Abstract
Background: Although the chondrocyte is a nonexcitable cell, there is strong interest in gaining detailed knowledge of its ion pumps, channels, exchangers, and transporters. In combination, these transport mechanisms set the resting potential, regulate cell volume, and strongly modulate responses of the chondrocyte to endocrine agents and physicochemical alterations in the surrounding extracellular microenvironment.
Materials and Methods: Mathematical modeling was used to assess the functional roles of energy-requiring active transport, the Na+/K+ pump, in chondrocytes.
Results: Our findings illustrate plausible physiological roles for the Na+/K+ pump in regulating the resting membrane potential and suggest ways in which specific molecular components of pump can respond to the unique electrochemical environment of the chondrocyte.
Conclusion: This analysis provides a basis for linking chondrocyte electrophysiology to metabolism and yields insights into novel ways of manipulating or regulating responsiveness to external stimuli both under baseline conditions and in chronic diseases such as osteoarthritis.
Keywords: articular cartilage, chondrocyte, Na+/K+ pump, Na+-K+-ATPase, electrogenic pumps, ion channels, mathematical model, osteoarthritis (OA), rheumatoid arthritis (RA)
Introduction
The biomechanical and physiological functions of mammalian articular joints are essential for locomotion, postural stability, proprioception, and motor learning.1–4 At the tissue and cellular levels, this results in requirements for a wide range of dynamic motion coupled with remarkable stability. An enabling component of this system is articular joint lubrication, made possible by coordinated activity and secretion of biological lubricants from the two principal cell types in synovial joints: chondrocytes and synovial fibroblasts.5,6
It is noteworthy that in the setting of chronic diseases of the articular joint, for example, rheumatoid arthritis (RA) or osteoarthritis (OA), significant changes in chondrocyte and synovial fibroblast function take place, and these contribute to reduced secretion of joint lubricants,7 attenuated boundary lubrication, and altered joint loading. Reduced tribology results in increased boundary friction, load-induced wear of articular cartilage, impaired joint function, and eventual loss of proprioception.8
The specific cell physiology-oriented focus of this study is the articular chondrocyte, which plays a key role in extracellular matrix (ECM) synthesis and degradation in all vertebrates.9 The presence of mature healthy chondrocytes and a full functional repertoire for these cells are essential for normal articular joint motion. Some of the important classes of transducer elements at the level of individual chondrocytes are ion channels, exchangers, or pumps that are expressed in the surface membrane.10–16 The significance of these integral membrane proteins, functioning individually or in concert, was first recognized with respect to the ability of chondrocytes to quickly and accurately volume regulate in response to even small changes in osmotic strength of the surrounding synovial fluid.17,18
Within the last decade, a number of different ion selective channels and exchangers have been identified and shown to play essential roles in chondrocyte physiology.16,19–24 Any such ion channel being functional in chondrocytes may seem somewhat surprising to researchers working on excitable cells, since the chondrocyte is considered to be a nonexcitable cell. However, despite chondrocytes being nonexcitable cells, their dynamic membrane potential serves many cellular roles,25 is metabolically active,16 especially during hypertrophy,26 and is capable of responses to commonly used drugs for treating hypertension.27 Metabolic activity occurs at lower levels, especially in fully differentiated and senescent chondrocytes.28,29 Indeed, chondrocyte precursors (i.e., the chondroblasts), which proliferate during joint development, and even some developmentally mature chondrocytes, are more metabolically active and are capable of robust ion channel mediated responses to biomechanical, pro-inflammatory,30–36 and immunometabolic factors.36,37 Essential to this fundamental physiological regulation is the ability of the chondrocyte to set and maintain an appropriate, stable resting membrane potential as mediated by ubiquitous ion channels, pumps, and exchangers in the plasma membranes of these cells.38
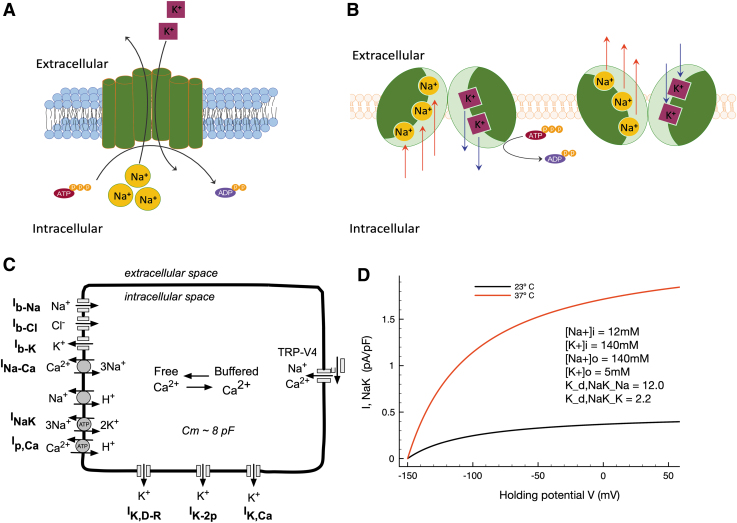
Our main goal in this study was to obtain additional information concerning the role of the electrogenic Na+/K+ pump (also known as the Na+, K+-ATPase) in regulating the resting potential of the mammalian chondrocyte and thus ensure robust and stable physiological responses. A key starting point and motivation for our study was the previous demonstration of the presence of Na+/K+ pump proteins in the chondrocyte surface membrane using immunological and autoradiographic techniques and the molecular characterization of its multiple α, β, and γ isoform subunits.39–41 This dataset, based on demonstration of relatively high affinity, saturable binding sites for ouabain (a classical sodium potassium pump antagonist), combined with more recent molecular studies that have identified transcripts and proteins of each of the known subunits (α, β, and γ) of this integral membrane protein,42 strongly suggests that in the mammalian chondrocyte the electrochemical gradients for sodium and potassium are established and maintained by this active or ATP-requiring pump mechanism.43–45 Very recent proteomic studies have taken an agnostic and unbiased molecular discovery approach to exploring the “surfaceome” of chondrocytes, confirming the presence of multiple Na+/K+ pump isoforms in these cells.24 These findings considered in the context of the extensive literature on Na+/K+ pump cell physiology46,47 make it very likely that in the chondrocyte the classical “coupled stoichiometry” that is characteristic of this enzyme is 3 Na+ pumped out of the cell, coupled with 2 K+ pumped into the chondrocyte cytoplasm (Fig. 1A). The resulting electrogenic current, oriented in the outward direction, would be expected to be one of the important factors in establishing the stable chondrocyte resting membrane potential. As shown in our previous article,38 when this consideration is combined with the fact that the chondrocyte is a functional single cell with exceptionally high membrane resistance, it is likely that the electrogenic current due the Na+/K+ pump (although it is very small) can provide a 10–20 mV contribution to the chondrocyte resting potential.
FIG. 1.
Modeling of the chondrocyte resting membrane potential and demonstration of the effects of temperature on the electrogenic current generated by the Na+/K+ pump in a human chondrocyte preparation. (A, B) Present an illustration of the structure and function of the electrogenic sodium-potassium ATPase whose role in chondrocyte electrophysiology is investigated here; each pump turnover results in 3 Na+ ions' expulsion from and 2 K+ ions' inclusion into the cell, generating a net outward current. (C) Shows an illustrated schematic of the mathematical model of the chondrocyte resting membrane potential used here, as previously published.38 (D) Explores the steady-state voltage dependence of the electrogenic pump Na+/K+ pump current density, given through our previously published model.38 Strong temperature dependence is revealed, and the steady-state current at healthy joint temperature (23°C, black trace), as well as at pathophysiological temperature (37°C, red trace), is illustrated. Both I-V curves also show the curvilinear waveform that is due to the intrinsic voltage dependence of the Na+/K+ pump in mammalian cells. Intracellular and extracellular ion concentrations as initial modeling conditions are assumed (as previously) to be those measured in synovial fluid (as shown in inset).
The mathematical modeling that forms the basis of this article represents a continuation of our studies of chondrocyte electrophysiology, with an emphasis on understanding the basis for the resting potential and its physiological implications for cartilage function. Modeling and simulation here serve as knowledge integrators, unifying results from diverse tissues and experimental sources to offer new insight and to advance new hypotheses. Theoretical studies of this type are thus essential components of ongoing efforts to address important gaps in the background knowledge of the chondrocyte phenotype, mechanisms for cell volume regulation, transmitter and paracrine modulation, responses to anabolic and pro-inflammatory mediators in the context of ECM turnover, identification of early disease markers in chondrocytes, and perhaps most importantly, drug development for OA.
Methods
Simulations essential for the illustrations in this article were performed using our previously published model of the resting membrane potential of the chondrocyte and related intracellular calcium homeostasis.38 In the present study, the physiological roles of the Na+/K+ pump are explored more fully by carrying out simulations in consideration of the unique environment of the chondrocyte in the synovial joint in terms of the temperature and the ionic composition of the extracellular milieu.
Temperature considerations
While temperature measurements of, for example, the human knee vary substantially dependent on the method, intrasurgical measurements suggest values between 31.5°C and 33.5°C for a healthy knee joint48–50; as these values arise in the likely context of trauma and/or inflammation, a truly healthy knee joint at rest might have a temperature closer to 25°C ± 3°C, as suggested by external measurement methods. In contrast, joint temperatures are elevated in the context of RA (34–36°C) and OA (30–37°C), as probably attributable to chronic inflammation in RA and low-grade inflammation in OA. Thus, we have here considered the basal, “room” temperature of the previously published model, 23°C, to reflect a near-physiological temperature for the healthy synovial joint and chondrocyte environment; simulations also consider, in contrast, an elevated joint capsule temperature of 37°C to consider downstream effects on Na+/K+ pump function and the chondrocyte in the pathophysiological context.
Extracellular ionic milieu
In addition, the parent model (Fig. 1C) has been modified such that it can generate data sets that take into account the exceptional ionic milieu of the immediate microenvironment of the chondrocyte (see, e.g., Fig. 2A inset). We use this expanded model to explore a putative role for the electrogenic Na+/K+ pump current in regulating the resting membrane in articular chondrocytes with particular focus on conditions likely to be found in vivo, that is, given the unique ionic environment of the chondrocyte matrix.
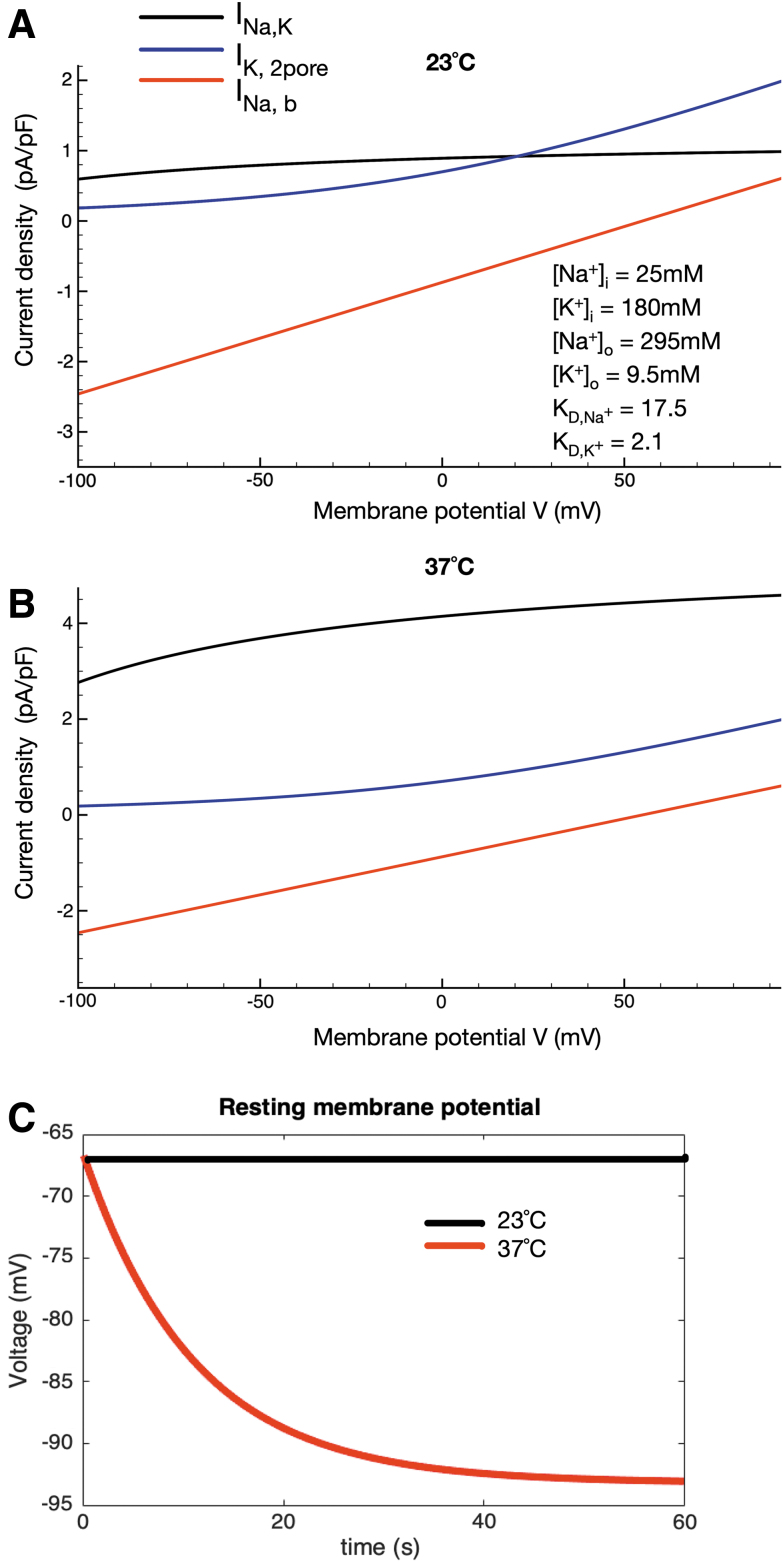
FIG. 2.
Temperature-dependent contribution of the Na+/K+ pump electrogenic current to the chondrocyte resting membrane potential. The superimposed I-V curves in (A) were generated using our published model of the chondrocyte resting potential as shown, assuming a temperature of 23°C and measured and estimated ionic concentrations within the chondrocyte matrix. The analogous superimposed I-V curves shown in (B) were generated using the same model after the Na+/K+ pump current was adjusted to pathophysiological temperature (37°C) based on a Q10 of 3.0 and the channel-mediated background currents were adjusted using a Q10 of 1.2. The two records of membrane potential in (C) illustrate the significant but relatively small effect of the electrogenic current due to the Na+/K+ pump at 23°C contrasted with the much larger hyperpolarization generated by this pump current at 37°C. As shown, the additional Na+/K+ pump current simulated at pathophysiological temperature in the chondrocyte matrix hyperpolarizes the chondrocyte resting membrane potential by ∼25 mV (approximately -67 mV at 23°C and approximately -92 mV at 37°C).
Differential subunit expression and pump function
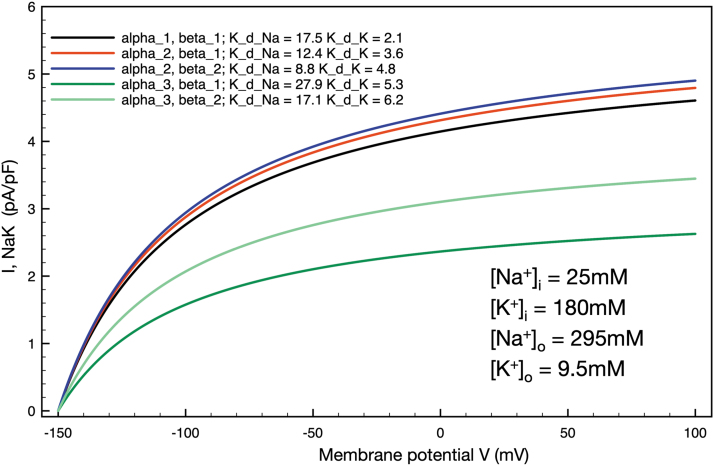
In addition, knowledge that chondrocytes express a number of different isoforms of the subunits that make up functional sodium potassium pumps is taken into account. The contributions of subtype-specific combinations of functional sodium potassium pumps are illustrated by adjusting the affinity constants for [Na+]i and [K+]o binding, as explored further in Figure 4. We illustrate and discuss the resulting profiles and patterns of electrogenic pump currents and related changes in membrane potential.
FIG. 4.
Simulations of the effects on steady-state electrogenic Na+/K+ pump currents due to assumed changes in the α and β subunit composition of the Na+/K+ pump complex in an adult mammalian chondrocyte. Illustrates five different combinations of α and β subunits and the corresponding changes in maximum steady state electrogenic currents and alterations in chondrocyte resting membrane potential. The steady-state voltage dependence and overall Na+/K+ pump expression held constant, the altered expression of α and β subunits (as specified in the inset, upper left), were modeled by changing the Na+/K+ pump affinities for [Na+-]i and [K+]o known to be associated with these isozymes.68 All ionic concentrations reflect the chondrocyte milieu, and all simulations were performed at 37°C to reflect a putative pathophysiological state, for example, OA. OA, osteoarthritis.
Overall, our findings can be used to inform ongoing and future patch clamp electrophysiological studies. They also have the potential to guide investigators in the design and implementation of novel planar recording methods.51,52 In the case of ion exchanger and pump activity generated by intracellular organelles, these approaches appear to be required for resolving and understanding these very small changes that can regulate fundamental properties of nonexcitable cells such as the chondrocyte.
More specific information as to the current simulations performed using the model, including details regarding implementation in Matlab and parameter sets, may be found in the Supplementary Data. The model itself may be accessed at https://github.com/mmaleck/chondrocyte
Results
The sodium potassium (Na+/K+) pump makes an important contribution to setting up and maintaining the membrane potential.53 The main goal of this study was to utilize mathematical modeling to explore the contribution of the electrogenic Na+/K+ pump to the resting membrane potential in chondrocytes isolated from healthy adult human articular joints. Na+/K+ pump expression has been demonstrated in primary bovine chondrocytes isolated from healthy joints,39 human chondrocytes in healthy and diseased joints in situ,41 and human chondrocyte-like cell lines.42 The functional presence of the Na+/K+ pump in chondrocytes has been further documented by related analyses of the molecular properties (isoform composition and expression levels),39 upregulation of the Na+/K+ pump in response to changes in extracellular Na+ concentration40,54 and activity as demonstrated by ouabain binding,40,43,44,54 and p-nitrophenylphosphatase activity in situ in both healthy and pathological samples.41 Importantly also, the basis for the chondrocyte resting potential has previously been analyzed using mathematical modeling.38,55–57
However, none of these studies focused on defining the functional roles of the electrogenic current generated by the Na+/K+ pump under pathophysiological conditions, for example, mimicking temperatures measured in OA (∼37°C), as opposed to the likely temperature found in healthy articular joints (∼23°C), also conditions that are typical of electrophysiological studies done using patch clamp methods under “room” temperature. Our first set of simulations involved making only one change to the parameters that govern the electrophysiological behavior of our human chondrocyte model. In this formulation, the mathematical expression for the electrogenic Na+/K+ pump includes the ability to account for temperature differences by changing the Q10 parameter that regulates the turnover rate of this enzyme. Based on the standard Q10 (3.0) for a membrane-bound integral membrane enzyme58 the change from the baseline conditions (23°C) in this model to pathophysiological conditions (37°C) resulted in a substantial increase in the steady-state electrogenic current (Fig. 1D). These two superimposed steady-state current-voltage (I-V) plots clearly demonstrate that over a broad range of membrane potentials, and in particular, in the range of membrane potentials (-70 to 0 mV) that is most relevant to chondrocyte physiology, the outward electrogenic current increases by a factor of approximately 3–4. The curvilinear characteristic of both steady-state I-V curves reflects the intrinsic voltage dependence of the enzymatic reactions that result in coupled net electrogenic (3 Na+ in for 2 K+ out) fluxes that produce the so-called “pump current.”59 These first findings strongly suggest that the Na+/K+ pump could modulate the resting membrane potential of the chondrocyte in health and disease.
The next set of mathematical simulations was performed in an attempt to illustrate the importance of the electrogenic current generated by the Na+/K+ pump for regulating or stabilizing the chondrocyte membrane potential. Figure 2A, based on our previously published mathematical model,38 illustrates our working hypothesis for the ionic basis for this resting membrane potential. In brief, a background Na+ current (see also Supplementary Data and Supplementary Fig. S1) interacts with outward currents generated by 2-pore K+ channels and the outward electrogenic Na+/K+ pump current. Current generated by the Na+/Ca2+ exchanger and a Cl- conductance are also present but these are not the focus of this study. Note that under room temperature conditions (23°C) this complement of currents balances to generate a resting potential of approximately -40 mV, considering synovial ionic concentrations and generic affinities of the sodium-potassium pump for sodium and potassium ions, as published previously.38 In the modified model used here (Supplementary Data), synovial electrolyte concentrations generate a chondrocyte resting membrane potential of approximately -60 mV. Note that the unique ionic milieu of the chondrocyte matrix may further hyperpolarize this resting membrane potential significantly with respect to the predictions using synovial ionic concentrations, even at room temperature/under healthy conditions (approximately -67 mV).
The analogous superimposed I-V plots that comprise Figure 2B were computed under conditions designed to mimic pathophysiological conditions (37°C) the chondrocyte may experience, for example, in OA. Specifically, the temperature dependence of the Na+/K+ pump was adjusted based on a Q10 value of 3.0, whereas the temperature dependence of the ion channel-mediated fluxes was adjusted using a Q10 value of 1.2 for each.60,61 The combination of the very small sizes of the baseline (resting conditions) channel-mediated current in this small and very high resistance cell and the strong temperature dependence of the electrogenic current of the Na+/K+ pump results in an increased outward current due mainly to the Na+/K+ pump. Although still relatively small, this current would be expected to result in a significant and maintained hyperpolarization of the resting potential (20–25 mV) in the chondrocyte under pathophysiological temperature; indeed, the chondrocyte resting membrane potential apparently can be hyperpolarized to approximately -92 mV at 37°C.
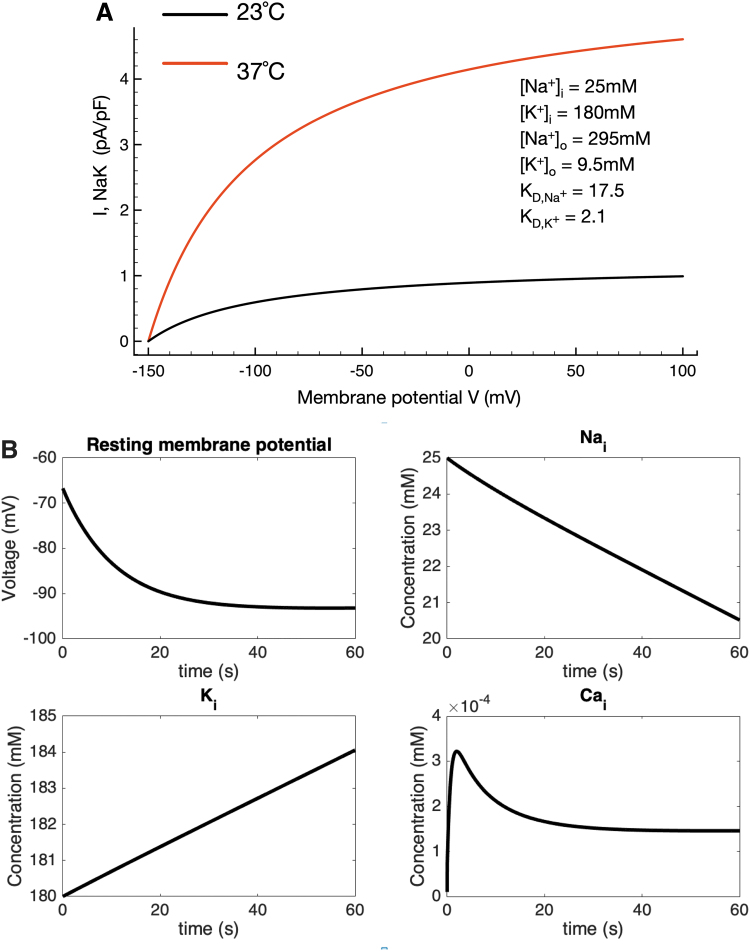
It is important to evaluate and understand overall homeostatic control of intracellular electrolytes and related changes in osmotic strength when studying active transport mechanisms in very small cells such as the chondrocyte.10,62 Accordingly, we have tracked and illustrated time-dependent changes in intracellular Na+ and Ca2+, as well as extracellular K+, in the in silico conditions in which our baseline mathematical model operates. Figure 3A confirms the steady-state changes in the Na+/K+ pump current due to a step change in temperature; four sets of time-dependent results are shown in Figure 3B. The upper left plot shows the time-dependent change in chondrocyte membrane potential that results from a step change in temperature (and hence pump turnover rate) from 23°C to 37°C. Corresponding changes in intracellular Na+ and K+ confirm that there are no significant changes from the starting conditions or baseline model values. Somewhat similarly, although intracellular Ca+ increases approximately twofold soon after the temperature change, this stabilizes very near the 0.2 mM value still characteristic of most healthy mammalian cells. In summary, therefore, although the electrogenic current can contribute to a very substantial hyperpolarization of the resting membrane potential, its activity does not significantly alter intracellular electrolyte homeostasis in this model. This is important since the electrochemical gradient for Na+ is a primary variable in regulating chondrocyte Na+/H+ ion exchange and hence intracellular pH and in modulating Na+/Ca2+ exchange in chondrocytes63 as it does in cardiomyocytes.64 It is known that changes in intracellular Ca2+ can alter Na+/K+ pump activity in most mammalian cells.65 In addition, the ability of the Na+/K+ pump to stabilize intracellular Na+ and thus contribute to medium- and long-term cellular volume regulation is well known.66
FIG. 3.
Intracellular electrolyte homeostasis in the setting of altered Na+/K+ pump activity/turnover rate due to a step change in temperature (23–37°C). As noted, the electrogenic Na+/K+ pump current is changed significantly when intracellular and extracellular ion concentrations are altered to reflect the ionic milieu of the chondrocyte matrix found in vivo (shown in inset, A); compare to simulations performed assuming a baseline synovial fluid environment (Fig. 1D). In (A) we illustrate steady-state voltage dependence and the temperature dependence of the Na+/K+ pump current. The steady-state current at 23°C (black trace), as well as at 37°C (red trace), is shown. The additional Na+/K+ pump current simulated at a pathophysiological temperature hyperpolarizes the chondrocyte resting membrane potential by ∼25 mV at 37°C in the chondrocyte milieu. (B) Also shows the time-dependent hyperpolarization of chondrocyte membrane potential resulting from a step change in temperature from 23°C to 37°C, as well as the corresponding alterations in intracellular Na+, K+, and Ca2+. In the cases of Na+ and K+ these changes are small: the shift in, for example, [Na+]i is about a 20% change over the time course measured and likely to be of small physiological relevance overall. The intracellular Ca2+ level changes transiently (perhaps due to the intrinsic voltage dependence of the Na+/Ca2+ exchanger or specific model-dependent features of intracellular Ca2+ buffering) but quickly stabilizes near 0.2 μM, an accepted level for a resting mammalian cell.
In the mammalian articular joint, electrolyte levels and particularly those for intracellular Na+ and K+ may deviate significantly from those in standard plasma or intracellular levels found in other tissues, for example, skin or muscle.10,67 These differences and the resulting changes in Na+/K+ pump activity and baseline chondrocyte conductances were a focus of our original paper38 and have been studied previously in physiological and pathophysiological settings.10,14,16,43,44 These considerations, when combined with the demonstration that the healthy bovine and human chondrocytes express a number of different isoforms of the α, β, and γ subunits of the Na+/K+ pump protein complex,39,41 raise important questions concerning the relative sizes of the electrogenic current and related changes in membrane potential that would be expected due to predominant expression of specific combinations of the α, β, and γ subunits.42,45 We have approached this by simulating several of the combinations of expression of α and β subunits that are specified in a comprehensive review of the molecular physiology of the Na+/K+ pump68 and more recent studies of the kinetic transitions of the movement of Na+ and K+ ions through the pump.69 Results expressed in terms of temperature-dependent development of steady-state electrogenic currents for five subsets of data are shown in Figure 4. The most common physiological condition, assuming an exclusive α1, β1, subunit composition with the affinity constants as shown in the inset, is shown in black. Note that the maximal current is ∼4.5 pA/pF and that the steady-state membrane potential is approximately -92 mV at 37°C (membrane potential traces not shown). The red trace illustrates an identical calculation done under the assumption of a predominant α2, β1, subunit expression. The corresponding higher affinity for the intracellular Na+ site on the Na+/K+ pump predictably results in a somewhat larger steady state electrogenic current (nearly 5.0 pA/pF) and larger hyperpolarization of the resting membrane potential to approximately -95 mV. In contrast, the dark green trace simulates the effect of predominant expression of the α3, β1, subunits; based on the markedly decreased affinity for intracellular Na+ of this combination, the maximal electrogenic current reaches ∼2.5 pA/pF and the chondrocyte resting membrane potential stabilizes at -78 mV.
Discussion
Main findings
This computational work, based on our published model of the healthy adult chondrocyte membrane potential,38 confirms that the electrogenic current generated by the Na+/K+ pump is strongly temperature dependent. The resulting net outward current is relatively small. However, it can have substantial hyperpolarizing influences on the resting membrane potential of the chondrocyte due mainly to the very high input resistance, ∼10 Giga-ohms14,55,56 of this cell. In both physiological and pathophysiological settings it is likely that the Na+/K+ pump in the chondrocyte is strongly activated due to the relatively high intracellular Na+ levels (∼20 mM or more) in this cell type.10,43,70
Previous findings
Hall et al. first reported evidence for energy-requiring active transport of K+ across the surface membrane of healthy adult mammalian chondrocytes.71 This initial observation was supported and put into a conventional cell physiology context by Mobasheri et al.39 who adapted 3H-labeled ouabain binding methods to demonstrate substantial expression of the Na+/K+ pump alpha subunit in mammalian chondrocytes. Mobasheri et al. then confirmed and extended these findings based on experimental 3H-labeled ouabain binding and confocal immunofluorescence microscopy work.39,40 They demonstrated regulation of surface expression by changes in levels of intra- and extracellular Na+ in isolated cells44,72 and in the ECM of articular cartilage from healthy bovine joints43 and also reported a scheme for overall Na+ regulation based on data that identified functional roles in the chondrocyte for Na+/Ca2+ exchange, Na+/H+ exchange, and antiporter exchange due to Na+/K+/Cl− expression.10 These articles and subsequent work have specified the essential role of the Na+/K+ pump and overall regulation of intracellular Na+ levels in volume regulation of the chondrocyte. It is also known that regulation of volume in the chondrocyte depends, in part, on the membrane potential of these cells.20,73
Physiological effects of the electrogenic Na+/K+ pump in chondrocytes
Insights gained from our mathematical modeling support the working hypothesis that the Na+/K+ pump can strongly regulate the resting membrane potential in chondrocytes from healthy and diseased adult articular cartilage. Specifically, it is plausible that due to its expression levels, turnover rates,74 and intrinsic voltage dependence,75,76 this pump mechanism produces a hyperpolarizing influence that can be as large as 30 mV. This hyperpolarization would be expected to modulate volume regulation; in addition, however, it is also likely to markedly alter the overall electrophysiological function or electrophysiological operating point of the chondrocyte.77 This is because a number of the other ion channels that are expressed in the chondrocyte, for example, L-type Ca2+ channels,15,27,78–80 delayed rectifier K+ channels,55,57 and 2-pore K+ channels56 exhibit strong intrinsic voltage dependence in the range -40 to -80 mV. Accordingly, the hyperpolarizing influence of the Na+/K+ pump significantly regulates the activation and/or deactivation of these (and perhaps other) ion channels in the chondrocyte.
We note, however, that the singular focus on the physiological and pathophysiological effects of the electrogenic current produced by the Na+/K+ pump in this study could be somewhat misleading. When the chondrocyte is stimulated by stretch, or activated by ligands such as histamine or ATP, agonist-induced ion fluxes through piezo,81 Cl−,77 or TRP channels14,77,82,83 will reduce the input resistance of the cell. The resulting parallel conductance will partially “shunt” the influence of the electrogenic current generated by the Na+/K+ pump. In addition, in both health and disease, the chondrocyte exists and functions in a relatively hypoxic environment.84–86 In this setting, the supply of ATP as the principal energy source of energy for chondrocytes87 may limit pump activity to a range that is less than the maximal currents shown in the Figure 1.88,89
Concluding remarks
Although we acknowledge the possibility that the Na+/K+ pump function in mammalian chondrocytes may be modulated by altered expression levels of selected isoforms of the α, β, and γ subunits, our analysis is not sufficiently complete to fully examine the consequences as has been done in other tissues, for example, skeletal muscle.74 It is known that the Na+/K+ pump can be strongly modulated by altered redox conditions90 such as those that occur in sterile inflammation or “low grade inflammation” in the context of chondrocyte biology and OA.91,92 Our work provides a basis for this type of analysis, but important pathophysiological effects such as this have not been studied. Finally, both classical findings and more recent detailed analyses have drawn attention to conditions under which changes in intracellular Ca2+ can markedly alter the function of the Na+/K+ pump.93 Our model, at its present state of development, cannot be used to simulate these Ca2+-dependent effects due to the simplistic formulations now used for intracellular Ca2+ buffering and Na+/Ca2+ exchange; in the absence of mathematical descriptors for Ca2+ pumps; and Ca2+-sensitive channels localized to the endoplasmic reticulum.94 These additions and other improvements will be needed before the mathematical modeling approach used in this study can be extended to analysis of ion homeostasis and the chondrocyte channelome16,22 in a more physiological context, specifically in chondron units, which represent the chondrocyte and its immediate pericellular environment.95 Further model development is also needed before our simulations can provide insights into the altered articular joint electrolyte homeostasis resulting from disease-producing point mutations in one or more of the Na+/K+ pump subunits or accessory proteins.69
From a tissue engineering perspective, there is ongoing interest in the Na+/K+ pump, ion transport, and the modulation of intracellular Na+ by pharmacological agents such as ouabain and bumetanide as in vitro treatments for altering intracellular ion concentrations as a viable method for manipulating ECM synthesis by chondrocytes and enhancing the mechanical properties of engineered articular cartilage.96
Finally, in the context of drug development and screening for diseases such as OA, which is known to be characterized by cellular senescence, or “chondrosenescence,”29,97 the Na+/K+ pump has already been identified as a candidate target for modulation by cardiac glycosides, re-entering the limelight as classical cardiotonic drugs reinvented as senolytic compounds.98 Thus, the modeling approaches described in this article can support drug development for OA and related osteoarticular disorders, as well as understanding of the basic cellular physiology and pathophysiology of joint tissues.
Supplementary Material
Acknowledgments
The authors acknowledge the members of our research teams and collaborators for their support and encouragement.
Authors' Contributions
W.R.G. drafted the article. M.M.M. performed the mathematical modeling. M.M.M., P.M.-V., and A.M. edited the article and contributed data. All authors made significant intellectual contribution to the concept proposed in this article. All coauthors reviewed and approved the article before submission and confirm that the article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist. The authors do not have any financial conflicts of interest in relation to this work.
Funding Information
A.M. has received funding from the following sources: The European Commission Framework 7 programme (EU FP7; HEALTH.2012.2.4.5-2, project No. 305815; Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). The Innovative Medicines Initiative Joint Undertaking under grant agreement number 115770, resources of which are composed of financial contribution from the European Union's Seventh Framework programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies' in-kind contribution. A.M. also wishes to acknowledge funding from the European Commission through a Marie Curie Intra-European Fellowship for Career Development grant (project No. 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF). A.M. also wishes to acknowledge financial support from the European Structural and Social Funds (ES Struktūrinės Paramos) through the Research Council of Lithuania (Lietuvos Mokslo Taryba) according to the activity “Improvement of researchers” qualification by implementing world-class R&D projects' of Measure number 09.3.3-LMT-K-712 (grant application code: 09.3.3-LMT-K-712-01-0157, agreement No. DOTSUT-215) and the new funding programme: Attracting Foreign Researchers for Research Implementation (2018–2022). M.M. would like to acknowledge funding from Norway's Ministry of Education and Research via her position at Simula Research Laboratory.
Supplementary Material
References
- 1.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol 2011;25:815–823. DOI: 10.1016/j.berh.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat 2006;208:491–512. DOI: 10.1111/j.1469-7580.2006.00546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui AY, McCarty WJ, Masuda K, et al. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med 2012;4:15–37. DOI: 10.1002/wsbm.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blalock D, Miller A, Tilley M, et al. Joint instability and osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord 2015;8:15–23. DOI: 10.4137/CMAMD.S22147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askary A, Smeeton J, Paul S, et al. Ancient origin of lubricated joints in bony vertebrates. Elife 2016;5:e16415. DOI: 10.7554/eLife.16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonacci JM, Schmidt TA, Serventi LA, et al. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: Role of hyaluronan. Arthritis Rheum 2012;64:2917–2926. DOI: 10.1002/art.34520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosinska MK, Ludwig TE, Liebisch G, et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One 2015;10:e0125192. DOI: 10.1371/journal.pone.0125192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoop J, Steultjens MPM, van der Leeden M, et al. Proprioception in knee osteoarthritis: A narrative review. Osteoarthr Cartil 2011;19:381–388. DOI: 10.1016/j.joca.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol 2003;35:401–404. DOI: 10.1016/s1357-2725(02)00301-1 [DOI] [PubMed] [Google Scholar]
- 10.Mobasheri A, Mobasheri R, Francis MJ, et al. Ion transport in chondrocytes: Membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol Histopathol 1998;13:893–910. DOI: 10.14670/HH-13.893 [DOI] [PubMed] [Google Scholar]
- 11.Mobasheri A, Carter SD, Martín-Vasallo P, et al. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int 2002;26:1–18. DOI: 10.1006/cbir.2001.0826 [DOI] [PubMed] [Google Scholar]
- 12.Wohlrab D, Lebek S, Krüger T, et al. Influence of ion channels on the proliferation of human chondrocytes. Biorheology 2002;39:55–61 [PubMed] [Google Scholar]
- 13.Mobasheri A, Lewis R, Maxwell JEJ, et al. Characterization of a stretch-activated potassium channel in chondrocytes. J Cell Physiol 2010;223:511–518. DOI: 10.1002/jcp.22075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-Jolley R, Lewis R, Fallman R, et al. The emerging chondrocyte channelome. Front Physiol 2010;1:135. DOI: 10.3389/fphys.2010.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matta C, Zákány R, Mobasheri A. Voltage-dependent calcium channels in chondrocytes: Roles in health and disease. Curr Rheumatol Rep 2015;17:43. DOI: 10.1007/s11926-015-0521-4 [DOI] [PubMed] [Google Scholar]
- 16.Mobasheri A, Matta C, Uzielienè I, et al. The chondrocyte channelome: A narrative review. Joint Bone Spine 2019;86:29–35. DOI: 10.1016/j.jbspin.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem 2002;86:290–301. DOI: 10.1002/jcb.10217 [DOI] [PubMed] [Google Scholar]
- 18.Hall AC. The role of chondrocyte morphology and volume in controlling phenotype-implications for osteoarthritis, cartilage repair, and cartilage engineering. Curr Rheumatol Rep 2019;21:38. DOI: 10.1007/s11926-019-0837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hdud IM, El-Shafei AA, Loughna P, et al. Expression of Transient Receptor Potential Vanilloid (TRPV) channels in different passages of articular chondrocytes. Int J Mol Sci 2012;13:4433–4445. DOI: 10.3390/ijms13044433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis R, Asplin KE, Bruce G, et al. The role of the membrane potential in chondrocyte volume regulation. J Cell Physiol 2011;226:2979–2986. DOI: 10.1002/jcp.22646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mobasheri A, Lewis R, Ferreira-Mendes A, et al. Potassium channels in articular chondrocytes. Channels 2012;6:416–425. DOI: 10.4161/chan.22340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis R, May H, Mobasheri A, et al. Chondrocyte channel transcriptomics: Do microarray data fit with expression and functional data? Channels 2013;7:459–467. DOI: 10.4161/chan.26071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis R, Feetham CH, Gentles L, et al. Benzamil sensitive ion channels contribute to volume regulation in canine chondrocytes. Br J Pharmacol 2013;168:1584–1596. DOI: 10.1111/j.1476-5381.2012.02185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeremiasse B, Matta C, Fellows CR, et al. Alterations in the chondrocyte surfaceome in response to pro-inflammatory cytokines. BMC Mol Cell Biol 2020;21:47. DOI: 10.1186/s12860-020-00288-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul Kadir L, Stacey M, Barrett-Jolley R. Emerging roles of the membrane potential: Action beyond the action potential. Front Physiol 2018;9:1661. DOI: 10.3389/fphys.2018.01661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada Y, Sakiyama H, Kuriiwa K, et al. Metabolic activities of partially degenerated hypertrophic chondrocytes: Gene expression of hyaluronan synthases. Cell Tissue Res 1999;298:317–325. DOI: 10.1007/s004419900082 [DOI] [PubMed] [Google Scholar]
- 27.Uzieliene I, Bernotiene E, Rakauskiene G, et al. The antihypertensive drug nifedipine modulates the metabolism of chondrocytes and human bone marrow-derived mesenchymal stem cells. Front Endocrinol (Lausanne) 2019;10:756. DOI: 10.3389/fendo.2019.00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trippel SB, Ehrlich MG, Lippiello L, et al. Characterization of chondrocytes from bovine articular cartilage: I. Metabolic and morphological experimental studies. J Bone Joint Surg Am 1980;62:816–820 [PubMed] [Google Scholar]
- 29.Mobasheri A, Matta C, Zákány R, et al. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015;80:237–244. DOI: 10.1016/j.maturitas.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Mobasheri A, Barrett-Jolley R, Carter SD, et al. Functional roles of mechanosensitive ion channels, β1 integrins and kinase cascades in chondrocyte mechanotransduction. In: Kamkin A, Kiseleva I, eds. Mechanosensitivity in Cells and Tissues. Moscow: Academia, 2005;434–451. [PubMed] [Google Scholar]
- 31.Wohlrab D, Wohlrab J, Reichel H, et al. Is the proliferation of human chondrocytes regulated by ionic channels? J Orthop Sci 2001;6:155–159. DOI: 10.1007/s0077610060155 [DOI] [PubMed] [Google Scholar]
- 32.Lee W, Leddy HA, Chen Y, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A 2014;111:E5114–E5122. DOI: 10.1073/pnas.1414298111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu QQ, Chen Q. Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: Ion-channel dependent transduction of matrix deformation signals. Exp Cell Res 2000;256:383–391. DOI: 10.1006/excr.2000.4847 [DOI] [PubMed] [Google Scholar]
- 34.Wohlrab D, Vocke M, Klapperstück T, et al. Effects of potassium and anion channel blockers on the cellular response of human osteoarthritic chondrocytes. J Orthop Sci 2004;9:364–371. DOI: 10.1007/s00776-004-0789-0 [DOI] [PubMed] [Google Scholar]
- 35.Yamamura H, Suzuki Y, Imaizumi Y. Physiological and pathological functions of Cl-channels in chondrocytes. Biol Pharm Bull 2018;41:1145–1151. DOI: 10.1248/bpb.b18-00152 [DOI] [PubMed] [Google Scholar]
- 36.Mobasheri A, Rayman MP, Gualillo O, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2017;13:302–311. DOI: 10.1038/nrrheum.2017.50 [DOI] [PubMed] [Google Scholar]
- 37.Sutton S, Clutterbuck A, Harris P, et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J 2009;179:10–24. DOI: 10.1016/j.tvjl.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 38.Maleckar MM, Clark RB, Votta B, et al. The resting potential and K+ currents in primary human articular chondrocytes. Front Physiol 2018;9:974. DOI: 10.3389/fphys.2018.00974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mobasheri A, Errington RJ, Golding S, et al. Characterization of the Na+, K(+)-ATPase in isolated bovine articular chondrocytes; molecular evidence for multiple alpha and beta isoforms. Cell Biol Int 1997;21:201–212. DOI: 10.1006/cbir.1997.0137 [DOI] [PubMed] [Google Scholar]
- 40.Mobasheri A, Hall AC, Urban JP, et al. Immunologic and autoradiographic localisation of the Na+, K(+)-ATPase in articular cartilage: Upregulation in response to changes in extracellular Na+ concentration. Int J Biochem Cell Biol 1997;29:649–657. DOI: 10.1016/s1357-2725(96)00150-1 [DOI] [PubMed] [Google Scholar]
- 41.Trujillo E, Alvarez de la Rosa D, Mobasheri A, et al. Sodium transport systems in human chondrocytes. I. Morphological and functional expression of the Na+,K(+)-ATPase alpha and beta subunit isoforms in healthy and arthritic chondrocytes. Histol Histopathol 1999;14:1011–1022. DOI: 10.14670/HH-14.1011 [DOI] [PubMed] [Google Scholar]
- 42.Mobasheri A, Trujillo E, Arteaga M-F, et al. Na(+), K(+)-ATPase subunit composition in a human chondrocyte cell line; evidence for the presence of α1, α3, β1, β2 and β3 isoforms. Int J Mol Sci 2012;13:5019–5034. DOI: 10.3390/ijms13045019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mobasheri A. Correlation between [Na+], [glycosaminoglycan] and Na+/K+ pump density in the extracellular matrix of bovine articular cartilage. Physiol Res 1998;47:47–52 [PubMed] [Google Scholar]
- 44.Mobasheri A. Regulation of Na+, K+-ATPase density by the extracellular ionic and osmotic environment in bovine articular chondrocytes. Physiol Res 1999;48:509–512 [PubMed] [Google Scholar]
- 45.Mobasheri A, Avila J, Cózar-Castellano I, et al. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep 2000;20:51–91 [DOI] [PubMed] [Google Scholar]
- 46.Glynn IM. Annual review prize lecture. “All hands to the sodium pump.” J Physiol (Lond) 1993;462:1–30. DOI: 10.1113/jphysiol.1993.sp019540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lingrel JB, Van Huysse J, O'Brien W, et al. Structure-function studies of the Na,K-ATPase. Kidney Int Suppl 1994;44:S32–S39 [PubMed] [Google Scholar]
- 48.Becher C, Springer J, Feil S, et al. Intra-articular temperatures of the knee in sports - an in-vivo study of jogging and alpine skiing. BMC Musculoskelet Disord 2008;9:46. DOI: 10.1186/1471-2474-9-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sánchez-Inchausti G, Vaquero-Martín J, Vidal-Fernández C. Effect of arthroscopy and continuous cryotherapy on the intra-articular temperature of the knee. Arthroscopy 2005;21:552–556. DOI: 10.1016/j.arthro.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 50.Zaffagnini S, Allen AA, Suh JK, et al. Temperature changes in the knee joint during arthroscopic surgery. Knee Surg Sports Traumatol Arthrosc 1996;3:199–201. DOI: 10.1007/BF01466616 [DOI] [PubMed] [Google Scholar]
- 51.Pintschovius J, Fendler K, Bamberg E. Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Cytoplasmic cation binding and release. Biophys J 1999;76:827–836. DOI: 10.1016/S0006-3495(99)77246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pintschovius J, Fendler K. Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Rapid solution exchange with a new technique. Biophys J 1999;76:814–826. DOI: 10.1016/S0006-3495(99)77245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassalle M. Contribution of the Na+/K+-pump to the membrane potential. Experientia 1987;43:1135–1140 [DOI] [PubMed] [Google Scholar]
- 54.Mobasheri A. The effect of the extracellular environment on sodium pump density in cartilage [dissertation]. Oxford, UK: The University of Oxford; 1996 [Google Scholar]
- 55.Clark RB, Hatano N, Kondo C, et al. Voltage-gated K+ currents in mouse articular chondrocytes regulate membrane potential. Channels 2010;4:179–191 [DOI] [PubMed] [Google Scholar]
- 56.Clark RB, Kondo C, Belke DD, et al. Two-pore domain K+ channels regulate membrane potential of isolated human articular chondrocytes. J Physiol (Lond) 2011;589(Pt 21):5071–5089. DOI: 10.1113/jphysiol.2011.210757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson JR, Duncan NA, Giles WR, et al. A voltage-dependent K+ current contributes to membrane potential of acutely isolated canine articular chondrocytes. J Physiol (Lond) 2004;557(Pt 1):93–104. DOI: 10.1113/jphysiol.2003.058883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hille B. Ion Channels of Excitable Membranes, 3rd Edition. Sunderland, MA: Sinauer Assoc. Inc., 2001 [Google Scholar]
- 59.Nakao M, Gadsby DC. Voltage dependence of Na translocation by the Na/K pump. Nature 1986;323:628–630. DOI: 10.1038/323628a0 [DOI] [PubMed] [Google Scholar]
- 60.Hille B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys J 1978;22:283–294. DOI: 10.1016/S0006-3495(78)85489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sterratt DC. Q10: The Effect of Temperature on Ion Channel Kinetics. In: Jaeger D, Jung R, eds. Encyclopedia of Computational Neuroscience. New York, NY: Springer, 2014. DOI: 10.1007/978-1-4614-7320-6_236-1 [DOI] [Google Scholar]
- 62.Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J 2002;82:720–727. DOI: 10.1016/S0006-3495(02)75434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trujillo E, Alvarez de la Rosa D, Mobasheri A, et al. Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl- cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol 1999;14:1023–1031. DOI: 10.14670/HH-14.1023 [DOI] [PubMed] [Google Scholar]
- 64.Shattock MJ, Ottolia M, Bers DM, et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol (Lond) 2015;593:1361–1382. DOI: 10.1113/jphysiol.2014.282319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasallo PM, Post RL. Calcium ion as a probe of the monovalent cation center of sodium, potassium ATPase. J Biol Chem 1986;261:16957–16962 [PubMed] [Google Scholar]
- 66.Hernández JA, Cristina E. Modeling cell volume regulation in nonexcitable cells: The roles of the Na+ pump and of cotransport systems. Am J Physiol 1998;275:C1067–C1080. DOI: 10.1152/ajpcell.1998.275.4.C1067 [DOI] [PubMed] [Google Scholar]
- 67.Browning JA, Saunders K, Urban JPG, et al. The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology 2004;41:299–308 [PubMed] [Google Scholar]
- 68.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am J Physiol 1998;275:F633–F650. DOI: 10.1152/ajprenal.1998.275.5.F633 [DOI] [PubMed] [Google Scholar]
- 69.Moreno C, Yano S, Bezanilla F, et al. Transient electrical currents mediated by the Na+/K+-ATPase: A tour from basic biophysics to human diseases. Biophys J 2020;119:236–242. DOI: 10.1016/j.bpj.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shapiro IM, Boyde A. Microdissection—Elemental analysis of the mineralizing growth cartilage of the normal and rachitic chick. Metab Bone Dis Relat Res 1984;5:317–326. DOI: 10.1016/0221-8747(84)90019-5 [DOI] [PubMed] [Google Scholar]
- 71.Hall AC, Starks I, Shoults CL, et al. Pathways for K+ transport across the bovine articular chondrocyte membrane and their sensitivity to cell volume. Am J Physiol 1996;270(5 Pt 1):C1300–C1310. DOI: 10.1152/ajpcell.1996.270.5.C1300 [DOI] [PubMed] [Google Scholar]
- 72.Mobasheri A. Brefeldin A influences the cell surface abundance and intracellular pools of low and high ouabain affinity Na+, K(+)-ATPase alpha subunit isoforms in articular chondrocytes. Histol Histopathol 1999;14:427–438. DOI: 10.14670/HH-14.427 [DOI] [PubMed] [Google Scholar]
- 73.Armstrong CM. The Na/K pump, Cl ion, and osmotic stabilization of cells. Proc Natl Acad Sci U S A 2003;100:6257–6262. DOI: 10.1073/pnas.0931278100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 2005;25:292–303. DOI: 10.1016/j.semnephrol.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 75.Stanley CM, Gagnon DG, Bernal A, et al. Importance of the voltage dependence of cardiac Na/K ATPase isozymes. Biophys J 2015;109:1852–1862. DOI: 10.1016/j.bpj.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mares LJ, Garcia A, Rasmussen HH, et al. Identification of electric-field-dependent steps in the Na(+),K(+)-pump cycle. Biophys J 2014;107:1352–1363. DOI: 10.1016/j.bpj.2014.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki Y, Yamamura H, Imaizumi Y, et al. K+ and Ca2+ channels regulate Ca2+ signaling in chondrocytes: An illustrated review. Cells 2020;9. [Epub ahead of print] DOI: 10.3390/cells9071577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wright M, Jobanputra P, Bavington C, et al. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: Evidence for the presence of stretch-activated membrane ion channels. Clin Sci 1996;90:61–71. DOI: 10.1042/cs0900061 [DOI] [PubMed] [Google Scholar]
- 79.Walker LM, Holm A, Cooling L, et al. Mechanical manipulation of bone and cartilage cells with “optical tweezers.” FEBS Lett 1999;459:39–42. DOI: 10.1016/s0014-5793(99)01169-2 [DOI] [PubMed] [Google Scholar]
- 80.Raizman I, De Croos JNA, Pilliar R, et al. Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium 2010;48:232–242. DOI: 10.1016/j.ceca.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 81.Lee W, Guilak F, Liedtke W. Role of piezo channels in joint health and injury. Curr Top Membr 2017;79:263–273. DOI: 10.1016/bs.ctm.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavenis K, Schumacher C, Schneider U, et al. Expression of ion channels of the TRP family in articular chondrocytes from osteoarthritic patients: Changes between native and in vitro propagated chondrocytes. Mol Cell Biochem 2009;321:135–143. DOI: 10.1007/s11010-008-9927-x [DOI] [PubMed] [Google Scholar]
- 83.Lieben L, Carmeliet G. The involvement of TRP channels in bone homeostasis. Front Endocrinol (Lausanne) 2012;3:99. DOI: 10.3389/fendo.2012.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coimbra IB, Jimenez SA, Hawkins DF, et al. Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthr Cartil 2004;12:336–345. DOI: 10.1016/j.joca.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 85.Pfander D, Cramer T, Swoboda B. Hypoxia and HIF-1alpha in osteoarthritis. Int Orthop 2005;29:6–9. DOI: 10.1007/s00264-004-0618-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pfander D, Swoboda B, Cramer T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res Ther 2006;8:104. DOI: 10.1186/ar1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J 1997;321 (Pt 1):95–102. DOI: 10.1042/bj3210095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Figtree GA, Keyvan Karimi G, Liu C-C, et al. Oxidative regulation of the Na(+)-K(+) pump in the cardiovascular system. Free Radic Biol Med 2012;53:2263–2268. DOI: 10.1016/j.freeradbiomed.2012.10.539 [DOI] [PubMed] [Google Scholar]
- 89.Engl E, Attwell D. Non-signalling energy use in the brain. J Physiol (Lond) 2015;593:3417–3429. DOI: 10.1113/jphysiol.2014.282517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White CN, Liu C-C, Garcia A, et al. Activation of cAMP-dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity. J Biol Chem 2010;285:13712–13720. DOI: 10.1074/jbc.M109.090225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 2017;29:79–85. DOI: 10.1097/BOR.0000000000000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther Adv Musculoskelet Dis 2013;5:77–94. DOI: 10.1177/1759720X12467868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu F-M, Deisl C, Hilgemann DW. Profound regulation of Na/K pump activity by transient elevations of cytoplasmic calcium in murine cardiac myocytes. Elife 2016;5. DOI: 10.7554/eLife.19267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee GM, Poole CA, Kelley SS, et al. Isolated chondrons: A viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthr Cartil 1997;5:261–274. DOI: 10.1016/s1063-4584(97)80022-2 [DOI] [PubMed] [Google Scholar]
- 96.Natoli RM, Skaalure S, Bijlani S, et al. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 2010;62:1097–1107. DOI: 10.1002/art.27313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Kraan P, Matta C, Mobasheri A. Age-related alterations in signaling pathways in articular chondrocytes: implications for the pathogenesis and progression of osteoarthritis—A mini-review. Gerontology 2017;63:29–35. DOI: 10.1159/000448711 [DOI] [PubMed] [Google Scholar]
- 98.Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, et al. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun 2019;10:4731. DOI: 10.1038/s41467-019-12888-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.