Abstract
Selective serotonin reuptake inhibitor (SSRI) drugs, targeting serotonin transport, are widely used. A puzzling and biomedically important phenomenon concerns the persistent sexual dysfunction following SSRI use seen in some patients. What could be the mechanism of a persistent physiological state brought on by a transient exposure to serotonin transport blockers? In this study, we briefly review the clinical facts concerning this side effect of serotonin reuptake inhibitors and suggest a possible mechanism. Bioelectric circuits (among neural or non-neural cells) could persistently maintain alterations of bioelectric cell properties (resting potential), resulting in long-term changes in electrophysiology and signaling. We present new data revealing this phenomenon in planarian flatworms, in which brief SSRI exposures induce long-lasting changes in resting potential profile. We also briefly review recent data linking neurotransmitter signaling to developmental bioelectrics. Further study of tissue bioelectric memory could enable the design of ionoceutical interventions to counteract side effects of SSRIs and similar drugs.
Keywords: SSRI, ion channels, bioelectricity, memory
Introduction: Long-Lasting Effects After Exposure to Serotonin Transporter Inhibitors
Selective Serotonin Reuptake Inhibitor (SSRI) drugs are widely used to treat depression and anxiety. Introduced around 1990, between 10% and 15% of the population of most Western countries now take them.1 Close to 100% of takers of a SSRI have a degree of genital sensory change within 30 min of taking. These effects consist primarily of a reduced sensitivity, often termed “numbing” by those affected but others have genital arousal (irritability). The reduced sensitivity is accompanied by an immediate delay of ejaculation in men and muting of orgasm in both men and women. After a period of treatment, orgasm may stop and there may be a loss of libido.2
The “numbing” effect produced by SSRIs has similarities to the effect of rubbing lidocaine into the genital area, which was a prior treatment for premature ejaculation, and SSRIs in single doses are used for premature ejaculation now. The effect is also described in terms of a loss of pleasurable sensation. In some cases, there is an actual genital numbing equivalent to that produced by lidocaine. These immediate onset sexual effects ordinarily lift when treatment stops. In 2006, reports appeared of a condition now termed Post-SSRI Sexual Dysfunction (PSSD), in which the genital numbing, pleasureless or absent ejaculation/orgasm, and loss of libido remain and may become more pronounced after treatment stops.3,4 PSSD can persist for decades afterward.2,5
In 2001, persistent genital arousal disorder (PGAD), an enduring disorder of irritable genital sensation, was described.6 This condition is not linked to enhanced libido and does not stem from psychological issues. At present PGAD appears to affect women more than men. This condition seems more likely to happen around the menopause and, while closely related to discontinuation from SSRI medication, can also occur following trauma to the genital area.2 These genital effects do not occur on antidepressants that do not inhibit serotonin reuptake; other antidepressants and psychotropic drugs can cause erectile dysfunction but not the syndromes of numbness, pleasureless orgasm, loss of libido, or persistent arousal.
Two other syndromes have been described which appear closely related to PSSD. One is postfinasteride syndrome (PFS). First described in 2011, this occurs in young men taking finasteride to stall hair loss.7 It also happens with other 5-alpha reductase inhibitors—dutasteride and saw palmetto. Genital anesthesia, loss of libido, and sexual dysfunction are features of this syndrome. Initial finasteride treatment can produce some sexual dysfunction, but this is less common than with SSRIs. It is unclear if the sexual dysfunction that appears on treatment is continuous with PFS or distinct from it.
A postretinoid sexual dysfunction (PRSD) has also been described.8 This also includes genital anesthesia, sexual dysfunction, and loss of libido. There can be some sexual dysfunction on initial treatment in patients taking isotretinoin for acne, but it is not clear what continuity there may be between this and PRSD. These enduring post-treatment syndromes may interface with tardive dyskinesia linked to antipsychotic drugs in the 1960s. Antipsychotics can cause dyskinesias on treatment, which ordinarily resolve when treatment is stopped. Dyskinesias can also emerge on withdrawal but clear up in time. Tardive dyskinesia is a syndrome that involves dyskinetic movements centered on the jaw and lower facial area, which can emerge on treatment but become more marked when treatment stops. The syndrome can endure for years or decades afterward.
These legacy effects of antidepressants and antipsychotics have some interface with withdrawal syndromes linked to these drugs. Withdrawal to opioids and alcohol is viewed as limited to a few weeks, having features not found during administration of the drug and as ordinarily responding to reinstitution of treatment. Antidepressant and antipsychotic withdrawal, however, is linked to dysthymia, which may appear continuous with the original problem but can be demonstrated in healthy volunteers given these drugs, as well as to other sensory and autonomic disturbances. These states can last for months or longer, opening up a possible link between enduring sexual syndromes and other legacy effects of antidepressants and antipsychotics.9 There are variations among antidepressants and antipsychotics in their likelihood of causing withdrawal problems and likelihood of causing tardive syndromes, but the basis for these differences is not understood.
PSSD happens in all ages, both sexes, and all ethnic groups. It can begin after a few doses of treatment or only become apparent after years of exposure. There are two issues to account for. One is the original sensory changes. These almost certainly extend beyond the genital area, but are more salient there perhaps because of the functional consequences. SSRIs also produce a more general dampening of reactivity—commonly termed emotional numbing. This may be linked to the pronounced sensory features that characterize the SSRI withdrawal syndrome, which can include spontaneous orgasms and can result in PGAD.
In this study, we hypothesize about a possible mechanism of this effect and provide data in a physiologically-amenable model system consistent with this possibility.
Possible Mechanisms: A Hypothesis
At present, there is no agreement as to how the sensory changes on SSRIs come about. Lidocaine, which also produces genital numbing, appears to do so through an action on late sodium currents,10 and serotonin reuptake inhibitors also have effects on late sodium currents.11 It is also the case that antidepressants with effects on sodium currents are used to treat neuropathic pain.
Aiming at finding a treatment, PSSD sufferers have tried a wide range of agents active on various dopamine and serotonin receptors along with phosphodiesterase inhibitors and other drugs, but these have no therapeutic effect for PSSD, PFS, or PRSD. PFS sufferers have focused on evidence for androgen insensitivity. It is also the case that SSRIs reduce testicular volume and sperm counts, but these effects appear to happen in the absence of PSSD. At present, no endocrine manipulations appear to make a difference in PFS, PSSD, or PGAD.
The treatment approaches adopted to date have been largely targeted at reversing the acute sexual effects rather than reversing the mechanism that leads to enduring effects. This is similar to research efforts on tardive dyskinesia which for four decades have focused on the dopamine system without finding an answer. A second issue therefore is one of pinpointing a mechanism that might underpin enduring effects like these. It does appear that with time (several years) a degree of spontaneous recovery happens in some cases. In other cases, there are brief remissions (days), often triggered by stopping a brief course of another drug such as an antibiotic. There are grounds to think therefore that these enduring effects do not stem from permanent damage.
Is this problem best seen as a physiological (bioelectric) or a pharmacological matter? Is the site at which the original sensory changes are affected central or peripheral? Do they arise in a central nucleus, at the dorsal root ganglion level, or do from local treatment effects on C-fibers? In this study, we explore one possible mechanism: ion channel- and pump-driven circuits that maintain tissue bioelectric state as a kind of persistent physiological memory.
Developmental bioelectricity is a field which studies how cells, neural and non-neural, propagate, store, and process information through the propagation of electrical states—specifically resting potential or Vmem.12–14 Recent work in this field has revealed the importance of bioelectric networks not only in embryogenesis but also in adult regeneration, stem cell biology, cancer, and immune system function.13–27 Importantly, recent computational models predicted that some bioelectric circuits exhibit a kind of memory, in which induced changes of Vmem are actively maintained.28–31
Research in model systems, such as regenerative planarian flatworms (an important model for human neurophysiology and pharmacology32–37), has revealed a remarkable long-term memory that can be induced by alterations to endogenous ion flows. Planaria whose bioelectric circuits are briefly modulated by small molecule drugs experience an alteration in bioelectric patterns within their tissues that are apparently permanent and persistently affect their cell- and tissue-level functions long after the original drugs are withdrawn.38–40 This occurs over many months and is (as far as is known) permanent, despite the rapid turnover of all of their somatic cells within a few weeks.
Importantly, however, the ion channel and gap junction networks that implement bioelectric control circuits are ubiquitous, being present and function across taxa, from bacterial biofilms to mammals.41–52 Effects on cell migration,53 proliferation,54 migration/pathfinding,55,56 and stem cell differentiation57 have been observed after modulation of neurotransmitter pathways. Even the microbiome, with many known roles in regulating mood and functioning of many organ systems, has been affected by neurotransmitter signaling.58,59 Taken together, these data suggest the possibility that persistent behavioral and physiological states, affecting the brain and numerous other organs, could be explained by long-lasting modulation of the bioelectricity-neurotransmitter axis through transient modulation by drugs. Thus, we hypothesized that SSRI application may alter Vmem in vivo, resulting in persistent changes to bioelectric circuit parameters in cells. This could be due to effects on the electrogenic serotonin transporter SERT or perhaps upon one or more of several ion channels.60–64 Because the basic bioelectric circuitry is highly conserved, animal models can serve as an important context within which to understand clinically relevant persistent physiological states induced by transient drug exposure. Thus, we examined the possibility of SSRI-induced long-term changes, which could provide a mechanism for the post-SSRI syndrome in human patients, in a tractable model system: planaria.
Experimental Test: Short-Term SSRI Soak Alters Long-Term Bioelectric Properties of Planarian Tissue
We experimentally examined the possibility of long-term effects of transient SSRI treatment on bioelectric state in intact planarian flatworms. We utilized the SSRI fluoxetine, because it has previously been shown in the literature to impact planarian regeneration.65 The serotonin neurotransmitter system in planaria has been characterized in numerous studies: an ortholog of the gene encoding the enzyme tryptophan hydroxylase which catalyzes the rate-limiting step in serotonin synthesis has been identified in Dugesia japonica,66 planarian serotonin receptors have been characterized,67,68 and multiples studies have found that SSRI treatment impacts planaria in numerous ways, including locomotion, light/dark preference (photophobic tendencies), DNA damage, and regenerative polarity.65,69–71
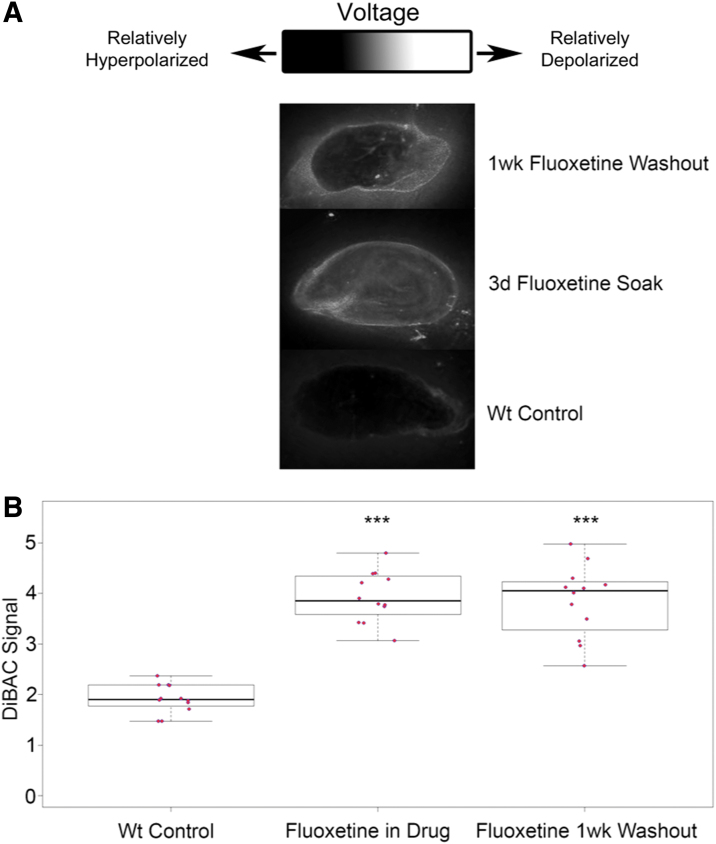
To assess the impact of transient fluoxetine treatment on bioelectric states, intact wild-type D. japonica were soaked in a 2 μM fluoxetine solution for 3 days, at which point the solution was washed out and the samples were placed in Poland Spring water for 1 week at 13°C to prevent fissioning (termed “1wk washout” group). Results are shown in Figure 1. Membrane polarization was then imaged using the voltage-sensitive dye bis-[1,3-dibarbituric acid]-trimethine oxonol [DiBAC4(3)].39,40,72 The 1wk washout group was compared directly to samples, which had been soaking in fluoxetine for 3 days (“fluoxetine soak” group) before imaging, and an age-matched H2O (“control”) group, which was never treated. Both the 1wk washout treated group and the 3d fluoxetine soak group were significantly depolarized relative to the controls (****p < 0.00001, Student's t-test, n = 14), while there were no differences in relative polarization between the 1wk washout group and the fluoxetine soak group (p = 0.16, Student's t-test, n = 14). This indicates that the depolarizing effect of fluoxetine is persistent far after the drug treatment has ended.
FIG. 1.
Fluoxetine exposure results in long-term physiological changes in planaria. (A) Planarian flatworms were exposed to the drug Fluoxetine, washed extensively, and then kept for 1 week in plain water. They were then imaged using a fluorescent voltage reporter dye (see Materials and Methods section) and compared to controls (exposed to vehicle only) or animals after 3 days of continuous fluoxetine exposure. (B) Quantification of the fluorescence signal revealed that even after 7 days in plain water, a brief exposure to Fluoxetine depolarizes the animal as much as does 3 days of continuous exposure, revealing a persistent voltage memory induced by SSRI treatment. SSRI, selective serotonin reuptake inhibitor. ***indicates significance to p < 0.01.
Conclusion
SSRIs' main target is the serotonin transporter, SERT. The well-known role of neurotransmitters in brain function has been suggested73 to be an evolutionary extension of a more ancient and ubiquitous role in developmental (preneural) morphogenetic systems.74–76 A number of neurotransmitter pathways have been identified as functioning in vertebrate development, for example, in the embryogenesis of the face,77 eye,78 and heart,79–87 as well as invertebrate regeneration65 and development.88,89 SERT, and serotonin signaling more broadly, has been identified as being part of bioelectric circuits in prior work. For example, voltage differences in early embryos drive the consistent left-right asymmetry of maternal serotonin molecules, which in turn control lateralized gene expression and visceral organ situs.90–93 Likewise, bioelectric controls of serotonin movement through gap junctions mediate the effect of ion channel drugs on ectopic innervation from transplanted organs94–96 and conversion of cells to a metastatic phenotype.95,97–99 However, prior work placed SERT downstream of voltage changes, and it was not known that SSRIs could also function upstream to alter Vmem of non-neural cells (relevant effects on neurons have been observed however100,101). In this study, we show this in planaria; moreover, the changes are persistent long after the SSRI is withdrawn.
Bioelectric circuits can maintain long-term and stable changes of state after relatively brief alterations of Vmem, and we have previously suggested bioelectric state to be a target of SSRIs and other psychoactive drugs in the context of developmental defects.102 Such alterations can plausibly affect neural (and non-neural) responses such as could be important for human sexual function, either directly on somatic cells or through indirect effects acting through the microbiome, immune system, or brain.12,103–105 Bioelectric memory has not yet been demonstrated in human patient tissues, representing an important area for subsequent work, which could be addressed in vivo and in human organoid systems in vitro.106 Paralleling the development of ion channel modulator drug cocktails, guided by computational models of bioelectric circuits to induce desired pro-regenerative states, it's possible that the negative effects of SSRI exposure could someday be mitigated by rationally designed cocktails of already human-approved drugs acting as ionoceuticals.107,108
Materials and Methods
Planaria husbandry
A clonal colony of D. japonica maintained in Poland Spring water at 13°C was used for the experiments in this study. All samples were starved for >7 days and continued to be starved throughout all experiments to control for metabolic variance in individual planaria. Colony care was performed as described in Oviedo et al.109
DiBAC membrane voltage assay
DiBAC4(3) (Invitrogen, Carlsbad, CA) was utilized to visualize membrane potential in samples. Whole intact samples were treated with a 2 μM fluoxetine solution for 3 days, at which point they were rinsed thrice to remove residual solution and placed in water. One week after the fluoxetine treatment, these samples were imaged along with planaria that had been soaked for 3 days in fluoxetine, as well as H2O controls. Samples were soaked in DiBAC, which had been dissolved in the appropriate solution for the treatment, for half an hour before imaging. Planaria were immobilized using a 2% low-melting point agarose and mounted on microscopy slides using a cold plate. Groups of fluoxetine 1wk washout, fluoxetine in drug, and H2O control samples were mounted in a single slide, ventral side up. Analysis of relative membrane potential was done using the measure function in ImageJ software.
Acknowledgments
The authors thank Catharina Faber for helpful discussions and ideas.
Authors' Contributions
All authors wrote the text; J.L. performed the experiments. All authors have reviewed and approved of the article before submission. The article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
M.L. and J.L. gratefully acknowledge support by an Allen Discovery Center award from the Paul G. Allen Frontiers Group (No. 12171).
References
- 1.Healy D.Psychiatric Drugs Explained. Edinburgh: Elsevier, 2016. [Google Scholar]
- 2.Healy D, Le Noury J, Mangin D. Enduring sexual dysfunction after treatment with antidepressants, 5alpha-reductase inhibitors and isotretinoin: 300 cases. Int J Risk Saf Med 2018;29:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csoka AB, Shipko S. Persistent sexual side effects after SSRI discontinuation. Psychother Psychosom 2006;75:187–188 [DOI] [PubMed] [Google Scholar]
- 4.Bahrick AS.Post SSRI sexual dysfunction. American Society for the Advancement of Pharmacotherapy Tablet, 2006;7:2-3, 10–11. [Google Scholar]
- 5.Healy D.Citizen petition: Sexual side effects of SSRIs and SNRIs. Int J Risk Saf Med 2018;29:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiblum SR, Nathan SG. Persistent sexual arousal syndrome: a newly discovered pattern of female sexuality. J Sex Marital Ther 2001;27:365–380 [DOI] [PubMed] [Google Scholar]
- 7.Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med 2011;8:1747–1753 [DOI] [PubMed] [Google Scholar]
- 8.Hogan C, Le Noury J, Healy D, et al. One hundred and twenty cases of enduring sexual dysfunction following treatment. Int J Risk Saf Med 2014;26:109–116 [DOI] [PubMed] [Google Scholar]
- 9.Healy D, Tranter R. Pharmacological stress diathesis syndromes. J Psychopharmacol 1999;13:287–290 [DOI] [PubMed] [Google Scholar]
- 10.Johannesen L, Vicente J, Mason JW, et al. Late sodium current block for drug-induced long QT syndrome: Results from a prospective clinical trial. Clin Pharmacol Ther 2016;99:214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang GK, Mitchell J, Wang SY. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol 2008;222:79–90 [DOI] [PubMed] [Google Scholar]
- 12.Mathews J, Levin M. The body electric 2.0: Recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr Opin Biotechnol 2018;52:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin KA, Levin M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol 2018;433:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates E.Ion channels in development and cancer. Annu Rev Cell Dev Biol 2015;31:231–247 [DOI] [PubMed] [Google Scholar]
- 15.Tuszynski J, Tilli TM, Levin M. Ion channel and neurotransmitter modulators as electroceutical approaches to the control of cancer. Curr Pharm Des 2017;23:4827–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore D, Walker SI, Levin M. Cancer as a disorder of patterning information: Computational and biophysical perspectives on the cancer problem. Converg Sci Phys Oncol 2017;3:043001 [Google Scholar]
- 17.Chernet B, Levin M. Endogenous voltage potentials and the microenvironment: Bioelectric signals that reveal, induce and normalize cancer. J Clin Exp Oncol 2013;Suppl 1:S1-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobikin M, Chernet B, Lobo D, et al. Resting potential, oncogene-induced tumorigenesis, and metastasis: The bioelectric basis of cancer in vivo. Phys Biol 2012;9:065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C, Levin M, Kaplan DL. Bioelectric modulation of macrophage polarization. Sci Rep 2016;6:21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundelacruz S, Levin M, Kaplan DL. Comparison of the depolarization response of human mesenchymal stem cells from different donors. Sci Rep 2015;5:18279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep 2009;5:231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One 2008;3:e3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbina A, Godoy-Silva R, Hoyos M, et al. Morphological and electrical disturbances after split-flow fractionation in murine macrophages. J Chromatogr A 2019;1590:104–112 [DOI] [PubMed] [Google Scholar]
- 24.Pare JF, Martyniuk CJ, Levin M. Bioelectric regulation of innate immune system function in regenerating and intact Xenopus laevis. NPJ Regen Med 2017;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purnell MC, Skrinjar TJ. Bioelectric field enhancement: The influence on membrane potential and cell migration in vitro. Adv Wound Care (New Rochelle) 2016;5:539–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul Kadir L, Stacey M, Barrett-Jolley R. Emerging roles of the membrane potential: action beyond the action potential. Front Physiol 2018;9:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017;31:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cervera J, Pietak A, Levin M, et al. Bioelectrical coupling in multicellular domains regulated by gap junctions: A conceptual approach. Bioelectrochemistry 2018;123:45–61 [DOI] [PubMed] [Google Scholar]
- 29.Pietak A, Levin M. Bioelectric gene and reaction networks: Computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation. J R Soc Interface 2017;14:20170425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietak A, Levin M. Exploring instructive physiological signaling with the bioelectric tissue simulation engine (BETSE). Front Bioeng Biotechnol 2016;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cervera J, Manzanares JA, Mafe S, et al. Synchronization of bioelectric oscillations in networks of nonexcitable cells: From single-cell to multicellular states. J Phys Chem B 2019;123:3924–3934 [DOI] [PubMed] [Google Scholar]
- 32.Pagan OR, Baker D, Deats S, et al. Planarians in pharmacology: Parthenolide is a specific behavioral antagonist of cocaine in the planarian Girardia tigrina. Int J Dev Biol 2012;56:193–196 [DOI] [PubMed] [Google Scholar]
- 33.Pagan OR, Rowlands A, Fattore A, et al. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol 2009;615:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowlands AL, Pagan OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol 2008;583:170–172 [DOI] [PubMed] [Google Scholar]
- 35.Pagan OR, Rowlands A, Azam M, et al. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav 2008;89:160–170 [DOI] [PubMed] [Google Scholar]
- 36.Rawls SM, Gerber K, Ding Z, et al. Agmatine: Identification and inhibition of methamphetamine, kappa opioid, and cannabinoid withdrawal in planarians. Synapse 2008;62:927–934 [DOI] [PubMed] [Google Scholar]
- 37.Rawls SM, Gomez T, Stagliano GW, et al. Measurement of glutamate and aspartate in Planaria. J Pharmacol Toxicol Methods 2006;53:291–295 [DOI] [PubMed] [Google Scholar]
- 38.Oviedo NJ, Morokuma J, Walentek P, et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 2010;339:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durant F, Bischof J, Fields C, et al. The role of early bioelectric signals in the regeneration of planarian anterior/posterior polarity. Biophys J 2019;116:948–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durant F, Morokuma J, Fields C, et al. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys J 2017;112:2231–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Martinez-Corral R, Prindle A, et al. Coupling between distant biofilms and emergence of nutrient time-sharing. Science 2017;356:638–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DD, Prindle A, Liu J, et al. SnapShot: Electrochemical communication in biofilms. Cell 2017;170:214–214.e1. [DOI] [PubMed] [Google Scholar]
- 43.Humphries J, Xiong L, Liu J, et al. Species-independent attraction to biofilms through electrical signaling. Cell 2017;168:200–209e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prindle A, Liu J, Asally M, et al. Ion channels enable electrical communication in bacterial communities. Nature 2015;527:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belus MT, Rogers MA, Elzubeir A, et al. Kir2.1 is important for efficient BMP signaling in mammalian face development. Dev Biol 2018;444(Suppl 1):S297–S307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RS, Kenny CJ, Ganesh V, et al. Sodium channel SCN3A (NaV1.3) regulation of human cerebral cortical folding and oral motor development. Neuron 2018;99:905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer CK, Calligari P, Radio FC, et al. Mutations in KCNK4 that affect gating cause a recognizable neurodevelopmental syndrome. Am J Hum Genet 2018;103:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masotti A, Uva P, Davis-Keppen L, et al. Keppen-Lubinsky Syndrome Is Caused by Mutations in the inwardly rectifying K(+) channel encoded by KCNJ6. Am J Hum Genet 2015;96:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litan A, Langhans SA. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front Cell Neurosci 2015;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kortüm F, Caputo V, Bauer CK, et al. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat Genet 2015;47:661–667 [DOI] [PubMed] [Google Scholar]
- 51.Côté F, Fligny C, Bayard E, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A 2007;104:329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ori M, De Lucchini S, Marras G, et al. Unraveling new roles for serotonin receptor 2B in development: Key findings from Xenopus. Int J Dev Biol 2013;57:707–714 [DOI] [PubMed] [Google Scholar]
- 53.Gustafson T, Toneby MI. How genes control morphogenesis. Am Sci 1971;59:452–462 [PubMed] [Google Scholar]
- 54.Yuan I, Horng CT, Chen VCH, et al. Escitalopram oxalate inhibits proliferation and migration and induces apoptosis in non-small cell lung cancer cells. Oncol Lett 2018;15:3376–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnin A, Torii M, Wang L, et al. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci 2007;10:588–597 [DOI] [PubMed] [Google Scholar]
- 56.Poopalasundaram S, Chambers D, Graham A, et al. Serotonin receptor 1A (HTR1A), a novel regulator of GnRH neuronal migration in chick embryo. Endocrinology 2016;157:4632–4640 [DOI] [PubMed] [Google Scholar]
- 57.Kruk JS, Bermeo S, Skarratt KK, et al. The effect of antidepressants on mesenchymal stem cell differentiation. J Bone Metab 2018;25:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oleskin AV, Kirovskaia TA, Botvinko IV, et al. Effect of serotonin (5-hydroxytryptamine) on the growth and differentiation of microorganisms [in Russian]. Mikrobiologiia 1998;67:305–312 [PubMed] [Google Scholar]
- 59.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oswald I, Lewis SA, Dunleavy DLF, et al. Drugs of dependence though not of abuse: Fenfluramine and imipramine. Br Med J 1971;3:70–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aldana BI, Sitges M. Sertraline inhibits pre-synaptic Na(+) channel-mediated responses in hippocampus-isolated nerve endings. J Neurochem 2012;121:197–205 [DOI] [PubMed] [Google Scholar]
- 62.Lee HA, Kim KS, Hyun SA, et al. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. Korean J Physiol Pharmacol 2012;16:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HS, Li H, Kim HW, et al. Selective serotonin reuptake inhibitor sertraline inhibits voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. J Biosci 2016;41:659–666 [DOI] [PubMed] [Google Scholar]
- 64.Lee HM, Hahn SJ, Choi BH. Blockade of Kv1.5 channels by the antidepressant drug sertraline. Korean J Physiol Pharmacol 2016;20:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan JD, Agbedanu PN, Zamanian M, et al. ‘Death and axes’: Unexpected Ca(2+) entry phenologs predict new anti-schistosomal agents. PLoS Pathog 2014;10:e1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishimura K., Kitamura Y, Inoue T, et al. Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesia japonica. Neurosci Res 2007;59:101–106 [DOI] [PubMed] [Google Scholar]
- 67.Saitoh O, Yuruzume E, Nakata H. Identification of planarian serotonin receptor by ligand binding and PCR studies. Neuroreport 1996;8:173–178 [DOI] [PubMed] [Google Scholar]
- 68.Creti P, Capasso A, Grasso M, et al. Identification of a 5-HT1A receptor positively coupled to planarian adenylate cyclase. Cell Biol Int Rep 1992;16:427–432 [DOI] [PubMed] [Google Scholar]
- 69.Ofoegbu PU, Lourenço J, Mendo S, et al. Effects of low concentrations of psychiatric drugs (carbamazepine and fluoxetine) on the freshwater planarian, Schmidtea mediterranea. Chemosphere 2019;217:542–549 [DOI] [PubMed] [Google Scholar]
- 70.Cho M, Nayak SU, Jennings T, et al. Predator odor produces anxiety-like behavioral phenotype in planarians that is counteracted by fluoxetine. Physiol Behav 2019;206:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welsh JH, Williams LD. Monoamine-containing neurons in planaria. J Comp Neurol 1970;138:103–115 [DOI] [PubMed] [Google Scholar]
- 72.Oviedo NJ, Nicolas CL, Adams DS, et al. Live imaging of planarian membrane potential using DiBAC4(3). CSH Protoc 2008;2008:pdb..prot5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fields C, Bischof J, Levin M. Morphological coordination: Unifying neural and non-neural signaling. Physiology 2020;35:16–30 [DOI] [PubMed] [Google Scholar]
- 74.Levin M, Buznikov GA, Lauder JM. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci 2006;28:171–185 [DOI] [PubMed] [Google Scholar]
- 75.Buznikov GA, Peterson RE, Nikitina LA, et al. The pre-nervous serotonergic system of developing sea urchin embryos and larvae: Pharmacologic and immunocytochemical evidence. Neurochem Res 2005;30:825–837 [DOI] [PubMed] [Google Scholar]
- 76.Buznikov G, Shmukler Y, Lauder J. From oocyte to neuron: Do neurotransmitters function in the same way throughout development? Cell Mol Neurobiol 1996;16:537–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan KG, Levin M. Neurotransmitter signaling pathways required for normal development in Xenopus laevis embryos: A pharmacological survey screen. J Anat 2016;229:483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Lucchini S, Ori M, Cremisi F, et al. 5-HT2B-mediated serotonin signaling is required for eye morphogenesis in Xenopus. Mol Cell Neurosci 2005;29:299–312 [DOI] [PubMed] [Google Scholar]
- 79.Ghavamabadi RT, Taghipour Z, Hassanipour M, et al. Effect of maternal fluoxetine exposure on lung, heart, and kidney development in rat neonates. Iran J Basic Med Sci 2018;21:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vichier-Guerre C, Parker M, Pomerantz Y. Impact of selective serotonin reuptake inhibitors on neural crest stem cell formation. Toxicol Lett 2017;281:20–25 [DOI] [PubMed] [Google Scholar]
- 81.Sari Y, Zhou FC/Serotonin and its transporter on proliferation of fetal heart cells. Int J Dev Neurosci 2003;21:417–424 [DOI] [PubMed] [Google Scholar]
- 82.Nebigil CG, Maroteaux L. A novel role for serotonin in heart. Trends Cardiovasc Med 2001;11:329–335 [DOI] [PubMed] [Google Scholar]
- 83.Nebigil CG, Hickel P, Messaddeq N, et al. Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation 2001;103:2973–2979 [DOI] [PubMed] [Google Scholar]
- 84.Nebigil CG, Etienne N, Schaerlinger B, et al. Developmentally regulated serotonin 5-HT2B receptors. Int J Dev Neurosci 2001;19:365–372 [DOI] [PubMed] [Google Scholar]
- 85.Yavarone MS, Shuey DL, Tamir H, et al. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology 1993;47:573–584 [DOI] [PubMed] [Google Scholar]
- 86.Shuey DL, Sadler TW, Tamir H, et al. Serotonin and morphogenesis. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol 1993;187:75–85 [DOI] [PubMed] [Google Scholar]
- 87.Shuey DL, Yavarone M, Sadler TW, et al. Serotonin and morphogenesis in the cultured mouse embryo. Adv Exp Med Biol 1990;265:205–215 [DOI] [PubMed] [Google Scholar]
- 88.Colas JF, Launay JM, Vonesch JL, et al. Serotonin synchronises convergent extension of ectoderm with morphogenetic gastrulation movements in Drosophila. Mech Dev 1999;87:77–91 [DOI] [PubMed] [Google Scholar]
- 89.Colas JF, Launay JM, Kellermann O, et al. Drosophila 5-HT2 serotonin receptor: Coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci U S A 1995;92:5441–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol 2005;15:794–803 [DOI] [PubMed] [Google Scholar]
- 91.Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci 2005;27:349–363 [DOI] [PubMed] [Google Scholar]
- 92.Adams DS, Robinson KR, Fukumoto T, et al. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 2006;133:1657–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vandenberg LN, Lemire JM, Levin M. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis Model Mech 2012;6:261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blackiston DJ, Vien K, Levin M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. NPJ Regen Med 2017;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lobikin M, Lobo D, Blackiston DJ, et al. Serotonergic regulation of melanocyte conversion: A bioelectrically regulated network for stochastic all-or-none hyperpigmentation. Sci Signal 2015;8:ra99. [DOI] [PubMed] [Google Scholar]
- 96.Blackiston DJ, Anderson GM, Rahman N, et al. A novel method for inducing nerve growth via modulation of host resting potential: Gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics 2015;12:170–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lobo D, Lobikin M, Levin M. Discovering novel phenotypes with automatically inferred dynamic models: A partial melanocyte conversion in Xenopus. Sci Rep 2017;7:41339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lobikin M, Paré JF, Kaplan DL, et al. Selective depolarization of transmembrane potential alters muscle patterning and muscle cell localization in Xenopus laevis embryos. Int J Dev Biol 2015;59:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blackiston D, Adams DS, Lemire JM, et al. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech 2011;4:67–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakatani Y, Amano T. Functional modulation of Nav1.2 voltage-gated sodium channels induced by escitalopram. Biol Pharm Bull 2018;41:1471–1474 [DOI] [PubMed] [Google Scholar]
- 101.Hong DH, Li H, Kim HS, et al. The effects of the selective serotonin reuptake inhibitor fluvoxamine on voltage-dependent K(+) channels in rabbit coronary arterial smooth muscle cells. Biol Pharm Bull 2015;38:1208–1213 [DOI] [PubMed] [Google Scholar]
- 102.Hernandez-Diaz S, Levin M. Alteration of bioelectrically-controlled processes in the embryo: A teratogenic mechanism for anticonvulsants. Reprod Toxicol 2014;47:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Norris GT, Kipnis J. Immune cells and CNS physiology: Microglia and beyond. J Exp Med 2019;216:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee FJ, Williams KB, Levin M, et al. The bacterial metabolite indole inhibits regeneration of the planarian flatworm Dugesia japonica. iScience 2018;10:135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levin M, Martyniuk CJ. The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems 2018;164:76–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang-Schomer MD, White JD, Tien LW, et al. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci U S A 2014;111:13811–13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Churchill CDM, Winter P, Tuszyunski JA, et al. EDEn—Electroceutical Design Environment: An ion channel database with small molecule modulators and tissue expression information. iScience 2018;11:42–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pai VP, Pietak A, Willocq V, et al. HCN2 rescues brain defects by enforcing endogenous voltage pre-patterns. Nat Commun 2018;9:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oviedo NJ, Nicolas CL, Adams DS, et al. Establishing and maintaining a colony of planarians. CSH Protoc 2008;2008:pdb..prot5053. [DOI] [PMC free article] [PubMed] [Google Scholar]