4000. [DOI] [PubMed] [Google Scholar]
- 11.Roy ME, Peppers MP, Whiteside LA, Lazear RM. 2014. Vancomycin concentration in synovial fluid: direct injection into the knee vs. intravenous infusion. J Arthroplasty 29:564–568. 10.1016/j.arth.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Argenson JN, Arndt M, Babis G, Battenberg A, Budhiparama N, Catani F, Chen F, de Beaubien B, Ebied A, Esposito S, Ferry C, Flores H, Giorgini A, Hansen E, Hernugrahanto KD, Hyonmin C, Kim TK, Koh IJ, Komnos G, Lausmann C, Loloi J, Lora-Tamayo J, Lumban-Gaol I, Mahyudin F, Mancheno-Losa M, Marculescu C, Marei S, Martin KE, Meshram P, Paprosky WG, Poultsides L, Saxena A, Schwechter E, Shah J, Shohat N, Sierra RJ, Soriano A, Stefansdottir A, Suleiman LI, Taylor A, Triantafyllopoulos GK, Utomo DN, Warren D, Whiteside L, Wouthuyzen-Bakker M, Yombi J, Zmistowski B. 2019. Hip and knee section, treatment, debridement and retention of implant: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34(Suppl):S399–S419. 10.1016/j.arth.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Sweet FA, Forsthoefel CW, Sweet AR, Dahlberg RK. 2018. Local versus systemic antibiotics for surgical infection prophylaxis in a rat model. J Bone Joint Surg 100:e120. 10.2106/JBJS.18.00105. [DOI] [PubMed] [Google Scholar]
- 14.Zebala LP, Chuntarapas T, Kelly MP, Talcott M, Greco S, Riew KD. 2014. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. J Bone Joint Surg Am 96:46–51. 10.2106/JBJS.L.01257. [DOI] [PubMed] [Google Scholar]
- 15.Shimazaki T, Miyamoto H, Ando Y, Noda I, Yonekura Y, Kawano S, Miyazaki M, Mawatari M, Hotokebuchi T. 2010. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J Biomed Mater Res B Appl Biomater 92:386–389. 10.1002/jbm.b.31526. [DOI] [PubMed] [Google Scholar]
- 16.Lovati AB, Romano CL, Bottagisio M, Monti L, De Vecchi E, Previdi S, Accetta R, Drago L. 2016. Modeling Staphylococcus epidermidis-induced non-unions: subclinical and clinical evidence in rats. PLoS One 11:e0147447. 10.1371/journal.pone.0147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovati AB, Bottagisio M, de Vecchi E, Gallazzi E, Drago L. 2017. Animal models of implant-related low-grade infections. a twenty-year review. Adv Exp Med Biol 971:29–50. 10.1007/5584_2016_157. [DOI] [PubMed] [Google Scholar]
- 18.de Beaubien B, Belden K, Bell K, Boyle KK, Cordero-Ampuero J, Della Valle CJ, Eijer H, Ferry C, Janz V, Kessler B, Kratky A, Lachiewicz A, Martin KE, Murillo O, Nijhof M, Nodzo SR, Petrie MJ, Stockley I, Suleiman LI. 2019. Hip and knee section, treatment, antimicrobials: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34(Suppl):S477–S482. 10.1016/j.arth.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Whiteside LA, Roy ME, Nayfeh TA. 2016. Intra-articular infusion: a direct approach to treatment of infected total knee arthroplasty. Bone Joint J 98B:31–36. 10.1302/0301-620X.98B.36276. [DOI] [PubMed] [Google Scholar]
- 20.Anemuller R, Belden K, Brause B, Citak M, Del Pozo JL, Frommelt L, Gehrke T, Hewlett A, Higuera CA, Hughes H, Kheir M, Kim KI, Konan S, Lausmann C, Marculescu C, Morata L, Ramirez I, Rossmann M, Silibovsky R, Soriano A, Suh GA, Vogely C, Volpin A, Yombi J, Zahar A, Zimmerli W. 2019. Hip and knee section, treatment, antimicrobials: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 34(Suppl):S463–S475. 10.1016/j.arth.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Whiteside LA, Peppers M, Nayfeh TA, Roy ME. 2011. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusion. Clin Orthop Relat Res 469:26–33. 10.1007/s11999-010-1313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteside LA, Roy ME. 2017. One-stage revision with catheter infusion of intraarticular antibiotics successfully treats infected THA. Clin Orthop Relat Res 475:419–429. 10.1007/s11999-016-4977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji B, Zhang X, Xu B, Guo W, Mu W, Cao L. 2017. Single-stage revision for chronic fungal periprosthetic joint infection: an average of 5 years of follow-up. J Arthroplasty 32:2523–2530. 10.1016/j.arth.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Ji B, Li G, Zhang X, Wang Y, Mu W, Cao L. 2020. Effective treatment of single-stage revision using intra-articular antibiotic infusion for culture-negative prosthetic joint infection. Bone Joint J 102B:336–344. 10.1302/0301-620X.102B3.BJJ-2019-0820.R1. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell JN, Rhodes NJ, Miglis CM, Catovic L, Liu J, Cluff C, Pais G, Avedissian S, Joshi MD, Griffin B, Prozialeck W, Gulati A, Lodise TP, Scheetz MH. 2018. Dose, duration, and animal sex predict vancomycin-associated acute kidney injury in preclinical studies. Int J Antimicrob Agents 51:239–243. 10.1016/j.ijantimicag.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. 2012. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 40:1031–1048. 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 28.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabler IM, Berkovitch M, Sandbank J, Kozer E, Dagan Z, Goldman M, Bahat H, Stav K, Zisman A, Klin B, Abu-Kishk I. 2016. Exposure to hyperbaric oxygen intensified vancomycin-induced nephrotoxicity in rats. PLoS One 11:e0152554. 10.1371/journal.pone.0152554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pais GM, Liu J, Avedissian SN, Hiner D, Xanthos T, Chalkias A, d’Aloja E, Locci E, Gilchrist A, Prozialeck WC, Rhodes NJ, Lodise TP, Fitzgerald JC, Downes KJ, Zuppa AF, Scheetz MH. 2020. Lack of synergistic nephrotoxicity between vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother 75:1228–1236. 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farber BF, MoelleringRC, Jr.. 1983. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 23:138–141. 10.1128/AAC.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellor JA, Kingdom J, Cafferkey M, Keane CT. 1985. Vancomycin toxicity: a prospective study. J Antimicrob Chemother 15:773–780. 10.1093/jac/15.6.773. [DOI] [PubMed] [Google Scholar]
- 33.Sorrell TC, Collignon PJ. 1985. A prospective study of adverse reactions associated with vancomycin therapy. J Antimicrob Chemother 16:235–241. 10.1093/jac/16.2.235. [DOI] [PubMed] [Google Scholar]
- 34.Ayoade F, Li DD, Mabrouk A, Todd JR. 2021. Prosthetic joint infection. StatPearls, Treasure Island, FL. [Google Scholar]
- 35.Bistolfi A, Ferracini R, Albanese C, Verne E, Miola M. 2019. PMMA-based bone cements and the problem of joint arthroplasty infections: status and new perspectives. Materials (Basel) 12:4002. 10.3390/ma12234002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop AR, Kim S, Squire MW, Rose WE, Ploeg HL. 2018. Vancomycin elution, activity and impact on mechanical properties when added to orthopedic bone cement. J Mech Behav Biomed Mater 87:80–86. 10.1016/j.jmbbm.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 37.Hsu YH, Hu CC, Hsieh PH, Shih HN, Ueng SW, Chang Y. 2017. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic joint infection. J Bone Joint Surg Am 99:223–231. 10.2106/JBJS.16.00290. [DOI] [PubMed] [Google Scholar]
- 38.Belmatoug N, Cremieux AC, Bleton R, Volk A, Saleh-Mghir A, Grossin M, Garry L, Carbon C. 1996. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic, and magnetic resonance imaging characterization. J Infect Dis 174:414–417. 10.1093/infdis/174.2.414. [DOI] [PubMed] [Google Scholar]
- 39.Soe NH, Jensen NV, Nurnberg BM, Jensen AL, Koch J, Poulsen SS, Pier G, Johansen HK. 2013. A novel knee prosthesis model of implant-related osteomyelitis in rats. Acta Orthop 84:92–97. 10.3109/17453674.2013.773121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig MR, Poelstra KA, Sherrell JC, Kwon MS, Belzile EL, Brown TE. 2005. A novel total knee arthroplasty infection model in rabbits. J Orthop Res 23:1100–1104. 10.1016/j.orthres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Carli AV, Bhimani S, Yang X, Shirley MB, de Mesy Bentley KL, Ross FP, Bostrom MP. 2017. Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg 99:e25. 10.2106/JBJS.16.00815. [DOI] [PubMed] [Google Scholar]
- 42.Lovati AB, Bottagisio M, Maraldi S, Violatto MB, Bortolin M, De Vecchi E, Bigini P, Drago L, Romano CL. 2018. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin Orthop Relat Res 476:1324–1338. 10.1097/01.blo.0000534692.41467.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA. 2013. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283. 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 44.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 45.Yokoe DS, Anderson DJ, Berenholtz SM, Calfee DP, Dubberke ER, Ellingson KD, Gerding DN, Haas JP, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle LE, Salgado CD, Bryant K, Classen D, Crist K, Deloney VM, Fishman NO, Foster N, Goldmann DA, Humphreys E, Jernigan JA, Padberg J, Perl TM, Podgorny K, Septimus EJ, VanAmringe M, Weaver T, Weinstein RA, Wise R, Maragakis LL. 2014. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 35:967–977. 10.1086/677216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelstein AI, Weiner JA, Cook RW, Chun DS, Monroe E, Mitchell SM, Kannan A, Hsu WK, Stulberg SD, Hsu EL. 2017. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg Am 99:232–238. 10.2106/JBJS.16.00127. [DOI] [PubMed] [Google Scholar]
- 47.Avedissian SN, Pais GM, O'Donnell JN, Lodise TP, Liu J, Prozialeck WC, Joshi MD, Lamar PC, Becher L, Gulati A, Hope W, Scheetz MH. 2019. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother 74:2326–2334. 10.1093/jac/dkz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cray C, Zaias J, Altman NH. 2009. Acute phase response in animals: a review. Comp Med 59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 49.Mestriner FL, Spiller F, Laure HJ, Souto FO, Tavares-Murta BM, Rosa JC, Basile-Filho A, Ferreira SH, Greene LJ, Cunha FQ. 2007. Acute-phase protein α-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci U S A 104:19595–19600. 10.1073/pnas.0709681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto K, Nishi K, Kikuchi M, Kadowaki D, Tokutomi Y, Tokutomi N, Nishi K, Suenaga A, Otagiri M. 2007. α1-Acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol Pharm Bull 30:1226–1230. 10.1248/bpb.30.1226. [DOI] [PubMed] [Google Scholar]
- 51.Aronoff GR, Sloan RS, DinwiddieCB, Jr, Glant MD, Fineberg NS, Luft FC. 1981. Effects of vancomycin on renal function in rats. Antimicrob Agents Chemother 19:306–308. 10.1128/AAC.19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. 2010. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One 5:e12580. 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris JL, Letson HL, Grant A, Wilkinson M, Hazratwala K, McEwen P. 2019. Experimental model of peri-prosthetic infection of the knee caused by Staphylococcus aureus using biomaterials representative of modern TKA. Biol Open 8:bio045203. 10.1242/bio.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]