Abstract
This review covers the use of pulsed electric fields in cancer therapy. It is organized into three sections based on pulse length, millisecond domain, microsecond domain, and nanosecond domain. The predominant application of pulsed electric fields is the modification of the permeability of cellular membranes, sometimes referred to as electroporation. This has been used in many different ways for cancer treatment. These include introducing genes into the tumor cells to activate an immune response, introducing poisons into the tumor cells, initiating necrosis using irreversible electroporation, and initiating immunogenic cell death with nanopulse stimulation.
Keywords: electroporation, pulsed electric fields, electrochemotherapy, gene electrotransfer, irreversible electroporation, Nano-Pulse Stimulation
The field of bioelectricity encompasses both elucidating the role of endogenous electrical signals used by cells and tissues in their daily functions and utilizing imposed electrical signals to influence or modify cell and tissue function. This brief overview concentrates on the latter application focusing on the use of very short pulsed electric fields in cancer therapies.
The cellular responses to pulsed electric fields fall generally into three different pulse duration domains: millisecond, microsecond, and nanosecond. Investigations of the effects of pulsed fields on cells have a long history and encompass thousands of publications over the past four decades. A comprehensive book, Bioelectrics, covering most of the applications of pulsed electric fields has recently been published.1 In this comprehensive collection, there is a chapter entitled “Medical Applications” that includes a section written by Richard Heller on the use of millisecond and microsecond pulses for gene electrotransfer, as well as another section on using microsecond pulses for electrochemotherapy (ECT) by Julie Gehl, Gregor Sersa, and Lluis Mir, and two sections describing nanosecond pulses by Beebe, Hargrave, and Nuccitelli.
The predominant application of pulsed electric fields is the modification of the permeability of cellular membranes. It has long been known that a voltage gradient of 500 mV across a bilayer membrane is sufficient to generate water-filled defects through the bilayer. This was referred to as “punch-through” by Coster,2 “reversible dielectric breakdown” by Zimmerman et al.,3 and “electroporation” by Neumann et al.4,5 Generally speaking, the longer the pulse, the larger the pore size that it generates in a biological membrane. Molecular dynamics models of cellular membranes have demonstrated that fields of this magnitude push charged water dipoles into the hydrophobic lipid bilayer and form a water-filled defect that spans the membrane.6 Such defects have many different applications to cancer therapy that vary with the duration of the pulse.
Pulses in the Millisecond Domain
These relatively long pulses are often used to stimulate the uptake of nucleic acids such as DNA plasmids into cells. The plasmids are added extracellularly and bind to the plasma membrane when the electric field is present. Aggregates form and are endocytosed into the cell.7 This process is now called gene electrotransfer or “GET.” The ultimate goal of GET is to transform cells and tissues to overcome metabolic and genetic disorders. The best measure of success is the amount of gene expression obtained and this varies greatly depending on pulse parameters such as amplitude and duration. Although much work has been done using shorter microsecond pulses at higher field strengths, this usually results in relatively low expression, whereas lower field strengths usually result in higher gene expression,8 although there are exceptions based on tissue type. As this topic is covered extensively in Richard Heller's chapter 5.5 in Bioelectrics,1 GET is not covered very deeply here.
The cancer connection comes when plasmids coding for genes that could stimulate an immune response are introduced into tumors. The most successful example is the treatment of melanoma using a plasmid containing the gene for interleukin 12 (IL-12).9,10 This cytokine stimulates the differentiation of naive T cells into Th1 cells as well as the production of interferon-gamma and tumor necrosis factor-alpha. The clinical trial treating melanoma was quite promising and indicated that the GET of the IL-12 plasmid eliminated the primary tumor along with some neighboring untreated tumors. This therapy is continuing to be developed and applied in ongoing clinical trials with melanoma and triple negative breast cancer sponsored by OncoSec Medical as listed on Clinicaltrials.gov (NCT03132675; NCT02531425).
Pulses in the Microsecond Domain
Pulses in the microsecond domain are most commonly used to permeabilize cells by generating pores large enough to allow the transport of small molecules across the plasma membrane. These fields can generate both reversible and irreversible electroporation (IRE) depending on the amplitude of the pulsed electric field. Pulses in this domain have been used quite extensively in cancer therapy. The two main therapies utilizing pulses of 100-μs duration are ECT and IRE.
Electrochemotherapy
ECT is the oldest of the electroporation therapies with its discovery in the mid-1980s.11,12 The first clinical use of this technology occurred in the early 1990s when 100 μs long pulsed electric fields in the 1–1.5 kV/cm range were used to deliver bleomycin to treat squamous cell carcinoma.13 Bleomycin is hydrophilic so it does not normally cross the hydrophobic membrane barrier to enter cells. However, it can be injected into the blood to be delivered locally to cells transiently permeabilized by ECT. Once it is in the cell cytoplasm, it acts as an enzyme that causes strand breaks in DNA, which leads to cell death. Many clinical trials have been performed by evaluating ECT of several cutaneous tumor targets, including melanoma, basal cell carcinoma, breast cancer, and Kaposi's sarcoma.14,15 This therapy has proven to be quite effective for most of these cutaneous lesions, exhibiting an overall efficiency of 75% with a 47% complete remission rate.16 In addition, there is some evidence that ECT stimulates the release of danger-associated molecular patterns (DAMPs) that attract dendritic cells to the treated tumor to phagocytose those cells and present any neoantigens to the immune system to stimulate an adaptive immune response.17 This process is referred to as immunogenic cell death (ICD). ECT has been approved for human therapy in several European countries, and bleomycin and cisplatin are the most commonly used drugs with this therapy.18 ECT is still not approved for human use in the United States.
Recently Dr. Julie Gehl and colleagues have demonstrated ECT's effectiveness when replacing bleomycin with high levels of Ca2+ injected into the region of ECT.19–21 This has the advantage that Ca2+ is more readily available and less expensive than bleomycin. However, Ca2+ cannot be delivered systemically as such high levels of Ca2+ in the blood would be toxic.
Irreversible electroporation
IRE was first discovered in 2005 when the amplitude of the applied 100 μs long electric field pulse was raised to 2 kV/cm or greater.22 Under the higher electric field, the cell's pores do not reseal, so irreversible damage results.23 These pores are large enough to allow adenosine triphosphate (ATP) and many other important cellular molecules to leak out of the cell, which leads very quickly to necrosis. One important advantage of IRE is that it is nonthermal, so it preserves some cellular structures such as blood vessels.24 In addition, because it does not rely on heat to achieve its destructive effect, it can be used effectively in regions with heat sinks, such as large blood vessels where classical thermal ablation often fails. IRE was approved in the United States for soft tissue ablation and is marketed by AngioDynamics Inc. Preclinical data on canine prostate and brain tumor ablations indicate that nerves recovered fully in the prostate25 and IRE has demonstrated effectiveness in treating canine gliomas.26 Several clinical trials sponsored by AngioDynamics are underway for the treatment of pancreatic cancer (NCT01790451), colorectal liver metastases (NCT02082782), unresectable renal tumors (NCT02335827), and rectal neoplasms (NCT02425059).
Unlike ECT or nanopulse stimulation (NPS™; discussed hereunder), IRE treatment does not generate a strong immune response to the tumor. Rechallenge tumor growth is only delayed rather than prevented.27
Pulses in the Nanosecond Domain
This shorter nanosecond pulse domain has only been studied for the past two decades, but has unique properties not observed with the longer pulses already described. Largely due to these unique properties, it is often referred to as NPS. There are two main properties that distinguish it from the microsecond-domain pulses: intracellular penetration and large electric field amplitude.
Intracellular penetration
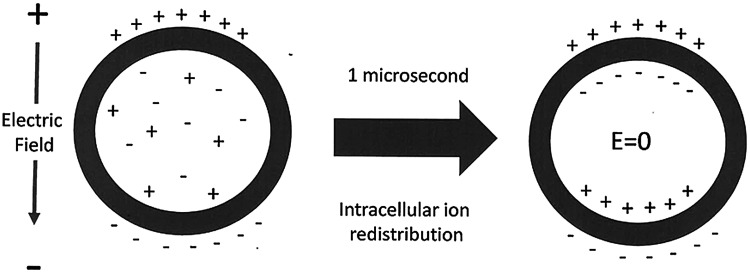
Ultrashort nanosecond-domain pulses can exhibit unique intracellular effects because they are able to penetrate into cells and organelles. This ability to penetrate beyond the plasma membrane is due to their pulse rise time reaching full amplitude in the nanosecond range. This is much faster than the time required for intracellular and intraorganellar charges to redistribute to cancel the imposed field. We know this from experiments studying the membrane charging time of cells. When a conductor is placed in an external electric field, the mobile charges in the conductor redistribute to generate an equal and opposite field so that the net field in the conductor is always zero. Cells can be modeled as a conductor surrounded by a resistive membrane bilayer, so when placed in an imposed field, positive ions in the cytoplasm will move toward the negative pole of the field and negative ions will move toward the positive pole until they encounter the outer membrane and cannot move further (Fig. 1).
FIG. 1.
Redistribution of ions in the cell cytoplasm after the application of an external electric field takes about 1 μs for cells packed together in a tissue. After this redistribution, the internal electric field is 0 kV/cm. However, imposed electric fields with rise times shorter than a microsecond can penetrate into the cell and organelles until this ion redistribution is complete.
This charges the membrane capacitance and generates a field that is equal and opposite to the imposed external electric field. This charge redistribution takes time that will vary with the access resistance to the cells. When cells are tightly packed in a tissue, this redistribution will typically take as long as 1 μs.28 Therefore, imposed fields with rise times faster than 1 μs will be able to penetrate tissues to exert their force on intracellular organelle membranes. This cytoplasm-penetrating property was demonstrated for nanosecond-domain fields in 2001.29 Since then many additional examples of small organelles permeabilized by NPS have appeared, including vesicles,30 mitochondria,31 endoplasmic reticulum,32,33 and nuclei.34
The penetration of the cytoplasm will occur for longer pulses as well if their rise time is <1 μs. Indeed, 100 μs pulses with fast rise times have been shown to permeabilize very long organelles that nearly span the cell, such as the endoplasmic reticulum. For long organelles, the imposed field does not have to be so large and the relatively small amplitude microsecond fields with fast rise times can also selectively permeabilize them.35 However, to permeabilize smaller organelles, much higher field strengths are required and such fields would introduce too much thermal energy if they were applied for microseconds as discussed next.
Electric field amplitude
When electric pulses are applied to cells or tissues, they will introduce “joule heating,” which can be calculated based on the current (I), voltage (V), and duration of the pulse (t):
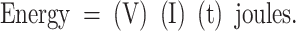 |
Since a joule of energy will heat 1 mL of water by 0.24°C, it is important to keep the energy delivered by the pulsed electric field in mind to avoid thermal effects. This is very easy for nanosecond-range pulses because they are 1000 times shorter than those in the microsecond domain. Since the total energy delivered is proportional to the product of the pulse duration, current, and voltage, the shorter pulse duration makes it possible to increase the applied voltage by this factor of 1000, so the applied voltage and current can be much larger while introducing less energy. A larger voltage gradient is critical for permeabilizing the membranes surrounding small intracellular organelles. The initial targets of pulsed electric fields are the cell membranes because they are the most resistive elements in the cells and tissues. The transmembrane voltage difference required to permeabilize a lipid bilayer membrane is 500 mV. To generate a voltage gradient of 500 mV across both end membranes perpendicular to the field lines in a 10 μm wide cell, a field of 1 V/10 μm (1 kV/cm) is required and this is the typical field strength used for ECT discussed earlier. However, to permeabilize smaller intracellular organelles, higher fields are required. For example, a typical mitochondrion is only 0.5 μm wide and will require 1 V/0.5 μm or 20 kV/cm to permeabilize it. If a 100 ns, 20 kV/cm pulse is applied to a 200 ohm-cm load, the current flowing through the load will be 100 A-cm.
E = 20 kV/cm × 100 A-cm × 10–7 s = 0.2 J and this energy will raise the temperature of the target tissue by <0.05°C, assuming the heat capacity of water.
In contrast, for a 100 μs pulse having the same voltage and current, E = 200 J, which would heat the cells exposed by 50°C. Therefore, the ability to apply large voltage gradients across the cytoplasm of cells without significant joule heating is a very important benefit of nanosecond-domain pulses. Such large voltage gradients are critical for generating transient nanopores in small organelles.
These unique features of nanosecond-domain pulses, large amplitude and intracellular penetrating ability, make them useful to tease new responses out of cells and tissues, such as the initiation of vesicle secretion and ICD.
Unique properties of NPS
Using patch clamp experiments and molecular probes of known size, we have learned that a single NPS pulse generates millions of 1 nm-wide pores in the plasma membrane36–38 as well as organelle membranes. These pores are too small for most biologically relevant molecules to traverse, but ions easily pass through them. The most important ion for cell signaling is Ca2+, and the cytoplasmic concentration of Ca2+ has been observed to transiently increase with every NPS pulse.32,33,39 Since Ca2+ spikes have been found to influence or control many cellular functions such as secretion and mitosis, it is not surprising that NPS can influence many different cellular functions. Some examples are listed as follows.
Vesicle secretion
Platelet activation involves degranulation or vesicle secretion and is normally triggered by thrombin. However, one of the earliest applications of NPS was to trigger platelet activation in the absence of thrombin.40 This has led to the NPS stimulation of platelet-rich plasma to improve wound healing and enhance blood flow.41–43
ICD and cancer treatment
The first indication that applying pulsed electric fields in the nanosecond domain could influence tumor growth came from the pioneering work of Beebe et al.44 treating subdermal murine fibrosarcoma allografts. Since then >60 articles have been published describing various aspects of tumor ablation by NPS. These articles found that tumors treated with NPS slowly disappear over a period of days to weeks, unlike IRE that generates necrotic death within hours. Furthermore, the treatment was nonthermal and drug-free and exhibited the characteristics of ICD, releasing DAMPs such as calreticulin translocation from the endoplasmic reticulum to the cell surface, ATP release, and HMGB1 release.45,46 More importantly, these DAMPs were found to generate a CD8-dependent adaptive immune response that prevented growth of a rechallenge tumor.46–52
This initiation of an adaptive immune response adds an exciting dimension to this tumor therapy and suggests the possibility that NPS treatment might reduce metastasis of the primary tumor. Some evidence for the inhibition of metastasis has recently appeared from NPS treatment of canine osteosarcoma53 and murine breast cancer.46
Additional advantages of NPS therapy are that it is drug-free, very fast, and leaves no scar from treated skin lesions.54 To eliminate human basal cell carcinoma, only a single treatment of 100 pulses at 100 ns and 30 kV/cm applied in 33 s is needed. One disadvantage is the large electric field of 30 kV/cm required to achieve the desired ICD. Owing to the challenges of applying pulses >15 kV to tissues, the size of the ablation zone is limited to about 1 cc so that larger tumors will require multiple applications to cover the entire tumor.
Summary
Pulsed electric fields have been used in cancer therapy for the past three decades. For the treatment of surface skin lesions, millisecond- and microsecond-domain pulses have been used to treat thousands of patients in many European countries. Nanosecond pulsed fields have been found to be effective in treating skin lesions but have not yet been approved for cancer therapy. They present the promise of the dual effect of eliminating the primary tumor while triggering an adaptive immune response that could eliminate metastases as well. This is an exciting time for the application of bioelectricity to cancer therapy.
Author Disclosure Statement
R.N. is the Chief Science Officer of Pulse Biosciences and is on the payroll.
References
- 1.Akiyama H, Heller R. Bioelectrics. Tokyo, Japan: Springer, 2017 [Google Scholar]
- 2.Coster HG. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of “punch-through”. Biophys J 1965;5:669–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann U, Pilwat G, Riemann F. Dielectric breakdown of cell membranes. Biophys J 1974;14:881–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann E, Schaefer-Ridder M, Wang Y, et al. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1982;1:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol 1972;10:279–290 [DOI] [PubMed] [Google Scholar]
- 6.Tokman M, Lee JH, Levine ZA, et al. Electric field-driven water dipoles: Nanoscale architecture of electroporation. PLoS One 2013;8:e61111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosazza C, Meglic SH, Zumbusch A, et al. Gene electrotransfer: A mechanistic perspective. Curr Gene Ther 2016;16:98–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigey P, Bureau MF, Scherman D. In vivo plasmid DNA electrotransfer. Curr Opin Biotechnol 2002;13:443–447 [DOI] [PubMed] [Google Scholar]
- 9.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther 2010;10:312–317 [DOI] [PubMed] [Google Scholar]
- 10.Daud AI, DeConti RC, Andrews S, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008;26:5896–5903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir LM, Banoun H, Paoletti C. Introduction of definite amounts of nonpermeant molecules into living cells after electropermeabilization: Direct access to the cytosol. Exp Cell Res 1988;175:15–25 [DOI] [PubMed] [Google Scholar]
- 12.Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res 1987;78:1319–1321 [PubMed] [Google Scholar]
- 13.Mir LM, Belehradek M, Domenge C, et al. [Electrochemotherapy, a new antitumor treatment: First clinical trial]. C R Acad Sci III 1991;313:613–618 [PubMed] [Google Scholar]
- 14.Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol 2011;50:621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998;83:148–157 [DOI] [PubMed] [Google Scholar]
- 16.Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J Clin Oncol 2014;32:3144–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvet CY, Famin D, Andre FM, et al. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014;3:e28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehl J, Sersa G, Matthiessen LW, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol 2018;57:874–882 [DOI] [PubMed] [Google Scholar]
- 19.Gehl J. Electroporation for drug and gene delivery in the clinic: Doctors go electric. Methods Mol Biol 2008;423:351–359:351–359. [DOI] [PubMed] [Google Scholar]
- 20.Miklavcic D, Sersa G, Brecelj E, et al. Electrochemotherapy: Technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput 2012;50:1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk H, Matthiessen LW, Wooler G, et al. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol 2018;57:311–319 [DOI] [PubMed] [Google Scholar]
- 22.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–231 [DOI] [PubMed] [Google Scholar]
- 23.Onik G, Mikus P, Rubinsky B. Irreversible electroporation: Implications for prostate ablation. Technol Cancer Res Treat 2007;6:295–300 [DOI] [PubMed] [Google Scholar]
- 24.Phillips MA, Narayan R, Padath T, et al. Irreversible electroporation on the small intestine. Br J Cancer 2012;106:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Fan Q, Ji Z, et al. The effects of irreversible electroporation (IRE) on nerves. PLoS One 2011;6:e18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossmeisl JH, Jr., Garcia PA, Pancotto TE, et al. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J Neurosurg 2015;123:1008–1025 [DOI] [PubMed] [Google Scholar]
- 27.Neal RE, 2nd, Rossmeisl JH, Jr., Robertson JL, et al. Improved local and systemic anti-tumor efficacy for irreversible electroporation in immunocompetent versus immunodeficient mice. PLoS One 2013;8:e64559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenbach KH. Bioelectric effect of intense nanosecond pulses. In: Pakhomov AG, Miklavcic D, Markov MS, eds. Advanced Electroporation Techniques in Biology and Medicine. Boca Raton, FL: Taylor and Francis Group, 2010:19–50 [Google Scholar]
- 29.Schoenbach KH, Beebe SJ, Buescher ES. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics 2001;22:440–448 [DOI] [PubMed] [Google Scholar]
- 30.Tekle E, Oubrahim H, Dzekunov SM, et al. Selective field effects on intracellular vacuoles and vesicle membranes with nanosecond electric pulses. Biophys J 2005;89:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vernier PT. Mitochondrial membrane permeabilization with nanosecond electric pulses. Conf Proc IEEE Eng Med Biol Soc 2011;2011:743–745 [DOI] [PubMed] [Google Scholar]
- 32.Vernier PT, Sun Y, Marcu L, et al. Calcium bursts induced by nanosecond electric pulses. Biochem Biophys Res Commun 2003;310:286–295 [DOI] [PubMed] [Google Scholar]
- 33.White JA, Blackmore PF, Schoenbach KH, et al. Stimulation of capacitative calcium entry in HL-60 cells by nanosecond pulsed electric fields. J Biol Chem 2004;279:22964–22972 [DOI] [PubMed] [Google Scholar]
- 34.Thompson GL, Roth CC, Kuipers MA, et al. Permeabilization of the nuclear envelope following nanosecond pulsed electric field exposure. Biochem Biophys Res Commun 2016;470:35–40 [DOI] [PubMed] [Google Scholar]
- 35.Hanna H, Denzi A, Liberti M, et al. Electropermeabilization of inner and outer cell membranes with microsecond pulsed electric fields: Quantitative study with calcium ions. Sci Rep 2017;7:13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pakhomov AG, Shevin R, White JA, et al. Membrane permeabilization and cell damage by ultrashort electric field shocks. Arch Biochem Biophys 2007;465:109–118 [DOI] [PubMed] [Google Scholar]
- 37.Pakhomov AG, Kolb JF, White JA, et al. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics 2007;28:655–663 [DOI] [PubMed] [Google Scholar]
- 38.Sozer EB, Levine ZA, Vernier PT. Quantitative limits on small molecule transport via the electropermeome—Measuring and modeling single nanosecond perturbations. Sci Rep 2017;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semenov I, Xiao S, Kang D, et al. Cell stimulation and calcium mobilization by picosecond electric pulses. Bioelectrochemistry 2015;105:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Blackmore PF, Hargrave BY, et al. Nanosecond pulse electric field (nanopulse): A novel non-ligand agonist for platelet activation. Arch Biochem Biophys 2008;471:240–248 [DOI] [PubMed] [Google Scholar]
- 41.Hargrave B, Li F. Nanosecond pulse electric field activation of platelet-rich plasma reduces myocardial infarct size and improves left ventricular mechanical function in the rabbit heart. J Extra Corpor Technol 2012;44:198–204 [PMC free article] [PubMed] [Google Scholar]
- 42.Hargrave B, Li F. Nanosecond pulse electric field activated-platelet rich plasma enhances the return of blood flow to large and ischemic wounds in a rabbit model. Physiol Rep 2015;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hargrave B, Varghese F, Barabutis N, et al. Nanosecond pulsed platelet-rich plasma (nsPRP) improves mechanical and electrical cardiac function following myocardial reperfusion injury. Physiol Rep 2016;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beebe SJ, Fox P, Rec LJ, et al. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans Plasma Sci 2002;30:286–292 [Google Scholar]
- 45.Nuccitelli R, McDaniel A, Anand S, et al. Nano-pulse stimulation is a physical modality that can trigger immunogenic tumor cell death. J Immunother Cancer 2017;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo S, Jing Y, Burcus NI, et al. Nano-pulse stimulation induces potent immune responses, eradicating local breast cancer while reducing distant metastases. Int J Cancer 2018;142:629–640 [DOI] [PubMed] [Google Scholar]
- 47.Beebe SJ, Lassiter BP, Guo S. Nanopulse stimulation (NPS) induces tumor ablation and immunity in orthotopic 4T1 mouse breast cancer: A review. Cancers 2018;10:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassiter BP, Guo S, Beebe SJ. Nano-pulse stimulation ablates orthotopic rat hepatocellular carcinoma and induces innate and adaptive memory immune mechanisms that prevent recurrence. Cancers 2018;10:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nuccitelli R, Berridge JC, Mallon Z, et al. Nanoelectroablation of murine tumors triggers a CD8-dependent inhibition of secondary tumor growth. PLoS One 2015;10:e0134364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R, Sain NM, Harlow KT, et al. A protective effect after clearance of orthotopic rat hepatocellular carcinoma by nanosecond pulsed electric fields. Eur J Cancer 2014;50:2705–2713 [DOI] [PubMed] [Google Scholar]
- 51.Nuccitelli R, Tran K, Lui K, et al. Non-thermal nanoelectroablation of UV-induced murine melanomas stimulates an immune response. Pigment Cell Melanoma Res 2012;25:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skeate JG, Da Silva DM, Chavez-Juan E, et al. Nano-pulse stimulation induces immunogenic cell death in human papillomavirus-transformed tumors and initiates an adaptive immune response. PLoS One 2018;13:e0191311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Chen Y, Jiang J, et al. Nano-pulse stimulation (NPS) ablate tumors and inhibit lung metastasis on both canine spontaneous osteosarcoma and murine transplanted hepatocellular carcinoma with high metastatic potential. Oncotarget 2017;8:44032–44039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nuccitelli R, Wood R, Kreis M, et al. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: Proof of method. Exp Dermatol 2014;23:135–137 [DOI] [PMC free article] [PubMed] [Google Scholar]