Abstract
Neural stem and progenitor cells (i.e., neural precursors) are found within specific regions in the central nervous system and have great regenerative capacity. These cells are electrosensitive and their behavior can be regulated by the presence of electric fields (EFs). Electrical stimulation is currently used to treat neurological disorders in a clinical setting. Herein we propose that electrical stimulation can be used to enhance neural repair by regulating neural precursor cell (NPC) kinetics and promoting their migration to sites of injury or disease. We discuss how intrinsic and extrinsic factors can affect NPC migration in the presence of an EF and how this impacts electrode design with the goal of enhancing tissue regeneration. We conclude with an outlook on future clinical applications of electrical stimulation and highlight technological advances that would greatly support these applications.
Keywords: electrical stimulation, cell migration, electrode design
Introduction
Neurological disorders, including stroke, Alzheimer's, and Parkinson's disease, are the leading cause of disability and the second leading cause of death globally.1 Recent advancements in bioelectricity research, conductive polymers, and carbon-based materials have the field poised to treat these neurological disorders using electrical stimulation by way of enhancing endogenous neural repair. The opportunity is afforded by the presence of electrosensitive resident neural stem and progenitor cells (termed neural precursor cells, NPCs) in the brain and the innovative approaches underlying novel electrode designs. New materials with improved mechanical, electrical, and chemical properties, including greater flexibility, conductivity, and biocompatibility, provide researchers with new options to implant and deliver electrical stimulation and promote neural repair.
Bioelectricity was discovered over 200 years ago and, since then, researchers have discovered that endogenous electric fields (EFs) are vital for proper development and wound healing. Disruption or reversal of these fields can cause developmental deformations and prevent tissue repair.2,3 Decades of investigation have examined the role of electrical stimulation in enhancing wound healing, particularly for skin and bone in animal models and clinical trials.4–6 The field of bioelectricity has highlighted our understanding of the diverse and profound responses of cells to EF application upon which to build our regenerative strategies.
Technology in the 1960's and 1970's focused on implanting devices into the central nervous system (CNS) to deliver electrical stimulation specifically for pain.7 Now, deep brain stimulation (DBS), spinal cord stimulation, peripheral nerve stimulation, vagus nerve stimulation, transcranial magnetic stimulation, and functional electrical stimulation are all examples of the successful application of clinical electrical stimulation to benefit patients.8–13 These techniques are widely available globally, with well-measured clinical outcomes, and in some cases are now standard of care. While the outcomes are well understood, the mechanisms underlying the success of neuromodulation therapies are less well-defined, although modification of neural circuits and action potential-generating cells has been shown to result from these interventions. DBS is standard of care for the treatment of appropriately selected patients in movement disorders and epilepsy and may be an option for patients with certain types of pain syndromes.9 Spinal cord stimulation has been used for decades to reduce chronic neuropathic pain.8 More recently, transcranial magnetic stimulation has been approved to treat depression12; and functional electrical stimulation has been used for decades to restore motor and sensory functions following CNS injury.13 Another treatment that uses electrical stimulation is tumor treating fields. These EFs do not focus on neuroplasticity or modifying neural circuits but instead focus on disrupting tumor cell mitosis through high-frequency electrical stimulation. Tumor treating fields were FDA approved in 2011 to treat glioblastoma multiforms.14 These varied uses with considerable success demonstrate the versatility of treatments using electrical stimulation.
We hypothesize that neural repair ensues upon application of electrical stimulation as a result of EF generation that modulates the behavior of nonaction potential-generating cells. This could include glial cells and vascular endothelial cells but most promising is the activation of electrosensitive resident NPCs. It has been demonstrated that NPCs are highly responsive to EF application and are activated to proliferate, differentiate, and migrate in response to EF application.15 Migration due to EFs has been extensively demonstrated in vitro, and more recent studies show the ability of applied EFs to promote NPC migration along migratory paths in vivo in the rodent brain.15–18 Differentiation and proliferation kinetics can also be modified by electrical stimulation, and this has been demonstrated both in vitro and in vivo.19–21 Optimization of these EFs to better control NPC behavior is still required, but manipulating NPC behavior affords great promise in the field of regenerative medicine.
As we consider the goal of developing novel therapeutics for brain repair, herein we will discuss the cellular outcomes following EF application and ongoing work designed to fully understand the response of CNS tissue to EFs. We will consider not only the ways to maximize the cell-based response (from genes to migration) but also importantly we will consider the optimization of EF-based activation strategies and how cellular outcomes will feed into the design elements of the electrodes. We will highlight the response of resident NPCs to EF application in terms of survival and neurogenesis and focus more specifically on EF-induced migration (galvanotaxis), as this is a critical step to ensuring that sufficient numbers of cells are available to contribute to neural repair. The various electrode materials and geometry design for optimizing galvanotaxis will be discussed in detail. Finally, we will conclude with some exciting potential clinical applications and technological advances.
What Influences Galvanotaxis? Nature Versus Nurture
Endogenous NPCs are rare, comprising less than 10% of the periventricular cells in a three to five cell layer thick region lining the lateral ventricles in the adult forebrain. These NPCs are highly responsive to EF application, expanding in number through proliferation and enhanced cell survival, as well as differentiating into newborn neurons.19 Most striking, NPCs are activated to migrate in a rapid and directed manner in the presence of an applied EF using well-established in vitro assays and live cell imaging.15 Together, these NPC behaviors provide promise for the design and implementation of regenerative medicine strategies that aim to replace lost or damaged cells following injury or disease, yet several important questions remain unanswered that are pivotal to understanding how to optimize the NPC response. For instance, how does a cell sense the EF? What is the intracellular signaling cascade(s) that dictates the EF-induced cell behavior? Indeed, electrosensitive cells can differ in their migratory response to the same EF application by migrating in different directions (cathodal vs. anodal), with different speeds and distinct migratory pathways (tortuosity). These behaviors are not only cell-dependent but also regulated by the stimulation paradigm and the microenvironment. Considering these factors together, one can envision that EF application would lead to a highly interactive, niche-dependent cellular response in injured or diseased tissue.
Nature: intrinsic cell migration mechanisms and responses to stimulation
The same EF application can lead to specific responses in distinct cell populations.22 Indeed, cells display directedness in an EF, migrating toward the cathode (negative) or anode (positive) depending on the cell type. What mechanisms may underlie these different responses? For a cell to start migrating in one direction, the cell needs to first sense the EF which will ultimately lead to asymmetry within the cell through signaling cascades that enhance migration in one direction (e.g., extension of the cytoskeleton). This galvanotactic response can involve the electrophoresis of charged membrane proteins following electrical stimulation, which creates a ligand gradient along the cell membrane, thereby generating asymmetry within the cell.23 Another response to the EF is the polarization of charged molecules within the cell, which can lead to asymmetry by binding and blocking channels on the cell surface.22
An equally plausible hypothesis is the presence of multiple EF-sensing mechanisms and signaling cascades that could, in theory, underlie migration in opposite directions from a resulting “tug-of-war” between mechanisms within a single cell. An example of a pathway involved in translating EF signals into migration is the phosphoinositol-3 kinase (PI3K) pathway. PI3K is a central enzyme involved in the signal transduction of stimuli, including growth factors and cytokines. Blocking PI3K significantly decreases galvanotactic response in many cell populations suggesting its important role in sensing the EF.16,24–26 Furthermore, studies have demonstrated that blocking guanylyl cyclase, an enzyme involved in the signal transduction of many cell processes like proliferation and migration, can completely reverse the direction of migration resulting in a cathodally-migrating cell becoming an anodally-migrating cell.25 Dissociating the speed of migration and the direction of migration highlights the complexity of the response and the presence of more than one signaling cascade underlying the galvanotactic response.
In general, increasing EF strength results in a graded increase in speed of migration until the cells undergo cell death from the high EF strength. Human NPCs will migrate in a directed manner in an EF strength of 250–350 mV/mm and undergo rapid cell death in higher EF strengths.27 Most interesting, the species from which the NPCs are derived can influence their migratory response. For instance, mouse-derived NPCs migrate to the cathode, while human-derived NPCs migrate to the anode in the same EF strength and when placed on the same substrate.15,27 NPCs derived from human embryonic stem cells or directly reprogrammed from mature human bone marrow can migrate toward the cathode in the presence of an EF.18,27 These findings suggest that galvanotaxis is a common feature of NPCs, irrespective of the origin of the cells. Bovine-derived epithelial cells are another example of a cell population that undergoes galvanotaxis but the direction of migration is dependent on the strength of the applied EF. These studies highlight the fact that different mechanisms appear to underlie EF induced migration of distinct cell populations.28 When considering in vivo application, it is important to consider that the vast majority of in vitro studies use direct current electrical stimulation. The use of direct current electrical stimulation requires that the electrodes and cells be placed in separate chambers to prevent toxic by-products generated from the electrode–electrolyte interface from influencing the cells.
For in vivo application, toxic by-products at the interface are reduced by stimulating with a charge-balanced pulse (i.e., the amount of charge injected into the tissue will equal the amount of charge drawn out of the tissue). Toward the goal of in vivo application, the effects of charge-balanced biphasic monopolar stimulation on NPC migration in vitro were examined and it was found that the frequency was a key element of NPC galvanotaxis.29 Higher frequencies were effective in promoting migration, whereas lower frequencies were not. We postulate that the increased time between pulses (lower frequency) allows the polarized or electrophoresed membrane proteins to move back to baseline conditions eliminating the asymmetrical activation of intracellular signaling cascades required for enhancing migration in one direction.
Biphasic stimulation is yet to be optimized in vitro, as is the applied electrical stimulation required to deliver EF strengths in vivo to promote galvanotaxis. However, promising recent work has demonstrated that biphasic in vivo stimulation can enhance NPC migration and regulate cell behavior.17,19 Transferring the in vitro parameters to in vivo settings will be a challenge as the microenvironment also has profound effects on galvanotaxis. The microenvironment plays an important role in what the cell perceives and as such, the galvanotactic response is highly sensitive, yet malleable, with the outcome dependent on the EF parameters such as strength and frequency and the cell's environment (e.g., extracellular matrix [ECM] and nearest neighbors, discussed next).
Nurture: extrinsic microenvironment factors influencing migration responses
The microenvironment is a complex combination of cues, which include cell–cell interactions and interactions with extracellular matrices and soluble and tethered physical factors (Fig. 1A). Different microenvironments are found during aging, disease, and injury, including altered extracellular membrane proteins, changes in pH, the presence of infiltrating blood cells, and changes in neighboring cell phenotypes (e.g., formation of a glial scar by activated astrocytes; activation of microglia which are the resident immune cells in the CNS), which ultimately alter cell behavior in response to electrical stimulation.15,30,31 For example, after injury, the ECM becomes more flexible, the pH is reduced, and pro-inflammatory factors are expressed near the injury site.32–34 These are all factors which can affect cell migration. It is important to understand how different brain microenvironments may affect the efficacy of galvanotaxis in a clinical setting and further to consider how altering the microenvironment through implanted electrodes may impact the galvanotactic response.
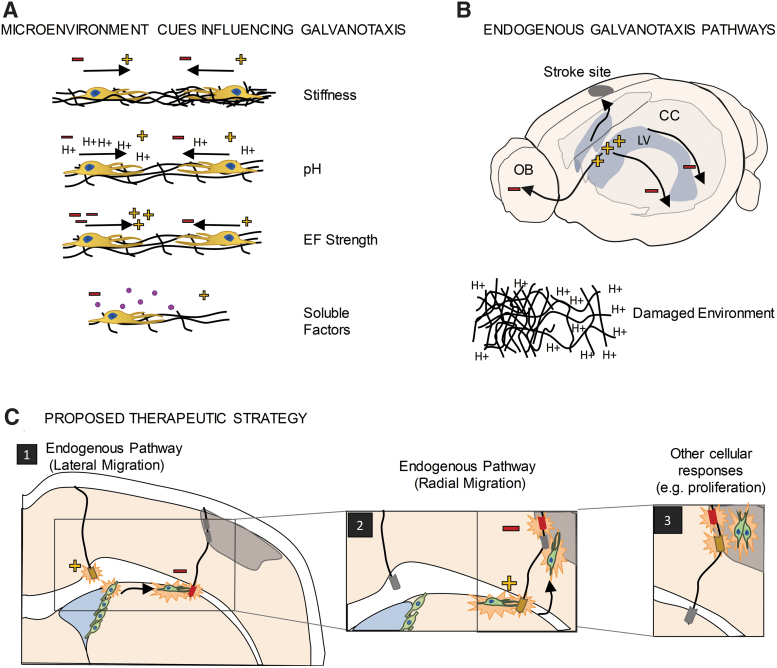
FIG. 1.
Galvanotaxis. (A) Differences in the microenvironment can affect the galvanotactic response, including reversing the direction and speed of galvanotaxis. (B) Endogenous electric potential differences are found in vivo and these are consistent with migration pathways found in vivo. Damaged tissue will have different microenvironments which could either affect galvanotactic response or serve as a migratory cue. Harnessing these pathways could facilitate electrical stimulation therapies. (C) Proposed therapeutic strategy utilizing endogenous pathways and fine-tuning the electrical stimulation to elicit different cellular responses. Minus (−), cathode; plus (+), anode; CC, corpus callosum; EF, electric field; LV, lateral ventricle; OB, olfactory bulb.
Altered levels of mitogens or increased cytokine release are good examples of factors that are affected by injury or disease and can impact NPC migration.35 For instance, the mitogen EGF is critical for the rapid and cathodally-directed galvanotaxis of murine NPCs such that blocking EGF signaling leads to slower cell migration, with no change in directionality.15 After ischemic injury, EGF is upregulated in damaged tissue, as well as the NPC niches in the brain.36 Injuries can also activate and recruit inflammatory cells leading to the release of cytokines which alter calcium and pH levels which are known to impact galvanotaxis.37,38 Indeed, extracellular pH can completely reverse the direction of migration (i.e., from cathodal to anodal) in keratinocytes.30 This is thought to be due to changes in ion channel activity such as potassium channel Kir4.2, which has been shown to be instrumental in sensing EFs. Hence, the regulation of the galvanotactic response is highly sensitive to the microenvironment, and the different migratory parameters (speed, direction) can be independently regulated by specific cues. Innovations in electrode design could include the delivery of molecules to regulate the microenvironment to control galvanotaxis.
Perhaps most compelling is some recent work highlighting the role of the ECM in galvanotaxis. Ahmed et al., studied human derived NPCs in the presence of an applied EF and reported that substrate stiffness was sufficient to completely reverse the direction of migration.27 Whether the substrate was a cell monolayer or fibrous protein, the direction of migration of human NPCs was dictated by the stiffness of the substrate, while the speed was unaffected. Considering the physical properties of the different regions of the brain (i.e., white matter axon tracts vs. gray matter neuronal cell bodies), as well as the scar formation after injury (composed of activated glial cells, which are less stiff than uninjured brain tissue), the impact on NPC based neuroplasticity is significant.
Another consideration for the development of electrical stimulation therapy is the endogenous EFs present within the tissue (Fig. 1B). Indeed, in the mature CNS endogenous EFs have been shown to play a role in NPC migration under baseline conditions.17,39 An endogenous EF exists along the rostral–caudal axis, which is a pathway for NPC to migrate to the olfactory bulb where they generate new olfactory bulb interneurons throughout life. The small endogenous EF (∼3 mV/mm) is thought to be the result of ion distribution in the extracellular space and differential ion pump distribution on the apical and basal surface of epithelial cells comprising the NPC niche.39 Reversing this endogenous EF causes cells to migrate in the opposite direction along this same rostro-caudal axis, supporting its role in migration.18,39
More recently, an electric potential difference was identified in the mature CNS along the medial-lateral axis, specifically along the corpus callosum (the largest white matter tract in the forebrain). This endogenous EF was coincident with the lateral migration of transplanted NPCs on the corpus callosum and, again, reversing the electric potential resulted in NPC migration in the opposite direction.17 Hence, enhancing cell migration to a site of injury or disease will also need to consider the presence of endogenous EFs that persist, or are generated, in response to injury, and may need to be overcome to enhance targeted migration.40,41
Currently, there are limited in vivo studies that have investigated transplanted NPC galvanotaxis in the rodent brain along these migratory paths.17,18 In these studies, fluorescent NPCs were visualized through immunohistochemistry, which provided snapshots of their migration. The respective electrical stimulation paradigms revealed migration ranging from ∼100 μm over 3 days to as much as 6 mm over the course of months. Details regarding migration path and speed in the brain's three-dimensional (3D) microenvironment were not determined as this was a limitation of using immunohistochemistry at single time points to evaluate the cellular response. Next steps to acquire higher spatial and temporal resolution will provide insight into these important aspects of in vivo galvanotaxis.
Nevertheless, together, these studies support the hypothesis that exogenous application of EFs will provide cues that can regulate NPC behavior and support neural repair (Fig. 1C). Notably, electrical stimulation can elicit other cellular responses, such as cell proliferation.19 Interestingly, electrical stimulation has been shown to increase the number of blood vessels in the injured brain,42 modulate blood–brain barrier permeability,43,44 and modulate numbers of microglia and astrocytes.45–48 The ability to affect the microenvironment creates the possibility of “side effects” such as modulation of the number of astrocytes and microglia but with more insight into the effects of EFs on tissue responses; these “side effects” could be purposely controlled to create an environment more amenable to tissue repair.45–48 Development of this therapy requires exquisite attention to design parameters to manufacture novel electrodes that will function in a range of microenvironments to facilitate the desired galvanotaxis response.
What Do You Need in an Electrode? Stimulation, Flexibility, and Compatibility
Design of electrodes for producing the EFs to augment NPC behaviors for neural repair requires the consideration of a number of factors. Current electrodes are rigid, and the implantation can serve as a source of tissue injury, ultimately impacting NPC migration, as described. Therefore, to successfully deliver this therapy, it is important to develop flexible electrodes with novel biocompatible materials that can be tuned to deliver appropriate electrical stimulation. Considerations such as electrode geometry can also be refined to provide additional customizable parameters depending on the location of the implants and the age, injury, or disease state of patients in need of neural repair.
Electrical stimulation: how shocking is it?
Characterization of the electrical properties of the electrode and the tissue is required to predict what EF cells are experiencing and to predict the outcomes. An important parameter is impedance, of both the electrode and the tissue. Impedance is the frequency-dependent current-voltage response that describes the dynamic electrical properties of a system. It is commonly defined as the opposition to alternating current and has two components: resistance and reactance. Resistance is the frequency-independent opposition to current, while reactance is the frequency-dependent combination of capacitance and inductance that oppose alternating current.49,50
The most common techniques used to characterize the electrical properties of an electrode are electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV). EIS measures electrical impedance for a wide range of different frequencies, while CV measures current density for a range of potentials. These can be tailored to values that elicit a biological response and used to characterize the electrode and the electrode-tissue interfacial properties, which are critical for in vivo application. The results can also be derived through an electrical model that represents the electrode as an equivalent circuit and are dependent on the stimulus parameters such as pulse amplitude, frequency, and pulse duration.51,52 For neural stimulation, a biphasic electrical stimulation is typically applied to prevent charge accumulation, which is associated with pH changes and overpotential.53 Significantly, it is only of late that charge-balanced electrical stimulation was shown to induce NPC migration in vitro and now in vivo.17,29 This is an exciting and positive step when considering EF application for NPC-based neural repair strategies.
Monitoring impedance is an important way to determine the efficacy of implanted electrodes as the degree of impedance (i.e., too large or too small) can indicate problems with the design or equipment.54 In general, for implanted stimulating electrodes, high current densities while operating are usually required and, as such, benefit from lower impedances.55 The impedance will vary depending on the stimulation parameters, the tissue environmental parameters such as temperature, and the electrode's material, surface area, and geometry.56 Even different regions of the brain, white matter, gray matter, and cerebral spinal fluid, have different electrical impedances which can further change through aging and disease. Indeed, the time of implantation relative to stimulation can also affect the impedance observed51,52,56–58; thus, it is critical to generate a comprehensive model to provide a clear understanding of the EF perceived by the NPCs in an applied EF.
Be flexible and biocompatible: fitting in
Materials such as platinum and its alloys, iridium oxide and titanium nitride, have been used extensively for creating implantable stimulation electrodes for the nervous system due to their low impedance and biocompatibility. These conventional metal-based electrodes have been reviewed previously, detailing information on electrode performance and potential drawbacks due to electrode degradation.59
One of the features of these currently used electrodes is their inflexible nature and the damage that can ensue following implantation, including mechanical disruption and tissue inflammation, ultimately resulting in changes to the microenvironment that can alter NPC behavior. To reduce the perturbation to the microenvironment, a biocompatible material that closely matches the stiffness of the brain would be ideal. Toward this end, a set of novel materials, including conducting polymers and carbon-based nanoparticles, have been used for adaptation as neural stimulation electrodes. These novel electrode materials afford benefits such as high charge injection density, high electrical conductivity, high flexibility, low toxicity, and electrical tunability. Most of these materials have been tested in vitro for biocompatibility and some have been tested for their ability to elicit an NPC behavioral response.60–62 Materials discussed are summarized in Table 1.
Table 1.
A Review of Materials for Neural Electrical Stimulation, Their Properties, Advantages, and Disadvantages
| Electrode materials | Fabrication process | Geometry | Electrical resistivity/conductivity | Charge injection density | Biocompatibility | Flexibility | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|---|---|
| PPy | Template-assisted electro-deposition | Flat planar design | 190 S/cm; doped with PSS; 19.84 S/cm | 5 mC/cm2 | Positive biocompatibility profile in vivo; increased neuron adhesion | Highly flexible | Flexibility; high electrical conductivity; biocompatible | Fragile mechanical properties; coating is thin; degradation possible | 76–78 |
| PEDOT:PSS | Crosslinked | 3D printed micropillars | 5.8 S/m | 1.2–3.9 mC/cm2 | Indirect and direct cytotoxic tests ISO 10993-5 | Highly flexible | High electrical conductivity; transparency; biocompatible; neural stimulation demonstrated | Water soluble; long-term unstable | 64,79 |
| PPy/PSS layered with MWNTs | Layering and codeposition | N/A | 30 S/cm | 7.5 mC/cm2 | Cell growth inhibition assay | Highly flexible | Electrochemically stable; high electrical conductivity | Requires process optimization; toxicity needs to be further verified | 78,80 |
| CNT | Low-pressure chemical vapor deposition | Vertically aligned pillars | 1.8 × 107 S/m | 1.0 − 1.6 mC/cm2 | Uncertain | Flexible | Highly electrically conductive; versatile | Surface modification required for biocompatibility; poor dispersion in composites | 70,71 |
| Porous graphene | Laser reduction | Coating | 303 S/m | 3.1 mC/cm2 | Live/dead cell analysis | Flexible | Mechanically flexible; biocompatible; high electrical conductivity | May be fragile; requires surface modification to enhance hydrophilicity | 73,81,82 |
| Pt | Extrusion/drawing | Cylindrical and wire | 9.6 × 106 S/m | 0.10–0.30 mC/cm2 | MTT proliferation assay; smaller scar thickness | Rigid | Good mechanical properties; good biocompatibility; used extensively; chemically inert; high electrical conductivity | Cell death possible; possible corrosion; may undergo irreversible dissolution producing toxic by-product | 83,84 |
| Chemical vapor deposition | Circle | 295.07 μC/cm2 | 83,85,86 | ||||||
| Fractal | 510.50 μC/cm2 | ||||||||
| Serpentine | 318.82–359.53 μC/cm2 | ||||||||
| Iridium oxide | Electrodeposition | Thin film | 0.75 × 10−3 to 1.67 × 10−3 Ω cm | 1.2 mC/cm2 | Glial scar assay; neuron adhesion; MTT cell viability | Rigid | Good mechanical and electrical properties; high charge injection capacity | Over-pulsing can cause degradation; chronic usage may lead to inconsistency; less biocompatible compared to Pt | 86–89 |
| Titanium nitride | Sputtering | Thin film, coating | 25 × 10−6 to 800 × 10−6 Ω cm | 0.87 mC/cm2 @ 0.2 ms | MTT proliferation assay; live/dead cell analysis | Flexible coating | High surface areas; ease of fabrication | Potentially increased cell death; oxidation possible | 90–93 |
3D, three-dimensional; CNT, carbon nanotube; MTT, 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide; MWNTs, multiwalled carbon nanotubes; PEDOT:PSS, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate; PPy, polypyrrole; PPy/PSS, polypyrrole polystyrene sulfonate; Pt, platinum.
Intrinsically conducting polymers have found their application in neural stimulation in vitro due to their flexible mechanical nature, surface biocompatibility, and their tunable electrical conductivity. As an additional benefit these electrodes can provide varied EFs along the surface of the electrode unlike conventional metal electrodes. This was demonstrated using polypyrrole (PPy) with dodecyl benzene sulfonate as the dopant.63 The electrode contained regions with higher electrical conductivity and the neuronal and glial cells adhered more to those regions. Cross-linked poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (xPEDOT:PSS) is another conductive polymer that stimulated NPC proliferation and differentiation in vitro with defined EF parameters.64 Furthermore, these intrinsically conducting polymers can be doped with other bioactive molecules to improve the microenvironment of damaged or diseased tissue, affording a combinatorial strategy to promote neural repair. One potential drawback is that these polymers may degrade in certain environments (e.g., higher pH at an injury site), which would require additional tuning of the parameters to support galvanotaxis.
Another important consideration for the design and implementation of novel stimulating electrodes is the method of fabrication. Conducting polymers can be 3D printed (Fig. 2A) which supports the production of easily customizable shapes for electrodes.65 The ease and affordability of 3D printing have already been demonstrated with printed electrode connectors.66 A second method of fabrication is electrospinning. Electrospinning allows nonwoven fibrous composite electrodes to be fabricated by encapsulating conducting particles within biocompatible polymers while retaining its nanofibrous morphology. The conductive portions of the electrode are woven into the polymer, and this provides new surface geometry, further enabling different biocompatible polymers to be used. Yan et al. have electrospun hybrid fibrous electrode by integrating different concentrations of polyaniline tetramer with polycaprolactone and showed that NPCs were responsive to the stimulation and exhibited increased proliferation.67
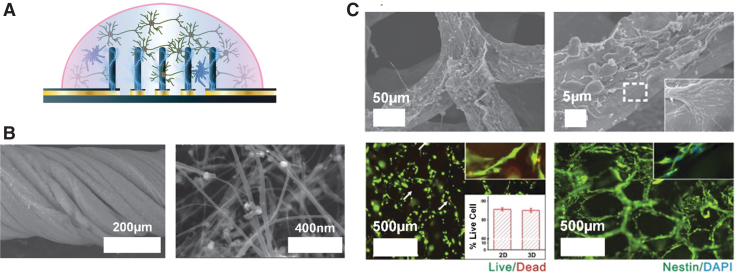
FIG. 2.
(A) PEDOT:PSS pillars were successfully 3D printed using a novel direct-write method, and electrical stimulation was applied to enhance NPC proliferation65; (B) CNT-based electrodes twisted into a rope morphology, which can be effectively utilized for neural stimulation. The graph on the right is the microscopy image of the synthesized CNTs at a higher magnification68; and (C) 3D graphene foam formation utilized as electrically conducting and biocompatible neural scaffolds for NPCs. The top two microscopy images show the structure of the graphene foams in detail and the region outlined with, while dashed line in the right image indicates the interaction between the cell and graphene foam surface. The bottom left image is a cell viability test with NPCs seeded on the graphene foam structure after 5 days of culturing, and the inset is the percentage live cell data (live cells—green, dead cells—red, and the arrows are indicating the dead cells). The bottom right image is a fluorescence image of NPC proliferation on the graphene foam surface (nestin for NPCs—green and DAPI for nuclei—blue).69 3D, three-dimensional; CNTs, carbon nanotubes; NPC, neural precursor cell; PEDOT:PSS, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate. Reproduced with permission. Copyright Wiley (A, B) and Nature (C).
Carbon nanotubes (CNTs) have unique properties in that they are structurally stable and very small, which are promising for neural electrode design. The smaller size leads to less tissue displacement upon implantation. Wang et al. fabricated CNT-based vertically aligned micropillars with small diameters of ∼50 μm for neural stimulation electrode arrays.70 Fabrication of CNTs is also advantageous as they can be twisted into a rope for increased contact area between the electrode and the tissue (Fig. 2B).68 Biocompatibility studies measuring the quantity of cytosolic enzyme lactate dehydrogenase, a marker of cell lysis, have shown that the CNT does not affect NPC survival. Most interestingly, the electrode design has unique surface properties that can impact cell interactions. Furthermore, functionalization of the CNT permits hydrophilic surfaces to form providing a safer electrode/tissue interface with high charge injection densities as a result of smaller interfacial resistance. While exciting, one concern is that CNTs may have biocompatibility issues in vivo. Therefore, simple surface modifications may be required to utilize CNTs to their full potential.71
Finally, graphene and graphene-oxide based electrodes are interesting platforms for electrical stimulation based on their inherent flexibility and biocompatibility.72 As shown in Figure 2C, Li et al. utilized graphene foams (GF) to provide an improved neural bioelectronic interface and stimulation scheme to regulate NPC migration and proliferation.69 It was further demonstrated that 3D GF could further enhance the NPC differentiation compared to its two-dimensional counter parts, if this is the desired outcome in vivo. The electrical conductivity of the GF structure decreased minimally in culture and provided control over the NPC migration and proliferation. Similar to the concerns for CNTs, graphene may still need surface modification to further improve biocompatibility in vivo.73,74
Composite neural electrode systems are constructed to combine the desired electrical properties for effective and safe stimulation, with a supporting mechanical scaffold potentially for cell adhesion. Recently, Fu et al. have created a poly(l-lactic-co-glycolic acid) (PLGA)/graphene oxide (GO) composite film to be used as neural stimulation platform.75 The PLGA/GO system was sufficient to promote stem cell proliferation and neurite elongation in differentiated neurons making it a promising material for future in vivo studies.
Overall, there exist a variety of flexible and biocompatible materials that are capable of stimulating NPCs and modifying their survival, proliferation kinetics, and differentiation profiles in vitro. The challenge lies in translating them to in vivo electrodes that will minimize damage to the microenvironment and provide electrical stimulation sufficient to promote migration to the target locations.
Surface and Geometry: Is the Solution Shaping Up?
The surface and geometry of implanted electrodes can play important roles in modulating galvanotaxis for neurostimulation since electrode surface features and overall geometry impact on electrode parameters such as electrical impedance, charge injection capacity, and stimulation efficiency.94 Surface and geometry should be optimized to maximize the clinical effectiveness while minimizing the risks associated with electrical stimulation of the brain or tissue trauma during electrode implantation. In this section, the effect of surface morphology and geometrical features on neurostimulation is outlined.
The effect of surface features on the performance of the commercial DBS electrode designs (Fig. 3) has been explored with the understanding that electrical properties of electrodes could be improved by considering surface morphology, shape, and size optimization. For instance, Yamagiwa et al. compared porous and flat metal electrodes made of the same material (titanium nitride and iridium oxide) and showed that rough electrodes have lower impedance and higher charge-injection capacity than flat ones, a beneficial property for stimulation electrodes.95 In another study, the surface roughness was increased to enhance capacitive characteristics by electroplating of iridium on gold electrode microarrays (Fig. 4A).96 There are a number of benefits to porous electrode surfaces; however, one caveat is the possibility of biofilm formation which can increase the risk of infection. Surface modifications such as nanotechnologies to prevent biofilm formation are worthy of consideration.97,98
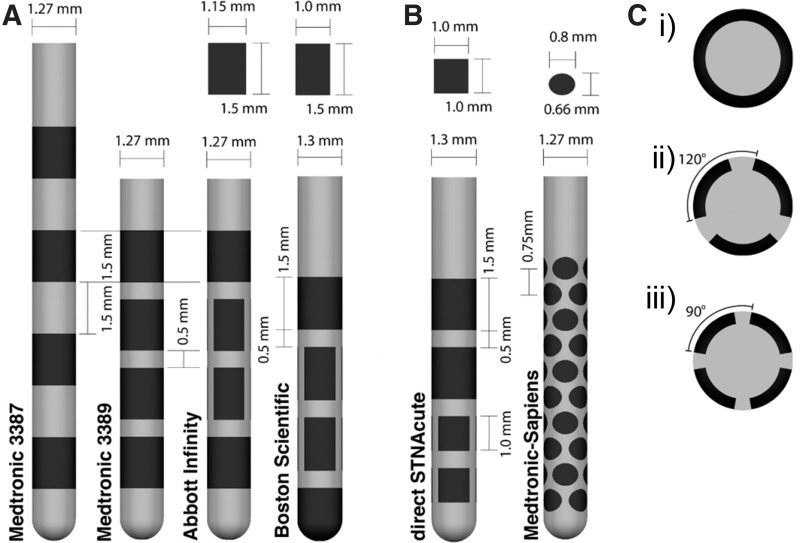
FIG. 3.
(A) FDA-approved commercial cylindrical DBS electrodes are mainly simple rectangular shapes with quadripolar arrangements. Featured here: Medtronic 3387/3389, the Abbott Infinity lead and the Boston Scientific lead. For the Abbott Infinity and Boston Scientific lead, the dimensions of the three contacts around the circumference of the middle two rows can be found above. (B) Different geometries and arrangements are featured in the direct STNAcute and Medtronic-Sapiens lead that have been implanted in human patients for testing but not approved by the FDA. (C) Cross-sections of the DBS electrodes: (C.i) Medtronic 3387/3389 and other cylindrical contacts on directional leads. (C.ii) Three segments on the Abbott directional lead, Boston Scientific directional lead, and the direct STNAcute. (C.iii) Four segments on the Medtronic-Sapiens. Dark regions on the implants correspond to the active electrode placements, while the gray regions indicate the supporting structures. DBS, deep brain stimulation. Figure reproduced under the terms of the Creative Commons Attribution 3.0 License.103
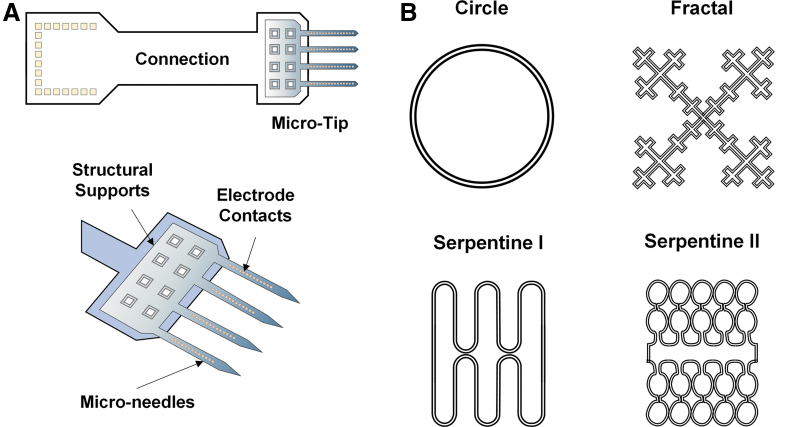
FIG. 4.
(A) Gold microelectrode probes with an array of 32 channels. Surface roughness was increased by electroplating iridium on gold electrode sites to enhance the capacitive characteristics.96 (B) Geometries with identical surface areas but different perimeters. Reproduced with permission. Copyright Nature (B).
Lee and colleagues studied the role of geometries on electrode electrical properties by fabricating fractal and serpentine-shaped platinum electrodes and compared them with traditional circular electrodes (Fig. 4B). Under the same area, Serpentine II had the highest perimeter and thus exhibited the greatest charge storage capacity and total delivered current. In addition, the fractal-shaped electrode exhibited an outstanding charge injection capacity and potential penetration capability due to greater Faradaic and non-Faradaic electrochemical processes.99 Fractal shapes have also been shown to improve the energy efficiency requiring only 78% of the original input power needed to maintain the same level of neurostimulation.100 Although noncircular electrodes provide better electrical performance, risks of damage due to sharp edges have not been examined in vivo. Nevertheless, these geometry patterns can be incorporated to traditional cylindrical electrodes and flexible electrode microarrays to promote effective neurostimulation while maintaining their signal quality and biocompatibility.
Reduction of electrode lead size from millimeters to micrometers effectively decreases the volume of the implant, which will decrease mechanical strain at the implantation site and reduce the inflammatory response. For instance, polypropylene fibers with micrometer-sized diameter exhibited significantly smaller macrophage density compared to larger diameter fibers. One caveat is the greater risk of breakage with the smaller diameter fibers.101 These results are consistent with another study, which compared two different sizes of stainless-steel electrode coated with poly-glycolic acid at 4 weeks postimplantation. Smaller diameter (12 μm) electrodes resulted in significantly less glial scar formation than the larger diameter (25 μm) electrode102 supporting the conclusion that size optimization should carefully be considered for electrode design.
Overall, theoretical and experimental studies indicate that the geometry has a tremendous effect on neurostimulation. However, none have looked at the effects of these changes on electrosensitive NPCs in the brain. In the future, these studies will be critical for exploiting EFs as a means to promote neural repair.
A Clinical Perspective: A Stimulating Future Outlook
Together, the developments discussed above could enable the use of EFs for applications that go well beyond the circuit modulation paradigm currently targeted by DBS protocols. Three clinical applications are of particular interest: galvanotaxis, neuroprotection, and spatiotemporal control of delivery of therapeutics, including drugs and viral-mediated gene therapies. These clinical applications could provide new therapies for the many individuals that are living with neurological disorders. Finally, we highlight two technological advances that can be used for these clinical applications: electrode modifications and EF modeling.
Galvanotaxis
The success of stem cell-based clinical trials in stroke or spinal cord injury has been hampered, in part, by the realization that NPCs need an appropriate environment to reach the diseased target, to differentiate into appropriate cell types, and organize into functional networks.104 Promising work has shown that murine NPC survival, migration, and differentiation can be modified by EF application. Indeed, human trials using scaffolds have been conducted to address these issues105,106; however, a major limitation in their utility is that artificial channels cannot be adjusted after implantation. If properly controlled, galvanotaxis could provide a means to direct NPC migration through more permissive mediums, such as hydrogels, or to enhance NPC migration along endogenous migration paths such as the corpus callosum (Fig. 1B). Furthermore, dynamic adjustment of the EF as the target tissue is being regenerated could allow for improved neuroplasticity, including synaptic reconstitution and network restoration.
Neuroprotection
Although we primarily discussed the potential of electrical stimulation to promote NPC migration for tissue regeneration, evidence is accumulating that DBS may have direct neuroprotective, disease-modifying effects outside of migratory responses.107 A number of reports of subthalamic nucleus DBS for Parkinson's Disease reveal a trend toward motor score stabilization or improvement when off-medication/off-stimulation in chronically stimulated patients.108 While the neurological substrate of this stabilization is yet to be determined in humans, animal studies have shown that chronic subthalamic nucleus DBS protected the dopaminergic neurons from cell toxicity in Parkinson's Disease in a number of animal models.109–113 Another example is Alzheimer's disease where DBS of the fornix leads to a reduction in the rate of hippocampal atrophy.114 The mechanism of action in both diseases is unknown, but is consistent with a reduction of excitotoxicity from hyperactive glutamatergic projections111,115,116 or the induction of neurotrophic factor release.117–119 In neurodegenerative diseases where specific structures are preferentially affected, targeted neuroprotective stimulation in the early stages of the disease could halt disease propagation across the affected neural network.
Spatiotemporal control of other therapeutics
While EFs can modulate cellular function with exquisite spatial and temporal resolution, they are hardly tissue selective. Targeting dopaminergic neurons without affecting colocalized cholinergic projections, for example, cannot be reliably achieved with current DBS programming paradigms. A promising approach to obtain tissue selectivity is through the use of drugs or viral vectors targeting tissue-specific receptors on the cells. Indeed, using electroresponsive drugs or viral mediated gene therapies could provide the best of both worlds by enabling EFs to exert spatiotemporal control over the activation of tissue-selective drugs or gene-specific promoters. This approach is currently being used with considerable success in optogenetics studies,120 although light penetration through tissue limits the size and location of optogenetic targets.121 It is exciting to speculate that “electrogenetics” could circumvent these limitations and, indeed, proof of concept studies are underway.122
Adaptable electrodes
Modified approaches to enhance neural repair using shape-memory polymers, for example, could provide adaptive control over the NPC migration path as cells migrate enabling the “best” cell migration performance to be achieved. Customizable electrodes could adapt to the patient-specific microenvironments and even change shape to create a better fit to ensure the EFs are effectively applied.123 Additional concepts of adaptable electrodes also include tailorable electrical conductivity and mechanical flexibility based on the in vivo environment, allowing the most effective electrical stimulation to be applied.123,124 Furthermore, it is possible to create electrodes that undergo harmless degradation and residual absorption once electrical stimulation is completed, which is an exciting concept that would effectively eliminate the need for further surgery to remove the electrode after tissue regeneration.125
EF modeling
Finally, a better understanding of the EF distribution in vivo is necessary to better predict outcomes related to these novel therapeutic interventions. Multiphysics modeling tools using measured electrical conductivities of the cerebral cortex, corpus callosum, and the lateral ventricles (e.g.) would facilitate an understanding of the EF lines in the brain in vivo.126 Customization of these conductivities and modeling could be performed to consider hydration status, disease progression, and even mental state of the participants using close-looped systems measuring local field potentials to adjust the state of the stimulation accordingly.127 Using these analyses, the optimal electrode configuration and customized stimulation paradigms can be predicted to provide the best stimulation performance. The directionality and strength of the applied EF in vivo and the electrodes' interactions with the adjacent neural tissues will offer valuable insights in the fundamental mechanisms underlying the results of the stimulation of these new clinical applications.
Conclusion
We have highlighted the promise of EF application for the goal of improving neuroplasticity and promoting neural repair. The presence of endogenous NPCs has generated excitement about their use in neural regeneration strategies. We propose that harnessing their electrosensitive properties is a novel approach to treat the injured or diseased CNS. NPCs respond to electrical stimulation through migration, proliferation, and differentiation. Controlling their behavior requires a sound knowledge of the impact of EFs on brain tissue, brain pathology, and how the brain perceives the EF. The design and manufacturing of stimulating electrodes specifically created for the purpose of neural repair are underway. To enable more effective future neural stimulation for tissue repair, next generation materials and optimized geometries will also need to be considered. The future is just around the corner.
Authors' Contributions
S.N.I., H.H.S., S.K.K., M.R.P., H.E.N., and C.M.M., planned the content. S.N.I. planned the organization of the article, wrote, revised, and edited the Introduction and What Influences Galvanotaxis? Nature Versus Nurture sections, and created and revised Figure 1. H.H.S. wrote, revised, and edited the What Do You Need in an Electrode? Stimulation, Flexibility, and Compatibility section and portions of Table 1, and created and edited Figures. S.H.H. and T.C. wrote, revised, and edited the Surface and Geometry: Is the Solution Shaping Up? and portions of Table 1. M. M.-C. wrote, revised, and edited the Electrical stimulation: how shocking is it? section. S.N.I., H.H.S., S.H.H., T.C., M.M.-C., and C.I.-M. wrote, revised, and edited the A Clinical Perspective: A Stimulating Future Outlook section. S.N.I., H.H.S., S.H.H., C.I.-M., S.K.K, M.R.P., H.E.N., and C.M.M., reviewed and edited the full article. All authors reviewed and approved the submission of the final version of the article. This review has been submitted only to Bioelectricity and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This article was not directly supported by external funding.
References
- 1.Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalf MEM, Borgens RB. Weak applied voltages interfere with amphibian morphogenesis and pattern. J Exp Zool 1994;268:323–338 [Google Scholar]
- 3.Zhao M.Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol 2009;20:674–682 [DOI] [PubMed] [Google Scholar]
- 4.Chiang M, Cragoe EJ, Vanable JW. Intrinsic electric fields promote epithelization of wounds in the newt, Notophthalmus viridescens. Dev Biol 1991;146:377–385 [DOI] [PubMed] [Google Scholar]
- 5.Houghton PE.Electrical stimulation therapy to promote healing of chronic wounds: A review of reviews. Chronic Wound Care Manage Res 2017;4:25–44 [Google Scholar]
- 6.Griffin M, Bayat A. Electrical stimulation in bone healing: Critical analysis by evaluating levels of evidence. Eplasty 2011;11:e34. [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner J.A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Soc Stud Sci 2013;43:707–728 [Google Scholar]
- 8.Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): A double-blind, randomised, controlled trial. Lancet Neurol 2020;19:123–134 [DOI] [PubMed] [Google Scholar]
- 9.Lozano AM, Lipsman N, Bergman H, et al. Deep brain stimulation: Current challenges and future directions. Nat Rev Neurol 2019;15:148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charkhkar H, Christie BP, Pinault GJ, et al. A translational framework for peripheral nerve stimulating electrodes: Reviewing the journey from concept to clinic. J Neurosci Methods 2019;328:108414. [DOI] [PubMed] [Google Scholar]
- 11.Howland RH.Vagus nerve stimulation. Curr Behav Neurosci Rep 2014;1:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeken C, Brem AK, Arns M, et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: Current knowledge and future directions. Curr Opin Psychiatry 2019;32:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquez-Chin C, Popovic MR. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: A review. Biomed Eng Online 2020;19:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson KD, Lok E, Wong ET. An overview of alternating electric fields therapy (NovoTTF Therapy) for the treatment of malignant glioma. Curr Neurol Neurosci Rep 2016;16:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babona-Pilipos R, Droujinine IA, Popovic MR, et al. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS One 2011;6:e23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X, Arocena M, Penninger J, et al. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol 2011;227:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasa SN, Rashidi A, Sefton E, et al. Charge-balanced electrical stimulation can modulate neural precursor cell migration in the presence of endogenous electric fields in mouse brains. eNeuro 2019;6:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J-F, Liu J, Zhang L, et al. Electrical guidance of human stem cells in the rat brain. Stem Cell Rep 2017;9:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sefton E, Iwasa SN, Morrison TJ, et al. Electric field application in vivo regulates nueral precursor cell behaviour in the adult mammalian forebrain. eNeuro 2020;7:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ariza CA, Fleury AT, Tormos CJ, et al. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev Rep 2010;6:585–600 [DOI] [PubMed] [Google Scholar]
- 21.Chang HF, Lee YS, Tang TK, et al. Pulsed DC electric field-induced differentiation of cortical neural precursor cells. PLoS One 2016;11:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima K, Zhu K, Sun YH, et al. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat Commun 2015;6:8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y-J, Schiapparelli P, Kozielski K, et al. Electrophoresis of cell membrane heparan sulfate regulates galvanotaxis in glial cells. J Cell Sci 2017;130:2459–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Do H, Gao J, et al. Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr Biol 2013;23:569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato MJ, Kuwayama H, van Egmond WN, et al. Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci U S A 2009;106:6667–6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006;442:457–460 [DOI] [PubMed] [Google Scholar]
- 27.Ahmed U, Iwasa SN, Poloni L, et al. Substrate-dependent galvanotaxis of directly reprogrammed human neural precursor cells. Bioelectricity 2020;2:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang E, Zhao M, Forrester JV, et al. Bi-directional migration of lens epithelial cells in a physiological electrical field. Exp Eye Res 2003;76:29–37 [DOI] [PubMed] [Google Scholar]
- 29.Babona-Pilipos R, Pritchard-Oh A, Popovic MR, et al. Biphasic monopolar electrical stimulation induces rapid and directed galvanotaxis in adult subependymal neural precursors. Stem Cell Res Ther 2015;6:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saltukoglu D, Grünewald J, Strohmeyer N, et al. Spontaneous and electric field-controlled front-rear polarization of human keratinocytes. Mol Biol Cell 2015;26:4373–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruddy RM, Morshead CM. Home sweet home: The neural stem cell niche throughout development and after injury. Cell Tissue Res 2018;371:125–141 [DOI] [PubMed] [Google Scholar]
- 32.Moeendarbary E, Weber IP, Sheridan GK, et al. The soft mechanical signature of glial scars in the central nervous system. Nat Commun 2017;8:14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 2006;24:739–747 [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, McNamara JO. Ischemic stroke: “Acidotoxicity” is a perpetrator. Cell 2004;118:665–666 [DOI] [PubMed] [Google Scholar]
- 35.O'Keeffe GC, Tyers P, Aarsland D, et al. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A 2009;106:8754–8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Addington CP, Roussas A, Dutta D, et al. Endogenous repair signaling after brain injury and complementary bioengineering approaches to enhance neural regeneration: Supplementary issue: Stem cell biology. Biomark insights 2015;10:43–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber JT.Altered calcium signaling following traumatic brain injury. Front Pharmacol 2012;3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babona-Pilipos R, Liu N, Pritchard-Oh A, et al. Calcium influx differentially regulates migration velocity and directedness in response to electric field application. Exp Cell Res 2018;368:202–214 [DOI] [PubMed] [Google Scholar]
- 39.Cao L, Wei D, Reid B, et al. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep 2013;14:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faiz M, Sachewsky N, Gascon S, et al. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell 2015;17:624–634 [DOI] [PubMed] [Google Scholar]
- 41.Kovac S, Speckmann EJ, Gorji A. Uncensored EEG: The role of DC potentials in neurobiology of the brain. Prog Neurobiol 2018;165–167:51–65 [DOI] [PubMed] [Google Scholar]
- 42.Zheng Z-T, Dong X-L, Li Y-D, et al. Electrical stimulation improved cognitive deficits associated with traumatic brain injury in rats. Brain Behav 2017;7:e00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YC, Zhu GY, Wang X, et al. Anterior thalamic nuclei deep brain stimulation reduces disruption of the blood–brain barrier, albumin extravasation, inflammation and apoptosis in kainic acid-induced epileptic rats. Neurol Res 2017;39:1103–1113 [DOI] [PubMed] [Google Scholar]
- 44.Watanabe Y, Nik-Mohd-Afizan NAR, Takashima I. Blood-brain barrier derangement after electrical brain stimulation. J Neurol Neuromed 2017;2:1–5 [Google Scholar]
- 45.Baba T, Kameda M, Yasuhara T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/akt signaling pathway. Stroke 2009;40:2–5 [DOI] [PubMed] [Google Scholar]
- 46.Morimoto T, Yasuhara T, Kameda M, et al. Striatal stimulation nurtures endogenous neurogenesis and angiogenesis in chronic-phase ischemic stroke rats. Cell Transplant 2011;20:1049–1064 [DOI] [PubMed] [Google Scholar]
- 47.Pikhovych A, Stolberg NP, Jessica Flitsch L, et al. Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells Int 2016;2016:2715196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gellner A-K, Reis J, Fritsch B. Glia: A neglected player in non-invasive direct current brain stimulation. Front Cell Neurosci 2016;10:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 2010;47:888–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macdonald JR, Barsoukov E. Impedance Spectroscopy Theory, Experiment, and Applications. Hoboken, NJ: John Wiley & Sons, Inc., 2005: 595
- 51.Clark JW Jr., Neuman MR, Olson WH, et al. Medical Instrumentation: Application and Design. Hoboken, NJ: John Wiley & Sons, Inc., 2010
- 52.Geddes LA.Historical evolution of circuit models for the electrode-electrolyte interface. Ann Biomed Eng 1997;25:1–14 [DOI] [PubMed] [Google Scholar]
- 53.Davidovics NS, Fridman GY, Chiang B, et al. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve. IEEE Transact Neural Syst Rehabil Eng 2010;19:84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol 2006;117:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ragheb T, Geddes LA. Electrical properties of metallic electrodes. Med Biol Eng Comput 1990;28:182–186 [DOI] [PubMed] [Google Scholar]
- 56.Satzer D, Lanctin D, Eberly LE, et al. Variation in deep brain stimulation electrode impedance over years following electrode implantation. Stereotact Funct Neurosurg 2014;92:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly A, Ballerini L, Lowery M, et al. 7.32 Engineering the Neural Interface. Elsevier Ltd., 2017: 642–660
- 58.Campbell A, Wu C. Chronically implanted intracranial electrodes: Tissue reaction and electrical changes. Micromachines 2018;9:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cogan SF.Neural stimulation and recording electrodes. Annu Rev Biomed Eng 2008;10:275–309 [DOI] [PubMed] [Google Scholar]
- 60.Wellman SM, Eles JR, Ludwig KA, et al. A materials roadmap to functional neural interface design. Adv Funct Mater 2018;28:1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holthoff EL, Marcus LS, Pellegrino PM. Towards the realization of a MEMS-based photoacoustic chemical sensor using ultracompact EC-QCL (SpriteIR). In: SENSORS, 2013 IEEE. Baltimore, MD: IEEE, 2013: 1–4
- 62.Thompson CH, Ti'Air ER, Patel PR, et al. Toward guiding principles for the design of biologically-integrated electrodes for the central nervous system. J Neural Eng 2020;17:021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart E, Kobayashi NR, Higgins MJ, et al. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: A biocompatible platform for translational neural tissue engineering. Tissue Eng Part C Methods 2015;21:385–393 [DOI] [PubMed] [Google Scholar]
- 64.Pires F, Ferreira Q, Rodrigues CA, et al. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim Biophys Acta 2015;1850:1158–1168 [DOI] [PubMed] [Google Scholar]
- 65.Tomaskovic-Crook E, Zhang P, Ahtiainen A, et al. Human neural tissues from neural stem cells using conductive biogel and printed polymer microelectrode arrays for 3D electrical stimulation. Adv Healthc Mater 2019;8:1900425. [DOI] [PubMed] [Google Scholar]
- 66.Morrison TJ, Sefton E, Marquez-Chin M, et al. A 3D printed device for low cost neural stimulation in mice. Front Neurosci 2019;13:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan H, Wang Y, Li L, et al. A micropatterned conductive electrospun nanofiber mesh combined with electrical stimulation for synergistically enhancing differentiation of rat neural stem cells. J Mater Chem B 2020;8:2673–2688 [DOI] [PubMed] [Google Scholar]
- 68.Huang YJ, Wu HC, Tai NH, et al. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small 2012;8:2869–2877 [DOI] [PubMed] [Google Scholar]
- 69.Li N, Zhang Q, Gao S, et al. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep 2013;3:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang K, Fishman HA, Dai H, et al. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett 2006;6:2043–2048 [DOI] [PubMed] [Google Scholar]
- 71.Lee J-R, Ryu S, Kim S, et al. Behaviors of stem cells on carbon nanotube. Biomater Res 2015;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi HH, Jang S, Naguib HE. Freestanding laser-assisted reduced graphene oxide microribbon textile electrode fabricated on a liquid surface for supercapacitors and breath sensors. ACS Appl Mater Interfaces 2019;11:27183–27191 [DOI] [PubMed] [Google Scholar]
- 73.Sahni D, Jea A, Mata JA, et al. Biocompatibility of pristine graphene for neuronal interface. J Neurosurg Pediatr 2013;11:575–583 [DOI] [PubMed] [Google Scholar]
- 74.Pinto AM, Goncalves IC, Magalhaes FD. Graphene-based materials biocompatibility: A review. Colloids Surf B Biointerfaces 2013;111:188–202 [DOI] [PubMed] [Google Scholar]
- 75.Fu C, Pan S, Ma Y, et al. Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif Cells Nanomed Biotechnol 2019;47:1867–1876 [DOI] [PubMed] [Google Scholar]
- 76.George PM, Lyckman AW, LaVan DA, et al. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005;26:3511–3519 [DOI] [PubMed] [Google Scholar]
- 77.Machida S, Miyata S, Techagumpuch A. Chemical synthesis of highly electrically conductive polypyrrole. Synth Met 1989;31:311–318 [Google Scholar]
- 78.Green R, Williams C, Lovell N, et al. Novel neural interface for implant electrodes: Improving electroactivity of polypyrrole through MWNT incorporation. J Mater Sci Mater Med 2008;19:1625–1629 [DOI] [PubMed] [Google Scholar]
- 79.Green R, Matteucci P, Hassarati R, et al. Performance of conducting polymer electrodes for stimulating neuroprosthetics. J Neural Eng 2013;10:016009. [DOI] [PubMed] [Google Scholar]
- 80.Lu Y, Li T, Zhao X, et al. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials 2010;31:5169–5181 [DOI] [PubMed] [Google Scholar]
- 81.Lu Y, Lyu H, Richardson AG, et al. Flexible neural electrode array based-on porous graphene for cortical microstimulation and sensing. Sci Rep 2016;6:33526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang L, Fan Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014;6:1922–1945 [DOI] [PubMed] [Google Scholar]
- 83.Lago N, Yoshida K, Koch KP, et al. Assessment of biocompatibility of chronically implanted polyimide and platinum intrafascicular electrodes. 2007;54:281–290 [DOI] [PubMed] [Google Scholar]
- 84.Chen Y-M, Chung T-W, Wu P-W, et al. A cost-effective fabrication of iridium oxide films as biocompatible electrostimulation electrodes for neural interface applications. J Alloys Compd 2017;692:339–345 [Google Scholar]
- 85.Park H, Takmakov P, LeeHJ, Sr.. Electrochemical evaluations of fractal microelectrodes for energy efficient neurostimulation. Sci Rep 2018;8:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ereifej ES, Khan S, Newaz G, et al. Comparative assessment of iridium oxide and platinum alloy wires using an in vitro glial scar assay. Biomed Microdevices 2013;15:917–924 [DOI] [PubMed] [Google Scholar]
- 87.Kawar R, Chigare P, Patil P. Substrate temperature dependent structural, optical and electrical properties of spray deposited iridium oxide thin films. Appl Surf Sci 2003;206:90–101 [Google Scholar]
- 88.Cogan SF, Plante T, Ehrlich J. Sputtered iridium oxide films (SIROFs) for low-impedance neural stimulation and recording electrodes. In: The 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. San Francisco, CA: IEEE, 2004: 4153–4156 [DOI] [PMC free article] [PubMed]
- 89.Weiland JD, Anderson DJ. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Transact Biomed Eng 2000;47:911–918 [DOI] [PubMed] [Google Scholar]
- 90.Gwo S, Yeh C-L, Chen P-F, et al. Local electric-field-induced oxidation of titanium nitride films. Appl Phys Lett 1999;74:1090–1092 [Google Scholar]
- 91.Guenther E, Tröger B, Schlosshauer B, et al. Long-term survival of retinal cell cultures on retinal implant materials. Vision Res 1999;39:3988–3994 [DOI] [PubMed] [Google Scholar]
- 92.Hikov T, Krasteva N, Hristova-Panusheva K, et al. Study on the biocompatibility of TiN/TiO2 bilayer coatings deposited by DC magnetron sputtering on stainless steel. In: AIP Conference Proceedings. Sofia, Bulgaria, AIP Publishing, LLC, 2019: 160022
- 93.Weiland JD, Anderson DJ, Humayun MS. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. IEEE Transact Biomed Eng 2002;49:1574–1579 [DOI] [PubMed] [Google Scholar]
- 94.Latif T, McKnight M, Dickey MD, et al. In vitro electrochemical assessment of electrodes for neurostimulation in roach biobots. PLoS One 2018;13:e0203880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamagiwa S, Fujishiro A, Sawahata H, et al. Layer-by-layer assembled nanorough iridium-oxide/platinum-black for low-voltage microscale electrode neurostimulation. Sensors Actuat B Chem 2015;206:205–211 [Google Scholar]
- 96.Fomani AA, Mansour RRJS, Physical AA. Fabrication and characterization of the flexible neural microprobes with improved structural design. Sensors Actuat A Phys 2011;168:233–241 [Google Scholar]
- 97.Braem A, Van Mellaert L, Mattheys T, et al. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J Biomed Mater Res A 2014;102:215–224 [DOI] [PubMed] [Google Scholar]
- 98.Braem A, Van Mellaert L, Hofmans D, et al. Bacterial colonisation of porous titanium coatings for orthopaedic implant applications—Effect of surface roughness and porosity. Powder Metall 2013;56:267–271 [Google Scholar]
- 99.Watterson W, Montgomery R, Taylor R. Fractal electrodes as a generic interface for stimulating neurons. Sci Rep 2017;7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Golestanirad L, Elahi B, Molina Arribere A, et al. Analysis of fractal electrodes for efficient neural stimulation. Front Neuroeng 2013;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alonso F, Latorre MA, Göransson N, et al. Investigation into deep brain stimulation lead designs: A patient-specific simulation study. Brain Sci 2016;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei XF, Grill WM. Analysis of high-perimeter planar electrodes for efficient neural stimulation. Front Neuroeng 2009;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson DN, Osting B, Vorwerk J, et al. Optimized programming algorithm for cylindrical and directional deep brain stimulation electrodes. J Neural Eng 2018;15:026005. [DOI] [PubMed] [Google Scholar]
- 104.Marei HE, Hasan A, Rizzi R, et al. Potential of stem cell-based therapy for ischemic stroke. Front Neurol 2018;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nomura H, Zahir T, Kim H, et al. Extramedullary chitosan channels promote survival of transplanted neural stem and progenitor cells and create a tissue bridge after complete spinal cord transection. Tissue Eng Part A 2008;14:649–665 [DOI] [PubMed] [Google Scholar]
- 106.Jin MC, Medress ZA, Azad TD, et al. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg Focus 2019;46:E10. [DOI] [PubMed] [Google Scholar]
- 107.McKinnon C, Gros P, Lee DJ, et al. Deep brain stimulation: Potential for neuroprotection. Ann Clin Transl Neurol 2019;6:174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krack P, Martinez-Fernandez R, Del Alamo M, et al. Current applications and limitations of surgical treatments for movement disorders. Mov Disord 2017;32:36–52 [DOI] [PubMed] [Google Scholar]
- 109.Temel Y, Visser-Vandewalle V, Kaplan S, et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res 2006;1120:100–105 [DOI] [PubMed] [Google Scholar]
- 110.Harnack D, Meissner W, Jira JA, et al. Placebo-controlled chronic high-frequency stimulation of the subthalamic nucleus preserves dopaminergic nigral neurons in a rat model of progressive Parkinsonism. Exp Neurol 2008;210:257–260 [DOI] [PubMed] [Google Scholar]
- 111.Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 2007;130:2129–2145 [DOI] [PubMed] [Google Scholar]
- 112.Shaw VE, Keay KA, Ashkan K, et al. Dopaminergic cells in the periaqueductal grey matter of MPTP-treated monkeys and mice; patterns of survival and effect of deep brain stimulation and lesion of the subthalamic nucleus. Parkinsonism Relat Disord 2010;16:338–344 [DOI] [PubMed] [Google Scholar]
- 113.Musacchio T, Rebenstorff M, Fluri F, et al. Subthalamic nucleus deep brain stimulation is neuroprotective in the A53T α-synuclein Parkinson's disease rat model. Ann Neurol 2017;81:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sankar T, Chakravarty MM, Bescos A, et al. Deep brain stimulation influences brain structure in Alzheimer's disease. Brain Stimul 2015;8:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodriguez M, Obeso J, Olanow CW. Subthalamic nucleus-mediated excitotoxicity in Parkinson's disease: A target for neuroprotection. Ann Neurol 1998;44:S175–S188 [DOI] [PubMed] [Google Scholar]
- 116.Maesawa S, Kaneoke Y, Kajita Y, et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: Neuroprotection of dopaminergic neurons. J Neurosurg 2004;100:679–687 [DOI] [PubMed] [Google Scholar]
- 117.Spieles-Engemann A, Behbehani M, Collier T, et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis 2010;39:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spieles-Engemann AL, Steece-Collier K, Behbehani MM, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis 2011;1:123–136 [PMC free article] [PubMed] [Google Scholar]
- 119.Hotta H, Kagitani F, Kondo M, et al. Basal forebrain stimulation induces NGF secretion in ipsilateral parietal cortex via nicotinic receptor activation in adult, but not aged rats. Neurosci Res 2009;63:122–128 [DOI] [PubMed] [Google Scholar]
- 120.Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017;18:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohanty SK, Lakshminarayananan V. Optical techniques in optogenetics. J Mod Opt 2015;62:949–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fomenko A, Lee DJ, McKinnon C, et al. Deep brain stimulation of the medial septal nucleus induces expression of a virally delivered reporter gene in dentate gyrus. Front Neurosci 2020;14:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pol S, Temel Y, Jahanshahi A.. A custom made electrode construct and reliable implantation method that allows for long-term bilateral deep brain stimulation in mice. Neuromodulation 2020. [Epub ahead of print]; DOI: 10.1111/ner.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bingham CS, Loizos K, Gene JY, et al. Model-based analysis of electrode placement and pulse amplitude for hippocampal stimulation. IEEE Transact Biomed Eng 2018;65:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nguyen HT, Sapp S, Wei C, et al. Electric field stimulation through a biodegradable polypyrrole-co-polycaprolactone substrate enhances neural cell growth. J Biomed Mater Res Part A 2014;102:2554–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klooster DC, de Louw AJ, Aldenkamp A, et al. Technical aspects of neurostimulation: Focus on equipment, electric field modeling, and stimulation protocols. Neurosci Biobehav Rev 2016;65:113–141 [DOI] [PubMed] [Google Scholar]
- 127.Pelot NA, Thio BJ, Grill WM. Modeling current sources for neural stimulation in COMSOL. Front Comput Neurosci 2018;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]