Abstract
Roles of bioelectrical signals are increasingly recognized in excitable and nonexcitable non-neural tissues. Diverse ion-selective channels, pumps, and gap junctions participate in bioelectrical signaling, including those transporting calcium ions (Ca2+). Ca2+ is the most versatile transported ion, because it serves as an electrical charge carrier and a biochemical regulator for multiple molecular binding, enzyme, and transcription activities. We aspire to learn how bioelectrical signals crosstalk to biochemical/biomechanical signals. In this study, we review four recent studies showing how bioelectrical currents and Ca2+ signaling affect collective dermal cell migration during feather bud elongation, affect chondrogenic differentiation in limb development, couple with mechanical tension in aligning gut smooth muscle, and affect mitochondrial function and skeletal muscle atrophy. We observe bioelectrical signals involved in several developmental and pathological conditions in chickens and mice at multiple spatial scales: cellular, cellular collective, and subcellular. These examples inspire novel concept and approaches for future basic and translational studies.
Keywords: collective cell migration, chondrogenic differentiation, smooth muscle alignment, neuromuscular degenerative disease
Introduction
Eight decades after the first measurements of action potentials in squid giant axon were published,1 bioelectrical signals in the form of flowing ions, including Ca2+, H+, Na+, K+, Cl− and charged large molecules (such as serotonin and butyrate) are recognized to play roles in a myriad of biological processes that have long-lasting impact on tissue/organ structure and function, which are beyond the scope of classical electrophysiology. For example, functional perturbation of a series of ion channels and gap junctions resulted in permanent changes of vein patterns developed in Drosophila wings and in the anterior/posterior body axis in planaria upon regeneration.2–4
Gap junctions are intercellular channels formed by two end-to-end hexameric hemichannels (composed of connexin protein in chordates) on closely apposed plasma membrane.5 The channels allow passages of small molecules up to 1 kDa, such as Ca2+, IP3, glutamate, glutathione, ADP, ATP,6 and serotonin.7–10 Electrical coupling between cells by gap junctions have been demonstrated to be crucial for large-scale patterning processes, such as left/right symmetry breakage, brain region specification during Xenopus embryogenesis,7–11 and bone growth in the fins of zebrafish.12
The cell behaviors modulated by bioelectrical signals, beside fate specification and proliferation in the processes mentioned above, also include migration/shape change. For example, biofilms formed by Bacillus subtilis were recently found to emanate self-sustained electrical field oscillations due to K+ currents, which attract other species of bacteria over long distances.13 In eukaryotic cells, not only could applied electrical fields reorient the growth cones, axons, and even the direction of migration of cultured neurons/neural progenitor cells in vitro,14–17 naturally occurring electrical fields in vivo under physiological and pathological conditions also could serve as axon guidance cues.16,18 Additionally, chemically manipulating membrane potential (Vmem) in cellular collectives, which results in altered tissue electrical field, could alter axon growth, as in ivermectin-induced depolarization, which enhances axon growth toward eye primordia transplanted to ectopic locations in tadpoles.19
The electrotaxis phenomenon is not restricted to excitable cells—keratinocytes, corneal epithelial cells, fibroblasts, adipose-derived stromal cells, osteoblasts and osteoclasts, lymphocytes, macrophages, and endothelial cells have all been reported to exhibit directed migration in applied electrical fields in vitro.20 Naturally occurring electrical signals, such as contact-dependent depolarization in zebrafish pigment cells, are involved in the repulsion of the xanthophores (yellow pigmented) from melanophores (black pigmented).21 On the other hand, wound-induced electrical fields in cornea attract corneal epithelial cells to the wounded area.22 More intriguingly, the strength of the wound-induced current is correlated with the rate of wound healing in both animal models and patients.22,23 A recent study reported bacterial (Salmonella) infection-induced reversal of gut electrical fields in follicle-associated epithelium, providing electrotactic or electrorepulsive cues for macrophages based on their surface electrical properties.24
Thus, although less well explored compared with neurons and muscles, the importance of bioelectrical signaling in nonexcitable tissue cannot be ignored. This new frontier is gaining increasing attention due to both its basic research and its translational values. In this review, we will use several recent studies to demonstrate how bioelectrical signals engage Ca2+ transients in developmental processes of organogenesis in both excitable and nonexcitable tissue and the pathological process of muscle atrophy during neuromuscular degenerative disease.
Ca2+-Mediated Crosstalk Between Bioelectrical and Biochemical Signaling
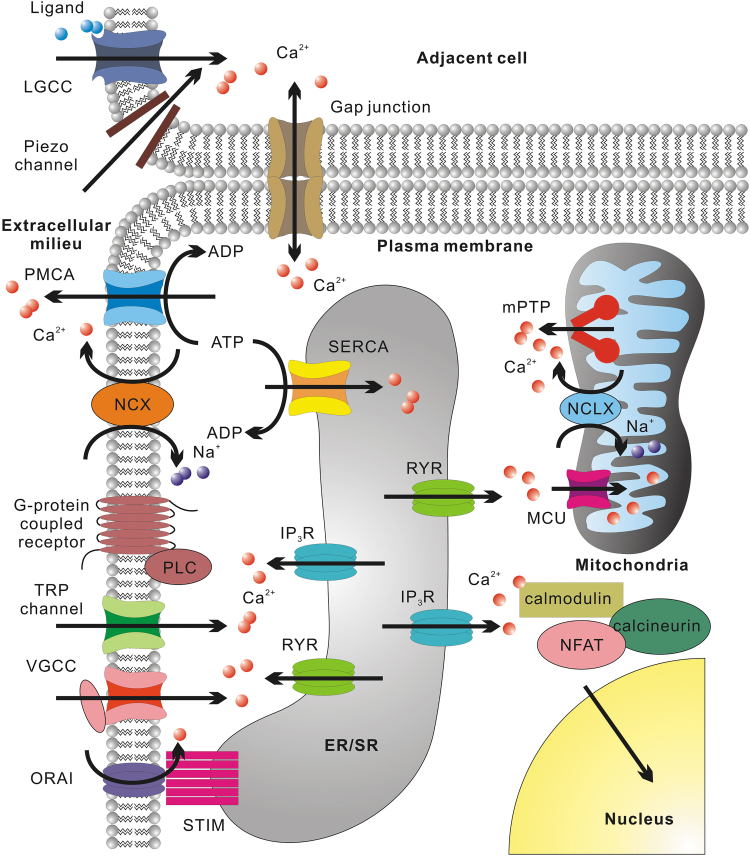
Bioelectrical signals are commonly observed to involve Ca2+ ions, either as the carrier of the electrical signal or as a downstream second messenger. Ca2+ ions are charged, rapidly diffusing, capable of passing through gap junctions between cells,25–27 and can be translocated among the cytosol, organelles, and extracellular milieu using different channels and transporters (Fig. 1).
FIG. 1.
Schematic representation of key regulators of cytosolic Ca2+. Ca2+ enters cytosol from the extracellular milieu through channels situated on the plasma membrane, such as LGCCs that are permeable to Ca2+ (such as purinergic receptors, CNG channels, AChRs, AMPARs, NMDARs, KARs), mechanosensitive Piezo1 and Piezo2 channels, VGCCs, TRP channels, and ORAI (the plasma membrane part of CRAC). ORAI is activated by STIM upon ER/SR Ca2+ depletion. Ca2+ efflux can occur through PMCA or NCX. Intercellular Ca2+ exchange can occur through gap junction channels. ER/SR Ca2+ release through RYR can be activated by cytosolic Ca2+ elevation or VGCC conformation change. IP3R Ca2+ release can be initiated by cytosolic Ca2+ elevation or IP3 produced by PLC upon the activation of G-protein-coupled receptors. Ca2+ influx into ER/SR can be mediated by SERCA. Mitochondrial Ca2+ intake mainly occur through MCU, while the efflux can occur through NCLX or mPTP formed by dimers of ATP synthase. For nuclear Ca2+ signaling, a representative example is the dephosphorylation of NFAT by calcineurin and its nuclear translocation. Calcineurin is activated by calmodulin upon Ca2+ binding. AChR, acetylcholine receptor; AMPAR, AMPA receptor; CNG, cyclic nucleotide-gated; CRAC, Ca2+ release-activated channel; ER/SR, endoplasmic reticulum/sarcoplasmic reticulum; KAR, kainate receptor; LGCCs, ligand-gated cation channel; MCU, mitochondrial Ca2+ uniporter; mPTP, mitochondrial permeability transition pore; NCLX, Na+/Ca2+/Li+ exchanger; NCX, Na+/Ca2+ exchanger; NMDAR, N-methyl-D-aspartate receptor; ORAI, Ca2+ release-activated Ca2+ channel protein; PLC, phospholipase C; PMCA, plasma membrane Ca2+ ATPase; RYR, ryanodine receptor; SERCA, sarcoendoplasmic reticulum Ca2+ pump; STIM, stromal interaction molecule; TRP, transient receptor potential; VGCC, voltage-gated Ca2+ channel.
More specifically, Ca2+ influx into the cells can occur through at least five routes: (1) Ligand-gated cation channels permeable to Ca2+, such as purinergic (ATP) receptor channels, cyclic nucleotide-gated channels28; acetylcholine receptor channels, N-methyl-D-aspartate receptor channels, AMPA receptor channels, and kainate receptor channels29–33; (2) Mechanosensitive Piezo1 and Piezo2 channels34–36; (3) Voltage-gated Ca2+ channels (VGCCs)37; (4) transient receptor potential (TRP) channels (gated by either ligands, mechanical force, or temperature variations)38; (5) Ca2+ release-activated channels (CRACs) composed of Ca2+ release-activated Ca2+ channel protein (ORAI) on the plasma membrane and stromal interaction molecule (STIM) on the endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR). They open upon ER/SR Ca2+ store depletion.39,40
Ca2+ efflux from cells can be carried out by plasma membrane Ca2+ ATPases (PMCA) or Na+/Ca2+ exchangers.41 Additionally, intracellular Ca2+ level is heavily influenced by Ca2+ release from ER/SR through the activation of ryanodine receptors (RYR) or inositol 1,4,5-trisphosphate receptors (IP3R).42 Ca2+ influx from the extracellular milieu can activate both RYRs and IP3Rs, leading to Ca2+-induced Ca2+ release .42
Under pathological conditions, cardiomyocyte SR can spontaneously release Ca2+ due to Ca2+ overload, leading to a phenomenon known as store-overload-induced Ca2+ release.43–49 IP3Rs can also be activated by IP3, whose production is catalyzed by phospholipase C upon the activation by G-protein-coupled receptors on the plasma membrane.50,51 This is one mechanism underlying purinergic receptor-mediated Ca2+ wave propagation.52–54 Reuptake of cytosolic Ca2+ into ER/SR is primarily carried out by the sarcoendoplasmic reticulum Ca2+ pump (SERCA).55
Cytosolic Ca2+ signaling may be further modulated by mitochondria.56,57 After influx into the mitochondrial matrix through mitochondrial Ca2+ uniporters (MCU),58 Ca2+ may be reversibly sequestered by phosphate.59 This Ca2+ buffering effect may be more prominent in cells with larger mitochondrial volume, such as differentiated cardiomyocyte (30–40% of the cell volume).60 Export of mitochondrial Ca2+ may be through Na+/Ca2+/Li+ exchangers or the mysterious permeability transition pore (PTP).50,61 The molecular nature of PTP has been under debate for many years and recent studies indicate that it forms from dimers of ATP synthase.62
Due to the presence of such complex regulatory machinery, it is not surprising that Ca2+ transients exhibit both an extremely diverse spatial range (from multicellular to subcellular) and temporal dynamics (from hours to less than a millisecond).63–67 Meanwhile there exist numerous Ca2+ effector proteins that activate different biochemical pathways, such as α-actinin for actin filament crosslinking,68 troponin for actin/tropomyosin filament contraction,69 protein kinase C (PKC)-NF-κB, calmodulin/calcineurin/NFAT and Ca2+/calmodulin-dependent protein kinase II (CaMKII) for gene expression regulation.67,70 Based on Ca2+ association/dissociation kinetics, Ca2+-binding sites, and subcellular localization of these effector proteins, they can specifically respond to Ca2+ transients at a certain amplitude/frequency.67,71,72 Therefore, it is not surprising that Ca2+ signaling is involved in numerous biological processes, such as migration,71 contraction,73 endocytosis and exocytosis,74 mitochondrial respiratory function regulation,56 cell death,56 proliferation,75,76 and differentiation77).
It is worth noticing that fast (less than a second), “superficial” (usually close-to-plasma membrane) Ca2+ transients more likely induce temporary phenomena such as exocytosis and contraction, while more profound changes like the regulation of gene expression or cell proliferation usually requires relatively slow (minutes to hours) and global (whole-cell) Ca2+ level changes.67 For bioelectrical currents to generate long-lasting impact on tissue/organ structure, engaging the slow, global Ca2+ transients seem to be ideal. However, repetitive activation of the superficial Ca2+ transients of sufficient duration, may trigger other signaling mechanism (such as cyclical contraction induced nonautonomous cell rearrangement, which will be discussed later) or creating a pathological condition, which may also produce permanent structural/functional changes.
We believe the study of developmental, physiological, and pathological events involving both bioelectrical currents and Ca2+ signaling could yield critical information about how bioelectrical signals are linked to other signaling mechanisms, either biochemical, biomechanical, or transcriptional. We expect the elucidation of these crosstalk pathways will uncover a whole dimension of signaling in development and pathological conditions. The following four recent studies represent only the tips of the icebergs.
Transient Bioelectrical Currents and Ca2+ Signaling in Collective Mesenchymal Cell Migration During Feather Bud Elongation
The involvement of Ca2+ in the motility of individual cells is well established. Ca2+-binding proteins regulate actin filament assembly/disassembly, myosin activity and focal adhesion turnover.71 The leading edges of migrating fibroblasts exhibit transient, intracellular Ca2+ elevations in localized submembrane microdomains due to the activation of mechanosensitive TRPM7 channels, and these “Ca2+ flickers” are involved in steering the migration direction.64
Yet, in many developmental and pathological processes, such as gastrulation, organogenesis, wound healing, chronic inflammation, and cancer metastasis, cells move as a collective population.78,79 Long-distance migration as a large cohort is difficult to explain by chemotaxis alone. Migration up a concentration gradient toward the source of released chemokine, implies that the gradient is steep and that a large cohort of separate cells will “see” different concentrations depending on whether cells are located close to or far from the source, so chemokine-stimulated movement would not be expected to be uniform. What mechanisms would ensure a more uniform signal across a large cell population?
A recent study of chicken feather elongation implicates a tissue-wide gap junction network80 as a means to enable directional mesenchymal cell population migration in posterior/distal elongation of feather primordia.81 Gap junctional coupling enables rapid and long-distance electrical signaling. Voltage-gated Ca2+ and Na+ channels regenerate signal strength as the signal travels from cell to cell. Higher-density channel expression assures nondecrementing (e.g., action potential) propagation over distances in the cm range, while far lower densities may be sufficient to enable shorter distance (100s of micrometers) propagation. As noted above, Ca2+ serves double duty as both an electrical charge carrier and as a second messenger for biochemical pathways. Furthermore, other ions and molecules may traverse gap junctions, also serving as second messengers.
So, how does the signaling initiate in a gap junction-coupled syncytium? In heart muscle, the initial signal arises at a pacemaker that initiates an action potential, which then spreads outward like a wave. How about in the mesenchyme of feather primordia? How does current flow in mesenchyme initiate and spread? Before feather primordia elongation, a vibrating probe detects only inward electrical currents across the feather region.82 Following the onset of elongation, the bioelectrical currents at the anterior part of the feather reverse direction. A local standing current then envelopes each primordium, with currents entering the feather bud tip and exiting at the anterior.80 Inward currents are typically mediated by Na+ and/or Ca2+ channel currents, and outward currents are most often potassium channel currents.
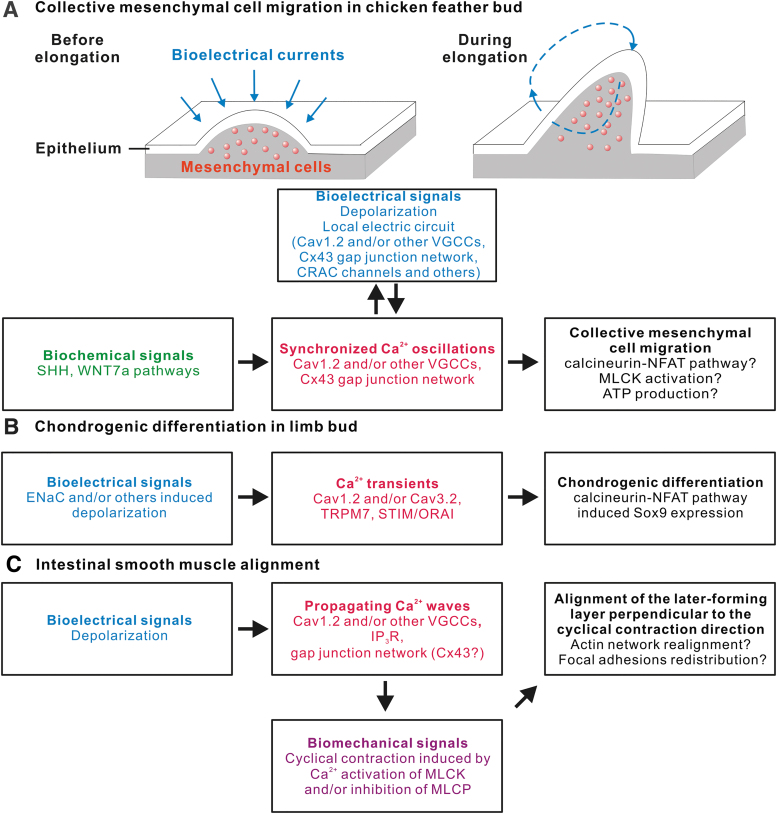
Through transcriptomic profiling, candidate gene expression analysis and functional perturbation experiments, we have shown that a connexin-43-based gap junction network, VGCCs (likely Cav1.2), and Icrac (ORAI/STIM) likely contribute to this electrical circuit within feather mesenchyme. The migration process is blocked upon inhibition of VGCCs (by nifedipine) or the gap junctions (by carbenoxolone, PMA or shRNA against connexin-43), whereas ectopic Ca2+ oscillations induced by photoactivatable CRACs enhanced this process.80 The expression of connexin-43 is regulated by the synergistic actions of Sonic Hedgehog and Wingless-INT signaling.80 Thus, the establishment of the feather bioelectrical circuit and Ca2+ oscillations rely on inputs from biochemical pathways (Fig. 2A).
FIG. 2.
Linkage of bioelectrical signals and Ca2+ transients to other signaling mechanisms in different biological processes. (A) Biochemical WNT and SHH pathways promote the establishment of Cx43 gap junction network, facilitating the completion of a bioelectric circuit around individual feather buds. The depolarization-induced VGCC activation, the gap junction network, and CRACs contribute to the synchronized Ca2+ oscillations in feather mesenchyme. These oscillations may promote cell migration either through transcriptional regulation (NFAT pathway), or MLCK activation to contract the actin/myosin network, or ATP production enhancement in mitochondria. (B) Early chondrogenic differentiation in limb involves ENaC-dependent depolarization, which initiates Cav1.2-dependent Ca2+ transients. Meanwhile, some other Ca2+ channels have also been reported to initiate Ca2+ transients during chondrogenic differentiation. These transients activate NFAT pathway and elevate Sox9 expression. (C) Spontaneous contractions of intestinal smooth muscle cells are driven by propagating waves of Ca2+. The formation of these waves requires depolarization-induced VGCC activation and gap junction network-based Ca2+ exchange between cells. The cyclic contraction serves as a biomechanical signal aligning newly forming smooth muscle layer along the direction perpendicular to it, likely through modulating actin network and focal adhesions. Cx43, connexin-43; ENaC, epithelial Na+ channel; MLCK, myosin light chain kinase; SHH, Sonic Hedgehog; WNT, wingless-INT.
Bioelectrical Currents and Ca2+ Signaling During Limb Chondrogenic Differentiation
Chondrogenesis is well known to be regulated by Ca2+ signaling, as the calmodulin/calcineurin/NFAT pathway that can activate the expression of Sox9, which is crucial for mesenchymal cell condensation and the expression of several cartilage-specific extracellular matrix molecules.83,84 Meanwhile Ca2+-calmodulin is also known to promote Sox9 nuclear localization.85 Rapid Ca2+ transients (∼1 min or less) have been observed in different chondrogenic models, while CRACs, TRP channels, as well as VGCCs (both L-type and T-type) have been demonstrated to contribute to these transients.83,84,86,87 The involvement of VGCCs implies Vmem changes, in other words bioelectrical signals, as an upstream event of chondrogenesis.
Indeed, a recent study of limb bud chondrogenesis used the potential sensitive fluorescent probe DiBAC4 to demonstrate a depolarization of Vmem in the chondrogenic region of the limb when chondrogenic differentiation starts.87 The spatiotemporal correlation of depolarization and chondrogenic differentiation was observed in both limb slice culture and micromass culture, in which Col2, a cartilage-specific extracellular matrix molecule, was used as the chondrogenic differentiation marker.87
The importance of depolarization to chondrogenesis is further supported by the phenomenon that pharmacological inhibition of epithelial Na+ channel, which is involved in depolarization, also blocks chondrogenic differentiation.87–89 Additionally, an artificially induced depolarization achieved by exposing cells to an external solution containing high K+ increased the frequency of Ca2+ transients in the micromass culture, while nifedipine treatment has the opposite effect. Yet the nifedipine-mediated inhibition of Ca2+ transients is limited to the first day of micromass culture, implying that VGCCs are important for the early but not late chondrogenic differentiation.87 Nifedipine treatment of the micromass culture significantly reduced Alcian Blue-positive cells and Sox9 expression, while the Ca2+ ionophore A23187 (Calcimycin) has the opposite effect.87
Finally, using a knockout mouse model, the authors pinpoint CaV1.2 as a VGCC critical for limb chondrogenesis and calmodulin/calcineurin/NFAT signaling is confirmed to be one of its downstream biochemical pathways that promote chondrogenic differentiation87 (Fig. 2B).
Bioelectrical Currents and Ca2+ Signaling Couple Mechanical Tension and Smooth Muscle Alignment in the Intestinal Wall
For gastrointestinal (GI) smooth muscle cells, excitation/contraction (E-C) initiates from a depolarization that can be triggered by multiple input sources, including enteric neurons and intestinal cells of Cajal.90,91 Depolarization-induced activation of VGCCs provides the majority of Ca2+ ions required to initiate the spontaneous contraction, which is supplemented by storage-operated Ca2+ release through IP3 receptors.90 Ca2+ activates kinases like myosin light chain kinase (MLCK), which phosphorylates the myosin light chain for contraction. Meanwhile Ca2+ has also been reported to inhibit myosin light chain phosphatase in a Rho kinase- and PKC-dependent manner, which also promotes contraction.90 Blocking VGCC by nicardipine reduced the contraction force in both the circular and longitudinal layer of the GI smooth muscle.90
Gap junctions were implicated in electrical coupling of the GI smooth muscle cells, as carbenoxolone treatment reduced the amplitude of contraction.91 However, the relationship between the spontaneous contraction and the alignment orientation of the smooth muscle has not been explored until recently, which reveals an intriguing coupling relationship between bioelectrical signals and mechanical force in tissue morphogenesis.92
The role of mechanical force in tissue morphogenesis has been recognized in the recent decade, as changes in the shape and arrangement of cells modify the mechanical forces, which may initiate signaling leading to changes in proliferation, differentiation, and gene expression.93 Previous in vitro experiments demonstrate that mesenchymal cells, such as fibroblasts and smooth muscle cells, could reorient themselves roughly perpendicular to the direction of cyclically applied mechanical stretches.94–98 During the development of intestinal smooth muscle, the circular layer forms earlier than the longitudinal layer and undergoes spontaneous contraction along the circumferential axis,99–103 bringing up the possibility that cyclical mechanical tension from the circular layer causes the longitudinal smooth muscle cells to differentiate later.
Consistent with this, the application of nifedipine, carbenoxolone, or ML-7 (an inhibitor of MLCK) to chicken intestinal explant culture before the formation of the longitudinal layer could all inhibit the longitudinal alignment. While treatment with the VGCC agonist (S)-(-)-BayK8644 had the opposite effect.92 Furthermore, the application of cyclical longitudinal stretch to the explant culture (while inhibiting the endogenous contraction with nifedipine) led to the circumferential alignment of the later-forming smooth muscle layer, implying that cyclical mechanical stimuli are not only necessary but also sufficient to align the smooth muscle cells.92 More interestingly, the authors also confirmed that alignment of the later-forming smooth muscle layer requires the spontaneous contraction of the earlier forming smooth muscle layer around the esophagus and ureter, which implies a broad applicability of this principle.92
In sum, the Vmem fluctuations and a gap junction network allow Ca2+-mediated contraction waves to occur in smooth muscle cells, producing cyclical mechanical stimuli as a tissue alignment signal (Fig. 2C).
Bioelectrical Currents and Ca2+ Signaling Affect Mitochondrial Function and Atrophy in the Skeletal Muscle
During development, mammalian skeletal muscles exhibit spontaneous Ca2+ sparks, which are also observed in smooth and cardiac muscles.104,105 However, in postnatal mammals, the sparks become rare (unless the muscle fibers are permeabilized), which may be explained by the loss of RYR3 and the maturation of t-tubule structure.105–108 In adult mammalian skeletal muscle, motor neuron impulses at neuromuscular junctions are generally considered the sole input signal to initiate muscular action potential and induce contraction, a process termed E-C coupling.109,110 Besides initiating contraction, bioelectrical signals (in the form of muscle action potential) also seem to be important for maintaining the skeletal muscle cytosolic Ca2+ levels, mitochondrial integrity, and muscle mass. This is exemplified by the linkage between neurodegenerative disease (such as spinal muscular atrophy and amyotrophic lateral sclerosis [ALS]) and muscle atrophy.111,112
In the human SOD1G93A-overexpressing mice (an animal model for familial ALS), the skeletal muscle fibers exhibited collapsed mitochondrial inner membrane potential, reduced mitochondrial Ca2+ uptake, and ectopic Ca2+ waves under osmotic stress.113,114 Furthermore, surgical denervation of the hindlimb skeletal muscle in rodent models triggered dramatic elevation of reactive oxygen species (ROS) production in the muscle mitochondria and ROS-related mitoflash events in just a few days, which posed a significant risk of oxidative damage to lipid, protein, and DNA.115–117 In 2 weeks, the denervated muscle lost more than 30% of its original mass.115,117 In contrast, electrical stimulation, as a physical therapy approach, has been shown to help preserve muscle mass and reverse the atrophy process in patients.118–121
Although the denervation-induced elevation of ROS production phenomenon has been known for more than a decade, the underlying molecular mechanism was poorly understood. Mitochondria are the major ATP provider for skeletal muscle, especially the slow oxidative muscle fibers, which heavily rely on oxidative phosphorylation for energy.122 Yet the oxidative phosphorylation process is also a major producer of ROS, which is exacerbated under conditions of mitochondrial Ca2+ overload.56 Long-term denervation or disuse of skeletal muscle was reported to elevate the resting cytosolic Ca2+ level.123–125 This could result from decreased PMCA/SERCA function due to handicapped mitochondrial ATP production,126 or Ca2+ leak-in from nicotinic acetylcholine receptors (nAChRs),30,127 as in denervated rodent models, nAChRs exhibit elevated expression and ectopic localization.128,129
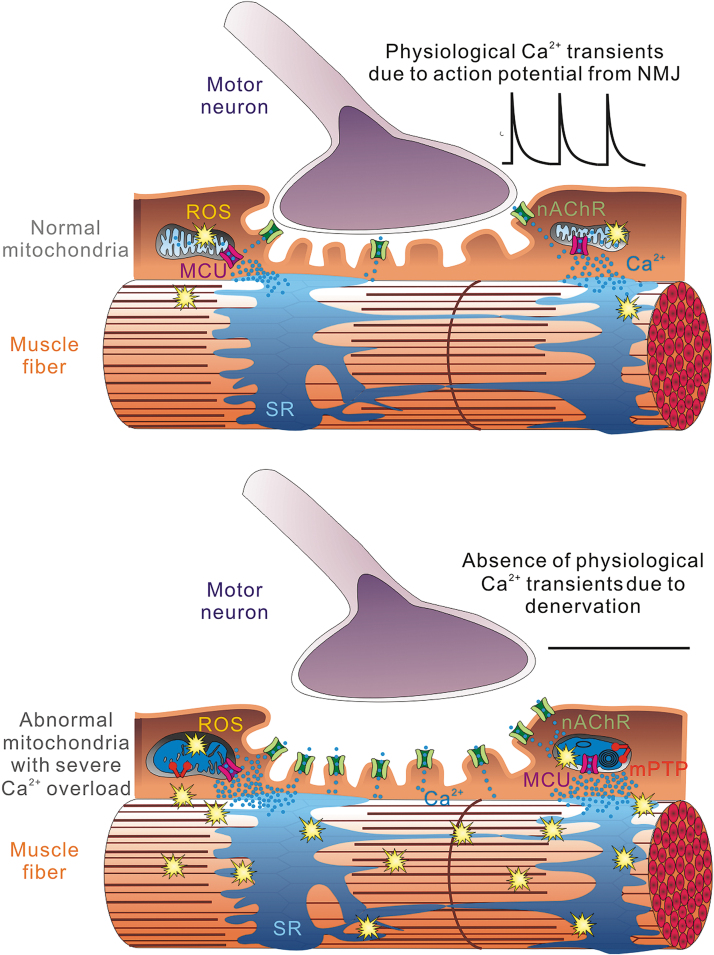
The elevated cytosolic Ca2+ likely increased resting mitochondrial Ca2+ load, which promoted mitochondrial ROS production, structural damage, and mitochondrial PTP opening116,130 (Fig. 3). In a recent study, when denervated muscles were exposed to electrical field stimulation, ROS-related mitoflash events were dramatically reduced. However, when mitochondrial Ca2+ transients induced by the electrical stimulation were blocked by the MCU antagonist Ru360, this reduction effect was abolished.116 More than a coincidence, virus-mediated overexpression of MCU was shown to have protective effect against denervation-induced skeletal muscle atrophy.131,132 These discoveries unveil a seemingly counterintuitive, yet possible scenario that while mitochondrial Ca2+ overload leads to excessive ROS production, the physiological mitochondrial Ca2+ transients/uptake induced by the motor neuron impulse are required to keep the ROS production in check.127
FIG. 3.
Physiological Ca2+ transients induced by motor neuron impulses help prevent mitochondrial Ca2+ overload and ROS overproduction. After denervation, elevated nAChR expression likely contributed to the increase of cytosolic Ca2+ in the skeletal muscle, which overloads mitochondrial matrix Ca2+ content. This promotes the production of ROS, increases structural damage to the mitochondria, and facilitates mPTP opening. However, the elevated ROS production could be reversed by the application of electric field stimulation that simulates physiological Ca2+ transients induced by neuronal impulses. nAChR, nicotinic acetylcholine receptor; ROS, reactive oxygen species.
Conclusions
The four examples highlighted above, although seemingly unrelated, share a core mechanism in common: the changes in membrane potential activate VGCCs, which trigger the downstream Ca2+-related physiological/pathological molecular processes. In each case, there are circumstantial evidences suggesting that VGCCs are crucial both for ensuring an electrical signal that coordinates events across populations of cells, and for generating intracellular changes in Ca2+ that elicit the developmental or pathological change. Both the electrical signal and the intracellular Ca2+ dynamics are thought to be necessary for proper development, differentiation, or in the last case, maintenance of tissue in a healthy state.
For the developmental process of organogenesis, understanding the cell/cell communication mechanisms coordinating collective behaviors of a population of cells is a major research direction and bears translational value. As shown in the feather mesenchyme and the intestinal smooth muscle studies, one possible solution for the large-scale intercellular communication is the establishment of the gap junction network.
In support of the idea that gap junctions are important for multicellular communications, studies of color stripe formation in zebrafish and Japanese quail have implicated gap junction channels in tuning the spatial organization of the pigmented cells.133–135 For example, the blockage of gap junction communication by carbenoxolone specifically reduced the width of yellow stripes formed by pheomelanin-producing melanocytes in Japanese quail embryonic skin explants, whereas overexpressing connexin-40 in melanocytes expanded the yellow stripes.133 Interestingly, besides playing a role in determining color patterning, the gap junctions may also contribute to feather branching morphogenesis as membrane-localized connexin-43 was detected in the hooklet, but not pennulum region of barbules in chicken plumes.136 Thus, the potential role of bioelectrical signals in the diversification of feather branching patterns for the adaptation to different ecospaces is worth future exploration among scientists interested in evo devo.
On the translational front, although electrical stimulation has become a well-established therapeutic approach,118–121 the correlation between muscle action potential, mitochondrial Ca2+ homeostasis, and ROS production is relatively underexplored. Further studies on the control of resting mitochondrial Ca2+ level and ROS production could provide inspiration for developing new therapeutic approaches against aging and neuromuscular diseases.
On the other hand, it is also worth noticing that ROS production is not completely bad. It is an important signal from wounded tissue used to attract immune cells and stimulate regeneration in multiple animal species.137–141 Recent studies also imply that ROS (more specifically H2O2) facilitates activation of voltage-gated Na+ channels, promoting the reversal of the electrical field around the wound tissue, which induces regeneration.137,138 We hope that insights from these studies will trigger more discoveries in this relatively unexplored new frontier.
Author Contributions
C-.M.C. and A.L. designed the outline of this article. A.L., J.Z., R.B.W., R.H.C., and C-.M.C. contributed to the writing and editing of this article. All coauthors have reviewed and approved of the article before submission.
Disclaimer
The article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors would like to acknowledge supports from NIH R01 AR047364 (C-.M.C., R.B.W.), AR060306 (C-.M.C., R.B.W), EY022931 (R.H.C), and NS105621 (J.Z.). C-.M.C. and R.B.W. are also partially supported by a research contract between China Medical University Hospital in Taiwan (CMUHJ) and USC (USC 5351285884), and C-.M.C. is a paid scientific advisor of CMUH. R.H.C. is also supported by NIH U01 MH098937 and NSF CBET1404089.
References
- 1.Hodgkin AL, Huxley AF. Action potentials recorded from inside a nerve fibre. Nature 1939;144:710 [Google Scholar]
- 2.George LF, Pradhan SJ, Mitchell D, et al. Ion channel contributions to wing development in Drosophila melanogaster. G3 2019;9:999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oviedo NJ, Morokuma J, Walentek P, et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 2010;339:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D, Chan JD, Nogi T, et al. Opposing roles of voltage-gated Ca2+ channels in neuronal control of regenerative patterning. J Neurosci 2011;31:15983–15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris AL.Emerging issues of connexin channels: Biophysics fills the gap. Q Rev Biophys 2001;34:325–472 [DOI] [PubMed] [Google Scholar]
- 6.Račkauskas M, Neverauskas V, Skeberdis VA. Diversity and properties of connexin gap junction channels. Medicina 2010;46:1. [PubMed] [Google Scholar]
- 7.McLaughlin KA, Levin M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol 2018;433:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol 2005;15:794–803 [DOI] [PubMed] [Google Scholar]
- 9.Vandenberg LN, Blackiston DJ, Rea AC, et al. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. Int J Dev Biol 2015;58:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Levin M. Particle tracking model of electrophoretic morphogen movement reveals stochastic dynamics of embryonic gradient. Dev Dyn 2009;238:1923–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai VP, Lemire JM, Paré J-F, et al. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J Neurosci 2015;35:4366–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iovine MK, Higgins EP, Hindes A, et al. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev Biol 2005;278:208–219 [DOI] [PubMed] [Google Scholar]
- 13.Humphries J, Xiong L, Liu J, et al. Species-independent attraction to biofilms through electrical signaling. Cell 2017;168:200–209. e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao L, McCaig CD, Zhao M. Electrical signals polarize neuronal organelles, direct neuron migration, and orient cell division. Hippocampus 2009;19:855–868 [DOI] [PubMed] [Google Scholar]
- 15.Li L, El-Hayek YH, Liu B, et al. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells 2008;26:2193–2200 [DOI] [PubMed] [Google Scholar]
- 16.McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci 2009;122:4267–4276 [DOI] [PubMed] [Google Scholar]
- 17.Gokoffski KK, Jia X, Shvarts D, et al. Physiologic electrical fields direct retinal ganglion cell axon growth in vitro. Invest Ophthalmol Vis Sci 2019;60:3659–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao L, Wei D, Reid B, et al. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep 2013;14:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackiston DJ, Anderson GM, Rahman N, et al. A novel method for inducing nerve growth via modulation of host resting potential: Gap junction-mediated and serotonergic signaling mechanisms. Neurotherapeutics 2015;12:170–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tai G, Tai M, Zhao M. Electrically stimulated cell migration and its contribution to wound healing. Burns Trauma 2018;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba M, Yamanaka H, Kondo S. Pigment pattern formation by contact-dependent depolarization. Science 2012;335:677. [DOI] [PubMed] [Google Scholar]
- 22.Reid B, Song B, McCaig CD, et al. Wound healing in rat cornea: The role of electric currents. FASEB J 2005;19:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Pfluger T, Ferreira F, et al. Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing. Sci Rep 2016;6:26525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Reid B, Ferreira F, et al. Infection-generated electric field in gut epithelium drives bidirectional migration of macrophages. PLoS Biol 2019;17:e3000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saez JC, Connor JA, Spray DC, et al. Hepatocyte gap junctions are permeable to the second messenger, inositol 1, 4, 5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A 1989;86:2708–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christ G, Moreno A, Melman A, et al. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol Cell Physiol 1992;263:C373–C383 [DOI] [PubMed] [Google Scholar]
- 27.Churchill G, Louis C. Roles of Ca2+, inositol trisphosphate and cyclic ADP-ribose in mediating intercellular Ca2+ signaling in sheep lens cells. J Cell Sci 1998;111:1217–1225 [DOI] [PubMed] [Google Scholar]
- 28.Leinders-Zufall T, Rand MN, Shepherd GM, et al. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: Spatiotemporal dynamics. J Neurosci 1997;17:4136–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers M, Dani JA. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J 1995;68:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decker E, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: The single-channel calcium influx is significant. J Neurosci 1990;10:3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflügers Archiv 1993;425:511–517 [DOI] [PubMed] [Google Scholar]
- 32.Burnashev N, Zhou Z, Neher E, et al. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol 1995;485:403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneggenburger R, Zhou Z, Konnerth A, et al. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron 1993;11:133–143 [DOI] [PubMed] [Google Scholar]
- 34.Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010;330:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syeda R, Florendo MN, Cox CD, et al. Piezo1 channels are inherently mechanosensitive. Cell Rep 2016;17:1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szczot M, Pogorzala LA, Solinski HJ, et al. Cell-type-specific splicing of Piezo2 regulates mechanotransduction. Cell Rep 2017;21:2760–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang S, Huang X-Y. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J Biol Chem 2005;280:27130–27137 [DOI] [PubMed] [Google Scholar]
- 38.Nilius B, Owsianik G, Voets T, et al. Transient receptor potential cation channels in disease. Physiol Rev 2007;87:165–217 [DOI] [PubMed] [Google Scholar]
- 39.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005;169:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006;312:1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawano S, Otsu K, Shoji S, et al. Ca2+ oscillations regulated by Na+–Ca2+ exchanger and plasma membrane Ca2+ pump induce fluctuations of membrane currents and potentials in human mesenchymal stem cells. Cell Calcium 2003;34:145–156 [DOI] [PubMed] [Google Scholar]
- 42.Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol 1990;6:715–760 [DOI] [PubMed] [Google Scholar]
- 43.Priori SG, Chen SW. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 2011;108:871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang D, Xiao B, Yang D, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc Natl Acad Sci U S A 2004;101:13062–13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang D, Wang R, Xiao B, et al. Enhanced store overload–induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res 2005;97:1173–1181 [DOI] [PubMed] [Google Scholar]
- 46.Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. In: Frank GB, Bianchi CP, Keurs H, eds. Excitation-Contraction Coupling in Skeletal, Cardiac, and Smooth Muscle. Springer, New York, NY, USA, 1992: 245–262 [DOI] [PubMed] [Google Scholar]
- 47.Lakatta EG.Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovasc Res 1992;26:193–214 [DOI] [PubMed] [Google Scholar]
- 48.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest 1986;78:1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orchard C, Eisner D, Allen D. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature 1983;304:735. [DOI] [PubMed] [Google Scholar]
- 50.Patergnani S, Suski JM, Agnoletto C, et al. Calcium signaling around mitochondria associated membranes (MAMs). Cell Commun Signal 2011;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clapham DE.Calcium signaling. Cell 2007;131:1047–1058 [DOI] [PubMed] [Google Scholar]
- 52.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature 1992;359:241. [DOI] [PubMed] [Google Scholar]
- 53.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: Fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 2000;20:1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 2006;26:1378–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Primeau JO, Armanious GP, M'Lynn EF, et al. The sarcoendoplasmic reticulum calcium ATPase. In: Hariis JR, Boekema EJ, eds. Membrane Protein Complexes: Structure and Function. Springer, Singapore, 2018: 229–258 [DOI] [PubMed] [Google Scholar]
- 56.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004;287:C817–C833 [DOI] [PubMed] [Google Scholar]
- 57.Szabadkai G, Duchen MR. Mitochondria: The hub of cellular Ca2+ signaling. Physiology 2008;23:84–94 [DOI] [PubMed] [Google Scholar]
- 58.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004;427:360. [DOI] [PubMed] [Google Scholar]
- 59.Nicholls DG.Mitochondria and calcium signaling. Cell Calcium 2005;38:311–317 [DOI] [PubMed] [Google Scholar]
- 60.Boengler K, Kosiol M, Mayr M, et al. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle 2017;8:349–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palty R, Silverman WF, Hershfinkel M, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 2010;107:436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giorgio V, Von Stockum S, Antoniel M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 2013;110:5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng H, Lederer W. Calcium sparks. Physiol Rev 2008;88:1491–1545 [DOI] [PubMed] [Google Scholar]
- 64.Wei C, Wang X, Chen M, et al. Calcium flickers steer cell migration. Nature 2009;457:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou J, Brum G, Gonzalez A, et al. Ca2+ sparks and embers of mammalian muscle. Properties of the sources. J Gen Physiol 2003;122:95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng H, Lederer MR, Lederer W, et al. Calcium sparks and [Ca2+] i waves in cardiac myocytes. Am J Physiol Cell Physiol 1996;270:C148–C159 [DOI] [PubMed] [Google Scholar]
- 67.Berridge M, Bootman M, Roderick H. Calcium signaling: Dynamics, homeostasis, and remodeling. Nature 2003;4:517–529 [DOI] [PubMed] [Google Scholar]
- 68.Noegel A, Witke W, Schleicher M. Calcium-sensitive non-muscle α-actinin contains EF-hand structures and highly conserved regions. FEBS Lett 1987;221:391–396 [DOI] [PubMed] [Google Scholar]
- 69.Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life 2002;54:323–333 [DOI] [PubMed] [Google Scholar]
- 70.Soboloff J, Rothberg BS, Madesh M, et al. STIM proteins: Dynamic calcium signal transducers. Nat Rev Mol Cell Biol 2012;13:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei C, Wang X, Zheng M, et al. Calcium gradients underlying cell migration. Curr Opin Cell Biol 2012;24:254–261 [DOI] [PubMed] [Google Scholar]
- 72.Parekh AB.Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 2011;36:78–87 [DOI] [PubMed] [Google Scholar]
- 73.Ríos E, Stern MD. Calcium in close quarters: Microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct 1997;26:47–82 [DOI] [PubMed] [Google Scholar]
- 74.Wu L-G, Hamid E, Shin W, et al. Exocytosis and endocytosis: Modes, functions, and coupling mechanisms. Annu Rev Physiol 2014;76:301–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berridge MJ.Calcium signalling and cell proliferation. Bioessays 1995;17:491–500 [DOI] [PubMed] [Google Scholar]
- 76.Lu KP, Means AR. Regulation of the cell cycle by calcium and calmodulin. Endocr Rev 1993;14:40–58 [DOI] [PubMed] [Google Scholar]
- 77.Gu X, Spitzer NC. Breaking the code: Regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci 1997;19:33–41 [DOI] [PubMed] [Google Scholar]
- 78.Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci 2013;70:3481–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009;10:445. [DOI] [PubMed] [Google Scholar]
- 80.Li A, Cho J-H, Reid B, et al. Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat Commun 2018;9:5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li A, Chen M, Jiang T-X, et al. Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation. Proc Natl Acad Sci U S A 2013;110:E1452–E1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc 2007;2:661. [DOI] [PubMed] [Google Scholar]
- 83.Fodor J, Matta C, Oláh T, et al. Store-operated calcium entry and calcium influx via voltage-operated calcium channels regulate intracellular calcium oscillations in chondrogenic cells. Cell Calcium 2013;54:1–16 [DOI] [PubMed] [Google Scholar]
- 84.Lin S-S, Tzeng B-H, Lee K-R, et al. Cav3. 2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc Natl Acad Sci U S A 2014;111:E1990–E1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanover JA, Love DC, Prinz WA. Calmodulin-driven nuclear entry: Trigger for sex determination and terminal differentiation. J Biol Chem 2009;284:12593–12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qian N, Ichimura A, Takei D, et al. TRPM7 channels mediate spontaneous Ca2+ fluctuations in growth plate chondrocytes that promote bone development. Sci Signal 2019;12:eaaw4847. [DOI] [PubMed] [Google Scholar]
- 87.Atsuta Y, Tomizawa RR, Levin M, et al. L-type voltage-gated Ca2+ channel CaV1. 2 regulates chondrogenesis during limb development. Proc Natl Acad Sci U S A 2019;116:21592–21601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrik D, Myoga MH, Grade S, et al. Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell 2018;22:865–878. e868. [DOI] [PubMed] [Google Scholar]
- 89.Chifflet S, Hernández JA, Grasso S. A possible role for membrane depolarization in epithelial wound healing. Am J Physiol Cell Physiol 2005;288:C1420–C1430 [DOI] [PubMed] [Google Scholar]
- 90.Sanders KM.Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil 2008;20:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schultz T, Daniel V, Daniel E, et al. Does ICC pacing require functional gap junctions between ICC and smooth muscle in mouse intestine? Neurogastroenterol Motil 2003;15:129–138 [DOI] [PubMed] [Google Scholar]
- 92.Huycke TR, Miller BM, Gill HK, et al. Genetic and mechanical regulation of intestinal smooth muscle development. Cell 2019;179:90–105. e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heisenberg C-P, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell 2013;153:948–962 [DOI] [PubMed] [Google Scholar]
- 94.Buck RC.Reorientation response of cells to repeated stretch and recoil of the substratum. Exp Cell Res 1980;127:470–474 [DOI] [PubMed] [Google Scholar]
- 95.Faust U, Hampe N, Rubner W, et al. Cyclic stress at mHz frequencies aligns fibroblasts in direction of zero strain. PLoS One 2011;6:e28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanda K, Matsuda T, Oka T. Two-dimensional orientational response of smooth muscle cells to cyclic stretching. ASAIO J 1992;38:M382–M385 [DOI] [PubMed] [Google Scholar]
- 97.Kim B-S, Nikolovski J, Bonadio J, et al. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 1999;17:979. [DOI] [PubMed] [Google Scholar]
- 98.Livne A, Bouchbinder E, Geiger B. Cell reorientation under cyclic stretching. Nat Commun 2014;5:3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gabella G. Development of visceral smooth muscle. In: Brand-Saberi B, ed. Vertebrate Myogenesis. Springer, Germany, 2002: 1–37 [DOI] [PubMed] [Google Scholar]
- 100.Kedinger M, Simon-Assmann P, Bouziges F, et al. Smooth muscle actin expression during rat gut development and induction in fetal skin fibroblastic cells associated with intestinal embryonic epithelium. Differentiation 1990;43:87–97 [DOI] [PubMed] [Google Scholar]
- 101.McHugh KM.Molecular analysis of smooth muscle development in the mouse. Dev Dyn 1995;204:278–290 [DOI] [PubMed] [Google Scholar]
- 102.Chevalier N, Fleury V, Dufour S, et al. Emergence and development of gut motility in the chicken embryo. PLoS One 2017;12:e0172511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts RR, Ellis M, Gwynne RM, et al. The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. J Physiol 2010;588:1153–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klein MG, Schneider MF. Ca2+ sparks in skeletal muscle. Prog Biophys Mol Biol 2006;92:308–332 [DOI] [PubMed] [Google Scholar]
- 105.Conklin MW, Barone V, Sorrentino V, et al. Contribution of ryanodine receptor type 3 to Ca2+ sparks in embryonic mouse skeletal muscle. Biophys J 1999;77:1394–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pouvreau S, Royer L, Yi J, et al. Ca2+ sparks operated by membrane depolarization require isoform 3 ryanodine receptor channels in skeletal muscle. Proc Natl Acad Sci U S A 2007;104:5235–5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weisleder N, Zhou J, Ma J. Detection of calcium sparks in intact and permeabilized skeletal muscle fibers. In: DeMario, JX, ed. Myogenesis. Humana Press, Totowa, NJ, USA, 2012:. 395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou J, Yi J, Royer L, et al. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol 2006;290:C539–C553 [DOI] [PubMed] [Google Scholar]
- 109.Dulhunty A.Excitation—Contraction coupling from the 1950s into the new millennium. Clin Exp Pharmacol Physiol 2006;33:763–772 [DOI] [PubMed] [Google Scholar]
- 110.Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1995;1241:59–116 [DOI] [PubMed] [Google Scholar]
- 111.Monani UR.Spinal muscular atrophy: A deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron 2005;48:885–895 [DOI] [PubMed] [Google Scholar]
- 112.Pansarasa O, Rossi D, Berardinelli A, et al. Amyotrophic lateral sclerosis and skeletal muscle: An update. Mol Neurobiol 2014;49:984–990 [DOI] [PubMed] [Google Scholar]
- 113.Zhou J, Yi J, Fu R, et al. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem 2010;285:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yi J, Ma C, Li Y, et al. Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (EC) coupling. J Biol Chem 2011;286:32436–32443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muller FL, Song W, Jang YC, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 2007;293:R1159–R1168 [DOI] [PubMed] [Google Scholar]
- 116.Karam C, Yi J, Xiao Y, et al. Absence of physiological Ca 2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skelet Muscle 2017;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adhihetty PJ, O'Leary MF, Chabi B, et al. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 2007;102:1143–1151 [DOI] [PubMed] [Google Scholar]
- 118.Kern H, Salmons S, Mayr W, et al. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve 2005;31:98–101 [DOI] [PubMed] [Google Scholar]
- 119.Kern H, Hofer C, Loefler S, et al. Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res 2017;39:660–666 [DOI] [PubMed] [Google Scholar]
- 120.Gerovasili V, Stefanidis K, Vitzilaios K, et al. Electrical muscle stimulation preserves the muscle mass of critically ill patients: A randomized study. Crit Care 2009;13:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ragnarsson K.Functional electrical stimulation after spinal cord injury: Current use, therapeutic effects and future directions. Spinal Cord 2008;46:255. [DOI] [PubMed] [Google Scholar]
- 122.Crupi AN, Nunnelee JS, Taylor DJ, et al. Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging. Aging (Albany NY) 2018;10:3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tischler ME, Rosenberg S, Satarug S, et al. Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metabolism 1990;39:756–763 [DOI] [PubMed] [Google Scholar]
- 124.Ingalls CP, Warren GL, Armstrong R. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol 1999;87:386–390 [DOI] [PubMed] [Google Scholar]
- 125.Squecco R, Carraro U, Kern H, et al. A subpopulation of rat muscle fibers maintains an assessable excitation-contraction coupling mechanism after long-standing denervation despite lost contractility. J Neuropathol Exp Neurol 2009;68:1256–1268 [DOI] [PubMed] [Google Scholar]
- 126.Joffe M, Savage N, Isaacs H. Biochemical functioning of mitochondria in normal and denervated mammalian skeletal muscle. Muscle Nerve 1981;4:514–519 [DOI] [PubMed] [Google Scholar]
- 127.Zhou J, Li A, Li X, et al. Dysregulated mitochondrial Ca2+ and ROS signaling in skeletal muscle of ALS mouse model. Arch Biochem Biophys 2019;663:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldman D, Staple J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron 1989;3:219–228 [DOI] [PubMed] [Google Scholar]
- 129.Kostrominova TY, Macpherson PC, Carlson BM, et al. Regulation of myogenin protein expression in denervated muscles from young and old rats. Am J Physiol Regul Integr Comp Physiol 2000;279:R179–R188 [DOI] [PubMed] [Google Scholar]
- 130.Abruzzo PM, Di Tullio S, Marchionni C, et al. Oxidative stress in the denervated muscle. Free Radic Res 2010;44:563–576 [DOI] [PubMed] [Google Scholar]
- 131.Chemello F, Mammucari C, Gherardi G, et al. Gene expression changes of single skeletal muscle fibers in response to modulation of the mitochondrial calcium uniporter (MCU). Genom Data 2015;5:64–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mammucari C, Gherardi G, Zamparo I, et al. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 2015;10:1269–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Inaba M, Jiang T-X, Liang Y-C, et al. Instructive role of melanocytes during pigment pattern formation of the avian skin. Proc Natl Acad Sci U S A 2019;116:6884–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watanabe M, Kondo S. Changing clothes easily: Connexin41. 8 regulates skin pattern variation. Pigment Cell Melanoma Res 2012;25:326–330 [DOI] [PubMed] [Google Scholar]
- 135.Watanabe M, Sawada R, Aramaki T, et al. The physiological characterization of connexin41. 8 and connexin39. 4, which are involved in the striped pattern formation of zebrafish. J Biol Chem 2016;291:1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang W-L, Wu H, Chiu Y-K, et al. The making of a flight feather: Bio-architectural principles and adaptation. Cell 2019;179:1409–1423. e1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferreira F, Luxardi G, Reid B, et al. Early bioelectric activities mediate redox-modulated regeneration. Development 2016;143:4582–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ferreira F, Raghunathan V, Luxardi G, et al. Early redox activities modulate Xenopus tail regeneration. Nat Commun 2018;9:4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niethammer P, Grabher C, Look AT, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Love NR, Chen Y, Ishibashi S, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol 2013;15:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Q, Wang Y, Man L, et al. Reactive oxygen species generated from skeletal muscles are required for gecko tail regeneration. Sci Rep 2016;6:20752. [DOI] [PMC free article] [PubMed] [Google Scholar]