ABSTRACT
We assessed the pharmacokinetics and safety of XAV-19, a swine glyco-humanized polyclonal antibody against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in coronavirus disease 2019 (COVID-19)-related moderate pneumonia. The objective was to evaluate the optimal dose and safety of XAV-19 during this first administration to patients with COVID-19-related moderate pneumonia. In this phase IIa trial, adults with COVID-19-related moderate pneumonia with a duration of ≤10 days were randomized to receive an infusion of XAV-19 at 0.5 mg/kg of body weight at day 1 and day 5 (group 1), 2 mg/kg at day 1 and day 5 (group 2), or 2 mg/kg at day 1 (group 3) or placebo. Eighteen patients (n = 7 for group 1, n = 1 for group 2, n = 5 for group 3, and n = 5 for placebo) were enrolled. Baseline characteristics were similar across groups; median XAV-19 serum concentrations (ranges) at the time of the maximum serum concentration of the drug (Cmax) and at day 8 were 9.1 (5.2 to 18.1) and 6.4 (2.8 to 11.9) μg/ml, 71.5 and 47.2 μg/ml, and 50.4 (29.1 to 55.0) and 20.3 (12.0 to 22.7) μg/ml for groups 1, 2, and 3, respectively (P = 0.012). The median terminal half-life (range) was estimated at 11.4 (5.5 to 13.9) days for 2 mg/kg of XAV-19 at day 1. Serum XAV-19 concentrations were above the target concentration of 10 μg/ml (2-fold the in vitro 100% inhibitory concentration [IC100]) from the end of perfusion to more than 8 days for XAV-19 at 2 mg/kg at day 1. No hypersensitivity or infusion-related reactions were reported during treatment, and there were no discontinuations for adverse events and no serious adverse events related to the study drug. A single intravenous dose of 2 mg/kg of XAV-19 demonstrated high serum concentrations, predictive of potent durable neutralizing activity with good tolerability. (This study has been registered at ClinicalTrials.gov under identifier NCT04453384.)
KEYWORDS: pneumonia, COVID-19, phase IIa, polyclonal glyco-humanized anti-SARS-CoV-2 antibody, XAV-19
INTRODUCTION
Since the first identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from patients with bilateral pneumonia in Wuhan, China, during December 2019, the ongoing pandemic of coronavirus disease 2019 (COVID-19) has affected more than 127 million people and caused 2.8 million deaths (1, 2). Among patients hospitalized for COVID-19, 15 to 20% develop severe respiratory failure requiring admission to the intensive care unit (ICU) (3). Corticosteroids and anticoagulant therapy have improved the prognosis of patients requiring respiratory support for severe or critical pneumonia (4, 5). Multitargeted interventions in severe COVID-19, combining a potent antiviral(s), steroids, anticoagulants, and, in the most severe cases, adjunctive immune-based therapy such as tocilizumab, could constitute an optimized cocktail to halt further progression to respiratory failure, acute respiratory distress syndrome (ARDS), multiorgan dysfunction, and death (6–8).
Animal-derived heterologous polyclonal antibodies, used as passive heterologous immunotherapy, could represent a highly efficient alternative to the use of monoclonal antibodies in COVID-19 by targeting multiple antigen epitopes. However, conventional polyclonal heterologous antibodies induce natural human xenogeneic antibody responses leading to immune complexes and a high risk of serum sickness. To avoid these concerns, we engineered experimental pigs deficient in the CMP-N-acetylneuraminic acid hydroxylase (CMAH)-encoding genes and the enzyme α1,3-galactosyltransferase (GGTA1) to produce glyco-humanized polyclonal antibodies (GH-pAbs) lacking Neu5Gc and α-Gal epitopes. XAV-19 is a purified polyclonal IgG fraction, first-in-class drug to treat COVID-19, based on immunization with the SARS-CoV-2 spike receptor binding domain (RBD) of CMAH/GGTA1 double-knockout pigs to obtain neutralizing polyclonal antibodies.
In an ongoing trial, we are investigating XAV-19, a swine glyco-humanized polyclonal SARS-CoV-2-neutralizing antibody (9), in hospitalized patients with COVID-19 pneumonia requiring low-flow oxygen supplementation (10). In agreement with French national authorities, it was agreed that the first-in-human study was to be performed as a phase IIa dose selection study in COVID-19 patients, to gather safety information for this population and allow rollover without a delay in a phase III study.
The main hypothesis is that reducing the viral burden and improving specific immunity by passive antibody administration at hospital entry of patients hospitalized for COVID-19-related moderate pneumonia within 10 days of first symptom onset could lead to clinical benefit. The dose rationale for the administration of XAV-19 was based on an assessment of the in vitro inhibitory potency of XAV-19 against SARS-CoV-2. The in vitro inhibitory potency of XAV-19 was determined at a concentration of <1 μg/ml by inhibiting the binding of the COVID-19 spike protein to the ACE-2 receptor by a competitive enzyme-linked immunosorbent assay (ELISA) and a cytopathogenic effect assay using infection of human Vero cells with SARS-CoV-2 (9, 11). Based on these findings, the target serum concentration was established at 10 μg/ml. Considering the volume of distribution and elimination half-life (t1/2) of swine glyco-humanized IgG measured in primates, an infusion of XAV-19 above 0.5 mg/kg of body weight was estimated to be necessary to obtain serum neutralizing antibody titers maintained for 10 days.
Here, we report the results of the first part of this ongoing clinical trial involving patients hospitalized for COVID-19-related moderate pneumonia requiring oxygen supplementation. In this phase IIa trial, the objectives of this first-in-human administration of XAV-19 were to assess its pharmacokinetics (PK) and safety.
RESULTS
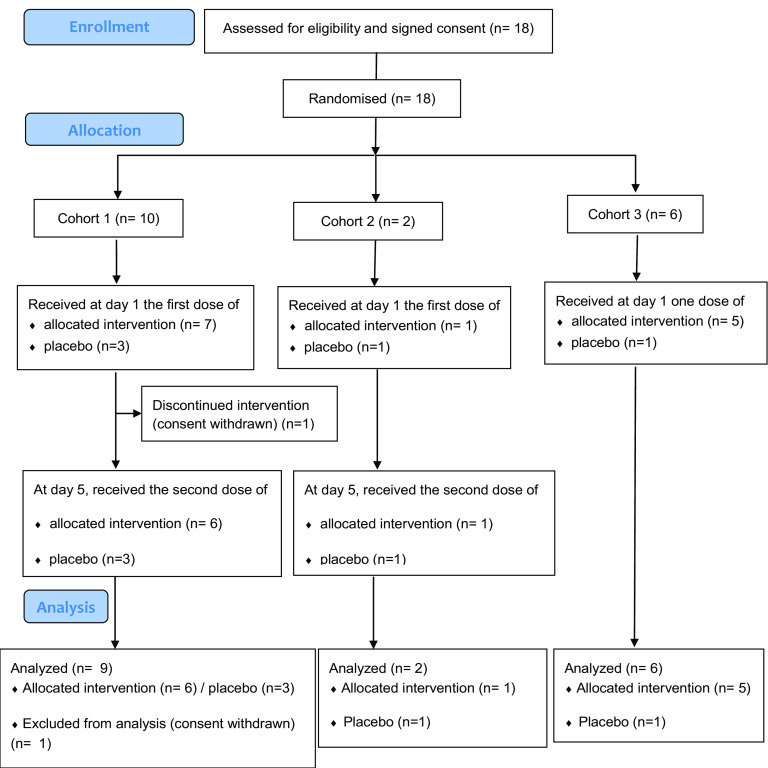
Between 30 August 2020 and 7 December 2020, 18 patients with COVID-19-related moderate pneumonia were randomized: 1 withdrew consent before the day 5 second infusion (cohort 1), and 17 completed all study visits (Fig. 1). Hence, the safety population was constituted of 18 patients, whereas the intent-to-treat exposed (ITT-E) population was constituted of 17 patients. Demographics and baseline characteristics of the 17 patients were similar across groups (Table 1). Of the 17 analyzed patients, 12 were treated with XAV-19 (0.5 mg/kg at days 1 and 5 [n = 6], 2 mg/kg at days 1 and 5 [n = 1], and 2 mg/kg at day 1 [n = 5]); 5 were treated with placebo; 11 were males (64.7%); the median age was 71 years (interquartile range [IQR], 51 to 75 years); and median body mass index (BMI) was 27.4 (IQR, 25.3 to 31.2) kg/m2. The majority (14/17) of patients had at least one comorbidity, including 3 with immunodeficiency disease (Table 1). At screening, all patients were hospitalized, requiring supplemental oxygen by nasal prongs or a mask with low-oxygen delivery (WHO scale 4). Associated COVID-19 therapies were steroids in all patients and remdesivir in 8 (47%) patients. The median time from symptom onset to the initial infusion of the investigational drug was 8 days (IQR, 6 to 9 days).
FIG 1.
Flow diagram.
TABLE 1.
Baseline characteristics of patients and clinical outcomes
| Characteristic | Value for group |
|||||
|---|---|---|---|---|---|---|
| XAV-19 (n = 12) |
All XAV-19-treated patients (n = 12) | Placebo (n = 5) | Total (n = 17) | |||
| 0.5 mg/kg, days 1–5 (n = 6) | 2 mg/kg, days 1–5 (n = 1) | 2 mg/kg, day 1 (n = 5) | ||||
| Median age (yrs) (IQR) | 68 (49.8; 77.3) | 75 | 74 (68; 76) | 75 (58.2; 76.5) | 63 (51; 71) | 71 (51; 75) |
| No. (%) of male patients | 3 (50) | 1 | 4 (80) | 8 (67) | 3 (60) | 11 (64.7) |
| Median BMI (kg/m2) (IQR) | 25.1 (23.6; 29.8) | 39.2 | 27.4 (27.4; 28.4) | 27.4 (25.2; 32) | 29.3 (26.1; 29.8) | 27.4 (25.3; 31.2) |
| No. (%) of patients with chronic underlying disease | ||||||
| Asthma | 2 (33.3) | 0 | 0 | 2 (16.7) | 1 (20) | 3 (17.6) |
| Cardiovascular/cerebrovascular disease | 1 (16.7) | 1 | 1 (20) | 3 (25) | 2 (40) | 5 (29.4) |
| Chronic kidney disease | 1 (16.7) | 0 | 0 | 1 (8.3) | 0 | 1 (5.9) |
| Diabetes | 0 | 0 | 1 (20) | 1 (8.3) | 1 (20) | 2 (11.8) |
| High blood pressure | 4 (66.7) | 1 | 2 (40) | 7 (58.3) | 1 (20) | 8 (47.1) |
| Obesity | 1 (16.7) | 0 | 1 (20) | 2 (16.7) | 1 (20) | 3 (17.6) |
| Solid-organ transplant | 1 (16.7) | 0 | 0 | 1 (8.3) | 0 | 1 (5.9) |
| Solid tumora | 1 (16.7) | 0 | 1 (20) | 2 (16.7) | 0 | 2 (11.8) |
| HIV infection | 1 (16.7) | 0 | 0 | 1 (8.3) | 0 | 1 (5.9) |
| Median duration of symptoms before 1st infusion (days) (IQR) | 7.5 (5.3; 9.8) | 5 | 8 (6; 9) | 7 (5.8; 9.3) | 8 (8; 8) | 8 (6; 9) |
| Symptom at infusion | ||||||
| No. (%) of patients hospitalized, without oxygen supplementation | 1 (16.7) | 0 | 0 | 1 (8.3) | 1 (20) | 2 (11.8) |
| No. (%) of patients hospitalized, low-oxygen delivery | 5 (83.3) | 1 | 4 (80) | 10 (83.3) | 4 (80) | 14 (82.4) |
| No. (%) of patients hospitalized, on noninvasive ventilation or high-flow oxygen | 0 | 0 | 1 (20) | 1 (8.3) | 0 | 1 (5.9) |
| Median respiratory rate (breaths/min) (IQR) | 27 (22.5; 31.5) | 26 | 22 (20; 28) | 25 (22; 30) | 24 (24; 28) | 24 (22; 30) |
| Median SpO2 (IQR) | 94 (93.3; 94.8) | 96 | 92 (91; 94) | 94 (92; 94.3) | 94 (93; 94) | 94 (92; 92) |
| Median National Early Warning Score 2 (IQR) | 6.5 (6; 7.8) | 6 | 7 (5; 8) | 6.5 (5.8; 8) | 6 (6; 6) | 6 (5; 7) |
| No. (%) of patients with concomitant medication for COVID-19 | ||||||
| Antibiotics | 4 (66.7) | 0 | 2 (40) | 6 (50) | 2 (40) | 8 (47.1) |
| Steroids | 6 (100) | 1 | 5 (100) | 12 (100) | 5 (100) | 17 (100) |
| Remdesivir | 2 (33.3) | 1 | 1 (20) | 4 (33.3) | 4 (80) | 8 (47.1) |
| Anticoagulant | 5 (83.3) | 1 | 5 (100) | 11 (91.7) | 5 (100) | 16 (94.1) |
| Median laboratory parameter at day 1 (IQR) | ||||||
| White blood cells/mm3 | 7.9 (7.6; 10.3) | 4.2 | 7.2 (6.9; 7.5) | 7.2 (6.6; 9.1) | 6.5 (6.2; 6.7) | 7.1 (6.5; 7.9) |
| Hemoglobin, g/dl | 11.8 (11; 12.5) | 12.9 | 12.7 (11.9; 13.4) | 12.6 (11.3; 13.5) | 14·1 (13.2; 15) | 12·4 (11.3; 13.3) |
| Platelets/mm3 | 314 (238.8; 407.5) | 163 | 257 (237; 276) | 240 (170; 314) | 245 (191; 297) | 261 (172; 350) |
| Creatinine, µmol/literb | 85 (76.3; 92.8) | 86 | 65.5 (64; 67) | 75.5 (61.3; 86) | 76 (71.5; 80.5) | 84 (67; 86) |
| No. (%) of patients with ICU admission | 0 | 0 | 2 (40) | 2 (16.7) | 1 (20) | 3 (17.6) |
| Median hospital length of stay (days) (IQR) | 5.5 (5; 11.3) | 10 | 11 (7; 12) | 8.5 (5.8; 12.3) | 7 (7; 12) | 7 (6; 12) |
| 60-day mortality rate [no. (%) of patients] | 1 (14.3) | 0 | 0 | 1 (8.3) | 0 | 1 (5.6) |
One patient was receiving treatment for diffuse large B cell lymphoma (DLBCL) diagnosed in 2019, and the other one presented a history of solid lung tumor diagnosed in 2001, considered not active.
Missing data for the 0.5-mg/kg group at days 1 and 5 (n = 2), the 2-mg/kg group at day 1 (n = 3), and the placebo group (n = 3).
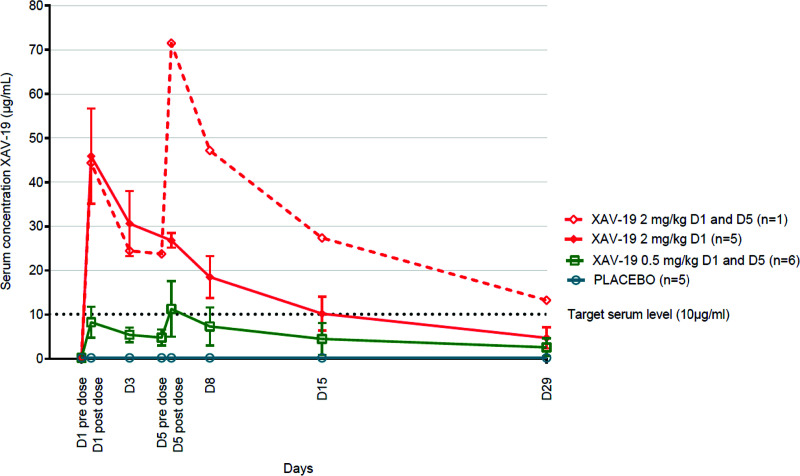
Pharmacokinetic parameters of XAV-19 are presented in Table 2. Median XAV-19 serum concentrations (ranges) at day 8 were 6.4 μg/ml (2.8 to 11.9 μg/ml) in patients who received 0.5 mg/kg of XAV-19 at days 1 and 5, 20.3 μg/ml (12.0 to 22.7 μg/ml) for those who received 2 mg/kg at day 1, and 47.2 μg/ml for the patient who received 2 mg/kg at days 1 and 5 (P = 0.012 between groups) (Table 2). Serum concentrations were above 29 μg/ml in all patients at day 1 after infusion of 2 mg/kg of XAV-19 (Table 2). Based on a target neutralization threshold of 10 μg/ml of XAV-19, all patients treated with 2 mg/kg (1 or 2 infusions) had serum concentrations above the target at day 8. A proportional relationship between the cumulative administered dose and the area under the concentration-time curve from 0 h to infinity (AUC0–∞) was found, suggesting a linear pharmacokinetic for XAV-19 between these doses. According to this linearity, no significant difference between t1/2 values was observed between the groups of patients. The median half-life (range) (n = 12) for all XAV-19 doses was 13.0 (0.7 to 19.4) days, which allowed maintenance of the serum concentration of XAV-19 above the previously defined target serum level of 10 μg/ml (2-fold the 100% neutralization activity in vitro) during 15 days after a single 2-mg/kg administration for most patients (Fig. 2) (9). After a single infusion of the 2-mg/kg dose, the median volume of distribution and clearance (ranges) (n = 5) were 4.9 (4.0 to 8.7) liters and 0.015 (0.010 to 0.021) liters/h, respectively.
TABLE 2.
XAV-19 pharmacokinetic parameter estimates according to dose and schedule of administrationa
| Variable | Value for XAV-19 treatment group (n = 12) |
P value | ||
|---|---|---|---|---|
| 0.5 mg/kg, days 1 and 5 (n = 6) | 2 mg/kg, days 1 and 5 (n = 1) | 2 mg/kg, day 1 (n = 5) | ||
| Median concn after day 1 infusion (min–max) | 6.90 (3.29–10.9) | 47.35 (29.10–55)b | 0.0022 | |
| Median serum concn at day 8 (μg/ml) (min–max) | 6.4 (2.8–11.9) | 47.2 | 20.3 (12.0–22.7) | 0.012 |
| Median AUC0–∞ (μg/ml · h) (min–max) | 4,564 (987–7,832) | 25,638 | 12,519 (7,515–14,314) | 0.018 |
| Median Cmax (μg/ml) (min–max) | 9.1 (5.2–18.1) | 71.5 | 50.4 (29.1–55.0) | 0.012 |
| Tmax (day postdose) | 5 | 5 | 1 | |
| Median t1/2 (days) (min–max) | 14.5 (0.7–19.4) | 11.88 | 11.4 (5.5–13.9) | 0.211 |
AUC, area under the serum concentration-time curve; Cmax, maximum serum concentration; Tmax, time when a drug is present at the maximum concentration in serum; t1/2, half-life.
Values for all day 1 postinfusion measures at a dose of 2 mg/kg (n = 6).
FIG 2.
Time course of mean XAV-19 serum concentrations (± standard deviations) in the groups of patients. D1, day 1.
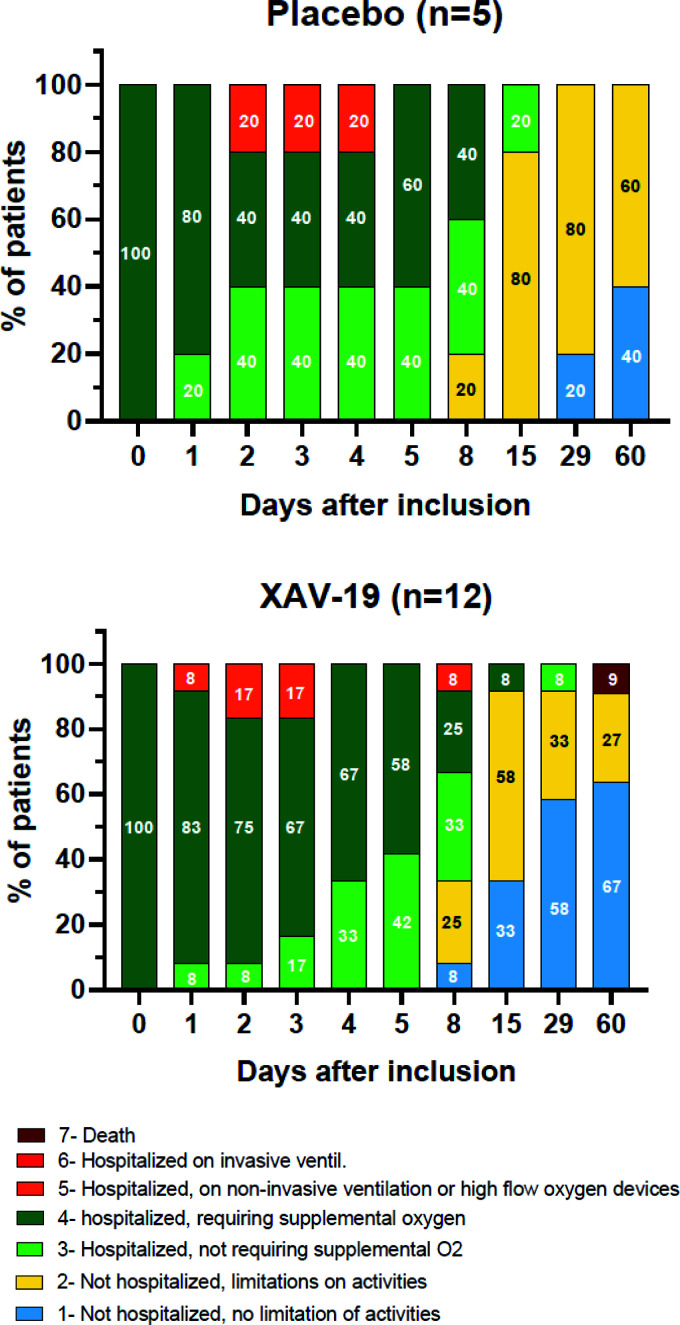
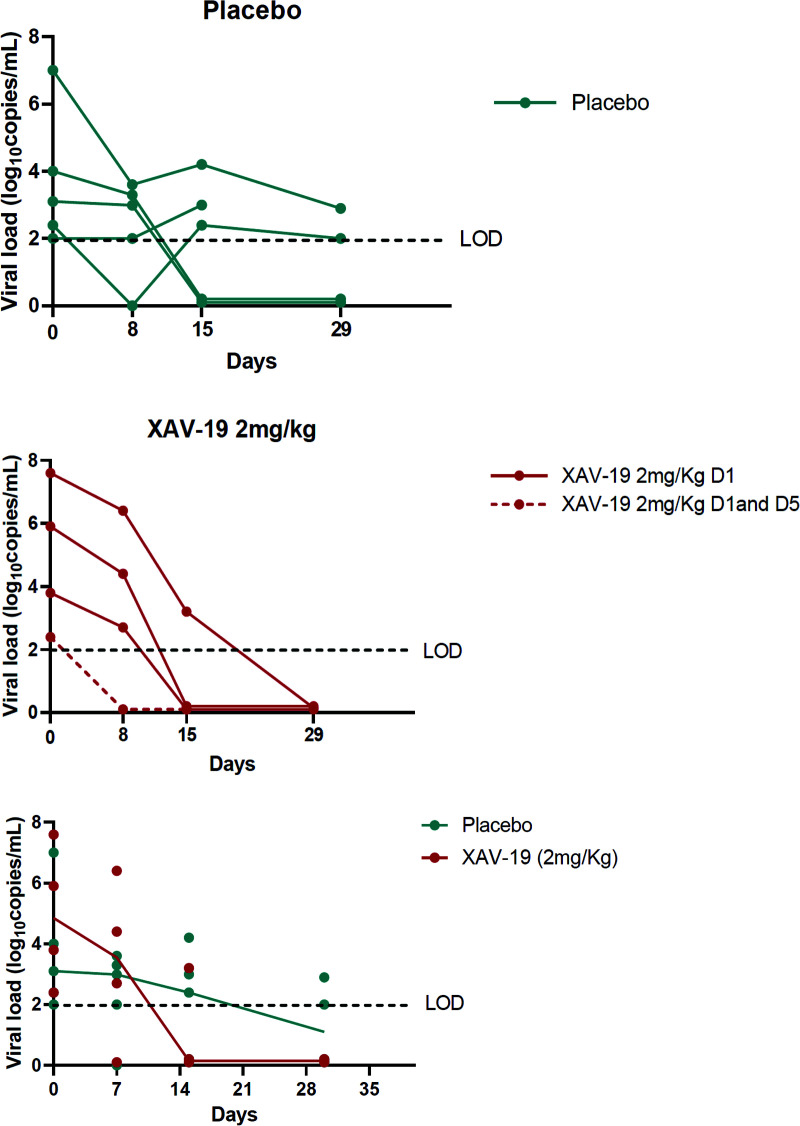
A summary of the adverse events is presented in Table 3. XAV-19 was well tolerated, with no differences in the nature and rate of adverse events between the placebo and XAV-19 groups and no serious adverse events related to the study drug. Only two patients experienced a drug-related grade 1 adverse event (one had lymphadenopathy in the placebo group, and one had localized transient ecchymotic rash at day 15 in the 0.5-mg/kg group). Severe adverse events occurred in three patients in the placebo group, with two grade 3 events (worsening asthenia and increased dyspnea) and one grade 4 event (respiratory distress), and in five patients in the XAV-19 group, with two grade 4 events (worsening respiratory distress) and three grade 3 events (respiratory failure and two hepatobiliary disorders) (see Tables S1 and S2 in the supplemental material). No hypersensitivity or infusion-related reactions were reported during treatment, and there were no treatment discontinuations due to adverse events. Of the 17 patients included in the ITT-E population, 3 (17.6%) developed respiratory failure requiring high-flow ventilation: 1 (20%) in the placebo group and 2 (16.6%) patients who received XAV-19. At day 8, 11/17 patients were free of oxygen: 3/5 in the placebo group and 8/12 in the XAV-19 group (Table 4). At the last follow-up visit on day 60, one patient in the 0.5-mg/kg group had died at day 58 from complications of comorbidities, and all other patients showed clinical improvement and had been discharged from the hospital (Fig. 3). Longitudinal assessment of quantitative viral loads in nasopharyngeal swabs was available for four patients in the 2-mg/kg XAV-19 groups and for five patients in the placebo group. The median decreases of viral loads from baseline were −0.6 and −2.8 log10 copies/ml at days 8 and 15, respectively, in the placebo group and −1.35 and −4.05 log10 copies/ml, respectively, in the XAV-19 2-mg/kg group (Fig. 4).
TABLE 3.
Adverse events (safety analysis population) over 29 days, with at least 1 event in the class
| Parameter | Value for group |
||||
|---|---|---|---|---|---|
| XAV-19 (n = 13) |
All XAV-19-treated patients (n = 13) | Placebo (n = 5) | |||
| 0.5 mg/kg, days 1 and 5 (n = 7) | 2 mg/kg, days 1 and 5 (n = 1) | 2 mg/kg, day 1 (n = 5) | |||
| Mean no. of any adverse events (per patient) (SD) | 3.7 (2.7) | 5 | 3 (2.6) | 3.5 (2.5) | 5.6 (4) |
| No. (%) of patients with adverse event of grade: | |||||
| 1 or 2 | 7 (100) | 1 (100) | 4 (80) | 12 (92.3) | 5 (100) |
| 3 or 4 | 2 (28.6) | 0 | 2 (40) | 4 (30.8) | 3 (60) |
| No. (%) of patients with adverse event related to study drug | 1 (14.3) | 0 | 0 | 1 (7.7) | 1 (20) |
| No. (%) of patients with ≥1 adverse event by class according to MedDRA (system organ class) | |||||
| Blood and lymphatic system disorders | 3 (42.9) | 0 | 0 | 3 (23.1) | 1 (20) |
| Cardiac disorders | 0 | 1 (100) | 0 | 1 (7.7) | 0 |
| Gastrointestinal disorders | 1 (14.3) | 0 | 0 | 1 (7.7) | 2 (40) |
| General disorders and administration site conditions | 1 (14.3) | 1 (100) | 1 (20) | 3 (23.1) | 3 (60) |
| Hepatobiliary disorders | 1 (14.3) | 1 (100) | 2 (40) | 4 (30.8) | 3 (60) |
| Infections and infestations | 3 (42.9) | 0 | 0 | 3 (23.1) | 0 |
| Injury, poisoning, and procedural complications | 1 (14.3) | 0 | 0 | 1 (7.7) | 0 |
| Musculoskeletal and connective tissue disorders | 2 (28.6) | 0 | 0 | 2 (15.4) | 1 (20) |
| Neurological/psychiatric system disorders | 1 (14.3) | 0 | 1 (20) | 2 (15.4) | 2 (40) |
| Renal and urinary disorders | 4 (57.1) | 0 | 1 (20) | 5 (38.5) | 0 |
| Respiratory, thoracic, and mediastinal disorders | 1 (14.3) | 1 (100) | 3 (60) | 5 (38.5) | 3 (60) |
| Skin and subcutaneous tissue disorders | 1 (14.3) | 0 | 0 | 1 (7.7) | 2 (40) |
TABLE 4.
Clinical status according to the 7-category WHO ordinal scale between screening (day 0) and day 29 in the placebo (n = 5) and XAV-19 (n = 12) groups
| 7-point ordinal scale score (description) | No. (%) of patients |
|
|---|---|---|
| XAV-19 (n = 12) | Placebo (n = 5) | |
| Baseline | ||
| 4 (hospitalized, requiring supplemental oxygen) | 12 (100) | 5 (100) |
| Day 3 | ||
| 3 (hospitalized, not requiring supplemental oxygen) | 2 (17) | 2 (40) |
| 4 (hospitalized, requiring supplemental oxygen) | 8 (67) | 2 (40) |
| 5 (hospitalized, on noninvasive ventilation or high-flow O2 devices) | 2 (17) | 1 (20) |
| Day 8 | ||
| 1 (not hospitalized, no limitations on activities) | 1 (8) | 0 |
| 2 (not hospitalized, limitations on activities) | 3 (25) | 1 (20) |
| 3 (hospitalized, not requiring supplemental oxygen) | 4 (33) | 2 (40) |
| 4 (hospitalized, requiring supplemental oxygen) | 3 (25) | 2 (40) |
| 5 (hospitalized, on noninvasive ventilation or high-flow O2 devices) | 1 (8) | 0 |
| Day 15 | ||
| 1 (not hospitalized, no limitations on activities) | 4 (33) | 0 |
| 2 (not hospitalized, limitations on activities) | 7 (58) | 4 (80) |
| 3 (hospitalized, not requiring supplemental oxygen) | 0 | 1 (20) |
| 4 (hospitalized, requiring supplemental oxygen) | 1 (8) | 0 |
| Day 29 | ||
| 1 (not hospitalized, no limitations on activities) | 7 (58) | 1 (20) |
| 2 (not hospitalized, limitations on activities) | 4 (33) | 4 (80) |
| 3 (hospitalized, not requiring supplemental oxygen) | 1 (8) | 0 |
FIG 3.
Clinical status according to the 7-category WHO ordinal scale between screening (day 0) and last follow-up (day 60) in the placebo (n = 5) and XAV-19 (n = 12) groups.
FIG 4.
Nasopharyngeal viral loads over time in the placebo (n = 5) and XAV-19 2-mg/kg (n = 4) groups. The evolution of median viral loads over time is shown. D1, day 1; LOD, limit of detection (2.2 log10 copies/ml); 0, no signal detected.
DISCUSSION
This study was a randomized, double-blind, placebo-controlled, multicenter, phase IIa trial evaluating the optimal dose and the safety of XAV-19, a swine-derived glyco-humanized polyclonal SARS-CoV-2-neutralizing antibody, in patients admitted to the hospital with COVID-19-related moderate pneumonia. The results of this first human trial are consistent with the a priori-estimated dose required to obtain an effective antibody titer in humans and have shown that a single infusion of XAV-19 at 2 mg/kg maintained the serum concentration of XAV-19 well above the predicted neutralization target concentration of 10 μg/ml during at least 8 days postinfusion (9). The median elimination half-life for XAV-19 at 2 mg/kg was estimated to be 11.4 days, which provides a rationale for a single infusion to maintain in vivo neutralizing activity for at least 8 days. Patients with acute COVID-19 requiring oxygen supplementation because of progression to severe COVID-19 require therapeutic interventions that prevent further worsening that occurs rapidly, usually within the first 8 to 10 days following hospitalization. Indeed, steroids in this setting are used for no longer than 10 days (5). Our data also suggest that XAV-19 has an antiviral effect in vivo, as the numerical reduction of nasopharyngeal viral loads was greater with XAV-19 than with placebo, although our analysis was exploratory and performed on a limited number of patients. Studies have shown that symptom onset is not a sufficient predictor of the viral load in respiratory secretions, as high viral loads might persist for 2 weeks, nor is it a sufficient predictor of individual innate immune responses or the ability of the immune response to control ongoing viral replication (12–14). Thus, although a maximal benefit of any antiviral therapy, either specific antiviral or neutralizing antibodies, is expected when treatment is started earlier in the illness, the benefit could also persist in treated patients with a longer duration of symptoms, as demonstrated with remdesivir (6).
XAV-19 maintains neutralization activity against the most predominant SARS-CoV-2 variants, including the initial Wuhan strain and the 501Y.V1 and 501Y.V2 variants, while some anti-SARS-CoV-2 monoclonal antibodies exhibit reduced activity against the new variants (11). Indeed, bamlanivimab has a slight loss of activity against the B.1.1.7 variant, while both bamlanivimab and etesevimab have a complete loss of activity against the B.1.351 variant; casirivimab has a large loss of activity against the B.1.351 variant, and imdevimab retains similar activity against all strains. Interim results of recent trials have suggested that among nonhospitalized patients with mild to moderate COVID-19 illness not requiring oxygen supplementation, treatment with a combination of 2 anti-spike neutralizing monoclonal antibodies was associated with an antiviral effect. To what extent such an effect translates into a clinical benefit by reducing worsening and the requirement for hospitalization is being assessed in phase III trials, especially in high-risk patients (15–17). As new strains of SARS-CoV-2 of epidemiological importance might continue to emerge, it will be necessary to carefully determine both in vitro and in vivo the neutralizing activity of cocktails of monoclonal antibodies and of XAV-19. Polyclonal antibodies offer the advantages over monoclonal antibodies of covering the different epitopes of the target antigen and mimicking natural responses to the antigen, with a lower cost.
XAV-19 was well tolerated, and no major safety issues or dose-related trends were identified. The clinical outcomes of COVID-19 were not different in both groups, but the numbers were too small in this phase IIa study to see any trend. The rate of worsening of respiratory failure, based on the WHO scale, was within the expected range based on the characteristics of the enrolled population, and the death observed at day 59 was unrelated to the study drug or COVID-19 (18, 19). No immediate hypersensitivity reactions or infusion-related reactions were reported in our study, in contrast to reports and warnings with cocktails of anti-spike monoclonal antibodies (17).
An important limitation of this phase IIa portion of our trial is the small sample size, which did not allow us to determine if XAV-19 was associated with improved outcomes. This question will be rigorously tested in the analysis of the phase III part of this ongoing trial. Second, higher doses of XAV-19 were not explored. However, the 2-mg/kg dose achieved sustained active concentrations.
In conclusion, XAV-19 was well tolerated in patients admitted to the hospital for COVID-related moderate pneumonia requiring low-flow oxygen supplementation. The pharmacokinetic results for a single infusion of 2 mg/kg suggest that this dose has the potential to successfully block viral diffusion in humans and support the selection of this regimen for the ongoing multicenter, randomized (1:1), double-blind, placebo-controlled, phase III trial (ClinicalTrials.gov identifier NCT04453384) involving 400 patients. This novel therapeutic strategy based on xenoantibodies offers new perspectives for the management of infectious and emerging diseases, as it allows the fast and efficient production of targeted polyclonal antibodies against emerging pathogens.
MATERIALS AND METHODS
Study design and participants.
This is an ongoing, multicenter, randomized, double-blind, placebo-controlled, phase IIa to III clinical trial involving hospitalized patients with COVID-19-related moderate pneumonia requiring low-flow oxygen supplementation (10). The first part was conducted as a phase IIa, first-in-human, dose-ranging study at four sites in France to assess the pharmacokinetics and safety of XAV-19 and to select the optimal dose for the second part of the trial, designed as a phase III trial, for which recruitment is ongoing.
This study was conducted in accordance with good clinical practice procedures, all applicable regulatory requirements, and the guiding principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee OUEST VI (approval number 20.06.15.31306). All patients provided written informed consent before the entry into the study. The study is registered under ClinicalTrials.gov identifier NCT04453384.
We prospectively identified hospitalized adults aged between 18 and 85 years with SARS-CoV-2 infection confirmed by positive reverse transcription-PCR (RT-PCR) and the onset of first symptoms less than 10 days prior to enrollment. Inclusion criteria included COVID-19-related moderate pneumonia defined by an oxygen saturation (SpO2) concentration of ≥92% on oxygen supplementation at ≤6 liters/min by a low-flow nasal cannula or mask (score of 4 on the World Health Organization 7-point clinical progression scale [WHO-CPS]) and evidence of pulmonary involvement upon lung examination (rales/crackles) and/or chest imaging (chest X ray or computed tomography [CT]). Exclusion criteria were evidence of multiorgan failure; receipt of immunoglobulins or any blood products in the past 30 days; psychiatric or cognitive illness or recreational drug/alcohol use that would affect subject safety or compliance; end-stage renal disease (estimated glomerular filtration rate [eGFR] of <15 ml/min/1.73 m2); Child-Pugh C-stage liver cirrhosis; decompensated cardiac insufficiency; known allergy, hypersensitivity, or intolerance to the study drug or any of its components; life expectancy estimated to be less than 6 months; patient under guardianship or trusteeship; or pregnancy or lack of effective contraception in women of childbearing potential.
Randomization and masking.
Eligible participants were assigned to two consecutive groups of approximately eight patients per cohort of ascending doses, in a 1:1 randomization scheme per dose for the first two patients and a five-active and one-placebo randomization scheme per dose for the six following participants of each cohort. The two doses of XAV-19 were 0.5 mg/kg and 2 mg/kg administered over a 1-h intravenous infusion at days 1 and 5. After the inclusion of the first two participants in each cohort, the safety and tolerability of the treatment were assessed up to 8 days postinfusion by an independent data-monitoring committee before the continuation of enrollment. Following pharmacokinetic analysis of the first cohort (0.5 mg/kg at day 1 and day 5) and the first two patients (one active and one placebo) of the second cohort (2 mg/kg at day 1 and day 5), the remaining patients of the second cohort received a single infusion of 2 mg/kg of XAV-19 at day 1. All patients also received therapy for COVID-19 according to the standard of care (SOC) at the participating centers. This SOC included the use of dexamethasone and other treatments according to local practice and national guidelines at the time of the study, which may include, not exclusively, antibiotics, antiviral treatment, immune therapies not based on antibody administration, and anticoagulants. Simple randomization was done using Web-based simple (unstratified) allocation by trained clinical research staff. Study investigators, all research and analysis teams, and patients were masked to treatment allocation. The study medications were prepared and dispensed by the hospital pharmacy and presented as ready-to-use aqueous solutions in prelabeled infusion kits according to regulatory requirements. The study medication was then administered to the patients by the medical ward nurses upon receipt.
Procedures.
Medical history and demographic data were collected at screening. Before dosing, patients underwent an evaluation of vital signs; physical examination; assessments for pneumonia, blood hematology, and chemistry; as well as nasopharyngeal swab sampling for SARS-CoV-2 RT-PCR. Patients also had a 12-lead electrocardiogram and, if clinically required, chest X rays and/or CT scans.
During and for the 2 h after the study treatment infusion, vital signs (temperature, respiratory rate, heart rate, systolic and diastolic blood pressures, and oxygen saturation) were monitored every 30 min, and particular attention was paid to the occurrence of hypersensitivity or infusion-related reactions. Patients were monitored for 2 months after the first infusion, with clinical assessment including respiratory status and the 7-point ordinal scale, daily during hospitalization and at days 8, 15, 29, and 60. Blood hematology and chemistry data were collected at each visit. Samples to measure drug serum concentrations were collected from all patients at days 1 (predose and postdose), days 3 and 5 (predose and postdose, if appropriate), and days 8, 15, and 29. The drug concentration was assessed at Charles River Laboratories, Evreux, France, with a validated swine IgG-specific sandwich ELISA, presenting a lower limit of quantification of approximately 50 ng/ml in human serum. The use of concomitant medications was recorded throughout the study. Patients were also regularly assessed for adverse events and possible relation to the investigational medicinal product.
Outcomes.
The two primary outcomes were the pharmacokinetic measurement of the serum concentration of XAV-19 at day 8 and tolerability over 29 days, comparing the XAV-19-treated participants and the placebo group.
Pharmacokinetic parameters of XAV-19 that were evaluated were serum drug concentrations determined immediately at the end of infusion: maximum serum concentration (Cmax); time to maximum serum concentration (Tmax); area under the serum concentration-time curve extrapolated to infinity (AUC0–∞), which was calculated using the AUC0–29 (area under the serum concentration-time curve measured to the concentration at day 29 using the trapezoidal rule), and serum concentration at day 29/Ke (Ke is the apparent first-order terminal rate constant calculated from a semilog plot of the serum concentration-versus-time curve); terminal half-life (t1/2), as determined by the quotient 0.693/Ke; clearance, as determined by the quotient dose/AUC0–∞; and volume of distribution, as determined by the quotient clearance/Ke.
Safety parameters were evaluated over 29 days by the onset of all adverse events suspected to be related to XAV-19 and the incidences of serious adverse events, treatment-related adverse events leading to discontinuation of the study drug, hypersensitivity reactions, and infusion-related reactions.
Secondary and additional outcomes included pharmacokinetics of XAV-19 up to day 29 and clinical outcomes (ICU transfer, 7-point ordinal scale, and length of hospital stay). In an exploratory analysis, SARS-CoV-2 nasopharyngeal viral load changes were assessed in an exploratory subcohort of patients treated with XAV-19 in comparison to the placebo group. Nasopharyngeal swabs were collected in 3 ml of viral transport medium (Yocon, China). RNA extraction was done with the EZ1 DSP virus kit (Qiagen) according to the manufacturer’s instructions. Viral nucleic acids were detected using multiplex SARS-CoV-2 RT-quantitative PCR (RT-qPCR), using RdRp-IP2 and RDRP IP4, adapted from methods of the Charité Protocol and National Reference Centre for Respiratory Viruses, Institute Pasteur (20, 21). When a sample was positive, quantification of the number of RNA copies was done by RT-qPCR using a specific in vitro-transcribed RNA, according to a scale ranging from 2.2 to 10 log10 copies/ml.
Statistical analysis.
The safety population included all subjects randomized into the study who received at least one dose of the study drug. The intent-to-treat exposed (ITT-E) population was defined as all subjects who met study criteria and were randomized into the study with documented evidence of having received at least one dose of randomized treatment and at least one postbaseline measurement of serum XAV-19 titers. The per-protocol population was defined as all subjects included in the ITT-E population excluding those who had at least one major protocol deviation. Placebo patients were pooled for the purpose of analysis. The PK concentration population included all subjects who completed the study drug schedule. Categorical variables were summarized by percent, and comparisons were assessed using a Fisher exact test. Continuous variables were summarized by means and standard errors and by medians and interquartile ranges, and comparisons between groups were done using a Kruskal-Wallis test. The primary endpoint was evaluated for the ITT-E population by a Kruskal-Wallis test between placebo patients and treated patients. The numbers of adverse events were compared between groups of patients using a Fisher exact test. Comparisons of pharmacokinetic parameters were conducted using a Kruskal-Wallis test. The relationship between the AUC0–∞ and the cumulative administered dose of XAV-19 was explored by a linear regression model. Treatment groups were described according to variables of the secondary endpoints. A P value of <0.05 was defined as significant.
Data availability.
The data analyzed and presented in this study are available from the corresponding author upon reasonable request, provided that the request meets local ethical and research governance criteria after publication. Data collected during the study may be processed electronically, in accordance with the requirements of the CNIL (compliance with reference methodology MR001).
ACKNOWLEDGMENTS
This work was funded by the Nantes University Hospital Research Department with support from the Public Investment Bank (BPI France) in the framework of the Investment for the Future program (Programme d’Investissements d’Avenir) and Xenothera. The funder of the study had a role in study design, data collection, and data analysis.
The corresponding author and coauthors interpreted the data, wrote the report, had full access to all the data in the study, and had final responsibility for the decision to submit the work for publication. B.G. and F.R. conceptualized the study. B.G., B.V., R.J., S.B., O.D., A.O., L.B., and F.R. were involved in finalizing the protocol of the study. B.G., F.R., L.B., and A.O. supervised the study. B.G., K.L., V.D., F.A., and F.R. were involved in the clinical care of the patients. A.C., A.J., and L.B. were involved in data curation. E.D., B.V., V.F., M.-A.V., R.J., S.B., R.D., A.L.T., A.C., and A.J. did formal analysis of data. L.F. was responsible for central depository and distribution of study drugs. B.G. and F.R. prepared the original draft of the manuscript. All authors were involved in writing, reviewing, and editing of the manuscript.
B.G. reports receipt of nonfinancial support from Gilead Sciences and MSD, outside the submitted work. B.V. is an employee and chief scientific officer/operating officer of Xenothera and owns shares and holds share options in Xenothera. K.L. reports receipt of personal fees and nonfinancial support from Abbvie, Chiesi, Healthcare, Janssen, MSD, and ViiV, outside the submitted work. V.D. reports receipt of nonfinancial support from Gilead Sciences, MSD, and Sanofi-Pasteur, outside the submitted work. O.D. is a cofounder of Xenothera, is the CEO of Xenothera, and owns shares and holds share options in Xenothera. F.R. reports receipt of personal fees from Abbvie, Gilead Sciences, Janssen, MSD, and ViiV Healthcare, outside the submitted work. All other authors report no conflict of interest.
We thank the Biological Resource Centre for Biobanking (CHU Nantes, Nantes Université, Centre de ressources biologiques [BB-0033_00040], Nantes, France).
We thank clinical and research teams as well as the pharmacists and virologists at all the participating clinical centers who contributed to the management of the study patients for their commitment to providing optimal patient care.
Members of the POLYCOR Study Group are as follows. Members of the trial development team are Laetitia Berly, Sophie Brouard, Odile Duvaux, Laurent Flet, Benjamin Gaborit, Régis Josien, Alexandra Jobert, Aurélie Le Thuaut, Anne Omnès, François Raffi, Emilie Rebouilleau, Laurent Vacher, Bernard Vanhove, and Marie-Anne Vibet. Members of the trial management team are Anne Chiffoleau, Laetitia Berly, Benjamin Gaborit, Marion Gautier, François Raffi, Joseph Herault, Alexandra Jobert, Aurélie Le Thuaut, Anne Omnès, Ludivine Perrier, Emily Rebouilleau, Sandrine Renaud, and Marie-Anne Vibet. Members of the trial steering committee are Florence Ader, Odile Duvaux, Virginie Ferre, Benjamin Gaborit, Karine Lacombe, Anne Omnès, François Raffi, Bernard Vanhove, and Eric Vicaut. Members of the trial independent data-monitoring committee are Bruno Hoen, Laura Richert, Caroline Solas, and Astrid Vabret. Investigators are as follows: at Angers, Marc-Antoine Custaud, Valérie Daniel, Vincent Dubee, and Rafaël Mahieu; at Nantes, Cécile Braudeau, Marie Chauveau, Eric Dailly, Colin Deschanvres, Laurent Flet, Benjamin Gaborit, Matthieu Gregoire, Anne-sophie Lecomte, Maëva Lefebvre, Pascale Morineau Le Houssine, François Raffi, and Martine Tching-Sin; at Lyon, Florence Ader, Agathe Becker, Pierre Chauvelot, Anne Conrad, Tristan Ferry, Julianne Oddone, Thomas Perpoint, Cécile Pouderoux, Sandrine Roux, Claire Triffault-Filit, and Florent Valour; and at Paris Saint Antoine, Diane Bollens, Thibault Chiarabini, Anne Daguenel-Nguyen, Emmanuelle Gras, Patrick Ingiliz, Karine Lacombe, Bénédicte Lefebvre, Laura Levi, Zineb Ouazene, Jérôme Pacanowski, Laure Surgers, and Nadia Valin.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John Hopkins Coronavirus Resource Center. 2021. COVID-19 map. Johns Hopkins Coronavirus Resource Center, Baltimore, MD. [Google Scholar]
- 3.Wu Z, McGoogan JM. 2020. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323:1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.INSPIRATION Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, Sezavar SH, Payandemehr P, Dabbagh A, Moghadam KG, Jamalkhani S, Khalili H, Yadollahzadeh M, Riahi T, Rezaeifar P, Tahamtan O, Matin S, Abedini A, Lookzadeh S, Rahmani H, Zoghi E, Mohammadi K, Sadeghipour P, Abri H, Tabrizi S, Mousavian SM, Shahmirzaei S, Bakhshandeh H, Amin A, Rafiee F, Baghizadeh E, Mohebbi B, Parhizgar SE, Aliannejad R, Eslami V, Kashefizadeh A, Kakavand H, Hosseini SH, Shafaghi S, Ghazi SF, Najafi A, Jimenez D, Gupta A, Madhavan MV, Sethi SS, Parikh SA, Monreal M, Hadavand N, Hajighasemi A, Maleki M, Sadeghian S, Piazza G, Kirtane AJ, et al. 2021. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 325:1620–1630. 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-D, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members. 2020. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 383:1813–1826. 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 8.Gaborit B, Bergmann J, Mussini C, Arribas J, Behrens G, Walmsley S, Pozniak A, Raffi F. 2020. Plea for multitargeted interventions for severe COVID-19. Lancet Infect Dis 20:1122–1123. 10.1016/S1473-3099(20)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanhove B, Duvaux O, Rousse J, Royer P-J, Evanno G, Ciron C, Lheriteau E, Vacher L, Gervois N, Oger R, Jacques Y, Conchon S, Salama A, Duchi R, Lagutina I, Perota A, Delahaut P, Ledure M, Paulus M, So RT, Mok CK-P, Bruzzone R, Bouillet M, Brouard S, Cozzi E, Galli C, Blanchard D, Bach J-M, Soulillou J-P. 2021. High neutralizing potency of swine glyco-humanized polyclonal antibodies against SARS-CoV-2. Eur J Immunol 51:1412–1422. 10.1002/eji.202049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaborit B, Vanhove B, Vibet M-A, Le Thuaut A, Lacombe K, Dubee V, Ader F, Ferre V, Vicaut E, Orain J, Le Bras M, Omnes A, Berly L, Jobert A, Morineau-Le Houssine P, Botturi K, Josien R, Flet L, Degauque N, Brouard S, Duvaux O, Poinas A, Raffi F, POLYCOR Study Group. 2021. Evaluation of the safety and efficacy of XAV-19 in patients with COVID-19-induced moderate pneumonia: study protocol for a randomized, double-blinded, placebo-controlled phase 2 (2a and 2b) trial. Trials 22:199. 10.1186/s13063-021-05132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhove B, Marot SS, Gaborit B, Evanno G, Malet I, Ciron C, Royer P-J, Lheriteau E, Denie S, Raffi F, Duvaux O, Marcelin A-G, Calvez V. 2021. XAV-19, a novel swine glyco-humanized polyclonal antibody against SARS-CoV-2 spike, efficiently neutralizes B.1.1.7 British and B.1.351 South-African variants. bioRxiv 10.1101/2021.04.02.437747. [DOI]
- 12.Gutiérrez-Gutiérrez B, Del Toro MD, Borobia AM, Carcas A, Jarrín I, Yllescas M, Ryan P, Pachón J, Carratalà J, Berenguer J, Arribas JR, Rodríguez-Baño J, REIPI-SEIMC COVID-19 Group, COVID@HULP Groups. 2021. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: a multicentre cohort study. Lancet Infect Dis 21:783–792. 10.1016/S1473-3099(21)00019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo PA, Dogra P, Gray JI, Wells SB, Connors TJ, Weisberg SP, Krupska I, Matsumoto R, Poon MML, Idzikowski E, Morris SE, Pasin C, Yates AJ, Ku A, Chait M, Davis-Porada J, Guo XV, Zhou J, Steinle M, Mackay S, Saqi A, Baldwin MR, Sims PA, Farber DL. 2021. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 54:797–814.e6. 10.1016/j.immuni.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combes AJ, Courau T, Kuhn NF, Hu KH, Ray A, Chen WS, Chew NW, Cleary SJ, Kushnoor D, Reeder GC, Shen A, Tsui J, Hiam-Galvez KJ, Muñoz-Sandoval P, Zhu WS, Lee DS, Sun Y, You R, Magnen M, Rodriguez L, Im KW, Serwas NK, Leligdowicz A, Zamecnik CR, Loudermilk RP, Wilson MR, Ye CJ, Fragiadakis GK, Looney MR, Chan V, Ward A, Carrillo S, UCSF COMET Consortium, Matthay M, Erle DJ, Woodruff PG, Langelier C, Kangelaris K, Hendrickson CM, Calfee C, Rao AA, Krummel MF. 2021. Global absence and targeting of protective immune states in severe COVID-19. Nature 591:124–130. 10.1038/s41586-021-03234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD, Trial Investigators. 2021. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 384:238–251. 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM, BLAZE-1 Investigators. 2021. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 384:229–237. 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. 2021. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 325:632–644. 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan Q, Yang K, Wang W, Jiang L, Song J. 2020. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46:846–848. 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette J-L, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. 2020. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25:2000045. 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet J-C, Mentre F, Duval X, Descamps D, Malvy D, Timsit J-F, Lina B, van-der-Werf S, Yazdanpanah Y. 2020. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 20:697–706. 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2. Download AAC.01237-21-s0001.pdf, PDF file, 0.2 MB (195.6KB, pdf)
Data Availability Statement
The data analyzed and presented in this study are available from the corresponding author upon reasonable request, provided that the request meets local ethical and research governance criteria after publication. Data collected during the study may be processed electronically, in accordance with the requirements of the CNIL (compliance with reference methodology MR001).