Abstract
Over the past decade, electroconductive hydrogels, integrating both the biomimetic attributes of hydrogels and the electrochemical properties of conductive materials, have gained significant attention. Hydrogels, three-dimensional and swollen hydrophilic polymer networks, are an important class of tissue engineering (TE) scaffolds owing to their microstructural and mechanical properties, ability to mimic the native extracellular matrix, and promote tissue repair. However, hydrogels are intrinsically insulating and therefore unable to emulate the complex electrophysiological microenvironment of cardiac and neural tissues. To overcome this challenge, electroconductive materials, including carbon-based materials, nanoparticles, and polymers, have been incorporated within nonconductive hydrogels to replicate the electrical and biological characteristics of biological tissues. This review gives a brief introduction on the rational design of electroconductive hydrogels and their current applications in TE, especially for neural and cardiac regeneration. The recent progress and development trends of electroconductive hydrogels, their challenges, and clinical translatability, as well as their future perspectives, with a focus on advanced manufacturing technologies, are also discussed.
Keywords: hydrogels, scaffolds, conductive materials, tissue engineering, cardiac, neural
Introduction
Tissue engineering (TE) is the application of materials science, engineering, chemistry, and biology to replace, repair, or maintain biological tissues through the use of cells, scaffolds, and/or biochemical factors.1 Ideal TE scaffolds mimic the biological, chemical, and physical properties of the native extracellular matrix (ECM), which influences cell adhesion, differentiation, migration, and proliferation.2–4 Considering that several tissues in the body, including cardiac, cartilage, muscle, neural, and skin,5–12 require electrical synaptic interactions between cells to function, TE scaffolds for these specific tissues must also support bioelectrical signaling.2 For instance, constructs for cardiac and neural TE should mediate electrochemical communications between cardiomyocytes (CMs) for a synchronous beating of the heart or neurons for a functioning nervous system.13–15
Hydrogels, three-dimensional (3D) cross-linked networks consisting of hydrophilic natural and/or synthetic polymers, are excellent TE scaffolds since they inherently mimic aspects of the native ECM. Furthermore, their biological, mass transport, mechanical, and topological properties can be fine-tuned to fit a specific TE application.10,16–30 For instance, hydrogels can be synthesized from ECM components (e.g., collagen, fibronectin, and hyaluronic acid [HA]), providing cells with binding domains that dictate their behavior.3,31
Their architectures and porous structures can be adjusted to promote nutrient, waste, and solute diffusion and cell organization, attachment, and migration.2 In addition, their mechanical properties (e.g., elasticity, compressibility, and viscoelastic behavior) can regulate cell fate and function through mechanotransduction signaling.2,32 Finally, hydrogel topology, controlled by the incorporation of nanomaterials or microfabrication techniques, can influence cell adhesion and orientation.33 Although hydrogels are highly tunable, they typically have inadequate mechanical strength and are dielectric, limiting their widespread biomedical applications.2,33,34
Electroconductive hydrogels, biomaterials blended and/or hybridized with conductive materials, have recently been engineered to reinforce their mechanical and electroconductive properties while exhibiting some characteristics of biological tissues.31,34,35 Several types of electroconductive dopants have been used, including carbon-based materials (nanotubes, graphene), metallic nanoparticles (gold, silver), and polymers (polyaniline, polypyrrole (PPy), polythiophene, and their derivatives).34–36 However, most of these conductive materials are usually brittle, nonbiodegradable, and cytotoxic and need to be integrated into hydrogels.34 Electroconductive hydrogels are typically synthesized using the copolymerization of various polymers, including electroconductive polymers, or the blending of conductive particles within a nonconductive hydrophilic polymer network.37
In this review, we first discuss the latest progress on the rational design in electroconductive hydrogels for cardiac and neural TE (Table 1). We emphasize how electroconductive hydrogels have the potential to treat diseases of the cardiovascular and nervous system, encompassing a number of major causes of deaths globally. In the subsequent sections, we critically evaluate the challenges that need to be addressed for this class of biomaterials to be clinically relevant. Finally, we highlight a number of state-of-the-art manufacturing technologies applicable for the fabrication of advanced electroconductive hydrogels to further broaden their applications and expedite their bench-to-bedside translation.
Table 1.
Selected Studies Using Electroconductive Hydrogels for Cardiac and Neural Tissue Engineering
| Electroconductive material | Hydrogel composition | Findings | Refs. |
|---|---|---|---|
| Cardiac TE | |||
| Tetraaniline | Hyaluronic acid | Electroconductive gels loaded with plasmid DNA for eNOs nanocomplexes and ADSCs improved the electrical activity (QRS interval) and decreased the fibrosis area and infarct size of the heart in an MI rat model. The treatment also promoted vascularization (increased eNOs and VEGF-A expression). This work takes a unique approach by combining cardiac TE and gene therapy. | 54 |
| Aniline | PEGDA | Injectable PEGDA-based gels improved adipose-derived mesenchymal stem cell retention when injected into mice subcutaneously compared to PBS injections. The hydrogels synthesized in this study can spontaneously self-repair, an important feature for clinical translation. | 55 |
| PTAA | Gelatin | Compared to gels without PTAA, electroconductive gels increased Cx43 and cardiac troponin T expression of breast ADSCs in the presence of ES. | 56 |
| PEDOT:PSS | Collagen and alginate | Electroconductive gels promoted the synchronous beating of neonatal rat CMs, whereas nonconductive gels did not. These gels also induced a high beating rate and promoted cellular alignment, elongation, and a unidirectional orientation. Similar results were obtained with hiPSC-derived CMs. This study developed scaffolds that successfully differentiated hiPSCs into functional CMs. | 57 |
| Ppy | PEGDA, gelatin, and PETA | Electroactive gels that were painted onto infarcted mice hearts restored electrocardiogram wave patterns after 4 weeks. Mice treated with gels without PPy experienced an adverse cardiomegaly, whereas mice with electroconductive gels did not. The authors take a unique delivery approach by painting their material onto hearts, a technique less invasive than patching. | 59 |
| MWCNTs | Decellularized pericardial matrix | Gels with MWCNTs induced hiPSC synchronous beating, a faster beating rate, greater unidirectional orientation, Cx43 expression, and sarcomeric length compared to gels without MWCNTs and Matrigel. | 60 |
| MWCNTs | Decellularized pericardial matrix | Gels with MWCNTs increased Cx43 expression after 7 days of culture of CMs compared to gels without MWCNTs. | 61 |
| CNTs | GelMA | CMs cultured on a CNT-based network and encapsulated within a GelMA hydrogel increased CM uniaxial direction, Cx43 expression, and sarcomere length. Layered CNT-based networks within a GelMA hydrogel controlled distribution of CMs and endothelial cells within the scaffold. The scaffold in this study led to 3D cardiac anisotropy consisting of multiple cell types, which is highly relevant for clinical translation. | 62 |
| CNTs | Alginate and collagen | Electroconductive hydrogels improved human coronary artery endothelial cell attachment and elongation compared to nonconductive alginate gels. | 63 |
| Gold nanorods | GelMA | Gels with gold nanorods and nonconductive silica nanoparticles induced similar CM retention, expression of SAC, troponin I, and Cx43 and excitation thresholds. This study concluded that scaffold stiffness and topography play a major role in cardiac tissue function. | 64 |
| Gold nanorods | GelMA | Electroconductive gels induced improved uniaxial alignment and increased Cx43 expression compared to GelMA hydrogels. | 65 |
| Gold or silver nanoparticles | Collagen | Grafting gold nanoparticle and collagen patches onto the infarcted myocardium of rats stabilized electrical activity of the heart and increased vCM vasculogenesis (CD31+) and Cx43 expression after 7 days. | 66 |
| GO | Chitosan | Compared to chitosan gels, chitosan/GO gels increased H9C2 heart cell adhesion and Cx43 expression. | 67 |
| GO | Chitosan | Electroconductive gels increased human embryonic stem cell-derived fibroblasts and CM viability, proliferation, and beating rate compared to nonelectroconductive gels. | 68 |
| rGO | GelMA | rGO-containing GelMA hydrogels greatly increased the beating rate of CMs compared to pristine GelMA. These gels also had higher CM cell retention compared to GelMA gels with GO. This study demonstrated that rGO may be more effective in promoting cardiac tissue function than GO. | 69 |
| Bio-IL | GelMA or PEGDA | CMs and cardiac fibroblasts encapsulated within electroconductive GelMA gels had higher cell viability and metabolic activity compared to cells within GelMA gels after 7 days of culture. | 70 |
| Neural TE | |||
| PANI | PEGDA | Electroconductive gels supported proliferation and differentiation of NSCs but required ES for increased β3 tubulin and PMP22 expression. | 83 |
| MWCNT | PEGDA | A 3D-printed gel promoted NSC differentiation. PCR showed that differentiation was dependent on ES. | 85 |
| PPy | Collagen | Neuronal phenotypes were upregulated with or without ES when cultured on electroconductive collagen gels. The degree of differentiation depended on PPy content. This work demonstrated that neuronal differentiation may selectively be dependent on an exogenous ES. | 86 |
| PEDOT:PSS | PEGDA | It was determined that PEDOT:PSS could be 3D printed and used as a scaffold for DRG cells encapsulated in GelMA. PEDOT structures showed increased expression of neural phenotype proteins, but only with ES. | 90 |
| CAGNF | Alginate | CAGNF-functionalized alginate gels increased the number of neurite-bearing cells, as well as the neurite length, without ES. This study indicated that CAGNFs increase neurite proliferation with little-to-no inflammation response and do not require external stimulation, a promising result for clinical translation. | 91 |
| PEDOT | Chitosan | PEDOT-enhanced chitosan hydrogels increased spindle-shaped morphology and showed increased pseudopod presence on PC12 cells. | 92 |
| rGO and CNTs | PEG | Electroconductive PEG gels increased the number of neurite-bearing cells. This effect was enhanced by adding a positive charge with MTAC. This work demonstrated that in addition to electrical conductivity, neurite outgrowth can be synergistically enhanced when neuronal cells are exposed to positive charges. | 93 |
| Aniline | PGS | Aniline-containing hydrogels increased the expression of myelination genes (PMP22, NGF, BDNF, and Krox20) in Schwann cells. In addition, a neuritogenic media from Schwann cell-laden aniline gels increased PC12 neurite length, a result attributed to increased growth factor secretion (e.g., neurotrophin) by stimulated Schwann cells. | 94 |
| PPy | Alginate | PPy-alginate hydrogels significantly increased expression of neural differentiation genes Tuj1 and MAP2. Subcutaneous implantation for 8 weeks resulted in mild inflammation. | 96 |
3D, three-dimensional; ADSCs, adipose-derived stem cells; Bio-IL, bio-ionic liquid; CAGNF, citric acid-functionalized graphite nanofibers; CM, cardiomyocyte; CNT, carbon nanotube; Cx43, connexin 43; eNOs, endothelial nitric oxide synthase; ES, electrical stimulation; GelMA, gelatin methacryloyl; GO, graphene oxide; hiPSC, human induced pluripotent stem cell; MI, myocardial infarction; MTAC, 2-(methacryloyloxy)ethyltrimethylammonium chloride; MWCNT, multiwalled carbon nanotube; PANI, polyaniline; PCR, polymerase chain reaction; PEDOT:PSS, poly(3, 4-ethylenedioxythiophene):polystyrene sulfonate; PEG, polyethylene glycol; PEGDA, polyethylene glycol diacrylate; PETA, pentaerythritol triacrylate; PGS, poly(glycerol sebacate); PPy, polypyrrole; PTAA, poly(thiophene-3-acetic acid); rGO, reduced graphene oxide; SAC, sarcomeric α-actinin; TE, tissue engineering; VEGF-A, vascular endothelial growth factor A.
Cardiac TE
Cardiovascular disease (CVD) is the number one cause of death globally, responsible for 17.9 million deaths in 2017 and costing over $32 billion per year in U.S. health care costs.38,39 The most common type of CVD, coronary artery disease, typically leads to a myocardial infarction (MI), in which blood flow to the heart is restricted. MI-induced ischemia leads to the permanent loss of nearly a billion of CMs, cells known to have limited regenerative capacities, in humans.40 Furthermore, increased secretion of matrix metalloproteinases and inflammation result in nonfunctional scar tissue deposition, leading to decreased cardiac output, and often heart failure.41 In fact, MI prognosis is poor with a 50% mortality rate within 5 years of diagnosis.42 A heart transplantation is often required, a complex treatment that suffers from several postsurgery complications and a shortage of donor organs.43,44
A combination of global health policies and lack of therapies for MI have driven several treatments into the clinic, including cell therapy45,46 and tissue-engineered cardiac patches.47,48 These strategies aim to mitigate fibrosis and stiffening of the heart, restore contractile function, and enhance muscle regeneration. However, the results have been modest at best due to several challenges such as (1) poor cell survival, retention, and function, (2) invasive surgery, and (3) inadequate electromechanical integration of transplanted stem cells.49–53
Electroconductive hydrogels have the potential to address a number of these limitations and ultimately succeed in the clinic. The incorporation of electroconductive materials within hydrogels, including conductive polymers,54–59 nanomaterials (e.g., carbon nanotubes [CNTs] and metallic nanoparticles),60–66 graphene oxide (GO),67–69 and bionic liquids,70 can enhance their electroconductivity and ability to restore the contractile function of the heart. For example, Shin et al. incorporated reduced graphene oxide (rGO) sheets into gelatin methacryloyl (GelMA) hydrogels.69 rGO lowered the impedance values, and at a concentration of 1 mg/mL, rGO-containing hybrid hydrogels had a much lower impedance (∼4 kΩ) than hydrogels made with nonreduced GO (∼120 kΩ).
Based on sarcomeric α-actinin (SAC) and connexin 43 (Cx43) immunofluorescent stainings in primary rat CMs, rGO-containing hydrogels exhibited more uniaxially aligned sarcomeric structures and enhanced cell–cell coupling. In addition, GelMA hydrogels hybridized with 5 mg/mL of rGO induced a much higher beating rate of CMs than rGO-free GelMA hydrogels (∼80 bpm vs. ∼10 bpm at day 6, respectively).69
The incorporation of electroconductive nanomaterials into hydrogels not only influences the bulk electrical properties but also the topography, which has been shown to influence cell retention and biology.71 This was explored by Navaei et al., who incorporated nonconductive silica nanomaterials and electroconductive gold nanorods into GelMA hydrogels.64 Interestingly, unlike nonhybridized GelMA, both hybridized gel types had similar CM retention, expression of SAC, troponin I, and Cx43. This work indicated that nonconductive nanomaterials influenced gel topography and subsequently enhanced CM maturation and function.64
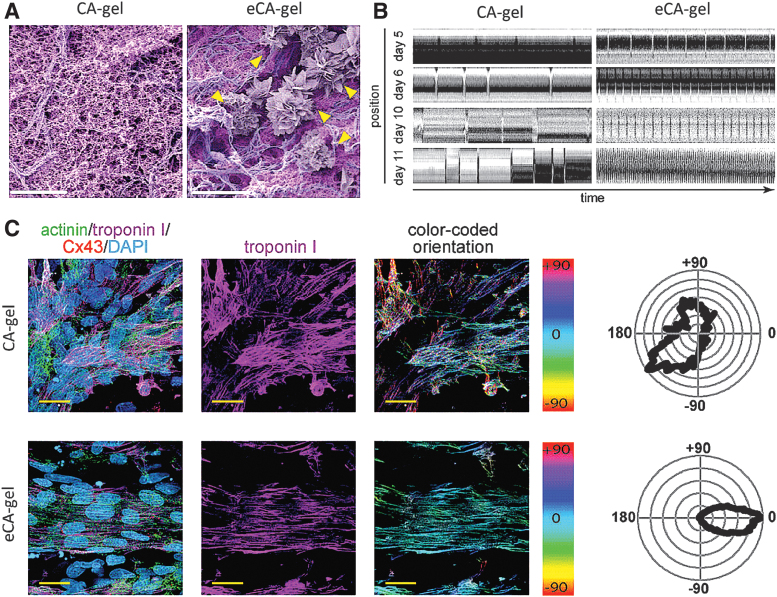
Arrhythmias, irregular or abnormal heartbeats, resulting from cell therapies and other cardiac TE strategies are also a major concern.72,73 Several studies, both in vitro and in vivo, have demonstrated that electroconductive hydrogels support synchronous beating of noncontacting CMs.57,60,66 For instance, Roshanbinfar et al. engineered electrically conductive hydrogels (eCA-gels) made with collagen, alginate, and poly(3, 4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS).57 The presence of PEDOT:PSS supported well-organized sand rose-like structures (Fig. 1A). eCA-gels promoted rhythmic beating of neonatal rat CMs, whereas PEDOT:PSS-free gels (CA-gels) did not (Fig. 1B).
FIG. 1.
Alginate, collagen, and PEDOT:PSS-based hydrogels promote maturation of primary rat CMs. (A) Colored SEM images of collagen/alginate hydrogels (CA-gel) and PEDOT:PSS/collagen/alginate hydrogels (eCA-gel). Yellow arrows indicate sand rose-like structures induced by PEDOT:PSS. (B) Kymograph analysis visualizing autonomous contractions (peaks) of neonatal rat CMs cultured on CA-gels and eCA-gels after 11 days. (C) Representative confocal images of neonatal rat CMs cultured on CA-gels or eCA-gels. Immunofluorescent staining was used to determine cell orientation distribution. Actinin: green, Troponin I: purple, Cx43: red, and DAPI, turquoise. Scale bars: 5 μm (A) and 100 μm (C). Adapted from Roshanbinfar et al.57 with permission from Wiley. CMs, cardiomyocytes; Cx43, connexin 43; PEDOT:PSS, poly(3, 4-ethylenedioxythiophene):polystyrene sulfonate.
Interestingly, eCA-gels induced beating frequencies of ∼220 per minute after 11 days of culture, equivalent to the resting heartbeat of newborn rats. Immunofluorescent staining of CMs on both gel types revealed that eCA-gels promoted cellular alignment, elongation, and a unidirectional orientation (Fig. 1C). Finally, similar results were obtained with human induced pluripotent stem cell-derived CMs, a more clinically relevant cell line.57
Injectable biomaterials, including injectable electroconductive hydrogels,54,55,58 obviate the need for open surgery and mitigate health care-associated infections and postsurgical complications.24,74 With respect to cardiac repair, injectable hydrogels may be deployed into the native myocardium, delivering cells with high viability and improved local retention. For example, Dong et al. demonstrated that injecting C2C12 myoblasts encapsulated within a composite chitosan and polyethylene glycol (PEG)-based hydrogel did not alter cell viability.55
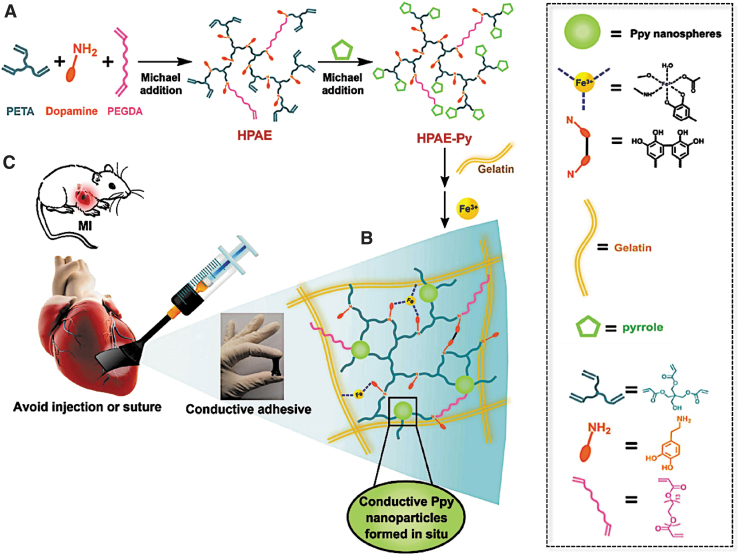
Going even further, Liang et al. used a two-step Michael addition reaction to synthesize composite hydrogels containing gelatin, polyethylene glycol diacrylate (PEGDA), and PPy (hyperbranched poly(amino ester) [HPAE]-Py/Gelatin). Their flowable biomaterial was directly painted onto the infarcted myocardium, circumventing potential damage from the injection or patch suturing (Fig. 2).59 The gelation time was ∼8 s, such that they could be applied to the myocardium without leaking. When tested in an MI rat model, HPAE-Py/Gelatin or HPAE/Gelatin hydrogels were applied to the infarcted area using a brush attached to a syringe.
FIG. 2.
Adhesive and electroconductive gelatin and PEGDA-based hydrogels can be directly painted onto infarcted myocardium. (A) Schematic depicting a two-step synthesis of HPAE-Py. First, dopamine hydrochloride, PETA, and PEGDA are reacted together using Michael addition to form HPAE. Next, pyrrole is reacted with HPAE using a second Michael addition reaction to form HPAE-Py. (B) Schematic showing Fe3+-mediated cross-linking of Gelatin with HPAE-Py, improving gel adhesion through catechol-Fe3+ complexation. (C) HPAE-Py/Gelatin hydrogels can be directly applied to infarcted myocardium using a brush-syringe system. Once applied, the gel rapidly adheres to the myocardium without leaking. Adapted from Liang et al.59 with permission from John Wiley and Sons. HPAE, hyperbranched poly(amino ester); PEGDA, polyethylene glycol diacrylate; PETA, pentaerythritol triacrylate.
After 4 weeks, both hydrogels were able to restore wave propagation, leading to a coordinated contraction and normal heartbeat. However, unlike nonconductive HPAE/Gelatin hydrogels, hearts treated with HPAE-Py/Gelatin hydrogels exhibited a lower infarct size, lower degree of fibrosis, higher left ventricle wall thickness, and avoided adverse cardiomegaly (e.g., enlarged heart).59
In another example, Wang et al. injected HA/PEG/tetraaniline-based hydrogels loaded with adipose-derived stem cells into the infarcted myocardium of rats.54 This therapy restored cardiac output and decreased fibrosis in the infarcted area. Equally important, this study addressed another major challenge facing clinical implementation, the lack of vascularization of transplanted cells or tissues.51,53 The hydrogels were loaded with endothelial nitric oxide synthase (eNOs) encoding plasmid DNA to promote angiogenesis. When tested in mice, the gene therapy successfully increased expression of eNOs and four myocardium-related genes: vascular endothelial growth factor A (VEGF-A), Angiopoietin 1 (Ang-1), Cx43, and Cadherin 2 (Cdh-2).55
To be clinically relevant, cardiac patches must withstand the dynamic stress environment on the surface of the heart during a cardiac cycle.75 The wall of a healthy heart can stiffen up to tenfold between diastole and systole due to the active mechanical properties of CMs. To this end, self-healing hydrogels have been engineered to recapitulate the ability of native tissues to regenerate through the formation of new chemical bonds.76 Jing et al. synthesized chitosan-based hydrogels hybridized with GO and reported their self-healing properties.68 When cut into two pieces, the hydrogel rapidly repaired itself and recovered its original precut mechanical properties. The self-healing properties were attributed to covalent bonds, supramolecular interactions, hydrogen bonds, and π-π stacking between chitosan and GO.68
Dong et al. described similar properties with chitosan-grafted-aniline and PEG composite hydrogels.55 Other studies have indicated that the incorporation of additional electroconductive materials into hydrogels can influence their elastic properties. For instance, Yang et al. engineered double network hydrogels based on gelatin and poly(thiophene-3-acetic acid).56 When tested for their mechanical properties, the Young's moduli values ranged from ∼20 to 500 kPa depending on the concentration of gelatin.56 In another example, Noshadi et al. synthesized GelMA or PEG diacrylate hydrogels, both covalently conjugated with conductive choline-based bio-ionic liquid (Bio-IL). Values for their Young's moduli ranged from ∼5–101 and 3–173 kPa, respectively.70 The mechanical properties were dependent on the polymer concentration and ratio of polymer to Bio-IL.70
Neural TE
Electroconductive hydrogels can also be applied toward repairing or regenerating neural tissues, supporting endogenous cell signaling or delivery of exogenous electrical stimulation (ES).77 Neural injury and associated diseases affect almost 1 billion people around the world.78 Unfortunately, current treatments for the central nervous system (CNS) and peripheral nervous system (PNS) are lacking.79
CNS injuries include traumatic brain and spinal cord injury, stroke, tumors, and neurodegenerative diseases such as Huntington's, prions, and Alzheimer's.80 Unlike the CNS, which has very limited regenerative capacity, the PNS is capable of self-regeneration although it has been associated with poor clinical outcomes.79 Common PNS injuries include traumas such as falls, motor vehicle accidents, violence, or occupational hazards.80 More rarely, PNS loss of function is associated with inflammatory or degenerative diseases such as Guillain-Barré, causing permanent damage to peripheral nerves.81
TE scaffolds have been proposed as a potential off-the-shelf method to facilitate neuroregeneration. For instance, soft hydrogels have been explored to promote nerve growth in vivo.80 When hybridized with conductive polymers, hydrogels have the potential to drive stem cell differentiation into neurons and encourage nerve repair.
Under ES, electroconductive hydrogels (e.g., PPy-based hydrogels) have shown to differentiate human neural stem cells (hNSCs) into neurons with longer neurites.82–85 Specifically, this approach increased gene expression of class III β-tubulin (Tuj1), a gene responsible for neuronal phenotype, over glial fibrillary acidic protein (GFAP), a gene associated with astrocyte phenotype. However, beyond the on/off exogenous stimulation, far more complex mechanisms may play a role in guiding and maintaining cell differentiation.
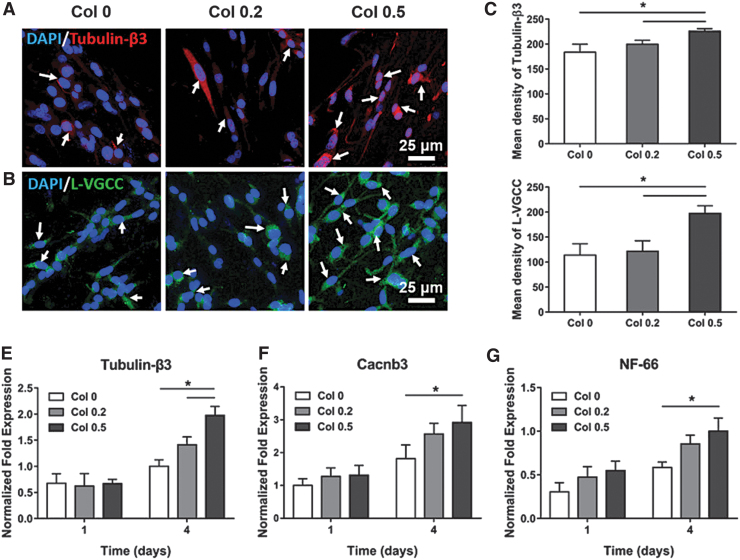
For example, another study showed that neurite outgrowth was dependent on the strength of the electric field applied, suggesting that neural differentiation is dependent upon the electroconductivity and external ES.84 Furthermore, the ES was able to increase nonspecific NSC differentiation toward neuronal and glial phenotypes.83 However, the differentiation may not depend solely on one or the other. Recent studies have indicated that the influence of collagen-PPy hydrogels on electrically stimulated PC12 cells, a classical neuronal cell model, could selectively differentiate these cells into a specific phenotype. Differentiated PC12 cells exhibited high expressions of tubulin-β3 and L-VGCC, two proteins indicative of neurogenesis (Fig. 3).86
FIG. 3.
Collagen-PPy hydrogel microfibers improve PC12 neurogenesis. (A, B) Immunofluorescent staining of Tubulin-β3 (red), L-VGCC (green), and DAPI (blue) of PC12 cells on several compositions of Collagen-PPy hydrogels, Arrows indicate immunostaining of Tubulin-β3 and L-VGCC. (C, D) Semiquantitation of Tubulin-β3 and L-VGCC expression. (E–G) Normalized fold changes of gene expression of Tubulin-β3, Cacnb3, and NF-66. * indicate significance at p < 0.05, n = 3. Adapted from Wu et al.86 with permission from American Chemical Society. PPy, polypyrrole.
In addition, the ES can also be harnessed to control the delivery of relevant drugs without altering neuron cell differentiation. Zarrintaj et al. cultured PC12 cells on a gelatin-aniline hydrogel which released embedded dexamethasone (an anti-inflammatory/immune suppressor) upon application of electrical current.87 Bagheri et al. built on this work using a chitosan-aniline gel, which also demonstrated superior biocompatibility and tunable drug release properties upon stimulation.88
To promote nerve regeneration, given the challenge and time-consuming nature of administering ES in vivo, stimulation-free conductive hydrogels are arguably more promising for clinical applications.89 Unfortunately, only a few stimulation-free hydrogels exhibiting such properties have been reported.90 Homaeigohar et al. showed that hydrogels functionalized with multiwalled carbon nanotubes (MWCNTs) promoted the total number of neurite-bearing cells.91 Lee et al. indicated that 3D-printed scaffolds with similar MWCNTs enhanced neurite length.85
Similarly, chitosan-based gels layered with PEDOT have also displayed a promising regenerative capacity.92 Recently, Liu et al. reported that formulating electroconductive hydrogels with poly(2-(methacryloyloxy)ethyl)trimethylammonium chloride, a hydrogen bond donor, significantly increased the population of neurite-bearing cells.93 In addition to enhancing neurite outgrowth, these hydrogels can also upregulate secretion of neurotrophic factors (e.g., neurotrophins) by Schwann cells for the survival, development, and function of neurons.94 Collectively, these properties present an opportunity for peripheral nerve regeneration following injuries.
Hydrogels, once blended with conductive polymers, tend to exhibit a higher mechanical strength. For instance, Xu et al. layered PEDOT on carboxymethyl chitosan to engineer a conductive scaffold with a Young's modulus reaching up to ∼12.5 kPa. This value is close to the human brain tissues (∼1.2 kPa),95 and the hydrogels displayed higher cell adhesion compared to their nonconductive counterparts.92 In addition, Yang et al. described that adding only 10 mM PPy to the alginate formulation resulted in a dramatic increase in gel stiffness, with a Young's modulus surging from 21 to 178 kPa.96
Interestingly, in another study, Homaeigohar et al. suggested that a scaffold with a higher Young's modulus compared with native neural tissues could enhance neurite extension. Their alginate-based hydrogels were reinforced when blended with graphite nanofilaments, resulting in a high Young's modulus (∼56 MPa) and improved neurite growth.91
Advanced Manufacturing Technologies
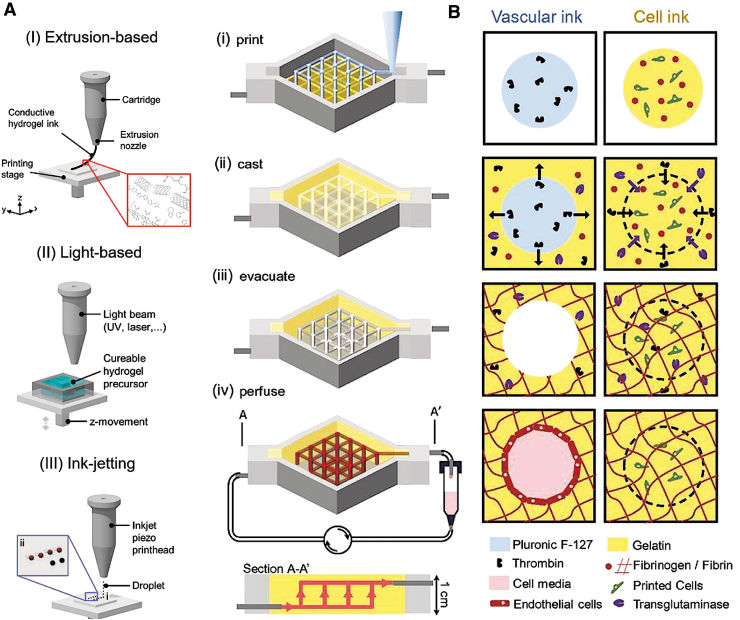
State-of-the-art 3D printing and sophisticated bioreactors represent advanced manufacturing technologies that can accelerate the clinical translatability of electroconductive hydrogels. Three-dimensional printing provides unprecedented control over scaffold design, including geometry, anisotropy, pore size, and topography.97,98 In this context, various techniques have been explored to print electroconductive hydrogels in 3D, such as extrusion-based printing, light-based printing, and ink jetting (Fig. 4A).37,97,98
FIG. 4.
Three-dimensional printing technology for the fabrication of electroconductive hydrogels and vascularized networks. (A) Various technologies used for 3D printing electroconductive hydrogels. (I) Extrusion-based printing utilizes pneumatic or mechanical pressure to depose the gel or gel precursors. (II) Laser-based printing uses either a light or laser to cross-link gel precursors in a defined pattern. (III) Ink-jetting involves the deposition of gel or gel precursors into a predefined pattern using thermal or piezoelectric energy. (B) Schematic describing the formation of a vascularized network within a 3D perfusion chip. (i) Print: Vascular ink, consisting of polyethylene oxide-polypropylene oxide-polyethylene oxide (Pluronic® F-127), and cell-laden ink (gelatin, fibrin, and cells) are printed onto silicone and glass-based chips. (ii) Cast: Next, an ECM-mimetic, which is similar to the cell-laden ink but also contains thrombin and TG, is cast onto the chip. Thrombin and TG induce fast polymerization of fibrinogen and slow polymerization of fibrinogen and gelatin, respectively, in both the ECM and cell ink. (iii) Evacuate: Chips are cooled down, causing a gel–fluid transition of the vascular ink, leaving behind a vascular network within the chip. (iv) Perfuse: The vascular network is then cellularized with endothelial cells using a perfusion system. (A) reproduced from Distler and Boccacini37 with permission from Elsevier. (B) Reproduced from Kolesky et al.108 with permission from National Academy of Sciences. 3D, three-dimensional; ECM, extracellular matrix; TG, transglutaminase.
Although some of these technologies were used for the fabrication of electroconductive hydrogels, such as those made with CNTs63,99–101 and conductive polymers,102–107 only a handful have been applied toward cardiac63 and neural99,103 TE. For instance, using stereolithography, Lee et al. encapsulated MWCNTs within 3D printed PEGDA hydrogels, while finely controlling their porous microarchitecture.99 They reported the beneficial effect of fine-tuning pore size on hNSC growth and length.99
Taking the technology one step forward, 3D printing has been leveraged to print biological materials (e.g., cells and bioinks). This technique, known as bioprinting, has been used to print cell-laden hydrogels while precisely controlling the spatial arrangement of growth factors and cells within the matrix.97,98 These attributes could address some of the challenges that electroconductive hydrogels are facing, including the homogenous distribution of cells within the gel constructs, the integration of various cell types in an anisotropic manner to better mimic native signal propagation, and the induction of neovascularization in vivo.
For instance, blood vessel formation has been facilitated by fine-tuning pore size and by incorporating pro-angiogenic growth factors.98,108–110 Using a sacrificial ink strategy, Kolesky et al. vascularized a 3D perfusion chip using a temperature-sensitive tri-block copolymer-based bioink that was removed by cooling, leaving behind a macroporous network (Fig. 4B).108 The construct was subsequently cellularized with human umbilical vein endothelial cells, a step required for differentiating human mesenchymal stem cells.108 A similar strategy could be used to create a vascularization network within electroconductive hydrogels. Although, to date, there is no report on bioprinted cell-laden electroconductive hydrogels, this technique has a tremendous potential to further advance the field.37
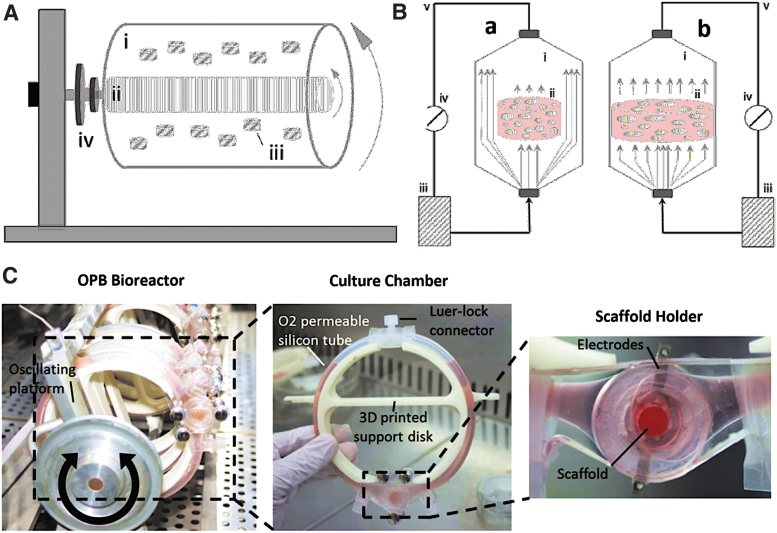
Bioreactors, another key manufacturing technology, represent a scalable, reproducible, automated, and sterile process to support the fabrication of new tissues in vitro (Fig. 5A, B). This technique allows a dynamic culture condition while mimicking the native physiological environment of cells by controlling oxygen tension, carbon dioxide concentration, pH, and nutrient levels.111,112 Bioreactors have also been designed to electrically stimulate cell-laden scaffolds to drive functional maturation of cells to the desired phenotype.113–115
FIG. 5.
TE bioreactors for dynamic cell culture integrating electroconductive hydrogels. (A) Schematic illustration of a rotary bioreactor consisting of outer (i) and inner (ii) cylinders, cell-laden scaffolds (iii), and the rotator support (iv). These systems are completely filled with liquid medium, in which gas is transferred using a silicon-rubber gas-transfer membrane. (B) Schematic illustration of indirect (a) and direct (b) perfusion bioreactors, including the culture chambers (i), cell-laden constructs (ii), culture medium (iii), peristaltic pumps (iv), and tubing (v). In indirect perfusion, perfusing medium circumvents the constructs, whereas in direct perfusion, constructs are tightly fitted within the chamber such that medium perfuses through them. (C) The OPB can host up to 18 cell-laden scaffolds simultaneously. The culture chamber, consisting of 3D-printed PDMS, promotes ES, perfusion of media, and real-time monitoring (digital microscopy) for each scaffold. (A, B) Reproduced from Sladkova et al. under the terms and conditions of the Creative Commons Attribution License 3.0.121 (C) Reproduced from Visone et al. under the terms and conditions of the Creative Commons Attribution License 4.0.115 OPB, oscillating perfusion bioreactor; PDMS, polydimethylsiloxane; TE, tissue engineering.
For instance, Visone et al. designed an oscillating perfusion bioreactor (OPB) that simultaneously provided bidirectional perfusion of nutrients, ES, and real-time monitoring of cell-laden constructs (Fig. 5C).115 When neonatal rat CMs were cultured in Matrigel-integrated OPB, cells exhibited high cell viability and differentiation as indicated by coordinated contraction, troponin I staining, and a lowered excitation threshold.115
Although bioreactors are well suited to endure ES, they have not been explored with electroconductive hydrogels yet. Increasing evidence suggests that leveraging electroconductive hydrogels with bioreactors may have a synergistic effect. In fact, combining both 3D bioprinting and bioreactor-assisted cell-laden scaffolds may hold the key to their translational therapeutic applications in the regeneration of cardiac and neural tissues.
Clinical Outlook and Future Perspectives
Over the past decade, tremendous progress has been achieved in the fields of cardiac and neural TE. One major milestone has been the utilization of electroconductive materials to recapitulate the conductive nature of myocardium and nervous tissues. Going one step further, the incorporation of conductive polymers and fillers into physiologically inspired hydrogels has addressed a number of their current limitations, particularly in alleviating biocompatibility concerns.
For cardiac TE, electroconductivity promotes cell–cell coupling (Cx43),57,60,62,66,69 cell elongation,57,60,62 and cell alignment,57,60,62 driving a mature CM phenotype. Interestingly, Navaei et al. found that nonconductive silica nanomaterials and conductive gold nanorods showed the same improvement with respect to CM cell–cell coupling, cell elongation, beating frequency, and excitation threshold.64 This finding suggests that the topographical effect of the substrates on CM fate needs to be further explored.
For neural TE, it has been established that electroconductivity can substantially improve neuron elongation,86,91,93 but additional measures of efficacy and toxicology are required before implementing electroconductive biomaterials into clinical applications. Since inclusion of electroconductive components can not only change the electrical properties but also the physical properties of hydrogels such as mechanics and topography, they provide a promising tool for TE and regeneration.
A number of reported electroconductive hydrogels for myocardium repair have performed remarkably well in a rat MI model,54,59 indicating their readiness for clinical consideration. However, to date, electroconductive hydrogels have several challenges that must be overcome. In terms of their biocompatibility, short- and long-term biocompatibility of electroconductive hydrogels should be carefully studied in representative animal models, for example, rat MI model. This is important, especially since some electroconductive materials are inherently cytotoxic.
In addition, in vivo studies are also required to evaluate their biodegradation, which may be significantly different from in vitro studies, due to our inability to emulate precisely the native microenvironment outside the human body. In fact, the biodistribution of hydrogels' degradation products needs to be carefully explored to evaluate the extent of systemic toxicity and immunogenicity. Furthermore, the electrochemical stability of these gels needs to be explored as electroconductivity is dependent on the environment and may change over time.
Finally, before being introduced into the human body, electroconductive hydrogels must be properly sterilized according to the Food and Drug Administration (FDA) guidelines. Recently, our group has demonstrated that various standard hydrogels do not withstand the extreme conditions of high-pressure steam sterilization such as autoclaving,116,117 a technique widely used in the field and FDA approved. As a result, other FDA-approved sterilization methods need to be investigated.116–118
With regards to cardiac TE, the amount of attention that has been placed toward electroconductivity needs to be equally given to hydrogel composition, stiffness, topography, and route of administration. In addition, the cell type selected (e.g., cardiac progenitor, mesenchymal stem cells, and bone marrow-derived stem cells) must be critically evaluated and considered on a case-by-case basis.50,119 Addressing one of the major challenges of TE, angiogenesis must be promoted to ensure blood supply and survival of transplanted cells.53 As a proof of concept, Wang et al. reported an increased survival of transplanted cells with angiogenic gene therapy.54 Yet, implementing this approach appends an additional degree of complexity to the current regulatory process.
For neural TE, a number of studies did not thoroughly evaluate the hydrogels' physical properties, which should be a prerequisite, given the importance of biomechanical cues on stem cell fate and function.82,84 Although conductivity appears to have predominantly more effect on neural phenotypes than mechanics, further investigations are required to decouple these confounding factors. In addition, future studies should examine more thoroughly not only neural cell differentiation but also protein and RNA expression.
Finally, it is recommended that follow-up in vivo studies are performed to confirm whether these electroconductive hydrogel candidates have the potential to promote nerve tissue regeneration in relevant medical conditions, such as for the treatment of PNS or CNS traumatic injuries. While outside the scope of this review, it is important to highlight that electroconductive hydrogels have great potential to improve the electrode-tissue integration and stability for brain–computer interface technologies.120
As Alzheimer's CNS and CVD remain among the major causes of death worldwide, the future of neural and cardiac TE holds great potential. Electroconductive hydrogels represent a very unique platform for treating these diseases and improving the quality of human life. The combination of several approaches such as 3D bioprinting, bioreactors, and stem cell engineering with electroconductive hydrogels could further advance the development of cardiac and neural tissues or their corresponding organs in their entirety.
Confirmation Statement
Z.J.R., M.P.Z., and S.A.B. conceived this topic and designed an outline. Z.J.R., M.P.Z., R.K., and S.A.B contributed to the writing and/or editing of this article. All coauthors have reviewed and approved of the article before submission. This article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work is supported by the National Science Foundation (DMR 1847843), Massachusetts Technology Transfer Center (MTTC) Acorn Innovation Award, and Northeastern University GapFund360 Award.
References
- 1.Vacanti JP, Vacanti CA. Chapter 1—The history and scope of tissue engineering. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 3–8 [Google Scholar]
- 2.Luo Y, Engelmayr G, Auguste DT, et al. Chapter 24—3D scaffolds. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 475–494 [Google Scholar]
- 3.Hubbell JA. Chapter 21—Matrix effects. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 407–421 [Google Scholar]
- 4.Saltzman WM, Kyriakides TR. Chapter 20—Cell interactions with polymers. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 385–406 [Google Scholar]
- 5.Eslahi N, Abdorahim M, Simchi A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016;17:3441–3463 [DOI] [PubMed] [Google Scholar]
- 6.Dong R, Ma PX, Guo B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020;229:119584. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Wu Y, Guo B, et al. Nanofiber yarn/hydrogel core–shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano 2015;9:9167–9179 [DOI] [PubMed] [Google Scholar]
- 8.Li M, Chen J, Shi M, et al. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem Eng J 2019;375:121999 [Google Scholar]
- 9.Liang Y, Chen B, Li M, et al. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules 2020;21:1841–1852 [DOI] [PubMed] [Google Scholar]
- 10.Memic A, Abudula T, Mohammed HS, et al. Latest progress in electrospun nanofibers for wound healing applications. ACS Appl Bio Mater 2019;2:952–969 [DOI] [PubMed] [Google Scholar]
- 11.Qu J, Zhao X, Liang Y, et al. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem Eng J 2019;362:548–560 [Google Scholar]
- 12.He J, Shi M, Liang Y, et al. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem Eng J 2020;394:124888 [Google Scholar]
- 13.Koppes AN, Keating KW, McGregor AL, et al. Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels. Acta Biomater 2016;39:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppes RA, Park S, Hood T, et al. Thermally drawn fibers as nerve guidance scaffolds. Biomaterials 2016;81:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer AR, Primbetova A, Koppes AN, et al. Electroconductive gelatin methacryloyl-PEDOT:PSS composite hydrogels: Design, synthesis, and properties. ACS Biomater Sci Eng 2018;4:1558–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bencherif SA, Sheehan JA, Hollinger JO, et al. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J Biomed Mater Res A 2009;90A:142–153 [DOI] [PubMed] [Google Scholar]
- 17.Gsib O, Deneufchatel M, Goczkowski M, et al. FibriDerm: Interpenetrated fibrin scaffolds for the construction of human skin equivalents for full thickness burns. IRBM 2018;39:103–108 [Google Scholar]
- 18.Gsib O, Duval J-L, Goczkowski M, et al. Evaluation of fibrin-based interpenetrating polymer networks as potential biomaterials for tissue engineering. Nanomaterials (Basel) 2017;7:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han M-E, Kim S-H, Kim HD, et al. Extracellular matrix-based cryogels for cartilage tissue engineering. Int J Biol Macromol 2016;93:1410–1419 [DOI] [PubMed] [Google Scholar]
- 20.Joshi Navare K, Eggermont L, Rogers Z, et al. Antimicrobial hydrogels: Key considerations and engineering strategies for biomedical applications. In: Li B, Moriarty TF, Webster Webster T, Xing M, eds. Racing for the Surface. Cham, Switzerland: Springer, 2020: 511–542 [Google Scholar]
- 21.Kim J, Bencherif SA, Li WA, et al. Cell-friendly inverse opal-like hydrogels for a spatially separated co-culture system. Macromol Rapid Commun 2014;35:1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin-Gibson S, Bencherif S, Antonucci JM, et al. Synthesis and characterization of poly(ethylene glycol) dimethacrylate hydrogels. Macromol Symp 2005;227:243–254 [Google Scholar]
- 23.Rogers JZ, Bencherif AS. Cryogelation and cryogels. Gels 2019;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Memic A, Colombani T, Eggermont LJ, et al. Latest advances in cryogel technology for biomedical applications. Adv Ther 2019;2:1800114 [Google Scholar]
- 25.Rezaeeyazdi M, Colombani T, Memic A, et al. Injectable hyaluronic acid-co-gelatin cryogels for tissue-engineering applications. Materials (Basel) 2018;11:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy S, Bencherif S, Norton D, et al. Rapid and extensive collapse from electrically responsive macroporous hydrogels. Adv Healthc Mater 2014;3:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Béduer A, Braschler T, Peric O, et al. A compressible scaffold for minimally invasive delivery of large intact neuronal networks. Adv Healthc Mater 2015;4:301–312 [DOI] [PubMed] [Google Scholar]
- 28.Bencherif SA, Braschler TM, Renaud P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J Periodontal Implant Sci 2013;43:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bencherif SA, Sands RW, Bhatta D, et al. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci U S A 2012;109:19590–19595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bencherif SA, Warren Sands R, Ali OA, et al. Injectable cryogel-based whole-cell cancer vaccines. Nat Commun 2015;6:7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Gao X, Ullah MW, et al. Electroconductive natural polymer-based hydrogels. Biomaterials 2016;111:40–54 [DOI] [PubMed] [Google Scholar]
- 32.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003;24:4337–4351 [DOI] [PubMed] [Google Scholar]
- 33.Trachtenberg JE, Kasper FK, Mikos AG. Chapter 22—Polymer scaffold fabrication. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 423–440 [Google Scholar]
- 34.Walker BW, Portillo Lara R, Mogadam E, et al. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog Polym Sci 2019;92:135–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu H, Zhang N, Ma M. Electroconductive hydrogels for biomedical applications. WIREs Nanomed Nanobiotechnol 2019;11:e1568. [DOI] [PubMed] [Google Scholar]
- 36.Mostafavi E, Medina-Cruz D, Kalantari K, et al. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity 2020;2:120–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Distler T, Boccaccini AR. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors—A review. Acta Biomater 2020;101:1–13 [DOI] [PubMed] [Google Scholar]
- 38.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Titler MG, Jensen GA, Dochterman JM, et al. Cost of hospital care for older adults with heart failure: Medical, pharmaceutical, and nursing costs. Health Serv Res 2008;43:635–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol 2005;23:845–856 [DOI] [PubMed] [Google Scholar]
- 41.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 2016;365:563–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Go Alan S, Mozaffarian D, Roger Véronique L, et al. Executive summary: Heart disease and stroke statistics—2013 Update. Circulation 2013;127:143–152 [DOI] [PubMed] [Google Scholar]
- 43.Alba AC, Bain E, Ng N, et al. Complications after heart transplantation: Hope for the best, but prepare for the worst. Int J Transplant Res Med 2016;2:022 [Google Scholar]
- 44.Gupta T, Krim SR. Cardiac transplantation: Update on a road less traveled. Ochsner J 2019;19:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Florea V, Rieger Angela C, DiFede Darcy L, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study). Circ Res 2017;121:1279–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karantalis V, DiFede Darcy L, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting. Circ Res 2014;114:1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chachques JC, Trainini JC, Lago N, et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM Trial): Clinical feasibility study. Ann Thorac Surg 2008;85:901–908 [DOI] [PubMed] [Google Scholar]
- 48.Menasché P, Vanneaux V, Hagège A, et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 2018;71:429–438 [DOI] [PubMed] [Google Scholar]
- 49.Broughton KM, Sussman MA. Cardiac tissue engineering therapeutic products to enhance myocardial contractility. J Muscle Res Cell Motil 2019. [Epub ahead of print]; DOI: 10.1007/s10974-019-09570-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broughton KM, Sussman MA. Empowering adult stem cells for myocardial regeneration V2.0: Success in small steps. Circ Res 2016;118:867–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorsey SM, Burdick JA. Hydrogels in cardiac tissue engineering. In: Abidian MR, Gurkan UA, Edalat F, eds. Gels Handbook. Hackensack, NJ: World Scientific, 2016: 323–361 [Google Scholar]
- 52.Tomov ML, Gil CJ, Cetnar A, et al. Engineering functional cardiac tissues for regenerative medicine applications. Curr Cardiol Rep 2019;21:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogle BM, Bursac N, Domian I, et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med 2016;8:342ps313–342ps313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W, Tan B, Chen J, et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018;160:69–81 [DOI] [PubMed] [Google Scholar]
- 55.Dong R, Zhao X, Guo B, et al. Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl Mater Interfaces 2016;8:17138–17150 [DOI] [PubMed] [Google Scholar]
- 56.Yang B, Yao F, Hao T, et al. Development of electrically conductive double-network hydrogels via one-step facile strategy for cardiac tissue engineering. Adv Healthc Mater 2016;5:474–488 [DOI] [PubMed] [Google Scholar]
- 57.Roshanbinfar K, Vogt L, Greber B, et al. Electroconductive biohybrid hydrogel for enhanced maturation and beating properties of engineered cardiac tissues. Adv Funct Mater 2018;28:1803951 [Google Scholar]
- 58.Komeri R, Muthu J. Injectable, cytocompatible, elastic, free radical scavenging and electroconductive hydrogel for cardiac cell encapsulation. Colloids Surf B Biointerfaces 2017;157:381–390 [DOI] [PubMed] [Google Scholar]
- 59.Liang S, Zhang Y, Wang H, et al. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv Mater 2018;30:1704235. [DOI] [PubMed] [Google Scholar]
- 60.Roshanbinfar K, Mohammadi Z, Sheikh-Mahdi Mesgar A, et al. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater Sci 2019;7:3906–3917 [DOI] [PubMed] [Google Scholar]
- 61.Roshanbinfar K, Hilborn J, Varghese OP, et al. Injectable and thermoresponsive pericardial matrix derived conductive scaffold for cardiac tissue engineering. RSC Adv 2017;7:31980–31988 [Google Scholar]
- 62.Wu Y, Wang L, Guo B, et al. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 2017;11:5646–5659 [DOI] [PubMed] [Google Scholar]
- 63.Izadifar M, Chapman D, Babyn P, et al. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods 2017;24:74–88 [DOI] [PubMed] [Google Scholar]
- 64.Navaei A, Rahmani Eliato K, Ros R, et al. The influence of electrically conductive and non-conductive nanocomposite scaffolds on the maturation and excitability of engineered cardiac tissues. Biomater Sci 2019;7:585–595 [DOI] [PubMed] [Google Scholar]
- 65.Navaei A, Moore N, Sullivan RT, et al. Electrically conductive hydrogel-based micro-topographies for the development of organized cardiac tissues. RSC Adv 2017;7:3302–3312 [Google Scholar]
- 66.Hosoyama K, Ahumada M, McTiernan CD, et al. Nanoengineered electroconductive collagen-based cardiac patch for infarcted myocardium repair. ACS Appl Mater Interfaces 2018;10:44668–44677 [DOI] [PubMed] [Google Scholar]
- 67.Jiang L, Chen D, Wang Z, et al. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl Biochem Biotechnol 2019;188:952–964 [DOI] [PubMed] [Google Scholar]
- 68.Jing X, Mi H-Y, Napiwocki B, et al. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017;125:557–570 [Google Scholar]
- 69.Shin SR, Zihlmann C, Akbari M, et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016;12:3677–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noshadi I, Walker BW, Portillo-Lara R, et al. Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties. Sci Rep 2017;7:4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carson D, Hnilova M, Yang X, et al. Nanotopography-induced structural anisotropy and sarcomere development in human cardiomyocytes derived from induced pluripotent stem cells. ACS Appl Mater Interfaces 2016;8:21923–21932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shiba Y, Fernandes S, Zhu W-Z, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012;489:322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chong JJH, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eggermont LJ, Rogers ZJ, Colombani T, et al. Injectable cryogels for biomedical applications. Trends Biotechnol 2020;38:418–431 [DOI] [PubMed] [Google Scholar]
- 75.Lin X, Liu Y, Bai A, et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat Biomed Eng 2019;3:632–643 [DOI] [PubMed] [Google Scholar]
- 76.Talebian S, Mehrali M, Taebnia N, et al. Self-healing hydrogels: The next paradigm shift in tissue engineering? Adv Sci 2019;6:1801664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertucci C, Koppes R, Dumont C, et al. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res Bull 2019;152:265–284 [DOI] [PubMed] [Google Scholar]
- 78.Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models. Annu Rev Pathol 2015;10:195–262 [DOI] [PubMed] [Google Scholar]
- 79.Gu X. Progress and perspectives of neural tissue engineering. Front Med 2015;9:401–411 [DOI] [PubMed] [Google Scholar]
- 80.Boni R, Ali A, Shavandi A, et al. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci 2018;25:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katona I, Weis J. Chapter 31—Diseases of the peripheral nerves. In: Kovacs GG, Alafuzoff I, eds. Handbook of Clinical Neurology. Amsterdam: Elsevier, 2018: 453–474. [DOI] [PubMed] [Google Scholar]
- 82.Stewart E, Kobayashi NR, Higgins MJ, et al. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng Part C Methods 2015;21:385–393 [DOI] [PubMed] [Google Scholar]
- 83.Xu B, Bai T, Sinclair A, et al. Directed neural stem cell differentiation on polyaniline-coated high strength hydrogels. Mater Today Chem 2016;1–2:15–22 [Google Scholar]
- 84.Pires F, Ferreira Q, Rodrigues CA, et al. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta 2015;1850:1158–1168 [DOI] [PubMed] [Google Scholar]
- 85.Lee SJ, Zhu W, Nowicki M, et al. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng 2018;15:016018. [DOI] [PubMed] [Google Scholar]
- 86.Wu C, Liu A, Chen S, et al. Cell-laden electroconductive hydrogel simulating nerve matrix to deliver electrical cues and promote neurogenesis. ACS Appl Mater Interfaces 2019;11:22152–22163 [DOI] [PubMed] [Google Scholar]
- 87.Zarrintaj P, Urbanska AM, Gholizadeh SS, et al. A facile route to the synthesis of anilinic electroactive colloidal hydrogels for neural tissue engineering applications. J Colloid Interface Sci 2018;516:57–66 [DOI] [PubMed] [Google Scholar]
- 88.Bagheri B, Zarrintaj P, Surwase SS, et al. Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf B Biointerfaces 2019;184:110549. [DOI] [PubMed] [Google Scholar]
- 89.Green RA, Lovell NH, Wallace GG, et al. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008;29:3393–3399 [DOI] [PubMed] [Google Scholar]
- 90.Heo DN, Lee SJ, Timsina R, et al. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater Sci Eng C Mater Biol Appl 2019;99:582–590 [DOI] [PubMed] [Google Scholar]
- 91.Homaeigohar S, Tsai TY, Young TH, et al. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr Polym 2019;224:115112. [DOI] [PubMed] [Google Scholar]
- 92.Xu C, Guan S, Wang S, et al. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater Sci Eng C Mater Biol Appl 2018;84:32–43 [DOI] [PubMed] [Google Scholar]
- 93.Liu X, Miller AL, 2nd, Park S, et al. Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation. ACS Appl Mater Interfaces 2017;9:14677–14690 [DOI] [PubMed] [Google Scholar]
- 94.Wu Y, Wang L, Guo B, et al. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016;87:18–31 [DOI] [PubMed] [Google Scholar]
- 95.Fallenstein GT, Hulce VD, Melvin JW. Dynamic mechanical properties of human brain tissue. J Biomech 1969;2:217–226 [DOI] [PubMed] [Google Scholar]
- 96.Yang S, Jang L, Kim S, et al. Polypyrrole/alginate hybrid hydrogels: Electrically conductive and soft biomaterials for human mesenchymal stem cell culture and potential neural tissue engineering applications. Macromol Biosci 2016;16:1653–1661 [DOI] [PubMed] [Google Scholar]
- 97.Sears NA, Seshadri DR, Dhavalikar PS, et al. A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev 2016;22:298–310 [DOI] [PubMed] [Google Scholar]
- 98.Zhu W, Ma X, Gou M, et al. 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol 2016;40:103–112 [DOI] [PubMed] [Google Scholar]
- 99.Lee S-J, Zhu W, Nowicki M, et al. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng 2018;15:016018. [DOI] [PubMed] [Google Scholar]
- 100.Shin J, Choi EJ, Cho JH, et al. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules 2017;18:3060–3072 [DOI] [PubMed] [Google Scholar]
- 101.Deng Z, Hu T, Lei Q, et al. Stimuli-responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl Mater Interfaces 2019;11:6796–6808 [DOI] [PubMed] [Google Scholar]
- 102.Wu Y, Chen YX, Yan J, et al. Fabrication of conductive gelatin methacrylate–polyaniline hydrogels. Acta Biomater 2016;33:122–130 [DOI] [PubMed] [Google Scholar]
- 103.Heo DN, Lee S-J, Timsina R, et al. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater Sci Eng: C 2019;99:582–590 [DOI] [PubMed] [Google Scholar]
- 104.Pan L, Yu G, Zhai D, et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc Natl Acad Sci U S A 2012;109:9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Darabi MA, Khosrozadeh A, Mbeleck R, et al. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv Mater 2017;29:1700533. [DOI] [PubMed] [Google Scholar]
- 106.Fantino E, Roppolo I, Zhang D, et al. 3D Printing/interfacial polymerization coupling for the fabrication of conductive hydrogel. Macromol Mater Eng 2018;303:1700356 [Google Scholar]
- 107.Håkansson KMO, Henriksson IC, de la Peña Vázquez C, et al. Solidification of 3D printed nanofibril hydrogels into functional 3D cellulose structures. Adv Mater Technol 2016;1:1600096 [Google Scholar]
- 108.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 2016;113:3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Yu Y, Ozbolat IT. Direct bioprinting of vessel-like tubular microfluidic channels. J Nanotechnol Eng Med 2013;4:0210011–0210017 [Google Scholar]
- 111.Paez-Mayorga J, Hernández-Vargas G, Ruiz-Esparza GU, et al. Bioreactors for cardiac tissue engineering. Adv Healthc Mater 2019;8:1701504. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J, Griffin M, Cai J, et al. Bioreactors for tissue engineering: An update. Biochem Eng J 2016;109:268–281 [Google Scholar]
- 113.Barash Y, Dvir T, Tandeitnik P, et al. Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering. Tissue Eng Part C Methods 2010;16:1417–1426 [DOI] [PubMed] [Google Scholar]
- 114.Maidhof R, Tandon N, Lee EJ, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 2012;6:e12–e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Visone R, Talò G, Lopa S, et al. Enhancing all-in-one bioreactors by combining interstitial perfusion, electrical stimulation, on-line monitoring and testing within a single chamber for cardiac constructs. Sci Rep 2018;8:16944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Memic A, Rezaeeyazdi M, Villard P, et al. Effect of polymer concentration on autoclaved cryogel properties. Macromol Mater Eng 2020;305:1900824 [Google Scholar]
- 117.Villard P, Rezaeeyazdi M, Colombani T, et al. Injectable autoclaved cryogels: Autoclavable and injectable cryogels for biomedical applications (Adv. Healthcare Mater. 17/2019). Adv Healthc Mater 2019;8:1970069. [DOI] [PubMed] [Google Scholar]
- 118.Villard P, Rezaeeyazdi M, Colombani T, et al. Autoclavable and injectable cryogels for biomedical applications. Adv Healthc Mater 2019;8:1900679. [DOI] [PubMed] [Google Scholar]
- 119.Kunisaki SM, Fauza DO. Chapter 80—Current state of clinical application. In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering (Fourth Edition). Boston, MA: Academic Press, 2014: 1687–1696 [Google Scholar]
- 120.Yuk H, Lu B, Zhao X. Hydrogel bioelectronics. Chem Soc Rev 2019;48:1642–1667 [DOI] [PubMed] [Google Scholar]
- 121.Sladkova M, De Peppo MG. Bioreactor systems for human bone tissue engineering. Processes 2014;2:494–525 [Google Scholar]