Abstract
Voltage-gated potassium channels are transmembrane proteins that allow flow of potassium across the membrane to regulate ion homeostasis, cell proliferation, migration, cell volume, and specific processes such as muscular contraction. Aberrant function or expression of potassium channels can underlie pathologies ranging from heart arrhythmia to cancer; the expression of potassium channels is altered in many types of cancer and that alteration correlates with malignancy and poor prognosis. Targeting potassium channels therefore constitutes a promising approach for cancer therapy. In this review, we discuss strategies to target a particular family of potassium channels, the voltage-gated potassium channels (KV) where a reasonable structural understanding is available. We also discuss the possible obstacles and advantages of such a strategy.
Keywords: potassium channels, immunotherapy, antibodies, cancer
Introduction
During transformation, cancer cells acquire characteristics that provide improved survival rates and better defense against different hostile environments and death stimuli.1 For example, overexpression of oncogenes and the inhibition of tumor suppressor proteins allow aberrant regulation in the signal pathways that orchestrate cellular processes, such as proliferation, metabolism, cell death resistance, migration, and invasion. For decades, the main strategy for cancer treatment has been to defeat the fast proliferation of tumor cells, that is, to target only one of these aberrant processes.
Currently, better understanding of cell signaling regulation in cancer is leading to a paradigm shift, with new tools and new therapeutic targets, allowing researchers to design new strategies to treat cancer in a safer and more efficacious way. In recent years, it has become increasingly clear that many ion channels are aberrantly expressed in cancer cells and that they are crucial in the regulation of transformation, migration, proliferation, and invasion.2–6 Because ion channels are typically plasma membrane proteins, functional regions are accessible from the outside; thus, there is growing interest in designing a therapy using antibodies that block activity. Treatments that block expression are also becoming a focus in cancer treatment research. Here, we summarize recent findings, focusing on a particular family, the voltage-gated potassium channels (KV).
Potassium Channel Function and Regulation
KV channels allow transport of potassium across the plasma membrane in response to the electrochemical gradient.4 They are transmembrane proteins that belong to the structurally related voltage-gated ion channel superfamily.7 This group encompasses the products of 40 different genes and is divided into 12 subfamilies. Four monomers surround the permeation pathway, with each monomer having six transmembrane segments (termed S1–S6) and a pore loop between the S5 and S6. Both N- and C-termini are intracellular (with one exception, KCa1.1, which has an extracellular amino terminus and an additional transmembrane segment, S0); therefore, only three loops (S1–S2, S3–S4, and part of S5–S6) protrude into the extracellular milieu (frequently termed E1, E2, and E3).
For a long time, KV channels were studied almost exclusively from the point of view of their functions as modulators of excitability and controllers of membrane potential in excitable cells of the nervous and muscular systems. In these cells, potassium efflux causes hyperpolarization that counteracts the depolarization induced by sodium or calcium channels. Nevertheless, it is now generally acknowledged that KV channels are fundamental for many other natural cell processes, either dependent or independent of cellular ionic homeostasis. Events as crucial as cell proliferation, control of cell volume, or cell adhesion depend on the function of KV channels.8–12
As anticipated, due to the canonical roles of potassium channels, most identified mutations in KV channels lead to altered excitability of neurons and muscle cells. Loss-of-function mutations can cause hyperexcitability leading to epilepsy or to cardiac arrhythmias such as long QT syndrome (LQT; e.g., KV7.113). Mutations in KV7.2 or KV7.3 channels are correlated with neonatal epilepsy and benign familial neonatal convulsions,14,15 whereas KV7.4 is correlated with deafness16 (autosomal dominant 2a type). Aberrant function of members of the Kv1 family, in particular KV1.3 and KV1.5, correlates with central neural system disorders,17 atrial fibrillation,18 and immune abnormalities19–21; whereas loss of function or mutations in KV1.1 and KV1.2 induce convulsions, ataxia episodes, and myokymia disorders,22,23 and mutations in KV11.1 cause Type 2 LQT.24,25 The exquisite equilibrium between ion channels required for proper function of excitable cells is also reflected in the pathological effects of gain-of-function mutations. For example, such mutations in KV7.1 cause familial atrial fibrillation, short QT syndrome, or type 2 diabetes mellitus.26 Similarly, excessive activity of KV10.1 underlies developmental defects that occur with seizures and cognitive impairment, such as Temple–Baraitser and Zimmermann–Laband syndromes27–31; interestingly, excess of function of other potassium channels (SK3, KCa2.3) has been identified in other patients with Zimmermann–Laband syndrome.32
In some cases, a loss of ion selectivity as a result of a mutation can be the basis for pathological phenotypes. Although not reported for KV channels, this mechanism has been described for Kir3.4. It has been shown that a mutation in the structure of this channel results in loss of selectivity for K+ and a higher permeability for Na+, inducing severe aldosteronism33; nevertheless, the intimate molecular mechanism is still unclear.
Potassium Channels in Cancer
During growth and transformation, cancer cells survive in hostile environments and are able to invade surrounding tissue and to migrate from one tissue to another. These changes can be the result of an atypical phenotype caused by genetic and/or aberrant protein expression. Understanding how cancer cells work, the regulation of cellular processes involved in tumor development, and which proteins are involved in these processes, could help us to design new therapies with high affinity for tumor cells.
Altered potassium channel expression leads to modifications that can help the development and growth of virtually every tumor type, even those as apparently unrelated as medulloblastoma, gliomas, adrenal or breast cancer, and their blockage can therefore reduce tumor growth. The specific mechanisms leading to such a role in tumorigenesis are still under investigation and can vary from their role in volume regulation during mitosis34 to interactions with cell adhesion molecules and their signaling pathways.35 In most cases, it is not completely understood how cellular potassium channels undergo remodeling during proliferation, nutrient starvation, angiogenesis, migration, or invasion. The reader can find detailed recent reviews on these aspects elsewhere.6
Even for the case of those KV channels where a therapeutic approach has explicitly been proposed, the mechanistic knowledge is still incomplete. It has been shown that the expression of two members of the KV1 family, KV1.3 and KV1.5, channels activates proliferation, migration, and invasion36–38; both KV channels regulate crucial functions in leukocytes, such as cell proliferation, activation, migration, and apoptosis. KV1.3 is expressed in solid tumors such as breast, colon, lung, glioma, muscle, and prostate cancers39 and is downregulated in some types of leukemia. Nevertheless, its correlation with malignancy is still not clear. KV1.3 expression levels change during cancer development; in particular, it is expressed in early stages and downregulated in high-grade cases of prostate cancer.40 In contrast, its expression is increased in higher stage breast cancer compared with low-grade tumors.41 In fact, the available information about KV channels in cancer development suggests that this is a common phenomenon, and their correlation with malignance and poor prognosis depends on the stage of the disease and the type of tissue in most cases.
Until now, the role that KV1.3 channel plays in cancer development is mechanistically obscure. In some cases, KV1.3 is related to proliferation and cell death, but for some others, it is related to migration and adhesion. It is important to mention that KV1.3 is also expressed on the outer mitochondria membrane, where it regulates apoptosis, and its function in cancer could therefore be more related to cell survival.42 KV1.5 is expressed frequently in medulloblastoma43–45 and is correlated with aggressiveness for non-Hodgkin's lymphoma.46
Members of the KV3 family are also involved in cancer development. KV3.4 and KV3.1 are sensitive to hypoxia, a common condition in solid tumors; they are highly expressed in precancerous lesions and in oral squamous cell carcinoma compared with normal oral mucosa; KV3.4 is upregulated in other head and neck cancers as well.40,47,48 It has been shown that KV3.4 and KV3.1 are involved in metastasis and invasion processes in A549 and MDA-MB-231 cells; an increased expression of KV3.4 and KV3.1 correlates with proliferation, metastasis, and invasion. The expression of both KV channels is regulated in a cell density-dependent manner, and the pattern is similar to those of hypoxia-inducible factor-1 (HIF-1) and reactive oxygen species.49 KV3.4 expression is also implicated in the survival strategies of irradiated leukemia cells.50
Another potassium channel related to oxygen homeostasis is KV11.1; in this case, by the activation of PI3K/Akt pathway, which induces HIF and vascular endothelial growth factor to promote gastric tumor progression.51 It is overexpressed in human primary cancers such as leukemia, esophageal, endometrial, pancreatic, colorectal, ovarian, and brain cancers52–54; it is also expressed in precancerous lesions of the esophagus, and it has a correlation with malignant progression for adenocarcinoma. Blockage of KV11.1 results in the inhibition of cell growth, angiogenesis, and metastasis.51,55 Nevertheless, KV11.1 has recently been correlated with glucose transporter 1 (GLUT-1), but in this case, its absence represents a negative prognostic factor for colorectal adenocarcinoma. The combination of KV11.1 and GLUT-1 deregulation can be used to identify the prostate cancer stage.56
Abnormal expression of KV10.1 is particularly frequent, being found in >70% of all types of cancer, and it can facilitate transformation and favor tumor progression.57–70 KV10.1 regulates proliferation processes in cancer cells,71 and due to its very restricted expression pattern (KV10.1 is only detected in neural tissue and cancer cells), it may represent a promising oncological target.72,73 KV10.1 stimulates vascularization through HIF activation,74,75 but it also promotes migration,76 is related to epithelial to mesenchymal transition,77 and is required for proper primary cilium disassembly and cell cycle progression after G2.78
Other potassium channels related to cancer are KV9.3 and KV2.1, correlated with cell cycle regulation, and KV4.1, related to proliferation. However, the mechanisms through which KV channels are regulating all these processes are still unclear.79,80
Antibodies Targeting KV Channels for Cancer Treatment
Based on their functional profile, KV channels constitute a very attractive target for cancer therapy: accessible, differentially expressed in cancer cells, and functionally related to pathogenesis. Nevertheless, exploiting these features is challenging. Although a large number of natural KV channel modulators are known, lack of selectivity is a major concern with the vast majority. The modulators usually belong to one of three major categories: metal ions, organic small molecules, and venom-derived peptides. Peptides and peptidomimetics are important candidates as potent and specific inhibitors,81 but lack of selectivity limits the applicability of the first two groups.
An alternative approach to circumvent this problem would be to take advantage of the exquisite selectivity of antibodies, which have been applied successfully to other target classes; in fact, there is a growing tendency toward investigation and application of antibodies to treat neurological diseases and cancer. Besides their high affinity and specificity, antibodies offer pharmacokinetic features that can be advantageous in a therapeutic setting. Avidity for the antigen results in the antibody being concentrated where the targets are found, and the plasma half-life is also high. Moreover, their activity is not limited to the particular effect of biding to the antigen since they can elicit immunological responses against the target cells. Furthermore, antibodies can be engineered to reduce antigenicity, manipulate biodistribution, and improve their immunogenic properties.82
Nevertheless, to date, only one ion channel antibody-based therapy has reached the level of clinical trials; this is a polyclonal sheep antibody against a nonfunctional form of P2X7, which is being evaluated for topical treatment of basal cell carcinoma.81,83
The selectivity and specificity of antibodies against KV channels can be also used for diagnostic and prognostic purposes, but we will limit our description to therapeutic strategies in cancer. The therapeutic potential of antibodies against ion channels in general has recently been exhaustively reviewed elsewhere.84,85 Of note, antibodies can modulate ion channels without directly inhibiting their function, for example, by inducing channel internalization as it is the case for KCNK986 or the calcium entry channel Orai1.87 Here, we focus on examples where inhibitory antibodies against KV channels relevant for cancer pathology have been generated, and we discuss possible causes and solutions for the low success rate in generating functional antibodies.
The simplest and most widely used approach for antibody generation, using peptide immunization, has shown limited success with ion channels. It seemed straightforward to obtain polyclonal antibodies able to inhibit KV channels. For example, KV1.3-BA and KV3.1-BA, rabbit polyclonal antibodies with high affinity, showed ability to reduce whole-cell current in HEK-293 and NG108-15 cells.88 KV3.1 has been correlated with important cellular processes such as proliferation and migration49 in oligodendrocyte progenitor cells in the nervous system. The use of antibodies derived from rabbit specific for KV3.1 inhibits proliferation and migration of oligodendrocytes.89 However, the limited availability and the limited suitability for modifications of rabbit polyclonal antibodies preclude seeking evidence that these agents have the desired effect in cancer cells. Similarly, monoclonal antibodies that specifically recognize individual KV channels are commonly available, but monoclonal functional antibodies are more elusive.
The selection of the antigen to obtain monoclonal antibodies able to inhibit ion channels is one of the major hurdles in the process. Several factors contribute to this difficulty, among others the high degree of conservation between channels at the extracellular loops. Over a decade ago, our laboratory took advantage of the (at the time only inferred, but then confirmed by cryo-EM investigations90,91) peculiar structure of the KCNH family of channels (KV10.x, KV11.x, and KV12.x), which show a particularly large extracellular loop between the transmembrane segments S5 and S6 (i.e., E3), immediately beside the selectivity filter.
Because KV10.1 shows a remarkably selective expression in cancer cells, and the overexpression of KV10.1 has been documented in 70% of all types of human solid tumors,2,4 the clinical potential of targeting this channel is high. Its inhibition reduces tumor growth in vivo, highlighting its potential as a therapeutic target in cancer. We therefore set out to produce a monoclonal antibody able to inhibit KV10.1 in intact cells while preserving KV10.2 and KV11 channels. The latter (most frequently termed HERG) poses a significant safety concern because its inhibition underlies a majority of drug-induced long QT events, which can produce malignant arrhythmia and sudden death. The antibody mAb56 was designed and obtained by immunization of mice using a fusion protein that incorporated the E3 region and the tetramerizing coiled-coil domain of KV10.1, followed by standard hybridoma methodology. mAb56 has a high affinity for KV10.1, and it is not able to bind to KV10.2. It inhibits KV10.1 currents in heterologous and native systems, and it has been demonstrated that mAb56 antibody is capable of inhibiting tumor growth, both in xenograft models of MDA-MB-435S human melanoma cells and in xenografted human pancreatic tumor fragments. Mice treated with a loading dose of 50 mg/kg of the antibody (once measurable tumors had developed), followed by 35 mg/kg weekly,92 showed reduced tumor progression in the range of 30–40%. The use of a control anti-KV10.1 antibody with an intracellular epitope did not modify tumor growth.
This approach has not been successful for other channels. A second look at the structures of both ion channels and antibodies, as sketched in Figure 1, offers an initial explanation for this difficulty. The image depicts KV10.1, which is actually a relatively large KV family member, and it is evident that monoclonal antibodies are very large molecules in comparison with ion channels; even moreso if one only takes into account the part of the protein exposed to the extracellular milieu. It is not easy to imagine how such a large molecule as an antibody could get deep enough into the extracellular funnel that leads to the selectivity filter to noticeably interfere with the current. The initial idea when generating the immunogen in Ref.92 was to obtain an antibody that binds close to the channel pore and thereby interferes with ion permeation. Nevertheless, another antibody resulting from the same screening (mAb62), which binds to the same region, 20 residues upstream in the sequence (Fig. 2), did not show any effects on ionic permeation through KV10.1, which indicates that it is unlikely that the mechanism of inhibition is a direct interference with ion permeation in the channel.
FIG. 1.
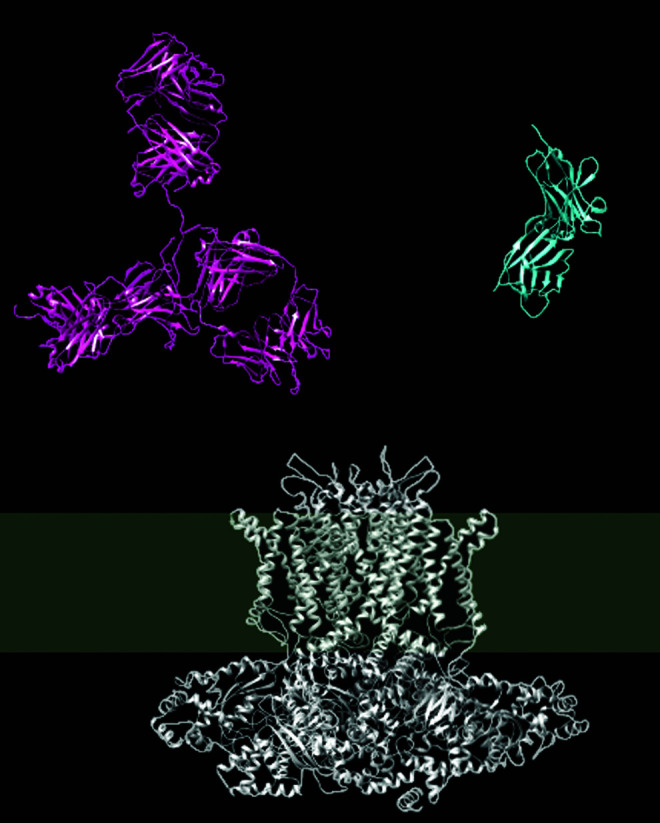
Comparison of the structures of an immunoglobulin (red; IgG1, PDB1HZH) and KV10.1 (white; PDB5K7L). A single-domain antibody (nanobody, blue; PBD5SV4) is depicted for comparison. The gray band indicates the approximate thickness of the membrane.
FIG. 2.
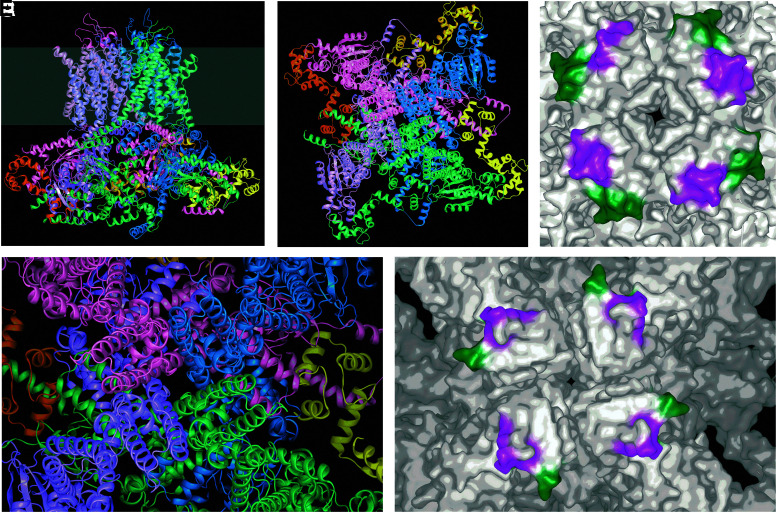
(A) Cryo-EM structure of KV10.1 (PDB ID 5k7l) represented as a side view of the tetramer; each monomer is indicated with a different color, and the gray band indicates the plasma membrane (extracellular is represented on the upper part). (B) Depicts the channel seen from the extracellular side at the same scale. (C) and (D) Zoom onto the pore area where mAb56 binds; in the surface representation (D), the epitopes for the blocking antibody (mAb56, magenta) and the nonblocking antibody mAb62 (green) are indicated. Note that both face away from the permeation pathway in the center of the structure. In the structure of the open KV11.1 (E; PDB ID 5va1), the corresponding regions still face toward the periphery of the channel.
The resolution of the structure of KV10.1 confirmed the initial suspicion, as the epitope for mAb56 lies far apart from the actual permeation pathway (Fig. 2), and is actually rather close to the epitope for mAb62 (Fig. 2D). The available structure corresponds to a channel in the activated state, but it has the pore gate closed through interaction with calmodulin. It is therefore possible that in the open channel the epitopes face toward the pore. Nevertheless, comparison with the structure of KV11.1, which is in the open state (Fig. 2E), reveals that the homologous regions still face outward with the gate open. We should point out that this is only an approximation, because parts of the relevant regions are missing in the structure of KV11.1, and the homology in the area is relatively low; this is actually what makes antibody selectivity possible.
Among the KV channels under study for therapeutic purposes, KV1.3 is likely the best studied case because of its relevance for T cell activation and its potential for the management of immune diseases. As a first approach, the blockage of KV1.3 with antibodies was conducted for autoimmune and inflammatory diseases such as ulcerative colitis, psoriasis, and type 1 diabetes. A program by Ablynx (a Belgian company now part of Sanofi) used DNA immunization to generate nanobodies, single domain antibodies endogenously found in camelids that are much smaller than regular mAbs. Nanobodies were able to block KV1.3 with low nanomolar affinity and at 10,000-fold higher binding to the closest KV1 family members, sodium channels, or KV11.1 (HERG). Interestingly, the predominant epitope appears to be the first extracellular loop rather than the pore region.93
A multi-platform approach using purified KV1.3 expressed in Tetrahymena thermophila at high levels and reconstituted into liposomes succeeded in generating several functional antibodies, both from chicken (by immunization) and from llama (by a phage display approach), although immunization with whole cells did not produce functional antibodies. The epitopes for the functional antibodies obtained in that study appear to be distributed across all three extracellular loops and are likely nonlinear, further indicating that the mechanism of inhibition exerted by antibodies is more likely to be due to interference with voltage-dependent gating rather than by blocking ionic permeation. The fact that only 1 in 19 nanobodies was functional, compared with 9 of 40 (50 if whole cell immunization is also considered), could also point to this notion, since nanobodies are much smaller but also monovalent and therefore are not able to bind the epitope in 2 of the subunits, thereby interfering with their movements relative to each other.
An alternative approach is the fusion of known active peptides (i.e., toxins) to the complementarity-determining regions of a known antibody specific to a particular channel. This has been carried out successfully in the case of KV1.3,94 resulting in a more powerful blockade by the toxin, although it is still unclear if the selectivity achieved comes from the antibody, from the toxin or both.
Despite the difficulty of obtaining functional antibodies, the differential expression of KV channels in tumor cells offers the possibility of targeting cancer cells based on the surface expression of these channels. Binding or blocking the channels is often not sufficient to eliminate the target cell; therefore, the antibody might better be used as a carrier for an active moiety, such as a cytotoxic drug or a cytokine. We could, for instance, find that a bifunctional antibody consisting of a fusion between an anti-KV10.1 single-chain variable fragment (scFv) antibody and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) (scFv62-TRAIL; Fig. 3) is able to induce apoptosis not only in tumor cells expressing KV10.1 on their surface but also on the surrounding tumor cells, while leaving nontumor cells unaffected.95
FIG. 3.
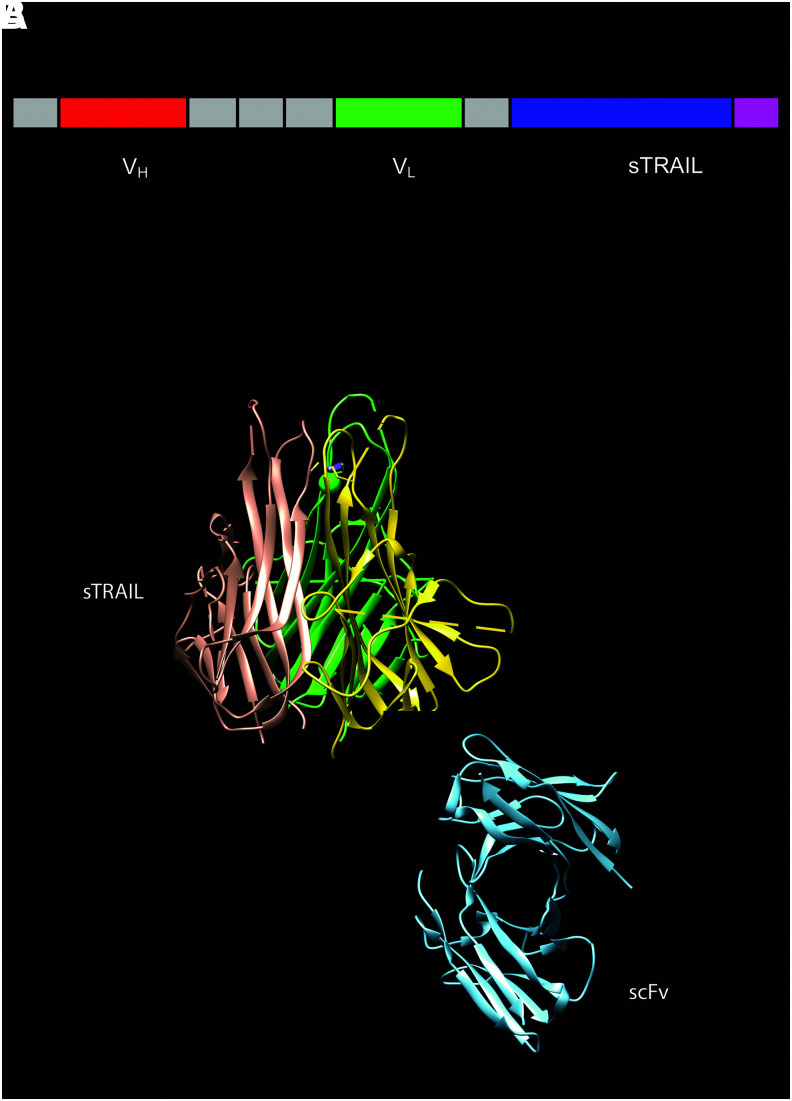
(A) Structure of the scFv62-TRAIL construct. The fusion consists of an scFv (the variable regions of both heavy [red] and light [green] chains bound by a flexible linker) and the full sequence of soluble TRAIL (purple). The cytokine drives formation of trimers. (B) The structures of a TRAIL trimer (PDB 1DG6) and an scFv monomer (PDB 14PI, tones of blue) are depicted. This is not a structural model of the construct; the molecules have been drawn together only to give an idea of the size of the trimeric construct.
The use of scFv62-TRAIL, in combination with the drugs etoposide, roscovitine, and doxorubicin, results in an increase of apoptosis in cancer cells with high endogenous expression of KV10.1,73 providing evidence of the use of antibodies that target potassium channels in cancer as a new and efficient therapy proposal. The effectiveness of combined targeting of a potassium channel and TRAIL can add an additional level of efficacy and specificity to the therapeutic strategy. Although not directly tested for KV channels, blockade of KCa3.1 by TRAM-34 sensitized melanoma cells to TRAIL treatment.96 It is therefore plausible that the fusion of TRAIL to a functional antibody increases the potency of the combined molecule.96
Because KV11.1 (HERG) is an attractive cancer target, its specific blockade in cancer cells would be of interest for therapeutic purposes, but manipulating its activity can result in a high risk of cardiotoxicity. In 2013, the group of Annarosa Arcangeli in Florence synthesized a conjugated of dicarboxylic acid-terminated PEG TiO2 nanocrystals (PEG-TiO2 NPs) and anti-Kv11.1 to obtain KV11.1-Mab-PEG-TiO2 NPs.97 This modified antibody recognizes the KV11.1 channel with high specificity in in vitro and in vivo models of pancreatic adenocarcinoma cancer cells89 but does not alter the current nor cause cytotoxicity in cancer cells. Despite its high specificity for KV11.1, this antibody did not cause cytotoxicity in cancer cells; however, it could be used for therapeutic purposes by exploiting the photocatalytic features of TiO2 with the ensuing ROS production98 that would occur upon illumination, or by introducing other new moieties or cytotoxic drugs.
Conclusion
The increasing body of knowledge on both the occurrence and the consequences of the expression of KV channels in tumors, together with the very detailed information on structure and function of these molecules and the possibility of detailed molecular studies, makes this family of channels an attractive candidate for the design of personalized therapies for oncological diseases. Although the attempts to generate blocking monoclonal antibodies using conventional approaches have shown a limited success, the insight that structural studies have provided in the last few years makes it possible to design alternative strategies with higher chances of success. This, in combination with the power of protein engineering approaches, allows researchers to combine the advantageous features of antibodies in their many forms with useful features of toxins, cytokines, and other active moieties and will certainly result in improved therapy alternatives in the not too distant future.
Authors' Contribution
All authors contributed to designing the study, collecting and evaluating the information, and writing the article. All co-authors have reviewed and approved the article before submission. The article has been submitted solely to this Journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funded by the Max Planck Society and a postdoctoral fellowship from the Mexican Consejo Nacional de Ciencia y Tecnología (CONACyT) to IHR (CVU, 290596).
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674 [DOI] [PubMed] [Google Scholar]
- 2.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 2009;8:982–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuhmer W, Pardo LA. K(+) channels as therapeutic targets in oncology. Fut Med Chem 2010;2:745–755 [DOI] [PubMed] [Google Scholar]
- 4.Pardo LA, Stühmer W. The roles of K+ channels in cancer. Nat Rev Cancer 2014;14:39–48 [DOI] [PubMed] [Google Scholar]
- 5.Urrego D, Tomczak AP, Zahed F, et al. Potassium channels in cell cycle and cell proliferation. Philos Trans Royal Soc B 2014;369:20130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol Rev 2018;98:559–621 [DOI] [PubMed] [Google Scholar]
- 7.Alexander SP, Striessnig J, Kelly E, et al. The concise guide to pharmacology 2017/18: Voltage-gated ion channels. Br J Pharmacol 2017;174Suppl 1:S160–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isom LL.The role of sodium channels in cell adhesion. Front Biosci 2002;7:12–23 [DOI] [PubMed] [Google Scholar]
- 9.Becchetti A.Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol 2011;301:C255–C265 [DOI] [PubMed] [Google Scholar]
- 10.Cidad P, Jimenez-Perez L, Garcia-Arribas D, et al. Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler Thromb Vasc Biol 2012;32:1299–1307 [DOI] [PubMed] [Google Scholar]
- 11.Eggermont J, Trouet D, Carton I, et al. Cellular function and control of volume-regulated anion channels. Cell Biochem Biophys 2001;35:263–274 [DOI] [PubMed] [Google Scholar]
- 12.Czarnecki A, Dufy-Barbe L, Huet S, et al. Potassium channel expression level is dependent on the proliferation state in the GH3 pituitary cell line. Am J Physiol Cell Physiol 2003;284:C1054–C1064 [DOI] [PubMed] [Google Scholar]
- 13.Kohling R, Wolfart J. Potassium channels in epilepsy. Cold Spring Harb Perspect Med 2016;6. DOI: 10.1101/cshperspect.a022871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science 1998;279:403–406 [DOI] [PubMed] [Google Scholar]
- 15.Charlier C, Singh NA, Ryan SG, et al. A pore mutation in a novel kqt-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998;18:53–55 [DOI] [PubMed] [Google Scholar]
- 16.Kubisch C, Schroeder BC, Friedrich T, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 1999;96:437–446 [DOI] [PubMed] [Google Scholar]
- 17.Perez-Verdaguer M, Capera J, Serrano-Novillo C, et al. The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin Therap Targets 2016;20:577–591 [DOI] [PubMed] [Google Scholar]
- 18.Ou XH, Li ML, Liu R, et al. Remodeling of Kv1.5 channel in right atria from Han Chinese patients with atrial fibrillation. Med Sci Monit 2015;21:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toldi G, Vasarhelyi B, Kaposi A, et al. Lymphocyte activation in type 1 diabetes mellitus: The increased significance of Kv1.3 potassium channels. Immunol Lett 2010;133:35–41 [DOI] [PubMed] [Google Scholar]
- 20.Nicolaou SA, Neumeier L, Takimoto K, et al. Differential calcium signaling and Kv1.3 trafficking to the immunological synapse in systemic lupus erythematosus. Cell Calcium 2010;47:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist 2005;11:550–562 [DOI] [PubMed] [Google Scholar]
- 22.Zuberi SM, Eunson LH, Spauschus A, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain 1999;122 (Pt 5):817–825 [DOI] [PubMed] [Google Scholar]
- 23.Brew HM, Gittelman JX, Silverstein RS, et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 2007;98:1501–1525 [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Furukawa T, Hirano Y, et al. Voltage-shift of the current activation in HERG S4 mutation (R534C) in LQT2. Cardiovasc Res 1999;44:283–293 [DOI] [PubMed] [Google Scholar]
- 25.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995;80:795–803 [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Zou A, Splawski I, et al. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem 1999;274:10113–10118 [DOI] [PubMed] [Google Scholar]
- 27.Simons C, Rash LD, Crawford J, et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat Genet 2015;47:73–77 [DOI] [PubMed] [Google Scholar]
- 28.Kortüm F, Caputo V, Bauer CK, et al. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat Genet 2015;47:661–667 [DOI] [PubMed] [Google Scholar]
- 29.Fukai R, Saitsu H, Tsurusaki Y, et al. De novo KCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures. J Hum Genet 2016;61:381–387 [DOI] [PubMed] [Google Scholar]
- 30.Mastrangelo M, Scheffer IE, Bramswig NC, et al. Epilepsy in KCNH1-related syndromes. Epileptic Disord 2016;18:123–136 [DOI] [PubMed] [Google Scholar]
- 31.Megarbane A, Al-Ali R, Choucair N, et al. Temple-Baraitser Syndrome and Zimmermann-Laband Syndrome: One clinical entity? BMC Med Genet 2016;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer CK, Schneeberger PE, Kortum F, et al. Gain-of-function mutations in KCNN3 encoding the small-conductance Ca(2+)-activated K(+) channel SK3 cause Zimmermann-Laband Syndrome. Am J Hum Genet 2019;104:1139–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011;331:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang X, Dubuc AM, Hashizume R, et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Gene Dev 2012;26:1780–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becchetti A, Petroni G, Arcangeli A. Ion channel conformations regulate integrin-dependent signaling. Trends Cell Biol 2019;29:298–307 [DOI] [PubMed] [Google Scholar]
- 36.Aissaoui D, Mlayah-Bellalouna S, Jebali J, et al. Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int J Biol Macromol 2018;111:1146–1155 [DOI] [PubMed] [Google Scholar]
- 37.Comes N, Bielanska J, Vallejo-Gracia A, et al. The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol 2013;4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardo LA.Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292 [DOI] [PubMed] [Google Scholar]
- 39.Comes N, Serrano-Albarrás A, Capera J, et al. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta 2015;1848:2477–2492 [DOI] [PubMed] [Google Scholar]
- 40.Abdul M, Hoosein N. Reduced Kv1.3 potassium channel expression in human prostate cancer. J Membr Biol 2006;214:99–102 [DOI] [PubMed] [Google Scholar]
- 41.Jang SH, Kang KS, Ryu PD, et al. Kv1.3 voltage-gated K(+) channel subunit as a potential diagnostic marker and therapeutic target for breast cancer. BMB Rep 2009;42:535–539 [DOI] [PubMed] [Google Scholar]
- 42.Szabo I, Bock J, Grassme H, et al. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc Natl Acad Sci U S A 2008;105:14861–14866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villalonga N, Ferreres JC, Argiles JM, et al. Potassium channels are a new target field in anticancer drug design. Recent Pat Anticanc Drug Discov 2007;2:212–223 [DOI] [PubMed] [Google Scholar]
- 44.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 2012;123:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felipe A, Bielanska J, Comes N, et al. Targeting the voltage-dependent K(+) channels Kv1.3 and Kv1.5 as tumor biomarkers for cancer detection and prevention. Curr Med Chem 2012;19:661–674 [DOI] [PubMed] [Google Scholar]
- 46.Vallejo-Gracia A, Bielanska J, Hernandez-Losa J, et al. Emerging role for the voltage-dependent K+ channel Kv1.5 in B-lymphocyte physiology: Expression associated with human lymphoma malignancy. J Leukoc Biol 2013;94:779–789 [DOI] [PubMed] [Google Scholar]
- 47.Jang SH, Choi C, Hong SG, et al. Silencing of Kv4.1 potassium channels inhibits cell proliferation of tumorigenic human mammary epithelial cells. Biochem Biophys Res Commun 2009;384:180–186 [DOI] [PubMed] [Google Scholar]
- 48.Menendez ST, Rodrigo JP, Allonca E, et al. Expression and clinical significance of the Kv3.4 potassium channel subunit in the development and progression of head and neck squamous cell carcinomas. J Pathol 2010;221:402–410 [DOI] [PubMed] [Google Scholar]
- 49.Song MS, Park SM, Park JS, et al. Kv3.1 and Kv3.4, are involved in cancer cell migration and invasion. Int J Mol Sci 2018;19. DOI: 10.3390/ijms19041061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palme D, Misovic M, Schmid E, et al. Kv3.4 potassium channel-mediated electrosignaling controls cell cycle and survival of irradiated leukemia cells. Pflugers Arch 2013;465:1209–1221 [DOI] [PubMed] [Google Scholar]
- 51.Crociani O, Lastraioli E, Boni L, et al. hERG1 channels regulate VEGF-A secretion in human gastric cancer: Clinicopathological correlations and therapeutical implications. Clin Cancer Res 2014;20:1502–1512 [DOI] [PubMed] [Google Scholar]
- 52.Pillozzi S, Brizzi MF, Balzi M, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 2002;16:1791–1798 [DOI] [PubMed] [Google Scholar]
- 53.Masi A, Becchetti A, Restano-Cassulini R, et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br J Cancer 2005;93:781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith GAM, Tsui HW, Newell EW, et al. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem 2002;277:18528–18534 [DOI] [PubMed] [Google Scholar]
- 55.Banderali U, Belke D, Singh A, et al. Curcumin blocks Kv11.1 (erg) potassium current and slows proliferation in the infant acute monocytic leukemia cell line THP-1. Cell Physiol Biochem 2011;28:1169–1180 [DOI] [PubMed] [Google Scholar]
- 56.Lastraioli E, Bencini L, Bianchini E, et al. hERG1 channels and glut-1 as independent prognostic indicators of worse outcome in stage I and II colorectal cancer: A pilot study. Transl Oncol 2012;5:105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardo LA, del Camino D, Sanchez A, et al. Oncogenic potential of EAG K+ channels. EMBO J 1999;18:5540–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farias LM, Ocana DB, Diaz L, et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res 2004;64:6996–7001 [DOI] [PubMed] [Google Scholar]
- 59.Patt S, Preussat K, Beetz C, et al. Expression of ether a go-go potassium channels in human gliomas. Neurosci Lett 2004;368:249–253 [DOI] [PubMed] [Google Scholar]
- 60.Hemmerlein B, Weseloh RM, de Queiroz FM, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer 2006;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding XW, Luo HS, Jin X, et al. Aberrant expression of Eag1 potassium channels in gastric cancer patients and cell lines. Med Oncol 2007;24:345–350 [DOI] [PubMed] [Google Scholar]
- 62.Ding XW, Yan JJ, An P, et al. Aberrant expression of ether a go-go potassium channel in colorectal cancer patients and cell lines. World J Gastroenterol 2007;13:1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spohr H, Radzun HJ, Stuhmer W, et al. Expression of Eag1 potassium channels in renal cell carcinomas. Pathol Res Pract 2007;203:311–312 [Google Scholar]
- 64.Spitzner M, Martins JR, Soria RB, et al. Eag1 and Bestrophin 1 are up-regulated in fast-growing colonic cancer cells. J Biol Chem 2008;283:7421–7428 [DOI] [PubMed] [Google Scholar]
- 65.Agarwal JR, Griesinger F, Stühmer W, et al. The potassium channel Ether à go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer 2010;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asher V, Khan R, Warren A, et al. The Eag potassium channel as a new prognostic marker in ovarian cancer. Diagn Pathol 2010;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menendez ST, Villaronga MA, Rodrigo JP, et al. Frequent aberrant expression of the human ether à go-go (hEAG1) potassium channel in head and neck cancer: Pathobiological mechanisms and clinical implications. J Mol Med (Berl) 2012;90:1173–1184 [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Zhong D, Fu X, et al. Silencing of Ether a go-go 1 by shRNA inhibits osteosarcoma growth and cell cycle progression. Int J Mol Sci 2014;15:5570–5581 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Sales TT, Resende FFB, Chaves NL, et al. Suppression of the Eag1 potassium channel sensitizes glioblastoma cells to injury caused by temozolomide. Oncol Lett 2016;12:2581–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mello de Queiroz F, Suarez-Kurtz G, Stühmer W, et al. Ether a go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer 2006;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardo LA, Bruggemann A, Camacho J, et al. Cell cycle-related changes in the conducting properties of r-eag K+ channels. J Cell Biol 1998;143:767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Napp J, Pardo LA, Hartung F, et al. In vivo imaging of tumour xenografts with an antibody targeting the potassium channel Kv10.1. Eur Biophys J 2016;45:721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartung F, Pardo LA. Guiding TRAIL to cancer cells through Kv10.1 potassium channel overcomes resistance to doxorubicin. Eur Biophys J 2016;45:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai Q, Wang T, Guo Q, et al. Positive correlation between the expression of hEag1 and HIF-1alpha in breast cancers: An observational study. BMJ Open 2014;4:e005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Downie BR, Sánchez A, Knötgen H, et al. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem 2008;283:36234–36240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hammadi M, Chopin V, Matifat F, et al. Human ether à-gogo K+ channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol 2012;227:3837–3846 [DOI] [PubMed] [Google Scholar]
- 77.Restrepo-Angulo I, Sanchez-Torres C, Camacho J. Human EAG1 potassium channels in the epithelial-to-mesenchymal transition in lung cancer cells. Anticancer Res 2011;31:1265–1270 [PubMed] [Google Scholar]
- 78.Urrego D, Movsisyan N, Ufartes R, et al. Periodic expression of Kv10.1 driven by pRb/E2F1 contributes to G2/M progression of cancer and non-transformed cells. Cell Cycle 2016;15:799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki T, Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Int J Oncol 2004;25:153–159 [PubMed] [Google Scholar]
- 80.Lee J-H, Park J-W, Byun JK, et al. Silencing of voltage-gated potassium channel KV9.3 inhibits proliferation in human colon and lung carcinoma cells. Oncotarget 2015;6:8132–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wulff H, Christophersen P, Colussi P, et al. Antibodies and venom peptides: New modalities for ion channels. Nat Rev Drug Discov 2019;18:339–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Almagro JC, Daniels-Wells TR, Perez-Tapia SM, et al. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front Immunol 2017;8:1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gilbert SM, Gidley Baird A, Glazer S, et al. A phase I clinical trial demonstrates that nfP2X7 -targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol 2017;177:117–124 [DOI] [PubMed] [Google Scholar]
- 84.Hutchings CJ, Colussi P, Clark TG. Ion channels as therapeutic antibody targets. MAbs 2019;11:265–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haustrate A, Hantute-Ghesquier A, Prevarskaya N, et al. Monoclonal antibodies targeting ion channels and their therapeutic potential. Front Pharmacol 2019;10:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun H, Luo L, Lal B, et al. A monoclonal antibody against KCNK9 K+ channel extracellular domain inhibits tumour growth and metastasis. Nat Commun 2016;7:10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox JH, Hussell S, Sondergaard H, et al. Antibody-mediated targeting of the Orai1 calcium channel inhibits T cell function. PLoS One 2013;8:e82944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou BY, Ma W, Huang XY. Specific antibodies to the external vestibule of voltage-gated potassium channels block current. J Gen Physiol 1998;111:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tiwari-Woodruff S, Beltran-Parrazal L, Charles A, et al. K+ channel KV3.1 associates with OSP/claudin-11 and regulates oligodendrocyte development. Am J Physiol Cell Physiol 2006;291:C687–C698 [DOI] [PubMed] [Google Scholar]
- 90.Whicher JR, MacKinnon R. Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 2016;353:664–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, MacKinnon R. Cryo-EM structure of the open human Ether-à-go-go -related K+ channel hERG. Cell 2017;169:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gómez-Varela D, Zwick-Wallasch E, Knötgen H, et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res 2007;67:7343–7349 [DOI] [PubMed] [Google Scholar]
- 93.Delanote V, Janssen D, Van Hoorick D.Characterization of anti-Kv1. 3 Nanobodies® and activity in inflammatory model systems. 12thAnnual Ion Channel Retreat. Vancouver, 2014 [Google Scholar]
- 94.Wang RE, Wang Y, Zhang Y, et al. Rational design of a Kv1.3 channel-blocking antibody as a selective immunosuppressant. Proc Natl Acad Sci U S A 2016;113:11501–11506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hartung F, Stühmer W, Pardo LA. Tumor cell-selective apoptosis induction through targeting of KV10.1 via bifunctional TRAIL antibody. Mol Cancer 2011;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quast S-A, Berger A, Buttstädt N, et al. General sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS One 2012;7:e39290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sette A, Spadavecchia J, Landoulsi J, et al. Development of novel anti-Kv 11.1 antibody-conjugated PEG-TiO2 nanoparticles for targeting pancreatic ductal adenocarcinoma cells. J Nanopart Res 2013;15:2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nosaka Y, Nosaka AY. Generation and detection of reactive oxygen species in photocatalysis. Chem Rev 2017;117:11302–11336 [DOI] [PubMed] [Google Scholar]