ABSTRACT
Rifamycins are widely used for treating mycobacterial and staphylococcal infections. Drug-drug interactions (DDI) caused by rifampicin (RIF) are a major issue. We used a model-based approach to predict the magnitude of DDI with RIF and rifabutin (RBT) for 217 cytochrome P450 (CYP) substrates. On average, DDI caused by low-dose RIF were twice as potent as those caused by RBT. Contrary to RIF, RBT appears unlikely to cause severe DDI, even with sensitive CYP substrates.
KEYWORDS: rifampicin, rifabutin, drug-drug interaction, pharmacokinetics
TEXT
Rifampicin (RIF [also known as rifampin]) is a first-line antimicrobial agent for various infectious diseases, such as tuberculosis (TB), brucellosis, and some staphylococcal infections, including infectious endocarditis and bone and joint infections. A major issue associated with rifampicin use is drug-drug interactions (DDI). Rifampicin is a potent inducer of several cytochrome P450 (CYP) and drug transporters, including P-glycoprotein (P-gP). Rifampicin may be responsible for strong DDI when coadministered with sensitive CYP substrate drugs (1). Rifabutin (RBT) is another rifamycin agent that shows a similar antimicrobial activity (2). It is considered an alternative to rifampicin for TB therapy (3, 4). In addition, increasing data suggest its potential for staphylococcal infections (2, 5–8). The induction potency of rifabutin is significant in vitro (9). However, rifabutin is considered a less potent drug inducer in vivo and should cause fewer strong DDI than rifampicin (10, 11), but comparative data are limited (12, 13). The aim of this study was to compare the magnitudes of DDI caused by rifampicin and rifabutin by using a modeling approach.
We used the In vivo Mechanistic Static Model (IMSM) implemented in the DDI-Predictor website (14–16) to calculate and compare the magnitudes of DDI caused by rifampicin (450 to 600 mg per day [RIF600]) and rifabutin (300 mg/day [RBT300]) for substrates of CYP3A4, CYP2C9, CYP2C19, and CYP1A2. The model implemented in the DDI-Predictor website has been previously validated for a large number of CYP substrates and interactors (17–20).
The metric used to quantify DDI magnitude was RAUC, defined as the ratio of area under the concentration-time curve of the substrate drug coadministered with the inducer (AUC*) over that of the substrate drug alone (AUC). The IMSM model for CYP induction can be summarized as
| (1) |
where CR is the contribution ratio of each CYP in the drug oral clearance, ranging from 0 to 1, and IC is the potency of induction, ranging from 0 to +∞ theoretically for each CYP involved. IC values estimated in a previous study (19) for rifabutin at 300 mg/day (RBT300) and rifampicin at 600 mg/day (RIF600) were 2.15 and 7.7, 0.67 and 1.22, 4.2 and 4.2, and 0.03 and 1.44 for CYP3A4, -2C9, -2C19, and -1A2, respectively. External validation of the model was performed by comparing the predicted RAUC to observed data reported in the literature for DDI caused by the two drugs.
Then, we predicted the RAUC for every drug recorded in the DDI-Predictor database, except those metabolized only by CYP2D6, as the activity of this CYP cannot be induced (17). The interactions were classified as weak (0.5 ≤ RAUC ≤ 1), moderate (0.2 < RAUC < 0.5), and strong (RAUC ≤ 0.2) (21). We compared the magnitudes of drug interactions caused by rifabutin at 300 mg/day (RBT300) and rifampicin at 450 to 600 mg/day (RIF600).
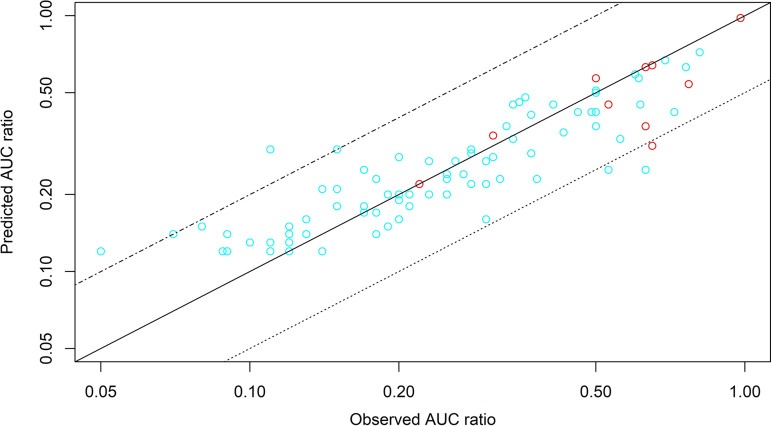
Detailed results of external validation are shown in the supplemental material. The supplemental material also provides data on CYP substrate drugs and other metabolism and transporter pathways (12, 13, 22–106). Figure 1 shows the plot of predicted versus observed AUC ratios. Model-based predictions correlated well with observed AUC ratios for both drugs.
FIG 1.
Predicted versus observed AUC ratio of substrate drugs for DDI caused by rifampicin and rifabutin reported in the literature. The solid line is the line of identity (y = x). The dotted line is y = 0.5x, and the combined dashed and dotted line is y = 2x. Abbreviations: RBT300, rifabutin at 300 mg/day (red circles); RIF600, rifampicin at 600 mg/day (cyan circles).
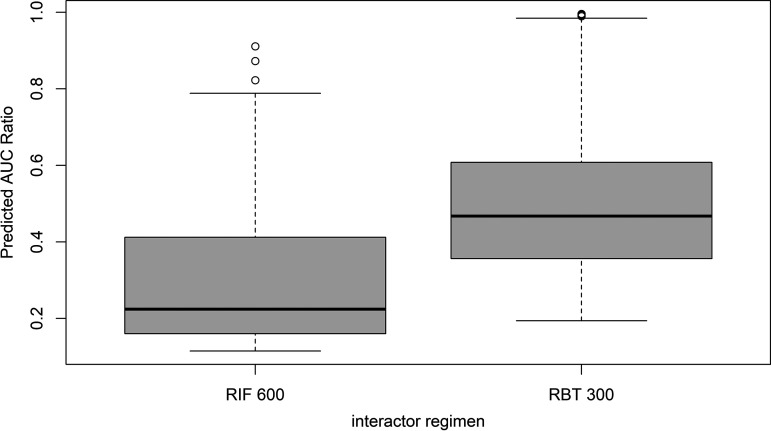
Model predictions for 217 substrates of the DDI-Predictor database are summarized in Fig. 2. For RIF600 and RBT300, the median (with interquartile range in parentheses) RAUC values were 0.22 (0.16 to 0.41) and 0.47 (0.36 to 0.61), respectively. On average, DDI caused by RIF600 were twice as potent than those caused by RBT300. Strong DDI were observed for 44% of substrates when coadministered with RIF600 and for only 1.05% of substrates when coadministered with RBT300. Moderate DDI were observed for 42% and 56% of substrates when coadministered with RIF600 and RBT300, respectively. Weak DDI were observed in 14% and 43% of cases with RIF600 and RBT300, respectively. Table 1 shows the proportion of DDI classified as strong, moderate, and weak when switching from RIF600 to RBT300, those dosages being considered equivalent, at least for TB therapy (107). The use of RBT300 instead of RIF600 would be associated with a lower magnitude of DDI for most CYP substrates.
FIG 2.
Box plot of predicted AUC ratios for 217 drug-drug interactions between CYP substrates and rifamycin agents. Abbreviations: RIF 600; rifampicin at 600 mg/day; RBT 300, rifabutin at 300 mg/day.
TABLE 1.
Compared classification of DDI caused by rifampicin and rifabutina
| DDI with RBT300 | No. (%) of DDI with RIF600 |
|
|---|---|---|
| Moderate (n = 91) | Strong (n = 95) | |
| Strong | 0 (0) | 1 (1) |
| Moderate | 28 (30.8) | 94 (99) |
| Weak | 63 (69.2) | 0 (0) |
RBT300, rifabutin at 300 mg/day; RIF600, rifampicin at 600 mg/day.
As an illustration, Table 2 shows the predicted AUC for a selection of 10 CYP substrate drugs when coadministered with RIF600 and RBT300. We selected some substrates highly selective of a given CYP pathway and others with a multiple-CYP metabolism. Predictions for all 217 substrates are available on the DDI-Predictor website (https://www.ddi-predictor.org/).
TABLE 2.
Predicted AUC ratios of DDI for a selection of 10 CYP substrate drugs when coadministered with RIF600 and RBT300a
| Substrate | CR of CYP to substrate oral clearance |
Mean RAUC (95% CI) withb: |
|||||
|---|---|---|---|---|---|---|---|
| CYP3A4 | CYP2D6 | CYP2C9 | CYP2C19 | CYP1A2 | RBT300 | RIF600 | |
| Acenocoumarol | 0.99 | 0.60 (0.40–0.90) | 0.45 (0.28–0.73) | ||||
| Agomelatine | 0.99 | 0.97 (0.73–1.29) | 0.41 (0.25–068) | ||||
| Gliclazide | 0.24 | 0.76 | 0.23 (0.12–0.43) | 0.22 (0.12–0.42) | |||
| Ibrutinib (fasting) | 0.98 | 0.32 (0.18–0.56) | 0.12 (0.06–0.23) | ||||
| Lansoprazole | 0.27 | 0.73 | 0.22 (0.11–0.40) | 0.16 (0.08–0.32) | |||
| Oxycodone | 0.54 | 0.2 | 0.46 (0.29–0.75) | 0.19 (0.10–0.37) | |||
| Risperidone | 0.25 | 0.75 | 0.65 (0.45–0.95) | 0.34 (0.20–0.59) | |||
| Simvastatin | 0.97 | 0.32 (0.19–0.57) | 0.12 (0.06–0.24) | ||||
| Tacrolimus | 0.91 | 0.34 (0.19–0.59) | 0.12 (0.06–0.25) | ||||
| Vortioxetine | 0.24 | 0.6 | 0.13 | 0.48 (0.30–0.77) | 0.29 (0.17–0.53) | ||
| Warfarin S | 0.99 | 0.60 (0.40–0.90) | 0.45 (0.28–0.73) | ||||
Abbreviations: CR, contribution ratio; CYP, cytochrome P450; RAUC, ratio of AUC* to AUC; RIF600, rifampicin at 600 mg/day; RBT300, rifabutin at 300 mg/day.
RAUC values are given as the mean with 95% confidence interval (CI) in parentheses.
Our model-based analysis confirmed that the magnitudes of DDI caused by rifampicin and rifabutin are quite different. Rifabutin at 300 mg/day has lower induction potency than the equivalent dosage of rifampicin (600 mg/day). Consequently, rifabutin is associated with much lower proportions of severe and moderate DDI. Indeed, rifampicin is the most potent CYP inducer in the DDI-Predictor database, and its induction potency is even greater when used at a higher dose of 1,200 mg/day (13), which is common in the therapy of bone and joint infections. Our results suggest that rifabutin could be more convenient and safer than rifampicin regarding DDI. While the predicted AUC ratio could be used for dosage adjustment of substrate drugs when coadministered with rifamycin agents, strong DDI with a predicted AUC ratio of ≤ 0.2 would require very large dose increases, which raises safety concerns. Such strong DDI are usually considered contraindications. As rifabutin can only cause weak to moderate DDI, rates of drug switch or dose increases of the substrate drug would be lower.
This study has several limitations. Only CYP pathways are formally incorporated in the model (equation 1). This means that the CR parameters and AUC ratios may be less accurate for drugs with non-CYP pathways that are also altered by RIF or RBT. However, because our approach is only based on in vivo data, the influence of drug inducers on other pathways may be indirectly quantified and considered. Indeed, the model prediction correlates well with observations, even for drugs that are known substrates of transporters and enzymes other than CYP, as shown in Table S1 in the supplemental material. It is noteworthy that rifabutin is also a substrate of CYP3A4, unlike rifampicin. Therefore, coadministration with CYP3A4 inducers and inhibitors may alter rifabutin pharmacokinetics and its induction potency. We only considered one dosage of rifabutin in our predictions, because no data were available to derive estimates for other dosages. It is possible that higher dosages of rifabutin could result in a greater magnitude of DDI.
Further clinical evaluation is necessary to assess whether rifabutin can be a safe and effective alternative to rifampicin. However, our model-based analysis confirms that rifabutin has a more favorable DDI profile than rifampicin. Contrary to rifampicin, rifabutin appears unlikely to cause strong DDI (i.e., with an RAUC of <0.2), even with sensitive CYP substrate drugs.
ACKNOWLEDGMENTS
This study was not supported by any private or sponsor funds. This work was performed as part of our routine activities that are supported by Hospices Civils de Lyon and Université Lyon 1.
N.B., L.B., M.T., and S.G. have contributed to the DDI-Predictor website, which is a free Web tool, without any profit for the authors. The authors have no conflicts of interest that are relevant to the content of this study.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivistö KT. 2003. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet 42:819–850. 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 2.Crabol Y, Catherinot E, Veziris N, Jullien V, Lortholary O. 2016. Rifabutin: where do we stand in 2016? J Antimicrob Chemother 71:1759–1771. 10.1093/jac/dkw024. [DOI] [PubMed] [Google Scholar]
- 3.Horne DJ, Spitters C, Narita M. 2011. Experience with rifabutin replacing rifampin in the treatment of tuberculosis. Int J Tuber Lung Dis 15:1485–1490. 10.5588/ijtld.11.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Montaner LJ, Natal S, Yongchaiyud P, Olliaro P, Abbate E, Mosca C, Casado G, Di Lonardo M, Gerhart-Filho G, Betjel I, Ferreira-Lima S, Narinho AP, Lopez R, Carlos-Moreira A, Santana A, Filho AC, Magahaes M, Marinho A, Cavabarra-Cardoso N, Branao-Montero R, Prijanonda B, Nuchprayoon C, Punnotok J, Chakorn T, Carpentieri M, Dolfi L, Maniero A. 1994. Rifabutin for the treatment of newly-diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus rifampicin. Tuber Lung Dis 75:341–347. 10.1016/0962-8479(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 5.Albano M, Karau MJ, Greenwood-Quaintance KE, Osmon DR, Oravec CP, Berry DJ, Abdel MP, Patel R. 2019. In vitro activity of rifampin, rifabutin, rifapentine, and rifaximin against planktonic and biofilm states of staphylococci isolated from periprosthetic joint infection. Antimicrob Agents Chemother 63:e00959-19. 10.1128/AAC.00959-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karau MJ, Schmidt-Malan SM, Albano M, Mandrekar JN, Rivera CG, Osmon DR, Oravec CP, Berry DJ, Abdel MP, Patel R. 2020. Novel use of rifabutin and rifapentine to treat methicillin-resistant Staphylococcus aureus in a rat model of foreign body osteomyelitis. J Infect Dis 222:1498–1504. 10.1093/infdis/jiaa401. [DOI] [PubMed] [Google Scholar]
- 7.Chambers HF. 2020. Rifabutin to the rescue? J Infect Dis 222:1422–1424. 10.1093/infdis/jiaa403. [DOI] [PubMed] [Google Scholar]
- 8.Abad L, Josse J, Tasse J, Lustig S, Ferry T, Diot A, Laurent F, Valour F. 2020. Antibiofilm and intraosteoblastic activities of rifamycins against Staphylococcus aureus: promising in vitro profile of rifabutin. J Antimicrob Chemother 75:1466–1473. 10.1093/jac/dkaa061. [DOI] [PubMed] [Google Scholar]
- 9.Dyavar SR, Mykris TM, Winchester LC, Scarsi KK, Fletcher CV, Podany AT. 2020. Hepatocytic transcriptional signatures predict comparative drug interaction potential of rifamycin antibiotics. Sci Rep 10:12565. 10.1038/s41598-020-69228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baciewicz AM, Chrisman CR, Finch CK, Self TH. 2008. Update on rifampin and rifabutin drug interactions. Am J Med Sci 335:126–136. 10.1097/MAJ.0b013e31814a586a. [DOI] [PubMed] [Google Scholar]
- 11.Baciewicz AM, Chrisman CR, Finch CK, Self TH. 2013. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin 29:1–12. 10.1185/03007995.2012.747952. [DOI] [PubMed] [Google Scholar]
- 12.Perucca E, Grimaldi R, Frigo GM, Sardi A, Mönig H, Ohnhaus EE. 1988. Comparative effects of rifabutin and rifampicin on hepatic microsomal enzyme activity in normal subjects. Eur J Clin Pharmacol 34:595–599. 10.1007/BF00615223. [DOI] [PubMed] [Google Scholar]
- 13.Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B, Worth A, Kearney BP, Mathias A. 2018. Cytochrome P450 3A induction predicts P-glycoprotein induction. Part 1. Establishing induction relationships using ascending dose rifampin. Clin Pharmacol Ther 104:1182–1190. 10.1002/cpt.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DDI-Predictor. 2021. Quantitative prediction of drug drug interactions—DDI-Predictor academic version. https://www.ddi-predictor.org/.
- 15.Goutelle S, Bourguignon L, Bleyzac N, Berry J, Clavel-Grabit F, Tod M, Genophar II Working Group. 2013. In vivo quantitative prediction of the effect of gene polymorphisms and drug interactions on drug exposure for CYP2C19 substrates. AAPS J 15:415–426. 10.1208/s12248-012-9431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellan A-C, Tod M, Gueyffier F, Audars M, Cambriels F, Kassaï B, Nony P, Genophar Working Group. 2013. Quantitative prediction of the impact of drug interactions and genetic polymorphisms on cytochrome P450 2C9 substrate exposure. Clin Pharmacokinet 52:199–209. 10.1007/s40262-013-0031-3. [DOI] [PubMed] [Google Scholar]
- 17.Tod M, Goutelle S, Clavel-Grabit F, Nicolas G, Charpiat B. 2011. Quantitative prediction of cytochrome P450 (CYP) 2D6-mediated drug interactions. Clin Pharmacokinet 50:519–530. 10.2165/11592620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel L, Tod M, Goutelle S. 2016. Quantitative prediction of drug interactions caused by CYP1A2 inhibitors and inducers. Clin Pharmacokinet 55:977–990. 10.1007/s40262-016-0371-x. [DOI] [PubMed] [Google Scholar]
- 19.Tod M, Pierrillas PB, Bourguignon L, Goutelle S. 2016. Comparison of the static in vivo approach to a physiologically based pharmacokinetic approach for metabolic drug-drug interactions prediction. Int J Pharmacokinet 1:25–34. 10.4155/ipk.16.2. [DOI] [Google Scholar]
- 20.Loue C, Tod M. 2014. Reliability and extension of quantitative prediction of CYP3A4-mediated drug interactions based on clinical data. AAPS J 16:1309–1320. 10.1208/s12248-014-9663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stout SM, Nemerovski CW, Streetman DS, Berg M, Hoffman J, Burke K, Bemben NM, Sklar SJ. 2021. Interpretation of cytochrome P-450 inhibition and induction effects from clinical data: current standards and recommendations for implementation. Clin Pharmacol Ther 109:82–86. 10.1002/cpt.1918. [DOI] [PubMed] [Google Scholar]
- 22.Xia B, Barve A, Heimbach T, Zhang T, Gu H, Wang L, Einolf H, Alexander N, Hanna I, Ke J, Mangold JB, He H, Sunkara G. 2014. Physiologically based pharmacokinetic modeling for assessing the clinical drug-drug interaction of alisporivir. Eur J Pharm Sci 63:103–112. 10.1016/j.ejps.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Schmider J, Brockmöller J, Arold G, Bauer S, Roots I. 1999. Simultaneous assessment of CYP3A4 and CYP1A2 activity in vivo with alprazolam and caffeine. Pharmacogenet Genomics 9:725–734. 10.1097/01213011-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ohnhaus EE, Brockmeyer N, Dylewicz P, Habicht H. 1987. The effect of antipyrine and rifampin on the metabolism of diazepam. Clin Pharmacol Ther 42:148–156. 10.1038/clpt.1987.125. [DOI] [PubMed] [Google Scholar]
- 25.Brockmeyer NH, Mertins L, Klimek K, Goos M, Ohnhaus EE. 1990. Comparative effects of rifampin and/or probenecid on the pharmacokinetics of temazepam and nitrazepam. Int J Clin Pharmacol Ther Toxicol 28:387–393. [PubMed] [Google Scholar]
- 26.Liu Y, Zhou S, Wan Y, Wu A, Palmisano M. 2014. The impact of co-administration of ketoconazole and rifampicin on the pharmacokinetics of apremilast in healthy volunteers. Br J Clin Pharmacol 78:1050–1057. 10.1111/bcp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Medicines Agency. 2018. Emend. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/emend.
- 28.Backman JT, Luurila H, Neuvonen M, Neuvonen PJ. 2005. Rifampin markedly decreases and gemfibrozil increases the plasma concentrations of atorvastatin and its metabolites. Clin Pharmacol Ther 78:154–167. 10.1016/j.clpt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK. 2013. Clinical pharmacology of axitinib. Clin Pharmacokinet 52:713–725. 10.1007/s40262-013-0068-3. [DOI] [PubMed] [Google Scholar]
- 30.van Heeswijk RPG, Dannemann B, Hoetelmans RMW. 2014. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother 69:2310–2318. 10.1093/jac/dku171. [DOI] [PubMed] [Google Scholar]
- 31.European Medicines Agency. 2018. Rxulti. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/rxulti.
- 32.Tugnait M, Gupta N, Hanley MJ, Sonnichsen D, Kerstein D, Dorer DJ, Venkatakrishnan K, Narasimhan N. 2020. Effects of strong CYP2C8 or CYP3A inhibition and CYP3A induction on the pharmacokinetics of brigatinib, an oral anaplastic lymphoma kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev 9:214–223. 10.1002/cpdd.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCance-Katz EF, Moody DE, Prathikanti S, Friedland G, Rainey PM. 2011. Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients. Drug Alcohol Depend 118:326–334. 10.1016/j.drugalcdep.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kivistö KT, Lamberg TS, Neuvonen PJ. 1999. Interactions of buspirone with itraconazole and rifampicin: effects on the pharmacokinetics of the active 1-(2-pyrhnidinyl)-piperazine metabolite of buspirone. Pharmacol Toxicol 84:94–97. 10.1111/j.1600-0773.1999.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen L, Holland J, Miles D, Engel C, Benrimoh N, O'Reilly T, Lacy S. 2015. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol 55:1012–1023. 10.1002/jcph.510. [DOI] [PubMed] [Google Scholar]
- 36.ASHP. 2020. Carbamazepine. Monograph for professionals. https://www.drugs.com/monograph/carbamazepine.html.
- 37.Johnson BM, Adams LM, Zhang K, Gainer SD, Kirby LC, Blum RA, Apseloff G, Morrison RA, Schutz RA, Lebowitz PF. 2010. Ketoconazole and rifampin significantly affect the pharmacokinetics, but not the safety or QTc interval, of casopitant, a neurokinin-1 receptor antagonist. J Clin Pharmacol 50:951–959. 10.1177/0091270009353761. [DOI] [PubMed] [Google Scholar]
- 38.Jayasagar G, Kumar MK, Chandrasekhar K, Rao YM. 2003. Influence of rifampicin pretreatment on the pharmacokinetics of celecoxib in healthy male volunteers. Drug Metabol Drug Interact 19:287–296. 10.1515/dmdi.2003.19.4.287. [DOI] [PubMed] [Google Scholar]
- 39.Zhao D, Chen J, Chu M, Long X, Wang J. 2020. Pharmacokinetic-based drug-drug interactions with anaplastic lymphoma kinase inhibitors: a review. Drug Des Devel Ther 14:1663–1681. 10.2147/DDDT.S249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.emc. 2018. Mycobutin—SmPC. (Summary of patient characteristics.) https://www.medicines.org.uk/emc/product/1088/smpc.
- 41.European Medicines Agency. 2018. Xalkori. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/xalkori.
- 42.European Medicines Agency. 2018. Daklinza. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/daklinza.
- 43.European Medicines Agency. 2018. Sprycel. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/sprycel.
- 44.Blode H, Zeun S, Parke S, Zimmermann T, Rohde B, Mellinger U, Kunz M. 2012. Evaluation of the effects of rifampicin, ketoconazole and erythromycin on the steady-state pharmacokinetics of the components of a novel oral contraceptive containing estradiol valerate and dienogest in healthy postmenopausal women. Contraception 86:337–344. 10.1016/j.contraception.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Aitio ML, Mansury L, Tala E, Haataja M, Aitio A. 1981. The effect of enzyme induction on the metabolism of disopyramide in man. Br J Clin Pharmacol 11:279–285. 10.1111/j.1365-2125.1981.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilby KJ, Eissa NA. 2018. Clinical pharmacokinetics and drug interactions of doravirine. Eur J Drug Metab Pharmacokinet 43:637–644. 10.1007/s13318-018-0497-3. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M, Wolf JL, Drolet DW, Fettner SH, Rakhit AK, Witt K, Lum BL. 2014. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol 73:613–621. 10.1007/s00280-014-2390-3. [DOI] [PubMed] [Google Scholar]
- 48.Kirigaya Y, Shiramoto M, Ishizuka T, Uchimaru H, Irie S, Kato M, Shimizu T, Nakatsu T, Nishikawa Y, Ishizuka H. 2020. Effects of itraconazole and rifampicin on the single-dose pharmacokinetics of the nonsteroidal mineralocorticoid receptor blocker esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol 86:2070–2079. 10.1111/bcp.14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barditch-Crovo P, Trapnell CB, Ette E, Zacur HA, Coresh J, Rocco LE, Hendrix CW, Flexner C. 1999. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther 65:428–438. 10.1016/S0009-9236(99)70138-4. [DOI] [PubMed] [Google Scholar]
- 50.Kakuda TN, Woodfall B, De Marez T, Peeters M, Vandermeulen K, Aharchi F, Hoetelmans RMW. 2014. Pharmacokinetic evaluation of the interaction between etravirine and rifabutin or clarithromycin in HIV-negative, healthy volunteers: results from two phase 1 studies. J Antimicrob Chemother 69:728–734. 10.1093/jac/dkt421. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra B, Sachse R, Wood N. 2009. Evaluation of drug-drug interactions with fesoterodine. Eur J Clin Pharmacol 65:551–560. 10.1007/s00228-009-0648-1. [DOI] [PubMed] [Google Scholar]
- 52.European Medicines Agency. 2018. Flibanserin. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-000085-pip01-07.
- 53.Scripture CD, Pieper JA. 2001. Clinical pharmacokinetics of fluvastatin. Clin Pharmacokinet 40:263–281. 10.2165/00003088-200140040-00003. [DOI] [PubMed] [Google Scholar]
- 54.Martin P, Gillen M, Millson D, Oliver S, Brealey C, Grossbard EB, Baluom M, Lau D, Sweeny D, Mant T, Craven K. 2016. Effects of CYP3A4 inhibitors ketoconazole and verapamil and the CYP3A4 inducer rifampicin on the pharmacokinetic parameters of fostamatinib: results from in vitro and phase I clinical studies. Drugs R D 16:81–92. 10.1007/s40268-015-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, Wild MJ. 2005. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet 44:1067–1081. 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- 56.Noh Y-H, Lim H-S, Jin S-J, Kim MJ, Kim YH, Sung HR, Choi HY, Bae K-S. 2012. Effects of ketoconazole and rifampicin on the pharmacokinetics of gemigliptin, a dipeptidyl peptidase-IV inhibitor: a crossover drug-drug interaction study in healthy male Korean volunteers. Clin Ther 34:1182–1194. 10.1016/j.clinthera.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT. 2001. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther 69:400–406. 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 58.Niemi M, Kivistö KT, Backman JT, Neuvonen PJ. 2000. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of glimepiride. Br J Clin Pharmacol 50:591–595. 10.1046/j.1365-2125.2000.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jong J, Skee D, Murphy J, Sukbuntherng J, Hellemans P, Smit J, de Vries R, Jiao JJ, Snoeys J, Mannaert E. 2015. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect 3:e00156. 10.1002/prp2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolton AE, Peng B, Hubert M, Krebs-Brown A, Capdeville R, Keller U, Seiberling M. 2004. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol 53:102–106. 10.1007/s00280-003-0722-9. [DOI] [PubMed] [Google Scholar]
- 61.Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, Lasseter K, Pearlman H, Rammelsberg D, Schmitt‐Hoffmann A, Yamazaki T, Desai A. 2017. Pharmacokinetic evaluation of CYP3A4-mediated drug-drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev 6:44–53. 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.European Medicines Agency. 2018. Kalydeco. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco.
- 63.Elgart A, Greenblatt DJ, Loupe PS, Zur AA, Weiss S, Mimrod D, Spiegelstein O. 2020. The effect of CYP3A induction and inhibition on the pharmacokinetics of laquinimod, a novel neuroimmunomodulator. Clin Pharmacol Drug Dev 9:1015–1024. 10.1002/cpdd.785. [DOI] [PubMed] [Google Scholar]
- 64.Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, Ginman K, Bergeron M, Pithavala YK. 2020. The effect of rifampin on the pharmacokinetics and safety of lorlatinib: results of a phase one, open-label, crossover study in healthy participants. Adv Ther 37:745–758. 10.1007/s12325-019-01198-9. [DOI] [PubMed] [Google Scholar]
- 65.Williamson KM, Patterson JH, McQueen RH, Adams KF, Pieper JA. 1998. Effects of erythromycin or rifampin on losartan pharmacokinetics in healthy volunteers. Clin Pharmacol Ther 63:316–323. 10.1016/S0009-9236(98)90163-1. [DOI] [PubMed] [Google Scholar]
- 66.Chiu Y-Y, Ereshefsky L, Preskorn SH, Poola N, Loebel A. 2014. Lurasidone drug-drug interaction studies: a comprehensive review. Drug Metabol Drug Interact 29:191–202. 10.1515/dmdi-2014-0005. [DOI] [PubMed] [Google Scholar]
- 67.Ridtitid W, Wongnawa M, Mahatthanatrakul W, Chaipol P, Sunbhanich M. 2010. Effect of rifampin on plasma concentrations of mefloquine in healthy volunteers. J Pharm Pharmacol 52:1265–1269. 10.1211/0022357001777243. [DOI] [PubMed] [Google Scholar]
- 68.Chung E, Nafziger AN, Kazierad DJ, Bertino JS. 2006. Comparison of midazolam and simvastatin as cytochrome P450 3A probes. Clin Pharmacol Ther 79:350–361. 10.1016/j.clpt.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Backman JT, Olkkola KT, Neuvonen PJ. 1996. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther 59:7–13. 10.1016/S0009-9236(96)90018-1. [DOI] [PubMed] [Google Scholar]
- 70.Fukumura K, Kawaguchi N, Ishibashi T, Kubota R, Tada Y, Ogura E. 2020. Clinical drug-drug interaction studies to evaluate the effects of a P-glycoprotein inhibitor, CYP3A inhibitors, and a CYP3A inducer on the pharmacokinetics of naldemedine in healthy subjects. Clin Drug Invest 40:529–540. 10.1007/s40261-020-00902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bui K, Zhou D, Sostek M, She F, Al‐Huniti N. 2016. Effects of CYP3A modulators on the pharmacokinetics of naloxegol. J Clin Pharmacol 56:1019–1027. 10.1002/jcph.693. [DOI] [PubMed] [Google Scholar]
- 72.Scheen AJ. 2007. Drug-drug and food-drug pharmacokinetic interactions with new insulinotropic agents repaglinide and nateglinide. Clin Pharmacokinet 46:93–108. 10.2165/00003088-200746020-00001. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Petrie ID, Levy RH, Ragueneau-Majlessi I. 2019. Mechanisms and clinical significance of pharmacokinetic-based drug-drug interactions with drugs approved by the U.S. Food and Drug Administration in 2017. Drug Metab Dispos 47:135–144. 10.1124/dmd.118.084905. [DOI] [PubMed] [Google Scholar]
- 74.Natale JJ, Spinelli T, Calcagnile S, Lanzarotti C, Rossi G, Cox D, Kashef K. 2016. Drug-drug interaction profile of components of a fixed combination of netupitant and palonosetron: review of clinical data. J Oncol Pharm Pract 22:485–495. 10.1177/1078155215586824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holtbecker N, Fromm MF, Kroemer HK, Ohnhaus EE, Heidemann H. 1996. The nifedipine-rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos 24:1121–1123. [PubMed] [Google Scholar]
- 76.Tanaka C, Yin OQP, Smith T, Sethuraman V, Grouss K, Galitz L, Harrell R, Schran H. 2011. Effects of rifampin and ketoconazole on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol 51:75–83. 10.1177/0091270010367428. [DOI] [PubMed] [Google Scholar]
- 77.ASHP. 2020. Olaparib. Monograph for professionals. https://www.drugs.com/monograph/olaparib.html.
- 78.European Medicines Agency. 2018. Ibrance. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/ibrance.
- 79.Kay L, Kampmann JP, Svendsen TL, Vergman B, Hansen JE, Skovsted L, Kristensen M. 1985. Influence of rifampicin and isoniazid on the kinetics of phenytoin. Br J Clin Pharmacol 20:323–326. 10.1111/j.1365-2125.1985.tb05071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.European Medicines Agency. 2018. Wakix. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/wakix.
- 81.Narasimhan NI, Dorer DJ, Davis J, Turner CD, Sonnichsen D. 2015. Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev 4:354–360. 10.1002/cpdd.182. [DOI] [PubMed] [Google Scholar]
- 82.Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M. 2002. Rifampin markedly decreases plasma concentrations of praziquantel in healthy volunteers. Clin Pharmacol Ther 72:505–513. 10.1067/mcp.2002.129319. [DOI] [PubMed] [Google Scholar]
- 83.Lee KH, Shin JG, Chong WS, Kim S, Lee JS, Jang IJ, Shin SG. 1993. Time course of the changes in prednisolone pharmacokinetics after co-administration or discontinuation of rifampin. Eur J Clin Pharmacol 45:287–289. 10.1007/BF00315399. [DOI] [PubMed] [Google Scholar]
- 84.McAllister WA, Thompson PJ, Al-Habet SM, Rogers HJ. 1983. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed) 286:923–925. 10.1136/bmj.286.6369.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herman RJ, Nakamura K, Wilkinson GR, Wood AJ. 1983. Induction of propranolol metabolism by rifampicin. Br J Clin Pharmacol 16:565–569. 10.1111/j.1365-2125.1983.tb02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGechan A, Wellington K. 2005. Ramelteon. CNS Drugs 19:1057–1067. 10.2165/00023210-200519120-00007. [DOI] [PubMed] [Google Scholar]
- 87.European Medicines Agency. 2018. Stivarga. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/stivarga.
- 88.European Medicines Agency. 2018. Kisqali. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/kisqali.
- 89.European Medicines Agency. 2018. Jakavi. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi.
- 90.Moyle GJ, Buss NE, Goggin T, Snell P, Higgs C, Hawkins DA. 2002. Interaction between saquinavir soft-gel and rifabutin in patients infected with HIV. Br J Clin Pharmacol 54:178–182. 10.1046/j.1365-2125.2002.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kyrklund C, Backman JT, Kivistö KT, Neuvonen M, Laitila J, Neuvonen PJ. 2000. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 68:592–597. 10.1067/mcp.2000.111414. [DOI] [PubMed] [Google Scholar]
- 92.Gardin A, Gray C, Neelakantham S, Huth F, Davidson AM, Dumitras S, Legangneux E, Shakeri-Nejad K. 2018. Siponimod pharmacokinetics, safety, and tolerability in combination with rifampin, a CYP2C9/3A4 inducer, in healthy subjects. Eur J Clin Pharmacol 74:1593–1604. 10.1007/s00228-018-2533-2. [DOI] [PubMed] [Google Scholar]
- 93.Bello C, Houk B, Sherman L, Misbah S, Sarapa N, Smeraglia J, Haung X. 2005. Effect of rifampin on the pharmacokinetics of SU11248 in healthy volunteers. J Clin Oncol 23:3078. 10.1200/jco.2005.23.16_suppl.3078. [DOI] [Google Scholar]
- 94.Wrishko RE, McCrea JB, Yee KL, Liu W, Panebianco D, Mangin E, Chakravarthy M, Martinez-Cantarin MP, Kraft WK. 2019. Effect of CYP3A inhibition and induction on the pharmacokinetics of suvorexant: two phase I, open-label, fixed-sequence trials in healthy subjects. Clin Drug Invest 39:441–451. 10.1007/s40261-019-00764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogilvie BW, Torres R, Dressman MA, Kramer WG, Baroldi P. 2015. Clinical assessment of drug-drug interactions of tasimelteon, a novel dual melatonin receptor agonist. J Clin Pharmacol 55:1004–1011. 10.1002/jcph.507. [DOI] [PubMed] [Google Scholar]
- 96.Shi J, Montay G, Bhargava VO. 2005. Clinical pharmacokinetics of telithromycin, the first ketolide antibacterial. Clin Pharmacokinet 44:915–934. 10.2165/00003088-200544090-00003. [DOI] [PubMed] [Google Scholar]
- 97.Backman JT, Granfors MT, Neuvonen PJ. 2006. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol 62:451–461. 10.1007/s00228-006-0127-x. [DOI] [PubMed] [Google Scholar]
- 98.Shoaf SE, Bricmont P, Mallikaarjun S. 2014. Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85:953–961. 10.1038/ki.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Villikka K, Kivistö KT, Backman JT, Olkkola KT, Neuvonen PJ. 1997. Triazolam is ineffective in patients taking rifampin. Clin Pharmacol Ther 61:8–14. 10.1016/S0009-9236(97)90176-4. [DOI] [PubMed] [Google Scholar]
- 100.European Medicines Agency. 2018. Esmya. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/esmya.
- 101.Martin P, Oliver S, Robertson J, Kennedy S-J, Read J, Duvauchelle T. 2011. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D 11:37–51. 10.2165/11586980-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen G, Lee R, Højer A-M, Buchbjerg JK, Serenko M, Zhao Z. 2013. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Invest 33:727–736. 10.1007/s40261-013-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villikka K, Kivistö KT, Luurila H, Neuvonen PJ. 1997. Rifampin reduces plasma concentrations and effects of zolpidem. Clin Pharmacol Ther 62:629–634. 10.1016/S0009-9236(97)90082-5. [DOI] [PubMed] [Google Scholar]
- 104.Villikka K, Kivistö KT, Lamberg TS, Kantola T, Neuvonen PJ. 1997. Concentrations and effects of zopiclone are greatly reduced by rifampicin. Br J Clin Pharmacol 43:471–474. 10.1046/j.1365-2125.1997.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mu S, Tang Z, Novotny W, Tawashi M, Li T-K, Ou Y, Sahasranaman S. 2020. Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton’s tyrosine kinase inhibitor) in Asian and non-Asian healthy subjects. Cancer Chemother Pharmacol 85:391–399. 10.1007/s00280-019-04015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B, Worth A, Kearney BP, Mathias A. 2018. Cytochrome P450 3A induction predicts P-glycoprotein induction. Part 2. Prediction of decreased substrate exposure after rifabutin or carbamazepine. Clin Pharmacol Ther 104:1191–1198. 10.1002/cpt.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGregor MM, Olliaro P, Wolmarans L, Mabuza B, Bredell M, Felten MK, Fourie PB. 1996. Efficacy and safety of rifabutin in the treatment of patients with newly diagnosed pulmonary tuberculosis. Am J Respir Crit Care Med 154:1462–1467. 10.1164/ajrccm.154.5.8912765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01043-21-s0001.pdf, PDF file, 0.2 MB (162.3KB, pdf)