Abstract
Remyelination failure contributes to axonal loss and progression of disability in multiple sclerosis. The failed repair process could be due to ongoing toxic neuroinflammation and to an inhibitory lesion microenvironment that prevents recruitment and/or differentiation of oligodendrocyte progenitor cells into myelin-forming oligodendrocytes. The extracellular matrix molecules deposited into lesions provide both an altered microenvironment that inhibits oligodendrocyte progenitor cells, and a fuel that exacerbates inflammatory responses within lesions. In this review, we discuss the extracellular matrix and where its molecules are normally distributed in an uninjured adult brain, specifically at the basement membranes of cerebral vessels, in perineuronal nets that surround the soma of certain populations of neurons, and in interstitial matrix between neural cells. We then highlight the deposition of different extracellular matrix members in multiple sclerosis lesions, including chondroitin sulphate proteoglycans, collagens, laminins, fibronectin, fibrinogen, thrombospondin and others. We consider reasons behind changes in extracellular matrix components in multiple sclerosis lesions, mainly due to deposition by cells such as reactive astrocytes and microglia/macrophages. We next discuss the consequences of an altered extracellular matrix in multiple sclerosis lesions. Besides impairing oligodendrocyte recruitment, many of the extracellular matrix components elevated in multiple sclerosis lesions are pro-inflammatory and they enhance inflammatory processes through several mechanisms. However, molecules such as thrombospondin-1 may counter inflammatory processes, and laminins appear to favour repair. Overall, we emphasize the crosstalk between the extracellular matrix, immune responses and remyelination in modulating lesions for recovery or worsening. Finally, we review potential therapeutic approaches to target extracellular matrix components to reduce detrimental neuroinflammation and to promote recruitment and maturation of oligodendrocyte lineage cells to enhance remyelination.
Keywords: multiple sclerosis, remyelination, extracellular matrix, CSPGs
Ghorbani and Wee Yong examine the role of the extracellular matrix in multiple sclerosis pathology. They describe the changes to ECM components seen in different lesion types, and consider how crosstalk between the ECM, immune responses and remyelination influences the recovery or worsening of lesions.
Introduction
Multiple sclerosis is an inflammatory condition of the CNS with demyelination and axonal loss, and oligodendrocyte and neuronal death. Following these destructive processes, recovery manifests as the regeneration of oligodendrocytes and the reformation of myelin. The success of regenerative processes is dependent on the balance of factors that promote the pathogenicity of the inflammatory responses,1 and availability of conditions that mitigate destructive neuroinflammation whilst promoting remyelination.2 Less well described is the altered extracellular matrix (ECM) in multiple sclerosis lesions that profoundly controls the injury and repair processes. In this review, we describe the ECM, highlight the changes to ECM members in multiple sclerosis lesions, consider reasons behind changes in ECM components in multiple sclerosis, and discuss the consequences of an altered ECM in multiple sclerosis lesions. In particular, we highlight the contributions of ECM components in promoting injurious neuroinflammation, and in enhancing or inhibiting attempts at repair of lesions. Finally, we consider approaches to normalize the altered ECM in multiple sclerosis lesions to enable recovery from insults.
The extracellular matrix in the uninjured adult CNS
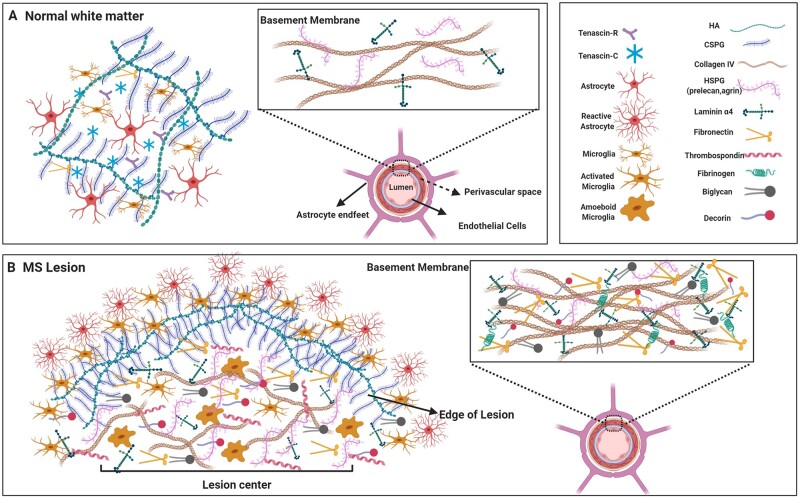
The ECM is a complex network of molecules distributed throughout the extracellular space of tissues. In the normal adult CNS, the ECM can be found in three principal compartments: the interstitial matrix, basement membrane around blood vessels, and the perineuronal nets surrounding neuronal soma.3,4 The interstitial matrix refers to the ECM components dispersed between cellular structures of the CNS and contributes to the structural support and signal transmission. The interstitial matrix of the uninjured white matter of the adult brain (Fig. 1) consists mainly of hyaluronan, tenascins and chondroitin sulphate proteoglycans (CSPGs), while it is low in fibronectin and fibrillar collagens common to other tissues.5 The basement membranes including endothelial and parenchymal basement membrane line the parenchymal side of cerebral microvessels and are composed of collagens (primarily collagen IV), laminins and heparan sulphate proteoglycans (HSPG) (e.g. perlecan and agrin) (Fig. 1). The basement membranes play a role in the maintenance of the blood–brain barrier integrity and angiogenesis.6,7 The perineuronal net is the dense extracellular matrix meshwork around certain populations of neuronal cell bodies, and it includes tenascins, hyaluronan bound with CSPGs, and link proteins. Perineuronal nets maintain the synaptic integrity, limit the synaptic plasticity and protect neurons against oxidative damage.8–10
Figure 1.
The ECM in the healthy CNS and in multiple sclerosis lesion. (A) In the uninjured white matter of the CNS, the neural interstitial matrix in the parenchyma primarily consists of CSPGs, hyaluronan (HA) and tenascins whereas collagens (principally type IV), laminins and some members of HSPGs are concentrated in the basement membranes that separate the perivascular space post-endothelial barrier. (B) Several members of the ECM are altered in multiple sclerosis lesions. High levels of CSPGs and hyaluronan accumulate and are prominent at the hypercellular edge as they have been deposited and then cleared from the centre of chronic active multiple sclerosis lesions. As well, fibrillar collagens (I, III, V), small leucine-rich repeat proteoglycans (biglycan, decorin), thrombospondin and the HSPG member (e.g. perlecan) are upregulated in the parenchyma of lesions. Moreover, the basement membranes in multiple sclerosis show a meshwork of ECM components including increased laminins, HSPGs, fibronectin, biglycan, decorin and collagens. Images were created using BioRender.
Hyaluronan, the most abundant ECM component in the interstitial matrix of the healthy CNS, is composed of long unbranched chains of repeating disaccharides called glycosaminoglycan.11 Hyaluronan is usually a large high molecular weight molecule that forms the framework for the structure of the CNS. Other ECM components such as sulphated proteoglycans can bind and cross-link high molecular weight hyaluronan. Sulphated proteoglycans are generally associated with a core protein that is covalently linked to one or more sulphated glycosaminoglycan chains. CSPGs are the highly expressed proteoglycans within the CNS. Based on the core protein and sulfation pattern of chains, CSPGs are divided into subfamilies that include the lecticans (i.e. aggrecan, brevican, neurocan and 4 isoforms of versicans), phosphacan and neuron-glial antigen-2 (NG2). Aggrecan is particularly enriched in perineuronal nets and forms large aggregates with hyaluronan.12 Small leucine-rich repeat proteoglycans, including decorin and biglycan, and other proteoglycans such as HSPGs participate mainly in protein-protein interactions and have diverse functions.13 Tenascins are the other subset of the CNS ECM proteins that form homodimers or trimers and can bind to the CSPG-hyaluronan aggregate, resulting in a highly complex substrate for cells and extracellular molecules.14 Several glycoproteins such as fibronectin, thrombospondin and vitronectin are also found within the brain ECM, albeit in low amounts. Osteopontin is a glycoprotein that defies easy classification as a bona fide ECM molecule: it exerts several distinct functions as a soluble molecule (e.g. cytokine and chemokine functions), and it can also be anchored to the ECM where it has particular activities.15 We will discuss its roles on the remyelination process where indicated, but we have not categorized it as a typical ECM molecule such as collagens or CSPGs.
Extracellular matrix changes in multiple sclerosis lesions
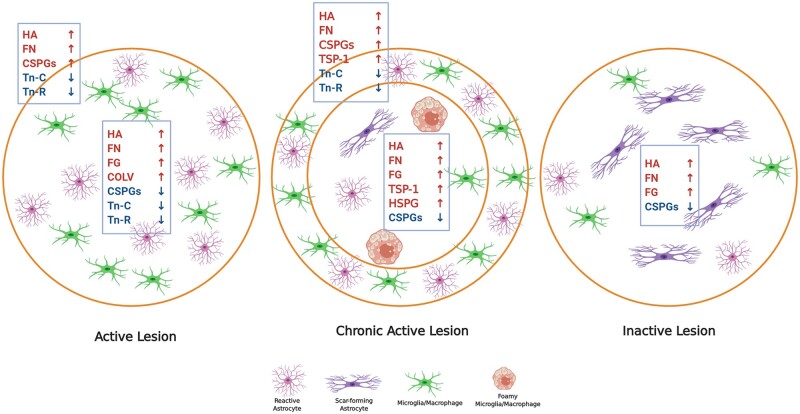
Multiple sclerosis lesions have been classified as active, chronic active and inactive lesions. In active lesions, immune cells are quite uniformly distributed throughout the plaque, correspondent with a recent injury. In chronic active lesions, the immune cells are at the edge of lesions, reflecting their migratory route outwards from an initial centre as the lesion expands. In inactive lesions, the immune cell activity has largely subsided. There is substantial evidence that several components of the ECM are altered in multiple sclerosis lesions (Figs 1 and 2). There is deposition of presumed blood-derived fibrinogen and fibronectin on and around endothelial cells in chronic active lesions, leading the authors to suggest their potential role in loss of integrity of the vessel walls followed by the infiltration of immune cells and plaque development.17 At sites of blood–brain barrier disruption, fibrinogen enters the CNS and is deposited as fibrin, which is a potent inflammatory factor including the activation of microglia.18 Fibrin deposition is a marker of blood–brain barrier disruption and occurs early in multiple sclerosis.19,20 Fibrinogen is abundant in the cortex of progressive multiple sclerosis and in both active and chronic multiple sclerosis lesions.21,22 Vitronectin is also elevated in the blood vessel walls of active multiple sclerosis lesions.23 While these observations provide insights into blood–brain barrier dysfunction in multiple sclerosis,24 revealed that ECM deposition could also occur in the parenchyma of multiple sclerosis lesions. They demonstrated the accumulation of laminin, HSPGs, fibronectin and collagen type IV in both the basement membranes of perivascular cuffs and in the parenchyma of active and chronic active lesions. These parenchymal deposits were associated with activated microglia and CD45+ leucocytes, indicating the probable involvement of the immune cells in the synthesis of these proteins in active multiple sclerosis lesions.24 Analyses of versican, aggrecan, neurocan and dermatan sulphate proteoglycans revealed an enhanced expression in the hypercellular edge but not the centre of chronic active multiple sclerosis lesions; there was decreased immunoreactivity in chronic plaques.16 Reduced levels of these proteoglycans at the centre of the chronic active lesion may be due to their removal by phagocytic cells following deposition, and the migration of these foamy macrophages outwards from the lesion centre as the lesion transitions to a chronic active plaque (Fig. 2).
Figure 2.
Expression of ECM proteins in different white matter multiple sclerosis lesions. Schematic shows ECM changes in distinct multiple sclerosis lesion types including active, chronic active and inactive lesions. Early demyelinating active lesions consist of hypercellular lesion centre containing reactive astrocytes and immune cells. Chronic active lesions are defined by a hypercellular inflammatory margin and a hypocellular centre with fibrous astrocytes and foamy (myelin-laden) microglia/macrophages. Inactive lesions show minimal signs of inflammation while containing mainly scar forming (fibrous) astrocytes. Each lesion type has a different ECM composition when compared among each other and to normal white matter. Refer to the main text for the capacity of individual ECM components to modulate the activity of immune cells. In active lesions, prominent sources of hyaluronan (HA), fibronectin (FN), CSPGs and Tenascin-C/R (Tn-C/R) appear to be reactive astrocytes while fibrinogen (FG) is deposited by leakage of serum into lesions. Sources of CSPGs are reactive astrocytes and macrophages/microglia although these cells are also removing the deposited CSPGs from the lesion centre as the immune cells move outwards.16 The accumulation of inhibitory ECM such as CSPGs in the lesion edge is thought to be a barrier to incoming progenitor cells such as OPCs that attempt to repair the lesion.3 COLV = collagen V. Images were created using BioRender.
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory animal model of multiple sclerosis. In C57BL/6 mice induced with myelin oligodendrocyte glycoprotein peptide (35-55), clinical signs commonly occur around Day 10 after immunization, with increasing clinical severity that reaches a peak about 5 days after, followed by a chronic phase with reduced clinical disability that does not completely resolve. At clinical onset and particularly at peak severity, active lesions of inflammation and demyelination adjacent to perivascular cuffs (aggregates of immune cells in postcapillary venules) in the spinal cord and cerebellar white matter, or adjacent to meninges in the spinal cord can be readily seen.25 Our immunohistochemical study of the spinal cords of mice afflicted with EAE showed increased V1 isoform of versican in active lesions and perivascular cuffs, closely associated with the infiltrating immune cells; there was no change of V2 versican isoform or aggrecan in the lesions.25 We corroborated the EAE findings by demonstrating that versican V1 was also present in the perivascular cuffs of active multiple sclerosis lesions. Given that the expression of versican V1 transcript has been documented in infiltrating CD45+ leucocytes and F4/80+ macrophages by in situ hybridization, we postulate that immune cells may be the main source of elevated levels of V1 isoform in lesions.25
Hyaluronan also accumulates in multiple sclerosis and EAE lesions,26 and we discuss its postulated roles in inhibiting remyelination below. Loss of tenascin-C and -R has been observed in active multiple sclerosis lesions, whereas their expression remained unaltered in chronic (inactive) lesions.27
Osteopontin is upregulated in the brain and serum of both multiple sclerosis patients and EAE mice during relapses of disease.28–30 Active multiple sclerosis lesion showed elevated osteopontin mRNA and protein levels compared to control white matter,31,32 although another gene expression study discovered a downregulation in osteopontin transcript levels.33
A study of RNA transcripts encoding 50 ECM components in multiple sclerosis plaques showed upregulation of more than 20 ECM members in both active and chronic lesions including fibrillar collagens (I, III and V), basement membrane collagen (IV), laminins, small leucine-rich repeat proteoglycans (biglycan, decorin), hyaluronan link proteins, thrombospondin and the HSPG member, perlecan.34 Fibrillar collagens, biglycan and decorin were localized as a meshwork in the perivascular space of blood vessels. In addition to the perivascular space, the parenchyma of active lesions had immunoreactivity for collagen V. That the altered ECM has consequences was demonstrated by fibrillar collagens preventing the production of the chemokine CCL2 by monocytes in culture, suggesting their potential role in limiting the enlargement of multiple sclerosis lesions through the inhibition of immune cell recruitment.34 Transcriptomic studies of astrocytes during EAE found higher levels of versican, fibronectin, fibulin-2, biglycan, decorin, collagen V, laminin α4 and α5 but reduced laminin α2, thrombospondin-1 and tenascin-C.35,36 The most expanded subpopulation of astrocytes in multiple sclerosis and EAE lesions had upregulated fibronectin, biglycan and laminin α4. It is of interest that during acute and chronic EAE, different astrocyte subpopulations with distinct ECM members were over-represented.36
From the above descriptions, it would appear that multiple sclerosis lesions have an altered expression pattern of ECM during their evolution from active to chronic active and inactive lesions (Fig. 2). However, this interpretation is largely based on separate studies reporting on specific ECM components in different lesion types. Future studies are needed to assess the deposition, consequence, cellular sources and clearance of ECM components systematically and comprehensively in multiple sclerosis lesions as they evolve from active to inactive plaques.
Since transcriptome profiling cannot accurately represent the protein content of the tissue, particularly their post-translational modifications, mass spectrometry would be advantageous to gain a better overview of the dynamic alterations in the ECM. A multiple sclerosis lesion-specific proteome profiling revealed a unique set of proteins in acute and chronic plaques, and implicated an important role of the coagulation cascade in the development of active lesions of multiple sclerosis.37 Further pathway analysis showed the abundance of ECM components, including collagen, fibronectin, fibulin, laminin, vitronectin, osteopontin, thrombospondin and heparan sulphate in the chronic active plaque proteome of multiple sclerosis.32
Finally, a recent comprehensive proteome map of the brain and spinal cord of EAE mice showed that cell adhesion characteristics are amongst the most enriched gene ontology terms for upregulated molecules; numerous ECM members are amongst the highly expressed proteins.38
Sources of altered extracellular matrix change expression in lesions
ECM remodelling is a response to injury and contributes to the repair process. Following an insult, reactive astrocytes change their pattern of secretion of ECM molecules to create a barrier called a glial scar, which likely represents an attempt to limit the extent of damage and leucocyte migration.39–41 Transforming growth factor beta (TGFβ) and epidermal growth factor signalling induce the elevation of ECM molecules in astrocytes.42,43
Another cause of increased ECM protein levels is damage to the blood–brain barrier and deposition of plasma derived ECM proteins such as fibrinogen and fibronectin. These members can further stimulate ECM production by promoting TGFβ signalling in astrocytes.44 Multiple cell types are known to be responsible for ECM upregulation within CNS, including astrocyte, neurons, oligodendrocytes and macrophages/microglia.45–48 As discussed earlier, immune cells are likely main sources of elevated versican V1 in lesions. Microglia may mediate the aberrant deposition of CSPGs directly or indirectly through regulation of other cells, consistent with the result from a recent study that depleted microglia in Huntington’s disease models.49
Interactions between extracellular matrix and immune cells within the CNS
The ECM and immune responses are intricately linked. In inflamed CNS, cytokines such as TGF-β, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) can increase ECM synthesis, turnover and protease secretion.4 In addition to reactive astrocytes, resident microglia and infiltrating macrophages contribute to the dysregulated remodelling of the ECM in multiple sclerosis lesions. Microglia and macrophages release cytokines and proteases including matrix metalloproteinases that degrade and remodel the ECM.50 The elevation of matrix metalloproteinases may be an important underlying reason for dysregulation of ECM molecules in multiple sclerosis.51 On the other hand, ECM components employ several mechanisms to modify immune responses. Increasing evidence indicates that the infiltration, activation, differentiation and survival of immune cells in the CNS are affected by aberrant expression of ECM components or production of ECM fragments. They are able to act as damage-associated molecular patterns (DAMPs) and interact directly with their specific pattern recognition receptors such as toll-like receptors (TLRs) (Fig. 3). Furthermore, they may play a role as chemoattractants and alter the properties of immune cells and their activities. In inflammatory conditions, activated macrophages synthesize and secrete several proteoglycans such as versican, biglycan and decorin. These ECM components can act as endogenous TLR ligands and shape the immune response in an autocrine and paracrine manner.52,53 Opposing pro- or anti-inflammatory outcomes from ECM-derived DAMPs interacting with pattern recognition receptors have resultant phenotypes that depend on unknown factors and contextual features.54,55 Whether altered ECM in inflamed tissue modulates inflammation further or contributes to the development of chronicity remains to be investigated. Here, we aim to review the known immunological aspects of ECM members. Where little is known about their immunological actions within the CNS, we refer to non-CNS tissues as guides where applicable.
Figure 3.
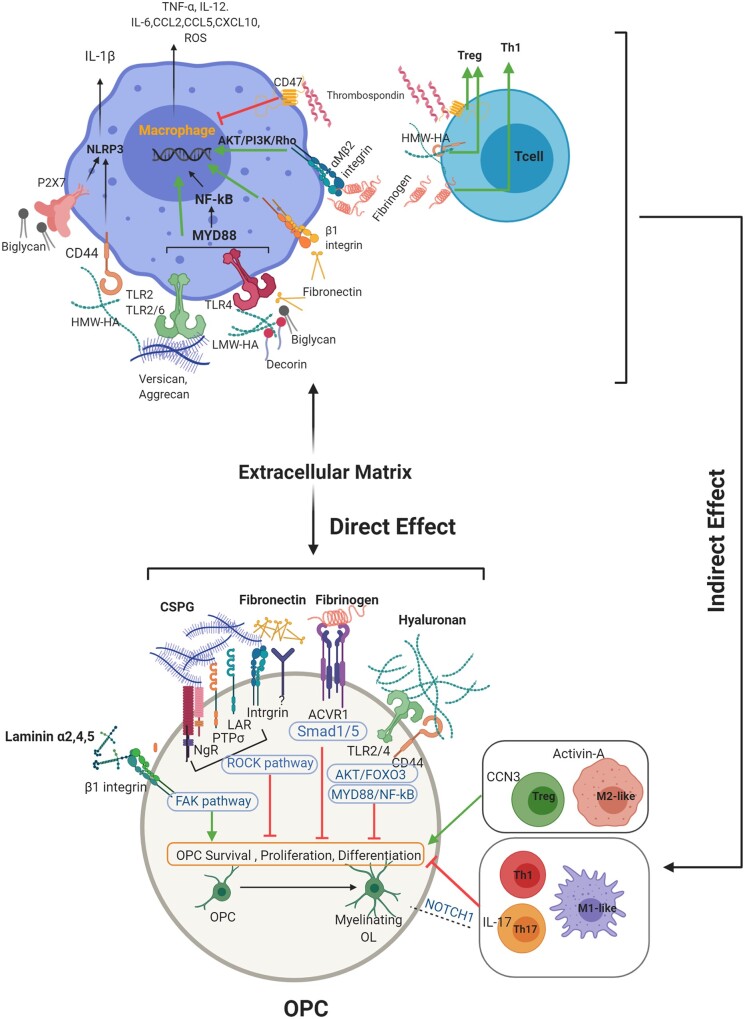
Direct interaction between ECM molecules and OPCs, and indirect effect of ECM on immune cells, affect OPCS and remyelination. This schematic aims to emphasize that remyelination is an outcome of the direct interplay between OPCs and ECM, and indirectly through the effects of ECM on immune cell activity. While CSPGs, hyaluronan, decorin, biglycan, fibrinogen and fibronectin promote an M1-like phenotype in macrophages, thrombospondin drives a regulatory response in both macrophages and T cells. The different phenotype of macrophages or T cells then contribute to oligodendrocyte development in diverse ways. Not strongly emphasized in this review, but also prominent, is that pro-inflammatory macrophages, Th1 and Th17 cells can directly impede OPCs, while a switch towards a regulatory phenotype such as M2-like macrophage and Treg improves tissue repair. ACVR1 = activin A receptor, type I; CCN3 = cellular communication network factor 3; FAK = focal adhesion kinase; Foxo3 = forkhead box O-3; HA = hyaluronan; HWA-HA = high molecular weight hyaluronan; LMW-HA = low molecular weight hyaluronan; LAR = leucocyte common antigen related; MYD88 = myeloid differentiation primary response 88; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells; NgR = Nogo receptor; NLRP3 = NLR family pyrin domain containing 3; NOTCH1 = Notch homolog 1, translocation-associated; PTPσ = protein tyrosine phosphatase sigma; ROCK = Rho-associated protein kinase; ROS = reactive oxygen species; Smad1/5 = small mothers against decapentaplegic 1/5. Images were created using BioRender.
Previous studies have mainly focused on the immunological role of both intact and degraded CSPGs such as versican and aggrecan, or small leucine-rich repeat proteoglycans including biglycan and decorin. Lewis lung carcinoma-derived versican can bind to TLR2/6 and engage myeloid differentiation primary response 88 (MYD88) adapter signalling, inducing an inflammatory response in macrophages.56 Versican enhances the migration of macrophages and production of pro-inflammatory cytokines such as TNF-α and interleukin (IL)-6.25,56,57 Moreover, versican affects the immune response indirectly through binding to hyaluronan and stabilizing CD44 signalling, resulting in enhanced leucocyte adhesion and retention.58,59 In support of the pro-inflammatory role of versican, fluorinated sugar analogues that block the production of CSPGs ameliorate inflammation and decrease clinical severity in the EAE model of multiple sclerosis through reducing the synthesis of versican.25,60,61
In contrast to the proinflammatory roles of versican, there are reports of its regulatory effect on immune responses. It has been suggested that macrophage-derived versican exerts anti-inflammatory roles, as it is a type I IFN-stimulated gene. Versican can be induced in macrophages by a signalling pathway involving the TIR-domain-containing adapter-inducing IFN-β (TRIF) adapter molecule and type I IFNs and their receptors.62 Type I IFN stimulated genes are important for the resolution of inflammation. This is consistent with another study in which versican: TLR2 interaction activated an anti-inflammatory response in dendritic cells through increased production of IL-6, IL-10 and IL-10R.63 In the context of lung inflammation in mice, versican deficiency in myeloid cells enhanced the recruitment of inflammatory cells and reduced the expression of type I IFN and IL-10, suggesting an immunomodulatory role for versican.62 However, general deficiency of versican showed a completely opposite effect with regards to leucocyte infiltration.57 Different cell types in response to stimuli use distinct signalling pathways to express versican which may be functionally diverse depending on the cellular source and signalling pathway invoked.64,65 The conflicting roles of versican could also arise from the different isotypes in various cell types.
Biglycan and decorin are mainly known as inflammatory mediators through direct interaction with TLR 2 and TLR4.66,67 Biglycan deficiency improves the severity of different inflammatory conditions and de novo overproduction of circulating biglycan in a transgenic mouse model elicits an innate immune response, highlighting the pro-inflammatory properties of biglycan.13,68
Biglycan impacts immune responses via various pathways. It promotes the recruitment of neutrophils and macrophages by induction of CXCL1 and CCl2 through TLR2/TLR4–MYD88 pathway. By activating the TLR4–TRIF signalling pathway, biglycan promotes CCL5 expression and T-cell infiltration.69,70 TRIF signalling induces the expression of type I IFN-stimulated genes which are considered to be important in the resolution of inflammation and the transition from innate to adaptive immune responses, suggesting biglycan as a link between innate and adaptive immunity. Biglycan is also involved in formation of the NLRP3 (NLR family pyrin domain containing 3) inflammasome complex, caspase-1 activation, and IL-1β maturation in macrophages. Although interaction of soluble biglycan with TLR2/4 and the purinergic receptor P2X7 induces the release of mature IL-1β, it may also play an anti-inflammatory role through inhibiting the synthesis of IL-1β.71,72
Decorin is another endogenous ligand for TLR2 and TLR4, and it can activate the mitogen-activated protein kinases (MAPKs) and NF-κB pathways to result in an acute inflammatory response via the synthesis of proinflammatory TNF-α and IL-12.66 Moreover, decorin is a known TGF-β-binding molecule and can sequester this regulatory cytokine or inhibit its signalling. Decorin may also contribute to inflammation by inducing programmed cell death 4 (PDCD4), which is a translational suppressor of anti-inflammatory IL-10. Overall, decorin forms a pro-inflammatory milieu by the induction of TNF-α, IL-12 and by the inhibition of TGF-β1 and IL-10.66
Hyaluronan is a non-sulphated glycosaminoglycan without a protein core whose molecular weight determines pro- or anti-inflammatory outcomes.69,73 Upon tissue injury, high molecular weight hyaluronan is degraded into low molecular weight hyaluronan fragments by inflammation-induced enzymes or reactive oxygen species. These low molecular weight hyaluronan fragments are capable of interacting with TLR2/4 and CD44 in macrophages to initiate MYD88 downstream signalling and NF-κB activation.74,75 In addition to the synthesis of pro-IL-1β, low molecular weight hyaluronan activates the NLRP3/ASC in inflammasome complex, resulting in maturation of IL-1β.75,76 Blockade of hyaluronan synthesis prevents and ameliorates EAE disease through enhancing regulatory immune cell populations.77,78 While low molecular weight hyaluronan promotes inflammation and acts as an activator of TLR2, high molecular weight hyaluronan inhibits TLR2 signalling and plays a protective role.79 It has been shown that high molecular weight hyaluronan promotes a regulatory T-cell response, thereby resolving inflammation.80,81
Fibrinogen is deposited in multiple sclerosis lesions as fibrin, which is a potent activator of innate immunity and a driver of T-cell infiltration and demyelination. In vivo imaging in EAE mice reveals that leakage of plasma fibrinogen into the CNS parenchyma before onset of neurological signs triggers perivascular microglial clustering and reactive oxygen species release, contributing to axonal damage. Fibrinogen promotes pro-inflammatory activation of microglia and macrophages.82,83 Injection of fibrinogen into the healthy CNS results in adaptive immune responses against myelin antigens that precede demyelination.83 The mechanism of fibrinogen in initiating CNS autoimmunity is attributed to CD11b/CD18 receptor (integrin αMβ2) activation in antigen presenting cells, since pharmacological blockade or genetic deletion of CD11b inhibits fibrinogen-driven demyelination.83 Depletion of fibrinogen using ancrod reduces inflammation and axonal damage, and results in ameliorated EAE and in other multiple sclerosis models.82,84–87
Substantial datasets indicate that fibronectin activates TLR signalling and promotes immune responses. Altered fibronectin domains resulting from unfolding, cleavage or alternative splicing processes induce pro-inflammatory cytokine expression through NF-κB and p38-MAPK-2 signalling axis.88–91 In addition to innate immune response, fibronectin enhances anti-CD3-induced T-cell proliferation via two receptors, very late antigen-4 (VLA-4) and VLA-5.92 The fibronectin extra domain A not only enhances dendritic cell maturation and inflammatory cytokine expression (IL-12, TNF-α), but also contributes to antigen presentation and acts as an antigen carrier, thereby augmenting cytotoxic T-cell responses.93 Based on proteomic analyses, fibronectin aggregates are potential points of attachment for other proteins, including Hsp70 and thrombospondin-1.94
HSPGs are also involved in shaping immune responses through regulating cell adhesion, cytokine and chemokine function and acting as TLR agonists.95–97 Based on a bioinformatics screen, it has been speculated that HSPGs may interact not only with TLR4 but also other TLRs containing the heparan sulphate-binding motif, such as TLR1, TLR2 or TLR6.97 Although HSPG binding to IFN-β, TNF-α and IFN-γ has been shown to result in sequestration of these cytokines and to reduce their bioavailability, HSPGs can protect IFN-γ from proteolytic cleavage, thereby enhancing the activity of IFN-γ.98,99
Thrombospondin-1 is commonly considered an anti-inflammatory molecule since it is a major activator of TGF-β in vivo100 and it promotes alternative activation in macrophages through interaction with CD47.94 Thrombospondin-1 is also able to impede the activation and induce tolerance of dendritic cells.101 Furthermore, thrombospondin-1:CD47 interaction skews T-cell polarization towards regulatory T cells.102 Prolonged inflammation in thrombospondin-1-deficient mice corroborates its positive effect on the resolution of inflammation.103,104 Additionally, given that it facilitates phagocytosis of damaged cells via binding to CD36, increased expression of thrombospondin-1 upon inflammation is a potential regulatory mechanism to control immune responses and tissue damage. However, opposite effects of thrombospondin-1 on inflammation have been discussed previously, and its high levels bind CD36 and increase IL-1 and IL-6 in macrophages.105
Osteopontin is considered a key inflammatory molecule that is secreted by various immune cell types in response to inflammation, including activated macrophages and T lymphocytes.106 Osteopontin-deficient mice developed milder EAE signs and were resistant to the progressive phase of disease.31,107 Osteopontin recruits immune cells to sites of inflammation, promotes Th1 and Th17 cell polarization and enhances their survival in the CNS.29,106,108–110 In contrast, in vitro studies document that osteopontin has an anti-inflammatory role in microglia.111
In summary, many of the ECM components elevated in multiple sclerosis lesions are pro-inflammatory and they enhance the inflammatory process through several mechanisms. However, some ECM molecules such as thrombospondin-1 may counter inflammatory processes. The regulatory roles of particular ECM components remain to be better defined.
Remyelination as an outcome of direct and indirect extracellular matrix–immune interactions
The process of myelin reformation (remyelination) begins with the generation of oligodendrocyte precursor cells (OPCs) and their maturation into oligodendrocytes whose processes contact and compact around axons to form myelin.2 Both innate and adaptive immunity contribute to oligodendrocyte development and remyelination. For macrophages/microglia, both pro-inflammatory and regulatory phenotypes promote remyelination.112,113 Pro-inflammatory macrophages/microglia facilitate the proliferation and recruitment of OPCs,113 in part through promoting the phagocytic removal of otherwise inhibitory myelin debris.114,115 Regulatory macrophages/microglia favour the maturation of OPCs into oligodendrocytes.113 For lymphocytes, the deficiency and selective depletion of CD4+ or CD8+ T cells reduce the levels of spontaneous remyelination in the lysolecithin model.116 The administration of regulatory T cells (Tregs) enhances, while their depletion inhibits, remyelination after lysolecithin or cuprizone demyelination; Tregs secrete cellular communication network factor 3 (CCN3), a matricellular growth factor associated with the ECM, to mediate oligodendrocyte differentiation.117 Conversely, pro-inflammatory Th1 and Th17 cells directly prevent OPCs and myelin formation, in vitro and in vivo.118,119 IL-17 from Th17 cells induces NOTCH1 (Notch homolog 1, translocation-associated) signalling in OPCs, resulting in apoptosis and reduced differentiation120 (Fig. 3). Overall, a properly harnessed inflammatory response can mediate oligodendrocyte repopulation and remyelination, and it remains to be characterized further whether there is involvement of the ECM in this process. Before addressing that, we next overview the direct effects of ECM components on oligodendroglial lineage cells briefly, as there several reviews on this topic.40,121–124
While the deposition of ECM components after injury appears to be an attempt to limit the spread of the lesion, the excessive and dysregulated deposition likely leads to a matrix that becomes unfavourable for repair (Fig. 3). In tissue culture studies, several ECM components inhibit OPC maturation and these include CSPGs, high molecular weight hyaluronan several collagens, aggregated fibronectin, tenascins and thrombospondin; conversely, pan-laminin, decorin and non-aggregated fibronectin promote OPC maturation (reviewed in Pu et al.).124In vivo, however, while most of the upregulated ECM molecules in lesions are considered inhibitory to remyelination,125 this has been addressed in experimental models only for CSPGs, hyaluronan, fibrinogen and aggregated fibronectin (see below).
Prominently, when OPCs are plated onto a mixed CSPGs preparation or onto purified aggrecan, they adhere poorly, and the cells that succeed in attachment do not elaborate profuse processes or mature properly into oligodendrocytes.60,126–128In vivo inhibition of CSPG synthesis, deposition or signalling enhances remyelination post-injury.60,126,129,130 CSPGs bind to cell surface receptors to activate growth-inhibitory pathways, but they also interact directly with growth factors, cytokines and guidance molecules to control their availability to differentiating neurons and glia.131–133 In contrast, CSPGs can play a possible pro-regenerative role through upregulation of trophic factors for OPC including insulin-like growth factor-1 (IGF1) and brain-derived neurotrophic factor (BDNF).134–136
Hyaluronan is another upregulated ECM component whose high and low molecular weight forms possess different roles for tissue repair. Back et al.26 have shown that high molecular weight hyaluronan injection limits robustly the amount of remyelination in lysolecithin lesions whereas mice receiving low molecular weight hyaluronan show the normal pattern of myelin repair. In contrast, another study by the same authors points out that hyaluronan-digestion products underlie deficient remyelination in lysolecithin lesions.137 More recent research by this group has speculated that low molecular weight hyaluronan fragments hinder the differentiation of late oligodendrocyte progenitors via an TLR/AKT/FoxO3 immune tolerance-like pathway that blocks pro-myelination signalling.138,139
Fibrinogen contributes to the non-permissive extracellular environment in demyelinating lesions. In addition to being a marker of blood–brain barrier disruption, fibrinogen initiates an inhibitory signalling involving inhibitor of DNA binding1-3 (Id1-3) to suppress differentiation of OPCs and remyelination.87 The outcome of this signalling pathway in OPCs is an arrest in maturation and myelin production. Fibrinogen also skews the OPC differentiation towards GFAP+ astrocyte-like cells instead of mature oligodendrocytes.87
The diverse roles of fibronectin have been also addressed. Fibronectin stimulates the migration and proliferation of OPCs via interaction with integrin receptors αvβ1 and αvβ3.140,141 Upregulation of astrocyte-derived rather than plasma fibronectin is thought to be a positive regulator of remyelination.142 However, more recent studies point to an inhibitory effect of fibronectin aggregates on oligodendrocyte differentiation and remyelination.143,144
Conversely, laminin-2 (merosin) promotes OPC survival and maturation while its in vivo effect on remyelination has yet to be defined precisely.145–147 A recent study has shown that after demyelination, pericytes foster OPC differentiation through the expression/secretion of laminin α2.148 Other findings suggest that in addition to laminin α2, two other chains, laminin α4 and α5, positively regulate migration and survival of OPCs through interaction with integrin α6β1 and downstream focal adhesion kinase (FAK) signalling.149
Less is known about the role of other ECM members in the context of remyelination. Increased type IV collagen in multiple sclerosis lesions is considered to be an inhibitor of OPC migration.24 Tenascin-C impedes oligodendrocyte from expressing myelin and its loss augments the level of OPC maturation and differentiation150,151; conversely, tenascin-R can potentially promote OPC adhesion and differentiation.152 In addition, neuronal precursors from thrombospondin-1-deficient mice yield significantly more OPCs and mature oligodendrocytes.153 Although indirect evidence indicate that decorin promotes repair processes by negative regulation of TGF-β and CSPGs, HSPGs might be involved in the suppression of OPC differentiation through interaction with fibroblast growth factor-2.124 Increased expression of osteopontin during the remyelination phase of toxin-induced demyelination in the mouse CNS and it ability to enhance proliferation and differentiation of OPCs in culture imply its possible role in remyelination.154,155 However, osteopontin deficiency did not affect remyelination, indicating that osteopontin may not play a critical role in myelin repair.155
In agreement with the evidence emphasizing the importance of ECM on OPC functions, several studies now highlight the role of mechanical properties of the extracellular milieu and ECM stiffness in OPC differentiation. OPCs are mechanosensitive and are able to sense the altered stiffness of their immediate surroundings and remain as precursor cells or differentiate into myelinating oligodendrocytes.156–158 Generally, a softer matrix is more permissive to OPC differentiation159 while the increased stiffness through elevated deposition of ECM components generally leads to remyelination failure in chronic demyelinated lesions.160 Recent findings in the ageing field raise the possibility that ageing ECM and niche stiffness may be an important factor impairing the function of OPCs, by signalling through the mechanosensitive ion channel PIEZO1 in OPCs; digesting the ECM of the ageing CNS using chondroitinase ABC enhances both OPC proliferation and differentiation.161
While it is clear that ECM components can directly affect OPCs, the evidence that the ECM alters immune responses that then impinge upon oligodendrocyte lineage cells and remyelination is still sparse. However, inferences can be drawn from the descriptions above where many ECM molecules tune immune responses that then affect oligodendrocytes. This is supported by results, for example, of impaired OPC differentiation following their exposure to conditioned medium from fibrin-treated macrophages.82,83 Fibrinogen depletion in mice decreases inflammation and augments myelinating oligodendrocyte repopulation and tissue repair.82,84–87
Overall, many of the ECM molecules that accumulate in multiple sclerosis lesions can directly affect oligodendrocyte lineage cells and remyelination, usually adversely. That ECM components alter immune responses in multiple sclerosis lesions that then affect OPCs favourably or otherwise is an area that deserves more research.
Targeting the extracellular matrix to enhance remyelination
Given that the extremely perturbed extracellular environment in multiple sclerosis plaques is considered to be a prominent remyelination-inhibiting factor in chronic lesions, targeting the ECM could have significant therapeutic potential to promote tissue repair (Fig. 4).
Figure 4.
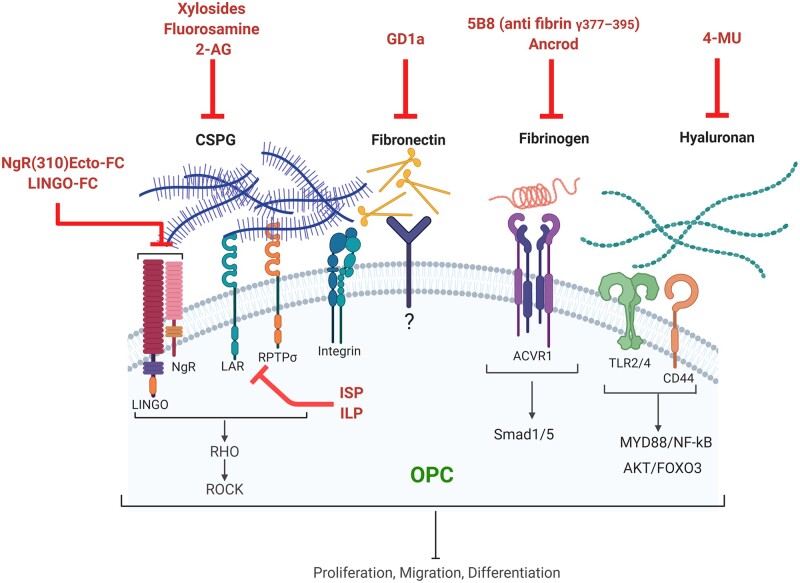
Targeting ECM components to promote OPCs and remyelination. This schematic depicts different approaches to overcome the inhibitory effect of dysregulated ECM molecules in multiple sclerosis. These methods include blocking hyaluronan synthesis by 4-methylumbelliferone (4-MU); blocking CSPGs synthesis by xylosides, fluorosamine (4-fluoro-N-acetylglucosamine) or 2-arachidonoylglycerol (2-AG); interfering with CSPG signalling through PTPσ/LAR-specific blocking peptides (ISP, ILP); antagonizing Nogo receptor complex using blocking antibodies; depleting fibrinogen by ancrod or blocking its activity using an anti-fibrin antibody; and neutralizing aggregated fibronectin by ganglioside GD1a. ACVR1 = activin A receptor, type I; Foxo3 = forkhead box O-3; ILP = intracellular LAR peptide; ISP = intracellular sigma peptide; LAR = leucocyte common antigen related; NgR = Nogo receptor; MYD88 = myeloid differentiation primary response 88; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells; PTPσ = protein tyrosine phosphatase Sigma; ROCK = Rho-associated protein kinase; Smad1/5 = small mothers against decapentaplegic1/5. Images were created using BioRender.
Several approaches have been applied to overcome the dysregulated CSPGs. These methods include degradation of deposited CSPGs using chondroitinase ABC, blocking CSPG synthesis by small-molecule inhibitors such as xylosides and fluorinated sugar analogues, and interfering with signalling through CSPG receptors. Enzymatic digestion of CSPGs by chondroitinase ABC has revealed promising effects on remyelination.126,128 To overcome the limitations of enzyme administration, a controlled chondroitinase ABC gene therapy has been developed for the treatment of spinal cord injury.162 Nonetheless, chondroitinase ABC leaves carbohydrate stubs attached to core protein which could be inflammatory and also hinder OPC functions.163,164 Another drawback of this approach is that not only upregulated CSPGs in the lesion but also those required for homeostatic functions in perineuronal nets are targeted. Inhibitors of glycosaminoglycan chain elongation such as xylosides or fluorinated glucosamine analogues block the synthesis and release of CSPGs. Use of a xyloside after injury to the spinal cord improves tissue repair and recovery.126,135 Previous work by our group has revealed the potency of fluorinated glucosamine analogues, particularly peracetylated N-acetyl,4-fluoro-N-acetylglucosamine (fluorosamine) at reducing injury-enhanced CSPG content; this reduction correlated with improved remyelination at 21 days post-injury in lysolecithin-induced demyelination.25,60,61 Recently, reduced CSPG accumulation and enhanced oligodendrocyte differentiation have been shown after targeting the endocannabinoid system using 2-arachidonoylglycerol (2-AG).165 As these compounds can affect CSPG synthesis throughout the body, developing CNS-targeted treatment is necessary to avoid potential off-target effects and peripheral toxicity.
Blocking the downstream signalling of CSPGs is another approach to overcome the repair-inhibitory properties of these molecules. CSPGs can interact with receptors, including protein tyrosine phosphatase sigma (PTPσ), leucocyte common antigen (LAR) and Nogo receptor (NgR1/3).166–168 Inhibition of LAR and PTPσ signalling using intracellular sigma peptide (ISP) and intracellular LAR peptide (ILP) following traumatic spinal cord injury promotes oligodendrogenesis as well as oligodendrocyte integrity, maturation, and myelination.129,168 Moreover, systemically delivered PTPσ-specific blocking peptide augments myelin repair and recovery in both toxin-induced demyelination and in chronic EAE.130 The Nogo receptor-1 and its co-receptor Lingo-1 bind to a broad range of interacting molecules such as Nogo A and also CSPGs.169 The interaction of Nogo receptor complex with its ligands results in negative growth and myelination signals.170,171 Therapies that target Lingo-1 lead to improved remyelination and functional recovery from EAE.172 Since some of these receptors are also expressed on immune cells, future studies should focus on how targeting these receptors affect the inflammatory response in the lesion. For example, as PTPσ is an important negative regulator for pro-inflammatory responses of dendritic cells and polarization of Th1 and Th17 cells, its suppression might result in deleterious autoimmune responses in EAE or multiple sclerosis while promoting remyelination.173 Therefore, it is of importance to develop cell specific approaches to target these receptors.
As mentioned earlier, fibronectin aggregates contribute to a remyelination-inhibitory environment in multiple sclerosis lesions.144 Fibronectin (aggregate) antagonists could thus be a potential therapeutic approach for remyelination. Moreover, the interaction between fibronectin and its cell surface receptors (integrins) can be hindered by gangliosides.174,175 There is some evidence that ganglioside GD1a, but not other gangliosides, overcomes the inhibitory effect of aggregated fibronectin on remyelination.143 GD1a rescues the inhibition of myelin membrane formation induced by aggregated fibronectin in primary oligodendrocyte cells and also neuron-oligodendrocyte cocultures. Furthermore, it promotes the repair process in demyelinated cerebellar slice cultures and in fibronectin-containing lesion after cuprizone demyelination. A specific GD1a-dependent activation of protein kinase signalling pathway is considered as an underlying mechanism for increased OPC maturation.143 These results suggest that GD1a could be a novel tool for remyelination therapy.
Hyaluronan contributes to several pathological conditions such as inflammation and inhibition of tissue repair as described earlier, so attempts have been conducted to inhibit its synthesis and accumulation. 4-methylumbelliferone (4-MU) is a derivate of coumarin, which is known for its anticoagulatory properties. 4-MU inhibits hyaluronan production through different mechanisms. It acts as a competitive substrate for UDP-glucuronosyltransferase enzyme which is required in hyaluronan synthesis.176,177 Moreover, 4-MU decreases mRNA levels of hyaluronan synthetase and other enzymes involved in hyaluronan synthesis, but the details of the mechanism are unclear.178,179 There are concerns that 4-MU affects other glycosaminoglycans, such as CSPGs and HSPGs.180 4-MU treatment has been reported to prevent and ameliorate EAE disease where it skews T-cell polarization towards Treg and Th2 cells and away from Th1.77,78 Given that hyaluronan fragments play a non-permissive role in myelin repair, whether using 4-MU would be beneficial in remyelination needs to be studied.
As discussed before, fibrinogen and fibrin impact various pathological mechanisms including inflammation and oligodendrocyte repair. To deplete fibrinogen and inhibit fibrin formation in the lesion, the defibrinogenating agent ancrod has been used in lysolecithin-induced demyelination and EAE.82,84–87 The results show that this anti-coagulant therapy is beneficial in both conditions. To avoid the anti-coagulant effect when targeting fibrinogen, an immunotherapy approach has been developed to selectively target fibrin-induced inflammation. Monoclonal antibody 5B8 against integrin binding site of fibrin reduces innate immune activation and neurodegeneration.181
Conclusions and future directions
Many ECM molecules are deposited in multiple sclerosis lesions where they affect oligodendrocyte functions and remyelination directly. Moreover, the deposited ECM is increasingly appreciated to form a second layer of regulation of remyelination through altering immune responses that then dictate repair or exacerbation of injury. The considerations of ECM components and functions must consider the individual ECM proteins, rather than lump them together, since different ECM components can exert permissive or inhibitory functions on oligodendroglial lineage cells and immune subsets, as described above. Nonetheless, ECM molecules interact with one another, and are often being cross-linked to one another through link proteins and other mechanisms, so while there is a need to consider ECM members separately, there is also a requirement to consider them as a whole, particularly since members with opposing functions are often in the mix. The permissive laminins for axon regrowth are often in the same lesion as the inhibitory CSPGs.182 It has been emphasized that the balance between permissive (laminins, unaggregated fibronectin) and inhibitory (CSPGs, tenascins, fibronectin aggregates) ECM molecules within and around a demyelinating lesion determines the degree of remyelination as an outcome.122 How one can elevate the levels of permissive molecules and simultaneously lower amounts of inhibitory ECM is a subject for future studies.
Regardless of our knowledge about the ECM changes in the CNS pathological conditions and also their role in the cellular processes such as differentiation or axonal growth, the precise functions of ECM in the CNS are still elusive. The potential role of ECM in remyelination has been highlighted using different strategies to target ECM members during CNS injury, but there is so little evidence for ECM in developmental myelination. Laminin alpha 2-deficiency resulted in myelination deficits or delays,183,184 while several other knockout mice e.g. neurocan, brevican, tenascin-C/R showed no CNS anatomical abnormalities but some effects on perineuronal nets.133,186 On the other hand, deficiency of some ECM molecules such as versican causes embryonic lethality.133 Lack of suitable animal models limits the study of the role of particular ECM components in the CNS. Moreover, complex and dynamic expression pattern of ECM during CNS development and disease, and also compensation mechanisms, hamper the elucidation of precise functions of particular ECM members. Future studies using conditional knock out mice would help to clarify the specific role of particular ECM components in the CNS in developmental myelination and in remyelination.
There are many other unknowns that are topics for future investigations. In this review we focused on the ECM content of white matter lesions because the ECM composition in grey matter lesions is not as well characterized. In one study of leukocortical lesions in multiple sclerosis, the grey matter region of the lesion did not have obvious versican elevation and showed better remyelination efficiency than the white matter part of the same lesion.187 Why and how ECM components differ in grey versus white matter, particularly after an injury, is not understood, and should be a subject for future studies. Moreover, while the loss of axons and synapses is an important feature of multiple sclerosis pathology, and abnormal perineuronal nets have been observed in Alzheimer’s disease and amyotrophic lateral sclerosis,188,189 we are unaware of reports of potential perineuronal net changes in cortical or other grey matter multiple sclerosis lesions. Given that perineuronal nets are protective against oxidative stress and neurodegeneration,190 their role in multiple sclerosis pathology is of interest. Overall, the potential of ECM changes in the grey matter and how this may affect the loss of neurons or their integrity and function in multiple sclerosis is an important area for future study.
It is now increasingly clear that different ECM components could be targeted separately; for instance, the repair inhibitory and pro-inflammatory CSPGs could be differentially affected while the pro-regenerative mechanisms of laminin could be simultaneously enhanced. In addition, each molecule could be distinctly targeted by specific means, such as the use of different inhibitors to the various receptors that interact with CSPGs.
Another issue that needs to be addressed is the effect of ECM-targeted therapies on other ECM members. Interaction between different ECM molecules and possible feedback loops add another layer of complexity to the situation. For example, when affecting hyaluronan synthesis with 4-MU, the CSPGs would be expected to be altered, as hyaluronan forms extensive cross-links with CSPGs. Moreover, since ECM members are broadly expressed in peripheral tissues, developing CNS-targeted treatments is necessary to avoid potential of off-target effects and peripheral toxicity.
One caveat in remyelination studies for multiple sclerosis is the lack of suitable animal model of multiple sclerosis for repair investigation. In spite of toxin-based models such as lysolecithin and cuprizone injury that are commonly used to study remyelination in the CNS, there is the absence of lymphocyte involvement and chronic immune responses which are characteristics of multiple sclerosis lesions.191 On the other hand, although EAE recapitulates many features of multiple sclerosis lesions, it is not an ideal model to assess remyelination due to unknown lesion localization and ambiguity in the stage of lesion evolution for a given plaque. To overcome these limitations, a new transgenic mouse that reveals newly formed oligodendrocytes, but not dying or spared oligodendrocytes, in lesions has been used.192 This model introduces a new approach to shed more light on the myelin repair during the ongoing neuroinflammation in EAE. The use of ECM-altering therapies should be encouraged in the EAE model to enable analyses of such perturbations not only for remyelination, but also on immune responses that impinge upon the repair potential.
In conclusion, the ECM in multiple sclerosis lesions is profoundly changed from the normal content. The altered ECM components have the capacity individually and collectively not only to directly affect oligodendrocytes and remyelination, but also the immune components that indirectly influence the success of lesional repair. These multiple axes of interactions lead us to consider the ECM as fine-tuning the subsequent outcome of repair or further injury to the lesion. While current disease modifying therapies used in multiple sclerosis are based on immunomodulatory approaches with low efficacy in promoting repair, more attention should be paid to ECM-targeted treatments due to their capacity to regulate both remyelination and neuroinflammation.
Funding
We thank the Canadian Institutes of Health Research and the multiple sclerosis Society of Canada for funding the authors’ studies. V.W.Y. acknowledges support from the Canada Research Chair program. S.G. acknowledges postdoctoral funding from the Rebecca Hotchkiss International Scholars Exchange funding of the Hotchkiss Brain Institute.
Competing interests
The authors report no competing interests.
Glossary
- CSPG
chondroitin sulphate proteoglycan
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- HSPG
heparan sulphate proteoglycan
- OPC
oligodendrocyte precursor cell
References
- 1.Croxford AL, Spath S, Becher B.. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 2015;36:651–662. [DOI] [PubMed] [Google Scholar]
- 2.Plemel JR, Liu WQ, Yong VW.. Remyelination therapies: a new direction and challenge in multiple sclerosis. Nat Rev Drug Discov. 2017;16:617–634. [DOI] [PubMed] [Google Scholar]
- 3.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW.. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722–729. [DOI] [PubMed] [Google Scholar]
- 4.Sorokin L.The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. [DOI] [PubMed] [Google Scholar]
- 5.Rauch U.Brain matrix: structure, turnover and necessity. Biochem Soc Trans. 2007;35:656–660. [DOI] [PubMed] [Google Scholar]
- 6.Iozzo RV.Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. [DOI] [PubMed] [Google Scholar]
- 7.Pozzi A, Yurchenco PD, Iozzo RV.. The nature and biology of basement membranes. Matrix Biol. 2017;57-58:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carulli D, Rhodes KE, Brown DJ, et al. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. [DOI] [PubMed] [Google Scholar]
- 9.Gogolla N, Caroni P, Luthi A, Herry C.. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. [DOI] [PubMed] [Google Scholar]
- 10.Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T.. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–315. [DOI] [PubMed] [Google Scholar]
- 11.Laurent TC, Fraser JR.. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 12.Rauch U.Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci. 2004;61:2031–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreth K, Iozzo RV, Schaefer L.. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aspberg A, Miura R, Bourdoulous S, et al. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci U S A. 1997;94:10116–10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giachelli CM, Steitz S.. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. [DOI] [PubMed] [Google Scholar]
- 16.Sobel RA, Ahmed AS.. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–1207. [DOI] [PubMed] [Google Scholar]
- 17.Sobel RA.The extracellular matrix in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1998;57:205–217. [DOI] [PubMed] [Google Scholar]
- 18.Davalos D, Akassoglou K.. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34:43–62. [DOI] [PubMed] [Google Scholar]
- 19.Lee NJ, Ha SK, Sati P, et al. Spatiotemporal distribution of fibrinogen in marmoset and human inflammatory demyelination. Brain. 2018;141:1637–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ.. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claudio L, Raine CS, Brosnan CF.. Evidence of persistent blood-brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta Neuropathol. 1995;90:228–238. [DOI] [PubMed] [Google Scholar]
- 22.Yates RL, Esiri MM, Palace J, Jacobs B, Perera R, DeLuca GC.. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann Neurol. 2017;82:259–270. [DOI] [PubMed] [Google Scholar]
- 23.Sobel RA, Chen M, Maeda A, Hinojoza JR.. Vitronectin and integrin vitronectin receptor localization in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1995;54:202–213. [DOI] [PubMed] [Google Scholar]
- 24.van Horssen J, Bo L, Dijkstra CD, de Vries HE.. Extensive extracellular matrix depositions in active multiple sclerosis lesions. Neurobiol Dis. 2006;24:484–491. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson EL, Mishra MK, Moussienko D, et al. Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain. 2018;141:1094–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. [DOI] [PubMed] [Google Scholar]
- 27.Gutowski NJ, Newcombe J, Cuzner ML.. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol Appl Neurobiol. 1999;25:207–214. [DOI] [PubMed] [Google Scholar]
- 28.Comabella M, Pericot I, Goertsches R, et al. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol. 2005;158:231–239. [DOI] [PubMed] [Google Scholar]
- 29.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L.. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. [DOI] [PubMed] [Google Scholar]
- 30.Vogt MH, Floris S, Killestein J, et al. Osteopontin levels and increased disease activity in relapsing-remitting multiple sclerosis patients. J Neuroimmunol. 2004;155:155–160. [DOI] [PubMed] [Google Scholar]
- 31.Chabas D, Baranzini SE, Mitchell D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. [DOI] [PubMed] [Google Scholar]
- 32.Satoh JI, Tabunoki H, Yamamura T.. Molecular network of the comprehensive multiple sclerosis brain-lesion proteome. Mult Scler. 2009;15:531–541. [DOI] [PubMed] [Google Scholar]
- 33.Koning N, Bo L, Hoek RM, Huitinga I.. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62:504–514. [DOI] [PubMed] [Google Scholar]
- 34.Mohan H, Krumbholz M, Sharma R, et al. Extracellular matrix in multiple sclerosis lesions: fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh N, Itoh Y, Tassoni A, et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc Natl Acad Sci U S A. 2018;115:E302–E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler MA, Clark IC, Tjon EC, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. 2020;578:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han MH, Hwang SI, Roy DB, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. [DOI] [PubMed] [Google Scholar]
- 38.Hasan M, Min H, Rahaman KA, et al. Quantitative proteome analysis of brain subregions and spinal cord from experimental autoimmune encephalomyelitis mice by TMT-based mass spectrometry. Proteomics. 2019;19:e1800355. [DOI] [PubMed] [Google Scholar]
- 39.Haylock-Jacobs S, Keough MB, Lau L, Yong VW.. Chondroitin sulphate proteoglycans: extracellular matrix proteins that regulate immunity of the central nervous system. Autoimmun Rev. 2011;10:766–772. [DOI] [PubMed] [Google Scholar]
- 40.Silver J, Miller JH.. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. [DOI] [PubMed] [Google Scholar]
- 41.Yiu G, He Z.. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jahan N, Hannila SS.. Transforming growth factor beta-induced expression of chondroitin sulfate proteoglycans is mediated through non-Smad signaling pathways. Exp Neurol. 2015;263:372–384. [DOI] [PubMed] [Google Scholar]
- 43.Properzi F, Carulli D, Asher RA, et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–390. [DOI] [PubMed] [Google Scholar]
- 44.Schachtrup C, Ryu JK, Helmrick MJ, et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW.. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beggah AT, Dours-Zimmermann MT, Barras FM, Brosius A, Zimmermann DR, Zurn AD.. Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience. 2005;133:749–762. [DOI] [PubMed] [Google Scholar]
- 47.Makatsori E, Lamari FN, Theocharis AD, et al. Large matrix proteoglycans, versican and perlecan, are expressed and secreted by human leukemic monocytes. Anticancer Res. 2003;23:3303–3309. [PubMed] [Google Scholar]
- 48.Martinez FO, Gordon S, Locati M, Mantovani A.. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. [DOI] [PubMed] [Google Scholar]
- 49.Crapser JD, Ochaba J, Soni N, Reidling JC, Thompson LM, Green KN.. Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington's disease. Brain. 2020;143:266–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloyd AF, Miron VE.. The pro-remyelination properties of microglia in the central nervous system. Nat Rev Neurol. 2019;15:447–458. [DOI] [PubMed] [Google Scholar]
- 51.de Jong JM, Wang P, Oomkens M, Baron W.. Remodeling of the interstitial extracellular matrix in white matter multiple sclerosis lesions: implications for remyelination (failure). J Neurosci Res. 2020;98:1370–1397. [DOI] [PubMed] [Google Scholar]
- 52.Toeda K, Nakamura K, Hirohata S, et al. Versican is induced in infiltrating monocytes in myocardial infarction. Mol Cell Biochem. 2005;280:47–56. [DOI] [PubMed] [Google Scholar]
- 53.Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF.. Interplay of extracellular matrix and leukocytes in lung inflammation. Cell Immunol. 2017;312:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asimakopoulos F, Hope C, Johnson MG, Pagenkopf A, Gromek K, Nagel B.. Extracellular matrix and the myeloid-in-myeloma compartment: balancing tolerogenic and immunogenic inflammation in the myeloma niche. J Leukoc Biol. 2017;102:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern R, Asari AA, Sugahara KN.. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. [DOI] [PubMed] [Google Scholar]
- 56.Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang I, Harten IA, Chang MY, et al. Versican deficiency significantly reduces lung inflammatory response induced by polyinosine-polycytidylic acid stimulation. J Biol Chem. 2017;292:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M.. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–35456. [DOI] [PubMed] [Google Scholar]
- 59.Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ.. Versican and the regulation of cell phenotype in disease. Biochim Biophys Acta. 2014;1840:2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keough MB, Rogers JA, Zhang P, et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun. 2016;7:11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephenson EL, Zhang P, Ghorbani S, et al. Targeting the chondroitin sulfate proteoglycans: evaluating fluorinated glucosamines and xylosides in screens pertinent to multiple sclerosis. ACS Cent Sci. 2019;5:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang MY, Kang I, Gale M Jr, et al. Versican is produced by Trif- and type I interferon-dependent signaling in macrophages and contributes to fine control of innate immunity in lungs. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1069–L1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS.. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating il-6 and il-10 receptor signaling. Cell Rep. 2015;13:2851–2864. [DOI] [PubMed] [Google Scholar]
- 64.Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HAM, Gosens R.. beta-Catenin signaling is required for TGF-beta1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;301:L956–L965. [DOI] [PubMed] [Google Scholar]
- 65.Rahmani M, Carthy JM, McManus BM.. Mapping of the Wnt/beta-catenin/TCF response elements in the human versican promoter. Methods Mol Biol. 2012;836:35–52. [DOI] [PubMed] [Google Scholar]
- 66.Merline R, Moreth K, Beckmann J, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popovic ZV, Wang S, Papatriantafyllou M, et al. The proteoglycan biglycan enhances antigen-specific T cell activation potentially via MyD88 and TRIF pathways and triggers autoimmune perimyocarditis. J Immunol. 2011;187:6217–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L.. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L.. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2014;35:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsieh LT, Frey H, Nastase MV, et al. Bimodal role of NADPH oxidases in the regulation of biglycan-triggered IL-1beta synthesis. Matrix Biol. 2016;49:61–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collins SL, Black KE, Chan-Li Y, et al. Hyaluronan fragments promote inflammation by down-regulating the anti-inflammatory A2a receptor. Am J Respir Cell Mol Biol. 2011;45:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor KR, Yamasaki K, Radek KA, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. [DOI] [PubMed] [Google Scholar]
- 76.Yamasaki K, Muto J, Taylor KR, et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuipers HF, Rieck M, Gurevich I, et al. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proc Natl Acad Sci U S A. 2016;113:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller AM, Yoon BH, Sadiq SA.. Inhibition of hyaluronan synthesis protects against central nervous system (CNS) autoimmunity and increases CXCL12 expression in the inflamed CNS. J Biol Chem. 2014;289:22888–22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu YY, Lee CH, Dedaj R, et al. High-molecular-weight hyaluronan-a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care. 2008;12:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bollyky PL, Lord JD, Masewicz SA, et al. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. [DOI] [PubMed] [Google Scholar]
- 81.Bollyky PL, Wu RP, Falk BA, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryu JK, Petersen MA, Murray SG, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun. 2015;6:8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams RA, Bauer J, Flick MJ, et al. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Akassoglou K, Adams RA, Bauer J, et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paterson PY.Experimental allergic encephalomyelitis: role of fibrin deposition in immunopathogenesis of inflammation in rats. Fed Proc. 1976;35:2428–2434. [PubMed] [Google Scholar]
- 87.Petersen MA, Ryu JK, Chang KJ, et al. Fibrinogen activates BMP signaling in oligodendrocyte progenitor cells and inhibits remyelination after vascular damage. Neuron. 2017;96:1003–1012.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McFadden JP, Basketter DA, Dearman RJ, Kimber IR.. Extra domain A-positive fibronectin-positive feedback loops and their association with cutaneous inflammatory disease. Clin Dermatol. 2011;29:257–265. [DOI] [PubMed] [Google Scholar]
- 89.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. [DOI] [PubMed] [Google Scholar]
- 90.You R, Zheng M, McKeown-Longo PJ.. The first type III repeat in fibronectin activates an inflammatory pathway in dermal fibroblasts. J Biol Chem. 2010;285:36255–36259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng M, Jones DM, Horzempa C, Prasad A, McKeown-Longo PJ.. The first type III domain of fibronectin is associated with the expression of cytokines within the lung tumor microenvironment. J Cancer. 2011;2:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis LS, Oppenheimer-Marks N, Bednarczyk JL, McIntyre BW, Lipsky PE.. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990;145:785–793. [PubMed] [Google Scholar]
- 93.Lasarte JJ, Casares N, Gorraiz M, et al. The extra domain A from fibronectin targets antigens to TLR4-expressing cells and induces cytotoxic T cell responses in vivo. J Immunol. 2007;178:748–756. [DOI] [PubMed] [Google Scholar]
- 94.Sikkema AH, Stoffels JMJ, Wang P, et al. Fibronectin aggregates promote features of a classically and alternatively activated phenotype in macrophages. J Neuroinflammation. 2018;15:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goodall KJ, Poon IK, Phipps S, Hulett MD.. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One. 2014;9:e109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson GB, Brunn GJ, Kodaira Y, Platt JL.. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. [DOI] [PubMed] [Google Scholar]
- 97.Simon DD, Parish CR.. Heparan sulfate: a ubiquitous glycosaminoglycan with multiple roles in immunity. Front Immunol. 2013;4:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gordts P, Foley EM, Lawrence R, et al. Reducing macrophage proteoglycan sulfation increases atherosclerosis and obesity through enhanced type I interferon signaling. Cell Metab. 2014;20:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lortat-Jacob H.Interferon and heparan sulphate. Biochem Soc Trans. 2006;34:461–464. [DOI] [PubMed] [Google Scholar]
- 100.Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. [DOI] [PubMed] [Google Scholar]
- 101.Doyen V, Rubio M, Braun D, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grimbert P, Bouguermouh S, Baba N, et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006;177:3534–3541. [DOI] [PubMed] [Google Scholar]
- 103.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A.. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. [DOI] [PubMed] [Google Scholar]
- 104.Punekar S, Zak S, Kalter VG, et al. Thrombospondin 1 and its mimetic peptide ABT-510 decrease angiogenesis and inflammation in a murine model of inflammatory bowel disease. Pathobiology. 2008;75:9–21. [DOI] [PubMed] [Google Scholar]
- 105.Yamauchi Y, Kuroki M, Imakiire T, et al. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002;21:441–448. [DOI] [PubMed] [Google Scholar]
- 106.Wang KX, Denhardt DT.. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. [DOI] [PubMed] [Google Scholar]
- 107.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H.. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. [DOI] [PubMed] [Google Scholar]
- 108.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. [DOI] [PubMed] [Google Scholar]
- 109.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H.. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steinman L.A molecular trio in relapse and remission in multiple sclerosis. Nat Rev Immunol. 2009;9:440–447. [DOI] [PubMed] [Google Scholar]
- 111.Yu H, Liu X, Zhong Y.. The Effect of osteopontin on microglia. Biomed Res Int. 2017;2017:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Butovsky O, Landa G, Kunis G, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miron VE, Boyd A, Zhao J-W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cunha MI, Su M, Cantuti-Castelvetri L, et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J Exp Med. 2020;217:e20191390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rawji KS, Young AMH, Ghosh T, et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 2020;139:893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bieber AJ, Kerr S, Rodriguez M.. Efficient central nervous system remyelination requires T cells. Ann Neurol. 2003;53:680–684. [DOI] [PubMed] [Google Scholar]
- 117.Dombrowski Y, O'Hagan T, Dittmer M, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baxi EG, DeBruin J, Tosi DM, et al. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J Neurosci. 2015;35:8626–8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moore CS, Cui QL, Warsi NM, et al. Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation. J Immunol. 2015;194:761–772. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, Zhang CJ, Martin BN, et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat Commun. 2017;8:15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gorter RP, Baron W.. Matrix metalloproteinases shape the oligodendrocyte (niche) during development and upon demyelination. Neurosci Lett. 2020;729:134980. [DOI] [PubMed] [Google Scholar]
- 122.Harlow DE, Macklin WB.. Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp Neurol. 2014;251:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marangon D, Boccazzi M, Lecca D, Fumagalli M.. Regulation of Oligodendrocyte Functions: targeting Lipid Metabolism and Extracellular Matrix for Myelin Repair. J Clin Med. 2020;9:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pu A, Stephenson EL, Yong VW.. The extracellular matrix: focus on oligodendrocyte biology and targeting CSPGs for remyelination therapies. Glia. 2018;66:1809–1825. [DOI] [PubMed] [Google Scholar]
- 125.Sherman LS, Back SA.. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. [DOI] [PubMed] [Google Scholar]
- 126.Lau LW, Keough MB, Haylock-Jacobs S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–432. [DOI] [PubMed] [Google Scholar]
- 127.Pendleton JC, Shamblott MJ, Gary DS, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPsigma. Exp Neurol. 2013;247:113–121. [DOI] [PubMed] [Google Scholar]
- 128.Siebert JR, Osterhout DJ.. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119:176–188. [DOI] [PubMed] [Google Scholar]
- 129.Dyck S, Kataria H, Alizadeh A, et al. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPsigma receptors promotes a beneficial inflammatory response following spinal cord injury. J Neuroinflammation. 2018;15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo F, Tran AP, Xin L, et al. Modulation of proteoglycan receptor PTPsigma enhances MMP-2 activity to promote recovery from multiple sclerosis. Nat Commun. 2018;9:4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schaefer L.Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–190. [DOI] [PubMed] [Google Scholar]
- 132.Sirko S, Akita K, Von Holst A, Faissner A.. Structural and functional analysis of chondroitin sulfate proteoglycans in the neural stem cell niche. Methods Enzymol. 2010;479:37–71. [DOI] [PubMed] [Google Scholar]
- 133.Zimmermann DR, Dours-Zimmermann MT.. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. [DOI] [PubMed] [Google Scholar]
- 134.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH.. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rolls A, Shechter R, London A, et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 2008;5:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.VonDran MW, Singh H, Honeywell JZ, Dreyfus CF.. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31:14182–14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Preston M, Gong X, Su W, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Srivastava T, Diba P, Dean JM, et al. A TLR/AKT/FoxO3 immune tolerance-like pathway disrupts the repair capacity of oligodendrocyte progenitors. J Clin Invest. 2018;128:2025–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Srivastava T, Sherman LS, Back SA.. Dysregulation of hyaluronan homeostasis during white matter injury. Neurochem Res. 2020;45:672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Blaschuk KL, Frost EE, Ffrench-Constant C.. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127:1961–1969. [DOI] [PubMed] [Google Scholar]
- 141.Milner R, Edwards G, Streuli C, Ffrench-Constant C.. A role in migration for the alpha V beta 1 integrin expressed on oligodendrocyte precursors. J Neurosci. 1996;16:7240–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stoffels JM, Hoekstra D, Franklin RJ, Baron W, Zhao C.. The EIIIA domain from astrocyte-derived fibronectin mediates proliferation of oligodendrocyte progenitor cells following CNS demyelination. Glia. 2015;63:242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qin J, Sikkema AH, van der Bij K, et al. GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: implications for Multiple Sclerosis. J Neurosci. 2017;37:9925–9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stoffels JM, de Jonge JC, Stancic M, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. [DOI] [PubMed] [Google Scholar]
- 145.Buttery PC, Ffrench-Constant C.. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. [DOI] [PubMed] [Google Scholar]
- 146.Colognato H, Baron W, Avellana-Adalid V, et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. [DOI] [PubMed] [Google Scholar]
- 147.Franco SJ, Muller U.. Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system. Dev Neurobiol. 2011;71:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De La Fuente AG, Lange S, Silva ME, et al. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suzuki N, Hyodo M, Hayashi C, et al. Laminin alpha2, alpha4, and alpha5 chains positively regulate migration and survival of oligodendrocyte precursor cells. Sci Rep. 2019;9:19882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Czopka T, Von Holst A, Schmidt G, Ffrench-Constant C, Faissner A.. Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway. Glia. 2009;57:1790–1801. [DOI] [PubMed] [Google Scholar]
- 151.Garwood J, Garcion E, Dobbertin A, et al. The extracellular matrix glycoprotein Tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur J Neurosci. 2004;20:2524–2540. [DOI] [PubMed] [Google Scholar]
- 152.Pesheva P, Gloor S, Schachner M, Probstmeier R.. Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J Neurosci. 1997;17:4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lu Z, Kipnis J.. Thrombospondin 1-a key astrocyte-derived neurogenic factor. FASEB J. 2010;24:1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Selvaraju R, Bernasconi L, Losberger C, et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol Cell Neurosci. 2004;25:707–721. [DOI] [PubMed] [Google Scholar]
- 155.Zhao C, Fancy SP, Ffrench-Constant C, Franklin RJ.. Osteopontin is extensively expressed by macrophages following CNS demyelination but has a redundant role in remyelination. Neurobiol Dis. 2008;31:209–217. [DOI] [PubMed] [Google Scholar]
- 156.Jagielska A, Norman AL, Whyte G, Vliet KJ, Guck J, Franklin RJ.. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev. 2012;21:2905–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lourenço T, Paes de Faria J, Bippes CA, et al. Modulation of oligodendrocyte differentiation and maturation by combined biochemical and mechanical cues. Sci Rep. 2016;6:21563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Urbanski MM, Kingsbury L, Moussouros D, et al. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci Rep. 2016;6:33751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, Pinto IM.. Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia. 2018;66:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Urbanski MM, Brendel MB, Melendez-Vasquez CV.. Acute and chronic demyelinated CNS lesions exhibit opposite elastic properties. Sci Rep. 2019;9:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Segel M, Neumann B, Hill MFE, et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573:130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burnside ER, De Winter F, Didangelos A, et al. Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain. 2018;141:2362–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cua RC, Lau LW, Keough MB, Midha R, Apte SS, Yong VW.. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia. 2013;61:972–984. [DOI] [PubMed] [Google Scholar]
- 164.Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ.. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci. 2014;34:16424–16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feliú A, Bonilla Del Río I, Carrillo-Salinas FJ, et al. 2-Arachidonoylglycerol reduces proteoglycans and enhances remyelination in a progressive model of demyelination. J Neurosci. 2017;37(35):8385–8398. doi:10.1523/JNEUROSCI.2900-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dickendesher TL, Baldwin KT, Mironova YA, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fisher D, Xing B, Dill J, et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shen Y, Tenney AP, Busch SA, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lang BT, Cregg JM, DePaul MA, et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mironova YA, Giger RJ.. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci. 2013;36:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chong SY, Rosenberg SS, Fancy SP, et al. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci U S A. 2012;109:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee JY, Kim MJ, Li L, et al. Nogo receptor 1 regulates Caspr distribution at axo-glial units in the central nervous system. Sci Rep. 2017;7:8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–1233. [DOI] [PubMed] [Google Scholar]
- 174.Ohtake Y, Saito A, Li S.. Diverse functions of protein tyrosine phosphatase sigma in the nervous and immune systems. Exp Neurol. 2018;302:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Merzak A, Koochekpour S, Pilkington GJ.. Adhesion of human glioma cell lines to fibronectin, laminin, vitronectin and collagen I is modulated by gangliosides in vitro. Cell Adhes Commun. 1995;3:27–43. [DOI] [PubMed] [Google Scholar]
- 176.Wang X, Sun P, Al-Qamari A, Tai T, Kawashima I, Paller AS.. Carbohydrate-carbohydrate binding of ganglioside to integrin alpha(5) modulates alpha(5)beta(1) function. J Biol Chem. 2001;276:8436–8444. [DOI] [PubMed] [Google Scholar]
- 177.Kakizaki I, Kojima K, Takagaki K, et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem. 2004;279:33281–33289. [DOI] [PubMed] [Google Scholar]
- 178.Vigetti D, Ori M, Viola M, et al. Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J Biol Chem. 2006;281:8254–8263. [DOI] [PubMed] [Google Scholar]
- 179.Kultti A, Pasonen-Seppanen S, Jauhiainen M, et al. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–1923. [DOI] [PubMed] [Google Scholar]
- 180.Vigetti D, Rizzi M, Viola M, et al. The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology. 2009;19:537–546. [DOI] [PubMed] [Google Scholar]
- 181.Clarkin CE, Allen S, Wheeler-Jones CP, Bastow ER, Pitsillides AA.. Reduced chondrogenic matrix accumulation by 4-methylumbelliferone reveals the potential for selective targeting of UDP-glucose dehydrogenase. Matrix Biol. 2011;30:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ryu JK, Rafalski VA, Meyer-Franke A, et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat Immunol. 2018;19:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Grimpe B, Silver J.. The extracellular matrix in axon regeneration. Prog Brain Res. 2002;137:333–349. [DOI] [PubMed] [Google Scholar]
- 184.Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T.. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Relucio J, Menezes MJ, Miyagoe-Suzuki Y, Takeda S, Colognato H.. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia. 2012;60:1451–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Quraishe S, Forbes LH, Andrews MR. The extracellular environment of the CNS: influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast. 2018;2018:2952386. Published 2018 Apr 18. doi:10.1155/2018/2952386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chang A, Staugaitis SM, Dutta R, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baig S, Wilcock GK, Love S.. Loss of perineuronal net N-acetylgalactosamine in Alzheimer's disease. Acta Neuropathol. 2005;110:393–401. Erratum in: Acta Neuropathol (Berl) 2006;112(1):113. [DOI] [PubMed] [Google Scholar]
- 189.Forostyak S, Homola A, Turnovcova K, Svitil P, Jendelova P, Sykova E.. Intrathecal delivery of mesenchymal stromal cells protects the structure of altered perineuronal nets in SOD1 rats and amends the course of ALS. Stem Cells. 2014;32:3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fawcett JW, Oohashi T, Pizzorusso T.. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–465. [DOI] [PubMed] [Google Scholar]
- 191.Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I.. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2011;18:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mei F, Lehmann-Horn K, Shen YA, et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5:e18246. [DOI] [PMC free article] [PubMed] [Google Scholar]