Abstract
Background
Allergies represent an important health problem in industrialized countries. Allergen sensitization is an important risk factor for the development of allergic diseases; thus, the identification of an individual’s allergen sensitization is essential for the diagnosis and treatment of diseases.
Objective
This review compares different modern methods applied for the analysis of allergens in various matrices (from 2015 to the end of September 2019).
Conclusions
Immunological methods are still most frequently used for detection of allergens. These methods are sensitive, but the lack of specificity and cross-reaction of some antibodies can still be a relevant source of errors. DNA-based methods are fast and reliable for determination of protein allergens, but the epitopes of protein allergens with posttranslational modifications and their changes, originated during various processing, cannot be identified through the use of this method. Methods based on application of biosensors are very rapid and easy to use, and can be readily implemented as screening methods to monitor allergens. Recent developments of new high-resolution MS instruments are encouraging and enable development in the analysis of allergens. Fast, very sensitive, reliable, and accurate detection and quantification of allergens in complex samples can be used in the near future. Mass spectrometry coupled with LC, GC, or electrophoretic methods bring additional advances in allergen analysis. The use of LC-MS or LC-MS/MS for the quantitative detection of allergens in various matrices is at present gaining acceptance as a protein-based confirmatory technique over the routinely performed enzyme-linked immunosorbent assays.
Allergies are an increasingly important public health problem, with implications for the general health status, economy, and legislation of a country (1). Allergy is a type of abnormal immune reaction, which is triggered by environmental antigens or allergens and mediated by Immunogloben E (IgE) antibodies. Allergic reactions to various allergens result from a dysfunction in, and the hypersensitivity (type I) of, the adaptive immune system against specific compounds. Theyrange from insignificant skin symptoms to anaphylactic shock. Exposure to allergens can invoke hives, vomiting, itching, nausea, and asthma in sensitive individuals. Allergy is one of the common causes of anaphylaxis, an acute and potentially deadly allergic reaction. Allergenic sources usually contain more than one allergenic substance e. g., protein. Within the same allergenic product there might be several substances capable of inducing allergic reactions. Small changes of allergen levels in foods or in the environment can lead to variations in allergenicity with potential life-threatening implications for allergic patients. There is currently no cure for allergies, and sufferers can only rely on the correct labelling of various products to avoid allergens. Contamination of food by hidden allergens is a major health problem for food allergic patients. Hence, it is important that methods for their detection and analysis are accurate, sensitive, reliable, robust, fast, reproducible, and standardized.
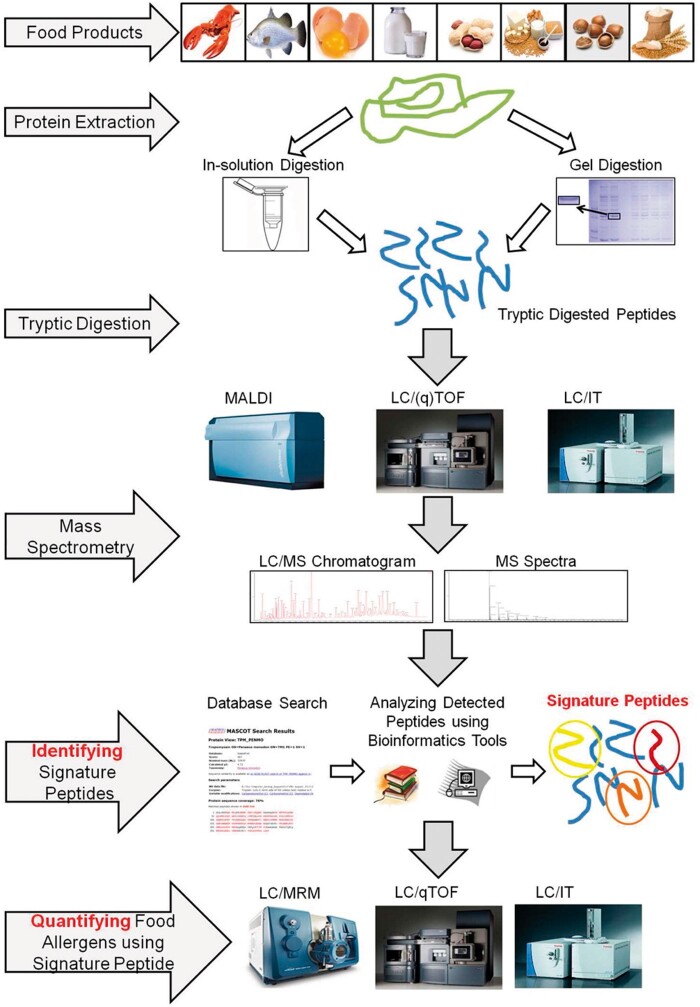
The analysis of allergens is important for correct diagnoses and treatment plans. Analysis is also a prerequisite for effective allergen avoidance. Specific and sensitive analytical methods, which allow for unequivocal identification and accurate quantification of allergenic components, are important tools in allergen risk management. Allergen analysis is also central to implementing and monitoring food allergen risk assessment and management processes by the food industry. Over the past several decades, many effective techniques have been applied to ensure the labelling and management of food allergens. Determination of food allergens are based on detection of either proteins or nucleic acids. Techniques based on protein detection include immunoblotting, enzyme-linked immune sorbent assay (ELISA), and chromatography most often coupled with MS or MS/MS. PCR is the technique based on nucleic acids determination. Currently, a combination of different methods is often applied for allergens analysis. A diagram of an example procedure for allergens analysis using different methods is shown in Figure 1 (2). The advantages of MS are that it is fast and can be used to analyze a number of allergens simultaneously, reducing total analysis time. The method is robust and stable and can easily be automated and standardized (3). However, the MS analysis of allergens requires additional steps that include protein digestion to generate peptides and their introduction into the MS system for analysis. An exemplary strategy for the analysis of allergens in food consists of a digestion stage, initial LC-MS or MS analysis, identification of signature peptides using bioinformatics tools, and quantification by LC-MS as is presented in Figure 2 (3).
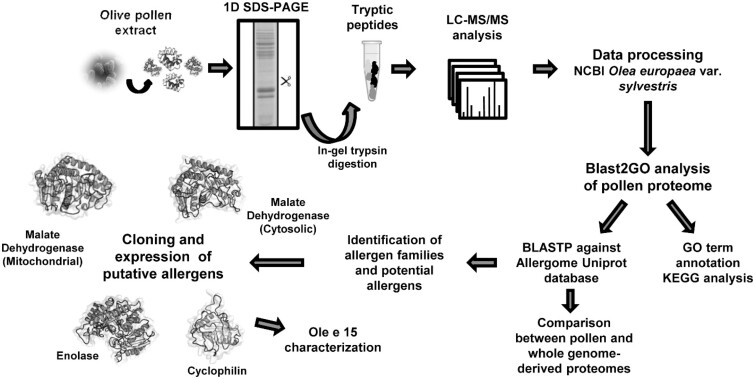
Figure 1.
Workflow of the study to delineate the olive pollen proteome and its allergenome and to describe Ole e 15 as a new relevant allergen implicated in cross-reactivity. 3D models of the proteins were created using 1SEV.1.A, 5.NUE.1.B, 1XSX.1.A, and 2MC9 PDB structures for malate dehydrogenase (mitochondrial), malate dehydrogenase (cytosolic), enolase, and cyclophilin, respectively (2). Reprinted with permission from San Segundo-Acosta, P., Oeo-Santos, C., Benedé, S., de los Ríos, V., Navas, A., Ruiz-Leon, B., Moreno, C., Pastor-Vargas, C., Jurado, A., Villalba, M., Barderas, R. (2019) J. Proteome Res. 18, 3052–3066. Copyright American Chemical Society.
Figure 2.
Signature peptide identity and characterization workflow for detection and quantification of food allergens (2). Reprinted with permission from San Segundo-Acosta, P., Oeo-Santos, C., Benedé, S., de los Ríos, V., Navas, A., Ruiz-Leon, B., Moreno, C., Pastor-Vargas, C., Jurado, A., Villalba, M., Barderas, R. (2019) J. Proteome Res. 18, 3052−3066. Copyright American Chemical Society.
Methods for the Detection and Analysis of Allergens
Immunological Methods
Many different types of immunoassays currently exist. Immunoassays typically utilize antibodies for the detection of specific allergenic proteins which serve as markers for allergens. Immunological characterization of allergens is widely based on the binding of IgE. In vivo skin prick testing (SPT) and in vitro serological assays are available to evaluate the presence of specific IgE to certain allergens in patients (4). Immunoassays, based on the specificity of antigen antibody interaction, are traditionally used for routine analysis, in particular in the format of sandwich or competitive enzyme-linked immunosorbent assay (ELISA).
Advantages and practical applications of ELISA and other immunological methods
Different classes of antibodies can be applied for determination of allergenic proteins, including monoclonal and polyclonal antibodies. Monoclonal antibodies are used for the recognition of specific antigens due to the recognition of one epitope, while polyclonal antibodies recognize multiple epitopes spread on the proteins. ELISA is a rapid diagnostic test that is often used for routine detection of allergens (2, 3). ELISA techniques use antibodies to selectively target allergens or specific marker proteins within investigated samples for detection. Once the allergen has been selectively bound by the antibody, an enzyme linked to these causes a proportional color change. This change can be measured to give highly sensitive results. ELISA relies mainly on immune-recognition, which includes the specific immune reaction between at least one antibody and the antigen. The antibody or antigen is firstly coated on the solid substrate. Next the solid substrate with coated antibody or antigen can be captured by specific recognition and immobilized on the supporting substrate. Next, the enzyme labeled antibody is added and incubated with the antigen to form a bioconjugation that can catalyze the reaction of the chromogenic reagents. ELISA kits utilizing polyclonal antibodies raised against different allergens are currently widely used for determination of various allergens, especially food allergens. The performance of quantitative ELISAs depends on the choice of extraction buffer, the binding of antibodies to its target, and the nature of the standards.
Nano-ELISA is increasingly applied for detection of allergens. Compared with traditional ELISA, nano-ELISA have advantages in terms of sensitivity, accuracy, operations, and cost. All kinds of nanomaterials such as nano-substrate, nanoprobes, nano-carriers, or even nano-coloring agents can be used to construct the nano-ELISA. However, the fundamental basis of ELISA settles upon the analyte’s separation through specific solvents and, frequently, it does not involve a sample pretreatment procedure; thus the endogenous components of the matrix are able to interact with the assay compounds or with the analyte. This outcome is called matrix effect and it is a sterling disadvantage of ELISA techniques.
In sandwich ELISA assays the antigen present in the investigated sample is captured by a specific antibody immobilised on a solid surface forming an antigen-antibody complex. The complex reacts with a second analyte-specific antibody which is conjugated to an enzyme, forming a sandwich, and the enzyme reacts with a specific substrate developing a color. The concentration of the antigen-antibody complex, measured by the absorbance of the colored product, is directly proportional to the amount of allergen present in the sample. For example, an ELISA kit for milk can target β-lactoglobulin or a different milk protein (e.g., casein) (5). Kobayashi et al. performed an investigation of the cross-reactivity of fish allergens applying ELISA experiments (6). Sera obtained from patients with fish allergies and from healthy control donors were examined for IgE reactivity to the purified Pacific mackerel parvalbumin and collagen and to the extract obtained from Anisakis simplex. In the ELISA test, authors demonstrated that pooled serum obtained from patients with fish parvalbumin-specific allergies exhibited IgE reactivity to the extracts obtained from most fish species, and pooled serum obtained from patients with fish collagen-specific allergies displayed IgE reactivity to the extracts of all types of fish.
Various ELISA-based methods were applied for determination of different allergens. Oyster allergen Cra g 1 was determined by ELISA (7). In the investigations, authors initially used different immunoinformatics strategies to predict oyster (Crassostrea gigas) tropomyosin epitopes. The potential epitopes were predicted by immunoinformatics tools and the resultant immunodominant epitopes were identified by inhibition ELISA with pooled sera and individual serum from oyster allergic patients. Surprisingly, homologous substitution of multiple amino acids led to an obvious decrease in affinity of IgE antibodies, but this method did not restrict binding completely. Five major linear epitopes were evenly distributed on the surface of a homology-based Cra g 1 model and hydrophilic residues appeared to be the most important for IgE binding. After combining prediction by immunoinformatics tools, potential epitope peptides were synthesized and validated by inhibition ELISA using the pooled sera and randomly selected individual serum from oyster allergic patients. Additionally, the crucial amino acids within each of the Cra g 1 epitope were determined. A homology-based three-dimensional model of Cra g 1 was constructed for the explanation of the positional distribution of IgE-binding epitopes in the allergen molecule. These results not only offer a better understanding of the molecular mechanism of interaction between Cra g 1 and oyster-specific IgE, but also have significance in clinical diagnosis and immunotherapy.
Detection of the food allergen glycinin, a soybean allergen in powdered milk, was performed by a lateral flow colloidal gold immunoassay strip test in a sandwich format with a colloidal gold-labeled mouse antiglycinin monoclonal antibody and a rabbit antiglycinin polyclonal antibody (8). The test strip developed by Wang et al. is composed of a sample pad, a conjugate reagent pad, an absorbent pad, and a test membrane containing a control line and a test line. The standard solution or sample extract is allowed to interreact with the colloidal gold-labeled mouse antiglycinin monoclonal antibody. The mixture then moves upward on the nitrocellulose membrane chromatographically via capillary action. For a positive sample, glycinin binds to the mouse antiglycinin monoclonal antibody, forming a gold/antigen/antibody complex, which binds to the rabbit antiglycinin polyclonal antibody and forms a red color band in the test region. A stronger line color indicates higher amounts of glycinin in the investigated sample. An optical density scanner that measures relative optical density was applied to quantify the color reaction. The LOD obtained by application of the test strip with an optical density scanner was 0.69 mg/kg.
An ELISA procedure was applied for determination of allergens in crab (Charybdis feriatus), one of the main causes of food allergy in Taiwan (9). Several proteins are recognized as crustacean allergens, and tropomyosin is known to be the major one. The allergic response between allergens in crucifix crab (C. feriatus) and specific-IgEs in patients’ sera was investigated, in search for a novel allergen. For quantification of topomyosin level Crustacean Tropomyosin ELISA KitSeveral was applied in the procedure. In addition, the effect of heating on tropomyosin levels in both raw and cooked crabs was examined.
Various undeclared food allergens in cumin were detected by multiplex methods: ELISA, SDS-PAGE protein electrophoresis and western blot, PCR, and LC-MS/MS analysis (10). In the investigation, ELISA was applied for determination of almond, hazelnut, and peanut allergens present in cumin samples. Initial analysis of the almond and peanut samples was undertaken using commercially available almond and peanut ELISAs (11). Further investigation was performed by HPLC-MS/MS. Antibody-based methodologies used to determine whether peanut was present in the cumin samples generated quantitative inconsistencies and a complex antigenic profile. It was necessary to apply DNA- and MS-based methods capable of detecting multiple biomarkers. However, accurate quantitation of peanut in the contaminated cumin was not possible. For example, if an immunoassay detected primarily Ara h 3 and used calibration standards that contained Ara h 3 as the predominant allergen protein, it would incorrectly assess the peanut content and potential allergenicity of the cumin samples. Obtained results demonstrate the limitations of single analyte-specific assays and the need for orthogonal multiplex methods to detect food allergens irrespective of varietal or other differences.
Parke et al. used quantitative ELISA kits for determination of egg, milk, and peanut allergens in baked goods (12). They compared the performance of commercial immunochemical assays: Morinaga Egg, Milk, and Peanut; Neogen BioKits Peanut; ELISA Systems; Neogen Veratox Egg, Milk, and Peanut; and R-Biopharm RIDASCREEN FAST Ei/Egg Protein, Milk, Peanut with that of a multi-allergen MS method for the detection and quantitation of allergens in baked good. The Morinaga ELISA and LC-MS/MS quantitative methods exhibited the highest recovery for all determined allergens, whereas the ELISA Systems, Neogen BioKits, Neogen Veratox, and R-Biopharm ELISA Kits underperformed in the determination of investigated allergens in the bakery products. An ELISA method was applied for the quantitative analysis of almond allergen protein residues in various food products, such as cookies, crackers, chocolate bars, cereals, beverages, and clean-in-place rinses (13). Quantification of almond protein residues was performed with Veratox for Almond Allergen kits ranging from 2.5 to 25 ppm.
Ecker et al. compared the suitability of two competitive ELISAs, one sandwich ELISA and a novel real-time PCR method for the detection of lupine in four different highly processed model foods (bread, biscuits, rice patties, and noodles) (14). All ELISA experiments were carried out in flat-bottom polystyrene microtiter plates. Authors compared results obtained by three in-house ELISAs including one sandwich ELISA and two competitive ELISAs. The sandwich ELISA proved to be the most sensitive method compared to a real-time PCR. The LODs obtained by repeatedly analyzing extracts from blank food matrices were in the range of 5 to 20 ppm (IgG ELISA) and 3 to 34 ppm lupine protein (IgY ELISA), corresponding to 17 to 77 ppm lupine and 11 to 126 ppm lupine, respectively. The LODs obtained by the sandwich ELISA were substantially lower (from 0.1 to 0.6 ppm lupine protein, corresponding to 0.4 to 2.3 ppm lupine). Based on the results, the authors concluded that the sandwich ELISA proved to be the most sensitive method applied in the investigations (competitive ELISAs, sandwich ELISA, and a novel real-time PCR) for determination of lupine in highly processed foods (14).
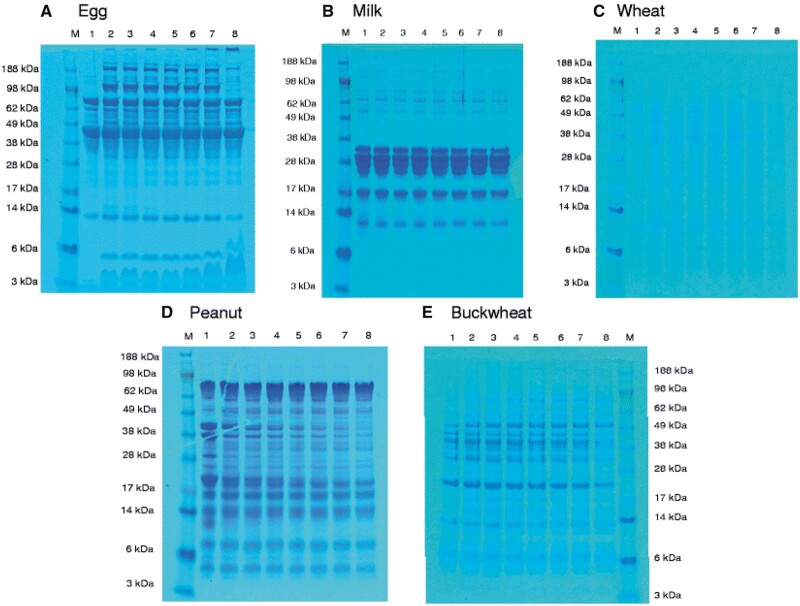
Ito et al. developed a novel human- and eco-friendly ELISA in which harmful reagents were eliminated (15). For determination of food allergens by this ELISA, sodium sulfite as potential reductant for the replacement of 2-mercaptoethanol (2-ME) was investigated. Next, the ELISA performance for egg, milk, wheat, peanut, and buckwheat was evaluated. The protein extraction capability of SDS/0.1 M sodium sulfite solution was similar to that of SDS/2-ME solution. The ELISA performance for egg, milk, wheat, peanut, and buckwheat was also estimated by using model-processed foods and commercially available food products. The food allergen recoveries of the novel SDS/0.1 M sulfite ELISAs corresponded with those of the SDS/2-ME ELISAs. Allergens from cashew nuts were separated by SDS-PAGE and analyzed by immunoblot and ELISA, with rabbit anti-cashew polyclonal sera and human serum IgE (16). Allergen soy proteins were detected by ELISA after separation by SDS-PAGE (17). Before SDS-PAGE procedure samples were prepared by microwave or ultrasound assisted extraction. The authors compared conventional extraction procedures, microwave, and ultrasound assisted extraction on protein recovery and concluded that the application of microwave and ultrasound assisted extraction techniques improve recovery of allergens from various soy matrices. The use of ultrasound assisted extraction and conventional extraction in combination with a stronger buffer like Laemmli, proved to be a very efficient extraction process especially for soy protein isolate and soy milk.
ELISA was also applied for determination of major mite allergens (18), gibberellin‐regulated allergen protein in orange (19), almond and hazelnut allergens (20), soybean allergen Gly m 4 (21), allergens from coconut pollen (22), protamine, the allergen from the milt of the large yellow croaker (Pseudosciaena crocea) (23).
Lu et al. identified the IgE binding proteins from soybean using sera from soy-allergic subjects and plasma from soy-sensitized subjects (24). In the procedure, proteins from gel were transferred to polyvinylidene difluoride membranes for immunoblots. The membranes were completely dried, fixed and stained. After the images, the membranes were rehydrated with methanol and blocked with 5% non-fat dry milk. Individual soybean allergic or non-allergic sera were diluted with soybean-specific IgE and incubated with the membranes overnight. Bound IgE was detected using monoclonal anti-human IgE. Bound anti-IgE was detected with SuperSignal West Dura chemiluminescent substrate (24).
IgE reactive recombinant egg white allergens expressed in Escherichia coli were determined by immunological analysis (25). In the investigations, produced IgE reactive recombinant egg allergens and their natural counterparts were compared. Egg white was separated from the egg yolk and was diluted with dH20. The diluted egg white was run on gels and blotted onto nitrocellulose. The blots were then cut into individual lane strips. The nitrocellulose strips with immobilized egg white proteins were then incubated with each diluted serum sample overnight at 4°C. Next, the strips were incubated with an anti-human IgE secondary antibody produced in a mouse. The detection was carried out using the WesternBreeze®Chromogenic kit.
SDS-PAGE were immunologically analyzed using egg yolk allergic patients’ sera (26). Western immunoassay performed with individual patients sera against crude egg yolk extract showed that multiple egg yolk proteins reacted with IgE from egg white allergic patients sera. In the study, the authors investigated whether sensitization to egg white is associated with reactivity to egg yolk as well. They suggest that the majority of patients sensitive to egg white are immunologically reactive towards yolk proteins as well. Therefore, in those patients, development of an allergy can be attributed to allergens derived from both the albumen and yolk. Furthermore, these results suggest that there are potentially undiscovered allergens within hens’ egg yolk. In the procedure, crude egg yolk in coating buffer was used to coat each well of an ELISA microtitre plate. After washing the wells with phosphate buffered saline with Tween 20, each well was blocked with bovine serum albumin in phosphate buffered saline for 2 h at 37°C. Next, the wells were triple washed with phosphate buffered saline with Tween-20, each coated well was incubated with control serum and serum pools which were pre-incubated with different amounts of rYGP42 for 1 h at room temperature. Following triple washing with phosphate buffered saline with Tween-20, all wells were incubated with monoclonal anti-human IgE mouse antibodies labelled with alkaline phosphatase for 1 h at 37°C. After washing the wells, alkaline phosphatase Yellow liquid substrate was added to each well and incubated at 37°C. After color development and addition of 3M NaOH the absorbance value was measured at 405 nm. A second ELISA was done on wells of the microtitre plate coated with rYGP42 protein in coating buffer. The test serum pool was pre-incubated in crude egg yolk. The absorbance was measured at 405 nm and the percentage of inhibition was calculated. Additionally, the IgE reactive protein bands were excised from a SDS-PAGE gel and determined by LC-MS/MS analysis to reveal their identity.
Preliminary investigations of egg allergens were also performed by ELISA comprising a monoclonal antibody generated via an analyte specific peptide antigen and sodium lauryl sulfate/sulfite solution (27). The aim of the investigation was to develop an egg allergen ELISA that can harmonize with LC-MS analysis. In the experiment a monoclonal antibody to an ovalbumin (OVA)-specific amino acid sequence was generated and developed by an ELISA combined with sodium lauryl sulfate (SDS)/sulfite reaction solution. Detection of OVA-specific tryptic peptide conforming to the target amino acid sequence of ELISA monoclonal antibody was detected by LC-MS/MS analysis. The comparison of LC-MS/MS and the new ELISA, which targets the amino acid sequence conforming to the LC-MS/MS detection peptide, showed a good compatibility.
For determination of allergens from Chinese shrimp (Penaeus chinensis), the IgE binding epitopes and critical amino acids of two major allergens were applied (28). The inhibitory dot-blot assay, indirect competition ELISA (icELISA), and LAD2 cell degranulation assay were used in the investigations to detect the binding affinity and antigenicity of the allergenic epitopes. The immunoinfo-CB method was applied to identify the critical amino acids of the confirmed allergenic epitopes. In the procedure, peptide icELISA, 100 μL × 0.5 mg/mL of tropomyosin or arginine kinase protein were coated on 96-well plates in carbonate buffer overnight at 4°C. After blocking with 3% bovine serum albumin/0.01 M pH 7.4 phosphate-buffered saline for 2 h at 37°C, the plates were incubated with individual serum samples and indicated peptide for 1.5 h at 37°C. Next, the plates were incubated with horseradish peroxidase goat antihuman IgE serum for 1.5 h at 37°C. The plates were then developed with tetramethylbenzidine substrate reagent, set in a dark environment for 15 min at 37°C and terminated by H2SO4. Absorbance was measured at 450 nm. Sandwich ELISA was used for quantification of Gly m 4, a soybean allergen (21). ELISA was carried out to determine the titers of goat sera, mouse sera, and the hybridoma cell lines.
Soy allergy is among the most common forms of food allergy. Soy allergens in food samples are currently detected in most cases using ELISAs based on antibodies raised against bulk soybean proteins or specifically targeting soybean trypsin inhibitor, conglycinin, or glycinin, but the results are often incorrect because the antibodies cross-react with other proteins. Ueberham et al. developed a monoclonal antibody-based sandwich ELISA targeting the soybean 2S albumin Gly m 8 soy allergen to detect traces of soy proteins in food samples (29). The various commercial ELISAs lack standardized reference material, and the results are often inaccurate because the antibodies cross-react with proteins from other legumes. Furthermore, the isolation of allergenic proteins involves laborious denaturing extraction conditions. The authors developed a novel sandwich ELISA based on monoclonal antibodies raised against the soybean 2S albumin Gly m 8 and a recombinant Gly m 8 reference protein with native-analogous characteristics. The antibodies do not cross-react with other legume proteins, and the extraordinary stability and solubility of Gly m 8 allows it to be extracted even from complex matrices after processing. In the ELISA procedure the capture antibody (mAb3) was immobilized onto 96-well plates carbonate buffer at 4°C overnight. The plates were washed three times with phosphate-buffered saline containing NaCl and Tween-20 and then blocked with Superblock blocking reagent at room temperature. The plates were then sealed, air-dried, shrink-wrapped, and stored at room temperature. In the next step, extracted samples were incubated for 10 min at room temperature, also in duplicate with Tween-20 and Superblock mixture. After three washes in Tween-20, the horseradish peroxidase-conjugated detection antibody (mAb8) was added, and the plates were incubated for 10 min at room temperature. Horseradish peroxidase activity was determined by incubating the plate with 3,3′,5,5′-tetramethylbenzidine substrate. The yellow color generated by treatment with sulfuric acid represented the quantity of bound detection antibodies and was measured at 450 nm. Surface plasmon resonance spectroscopy was used to detail the characterization of the activity and binding parameters of the antibodies. Application of this ELISA test, allows obtained LOD and LOQ values of Gly m 8 higher then 10 pg/mL and 65 pg/mL, respectively.
2-D immunoblot analysis was rarely applied for detection of allergens e.g., for determination of the catalase allergen from banana (30). For allergogram analysis banana protein extract was mixed with buffer containing Tris-HCl, pH 6.8, glycerol and bromphenolblue and incubated for 5 min at 95°C. Next, banana fruit proteins were resolved by 1-D SDS PAGE and then were electrotransferred onto a nitrocellulose membrane. The transfer was performed by using semi-dry transfer buffer containing 25 mM Tris, 192 mM glycine, 20% (v/v) methanol, 0.0375 (w/v) SDS, pH 8.3. After blocking with Tris buffered saline containing human serum albumin for 2 h at room temperature, IgE reactive proteins were detected by using individual sera of patients with suspected allergy to banana. The stripes were incubated with polyclonal goat anti-human IgE for 1 h, followed by 1 h of incubation with alkaline phosphatase-labeled polyclonal rabbit anti-goat IgG tertiary antibodies. Visualization of the reaction was achieved with 5-bromo-4-chloro-3-indolyl phosphate/4-nitroblue tetrazolium. IgE reactive banana protein species were determined in the wide range of molecular masses and isoelectric points.
ELISA was also applied for determination of coconut pollen allergens, lupine allergen, and milk protein residues in Cheddar cheese (22, 31, 32). The identification of IgE binding proteins is important to achieve a better understanding of allergens for allergy diagnosis, identification of possible immunotherapy reagents, and for risk assessment and risk management of products containing allergens. The multiplexing capacity of ELISA makes it highly effective in screening multiple allergenic foods such as peanuts and tree nuts.
However, ELISA-based methods for determination of allergens have some disadvantages. Antibody-based methods require the availability of either monoclonal or polyclonal antibodies, preferably both. Many antibodies are commercially available, however they are often weakly characterized. The performance of immunological methods such as ELISA can be unfavorably affected by issues of cross-reactivity, hook effects, and extensive food processing. A major reason for the variability of methods based on immuno tests are processing-induced modification of allergens and the physical form of allergenic ingredients together with the nature of the food matrix. Food processing or sample preparation can modify allergens, which then may not be recognized by the target antibody, leading to false-negative results. The various commercial ELISAs often lack standardized reference material, and the results can be inaccurate since the antibodies cross-react with other proteins. Quantitative results obtained for allergens analyzed in complex matrices by ELISAs sometimes exhibit differences which can arise due to limitations in protein extraction, lack of standard reference materials, variations in batch and cultivar sampling, or epitope modifications due to food processing (33). addition, ELISA-based methods are strongly limited in the detection of multiple allergens. Examples of application of ELISA for allergens determination are presented in Table 1.
Table 1.
Application of ELISA for allergen analysis
| Sample | Allergen | ELISA test | Determination | LOD | References |
|---|---|---|---|---|---|
|
Tropomyosin |
|
Quantification of topomyosin level | 0.09 ppb | (9) |
| Spice blend | Proteins from almond and peanut | AgraQuant Peanut and Almond ELISA test kits | Quantification of almond proteins and peanut proteins |
|
(11) |
| Cashew nut | Allergenic proteins |
|
Quantitative measure allergenic proteins from cashew nut | (16) | |
| Soy | Allergenic soy proteins | Neogen’s Veratox for Soy Allergen sandwich ELISA kit | Quantitative determination of total allergenic proteins from soy | 1.175 ppm | (17) |
| Soy | Gly m 4 | Sandwich ELISA for Gly m 4 | Gly m 4 (rGly m 4) protein | 2.1 ng/mL | (21) |
| Cumin | Peanut allergen proteins |
|
Quantification of peanut allergen proteins |
|
(10) |
|
|
Lupin residue from ELISA Systems Pty Ltd.; RIDASCREEN fast lupine from RBiopharm; AgraQuant lupine from Romer Labs® | Quantification of lupine allergen proteins | Different from various applied ELISA kit and kind of sample | (31) |
| Cheddar cheese | Milk allergen proteins |
|
Quantification of milk protein residues |
|
(32) |
| Roasted nut | Hazelnut and almond allergen proteins |
|
Quantification of hazelnut and almond allergen proteins |
|
(20) |
| Bakedfood samples | Casein, soy protein, and gluten | Veratox and BioKits ELISA kits | Quantification of casein, soy protein, and gluten | 1.6–25.6 ppm for casein, 1.25–25 ppm for soy protein, 5–80 ppm for gluten | (34) |
| Wheat, barley, rye, spelt, kamut, and einkorn | Gluten | R7021 ELISA kit | Quantification of gluten | From 5.5 ppm to >100ppm | (35) |
Detection of allergenic ingredients by DNA analysis
DNA-based methods offer an alternative to immunological methods. DNA-based methods do not analyze protein directly, but instead detect the gene which encodes for that protein. The DNA-based test involves the extraction of a specific allergen protein encoding fragment that is followed by amplification by PCR (33). The most frequently applied DNA-based methods are: PCR-ELISA; real-time PCR; PCR peptide nucleic acid HPLC; duplex PCR; and multiplex real-time PCR.
PCR
Nucleic acid-based PCR methods are often used for determination of allergens especially for qualitative and quantitative determination of food allergens. PCR is highly specific but may not be representative of the quantity of allergenic protein content of a sample.
Reverse transcriptase quantitative PCR (RT-qPCR) was applied for comparative analysis of allergen genes and pro-inflammatory factors in the pollen and fruit of apple varieties (36). Gene expression levels were analyzed by RT-qPCR, then applied with SYBR green based assays and specific primer pairs. The expression, production, and activation of sensitizing factors (specific allergens expression, ROS production, TGase, and PLA2 activity) were investigated in relation to the known allergenic potential of apple varieties (36).
Ladenburger et al. developed two competitive real-time PCR assays for the quantitative determination of trace amounts of two major food allergens, peanut and soybean based on competitive real-time PCR primer pairs and probes targeting species-specific mitochondrial sequences: atp6 in peanut and bait8 in soybean (37). In the procedure food samples were ground, aliquots were incubated with cetyltrimethylammonium bromide extraction buffer (2% cetyltrimethylammonium bromide, 1.4 M NaCl, 0.1 M Tris-HCl, and 20 mM EDTA; pH 8) for 90 min at 65°C with 0.6 mg proteinase K. The next samples were incubated and centrifuged. Supernatants were transferred to a mixture of chloroform and isoamylalcohol (24 + 1, v/v). After mixing, the samples were centrifuged and DNA containing aqueous phase were transferred into a reaction tube and mixed with isopropanol. After 30 min at room temperature, the DNA was precipitated by centrifugation. The supernatant was discarded and the resulting DNA pellet washed with 70% ethanol. After an additional centrifugation the supernatant was discarded again and the pellet air-dried at room temperature. The DNA pellet was resuspended in buffer containing 10mM Tris-HCl and 1 mM EDTA,(pH 8) supplemented with RNase A for 60 min at 50°C. The DNA extracts were purified on a column. After purification samples were eluted with elution buffer and the concentration of the DNA was determined by spectrometry and analyzed by competitive real-time PCR. Amplification reactions were performed with SsoAdvanced Universal Probes Supermix template and each primer pair. Coamplification of competitor DNA occurred in the presence of equimolar concentrations of competitor probes. Real-time PCR reactions were run on a CFX96 real-time PCR System with an initial denaturation step at 95°C for 3 min, followed by 45 two-step cycles at 95°C for 15 s and 60°C for 30 s. Relative fluorescence signals were recorded after each cycle. The higher detection sensitivity was obtained by determination of targeting mitochondrial DNA sequences for allergen detection compared to nuclear DNA determination. It was achieved by the fact that there are more mitochondrial DNA copies than nuclear DNA copies per cell can be obtained.
A real-time PCR method was also applied for detection of lupine in four different highly processed model foods (bread, biscuits, rice patties, and noodles) (14). Results obtained by real-time PCR were compared with those obtained by ELISA sandwich. The sensitivity of the methods was significantly different; lupine was detected in eight out of the 25 investigated products with the sandwich ELISA but not by real-time PCR (14). By serially diluting the lupine DNA extract the LOD of 20 pg/lL, corresponding to an amount of 100 pg lupine DNA was determined. The LODs obtained in the investigations by real-time PCR were significantly higher than those of the ELISAs especially sandwich ELISA. For this reason, the real-time PCR method targeting a sequence of the gene coding for the tRNA nucleotidyltransferase (CCA1) mRNA, was not recommended by authors for detecting traces of lupine in processed foods.
Various food allergens (almond, cashew, hazelnut, peanut, pistachio, and walnut) in cumin were determined by PCR (10). The DNA-based analysis consisted of a combination of gene-specific methods, such as PCR, real-time PCR, and Sanger sequencing using universal plant primers. Using PCR analysis, the peanut genes Ara h 1, Ara h 2, and Ara h 3 were determined in cumin samples, with the Ara h 3 amplicons confirmed by sequencing. LOQ obtained through the PCR method was 4 mg/kg. Sometimes inconsistencies among the various PCR analyses were observed. The authors explained that these inconsistencies were due to differences in assay sensitivity and/or primer sets that are not suitable for all nut varieties.
A multiplex PCR (MPCR) assay combined with capillary electrophoresis (CE) was developed for simultaneously detecting ten common food allergens from hazelnut, pistachio, oat, sesame, peanut, cashew, barley, wheat, soybean, and pecan (Figure 3) (38). The procedure of the MPCR method was applied for determination of allergens in 20 commercial food products, including cake, cookies, crackers, waffles, cocktail nuts, Quaker rolled oats, fruit juice, noodles, pistachios, chocolate, mixed nuts, milk, candy, soy milk, and powdered beef soup. The authors concluded that this MPCR assay could be used for routine simultaneous detection of multiple food allergens in various matrices. Prior to analysis, DNA was extracted and purified with a Plant Genomic DNA Isolation Kit according to the manufacturer’s instructions. Food allergens were tested and MPCR primers were designed based on published DNA sequences of selected allergen genes. First, simplex PCR amplification of each allergen was performed to check primer specificity and sequence authenticity of amplified target DNA. Next, the expected PCR amplicons of target allergen genes by simplex PCR were sequenced to confirm sequence authenticity. Subsequently, the final optimized multiplex PCR was carried out which contained PCR buffer, template DNA, and ten pairs of primers (pistachio, pecan, hazelnut, sesame, wheat, soybean, cashew, oat, peanut, and barley) at different concentrations. The multiplex PCR was performed with the following program: denaturation at 95°C for 10 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s; one final extension step at 72°C for 10 min. After amplification, 1 lL PCR product was examined using Aglient 2100 Bioanalyzer and Agilent DNA 1000 Reagents.
Figure 3.
Simultaneous detection of 10 food allergens by optimized decaplex PCR assay using mixed genomic DNA as template (2000 copies haploid genome of each allergen). Peak 1: Hazelnut; Peak 2: Pistachio; Peak 3: Oat; Peak 4: Sesame; Peak 5: Peanut; Peak 6: Cashew; Peak 7: Barley; Peak 8: Wheat; Peak 9: Soybean; Peak 10: Pecan (38). Reprinted with permission from Cheng, F., Wu, J., Zhang, J., Pan, A., Quan, S., Zhang, D., Kim, H.Y., Li, X., Zhou, S., Yang, L. (2016) Food Chem. 199, 799–808. Copyright Elsevier.
Multiplex real-time PCR is a variant of real-time PCR which enables amplification and quantification of multiple targets in one reaction using more than one pair of primers/probes. Compared to singleplex real-time PCR systems, multiplex real-time PCR assays offered multiple target detection in a single assay platform. A multiplex real-time PCR assay was applied for determination of citrus fruit allergens and for monitoring of the expression of citrus allergen genes (39). First, four optimal singleplex systems were established by selecting the primer and probe concentrations with a suitable amplification efficiency. Total RNA was isolated from plant materials (peels and pulps) and applied with Trizol reagent. Contaminating DNA was digested with DNase I. Total RNA was reverse transcribed with random hexamers using a Prime-Script First-Strand cDNA Synthesis Kit. The information on sweet orange allergens was obtained from the WHO-IUIS database. To identify new members of citrus allergen gene families, in silico analysis of the genome information, nucleotide, and protein sequences of citrus allergens were used to blast the sweet orange genome based on calculating the statistical significance of matches and protein domains were also an important determinant for new citrus allergens since they contained Cupin_1 domain, Profilin domain, and Tryp_alpha_amyl domain, respectively. TaqMan real-time PCR reactions were performed by an Applied Biosystems 7500 Real Time PCR System with application AceQ U+ Probe Master Mix containing heat-labile uracil−DNA glycosylase. The same primer sequences for the singleplex reactions were used for the multiplex PCR. Similar LOD values were obtained in simple and multiplex real-time PCR. LOD values obtained by both methods for primer Cit s 1.01 were 200 fg and for primers Cit s 2.01 and Cit s 3.01 were 20 fg. Reverse transcription PCR (RT-PCR) using One Step RT-PCR kit was performed for egg yolk allergens (26).
PCR is sensitive and can be used for the determination of allergens in trace concentrations. Real-time PCR is a fast, highly sensitive, and reproducible technique to study allergen gene expression. Recent developments of nucleic acid-based biosensors, their miniaturization, and increasing application of nanotechnology, has significantly supported further application of the strategies. However, lack of specificity and cross-reaction of some antibodies can be an important source of false results. The interference and enzyme inhibitors (giving rise to false negatives) and poor DNA recovery from samples can be adversely affected on the performance of PCR-based methods. Also, it is not possible to trace protein allergen epitopes with post-translational modifications and their changes arising during food processing using the method. Additionally, PCR is not tissue specific.
Biosensors and Chips
Another technology in allergen analysis is the application of biosensors consisting of an integrated receptor-transducer device, which is capable of providing selective quantitative or semi-quantitative information using a biological recognition element. Biosensors are an attractive alternative to traditional immunoassay methods offering comparable sensitivities and selectivity while allowing for on-site detection. A number of papers have reported the use of biosensors based on quartz crystal microbalance (QCM), surface plasmon resonance (SPR), and electrochemical sensors (40–42). SPR biosensors are based on a special mode of metal-dielectric waveguides, the surface plasmon, to measure the refractive index changes caused by the interaction of biomolecules with the surface of SPR biosensors. The surface plasmons are delocalized electron oscillations, which exist at the interface of a metal-dielectric medium. SPR is a highly sensitive optical sensing technology relying on the interactions of light with the free electrons in a semi-transparent noble metallic layer or chip and can realize the real-time monitoring of small changes in the effective refractive index of a metal-dielectric interface. molecule capable of hybridizing with an allergen-specific DNA fragment. Biosensors allow for the real-time detection of compounds by interact with an immobilized target molecule. This target molecule can be an antibody raised against an allergen or a single stranded DNA. DNA biosensors containing the immobilization of a DNA probe onto the transducer surface. Biosensors contain a biological recognition element (biochemical receptor) in direct spatial contact with a transducer, which converts the recognition event into a measurable chemical or physical signal, connected to a data acquisition and processing system. Biosensors contain a biological recognition component, a signal transduction (and signal amplification) device, that is connected to a computer for both data acquisition and processing. The reaction between the target and sensing molecule can be further sensed and amplified (33, 40, 42). The most frequently used amplification methods are nanomaterial-enhanced, enzyme based, and DNA-based amplifications (33, 42). Other methods often used for detection are based on voltammetry, amperometry, electrochemiluminescence, photoelectrochemistry, and impedance. Biosensors are an attractive alternative to traditional immunoassay methods offering comparable sensitivities and selectivity while allowing for on-site detection.
Based on a combination of an amperometric transduction, magnetic particles, and disposable screen-printed electrodes, a shrimp tropomyosin immunosensor having a sandwich structure composed of a capture antibody, a detection antibody and an horseradish peroxidase (HRP)-labeled secondary antibody has been developed and optimized for detection of trace amounts of shrimp (41). The sensor is based on the implementation of a sandwich immunoassay format on the surface of magnetic beads (MBs) and their coupling onto disposable screen- printed electrodes to register the amperometric response at 200mV vs Ag pseudo-reference electrode. H2O2 was applied as the enzymatic substrate and hydroquinone was used as the redox mediator. All the steps involved in the specific capture and labelling of the shrimp tropomyosin were performed with magnetic microbeads or in-house made magnetic nanoparticles, using a sandwich format. In the sensor, HOOC-modified magnetic particles were activated through carbodiimide/N-hydroxysuccinimide to covalently bind polyclonal rabbit anti shrimp tropomyosin capture antibodies; the unbound active sites were blocked using ethanolamine hydrochloride to avoid any succeeding nonspecific binding events. These functionalized magnetic microbeads and magnetic nanoparticles were then applied to specifically catch shrimp tropomyosin; finally, bound shrimp tropomyosin was sandwiched through a detector shrimp tropomyosin antibody and a secondary labeled antibody. The magnetic microbeads or magnetic nanoparticles with the sandwich immunocomplexes were transferred to the surface of the working electrode of the sensing electrodes to perform the amperometric detection upon the addition of the H2O2 enzymatic substrate.
For the detection of a-casein in rinse water samples of cleaning in place systems of food manufacturers an immunoassay based a surface plasmon resonance (SPR) sensor chip consisting of four sensing arrays was developed. The sensor allows for the measurement of samples and control allergen binding events simultaneously (42). Caseins are a significant fraction of milk protein and the detection of α-casein would be a useful marker for monitoring levels of milk during the cleaning-in-place system. A sensor chip was used to immobilize an α-casein-polyclonal antibody using an 1-ethyl-3-(3-dimethylaminopropyl)car-bodiimide/N-hydro-xysuccinimide coupling procedure. The sensor exhibited sub ppm sensitivity and good selectivity for α-casein detection. LOD obtained by application of the sensor was equal to 57.80 ng/ML, while LOD for commercially available ELISA kit is 2.5 ppm.
Hideshima et al. proposed a label-free field effect transistor (FET)-based biosensing system for the detection of a buckwheat allergenic protein, BWp16, by surfactant-induced signal amplification (43). BWp16 was detected by coupling with an anionic surfactant, sodium dodecyl sulfate use to receive the signal amplification. This coupling enhanced the net charge of the protein enough to be detected by FET biosensors (43). The conception of the signal amplification is based on the increase of total charges of target molecules by charged additives within the charge-detectable region for FET-based detection. Coupling BWp16 with SDS molecules is an effective way to induce enough additional charges to be detected by FET biosensors. The optimal SDS concentration for coupling with the target protein was optimized and was found to be 1% (w/w). The coupling of SDS molecules was verified by application of fluorescence spectroscopy. Authors observed a significant response when the allergen was coupled with SDS, while the responses were decreased or unchanged when it was coupled with a cationic or non-ionic surfactant. It indicating that the SDS coupling supports the antibody recognition ability of the target allergen. For this the application of SDS can be useful to increase the sensor responses. The application of the sensor with the signal amplification obtained LOD = 10 ng/mL.
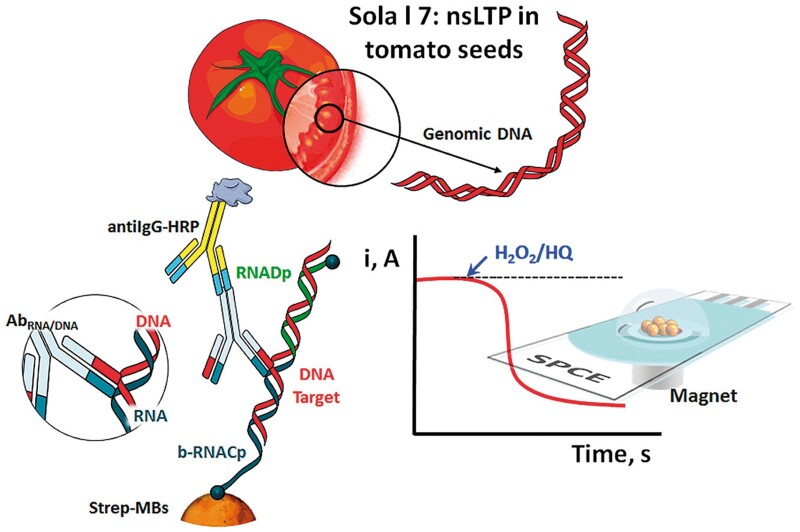
An electrochemical nucleic acid biosensor was applied for the sensitive and selective determination of PCR-free and selective detection of the Sola l 7 allergen by targeting a 60-mer specific fragment of its coding sequence in tomato seeds (44). A schematic display of the fundamentals involved in the nucleic acid biosensing platform prepared for the determination of a specific fragment of the Sola l 7 allergen coding sequence is presented in Figure 4. In the biosensor, DNA/RNA heterohybrids by sandwich hybridization of a specific fragment of the Sola l 7 allergen were formed. Labeling was performed with commercial antibodies specific to the heteroduplexes and secondary antibodies conjugated with HRP onto the surface of magnetic beads. Amperometric transduction was carried out upon magnetic capture of the resulting magnetic bioconjugates on screen-printed electrodes using the system H2O2/HQ. The biosensor offers the possibility of tailoring the sensitivity by varying the bioassay format, heterohybrid length, or labeling strategy using new low-cost, simple to fabricate, disposable, electrochemical mast cell-based paper.
Figure 4.
Schematic display of the magnetic beads (MBs)-based amperometric biosensing strategy developed at surface plasmon coupled emissions (SPCEs) for targeting a specific fragment of the Sola l 7 allergen coding sequence (44). Reprinted with permission from Pereira-Barros, M.A., Barroso, M.F., Martín-Pedraza, L., Vargas, E., Benedé, S., Villalba, M., Rocha, J.M., Campuzano, S., Pingarrón, J.M. (2019) Biosens. Bioelectron. 137, 171–177. Copyright Elsevier.
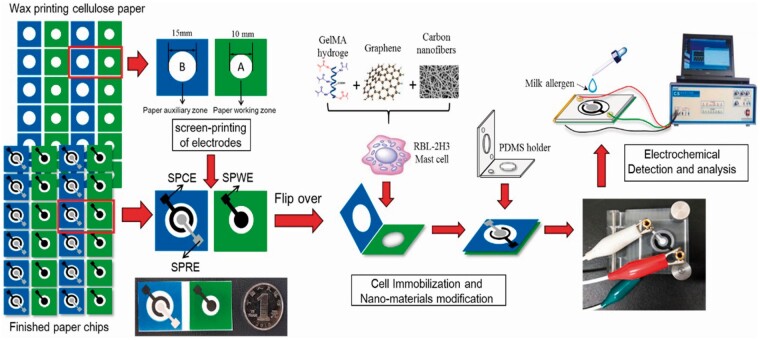
Jiang et al. developed an electrochemical mast cell sensor to determine the major milk allergen casein (45). In the sensor, carbon nanofiber/graphene-modified screen-printed electrodes formed an effective cell immobilization layer and allowed the immobilized mast cells to have high stability and bioactivity. The casein antibody-sensitized mast cells were immobilized on the paper fibers through the biological affinity of the Gelatin methacryloyl hydrogel. The sensor exhibited an irreversible voltammetric response related to the response of mast cells to the allergen and the peak current showed a positive relationship with the concentration of casein. Schematic representation of the fabrication and procedure of the electrochemical mast cell-based paper sensor is presented in Figure 5. The LOD obtained by application of the sensor for determination of casein was 3.2 × 10−8 g/mL.
Figure 5.
Schematic representation of the fabrication and assay procedure of the electrochemical mast cell-based paper sensor. Paper sheets were firstly patterned in bulk using a wax printer. After baking, three electrodes were screen-printed on wax-patterned sheet. The reference electrode and counter electrode were printed on the B zone, while the working electrode was printed on the A zone. The prepared sheet was cut to rectangular paper. After nano-materials modification and mast cell immobilization, the rectangular paper was folded and integrated with a device-holder for electrochemical assay (45). Reprinted with permission from Biosens. Bioelectron. 130, 299–306. Copyright Elsevier.
An elelectrochemical rat basophilic leukemia cell (RBL-2H3) cell sensor, based on fluorescent magnetic beads, has been developed for the determination of different allergens in foodstuffs (46). In the sensor, fluorescein isothiocyanate was fused inside the SiO2 layer of SiO2 shell-coated Fe3O4 nanoparticles. The as-synthesized fluorescent magnetic beads were then encapsulated with lipidosome to form cationic magnetic fluorescent nanoparticles for mast cell magnetofection. The cationic magnetic fluorescent nanoparticles were then transfected into rat basophilic leukemia cell (RBL-2H3) using a highly efficient, lipid-mediated magnetofection procedure. A magnetic glassy carbon electrode was then employed to adsorb the cationic magnetic fluorescent nanoparticles transfected RBL-2H3 cells activated by an allergen antigen for electrochemical assay. To demonstrate the utility of this mast cell-based biosensor for detection of real allergens in foodstuffs, Anti-Pena1IgE an dAnti-PV IgE activated cells were employed to quantify both shrimp allergen tropomyosin (Pena1) and fish allergen parvalbumin. The sensor can be applied for determination of different allergens in turn, including shrimp allergen Pena1, soybean allergen β-conglycinin, and peanut allergen Arah1A. A model antigen–dinitrophenol–bovine serum albumin was determined by application of the sensor in concentrations, ranging from 1 × 10−3 to 10 ng/mL, at a detection limit of 3.3 × 10−4 ng/mL. The method was also successfully used for quantification of fish PV allergen with a detection limit of 0.16 ng/mL and a shrimp allergen Pen a with a detection limit at 0.03 μg/mL.
A miniaturized silicon-based sensor chip combined with an advanced microfluidic module for the simultaneous, label-free immunochemical determination of four allergens, bovine milk protein, peanut protein, soy protein, and gliadin was applied (47). The sensor chip consists of an array of 10 broad-band Mach−Zehnder interferometers (BB-MZIs) integrated on silicon, along with their respective broad-band light sources. The BB-MZIs were biofunctionalized with the targeted allergens and their responses during immunoreaction were monitored by multiplexing their transmission spectra through an external miniaturized spectrometer. The analysis was performed by running mixtures of calibrators or samples with the antibodies against the four allergens followed by an antispecies specific antibodies solution. The detection limits obtained by application of the sensor were 0.04 μg/mL for bovine k-casein, 1.0 μg/mL for peanut protein, 0.80 μg/mL for soy protein, and 0.10 μg/mL for gliadin.
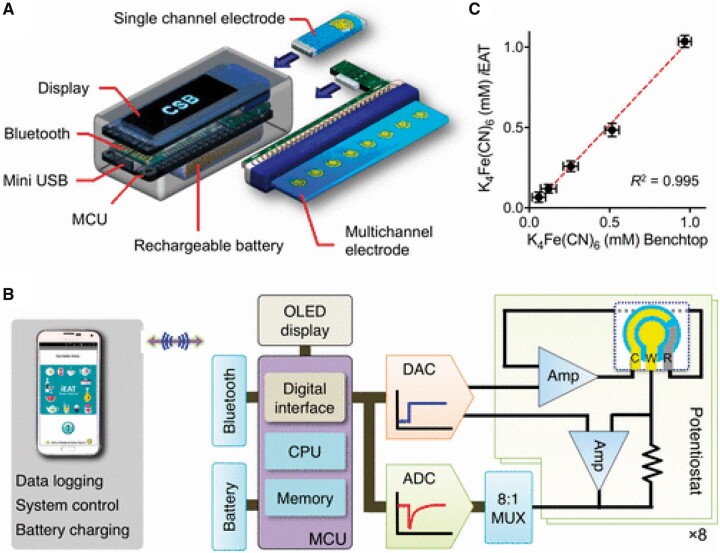
A magneto-chemical sensor was developed for on-site food allergen detection (48). A portable, point-of-use detection technology, termed integrated exogenous antigen testing (iEAT) was applied. The system consists of a disposable antigen extraction device coupled with an electronic keychain reader for rapid sensing and communication. Figure 6A shows the portable iEAT system comprising a keychain reader, an extraction kit, and a smartphone app. Figure 6B presents the details of the iEAT reader, Figure 6C the correlation of results obtained by ELISA and iEAT. The prototype iEAT system was optimized to detect five major food antigens in peanuts, hazelnuts, wheat, milk, and eggs. Using the magneto-chemical sensor, the following LOD values were obtained: 0.075 mg/kg for gliadin, 0.007 mg/kg for Ara h1, 0.089 mg/kg for Cor a1, 0.170 mg/kg for casein and 0.003 mg/kg for ovalbumin.
Figure 6.
iEAT reader. (A) System schematic. This pocket-size reader is designed for standalone operation with its own display, rechargeable battery, and wireless communication module. A single electrode or multiple electrodes are connected to the reader through a card-edge connector; the device automatically identifies the electrode type and configures the detection mode accordingly. (B) Functional blocks in the iEAT reader. Electrical currents are measured by potentiostats and digitized. The microcontroller unit (MCU) converts current levels to allergen concentrations according to preloaded lookup tables. The MCU also communicates with a smartphone to provide an extended user interface and to wirelessly charge the battery. ADC, analog-to-digital converter; DAC, digital-to-analog converter; Amp, amplifier; OLED, organic light-emitting diode; MUX, multiplexer. (C) Both the iEAT reader and a commercial electrochemical system were used to measure buffer solutions with varying concentrations of ferrocyanide in 0.1 M KCl solution. The correlation between the two systems’ performance was 0.995 (48). Reprinted with permission from Lin, H.-Y., Huang, C.-H., Park, J., Pathania, D., Castro, C.M., Fasano, A., Weissleder, R., Lee, H. (2017) ACS Nano 11, 10062–10069. Copyright American Chemical Society.
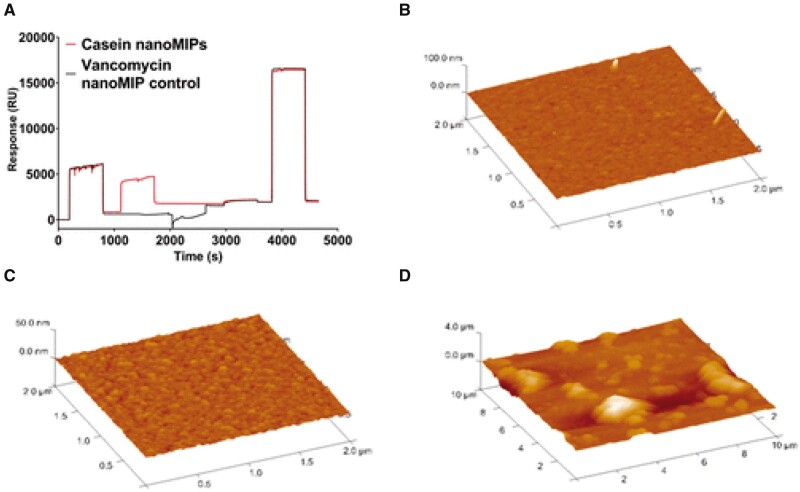
Molecularly imprinted polymer nanoparticles (nanoMIPs) α-casein detection using surface plasmon resonance (SPR) was successfully applied for a milk allergen sensor (49). NanoMIPs with a high affinity toward bovine α-casein were synthesized using a solid-phase imprinting method. The nanoMIPs were then characterized and incorporated into a label-free surface plasmon resonance (SPR) based sensor. In the described method, the protein was attached to the glass beads via an amine coupling reaction. NanoMIPs were synthesized with a secondary monomer containing a primary amine group which were eluted with adequate yields and diameters. Molecularly-imprinted polymer nanoparticles in biosensors are thermally stable, exhibit comparable binding kinetics, can exist in a wide pH range, and have a low cost. The detection limit obtained by the sensor for determination of α-casein was 127 ± 97.6 ng/mL. An example SPR sensorgram showing the immobilization of casein nanoMIPs and the control nanoMIP on the sensor surface is presented in Figure 7, with Atomic Force Microscopy (AFM) of (B) bare gold, (C) self-assembled monolayer (SAM) monolayer, and (D) covalently attached nanoMIPs.
Figure 7.
(A) SPR sensorgram showing the immobilization of casein nanoMIPs and the control nanoMIP on the sensor surface. atomic force microscope (AFM) of (B) bare gold, (C) Self-assembled monolayers (SAM) monolayer, and (D) covalently attached nanoMIPs (49). Reprinted with permission from Ashley, J., Shukor, Y., D’Aurelio, R., Trinh, L., Rodgers, T.L. Temblay, J., Pleasants, M., Tothill, I.E. (2018) ACS Sens. 3, 418–424. Copyright American Chemical Society.
Most sensors offer several advantages in terms of label-free detection, real-time measurements, and superior sensitivity compared to ELISA-based techniques. The application of functionalized magnetic microbeads in sensors allows the selective determination of the allergen concentration directly in complex matrices, without complicated sample preparation steps. However, biosensors which use antibody-based receptors can exhibit problems in terms of their limited shelf-lives and instability to testing conditions.
Detection of Allergens with Application of MS and MS/MS
A number of immunochemical-based methods have been developed for allergen detection. Commonly used methods for allergen analysis are antibody-based assays, although some drawbacks are encountered such as matrix/processing effects and epitope masking especially when dealing with products such as cookies, biscuits, and chocolate and other extensively processed foods. These contain complex matrices usually containing e.g., fats, carbohydrates, proteins, minerals, and other compounds that are known to interact with one another and influence the properties of allergens. The disadvantages of current immunological methods for allergen analyses are numerous, hence alternatives have been investigated in recent years. In particular, nonimmunological methods have been investigated and developed to overcome the drawbacks of ELISA (3). In the recent past, MS techniques have been successfully developed and applied for detection of allergens. An advantage of MS is that it confirms the presence of protein in investigated samples. As far as MS-based allergen detection is concerned, two methodological options are currently available: detection of the intact protein representative of the allergenic ingredient which is usually the most abundant in the proteomic profile, and detection of the target analytes—namely markers that are signature peptides, properly selected, resulting from the enzymatic digestion of the whole allergenic component (50). MS-based procedures are limited by the application of mass analyzers and the size of the protein or charge state.
The sensitivity and specificity of an ELISA can depend on the 3-D structure of allergens, whereas MS is based on the structurally independent amino acid sequence. Post-translational modifications can therefore have a huge impact on allergenicity of the protein, which can lead to false-positive or false-negative results in ELISA techniques. High resolving power and sensitivity coupled to this independence from structural changes allows MS to detect allergens in trace amounts (3). A crucial aspect of MS methods is the selection of proteotypic peptides that act as markers for the presence of the allergenic protein. Such peptide markers must be specific to the particular allergenic source and preferably originate from a recognized allergen molecule (1).
Through the performance offered by the latest generation of mass analyzers, new efforts have been situated on the development of MS methods capable of delivering both qualitative and quantitative information about allergenic proteins.
All mass spectrometers are composed of three different parts: ion source, mass analyzer, and detector. For the ion source, matrix-assisted laser desorption/ionization (MALDI) or electrospray ionization (ESI) are applied commonly. For the mass analyzer, time-of-flight (TOF) and ion trap (IT) are most often applied in MS used for allergen detection (3). Different coupling with various mass analyzers are available such as electrospray ionization-ion trap (ESI-IT), electrospray ionization-quadrupole time-of-flight (ESI-qTOF), or MALDI-TOF. Quantitative triple quadrupole and IT systems have the advantage of identification and quantification through fragmentation settings in the MS collision cell. The parallel reaction monitoring (PRM) option available on quadrupole-orbitrap (Q-Orbitrap) MS equipment acquires a full MS/MS fragmentation spectrum for a specific parent ion, providing the simultaneous monitoring of all the product ions at high accuracy and resolving power. The inherently high resolution of MS instruments combined with pairing of parent and fragment ion detection leads to the potential for high specificity (3).
MS methods based on selection reaction monitoring (SRM) offer an alternative to ELISA and PCR for determination of allergens. Such methods have the multiplexing capacity required for a multiallergen screening tool and the potential to provide a rigorous, orthogonal reference method for allergen analysis.
Before conducting MS analysis of allergens, allergen proteins are extracted from matrices and subsequently the digestion of the allergenic protein is most often performed. The conditions for the digestion process depend on the individual allergen structure. Optimally, complete digestion should be achieved in a very short time with a maximum of peptides generated and without missed cleavage sites.
One of the most efficient MS-based methods for allergen identification is based on electrophoresis separation (one- or two-dimensional) followed by western blotting detection using sera from allergic patients, combined with an additional gel used for immune-reactive proteins characterization. Digestion of the allergenic protein is commonly performed prior to MS analysis following the bottom-up strategy. Digestion cleaves large proteins onto smaller peptides, and thus potential matrix interferences and associated interactions with other proteins are reduced. These reductions remove complicating factors and make the analysis with LC−MS more reproducible. Optimally, a complete digestion is performed in a very short time with a maximum of peptides generated and without missed cleavage sites. The peptides generated should be stable and easily detected by MS (3). In the next step of experiments, allergens are determined by MS, MS/MS, or chromatographic techniques coupled with MS or MS/MS. MS/MS analysis (e.g., MALDI-TOF/TOF or ESI-MS/MS) determines structural information related to the sequence of peptides, rather than only their mass, making the search highly specific (3).
For analysis of allergens from sunflower (Helianthus annuus L.), spots from 2-D gel electrophoresis (2-DE) corresponding to the IgE reactive spots on a 2 D blot, were excised and subjected to in-gel trypsin digestion for sample preparation (51). After appropriate extraction procedure including gel trypsin digestion of spots from 2 D gel corresponding to the IgE reactive spots on 2 D blot samples were analysed by MALDI-TOF/TOF in positive ion mode (51). MS/MS spectra were acquired with a minimum of 4000 and a maximum of 8000 laser shots applied through the instrument calibration file. MALDI-TOF/TOF operated in positive ion mode was also applied for identification of allergens from Curvularia pallescens (52). In the procedure, before MS detection allergen proteins were separated by SDS-PAGE and 2D-PAGE. Following that, corresponding immunoblots were performed to detect IgE reaction.
Triosephosphate isomerase as an allergen in Octopus fangsiao was separated from matrix by SDS-PAGE or by 2-DE (53). After separation and purification the allergen was determined by MALDI TOF-MS (53).
MS methods based on selection reaction monitoring offer an alternative to ELISA and PCR for allergen analysis. The advantages of MS are ease of sample preparation, rapid analysis, and the ability to analyze many allergens simultaneously. An important advantage in analysis of peptides by MS is the excellent predictability of peptide fragmentation depending on the applied fragmentation mechanism. MS-based methods have the multiplexing capacity required for a multi-allergen screening tool and the potential to provide a rigorous, orthogonal reference method for allergen determination. Regarding accuracy, reliability, and sensitivity, mass spectrometry-based strategies bring important advantage over immunological and nucleic acid-based methods. MS is especially appropriate for allergen analysis in complex samples such as food matrices. The majority of published targeted MS-based allergen detection methods use QqQ instrumentation and make use of multiple-reaction monitoring (MRM) experiments for specific peptide identification and quantitation (54).
Recent developments of new high-resolution instruments have enabled advancement in MS-based methods for allergen analysis. MALDI-TOF-MS has been used in numerous procedures for the determination of food allergens, generally after one- or two-dimensional separation of proteins by gel electrophoresis. To enhance sensitivity and specificity, triple-stage MS (MS3)has been developed. This method is, in principle, available on QTrap instruments in which the third quadrupole necessary for the detection of fragment ions is exchanged for a quadrupole linear ion trap (LIT) (54). This allows for the accumulation and secondary fragmentation of ions in the linear trap. Accumulation of fragment ions in the LIT results in increased sensitivity, whereas the possibility of a secondary fragmentation experiment increases the specificity of the method (54). In the procedure, intact proteins were analyzed on a Q-TOF instrument, and sequence data were obtained after chymotryptic digestion in a bottom-up proteomics experiment. Obtained results were applied for allergen monitoring in food samples. A significant increase in sensitivity of multiple reaction monitoring cubed (MRM3) compared to MRM was observed (55). MS combined with appropriate sample preparation, liquid or gas chromatography and/or different electrophoretic methods brings an additional strategic advance. However, the MS analysis of allergen proteins requires a step of protein digestion to generate peptides for their detection by MS. The development of MS for allergen analysis should take into consideration the properties of the individual proteins and kind of matrices.
Chromatographic Methods Coupled with Different Detection Techniques
In chromatography the components in the investigated mixture are separated based on their relative affinity to the two phases. Molecules having a greater affinity to the stationary phase travel slower than the ones with lesser affinity. The separated molecules are then compared to known standards and identified. Chromatographic techniques especially coupled with MS or MS/MS detection are increasingly applied for allergen analysis.
Thin layer chromatography (TLC)
TLC is rarely applied for analysis of allergens. In TLC, different solvent systems and various stationary phases in a large range of polarity can be applied. Proteins in milk were separated by TLC on HPTLC silica gel plates with mobile phase containing 2-butanol–pyridine−ammonia–ddH2O (39:20:10:31; v/v/v/v), cellulose plates with the same mixture consisting of 2-butanol– pyridine–ammonia–ddH2O (32:30:11:25; v/v/v/v), C18 plates mixture of acetonitrile–TFA– ddH2O (50:3.75:46.25; v/v/v) (56). For protein/peptide specific derivatization, ninhydrin was used on cellulose stationary phase, while on reversed-phase (RP) and silica gel plates fluorescamine was applied. As an extension to an all-encompassing protein staining, a specific detection of single proteins using antibodies was developed. The first antibody possesses affinity towards the target protein and was applied following the initial blocking step. The secondary antibody was labeled with an enzyme (HRP) that exerts a reaction on the chromogenic substrate and therefore visualizes the first bound antibody. Following the chromatographic separation and evaporation of the mobile phase, the HPTLC plate was transferred into a small vessel. In order to inhibit unspecific binding of the antibodies to the surface, the plate was initially incubated with Tween20 as blocking reagent, containing Tween20, sodium chloride, and tris(hydroxymethyl)-aminomethane. After incubation the plates with blocking solution (twices for 15 min), blocking solution was removed and replaced by the primary antibody solution in Tris buffer. The plate was incubated for 2 h with primary antibodies and washed with buffer. Coating with the secondary antibody was performed for 1 h and washing. Prior to detection, the pH value was lowered by incubation of the plate for 1 min in a solution consisting of tris-HCl (pH 6.0). Finally, the detection was performed by incubating the plate in a dying solution containing 3,3′,5,5′-tetramethylbenzidine, dioctyl sulfosuccinate sodium salt, citric acid monohydrate, sodium hydrogen phosphate dihydrate, ethanol, and dihydrogen dioxide. The conducted incubation using the detection medium was carried out until blue bands appeared on a white background. Subsequently, the analytes were detected in white light and documented using a photodocumentation system. The procedure of HPTLC separation with the immunological detection of antigens was applied for analysis of allergens in real food samples (a commercially available milk powder and a wine-fining agent based on milk) (56).
HPLC and ultrahigh performance liquid chromatography (UPLC)
HPLC coupled with MS (LC-MS) and especially MS/MS (LC-MS/MS), have the ability to determine the protein sequence level. Chromatographic methods coupled with MS/MS are also able to provide relative or absolute quantitative data, which is particularly valuable in allergen protein analysis. The multiplexing capability of the method is especially attractive for multi-allergen detection given the increased complexity and diversity of complex matrices. Many reports on LC-MS and LC-MS/MS allergen analysis methods for individual food allergens have been published for the analysis of egg, milk, walnuts, peanut, and tree nut and various allergens in different matrices (57–59).
RP system on octadecyl (C18) stationary phase for analysis of allergens
RP system containing organic modifiers, water, and formic or trifluoroacetic acid
Most often acetonitrile or definitely less often methanol were applied as organic modifiers in RP system for analysis of allergens. The addition of a small amount of formic or acetic acid, or less rarely trifluoroacetic acid as components of mobile phase supports the ionization of ESI in positive mode (most often used in the procedures for allergen analysis) due to increased tendency to protonate analytes.
Daly et al. analyzed almond and peanut allergens in raw commodity as well as in processed products, such as chocolate, biscuits, snacks, and crackers, by LC- MS/MS on a peptide column with mobile phase containing acetonitrile, water, and formic acid (11). For separation of proteins, gradient elution was applied. For detection, a Q-Orbitrap mass spectrometer with a turbo spray ESI source operated in positive ionization mode was used. The LODs obtained by the procedure for almond peptides (GNLDFVQPPR and ALPDEVLANAYQISR) was 0.15 ppm. For peanut peptide GTGNLELVAVR, the LOD was 0.05 ppm andfor peanut peptide RPFYSNAPQEIFIQQGR the LOD was 0.5 ppm. LOQ for almond peptides (GNLDFVQPPR and ALPDEVLANAYQISR) was 0.5 ppm, for peanut peptide GTGNLELVAVR 0.15 ppm, and for peanut peptide RPFYSNAPQEIFIQQGR 1.0 ppm (11).
UPLC-MS/MS was applied for quantification of allergens from roasted walnuts (57). A UPLC system was connected with Orbitrap MS for detection of analytes. Additionally, the method was applied to confirm the presence of Maillard-type adducts on the walnut allergens (57). The LC-MS/MS data analysis incorporated label-free quantification of relevant allergens and Maillard adduct screening. The MS analysis results presented in this study revealed information about the molecular-level effects of roasting on walnut allergens. The authors also concluded that the LC-MS/MS detection and quantification of allergens from roasted walnuts was affected differentially depending on the individual allergen in question, the degree of heat treatment, and the sample preparation method (57).
Milk protein allergens from processed food were determined by LC-MS/MS on a ProtID-Chip-150 column (58). For LC-MS/MS analysis Q-TOF mass spectrometer and for targeted analytes. LC-MS/MS quantification a triple quadrupole mass spectrometer were applied in the method. Additionally, authors described a method for the extraction of food allergen proteins from solid matrices based on proteolytic digestion. The performance of the method was compared with the performance of methods reported in the literature. (58). Boo et al. simultaneously detected and quantified allergens of egg, milk, and peanuts in sugar cookies by UPLC-MS/MS (59). In the described investigations, extraction, concentration, and digestion of samples for candidate peptide markers were optimized. In the procedure, a linear gradient was applied for peptides separation. MRM-MS analysis was conducted using a Q-Orbitrap mass spectrometer operates in positive-ion mode. The UPLC-MS/MS MRM method was used for the monitoring of 102 peptide transitions (59).
Sometimes automated systems for sample preparation and chromatographic separation are applied for determination of allergens. Allergens in egg, skimmed milk, soy flour, ground hazelnut, and ground peanut in cookies were determined by LC-MS/MS (60). The sample preparation procedure was based on ultrasound-assisted solvent extraction followed by rapid size-exclusion chromatography. After enzymatic tryptic digestion by trypsin solution, peptides were transferred to on-line coupling of solid phase extraction (SPE) with a RP-HPLC system. Peptides were purified on SPE C18 cartridges and separated on a C18 analytical column with mobile phase containing acetonitrile, water, and formic acid. For detection, ESI interface connected to a dual pressure linear IT-MS was applied. The application of an on-line SPE resulted in higher sensitivity of the SRM detection (60). LODs obtained by the extraction, separation, and detection procedure were from 6 to 13 µg/g, LOQs from 19 to 40 µg/g.
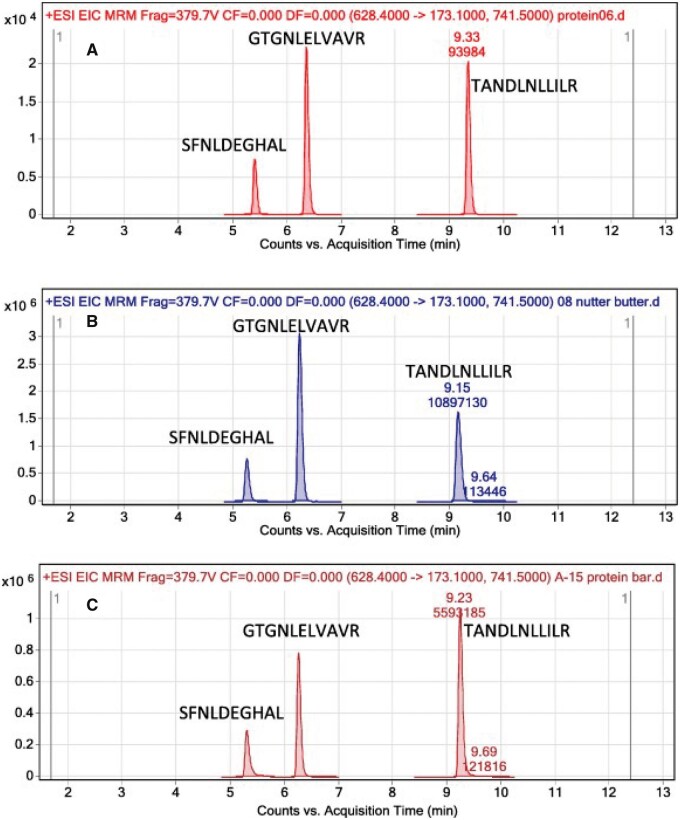
LC–ESI+-MS/MS was also applied for determination of peanut and tree nut allergen peptides (61). Allergen proteins from samples were extracted using TRIS and HCl (pH 7.5) and separated on an Agilent Poroshell 120 column with mobile phase containing acetonitrile, water, and trifluoroacetic acid. The described proteomic approach uses LC–MS/MS to specifically detect 12 tree nut allergens and peanut in one analysis. This multiplexed approach allows for accuracy and sensitivity in the testing of food for hidden allergenic compounds to satisfy both inspection and labelling purposes. Figure 8 shows a representative extracted ion current chromatogram for 3 peanut marker peptides in several foods that contain peanuts and in corn, which did not contain peanuts, illustrating the specificity that can be achieved with LC-ESI+-MS/MS (61).
Figure 8.
Extracted ion current chromatogram for three peanut marker peptides in: (A) unroasted peanuts 10 ppm standard, (B) nut butter (ingredients state peanuts present), (C) protein bar (ingredients state peanuts present), (D) nut crisps (peanuts detected and label says manufactured in a facility that processes tree nuts but did not mention peanuts), (E) 0.033 ppm peanut standard, and (F) corn blank (61). Reprinted with permission from Sealey-Voyksner, J., Zweigenbaum, J., Voyksner, R. (2016) Food Chem. 194, 201–211. Copyright Elsevier.
One of the current trends of advanced analytical chemistry is the miniaturization of analytical instruments. A micro HPLC system often leads to increased separation speed, reduced sample and reagent consumption, and a lower instrument cost compared to that of a traditional system. Additionally, there is an increasing demand for sensitive and high throughput MS-based methods for screening especially tailored to the detection of allergen contaminants in different food commodities. A challenging issue is represented by complex food matrices where commercial antibody-based kits might encounter objective limitations due to the masking of epitopes caused by the interference of compounds arising from the matrix. A performance of a method duly optimized for the extraction and simultaneous detection of soy, egg, and milk allergens in a cookie food matrix by micro HPLC–ESI-MS/MS, was reported by Monaci et al (62). The extraction procedures and different instrumental parameters were optimized in the investigations in order to obtain optimal conditions for analysis of these allergens. Based on the optimization, allergens from food samples were extracted with Tris–HCl buffer at pH = 8.2 and purified on a size exclusion column before protein enzymatic digestion was performed. For separation of peptides, a micro C18 column and mobile phase consisting acetonitrile, water, and formic acid was applied. Dual cell linear IT-MS/MS was used for detection of analytes. The use of dual cell linear IT-MS/MS allowed for determination of the most intense and reliable peptide markers in investigated food samples. A total of eleven peptides were monitored for confirmation purposes of each allergenic contaminant and the two most sensitive peptide markers/proteins were selected in order to perform quantitative determination (62). LODs obtained by the procedure were in range from 0.1 µg/g for milk,0.3 µg/g for egg, and 2 µg/g for soy.
HPLC-ESI+-MS/MS using a Qtrap was also applied for analysis of egg white, skim milk, peanut, soy, and tree nuts (almond, Brazil nut, cashew, hazelnut, pecan, pine nut, pistachio, and walnut) allergens (63). Before analysis, food samples were defatted and proteins were extracted with buffer containing urea, Trizma base, and octyl b-D-glucopyranoside. After extraction, reduction by tris(2-carboxyethyl)phosphine hydrochloride, alkylation by S-methyl methanethiosulfonate, and protein digestion by trypsin at 37°C were performed. The authors carried out peptide-mapping experiments to identify unique and selective peptides that could be used as signature markers of allergens. For this purpose, the tryptic digests of the allergen commodities were separated on a C18 column using mobile phase containing acetonitrile, water, and formic acid. A triple TOF mas spectrometer operated in positive ESI mode was applied for detection of analytes. For the quantitative analysis of allergenic peptides QTRAP MS was used. Data processing were performed using the MQ4 algorithm in MultiQuant v3.0.2 software. The LOD value obtained by the analytical procedure was 10 ppm (63). Next, a targeted MRM method was optimized and applied to screen marker peptides in food samples.
Gomaa et al. compared the performance of the flow cytometry method developed with LC–MS and ELISA for detection of allergens in food matrices containing either single or multiple allergens (34). The ELISA kits performed well in detecting allergens in the raw samples with recoveries of 91–108%, 88–127%, and 85–108% for casein, soy protein, and gluten, respectively. Recoveries were poor for the baked cookies (67–90%, 66–95%, and 66–88% for casein, soy protein, and gluten, respectively). The multiplex flow cytometry assay allows for multiple allergen detection in the raw samples, with the following recoveries based on soluble protein: casein, 95–107%; soy protein, 92–97%, and gluten, 96–99%. Recoveries for the baked cookies were as follows: casein, 84–90%; soy protein, 80–88%, and gluten, 80–90%. Allergen proteins were separated on a C18 column with mobile phase containing acetonitrile with trifluoroacetic acid and water with formic acid. Linear gradient elution was performed by increasing the mobile-phase composition from 3% to 40% of acetonitrile with trifluoroacetic acid over 45 min. Analytes were detected by a tandem Q-TOF mass spectrometer operated in positive ion mode. The application of a LC–MS technique led to detection of peptide markers that could be used to identify allergens in the baked food samples up to concentrations of 10 ppm for casein and soy protein, and 100 ppm for gluten (34). The mustard allergen Sin a 1 in food was determined by a LC-MS/MS-SRM-based method (64, 65). A triple quadrupole mass spectrometer operated in positive ion mode was applied for detection of investigated peptides. Samples of cashew nut were analyzed using LC-MS, SDS-PAGE, and immunoassay methods (16). The mass spectrum was simultaneously acquired using positive and negative ESI modes (16). Soybean allergens (Glycine max) were analyzed by LC-MS/MS after separation by 2-DE and extraction (24). Soybean proteins were initially separated by 2-DE. Next, proteins from identical gels were transferred to polyvinylidene difluoride membranes for immunoblots. Bound IgE was detected using monoclonal anti-human IgE, then washed to remove unbound detection antibodies. Bound anti-IgE was detected with chemiluminescent substrate. After trypsin digestion allergen peptides were analyzed by LC-MS/MS. Separation was performed on a C18 column with mobile phase containing acetonitrile, water, and formic acid. Q-TOF-MS with an ESI source was applied for detection of analytes. In another paper, a high-resolution qTOF mass spectrometer with electrospray ionization (LC-ESIqTOF) was applied for determination of sunflower allergens (51). Allergen proteins from large yellow croaker (Larimichthys crocea) (66), Glycyrrhiza uralensis (64), and hazelnut allergens (67), initially separated by SDS-PAGE, were analyzed on a C18 column with mobile phase containing acetonitrile, water, and formic acid and detected by MS/MS.
In a similar RP system, allergen tryptic peptides from egg were analyzed by LC-MS/MS after preliminary detection by ELISA (27). In the method, in order to successfully fitting immunoassay to LC-MS analysis, authors applied AASIA-ELISA, in which target amino acid sequence of the analyte is conformed to LC-MS/MS detection peptide. After separation by HPLC, the tryptic peptides were identified by a triple quadrupole MS for peptide quantification or by a TOF-MS for peptide identification (27).
Birch pollen allergens were analyzed by UPLC-MS (65). Chromatographic separation was performed on a C18 UPLC column with a mixture of acetonitrile, water, and formic acid as eluent. Detection of allergen peptides were carried out on a triple quadrupole mass spectrometer in MRM mode using positive ion ESI (65).
In another paper (12), a triple quadrupole mass spectrometer operated in positive ionization mode was coupled to a HPLC instrument for detection and quantification of egg, milk, and peanut in thermally processed bakery products (cereal bars and muffins). Data acquisition was performed in scheduled MRM mode. The comparison of the performance of commercial immunochemical assays with that of a multi-allergen MS method for the detection and quantitation of investigated allergens was also described (12).
Detection of allergen Glb33 in rice was performed on a triple quadrupole mass spectrometer operated in ESI positive ion mode after separation on a C18 column with mobile phase containing acetonitrile, water, and formic acid. The MS/MS data were collected in MRM mode Three mass transitions (precursor/fragment ion pairs) were selected for each peptide for quantitation and confirmation. Before LC-MS/MS analysis an enzymatic digestion of samples was performed after salt solution extraction of samples. A signature peptide from the tryptic digest was selected to represent the target protein, and an isotope-labeled signature peptide from the tryptic digest of the internal standard peptide was employed as the actual internal standard during MS analysis (68).
Soybean peptides were separated on a RP column using a 30 min gradient of 5% to 60% acetonitrile in water with 0.1% formic acid (69). MALDI-TOF-MS was applied for analyte detection. Before proper analysis, protein digestion was performed with modified porcine trypsin in ammonium bicarbonate buffer.
After SDS-PAGE and trypsin digestion, olive pollen allergens were analyzed by LC-MS/MS (2). For comprehensive proteomics analysis, the authors applied the wild olive genomics data (2). Before LC-MS/MS analysis, the proteins were suspended in buffer containing 100 mM Tris-HCl pH 7.3, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, and 1 mM phenylmethanesulfonyl fluoride with Complete Protease Inhibitor Cocktail. After centrifugation, the supernatant was analyzed by SDS-PAGE. Proteins were separated by 15% SDS-PAGE and stained with Coomassie Brilliant Blue G 250. Next, whole lanes were cut into four slices and in-gel trypsin digestion was performed. Then, excised bands were cut into small pieces and were separately treated with 50 mM ammonium bicarbonate with 50% acetonitrile, Next, the bands were dehydrated with acetonitrile, and dried. Samples were then reduced with 10 mM dithiothreitol in 25 mM ammonium bicarbonate and alkylated with iodoacetamide to a final concentration of 50 mM. The gel pieces were then dried, rehydrated with 12.5 ng/mL of trypsin in 50 mM ammonium bicarbonate, and incubated at 37°C. Next, peptides were extracted using 100% acetonitrile and 0.5% trifluoroacetic acid, purified using a Zip Tip with 0.6 μL of C18 resin, and dried. Obtained samples were reconstituted in 5 μL of 0.1% formic acid in 2% acetonitrile and analyzed by LC−MS/MS. Analytes were separated on a C18 column with the application of a gradient elution. The mobile phase contained acetonitrile, water, and 0.1% of formic acid. Hybrid Q-Orbitrap MS was used for allergen peptides detection in the procedure (2).
Multiplex food allergen (almond, brazil nut, cashew, hazelnut, pistachio, walnut, egg, lupin, milk, mustard, peanut, sesame, soy, and wheat) detection was performed by LC−MS/MS (70). Allergens were separated on a C18 column with mobile phase containing acetonitrile, water, and trifluoroacetic acid. Detection was performed on a triple quadrupole mass spectrometer. Authors also described a computational approach for addressing heterogeneous interference derived from diverse food matrices. Authors highlighted how a single multiplexed scheduled MRM method can overcome complex matrix interference and capture trends in allergen contamination (70).
To improve the sensitivity of LC-MS methods, nanoflow separation whereby the narrow diameter of the analytical channel from which the sample enters the ion source produces a smaller electrospray plume, can be applied. Conventional or microfluidic chromatographic separation with MS detection was applied for quantification of peanut allergens in a complex food matrix (71). In the procedure for sample preparation reduction was performed by addition of dithiothreitol, alkylation by application of iodoacetamide. Next, samples were digested by trypsin for 3h at 37 and were extracted by a simple buffer extraction with an acid-labile detergent. A C18 column and mobile phase containing acetonitrile, water, and formic acid were applied for separation of allergenic peptides. For detection of analytes, a triple quadrupole mass spectrometer operated in positive ion MRM mode was coupled to a HPLC system. Microfluidic separation provided greater sensitivity and increased ionization efficiency at low levels of allergenic peptide concentrations (71). Next, 2DE-IgE immunoblotting allergens of house dust mite (Dermatophagoides farinae) were chromatographed on an Acclaim PepMap column (72). A MicroToF-Q-MS with ESI was used for detection of allergen proteins.
Various undeclared food allergens in cumin were determined by UPLC-MS/MS (10). Peanut allergen proteins (Ara h 1, Ara h 2, Ara h 3, Ara h 6, Ara h 7, and Ara h 8) in cumin samples were separated on a C18 UPLC capillary column using mobile phase containing acetonitrile, water, and 0.1% of formic acid. For detection of analytes, a Hybrid Ion Trap-Orbitrap mass spectrometer was coupled to a UPLC system.
Almond and hazelnut allergens from roasted nuts were also determined by LC-MS/MS. Separation of allergen proteins was performed on a C18 column with mobile phase containing acetonitrile, water, and formic acid. Detection of allergens was performed on Q-Orbitrap-based MS systems operated in ipositive ionisation mode or on a Q-Orbitrap mass spectrometer operated in positive ionization mode. All measurements were performed using a scheduled MRM method with two transitions per selected peptide (20).
Analysis of gluten in processed foodstuffs by a multi-allergens and grain-specific UHPLC-MS/MS method has also been described (69). Before analysis, samples were ultrasonicated and centrifuged and the proteins were subsequently reduced with DL-dithiothreitol, alkylated with iodoacetamide, and enzymatically digested with trypsin. Peptide solutions were purified using C18 SPE columns. Analytes were separated on a C18 column with mobile phase containing acetonitrile, water, and formic acid. Detection was performed using a triple quadrupole mass spectrometer operated in positive ionization mode. The chromatographic method with MS/MS detection was compared to ELISA. The semiquantitative multi-allergen and grain-specific UHPLC-MS/MS method turned out to be complementary to ELISA kits (35).
Allergen peptides from hazelnut, cashew, almond, peanut, and walnut were analyzed by LC-MS/MS. A triple quadrupole mass spectrometer operated in positive ion electrospray mode was used for detection of analytes in various food samples. In the method, for sample preparation, urease to hydrolyze the urea in the extraction buffer was apllied. Therefore, no further cleanup of the extract before injecting was required. Before LC-MS/MS analysis in silico digestion with the enzyme trypsin was performed (73).
LC-MS/MS was also applied for determination of fish allergens (74). Allergens were separated on a C18 column with mobile phase containing acetonitrile, water, and formic acid. After separation analytes were detected on a qTOF mass spectrometer.
Organic modifier (acetonitrile or methanol)-water (RP) system
Quantitative analysis of nut allergens was performed by LC-MS/MS. Detection was performed by a ESI mass spectrometer operated in positive ionization mode. The important parameters influencing the quantitative analysis of food allergens via LC-MS (food matrix, sample history, and sample preparation) were investigated (75).
Mobile phases containing only organic modifier and water were used much less frequently for the analysis of allergens.
Before LC-MS/MS analysis the proteins of eggplant peel extract were separated on a phenyl-sepharose column and analyzed by skin prick test, ELISA, and IgE-immunoblotting. The components were analyzed for polyphenol oxidase activity, presence of protein–bound copper, and recognition by rabbit polyclonal anti-sweet potato polyphenol oxidase antiserum (76). In the next step of experiment, LC–MS/MS and in silico analysis were employed to identify the separated allergens and prediction of IgE epitopes. Eggplant allergens were separated into five components (PS1–PS5), of which component PS2 exhibited high specific Polyphenol Oxidase (PPO) activity (76).
In rare cases, HPLC-DAD has been applied for determination of allergens. Using this method, allergens are determined by comparison of retention times and UV-Vis spectra. HPLC-DAD was applied for analysis of hydroxycitronellal, coumarin, lyral, eugenol, citronellol, farnesol, and eighteen potentially allergenic substances in cosmetics. For sample preparation, an ultrasound-assisted emulsification microextraction technique was used, followed by solidification of the floating organic drop. Analytes were separated on a octadecyl (C18) stationary pahse with mobile phase containing acetonitrile and water.. Detection was performed by DAD coupled to a HPLC instrument. LODs obtained by the described procedure were from 0.013 µg/mL for citral to 6 µg/mL for hydroxycitronellal. HPLC–DAD chromatogram obtained at 200 nm for a standard mixture of the 24 potentially allergenic substances and UV–Vis spectra of the analytes are presented in Figure 9 (77).
Figure 9.
In black: HPLC–DAD chromatogram at 200 nm of a standard mixture of the 24 regulated potentially allergenic substances (PAS). Concentration level was 0.8 µg/mL for all the analytes except forhydroxycitronellal (136 µg/mL), lyral (8.37 µg/mL), and farnesol (1.98 µg/mL). Assignation of peaks: (1) anise alcohol; (2) benzyl alcohol; (3) cinnamyl alcohol; (4) cinnamal; (5) isoeugenol; (6) geraniol; (7) linalool; (8) citral; (9) methyl-2-octynoate; (10) amylcinnamyl alcohol; (11) benzyl benzoate; (12) benzyl salicylate; (13) benzyl cinnamate; (14) lilial; (15) amyl cinnamal; (16) alpha-isomethyl ionone; (17) hexyl cinnamal; (18) limonene; (a) hydroxycitronellal; (b) coumarin; (c) lyral; (d) eugenol, (e) citronellol; (f) farnesol. In grey: blank chromatogram obtained after applying all the procedures to a sample without analytes. b) Zoom-in of the intervals with problems of overlapped peaks and UV–vis spectra of the analytes present in them (78). Reprinted with permission from Pérez-Outeiral, J., Elcoroaristizabal, S., Amigo, J.M., Vidal, M. (2017) J. Chromatogr. A 1526, 82–92. Copyright Elsevier.
HPLC coupled with a variable-wavelength UV-Vis detector is sometimes applied for analysis of allergens. For example, protamines from different types of fish and target proteins were analyzed by HPLC-UV-Vis (23). Chromatography was performed to analyze the types of phenylthiohydantoin amino acids to obtain the protamine N-terminal sequence information. Separation was carried out on a octadecyl (C18) stationary phase with mobile phase containing acetonitrile and water. Allergen proteins were detected by a UV-Vis detector at λ=220 nm.
Korte et al. developed a MS approach for the detection of contamination with shrimp and lobster, two economically important types of crustaceans, in complex food matrices. Lobster and shrimp allergens in food samples were analyzed by LC coupled high-resolution MS/MS (LC−HRMS/MS) (55). Detection was carried out by a triple TOF spectrometer operating in positive MRM and MRM3 modes. The MS/MS scan was performed with high-sensitivity settings on the 25 most intense ions of each cycle with an accumulation time of 100 ms. A total of 2036 cycles were performed, thus an average of 11 cycles per chromatographic peak (55).
Maize allergens were separated by HPLC and detected by the application of Orbitrap MS (78). For sample preparation, protein was dissolved in a chaotrope-containing buffer (8 M urea, 50 mM Tris-HCl, pH 8.0) followed by quantification using the Bradford assay used to measure the concentration of total protein in a sample. Protein was portioned and spiked with AQUA peptides and reduced by adding dithiothreitol to 10 mM and incubated for 1 h at RT. Alkylation was performed by adding iodoacetamide and incubated for 1 h. Urea was neutralized by adding a solution containing 10 mM ammonium bicarbonate and dithiothreitol. Trypsin was added to sample protein at a 1:49 ratio and digestion proceeded for 18 h at 37°C. After digestion, samples were frozen at −80°C and evaporated to dryness. Dried protein digests were dissolved immediately prior to MS analysis to a concentration of 0.40 μg/μL with 5% (v/v) acetonitrile, 0.1% formic acid (v/v) in water. HPLC separation of analytes was performed using in-house fused silica nanospray needles, 10.5 cm length, (360 μm outer diameter, 150 μm inner diameter), that were packed with a C18 sorbent. Mobile phase consisted of acetonitrile and water (78).
Food allergens in milk, eggs, cod, shrimp, lobster, almonds, brazil nuts, cashew nuts, hazelnuts, walnuts, peanuts, wheat, and soybeans were separated on a C18 column with mobile phase containing acetonitrile and water (79). Detection was performed on a triple quadrupole mass spectrometer. Allergens were monitored by single reaction monitoring in positive ESI mode (79).
OTHER mobile and stationary phases
Mobile phases of a different composition than those discussed so far have also been infrequently used to analyze allergens.
Hydrophilic interaction chromatography (HILIC) is a liquid chromatography technique that uses a polar stationary phase—silica or a polar bonded phases in conjunction with a mobile phase containing a small amount of water combined with a higher proportion of a less polar solvent (often acetonitrile). HILIC is often applied for the separation of very polar analytes. An important feature of HILIC is the improved sensitivity with electrospray MS. HILIC has been infrequently used for the separation of allergens. For example, analysis of N-glycoforms of soybean allergenic glycoproteins previously separated by SDS-PAGE was performed by LC-MS/MS on an amide HILIC column with mobile phase containing acetonitrile and aqueous ammonium solution. Analytes were detected by linear IT-ESI-MS/MS. The method allows the separation of N-glycan isomers and obtaining information on the quantitative distribution of different types of N-glycans for each soy protein sample.
Allergen carbohydrate modification on glycoproteins from seeds of Ginkgo biloba were analyzed qualitatively and quantitatively by UPLC-MS/MS (80). Monosaccharides were separated on a C18 column with mobile phase containing acetonitrile, tetrahydrofuran, water, phosphoric acid, and n-butylamine. Analytes were detected by MALDI-TOF-MS. 1-Phenyl-3-methyl-5-pyrazolone was analyzed on a C18 UPLC column with a mixture of acetonitrile, water, and ammonium acetate. Detection in the procedure was performed by DAD. HILIC-UPLC analysis was also performed for N-glycan profiling and quantification. Separation was carried out on an ethylene-bridged hybrid (BEH) UPLC column. A mixture of acetonitrile, water, and ammonium acetate was used as mobile phase (80).
Plant N-glycans were separated by UPLC using an Acquity BEH Glycan column and mobile phase containing acetonitrile, water, and ammonium formate (81). Eluate fractions were manually collected to isolate the main N-glycan peak observed in the chromatogram. After solvent evaporation using vacuum centrifugation, samples were analyzed by MALDI/TOF-MS. Before UPLC-MS/MS analysis, samples of blended plant foodstuff were centrifuged to remove insoluble material. Glycoprotein pellets were obtained after precipitation of cleared supernatant with aqueous trichloroacetic acid solution and centrifugation and directly used for bacterial protein N-glycanase treatment. Samples were mixed with a labeling reagent and incubated for 2 h at 65°C. Prior to UPLC analysis, acetonitrile was added to the samples (81).
LC-MS has several proven advantages, including sensitivity, specificity, and the ability to detect a broad range of allergen concentrations that may span four or five orders of magnitude. LC-MS/MS has the desirable capability to function at the protein sequence level. Depending on the approach, LC-MS/MS has the potential to estimate changes in individual proteins or individual amino acids in a protein sequence.
Despite these advantages, the application of LC-MS to allergen analysis has some limitations such as: a lack of repositories of protein sequences for allergenic foods, especially those of plant origin, available genome sequences may not be in a format suitable for protein identification, and species-specific peptide markers need identification (1). Additionally, the separation of intact proteins from complex samples using LC is still extremely challenging. Generally, the LC-MS/MS detection and quantification of allergens differentiate significantly depending on the individual type of allergen, the degree of heat treatment, and the sample preparation method.
Examples of chromatographic and MS conditions are presented in Table 2.
Table 2.
LC-MS conditions for analysis of allergens in various samples
| Sample | Allergen | Stationary phase | Mobile phase | Method of detection | References |
|---|---|---|---|---|---|
| Soybean (G. max) |
|
C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Quadrupole TOF ESI-MS/MS | (24) |
| Egg | Ovalbumin peptide | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Triple quadrupole or TOF ESI+-MS/MS | (27) |
| Baked goods | Egg (lysozyme and ovalbumin), milk (αS1-casein and β-lactoglobulin), and peanut (Ara h 1, Ara h 2 and Ara h 3) allergen peptides | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Triple quadrupole ESI+-MS/MS | (12) |
| Olive pollen (O. europaea L.) |
|
C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Q-Orbitrap ESI+-MS/MS | (2) |
| Sugar cookies |
|
C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Triple Quadrupole Linear Ion Traps ESI+-MS/MS | (59) |
| Processed food | Milk allergen a-S1 casein | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Quadrupole TOF ESI+-MS/MS | (58) |
| Processed foods | Mustard allergen Sin a 1 | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Triple Quadrupole ESI+-MS | (82) |
| Large yellow croaker (L. crocea) | β-enolase and tropomyosin α-1 chain | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Ion Trap-Orbitrap MS | (66) |
| Glycyrrhiza uralensis | Allergen proteins from G. uralensis | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | Ion Trap MS | (64) |
| Hazelnut | Hazelnut allergens: Cor a 8, Cor a 9, Cor a 11, Cor a 12, Cor a 13, and Cor a 14 | C18 | Acetonitrile, water, and 1% formic acid (gradient elution) | Hybrid Ion Trap-Orbitrap ESI+-MS | (63) |
| Soybean seed | Allergen proteins β-conglycinin and glycinin (major allergens: P34, Gly m Bd 28K, SAM 22, 2S albumin) | C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | MALDI-TOF-MS | (64) |
| House dust |
|
C18 | Acetonitrile, water, and 0.1% formic acid (gradient elution) | MicroToF Q ESI-MS | (72) |
| Bakery products and chocolates | Pru du 6, Pru du 6, Pru du 6, Ana o 2, Ana o 2, Ana o 2, Cor a 9, Ara h 3, Pis v 5, Pis v 5, Jug r 2, Jug r 2, Jug r 4 | C18 | Acetonitrile and water (gradient elution) | QTRAP ESI+-MS | (75) |
| Lobster (Homarus americanus), giant tiger prawn (Penaeus monodon), and whiteleg shrimp (Litopenaeus vannamei) | Myosin light chain 2, slow muscle myosin S1 heavy chain, fast myosin heavy chain, arginine kinase, myosin heavy chain type 2, myosin heavy chain type b, myosin heavy chain type 1, myosin heavy chain type a | C18 | Acetonitrile and water (gradient elution) | TripleTOF or QTRAP MS | (55) |
| Thermally processed food | 13 food allergens (milk, eggs, cod, shrimp, lobster, almonds, brazil nuts, cashew nuts, hazelnuts, walnuts, peanuts, wheat, and soybeans) | C18 | Acetonitrile and water (gradient elution) | Triple Quadrupole ESI+-MS | (79) |
GC and multidimensional or coupled techniques (GC-GC, GC × GC, 2-D)
Allergens, especially those found in cosmetics, have been analyzed by GC methods usually coupled with MS or MS/MS. Often GC-GC-MS has been applied for analysis of allergens. A typical GC-GC-MS configuration consists of a monitoring flame ionization detector (FID) to detect the effluent of the first dimension column, while only the second dimension column is connected to the mass spectrometer. Comprehensive two-dimensional (2-D) gas chromatography coupled with MS (GC-MS) or tandem mass spectrometry (GC-MS/MS) is a effective technique for analysis of multicomponent mixtures of volatiles.
Rey et al. developed a GC-GC-FID-MS method for analysis of 56 (69 analytes including isomers) suspected chemically defined fragrance allergens in perfumes (83). Samples were not prepared before GC-GC-FID-MS analysis. The first dimension separation was carried out on a VF1-MS capillary column. The second dimension separation was performed on an LTM DB17-MS capillary column. The optimized condition was successfully applied for quantification of the target allergens in complex perfume matrices. The authors applied the combination of multiple heart-cut chromatography with a low thermal mass chromatography module applied to quantification of allergens. The low thermal mass chromatography produces independent and fast temperature gradients in the second dimension. Analytes were detected by quadrupole FID-MS.
A 2-D GC coupled with quadrupole MS (GC × GC-qMS) method was developed for the determination of 54 fragrance allergens in cosmetics (84). In the first dimension, SLB-5ms [silphenylene polymer virtually equivalent in polarity to poly(5%diphenyl/95% methyl siloxane)], in the second dimension SLB-35 [equivalent in polarity to poly(35% diphenyl/65% methyl siloxane)] columns were used. Quantification of allergens was performed by using extracted ions; target analyte identification was performed through measurement of ion ratios (qualifier/quantifier), full-scan MS database matching and the use of linear retention indices. The calculated LODs and LOQs obtained by the proposed procedure were in the ranges from 0.04 (methylsalicylate) to 0.30 mg/L (caryophyllene oxide, two farnesol isomers), and from 0.14 (methyl salicylate) to 1.00 mg/L(caryophyllene oxide, two farnesol isomers), respectively. The flow-modulation (FM) GC × GC–qMS chromatogram obtained for the allergen solution is shown in Figure 10.
Figure 10.
FM GC × GC–qMS chromatogram of the allergen solution at the 50 mg/L level (84). Reprinted with permission from Tranchida, P.Q., Maimone, M., Franchina, F.A. Bjerk, T.R., Zini, C.A., Purcaro, G., Mondello, L. (2016) J. Chromatogr. A 1439, 144–151. Copyright Elsevier.
Full evaporation dynamic headspace in combination with selectable one-dimensional (1-D) or 2-D gas chromatography coupled with MS was applied for the determination of suspected fragrance allergens in cosmetic products—shower gels or body creams (85). In the proposed method an alternative approach based on full evaporation dynamic headspace sampling was applied. For separation of allergens, a polar DB-Wax capillary column was applied in the first dimension and an apolar Rtx-5 (5/% phenyl methyl siloxane) capillary column was selected in the second dimension. The main advantage of the procedure is the non-discriminating injection of solutes covering a relatively large boiling point range, while non-volatile material such as salts and detergents are not recovered.
Fragrance allergens were analyzed by GC × 2GC-MS/FID with a dual-parallel secondary column and dual detection (86). The applied chromatographic system was equipped with a reverse-inject differential flow modulator consisting of one capillary flow technology plate connected to a three-way solenoid valve that receives a controlled supply of carrier gas (helium) from an auxiliary electronic pressure control module. In the first dimension, a polar (DB-Wax) capillary column was applied, and in the second dimension two parallel columns (OV-1701 and deactivated fused silica) were used.
Analysis of different personal care products to simultaneous determination of different families of personal care products including fragrance allergens, synthetic musks, phthalates, antioxidants, and UV filters in continental water, was also performed by GC-MS/MS (87). Before GC analysis, samples were prepared by solid-phase microextraction on poly-dimethylsiloxane/divinylbenzene (PDMS/DVB) fiber. GC separation was carried out on a Zebron ZB-Semivolatiles column with helium as the carrier gas. A triple quadrupole mass spectrometer operated in positive ionization mode was coupled to a GC instrument for detection of allergens. LODs obtained by the procedure were from 0.023 ng/L to 14 ng/L, and LOQs ranged between 0.078 ng/Lto 46 ng/L. The obtained chromatogram for a sample containing 19 of the target analytes is presented in Figure 11.
Figure 11.
SPME-GC-MS/MS reconstructed chromatogram for the real sample S5 containing 19 of the target analytes (87). Reprinted with permission from Celeiro, M., Lamas, J.P., Vila, M., Garcia-Jares, C., Homem, V., Ratola, N., Dagnac, T., Llompart, M. (2019) J. Chromatogr. A 1607, 460398. Copyright Elsevier.
Eighteen cosmetic products of different formulation, including creams, lotions, and gels were analyzed for the presence of undeclared allergenic fragrance ingredients by GC-MS (88). Separation was performed on a VF-5 ms column, detection on a single quadrupole MS detector. Authors proposed that a simple liquid extraction was developed for sample preparation before head space GC-MS method. The procedure was applied for simultaneous analysis of twenty four volatile allergenic fragrances in complex cosmetic formulations, such as hydrophilic and lipophilic creams, lotions and gels.
Application of electrophoresis to allergen analysis
SDS-PAGE
Gel electrophoresis followed by immunoblotting represents the standard procedure for allergen separation and identification. SDS-PAGE is the most commonly used method to fractionate allergen proteins according to their molecular weight. The SDS denatures and coats the proteins, giving them a strong negative charge. The proteins are separated according to their molecular mass irrespective of their original electrochemical charge. The separation medium is a discontinuous gel based on polyacrylamide, and an electric field is applied across the gel, causing the negatively charged proteins to migrate across the gel towards the anode. The longer the proteins are, the more they are retained in the gel. Changes in the molecular masses of the proteins can be identified by SDS. SDS cleaves non-covalent linked aggregates into monomers, whereas covalent disulfide bridges remain intact. Following electrophoresis, the gel may be stained with Coomassie Brilliant Blue to make the separated proteins visible.
To identify allergens separated by electrophoresis, in either 1- or 2-D versions, coupled to MS analysis are usually antibody labelling in blotting setup, using sera from allergic patients as a source of specific IgE are applied. Complex allergen–IgE is usually detected using a conjugated fluorescently labeled polyclonal anti-human IgE as the secondary antibody.
A method for determination of pyruvate kinase, an allergen in whiteleg shrimp (Litopenaeus vannamei), by specific IgE present in patients with a shrimp allergy, was described by Lee et al. (89). The sample protein extracts were separated by SDS-PAGE. The integrated protein lane on SDS-PAGE was excised manually, and transferred into Eppendorf tubes. After trypsin digestion and peptide extraction, target peptides were analyzed by nano-UPLC-MS. Authors also demonstrated that various allergens from raw or cooked shrimps sensitized patients with crustacean allergy.
SDS-PAGE was also applied for analysis of other food allergens (75). Before SDS-PAGE separation, allergen proteins were extracted by a chaotropic, high-molarity urea buffer in combination with a high-speed stirrer for a quick 2 min manual extraction. Following this procedure, extracted allergens were obtained in a denaturing buffer that can be directly applied to preparation of the tryptic digest without need for a buffer change or precipitation. Obtained extraction efficiency and SDS-PAGE analysis are presented in Figure 12. After these procedures, allergens were determined by LC-MS/MS.
Figure 12.
Extraction of allergenic nuts. (A) Extraction efficiency, calculated as the protein concentration of the extracts (via Bradford assay, given on secondary axis) divided by the protein content of the respective nut (via Kjeldahl method). (B) Laemmli SDS-PAGE (12%) analysis, 15 μg protein per lane (76). Reprinted with permission from Korte, R., Oberleitner, D., Brockmeyer, J. (2019) J. Proteomics 196, 131–140. Copyright Elsevier.
Extracted proteins from the rice allergens in rice bran were separated by SDS-PAGE and detected by the IgE-binding proteins containing the causative allergens in patients with a rice bran allergy (90). In the initial step, three kinds of purification methods were applied. To clarify the protein that needed to be targeted, products containing rice bran were examined; protein extraction of rice allergens were carried out for cosmetics and health foods containing rice bran. Extracted proteins were separated by SDS-PAGE using reducing conditions. Finally, the IgE-binding proteins containing the causative allergens in patients with a rice bran allergy were identified. The potential causative allergen of rice bran allergy was identified as a 52- kDa globulin. Western blot analysis using a rice-bran-allergic patient’s plasma showed that 52-kDa globulin was detected as an Ig57E-binding protein of rice bran and some rice bran-containing cosmetics and health foods.
Extracted allergen proteins from cashew nut were also separated by SDS-PAGE and then analyzed by immunoassay tests (16). Proteins from the milt of large yellow croaker (P. crocea) were analyzed by SDS-PAGE (23). Allergens separated by SDS-PAGE are often determined by immunological methods e.g., egg white allergens expressed in E. coli (25). Various allergen proteins, after separation by SDS-PAGE, were determined by LC-MS/MS e.g., large yellow croaker (L. crocea) allergens (66), allergen in eggplant (Solanum melongena L.) (76), allergen Glycyrrhiza uralensis (64), hazelnut allergens (78), olive pollen allergens (2), raw and roasted peanut allergens (71), N-glycoforms of soybean allergenic glycoproteins (91), allergens from seeds of Ginkgo biloba (80), and almond and hazelnut allergens from roasted nut (20). Immunological analysis such as immunoblotting was also applied after SDS-PAGE separation e.g., to egg yolk allergens (26), peanut allergens (92), Carya illinoinensis (Wangenh.) allergen (93), Gly m 4, a soybean allergen (21), citrus fruit allergens (94), and tree nuts (95).
SDS-PAGE was applied with ELISA for determination of allergens in food (egg, milk, wheat, peanut, and buckwheat) (15). A new extraction solution containing a human- and eco-friendly reductant sodium sulfite, tris(3-hydroxypropyl)phosphine, and mercaptoethylamine sodium sulfite was applied as a 2-mercaptoethanol substitute. In the procedure, food samples were added to SDS/2-ME or SDS/substitute extraction solutions containing sodium sulfite, Tris (3-hydroxypropyl) phosphine, or mercaptoethylamine hydrochloride in a centrifuge tube. The sample was extracted overnight in a shaker (100 rpm) at room temperature, and the solution was then centrifuged to obtain the supernatant. The ELISA procedure was then carried out by following the 2-D Quant kit instructions. A modified ELISA in which 2-mercaptoethanol was totally substituted with 0.1 M sodium sulfite was constructed to examine ELISA performance in model-processed and commercial food products. The ability of SDS/0.1 M sodium sulfite for protein extraction was compared to that of SDS/2-mercaptoethanol solution. The food allergen recoveries of the SDS/0.1 M sulfite ELISAs were compatible with those of the SDS/2-mercaptoethanol ELISAs. The results obtained by different SDS-PAGE for various food samples are shown in Figure 13. These two methods were also used for analysis of undeclared food allergens in cumin samples (10) and milk protein residues in Cheddar cheese (32).
Figure 13.
SDS-PAGE protein pattern of the food allergen reference materials. Each reference material, egg 0.2 g, milk 0.2 g, wheat 1 g, peanut 0.4 g, and buckwheat 1 g was extracted with 20 mL of the extraction solution containing only SDS, or SDS plus 0.02–0.5 M sodium sulfite. The resulting supernatant 7.5 μL was loaded on a lane of NuPAGE 12% gel. After electrophoresis, the protein pattern of the SDS-PAGE gel was visualized by a Rapid CBB staining kit; (A) egg; (B) milk; (C) wheat; (D) peanut; (E) buckwheat. Lane 1, PBS extract; Lane 2, SDS extract; Lane 3, SDS/2-ME extract; Lane 4, SDS/0.02 M sulphite extract; Lane 5, SDS/0.05 M sulfite extract; Lane 6, SDS/0.1 M sulphite extract; Lane 7, SDS/0.2 M sulfite extract; Lane 8, SDS/0.5 M sulphite extract; Lane M, see blue PLUS 2 prestained standard (15). Reprinted with permission from Ito, K., Yamamoto, T., Oyama, Y. et al. (2016) Anal. Bioanal. Chem. 408, 5973–5984. Copyright Springer Nature.
Details of some SDS-PAGE procedures for analysis of allergens are presented in Table 3.
Table 3.
Application of SDS-PAGE for allergen analysis
| Sample | Allergen | Gel chemistry | Loading buffer | Running conditions | Mode of separation | References |
|---|---|---|---|---|---|---|
| Recombinant egg white | Gal d 1 (ovomucoid), Gal d 2(ovalbumin), Gal d 3 (ovotransferrin), and Gal d 4 (lysozyme) | Tris-Glycine | Tris-Glycine SDS sample loading buffer | 1.5 hrs at 125 constant voltage | Molecular weight | (25) |
| Egg yolk | α-livetin (Gal d 5) and YGP42 (Gal d 6) | Tris-Glycine | Tris-Glycine SDS sample buffer | Constant voltage of 125 V for 90 min |
|
(26) |
| Soy | Soy protein | Tris-Glycine |
|
170 V for approximately 1 h | Molecular weight | (17) |
| Milt of large yellow croaker (P. crocea) | Protamine | Tricine |
|
30–270 V for 3–4 h | Molecular weight | (23) |
| Roasted nut | Hazelnut and almond allergen proteins | Bis-Tris | Lithium dodecyl sulfate at pH 8.5 with SERVA Blue G250 and phenol red | 200 V for 35 min | Molecular weight | (20) |
| Chocolate dessert and chocolate bar | Peanut allergens (Arah1, Arah2, Arah3, Arah6, Arah7) | Bis-Tris | Lithium dodecyl sulfate at pH 8.5 with SERVA Blue G250 and phenol red | 200 V for 35 min | Molecular weight | (71) |
| Bakery products and chocolates | Pru du 6, Pru du 6, Pru du 6, Ana o 2, Ana o 2, Ana o 2, Cor a 9, Ara h 3, Pis v 5, Pis v 5, Jug r 2, Jug r 2, Jug r 4 | Tricine | 0.01M Tris-HCl, pH 6.8, 0.1% SDS, and 0.1%–a 2-mercaptoethanol | Molecular weight | (75) | |
| Food containing cashew nut | Ara h 1 and Ara h 2/6 peanut allergens | Tris-Glycine | Lithium dodecyl sulfate at pH 8.5 with SERVA Blue G250 and phenol red | Molecular weight | (16) | |
| Peanut | Ara h 1, Ara h 2, Ara h 3, and Ara h 6 | Tris-Glycine | 5% β-mercaptoethanol | 1 h at 50 V followed by 1.5 h at 100 V | Molecular weight | (92) |
| Soybean | Gly m Bd 30K, Gly m lectin, Gly m Bd 28K, Gly m 5 consisting of α, α′, β, and β′ subunits of the 7S proteins, and Gly m 6 consisting of A1, A2, A3, A4, B1, B2, B3, and B4 subunits of 11S proteins | Tris-Glycine | 10 mM Tris-HCl, pH 8.0, 1% SDS, 40% glycerol, 0.1% DTT, and 0.05% bromophenol blue | 90 V in the stacking gel and 120 V in the separating gel for 3 h | Molecular weight | (91) |
| Olive (O. europaea L.) | Ole e 1, Ole e 2, and Ole e 5 | Tris-glycine | 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue and 0.125 M Tris HCl, pH approx. 6.8 | 300 and 1000 V for 1 h each followed by a linear increase from 1000 to 10 000 V, and finally 10 000 V | Molecular weight | (96) |
No data.
2-DE
2-DE is a technique for studying protein expression in biological materials. Several thousands of proteins can be visualized and quantified simultaneously, which allows comparisons under various experimental conditions. The principle of 2-DE is to separate proteins according to their isoelectric point in the first dimension and according to their molecular weight in the second dimension.
2-DE followed by immunoblotting was applied for analysis of snake venom allergens (97). Allergen proteins were separated by 2-DE with application of the pH gradient. In the procedure for total protein separation, the immobilized pH gradient strips were rehydrated passively for 13 h. The voltage settings for isoelectric focusing in the Protean system were 2 h at 250 V, 1 h at 1000 V, 3 h at 8000 V, and then kept at 8000 V until a total of 50 000 Vh was reached. After isoelectric focusing and equilibration, the second dimensional SDS-PAGE gels of 12.5% were run at 2 W/gel for 2 h and 12 W/gel for 1.5 h. For each sample, duplicate 2-D gels were run under the same conditions. Proteins were visualized from one gel by scanning after reaction with Ponceau S solution and on the other proteins were identified by multidimensional LC-IT-MS.
2-DE was applied for separation of proteins from banana fruit extract (30). In the procedure, banana fruit proteins were resolved by 1-D SDS-PAGE (4% stacking and 12% resolving gel) and then electrotransferred onto a nitrocellulose membrane. For identification of proteins resolved by 2-D, PAGE-MS and MS/MS were used. For analysis of allergens, the banana protein extract as mixed with a sample buffer containing Tris-HCl, pH 6.8, glycerol, and bromphenol blue. The mixture was incubated for 5 min at 95°C. Banana fruit proteins were resolved by 1-D SDS-PAGE and were electrotransferred onto a nitrocellulose membrane by using a semi-dry transfer buffer containing Tris, glycine, methanol, and SDS (pH 8.3). In the next step of the experiments, blocking was performed with Tris buffered saline containing human serum albumin for 2 h at room temperature, IgE reactive proteins were detected by using individual sera of patients with a suspected allergy to banana. The stripes were incubated with polyclonal goat anti-human IgE for 1 h, followed by 1 h of incubation with alkaline phosphatase-labeled polyclonal rabbit anti-goat IgG tertiary antibodies. Visualization of the reaction was performed with 5-bromo-4-chloro-3-indolyl phosphate/4-nitroblue tetrazolium.
Almond (Prunus dulcis) vicilin, a food allergen, was separated by SDS-PAGE using 4–20% polyacrylamide gels with a Tris-[4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (HEPES)-SDS running buffer (100 mM Tris, 100 mM HEPES, 3 mM SDS, pH 8.0) (98). The peptide bands of interest were excised and N-terminal amino acid sequencing was performed. Multiple SDS gels were used to assess IgE binding by western blot and for protein detection by Coomassie Brilliant Blue staining. For western blot, protein bands in the SDS gels were transferred to PVDF membranes.
Paramyosin, a prominent allergen in dust mite allergies, was initially determined by in vivo (prick test) and in vitro (serum specific IgE) tests (99). The allergen was then analysed by 2-D gel electrophoresis combined with immunoblotting and MS.
Analysis of low molecular weight allergens and putative allergen proteins in lentil (Lens culinaris) cultivars of Bangladesh was performed by SDS-PAGE on polyacrylamide gels (100). In the procedure, protein from seeds was extracted under denaturing conditions using a modified phenol partitioning protocol and precipitated with three different precipitation buffers: (1) 0.1 M ammonium acetate in methanol, (2) ice cold 75% acetone, and (3) ice cold 10% trichloroacetic acid, 20 mM dithiothreitol in acetone. Precipitated protein was resuspended in dissolution lysis buffer (8 M urea, 50 mM Tris-HCl, pH 8.0) and quantified using the BCA Protein Kit using bovine serum albumin as a standard. Protein lysate was diluted four-fold with 20 mM HEPES buffer, pH 8.0 prior to protein concentration measurement and trypsin digestion. Gel electrophoresis was performed under denaturing conditions in 13% polyacrylamide gels using 20 mA per gel. Gels were stained with colloidal Coomassie Brilliant Blue stain under standard conditions. LC–MS/MS was then performed on a C18 column. A mixture of acetonitrile, water, and acetic acid was applied for elution of analytes. Allergen proteins were detected by triple quadruple MS coupled to a HPLC system.
2-DE immunoblots and MS after 2-DE were applied for determination of putative soybean (G. max) allergens (24). Soybean protein extracts were diluted to a final volume by rehydration buffer containing 8 M urea, 2% 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate, 50 mM dithiothreitol, and 0.5% ampholyte (pH 3–10) for each 2-DE steps. The sample was applied to a non-linear 7 cm immobilized pH gradient strip pH 3–10. Active rehydration was performed at 50 V of direct current (vdc), 20°C for 12 h, followed by separation with rapid ramping up to 250 vdc for 15 min, gradual ramping up to 4000 vdc for 2 h, then a limit of 4000 vdc which was continued until 37 500 integrated volt × hours was obtained. The strips were reduced with dithiothreitol and alkylated with iodoacetamide equilibration buffer. The second dimension gel electrophoresis separation was completed using NuPAGE® Novex 4–12% Bis-Tris ZOOM® gels.
Natarajan et al. analyzed soybean allergen proteins by 2-DE (69). For the first dimension, isoelectric focusing of protein samples was performed using 13 cm pH 4−7 and 6−11 linear immobilized pH gradient (IPG) strips in the IPGphor system. For the second dimension, the IPG strips were incubated with 50 mM Tris-HCl pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 0.002% bromophenol blue, 1% DTT, and further acetylated with iodoacetamide.
Olive (Olea europaea L.) pollen allergens were determined by 2-DE with fluorescence-based detection methods (96). Using a combination of high-resolution 2-DE techniques with high-sensitive fluorescence-based detection methods, the identification of a high number of allergen forms were possible.
2-DE was often applied for separation of allergen peptides before further analysis by immunological methods (25, 77), MS, MS/MS (22, 51), or LC-MS/MS (51, 72). Allergens from sunflower (H. annuus L.), after separation by 2-DE, were analyzed by MS/MS and LC-MS/MS (51). After 2-DE by MS/MS were determined, allergens from Curvularia pallescens (CP) (52). Results obtained by SDS-PAGE, 2-D-PAGE, and 2-D immunoblot using pooled sera of patients obtained for C. pallescens allergen proteins are showed in Figure 14.
Figure 14.
SDS-PAGE and 2D PAGE of total protein of CP and corresponding immunoblots. (A). Molecular weight marker (M), total protein of CP (T) in 12% SDS-PAGE and 1D blot with 22 individual sera (lanes 1–22) (from left to right). (B). 2D gel of spore-mycelial proteome of CP. (C). 2D immunoblot using pooled sera of patients. (D). Four representative 2D immunoblots using 4 individual sera (patient numbers 1, 6, 9, and 18; from left to right) (52). Reprinted with permission from Dey, D., Saha, B., Sircar, G., Ghosal, K., Bhattacharya, S.G. (2016) Biochim. Biophys. Acta 1864, 869–879. Copyright Elsevier.
Examples of applications of SDS-PAGE for allergen analysis in various samples are presented in Table 1.
CE
CE is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions.
CE was applied with MPCR for simultaneous determination of 10 food allergens (hazelnut, pistachio, oat, sesame, peanut, cashew, barley, wheat, soybean and pecan) in cake, cookies, crackers, waffles, cocktail nuts, Quaker rolled oats, fruit juice, noodle, pistachio, chocolate, mixed nuts, milk, candy, soy milk, and powdered beef soup (38).
Capillary zone electrophoresis (CZE)-UV and transient capillary isotachophoresis (CZE-ESI-TOF-ESI+-MS) was performed for monitoring of deamidation and lanthionine formation in recombinant mugwort allergen (101). In the procedure, transient capillary isotachophoresis was applied for in-capillary preconcentration. Ammonium formate stock solution, pH 4.00, was applied as the leading electrolyte. The CZE system was coupled with TOF-MS for detection. A bare fused-silica capillary was installed in the CE system and aligned in the electrospray interface. The capillary conditioning protocol had the following steps: 1.00 mol/L NaOH (10 min), ultrapure water (15.0 min), 0.10 mol/L HCl (10 min), and background electrolyte (10 min) (all with 200.0 kPa at 35.0°C). After conditioning, the cleaned capillary was then aligned in the coaxial electrosprayer. For the final equilibration, the capillary was rinsed with background electrolyte (10 min, 200 kPa) followed by application of 25.0 kV (10 min with 1.0 min ramp time), all at 35.0°C. Obtained for investigated allergens, TIC electropherogram and MS spectra obtained for investigated allergens are presented in Figure 15.
Figure 15.
tCITP-CZE-ESI-TOF-MS monitoring of the development of modifications to rArt v 3.0201 at pH 7.3 unheated (A, A1) and heated to 95 °C for 15 min (B, B1−B3). (A, B) TIC electropherograms, (A1, B1−B3) extracted raw mass spectra, and deconvoluted mass spectra depicted as insets. Sample: 0.23mg/mL rArt v 3.0201 at pH 7.3 either unheated (control) or heated to 95 °C for 15 min with addition of 125 mmol/L ammonium formate, respectively. Injection: 3.45 kPa, 50.0 s. CZE separation: 25.0 kV (1.00 min ramp time, anode at the inlet side), 25.0 °C. Sheath liquid: methanol–water–FA 75%:24.9%:0.1% (v/v/v). Nebulizer gas: 0.4 bar. Drying gas: 4.0 L/min, 190 °C. End plate offset: −500 V. MS transfer capillary voltage: 4.5 kV (positive ion mode). Abbreviations in peak annotations represent: D = deamidation; +S = gain of sulfur; −S = loss of sulfur (LAN formation); O = oxidation (101). Reprinted with permission from Stock, L.G., Wildner, S., Regl, C., Gadermaier, G., Huber, C.G., Stutz, H. (2018) Anal. Chem. 90, 11933–11940. Copyright American Chemical Society.
Parvalbumin, a major allergen in fish was analyzed by CE after background suppression in ion-exchange chromatography (102). For CE, fused silica capillaries with dimensions of 75 μm i.d., 375 μm o.d. (20 cm effective length, 30.2 cm total length), borate-borax running buffer at concentration 25 mmol/L, and a separation voltage of 15 kV, were applied. A UV detector set at 214 nm was applied for analyte detection. Parvalbumin was determined in 2.8 min, with the LOD at 0.71 μg/mL.
Examples of application of modern analytical methods coupled with different detection techniques for allergens analysis in various samples, and the LOD and LOQ values are presented in Table 1.
Conclusions
Immunological methods are most frequently used for determination of allergens. They are sensitive and can be applied for determination of allergens in trace concentrations, but the lack of specificity and cross-reaction of some antibodies can be a relevant source of errors.
Owing to its advantages such as simplicity, good sensitivity, and ease of operation, enzyme-linked ELISA has been a powerful tool in allergen detection. However, the relatively low sensitivity and accuracy are the main limitations of traditional ELISA, which have hindered its application in allergen analysis. Another disadvantage is that most ELISA kits target only a single allergen from a single product. Till now, great progress has been made in boosting the accuracy, sensitivity, and stability to improve traditional ELISA procedures. Connection of ELISA or Nano-ELISA with other techniques such as PCR, DSD-PAGE, and HPLC-MS/MS are newly developed detection methods that can realize rapid and sensitive determination of allergens.
Nucleic acid-based methods are fast and reliable for determination of protein allergens. However, the epitopes of protein allergens with posttranslational modifications and the changes originating during various processing cannot be traced by use of this analytical strategy.
PCR technology is commonly used to monitor allergenic ingredients during food processing, with high specificity and a high degree of automation, since DNA is generally more stable than proteins in food processing. However, the application of PCR technology to food allergen detection is limited, since it is unsuitable for identifying target allergenic proteins with unidentified genes in food matrices. DNA-based allergen detection using PCR is useful and efficient. Sensitivity of multicopy-based detection is comparable to that obtained by application of immunoassays, but PCR offers extraordinary specificity in comparison to immunoassays. Currently, other emerging developments, such as digital PCR, are explored for the quantification of allergens.
The ELISA and real-time PCR methods have limitations in their application in quantifying allergens. The performance of immunoassays, particularly in the form of ELISA and PCR methods, can be highly variable and matrix dependent, making results difficult to understand and interpret. Therefore, the development of new methods for the analysis of allergens, which are reliable, comparable, robust, fast, stable, and easy to standardize, is necessary. Biosensors are considered a powerful technology to rapidly analyze various allergens with high sensitivity and selectivity. Biosensors demonstrated to be very rapid and easy to use and can be readily implemented as screening methods along to monitor allergens. However, biosensors often require expensive instruments and skilled operators. Hence, it is an urgent requirement to develop simple, accurate, sensitive, inexpensive, and easy-to-use methods to quantify allergens in complicated matrices. Biosensors also offer a viable alternative to traditional analytical techniques due to their potential for miniaturization and label-free online detection.
The field of targeted proteomics has advanced rapidly over the last few years, and a number of useful tools and resources for targeted method development are now available. MS methods for allergen detection and quantification have proved to be very useful, but require consideration over the complexity of allergen molecules and the presence of a complicated matrices. Assessing the recovery of allergens from matrices is one of the most difficult steps to overcome to enable the accurate quantification of protein allergens by both immunoassay and MS.
Recent developments of new high-resolution MS instruments are encouraging and enable development in the analysis of allergens. Consequently, fast, very sensitive, reliable, and accurate detection and quantification of allergens in complex samples and the application of MS in routine determination of allergens could be available in the near future. MS, and especially HRMS, is very importanct for the molecular characterization and analytical detection of allergens. With the dynamic technological advancement of mass spectrometers with novel hybrid instruments, the addition of further separation dimensions, such as ion mobility, or the renaissance of CE as a separation technique also taken into consideration, it is estimated that HRMS will be of growing importance in the future.
LC, GC, or electrophoretic methods coupled with MS brings additional advances in allergen analysis. The use of LC-MS or LC-MS/MS for the quantitative detection of allergens in various matrices is presently gaining acceptance as a protein-based confirmatory technique over the routinely performed enzyme-linked immunosorbent assays. Quantitative LC-MS or LC-MS/MS of allergens is a field under development, and more methods will be developed in the near future, including multi-allergen assays for the simultaneous determination of several major food allergens from complex processed food matrices.
Because fragrance allergens are usually very volatile, GC with different columns coupled to MS or MS/MS is the most popular technique for their analysis. Selected ion flow tube MS is also another novel technique that can be applied for fragrance analysis, because of its simplicity, non-destructive sample analysis, very low operating costs, and low maintenance requirements.
Often, an LC-MS/MS method is applicable for the detection and quantitation of food allergens in finished food products and ingredients. Predominantly, in proposed methods using triple quadrupole MS and selective MRM of characteristic transitions of precursor ions to fragment ions of multiple proteins and peptides to uniquely identify each allergen. It is important to characteristic signature peptides were chosen for each allergen, and MRM transitions for each signature peptide were determined based on their uniqueness compared to background proteins and their sensitivity of detection. Therefore, in the proposed methods, for each allergen multiple unique peptides were chosen from unique proteins, and two MRM transitions per peptide were chosen. The authors who described procedures for the analysis of selected allergens are convinced that these procedures can be easily extended to the detection of others allergens.
However, absolute quantification of food allergens requires proper calibration as well as suitable standards, to compensate effects on peptide detectability caused by the food matrix and processing. The use of stable isotopically labeled peptides, which are added to the tryptic digest prior to measurement, has been proposed for the exact quantification of allergenic contaminants. Another challenge for the exact quantification of food allergens is introduced through food processing, which might affect protein structure. The problem of suitable standards for analysis was also observed. Alternatively, the solution may be the establishment of a range of biomarkers and properties utilizing orthogonal multiplex analytical methods that generate sufficient data to detect allergens irrespective of varietal or other differences.
Parallel development of different analytical methodologies accompanied by effective test method validation contribute to improve the quality of allergen analysis and promote best practices in allergen analysis.
Gel electrophoresis, in both 1-D and 2-D variants, is a very useful, simple, cheap, and convenient method for the analysis of allergens. From a few hundred to one thousand protein species can be resolved in a single experiment. Future directions of gel electrophoresis could relate to technical aspects such as miniaturization, nanotechnology of gels, and application of a free-flow electrophoresis format.
Multidisciplinarity is important to ensure that analytical methods developed for allergen determination are clinically relevant.
Further research is needed to define signature peptides for most major allergens and to develop new reliable, robust, comparable, fast, stable, and easy-to-standardize methods for their analysis.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.DRAFT SCIENTIFIC OPINION, Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes, 1 EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), European Food Safety Authority (EFSA), Parma, Italy (2014)
- 2. San Segundo-Acosta P., Oeo-Santos C., Benedé S., de los Ríos V., Navas A., Ruiz-Leon B., Moreno C., Pastor-Vargas C., Jurado A., Villalba M., Barderas R. (2019) J. Proteome Res. 18, 3052–3066. doi:10.1021/acs.jproteome.9b00167 [DOI] [PubMed] [Google Scholar]
- 3. Koeberl M., Clarke D., Lopata A.L. (2014) J. Proteome Res. 13, 3499–3509. doi:10.1021/pr500247r [DOI] [PubMed] [Google Scholar]
- 4. Esteve C., Montealegre C., Marina M.L., García M.C. (2012) Talanta 92, 1–14. doi:10.1016/j.talanta.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 5. Do A.B., Khuda S.E., Sharma G.M. (2018) J. AOAC Int. 101, 23–35. doi:10.5740/jaoacint.17-0384 [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi Y., Huge J., Imamura S., Hamada-Sato N. (2016) Allergol. Int. 65, 272–279. 10.1016/j.alit.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 7. Fang L., Li G., Zhang J., Gu R., Cai M., Lu J. (2019) Clin. Immunol. 201, 20–29. 10.1016/j.clim.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y., Deng R., Zhang G., Li Q., Yang J., Sun Y., Li Z., Hu X. (2015) J. Agric. Food Chem. 63, 2172–2178. doi:10.1021/jf5052128 [DOI] [PubMed] [Google Scholar]
- 9. Wu C.-C., Lee C.-H., Tyan Y.-C., Huang E.S., Yu W.-T., Yu H.-S. (2019) Food Chem. 289, 413–418. 10.1016/j.foodchem.2019.03.074 [DOI] [PubMed] [Google Scholar]
- 10. Garber E.A.E., Parker C.H., Handy S.M., Cho C.Y., Panda R., Samadpour M., Reynaud D.H., Ziobro G.C. (2016) J. Agric. Food Chem. 64, 1202–1211. doi:10.1021/acs.jafc.5b05497 [DOI] [PubMed] [Google Scholar]
- 11. Daly M., Ansari P., Haubl G., Rogers A., Brunner K. (2018) J. AOAC Int. 101, 96–101. doi:10.5740/jaoacint.17-0398 [DOI] [PubMed] [Google Scholar]
- 12. Parker C.H., Khuda S.E., Pereira M., Ross M.M., Fu T.-J., Fan X., Wu Y., Williams K.M., DeVries J., Pulvermacher B., Bedford B., Zhang X., Jackson L.S. (2015) J. Agric. Food Chem. 63, 10669–10680. doi:10.1021/acs.jafc.5b04287 [DOI] [PubMed] [Google Scholar]
- 13. Slotwinski E., Almy D., Viator R., Abouzied M., Klein F., Rice J. (2018) J. AOAC Int. 101, 118–123. doi:10.5740/jaoacint.17-0401 [DOI] [PubMed] [Google Scholar]
- 14. Ecker C., Ertl A., Pulverer W., Nemes A., Szekely P., Petrasch A., Linsberger-Martin G., Cichna-Markl M. (2013) Food Chem. 141, 407–418. doi:10.1016/j.foodchem.2013.02.091 [DOI] [PubMed] [Google Scholar]
- 15. Ito K., Yamamoto T., Oyama Y., Tsuruma R., Saito E., Saito Y., Ozu T., Honjoh T., Adachi R., Sakai S., Akiyama H., Shoji M. (2016) Anal. Bioanal. Chem. 408, 5973–5984. doi:10.1007/s00216-016-9438-7 [DOI] [PubMed] [Google Scholar]
- 16. Mattison C.P., Cavalcante J.M., Gallão M.I., de Brito E.S. (2018) Food Chem. 240, 370–376. 10.1016/j.foodchem.2017.07.146 [DOI] [PubMed] [Google Scholar]
- 17. Amponsah A., Nayak B. (2016) J. Food Sci. 81, T2876–2885. doi:10.1111/1750-3841.13534 [DOI] [PubMed] [Google Scholar]
- 18. Mueller G.A., Randall T.A., Glesner J., Pedersen L.C., Perera L., Edwards L.L., DeRose E.F., Chapman M.D., London R.E., Pomes A. (2016) Clin. Exp. Allergy 46, 365–376. doi:10.1111/cea.12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inomata N., Miyakawa M., Ikeda N., Oda K., Aihara M. (2018) Clin. Exp. Allergy 48, 1509–1520. doi:10.1111/cea.13247 [DOI] [PubMed] [Google Scholar]
- 20. Perner S.P., Heupel L., Zimmermann L., Peters Y., Vongehr K.U., El-Bedewy H., Siebeneicher S., Weiß T., Hektor T., Lindemann B., Loos-Theisen S., Schneider K. (2019) J. AOAC Int. 102, 1271–1279. doi:10.5740/jaoacint.19-0055 [DOI] [PubMed] [Google Scholar]
- 21. Geng T., Liu K., Frazier R., Shi L., Bell E., Glenn K., Ward J.M. (2015) J. Agric. Food Chem. 63, 4947–4953. doi:10.1021/acs.jafc.5b00792 [DOI] [PubMed] [Google Scholar]
- 22. Saha B., Sircar G., Pandey N., Bhattacharya S.G. (2015) J. Proteome Res. 14, 4823–4833. doi:10.1021/acs.jproteome.5b00657 [DOI] [PubMed] [Google Scholar]
- 23. Liu Y.-Y., Chen X.-F., Hu J.-W., Chen Z.-W., Zhang L.-J., Cao M.-J., Liu G.-M. (2016) J. Agric. Food Chem. 64, 1999–2011. doi:10.1021/acs.jafc.5b05899 [DOI] [PubMed] [Google Scholar]
- 24. Lu M., Jin Y., Cerny R., Ballmer-Weber B., Goodman R.E. (2018) Food Chem. Toxicol. 116, 207–215. 10.1016/j.fct.2018.04.032 [DOI] [PubMed] [Google Scholar]
- 25. Dhanapala P., Doran T., Tang M.L.K., Suphioglu C. (2015) Mol. Immunol. 65, 104–112. 10.1016/j.molimm.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 26. De Silva C., Dhanapala P., Doran T., Tang M.L.K., Suphioglu C. (2016) Mol. Immunol. 71, 152–160. 10.1016/j.molimm.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 27. Nimata M., Okada H., Kurihara K., Sugimoto T., Honjoh T., Kuroda K., Yano T., Tachibana H., Shoji M. (2018) Anal. Bioanal. Chem. 410, 325–335. 10.1007/s00216-017-0721-z [DOI] [PubMed] [Google Scholar]
- 28. Fu L., Wang J., Ni S., Wang C., Wang Y. (2018) J. Agric. Food Chem. 66, 2944–2953. doi:10.1021/acs.jafc.7b06042 [DOI] [PubMed] [Google Scholar]
- 29. Ueberham E., Spiegel H., Havenith H., Rautenberger P., Lidzba N., Schillberg S., Lehmann J. (2019) J. Agric. Food Chem. 67, 8660–8667. doi:10.1021/acs.jafc.9b02717 [DOI] [PubMed] [Google Scholar]
- 30. Nikolić J., Nešić A., Kull S., Schocker F., Jappe U., Gavrović-Jankulović M. (2018) J. Proteomics 175, 87–94. 10.1016/j.jprot.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 31. Koeberl M., Sharp M.F., Tian R., Buddhadasa S., Clarke D., Roberts J. (2018) Food Chem. 256, 105–112. 10.1016/j.foodchem.2018.02.043 [DOI] [PubMed] [Google Scholar]
- 32. Ivens K.O., Baumert J.L., Hutkins R.L., Taylor S.L. (2017) J. Dairy Sci. 100, 1629–1639. 10.3168/jds.2016-11649 [DOI] [PubMed] [Google Scholar]
- 33. Andjelkovic U., Gavrovic-Jankulovic M., Martinovic T., Josic D. (2017) Trend. Anal. Chem. 96, 107–115. 10.1016/j.trac.2017.07.011 [DOI] [Google Scholar]
- 34. Goma A., Boye J. (2015) Food Chem. 175, 585–592. 10.1016/j.foodchem.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 35. Henrottin J., Planque M., Huet A.-C., Marega R., Lamote A., Gillard N. (2019) J. AOAC Int. 102, 1286–1302. doi:10.5740/jaoacint.19-0057 [DOI] [PubMed] [Google Scholar]
- 36. Paris R., Pagliarani G., Savazzini F., Aloisi I., Iorio R.A., Tartarini S., Ricci G., Del Duca S. (2017) Plant Sci. 264, 57–68. 10.1016/j.plantsci.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 37. Ladenburger E.-M., Dehmer M., Grunberg R., Waiblinger H.-U., Stoll D., Bergemann J. (2018) J. AOAC Int. 101, 170–184. doi:10.5740/jaoacint.17-0406 [DOI] [PubMed] [Google Scholar]
- 38. Cheng F., Wu J., Zhang J., Pan A., Quan S., Zhang D., Kim H.Y., Li X., Zhou S., Yang L. (2016) Food Chem. 199, 799–808. doi:10.1016/j.foodchem.2015.12.058 [DOI] [PubMed] [Google Scholar]
- 39. Wu J., Chen L., Lin D., Ma Z., Deng X. (2016) J. Agric. Food Chem. 64, 9089–9098. doi:10.1021/acs.jafc.6b03410 [DOI] [PubMed] [Google Scholar]
- 40. Zhou J., Qi Q., Wang C., Qian Y., Liu G., Wang Y., Fu L.. Biosens. Bioelectron. doi:10.1016/j.bios.2019.111449 [DOI] [PubMed] [Google Scholar]
- 41. Angulo-Ibanez A., Eletxigerra U., Lasheras X., Campuzano S., Merino S. (2019) Anal. Chim. Acta 1079, 94–102. 10.1016/j.aca.2019.06.030 [DOI] [PubMed] [Google Scholar]
- 42. Ashley J., Piekarska M., Segers C., Trinh L., Rodgers T., Willey R., Tothill I.E. (2017) Biosens. Bioelectron. 88, 109–113. 10.1016/j.bios.2016.07.101 [DOI] [PubMed] [Google Scholar]
- 43. Hideshima S., Saito M., Fujita K., Harada Y., Tsuna M., Sekiguchi S., Kuroiwa S., Nakanishi T., Osaka T. (2018) Sens. Actuators. B-Chem. 254, 1011–1016. 10.1016/j.snb.2017.07.187 [DOI] [Google Scholar]
- 44. Pereira-Barros M.A., Barroso M.F., Martín-Pedraza L., Vargas E., Benedé S., Villalba M., Rocha J.M., Campuzano S., Pingarrón J.M. (2019) Biosens. Bioelectron. 137, 171–177. 10.1016/j.bios.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 45. Jiang D., Ge P., Wang L., Jiang H., Yang M., Yuan L., Ge Q., Fang W., Ju X. (2019) Biosens. Bioelectron. 130, 299–306. 10.1016/j.bios.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 46. Jiang D., Zhu P., Jiang H., Ji J., Sun X., Gu W., Zhang G. (2015) Biosens. Bioelectron. 70, 482–490. 10.1016/j.bios.2015.03.058 [DOI] [PubMed] [Google Scholar]
- 47. Angelopoulou M., Petrou P.S., Makarona E., Haasnoot W., Moser I., Jobst G., Goustouridis D., Lees M., Kalatzi K., Raptis I., Misiakos K., Kakabakos S.E. (2018) Anal. Chem. 90, 9559–9567. doi:10.1021/acs.analchem.8b02321 [DOI] [PubMed] [Google Scholar]
- 48. Lin H.-Y., Huang C.-H., Park J., Pathania D., Castro C.M., Fasano A., Weissleder R., Lee H. (2017) ACS Nano 11, 10062–10069. doi:10.1021/acsnano.7b04318 [DOI] [PubMed] [Google Scholar]
- 49. Ashley J., Shukor Y., D’Aurelio R., Trinh L., Rodgers T.L., Temblay J., Pleasants M., Tothill I.E. (2018) ACS Sens. 3, 418–424. doi:10.1021/acssensors.7b00850 [DOI] [PubMed] [Google Scholar]
- 50. Monaci L., De Angelis E., Montemurro N., Pilolli R. (2018) Trend. Anal. Chem. 106, 21–36. 10.1016/j.trac.2018.06.016 [DOI] [Google Scholar]
- 51. Ghosh N., Sircar G., Saha B., Pandey N., Bhattacharya S.G. (2016) Data Brief 7, 735–739. 10.1016/j.dib.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dey D., Saha B., Sircar G., Ghosal K., Bhattacharya S.G. (2016) Biochim. Biophys. Acta 1864, 869–879. 10.1016/j.bbapap.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 53. Yang Y., Chen Z.-W., Hurlburt B.K., Li G.-L., Zhang Y.-X., Fei D.-X., Shen H.-W., Cao M.-J., Liu G.-M. (2017) Mol. Immunol. 85, 35–46. 10.1016/j.molimm.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 54. Brockmeyer J. (2018) J. AOAC Int. 101, 124–131. doi:10.5740/jaoacint.17-0402 [DOI] [PubMed] [Google Scholar]
- 55. Korte R., Monneuse J.-M., Gemrot E., Metton I., Humpf H.-U., Brockmeyer J. (2016) J. Agric. Food Chem. 64, 6219–6227. doi:10.1021/acs.jafc.6b02620 [DOI] [PubMed] [Google Scholar]
- 56. Morschheuser L., Mink K., Horst R., Kallinich C., Rohn S. (2017) J. Chromatogr. A 1526, 157–166. 10.1016/j.chroma.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 57. Downs M.L., Baumert J.L., Taylor S.L., Mills E.N.C. (2016) J. Proteomics 142, 62–69. 10.1016/j.jprot.2016.04.045 [DOI] [PubMed] [Google Scholar]
- 58. Groves K., Cryar A., Walker M., Quaglia M. (2018) J. AOAC Int. 101, 152–161. doi:10.5740/jaoacint.17-0214 [DOI] [PubMed] [Google Scholar]
- 59. Boo C.C., Parker C.H., Jackson L.S. (2018) J. AOAC Int. 101, 108–117. doi:10.5740/jaoacint.17-0400 [DOI] [PubMed] [Google Scholar]
- 60. Pilolli R., De Angelis E., Monaci L. (2017) Food Chem. 221, 1747–1753. 10.1016/j.foodchem.2016.10.110 [DOI] [PubMed] [Google Scholar]
- 61. Sealey-Voyksner J., Zweigenbaum J., Voyksner R. (2016) Food Chem. 194, 201–211. 10.1016/j.foodchem.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 62. Monaci L., Pilolli R., De Angelis E., Godula M., Visconti A. (2014) J. Chromatogr. A 1358, 136–144. 10.1016/j.chroma.2014.06.092 [DOI] [PubMed] [Google Scholar]
- 63. New L.S., Schreiber A., Stahl-Zeng J., Liu H.-F. (2018) J. AOAC Int. 101, 132–145. doi:10.5740/jaoacint.17-0403 [DOI] [PubMed] [Google Scholar]
- 64. An E.-J., Kim K.-H., Lee I.-S., Park J.Y., Kim Y., Jung W.S., Kwon D., Jang H.-J. (2018) Biochip J. 12, 75–82. doi:10.1007/s13206-017-2110-2 [Google Scholar]
- 65. Spiric J., Schulenborg T., Schwaben L., Engin A.M., Karas M., Reuter A. (2017) J. Proteome Res. 16, 3852–−3862. doi:10.1021/acs.jproteome.7b00490 [DOI] [PubMed] [Google Scholar]
- 66. Yang Y., Qing-Li G., Yang S., Jing-Yu Z., Zhi-Bo L., Wei L., Yan J., Meng L., Qian-Cheng Z., Yan-Xia Q. (2017) Chin. J. Anal. Chem. 45, e1713–e1718. doi:10.1016/S1872-2040(17)61016-8 [Google Scholar]
- 67. Korte R., Brälcker J., Brockmeyer J, Brockmeyer (2017) Mol. Nutr. Food Res. 61, 1700130.doi:10.1002/mnfr.201700130 [DOI] [PubMed] [Google Scholar]
- 68. Chen M.-X., Yang H., Ma Y.-N., Mou R.-X., Zhu Z.-W., Cao Z.-Y., Cheng F.-M. (2019) J. Agric. Food Chem. 67, 5026–5032. doi:10.1021/acs.jafc.8b06738 [DOI] [PubMed] [Google Scholar]
- 69. Natarajan S., Khan F., Song Q., Lakshman S., Cregan P., Scott R., Shipe E., Garrett W. (2016) J. Agric. Food Chem. 64, 1433–1445. doi:10.1021/acs.jafc.5b05172 [DOI] [PubMed] [Google Scholar]
- 70. Croote D., Braslavsky I., Quake S.R. (2019) Anal. Chem. 91, 9760–9769. doi:10.1021/acs.analchem.9b01388 [DOI] [PubMed] [Google Scholar]
- 71. Sayers R.L., Gethings L.A., Lee V., Balasundaram A., Johnson P.E., Marsh J.A., Wallace A., Brown H., Rogers A., Langridge J.I., Mills E.N.C. (2018) J. Proteome Res. 17, 647–655. doi:10.1021/acs.jproteome.7b00714 [DOI] [PubMed] [Google Scholar]
- 72. Choopong J., Reamtong O., Sookrung N., Seesuay W., Indrawattana N., Sakolvaree Y., Chaicumpa W., Tungtrongchitr A. (2016) J. Proteome Res. 15, 422–430. doi:10.1021/acs.jproteome.5b00663 [DOI] [PubMed] [Google Scholar]
- 73. Ruhland M., Klinger R. (2019) J. AOAC Int. 102, 1303–1308. doi:10.5740/jaoacint.19-0058 [DOI] [PubMed] [Google Scholar]
- 74. Fiorino G.M., Fresch M., Brümmer I., Losito I., Arlorio M., Brockmeyer J., Monaci L. (2019) J. AOAC Int. 102, 1339–1345. doi:10.5740/jaoacint.19-0062 [DOI] [PubMed] [Google Scholar]
- 75. Korte R., Oberleitner D., Brockmeyer J. (2019) J. Proteom. 196, 131–140. 10.1016/j.jprot.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 76. Babu B.N.H., Wilfred A., Venkatesh Y.P. (2017) Immunobiology 222, 155–163. 10.1016/j.imbio.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 77. Pérez-Outeiral J., Elcoroaristizabal S., Amigo J.M., Vidal M. (2017) J. Chromatogr. A 1526, 82–92. 10.1016/j.chroma.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 78. Stevenson S.E., McClain S., Thelen J.J. (2015) J. Agric. Food Chem. 63, 821–828. doi:10.1021/jf504708u [DOI] [PubMed] [Google Scholar]
- 79. Ogura T., Clifford R., Oppermann U. (2019) J. AOAC Int. 102, 1316–1329. doi:10.5740/jaoacint.19-0060 [DOI] [PubMed] [Google Scholar]
- 80. Wang T., Hu X.-C., Cai Z.-P., Voglmeir J., Liu L. (2017) J. Agric. Food Chem. 65, 7669–7679. doi:10.1021/acs.jafc.7b01690 [DOI] [PubMed] [Google Scholar]
- 81. Du Y.M., Xia T., Gu X.Q., Wang T., Ma H.Y., Voglmeir J., Liu L. (2015) J. Agric. Food Chem. 63, 10550–11055. doi:10.1021/acs.jafc.5b03633 [DOI] [PubMed] [Google Scholar]
- 82. Posada-Ayala M., Alvarez-Llamas G., Maroto A.S., Maes X., Muñoz-Garcia E., Villalba M., Rodríguez R., Perez-Gordo M., Vivanco F., Pastor-Vargas C., Cuesta-Herranz J. (2015) Food Chem. 183, 58–63. 10.1016/j.foodchem.2015.02.139 [DOI] [PubMed] [Google Scholar]
- 83. Rey A., Corbi E., Pérès C., David N. (2015) J. Chromatogr. A 1404, 95–103. 10.1016/j.chroma.2015.05.045 [DOI] [PubMed] [Google Scholar]
- 84. Tranchida P.Q., Maimone M., Franchina F.A., Bjerk T.R., Zini C.A., Purcaro G., Mondello L. (2016) J. Chromatogr. A 1439, 144–151. doi:10.1016/j.chroma.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 85. Devos C., Ochiai N., Sasamoto K., Sandra P., Davi F. (2012) J. Chromatogr. A 1255, 207–215. doi:10.1016/j.chroma.2012.01.082 [DOI] [PubMed] [Google Scholar]
- 86. Cordero C., Rubiolo P., Reichenbach S.E., Carretta A., Cobelli L., Giardina M., Bicchi C. (2017) J. Chromatogr. A 1480, 70–82. 10.1016/j.chroma.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 87. Celeiro M., Lamas J.P., Vila M., Garcia-Jares C., Homem V., Ratola N., Dagnac T., Llompart M. (2019) J. Chromatogr. A 10.1016/j.chroma.2019.460398 [DOI] [PubMed] [Google Scholar]
- 88. Desmedt B., Canfyn M., Pype M., Baudewyns S., Hanot V., Courselle P., De Beer J.O., Rogiers V., De Paepe K., Deconinck E. (2015) Talanta 131, 444–451. 10.1016/j.talanta.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 89. Lee C.-H., Wu C.-C., Tyan Y.-C., Yu W.-T., Huang E.S., Yu H.-S. (2018) Food Chem. 258, 359–365. 10.1016/j.foodchem.2018.03.088 [DOI] [PubMed] [Google Scholar]
- 90. Satoh R., Tsuge I., Tokuda R., Teshima R. (2019) Food Chem. 276, 761–767. 10.1016/j.foodchem.2018.10.080 [DOI] [PubMed] [Google Scholar]
- 91. Li L., Wang C., Qiang S., Zhao J., Song S., Jin W., Wang B., Zhang Y., Huang L., Wang Z. (2016) J. Agric. Food Chem. 64, 7367–7376. doi:10.1021/acs.jafc.6b02773 [DOI] [PubMed] [Google Scholar]
- 92. Meng S., Li J., Chang S., Maleki S.J. (2019) Food Chem. X 1, 100004. 10.1016/j.fochx.2019.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang Y., Lee B.R., Du W.-X., Lyu S.-C., Nadeau K.C., Grauke L.J., Zhang Y., Wang S., Fan Y., Yi J., McHugh T.H. (2016) J. Agric. Food Chem. 64, 4146–4151. doi:10.1021/acs.jafc.6b00884 [DOI] [PubMed] [Google Scholar]
- 94. Wu J., Deng W., Lin D., Deng X., Ma Z. (2018) J. Agric. Food Chem. 66, 1964–1973. doi:10.1021/acs.jafc.7b05722 [DOI] [PubMed] [Google Scholar]
- 95. L’Hocine L., Pitre M. (2016) Food Chem. 194, 780–786. 10.1016/j.foodchem.2015.08.031 [DOI] [PubMed] [Google Scholar]
- 96. Zienkiewicz K., de Dios Alche J., Zienkiewicz A., Tormo A., Castro A.J. (2015) Electrophoresis 36, 1043–1050. doi:10.1002/elps.201400508 [DOI] [PubMed] [Google Scholar]
- 97. Hu Y., Yang L., Yang H., He S., Wei J.-F. (2017) Toxicon 125, 13–18. 10.1016/j.toxicon.2016.11.251 [DOI] [PubMed] [Google Scholar]
- 98. Che H., Zhang Y., Lyu S.-C., Nadeau K.C., McHugh T. (2019) J. Agric. Food Chem. 67, 425–432. doi:10.1021/acs.jafc.8b05290 [DOI] [PubMed] [Google Scholar]
- 99. Conti A., Burastero G.J., Suli C., Banerjee S., Vrtala S., Alessio M., Burastero S.E. (2017) J. Proteom. 166, 19–26. 10.1016/j.jprot.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 100. Shaheen N., Halima O., Akhter K.T., Nuzhat N., Rao R.S.P., Wilson R.S., Ahsan N. (2019) Food Chem. 297, 124936. 10.1016/j.foodchem.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 101. Stock L.G., Wildner S., Regl C., Gadermaier G., Huber C.G., Stutz H. (2018) Anal. Chem. 90, 11933–11940. doi:10.1021/acs.analchem.8b02328 [DOI] [PubMed] [Google Scholar]
- 102. Fu L., Zhou J., Wang C., Zhang Y., Ma A., Wang Y. (2018) Food Chem. 261, 124–130. 10.1016/j.foodchem.2018.04.037 [DOI] [PubMed] [Google Scholar]