Abstract
This scientific commentary refers to ‘Cardiovascular brain impulses in Alzheimer’s disease’ by Rajna et al. (doi:10.1093/brain/awab144).
This scientific commentary refers to ‘Cardiovascular brain impulses in Alzheimer’s disease’ by Rajna et al. (doi:10.1093/brain/awab144).
While Alzheimer’s disease has long been defined by the accumulation of protein aggregates such as amyloid-β and tau, increasing research in recent years has also highlighted the role of neurovascular dysfunction in disease onset. Notably, cerebral blood flow declines years before the onset of cognitive decline, and the arteries stiffen and deteriorate with age.1,2 Work in rodents has shown that arterial pulsations and dilations are coupled to CSF flow and to the clearance of metabolic waste products, including amyloid-β.3,4 This suggests that declining vascular pulsatility may contribute directly to protein aggregation.
Techniques capable of assessing vascular pulsatility in the human brain may therefore be of great importance for understanding how neurovascular function is linked to Alzheimer’s disease. In this issue of Brain, Rajna et al.5 propose ultrafast 10 Hz magnetic resonance encephalography (MREG) as a non-invasive method for quantification of cardiovascular impulses in the human brain, and demonstrate striking differences in these impulses in patients with Alzheimer’s disease compared to age-matched controls.5
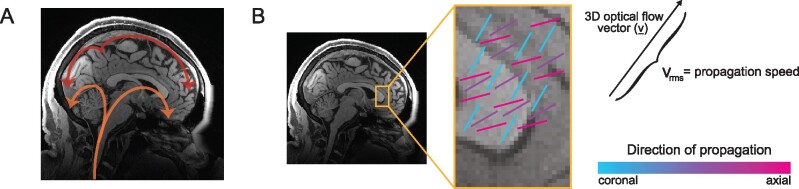
The arteries and tissue of the brain pulse with each heartbeat, and these cardiac-locked pulsations provided a target for the investigation in Rajna et al.5 As the wavefront of an arterial pulsation arrives in the brain (Fig. 1A), it induces a drop in the MREG signal. When images are acquired at a sufficiently fast rate (<300 ms per image) this technique enables imaging of brain pulsations at multiple time points within each cardiac cycle. Using this ultrafast MREG signal and previously established methods, Rajna et al.5 quantified the timing and propagation of cardiac-locked pulsations across the brain (Fig. 1B). They then generated maps of the propagation speed and direction of propagation, and compared them between patients with Alzheimer’s disease (n = 26; mean age 57; 62% female) and controls (n = 31; mean age 60; 58% female).
Figure 1.
Schematic overview of propagation of cardiovascular brain pulsations. (A) Illustration of the typical path of cardiovascular pulse propagation through the brain.10 (B) Example illustration of voxel-wise measurements of optical flow vector (v), and calculations of speed and direction.
Looking first at the speed and arrival time of cardiovascular brain impulses, Rajna et al.5 showed higher propagation speeds, and therefore shorter arrival latencies, in more peripheral vascular territories in patients compared with controls. Higher pulse wave velocity is indicative of increased arterial stiffness, and, intriguingly, prior studies have shown that it may also be associated with the conversion from mild cognitive impairment (MCI) to dementia.6
In addition, the authors detected substantial overlap between the spatial maps of cardiovascular impulse speed, and typical PET findings of increased amyloid-β accumulation in early Alzheimer’s disease. Previous work has suggested that accumulation of amyloid-β and tau filaments induces a narrowing of microvessels, leading to a reduction in impulse propagation.7,8 The exact relationship between increased cardiovascular impulse speed and amyloid-β accumulation—whether causal or correlative—and the physiological mechanisms underlying this relationship are not fully understood. Taken together, however, the results demonstrate convincingly that altered cardiac-locked brain pulsations are a feature of Alzheimer’s disease.
Another notable finding in the current study was that while the direction of impulse propagation was often similar between the control and Alzheimer’s disease groups, a subset of areas showed reversed propagation direction in patients. It is important to note that these reversed impulses should be interpreted as distinct spatiotemporal patterns of cardiac-locked dynamics in the Alzheimer’s disease brain, not as areas of reversed blood flow. Prior work has illustrated that the likelihood of reversed impulses increases in response to both stiffened arteries and high blood pressure and is associated with cognitive decline, brain atrophy, and amyloid-β accumulation.6,8
The zones of propagation reversal observed in the current study were mainly in periventricular areas and mesiotemporal structures, such as the hippocampus. Moreover, there were notable partial overlaps between these zones and group-level grey matter atrophy maps in the temporal and parietal lobes.9 Rajna et al.5 suggest that these partial overlaps may indicate a role of atrophy in altering impulse propagation dynamics in these regions in patients. However, whether this pattern is also apparent within individual-level grey matter atrophy is not yet known.
A key question raised by these findings is what impact the observed perturbations have on waste clearance and the glymphatic system. Rodent studies have shown that arterial pulsations play a role in driving flow of CSF through perivascular spaces in the brain, and that hypertension induces changes in vessel dynamics that decrease, and can even reverse, net CSF flow through perivascular spaces.3,4 Rajna et al.5 propose that the reversed pulse propagation and increased speeds seen in the Alzheimer’s disease group could impose local hydrostatic stress on blood vessel walls and disrupt CSF flow. An important next step will therefore be to understand how these altered pulsatile dynamics affect waste clearance and amyloid transport, and whether and how this may contribute to the development of Alzheimer’s disease.
While this study makes significant progress towards the ultimate goal of developing a non-invasive metric for early detection of physiological markers of Alzheimer’s disease, it also raises several questions to be addressed in future work.
As the authors note, local non-linear motion artefacts originating from cardiorespiratory pulsations represent a confounding factor in the results, as these signals cannot be corrected for with standard motion correction techniques. This issue is compounded by the low spatial resolution (4.5 mm at full-width at half-maximum), which introduces partial volume effects. Additionally, in the preprocessing pipeline, each subject’s data were resampled to an average standard space and the images were spatially smoothed, further reducing the spatial resolution of the analyses. These steps enabled assessment of group differences, but reduced the ability to extract precise, voxel-wise timings of cardiovascular impulse propagation that may appear within individuals and be of clinical relevance.
Future work could pursue an improvement in spatial resolution, combining improved motion correction techniques and smaller voxel sizes, along with an investigation of the features identified by Rajna et al.5 on an individual subject level. This would provide researchers with more precise insights into the physiological changes that disrupt cardiovascular brain pulsations in Alzheimer’s disease patient populations.
Looking at individual data could also help us understand how early these abnormalities in cardiovascular impulse propagation speed and direction arise, and whether their onset can be detected in individual patients. Another exciting future direction would be to compare PET and MREG imaging within individuals, to examine whether individual amyloid-β levels are linked to cardiovascular impulse propagation and local vascular changes. Such studies could help clarify whether the effects seen here are specific to Alzheimer’s disease pathology and if they could be used as early predictors of the disease. Addressing these questions will be important next steps in order to achieve the authors’ ambitious goal of using MREG as a screening method to facilitate early detection of Alzheimer’s disease.
In conclusion, the results presented by Rajna et al.5 show striking perturbations of cardiovascular brain impulse propagation in patients with Alzheimer’s disease. The work highlights the benefits of accelerated imaging techniques for assessing brain physiological dynamics, and suggests many promising future directions for understanding the role of neurovascular health in Alzheimer’s disease progression.
Funding
S.M.B. was supported by National Institutes of Health grant T32-GM008764. L.D.L. was supported by the Simons Collaboration on Plasticity in the Aging Brain and National Institutes of Health grant R01-AG070135.
Competing interests
L.D.L. is an inventor on a pending patent application for CSF flow imaging techniques.
References
- 1.Selkoe DJ.The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–498. [DOI] [PubMed] [Google Scholar]
- 2.Kisler K, Nelson AR, Montagne A, Zlokovic BV.. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18(7):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajna Z, Mattila H, Huotari N, et al. Cardiovascular brain impulses in Alzheimer’s disease. Brain. 2021;144(7):2214–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouch L, Cestac P, Sallerin B, et al. Pulse wave velocity is associated with greater risk of dementia in mild cognitive impairment patients. Hypertension. 2018;72(5):1109–1116. [DOI] [PubMed] [Google Scholar]
- 7.Hansra GK, Popov G, Banaczek PO, et al. The neuritic plaque in Alzheimer’s disease: Perivascular degeneration of neuronal processes. Neurobiol Aging. 2019;82:88–101. [DOI] [PubMed] [Google Scholar]
- 8.Hatada Y, Hashimoto M, Shiraishi S, et al. Cerebral microbleeds are associated with cerebral hypoperfusion in patients with Alzheimer’s disease. J Alzheimers Dis. 2019;71(1):273–280. [DOI] [PubMed] [Google Scholar]
- 9.Tuovinen T, Kananen J, Rajna Z, et al. The variability of functional MRI brain signal increases in Alzheimer’s disease at cardiorespiratory frequencies. Sci Rep. 2020;10(1):21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajna Z, Raitamaa L, Tuovinen T, Heikkila J, Kiviniemi V, Seppanen T.. 3D multi-resolution optical flow analysis of cardiovascular pulse propagation in human brain. IEEE Trans Med Imaging. 2019;38(9):2028–2036. [DOI] [PubMed] [Google Scholar]