ABSTRACT
Sporotrichosis has become an important zoonosis in Brazil, and Sporothrix brasiliensis is the primary species transmitted by cats. Improvement of animal treatment will help control and limit the spread and geographic expansion of sporotrichosis. Accordingly, buparvaquone, an antiprotozoal hydroxynaphthoquinone agent marketed as Butalex, was evaluated in vitro and in vivo against feline-borne isolates of S. brasiliensis. Buparvaquone inhibited in vitro fungal growth at concentrations 4-fold lower than itraconazole (the first-choice antifungal used for sporotrichosis) and was 408 times more selective for S. brasiliensis than mammalian cells. Yeasts treated with a subinhibitory concentration of buparvaquone exhibited mitochondrial dysfunction, reactive oxygen species and neutral lipid accumulation, and impaired plasma membranes. Scanning electron microscopy images also revealed buparvaquone altered cell wall integrity and induced cell disruption. In vivo experiments in a Galleria mellonella model revealed that buparvaquone (single dose of 5 mg/kg of body weight) is more effective than itraconazole against infections with S. brasiliensis yeasts. Combined, our results indicate that buparvaquone has a great in vitro and in vivo antifungal activity against S. brasiliensis, revealing the potential application of this drug as an alternative treatment for feline sporotrichosis.
KEYWORDS: antifungal, naphthoquinone, Sporothrix spp., zoonosis, cat, sporotrichosis, antifungal agents
INTRODUCTION
Sporothrix brasiliensis is a pathogenic dimorphic fungus and has become the main cause of zoonotic sporotrichosis outbreaks among cats and humans in Brazil, and it is also responsible for the spread and geographic expansion of the disease (1, 2).
Currently available feline treatments are excessively long and restricted to very few options (3). Itraconazole is the first-choice antifungal used to treat both human and feline sporotrichosis (1, 3); however, its oral administration is challenging for feline handling and is also associated with several side effects (3). Feline isolates of S. brasiliensis with low sensitivity to itraconazole have been reported in Brazil (4, 5). Optimization of the feline treatment will help control the spread of zoonotic sporotrichosis until a vaccine is available. An alternative to the development of new antifungal agents is the repurposing of drugs already available (6); therefore, medicines currently approved for other veterinary therapies could be successfully repositioned for the treatment of feline sporotrichosis.
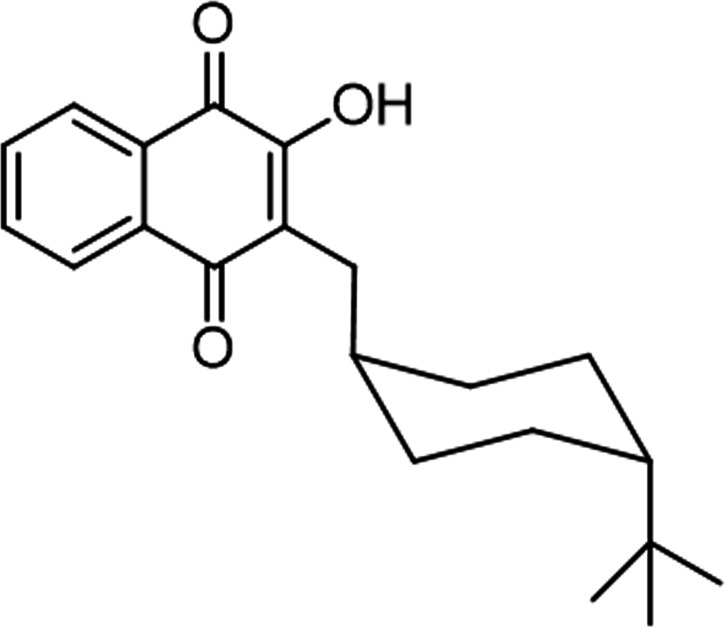
Buparvaquone (BPQ) (Fig. 1) is a safe antiprotozoal drug used to treat theileriosis, a cattle infection caused by Theileria species (7), and is commercially available as an intramuscular injection marketed as Butalex. BQP was first reported in the 1980s and exhibited moderate in vitro activity against Plasmodium spp. and Toxoplasma gondii but good activity against Theileria parva (8). Further studies reported its promising in vitro and in vivo activity as an antileishmanial agent (9–13).
FIG 1.
Molecular structure of buparvaquone (BQP).
Recently, in a screening of 400 molecules from the Pathogenic Box library, we identified that BPQ is able to inhibit S. brasiliensis growth at low concentrations (14). Based on this, we expanded these investigations to evaluate the in vitro activity of BPQ against a collection of feline-borne isolates of S. brasiliensis and confirmed its promising in vivo activity using the Galleria mellonella model (15). Additionally, we explored its possible mechanism of action in Sporothrix yeast cells.
RESULTS
BPQ exhibits high antifungal activity and selectivity against S. brasiliensis.
To determine the antifungal activity of BPQ against S. brasiliensis, MICs were evaluated for 20 isolates (reference and feline-borne strains) (Table 1). BPQ was more active than ITC (P = 0.0034), with MIC median of 0.02 μg/ml for BQP and 0.08 μg/ml for itraconazole (ITC), revealing that BPQ inhibited fungal growth at concentrations 4-fold lower than that for ITC (Table 1). However, BPQ was not able to kill fungal cells at higher tested concentrations, and similar data were obtained for ITC (minimum fungicidal concentration, ≥2.61 μg/ml) (Table 1). The reference Sporothrix schenckii isolate ATCC 16345 was included as a control in the susceptibility tests and exhibited a MIC of 0.16 μg/ml for BPQ and 0.02 μg/ml for ITC. Additionally, in an in vitro cytotoxicity assay, BPQ was 408 times more selective for S. brasiliensis than mammalian cells (Table 1).
TABLE 1.
Antifungal activity and selectivity of BPQ against Sporothrix brasiliensis compared to ITCa
| Compound | MICrange | MICmedian | MFCrange | LLC-MK2 cell CC50 | Selectivity index |
|---|---|---|---|---|---|
| BPQ | 0.005–0.16 | 0.02* | 2.61–>2.61 | 8.16 | 408 |
| ITC | 0.005–0.16 | 0.08 | 2.61–>2.61 | >70.5 | >881.25 |
Results are expressed in microgram per milliliter, n = 20. MIC, MIC that inhibits ≥50% of fungal growth. MFC, minimum fungicidal concentration that kills ≥99.9% of fungal cells. CC50, concentration that elicited 50% cytotoxicity. Selectivity index, ratio between CC50 and MICmedian. *, P = 0.0034 compared with MIC values for ITC (by Wilcoxon test).
BPQ induces pronounced morphophysiological alterations in S. brasiliensis yeasts.
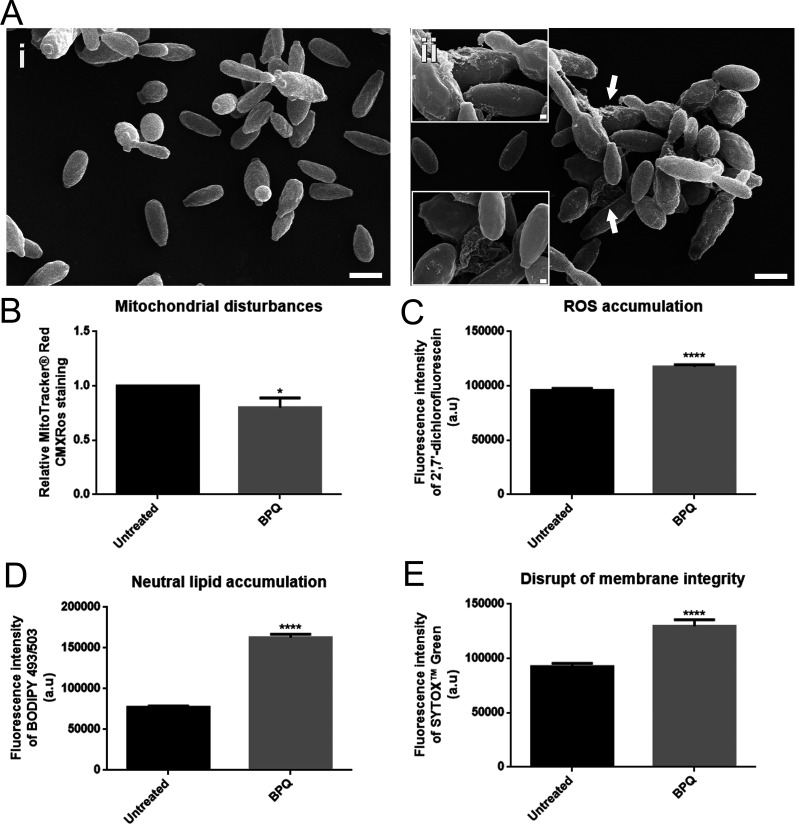
To evaluate how BPQ exerts its inhibitory activity against S. brasiliensis, yeasts of the reference strain CBS 133006 were exposed to 0.08 μg/ml BPQ (MIC) for 48 h and analyzed by scanning electron microscopy (SEM) and flow cytometry. SEM revealed that BPQ treatment induces fungal cell wall injuries and cell ruptures (Fig. 2A). Mitochondrial function in BPQ-treated yeasts was evaluated by flow cytometry using MitoTracker Red CMXRos stain and revealed a small decrease in fungal mitochondrial activity (Fig. 2B). The increased fluorescence intensity observed for 2′,7′-dichlorofluorescein, BODIPY 493/503, and SYTOX green in BPQ-treated yeasts also indicates reactive oxygen species (ROS) and neutral lipid accumulation and damaged plasma membranes, respectively (Fig. 2C to E).
FIG 2.
Effect of buparvaquone (BPQ) exposure on Sporothrix brasiliensis CBS 133006 strain. (A) Scanning electron microscopy images of yeasts treated with 0.08 μg/ml BPQ showed alterations in cell wall integrity and cell disruption (arrows and insets in image ii). Yeasts analyzed by flow cytometry exhibited a significant decrease in mitochondrial activity (B), increased cytoplasmic ROS (C), an increase in neutral lipid (D), and a disrupted plasma membrane (E). Scale bars, 2 μm and 200 nm (insets). *, P < 0.05; ****, P < 0.0001 by Student's t test. Data represent means ± standard errors of the means. a.u, arbitrary units.
BPQ is effective in vivo for the treatment of experimental S. brasiliensis infections.
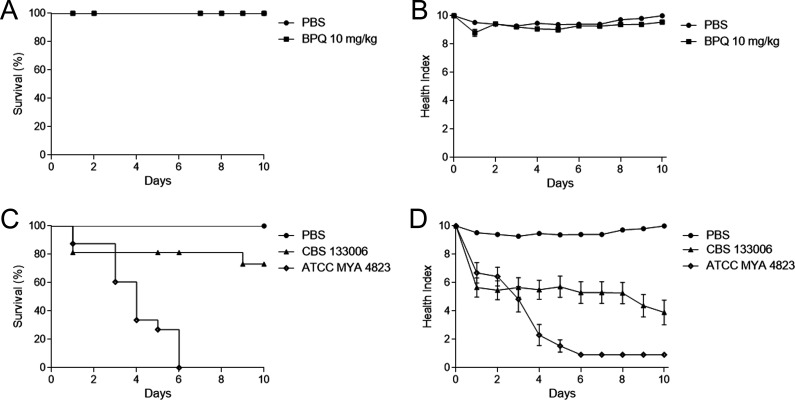
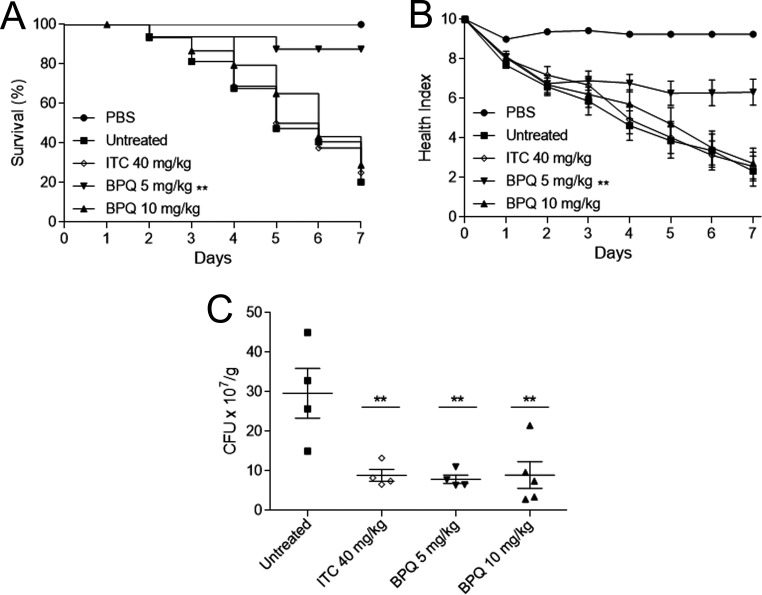
To estimate the potential application of BPQ for sporotrichosis treatment, we used an experimental model of S. brasiliensis infection in the Galleria mellonella model. First, we evaluated the toxicity of BPQ in G. mellonella larvae and observed no mortality or health index alterations after exposure to a single dose of 10 mg/kg of body weight (Fig. 3A and B). We also evaluated the virulence of the reference strain CBS 133006 and compared it with the ATCC MYA 4823 strain, previously reported as highly virulent in vivo (16). The ATCC MYA 4823 strain showed a high mortality rate in G. mellonella, while only 27% mortally was observed for larvae infected with the CBS 133006 strain (Fig. 3C and D). Therefore, we selected the ATCC MYA 4823 strain for the drug efficacy experiments, where BPQ activity was compared to the reference drug ITC. The larval survival profile for the group treated with 40 mg/kg ITC was similar to that of the infected-untreated group, with 25% of survival at the end of the experiment. However, ITC significantly reduced the fungal burden (P < 0.01). BPQ treatment at a lower dose (5 mg/kg) was able to contain S. brasiliensis infection, maintaining 87.5% of survival for 7 days postinfection, and also significantly improved the health index compared to that of the untreated group (P < 0.01). The higher dose of BPQ (10 mg/kg) did not enhance the survival and morbidity rates. Nevertheless, single-dose treatments with either 5 mg/kg or 10 mg/kg BPQ presented similar results and reduced the fungal burden three times in the larval tissue (P < 0.01), similar to the ITC-treated group (Fig. 4).
FIG 3.
Toxicity of buparvaquone (BPQ) and Sporothrix brasiliensis virulence in Galleria mellonella larval model. Survival (A) and morbidity (B) curves of Galleria mellonella larvae inoculated with 10 mg/kg BPQ. Survival (C) and morbidity (D) curves of larvae infected with yeasts from Sporothrix brasiliensis CBS 133006 or ATCC MYA 4823 strains. Control larvae were injected with PBS in both assays.
FIG 4.
Efficacy of buparvaquone (BPQ) treatment in Galleria mellonella after infection with the S. brasiliensis ATCC MYA 4823 strain. Survival (A) and morbidity (B) curves indicated that 5 mg/kg BPQ improves the survival of Galleria mellonella larvae. (C) BPQ reduced the fungal burden of larval tissue, similar to itraconazole (ITC). Data are represented by means ± standard errors of the means. **, P < 0.01 compared with the untreated group.
DISCUSSION
Sporotrichosis is currently considered an infection endemic to Brazil, with zoonotic transmission playing an important role in the spread and pathogenesis of this disease (1). In recent years, the reports that describe feline isolates having low sensitivity to itraconazole (ITC) increased (5), mainly in the south region of Brazil (4). Cats have a central role in the transmission and spread of sporotrichosis; therefore, improving feline treatment will positively impact the control of the disease and contribute to reducing its incidence. Here, we show that buparvaquone (BPQ), an antiparasitic drug, is more effective than ITC against S. brasiliensis, the main etiological agent of sporotrichosis in Brazil, both in vitro and in vivo.
The antifungal activity of BPQ was first reported when we identified that BPQ inhibited S. brasiliensis growth at 1 μM (14). Based on this, we increased the concentration to 8 μM (2.61 μg/ml) and expanded our investigations, testing concentrations ranging from 0.015 to 8 μM BPQ (equivalent to 0.005 to 2.61 μg/ml) to determine concentrations able to inhibit and kill 20 feline-borne isolates. The same concentrations, in micrograms per milliliter, were evaluated for ITC.
BPQ was highly active against feline-borne strains of S. brasiliensis and also inhibited S. schenckii (ATCC 16345). Most feline sporotrichosis cases in Brazil are due to infection by S. brasiliensis, but S. schenckii can also cause the disease in cats (17). Therefore, BPQ could be used as an alternative treatment for infections caused by the two most virulent species. Despite its great inhibitory activity on fungal growth, BPQ showed a fungistatic profile against S. brasiliensis, similar to what is observed for ITC.
BPQ is the main treatment against theileriosis, a tick-borne disease that occurs in parts of Europe, Asia, and Africa and affects livestock (7). This naphthoquinone also exhibits antiprotozoal activity against Leishmania spp. and Neospora caninum (9, 18). It is noteworthy that BPQ concentrations that inhibit T. parva (50% inhibitory concentration [IC50] = 0.0042 μM), N. caninum (IC50 = 0.0049 μM), and Leishmania donovani (IC50 = 0.05 μM) growth are lower than concentrations that demonstrated antifungal activity in this study (MIC median, 0.02 μg/ml [0.06 μM]) (9, 18, 19). These differences could be related in part to the presence of the cell wall in the fungus that decreases its permeability.
BPQ is a mitochondrial inhibitor, and, in protozoa, the disruption of mitochondrial activity results from an impaired respiratory chain due to the drug interaction with cytochrome b (20). Our data indicate that BPQ also disrupts mitochondrial activity in fungal cells (Fig. 2B). We also observed ROS and neutral lipid accumulation and cell damage as a result of BPQ treatment (Fig. 2C to E). Previous studies have associated mitochondrial metabolism and changes in ROS levels, and mitochondrial dysfunctions could impact the intracellular redox environment, increasing cytosolic ROS (21). Furthermore, BPQ is a naphthoquinone, a class of molecules able to interfere with cellular redox cycles and induce free radical production (22). Based on the data obtained in this study, we speculate that the increase in ROS levels in the cytoplasm of BPQ-treated yeasts is related to damage in the plasma membrane (Fig. 2A). Cellular responses to stress are intimately connected to cell metabolism. In eukaryotic cells, cellular stress can induce an increase of neutral lipid storage in the lipid droplets in the cytoplasm (23). Thus, the neutral lipid accumulation observed in treated yeasts could represent a response to the cellular stress induced by BPQ. Importantly, our data indicate that BPQ exerts antifungal activity through a different mechanism than antifungals used to treat systemic fungal infections (24), showing a distinct cellular target that could be useful to treat infections by species resistant to the currently available antifungals.
The fact that BPQ is more selective toward S. brasiliensis than mammalian cells (Table 1) stimulated our subsequent experiments using the G. mellonella model to investigate the in vivo applicability of BPQ as a treatment for sporotrichosis. BPQ is used for theileriosis treatment in a single or double injection of 2.5 mg/kg (Butalex leaflet). Since the in vitro MIC against S. brasiliensis was higher than the reported values for protozoa, we increase the BPQ dose to 5 and 10 mg/kg in our tests. We showed that a single dose of 5 mg/kg BPQ increased larva survival to better than 40 mg/kg ITC (Fig. 4). Even though 10 mg/kg BPQ did not show any toxic effect in the larvae (Fig. 3A and B), this dose was not better than 5 mg/kg (Fig. 4). BPQ has limited solubility in water that compromises the bioavailability and its penetration in the larva tissue, limiting the dose-response effects. This could be addressed by different BPQ formulations to increase drug bioavailability and tissue distribution. Further in vivo studies with vertebrate models also are required to confirm the efficacy of BPQ to treat sporotrichosis before its clinical use in cats, considering that no reports about its use for treating cats (dosage, effectiveness, and adverse effects) are available.
In conclusion, BPQ demonstrated great in vitro and in vivo antifungal activity against S. brasiliensis, and the data presented here highlight a potential application of BPQ as an alternative drug to treat feline sporotrichosis.
MATERIALS AND METHODS
Microorganisms.
This study used three reference isolates of S. brasiliensis (CBS 133006, ATCC MYA 4823, and ATCC MYA 4824) and 17 clinical isolates from feline sporotrichosis. The feline-borne isolates were obtained by the veterinarian group from the Veterinary School Clinic at Pontifícia Universidade Católica do Paraná (PUCPR) by following an approved protocol from the Animal Research Ethics Committee at PUCPR (CEUA/PUCPR protocol number 01197). Molecular identification based on the calmodulin gene was performed to confirm S. brasiliensis species for all feline-bone isolates.
Culture conditions.
Yeast cell morphology was used in all experiments and was obtained from the filamentous form. The filamentous form was cultivated in Sabouraud dextrose broth (Difco, United States), and conidia (105 CFU/ml) were inoculated into brain heart infusion broth (Difco, USA) supplemented with 2% glucose (pH 7.8). Both incubations were performed at 36°C with orbital shaking for 7 days.
Compounds.
Buparvaquone (BPQ) dissolved in dimethyl sulfoxide (DMSO) at 10 mM was kindly provided by Medicines for Malaria Venture (MMV; Switzerland) as a component of the Pathogen Box library (https://www.mmv.org/mmv-open/pathogen-box). Additional experiments were conducted using BPQ powder (Sigma-Aldrich Co., USA). Stock solutions prepared in DMSO at 10 and 1 mM were stored at −20°C and used in experiments. Itraconazole (ITC) (Sigma-Aldrich Co., USA) was used as a reference antifungal, and stock solutions (10 and 1 mM in DMSO) were kept at −20°C.
Susceptibility tests.
The in vitro broth microdilution technique was performed to determine MIC values (14, 25). Yeasts were added into flat-bottom 96-well microplates containing BPQ or ITC diluted in RPMI 1640 medium (Sigma-Aldrich Co., USA) (supplemented with 2% glucose and buffered to pH 7.2, with 0.165 M morpholinepropanesulfonic acid [MOPS]). The final concentration of drugs was 0.005 to 2.61 μg/ml (equivalent to 0.015 to 8 μM BPQ), and the final yeast concentration tested was 105 CFU/ml. Microplates were incubated at 35°C for 48 h in a 5% CO2 chamber. Sporothrix growth was analyzed by visual inspection in an inverted light microscope (Axiovert 100; Zeiss Company, Germany) and quantified by spectrophotometric readings at 492 nm (Emax Plus plate reader; Molecular Devices, USA). The MIC was the lowest concentration able to inhibit ≥50% of fungal growth and was calculated using the equation IC = 100 − (A × 100/C), where A was the absorbance of treated wells and C was the absorbance of untreated wells. After visual readings, 50-μl aliquots of fungal samples were plated into drug-free Sabouraud plates and incubated at 35°C for 5 days in a 5% CO2 chamber. The minimum fungicidal concentration (MFC) was the lowest drug concentration that killed >99.9% of fungal cells and indicates a fungicidal effect when MFC ≤ 4×MIC or fungistatic effect if MFC > 4×MIC (26). Experiments were performed in duplicate, and the quality control isolate Candida parapsilosis ATCC 22019 was included as a control (25). The Wilcoxon test was used to compare BPQ and ITC activities, and statistical significance was accepted at a P value of <0.05 (GraphPad Software, Inc., USA).
Cytotoxicity assay.
To determine the selectivity of BPQ toward S. brasiliensis, confluent monolayers of LLC-MK2 cells (monkey cell line ATCC CCL-7) were treated with several concentrations of BPQ or ITC for 48 h at 37°C and 5% CO2 (14). The final concentration of drugs ranged from 0.003 to 32.64 μg/ml for BPQ or 0.007 to 70.5 μg/ml for ITC (these concentrations are equivalent to 0.01 to 100 μM). Concentrations that elicited 50% cytotoxicity (CC50) were estimated by 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt reduction assay. The selectivity index was calculated using the following equation: CC50/MICmedian. Results are representative of three independent experiments, performed with triplicates.
Flow cytometry.
Yeasts (105 CFU/ml) of the reference isolate S. brasiliensis CBS133006 were exposed to the MIC (0.08 μg/ml) of BPQ for 48 h at 36°C, with orbital agitation, in RPMI 1640 medium (supplemented with 2% glucose and buffered to pH 7.2 using 0.165 M MOPS). Untreated and treated cells (107 cells/ml) were incubated for 30 min at room temperature in the dark with the following fluorescent marker: 20 μM MitoTracker red CMXRos (Thermo Fisher Scientific, USA), 50 μM 2′,7′-dichlorofluorescein diacetate (Sigma Chemical CO., USA), 20 μM BOPIDY 493/503 (Thermo Fisher Scientific, USA), or 20 μM SYTOX green (Thermo Fisher Scientific, USA). MitoTracker Red CMXRos stains mitochondria in live cells with accumulation dependent on membrane potential, while 2′,7′-dichlorofluorescein diacetate is a nonfluorescent probe that generates the fluorescent 2′,7′-dichlorofluorescein upon oxidation by ROS. BODIPY 493/503 stains neutral lipids, and SYTOX green does not cross intact plasmatic membranes but penetrates compromised membranes and stains cellular nucleic acids. Samples were analyzed in a BD Accuri C6 flow cytometer (BD Biosciences, USA) that counted 5,000 events per sample, and data were analyzed using BD Accuri C6 software. Experiments were repeated on three separate occasions. Fluorescence intensity data depicted in Results show the means and standard errors of the means from one representative experiment. Statistical analysis was performed using Student's t test, with a P value of <0.05 considered statistically significant (Graph Pad Software Inc., USA).
SEM.
S. brasiliensis CBS133006 yeast cells treated as described above were also analyzed by scanning electron microscopy. Untreated and treated cells were washed in phosphate-buffered saline (PBS) and fixed in 2.5% glutaraldehyde and 4% formaldehyde in 0.1 M cacodylate buffer for 1 h. Samples were washed in cacodylate buffer, adhered to poly-l-lysine-coated glass coverslips, dehydrated in a graded ethanol series, critical point dried in CO2, and coated with gold. Images were obtained in a Zeiss EVO 10 scanning electron microscope (Zeiss Company, Germany) and processed using Photoshop software (Adobe, USA).
BPQ toxicity in Galleria mellonella.
An aliquot of 10 μl of BPQ in a single dose of 10 mg/kg was injected into the last left proleg of the larvae (length from 2 to 2.5 cm and weight from 100 to 150 mg). BPQ-treated larvae were compared with a larval group that received only PBS (pH 7.4). Treated and untreated larvae (n = 16 larvae in each group) were incubated at 35°C and observed daily for 10 days after treatment to determine survival profile and health index (27).
Virulence of S. brasiliensis strains in Galleria mellonella.
The virulence assay of the S. brasiliensis strains ATCC MYA 4823 and CBS 133006 was assessed in larvae (length from 1 to 1.5 cm) infected with 10 μl of a standardized suspension of yeasts at 109 CFU/ml. The larval group that received only PBS was defined as the uninfected group. Infected and uninfected larvae (n = 16 larvae in each group) were incubated at 35°C and observed daily for 10 days after infection. The survival profile and the health index were determined (27).
Antifungal activity of BPQ in a Galleria mellonella model.
G. mellonella larvae (length, 2 to 2.5 cm) were infected with 10 μl of yeast suspension (109 CFU/ml) of S. brasiliensis ATCC MYA 4823. After 1 h, the larvae were treated with ITC (40 mg/kg) or BPQ (5 mg/kg or 10 mg/kg). The infected-untreated or uninfected groups (PBS group) were added as control groups. All groups (n = 16 larvae/group) were incubated at 35°C and observed daily for 7 days. The survival profile and the health index were determined (27). Additionally, the fungal burden in the larval tissue 48 h postinfection (4 larvae/group) was evaluated. Each larva was weighed and macerated. The homogenate was plated on Sabouraud dextrose agar containing 50 μg/ml chloramphenicol and incubated at 35°C, and the colonies were counted after 72 h for determination of the number of CFU by gram of larval tissue (CFU/g). Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, Inc., USA). Survival curves and fungal burden data were analyzed by unidirectional analysis of variance (one-way ANOVA), followed by Dunnett’s test. The morbidity curve was analyzed by bidirectional analysis of variance (two-way ANOVA). P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We are grateful to Medicine for Malaria Venture (MMV; Switzerland) for kindly providing the Pathogen Box library containing BPQ. We thank Centro Nacional de Biologia Estrutural e Bioimagem (CENABIO, UFRJ, Rio de Janeiro, Brazil) for facilities and support in the use of SEM equipment.
This study was supported by the Brazilian funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2017/19374-9).
We declare that there are no conflicts of interest.
REFERENCES
- 1.Rossow JA, Queiroz-Telles F, Caceres DH, Beer KD, Jackson BR, Pereira JG, Ferreira Gremião ID, Pereira SA. 2020. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi 6:247. doi: 10.3390/jof6040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gremião IDF, Oliveira MME, Miranda LHM, Freitas DFS, Pereira SA. 2020. Geographic expansion of sporotrichosis, Brazil. Emerg Infect Dis 26:621–624. doi: 10.3201/eid2603.190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gremião IDF, Martins da Silva da Rocha E, Montenegro H, Carneiro AJB, Xavier MO, de Farias MR, Monti F, Mansho W, de Macedo Assunção Pereira RH, Pereira SA, Lopes-Bezerra LM. 2021. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz J Microbiol 52:107–124. doi: 10.1007/s42770-020-00365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakasu CCT, Waller SB, Ripoll MK, Ferreira MRA, Conceição FR, Gomes AR, Osório LG, Faria RO, Cleff MB. 2021. Feline sporotrichosis: a case series of itraconazole-resistant Sporothrix brasiliensis infection. Braz J Microbiol 52:163–171. doi: 10.1007/s42770-020-00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waller SB, Dalla Lana DF, Quatrin PM, Ferreira MRA, Fuentefria AM, Mezzari A. 2021. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol 52:73–80. doi: 10.1007/s42770-020-00307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katragkou A, Roilides E, Walsh TJ. 2016. Can repurposing of existing drugs provide more effective therapies for invasive fungal infections? Expert Opin Pharmacother 17:1179–1182. doi: 10.1080/14656566.2016.1186647. [DOI] [PubMed] [Google Scholar]
- 7.Gharbi M, Darghouth MA, Elati K, Al-Hosary AAT, Ayadi O, Salih DA, El Hussein AM, Mhadhbi M, Khamassi Khbou M, Hassan SM, Obara I, Ahmed LS, Ahmed J. 2020. Current status of tropical theileriosis in Northern Africa: a review of recent epidemiological investigations and implications for control. Transbound Emerg Dis 67:8–25. doi: 10.1111/tbed.13312. [DOI] [PubMed] [Google Scholar]
- 8.Hudson AT, Randall AW, Fry M, Ginger CD, Hill B, Latter VS, McHardy N, Williams RB. 1985. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology 90:45–55. doi: 10.1017/S0031182000049003. [DOI] [PubMed] [Google Scholar]
- 9.Croft SL, Hogg J, Gutteridge WE, Hudson AT, Randall AW. 1992. The activity of hydroxynaphthoquinones against Leishmania donovani. J Antimicrob Chemother 30:827–832. doi: 10.1093/jac/30.6.827. [DOI] [PubMed] [Google Scholar]
- 10.Mäntylä A, Rautio J, Nevalainen T, Vepsälainen J, Juvonen R, Kendrick H, Garnier T, Croft SL, Järvinen T. 2004. Synthesis and antileishmanial activity of novel buparvaquone oxime derivatives. Bioorg Med Chem 12:3497–3502. doi: 10.1016/j.bmc.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Garnier T, Mantyla A, Jarvinen T, Lawrence J, Brown M, Croft S. 2007. In vivo studies on the antileishmanial activity of buparvaquone and its prodrugs. J Antimicrob Chemother 60:802–810. doi: 10.1093/jac/dkm303. [DOI] [PubMed] [Google Scholar]
- 12.da Costa-Silva TA, GalisteoAJ, Jr, Lindoso JA, Barbosa LR, Tempone AG. 2017. Nanoliposomal buparvaquone immunomodulates Leishmania infantum-infected macrophages and is highly effective in a murine model. Antimicrob Agents Chemother 61:e02297-16. doi: 10.1128/AAC.02297-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimão JQ, Colombo FA, Pereira-Chioccola VL, Tempone AG. 2012. Effectiveness of liposomal buparvaquone in an experimental hamster model of Leishmania (L.) infantum chagasi. Exp Parasitol 130:195–199. doi: 10.1016/j.exppara.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Borba-Santos LP, Vila T, Rozental S. 2020. Identification of two potential inhibitors of Sporothrix brasiliensis and Sporothrix schenckii in the Pathogen Box collection. PLoS One 15:e0240658. doi: 10.1371/journal.pone.0240658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemel S, Guillot J, Kallel K, Botterel F, Dannaoui E. 2020. Galleria mellonella for the evaluation of antifungal efficacy against medically important fungi, a narrative review. Microorganisms 8:390. doi: 10.3390/microorganisms8030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clavijo-Giraldo DM, Matínez-Alvarez JA, Lopes-Bezerra LM, Ponce-Noyola P, Franco B, Almeida RS, Mora-Montes HM. 2016. Analysis of Sporothrix schenckii sensu stricto and Sporothrix brasiliensis virulence in Galleria mellonella. J Microbiol Methods 122:73–77. doi: 10.1016/j.mimet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Kamal Azam NK, Selvarajah GT, Santhanam J, Abdul Razak MF, Ginsapu SJ, James JE, Suetrong S. 2020. Molecular epidemiology of Sporothrix schenkii isolates in Malaysia. Med Mycol 58:617–625. doi: 10.1093/mmy/myz106. [DOI] [PubMed] [Google Scholar]
- 18.Müller J, Aguado-Martínez A, Manser V, Wong HN, Haynes RK, Hemphill A. 2016. Repurposing of antiparasitic drugs: the hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Vet Res 47:32. doi: 10.1186/s13567-016-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyagwange J, Awino E, Tijhaar E, Svitek N, Pelle R, Nene V. 2019. Leveraging the Medicines for Malaria Venture malaria and pathogen boxes to discover chemical inhibitors of East Coast fever. Int J Parasitol Drugs Drug Resist 9:80–86. doi: 10.1016/j.ijpddr.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharifiyazdi H, Namazi F, Oryan A, Shahriari R, Razavi M. 2012. Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet Parasitol 187:431–435. doi: 10.1016/j.vetpar.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Murphy MP. 2013. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab 18:145–146. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Futuro DO, Ferreira PG, Nicoletti CD, Borba-Santos LP, Silva FCD, Rozental S, Ferreira VF. 2018. The antifungal activity of naphthoquinones: an integrative review. An Acad Bras Cienc 90:1187–1214. doi: 10.1590/0001-3765201820170815. [DOI] [PubMed] [Google Scholar]
- 23.Geltinger F, Schartel L, Wiederstein M, Tevini J, Aigner E, Felder TK, Rinnerthaler M. 2020. Friend or foe: lipid droplets as organelles for protein and lipid storage in cellular stress response. Aging and Dis Molecules 25:5053. doi: 10.3390/molecules25215053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins N, Wright GD, Cowen LE. 2016. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr 4:1–20. doi: 10.1128/microbiolspec.FUNK-0002-2016. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed. CLSI standard M27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 26.Pfaller MA, Sheehan DJ, Rex JH. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin Microbiol Rev 17:268–280. doi: 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loh JM, Adenwalla N, Wiles S, Proft T. 2013. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 4:419–428. doi: 10.4161/viru.24930. [DOI] [PMC free article] [PubMed] [Google Scholar]