Abstract
Although multiple sclerosis has traditionally been considered a white matter disease, extensive research documents the presence and importance of grey matter injury including cortical and deep regions. The deep grey matter exhibits a broad range of pathology and is uniquely suited to study the mechanisms and clinical relevance of tissue injury in multiple sclerosis using magnetic resonance techniques. Deep grey matter injury has been associated with clinical and cognitive disability. Recently, MRI characterization of deep grey matter properties, such as thalamic volume, have been tested as potential clinical trial end points associated with neurodegenerative aspects of multiple sclerosis. Given this emerging area of interest and its potential clinical trial relevance, the North American Imaging in Multiple Sclerosis (NAIMS) Cooperative held a workshop and reached consensus on imaging topics related to deep grey matter. Herein, we review current knowledge regarding deep grey matter injury in multiple sclerosis from an imaging perspective, including insights from histopathology, image acquisition and post-processing for deep grey matter. We discuss the clinical relevance of deep grey matter injury and specific regions of interest within the deep grey matter. We highlight unanswered questions and propose future directions, with the aim of focusing research priorities towards better methods, analysis, and interpretation of results.
Keywords: multiple sclerosis, deep grey matter, thalamus, atrophy, MRI
On behalf of the North American Imaging in Multiple Sclerosis Cooperative, Ontaneda et al. review the imaging of deep grey matter injury in multiple sclerosis. The pathological mechanisms and clinical relevance of deep grey matter injury are discussed, along with technical issues related to image acquisition and processing.
Introduction
Multiple sclerosis has traditionally been viewed as a white matter disease, with focal lesions resulting in neuronal injury and tissue destruction.1,2 However, detailed neuropathological examination also reveals extensive pathology in cortical and deep grey matter (DGM).3 While the mechanisms of DGM injury vary across structures, the net effect of this pathology can be examined in vivo using MRI. Volume loss/atrophy of the thalamus and other DGM structures has been well-documented in multiple sclerosis using MRI and is clinically relevant.4-9 The precise mechanisms of DGM loss remain to be fully elucidated and likely represent a complex interplay between the various aspects of pathology in multiple sclerosis.
Conventional MRI can be used to measure DGM volume and lesions. However, DGM lesions are best visualized at 7 T,10,11 are more difficult to visualize at conventional field strengths, and have even been considered a red flag for a diagnosis of multiple sclerosis.12 Advanced techniques, such as functional MRI (fMRI), diffusion tensor MRI, relaxometry and magnetic resonance (MR) spectroscopy, can quantify additional changes in DGM structures.13–15 With recent advances in imaging, there is an opportunity to satisfy an unmet need to fully characterize DGM injury in multiple sclerosis and develop measures for use in clinical care and research. A better understanding of the mechanisms of DGM injury will provide fundamental knowledge about the pathophysiology of multiple sclerosis and may enable more efficient clinical trial design for neuroprotective therapies.
Materials and methods
The North American Imaging in Multiple Sclerosis Cooperative (NAIMS) initially met to discuss DGM injury in San Diego, California in February 2018. Topics included: pathological mechanisms of DGM injury in multiple sclerosis, measurement of DGM volume, clinical relevance and regions of interest, and DGM metrics as clinical trial outcomes. Consensus points were drafted after completion of each topic presentations with input from all attendees. Consensus opinions were presented to the group at the completion of the meeting, and these were further refined by meeting presenters during teleconferences conducted after the meeting. Finally, all authors approved the final version of the manuscript. The goals of this paper are to summarize the proceedings, update and review the current understanding of DGM imaging with a focus on lesions and atrophy based on conventional MRI sequences, and provide recommendations for future research.
Pathological mechanisms of deep grey matter injury in multiple sclerosis
Deep grey matter lesions
Focal DGM lesions have been well-documented in multiple sclerosis and differ quantitatively and qualitatively from white matter lesions.5 Focal lesions occur in all DGM nuclei but are more common in the caudate, thalamus, and hypothalamus (Table 1).16 DGM lesions are characterized by demyelination with varying degrees of inflammatory changes.16 Similar to white matter lesions, DGM lesions can be classified into ‘active’, ‘chronic active’, and ‘chronic inactive’.16,33,34 Active DGM lesions show increased perivascular cuffs and lymphocytic infiltrates.16 Chronic active lesions are characterized by a rim of activated microglia/macrophages, and are common in DGM.16 When compared to white matter lesions (more inflammation) and cortical lesions (less inflammation), DGM lesions have an intermediate inflammatory pattern.16
Table 1.
Histopathological and MRI findings in DGM and related grey matter regions in multiple sclerosis
| Histopathological findings |
MRI findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lesions | Normal-appearing tissue |
||||||||
| DGM Structure | Focal lesions present16–18 | Diffuse inflammatory infiltrate16,17 | Activated microglia16–18 | Iron deposition16,* | Neuronal loss16,17 | Markers of oxidative stress16 | Early volume loss19–21 | DGM lesions MRI-visible22–24 | Iron accumulation25–27 |
| Thalamus16–20,22 | +++ | + | + | + | + | + | +++ | +++ | + |
| Caudate16,17,20,23 | +++ | + | + | + | + | + | ++ | + | + |
| Putamen16,17,19,20,23 | ++ | + | + | + | + | + | +++ | + | + |
| Globus pallidus16,17 | ++ | + | + | + | + | + | NA | NA | + |
| Substantia nigra17 | + | + | NA | NA | NA | NA | NA | NA | NA |
| Amygdala17 | + | + | NA | NA | NA | NA | NA | NA | NA |
| Hypothalamus17,28 | +++ | + | + | NA | + | NA | NA | NA | NA |
| Hippocampus29–32 | +++ | NA | + | NA | + | NA | + | +++ | NA |
NA = not available.
Did not reach statistical significance.
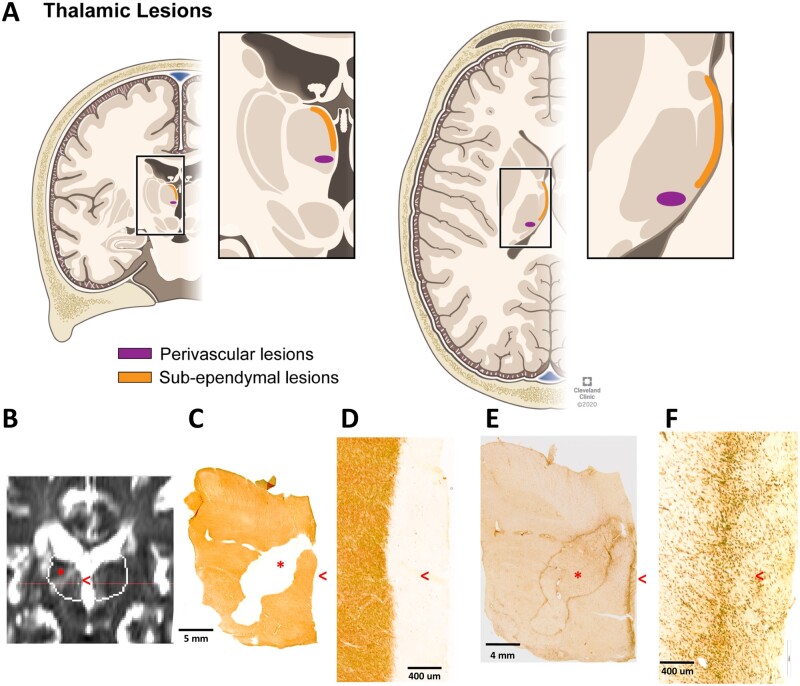
The most extensively studied DGM structure in multiple sclerosis is the thalamus. Focal thalamic lesions can be identified in over two-thirds of multiple sclerosis cases using microscopy and high-field MRI.10,11 Pathologically, focal thalamic lesions exhibit neuronal loss,17 demyelination, and axonal transection.35,36 MRI and histopathological studies have identified two thalamic lesion types: perivascular lesions, which are typically ovoid in shape, and subependymal lesions, which are thin bands of demyelination that line the third ventricle18 (Fig. 1). The presence of ovoid, perivascular, thalamic lesions shows conflicting results regarding correlation to cortical lesions.10,11 The appearance of subependymal thalamic lesions resembles that of subpial cortical lesions, and close proximity to the ventricle suggest a soluble or diffusible factor present in the CSF, perhaps with a role of inflammatory cells.37,38 The exact reasons for the variability in lesion frequency across DGM structures is unknown, but may relate to myelin content, contact with CSF spaces, and/or vascular distribution.
Figure 1.
Demonstration of thalamic demyelinating lesion types. (A) Thalamic lesions are represented in coronal (left) and axial (right) planes. Lesions can either border the lateral and third ventricles (termed subependymal lesions, in orange) or have an ovoid appearance around blood vessels (termed perivascular, in purple). (B) Thalamic lesions (perivascular labelled with an asterisk and subependymal with a red open arrowhead) on T2-weighted coronal 3 T MRI from a post-mortem multiple sclerosis case, and matching histological images from the same case (C–F), highlighting that subependymal lesions are more difficult to visualize. Myelin proteolipid protein immunohistochemistry demonstrates demyelination in perivascular and subependymal lesions (low magnification in C; higher magnification of subependymal lesion in D). Activated microglia/macrophages (MHC class II) immunohistochemistry demonstrating a chronic-active perivascular lesion and a rim at the border of the subependymal lesion (low magnification in E, higher magnification in F). Panel A is reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Non-lesional pathology in deep grey matter
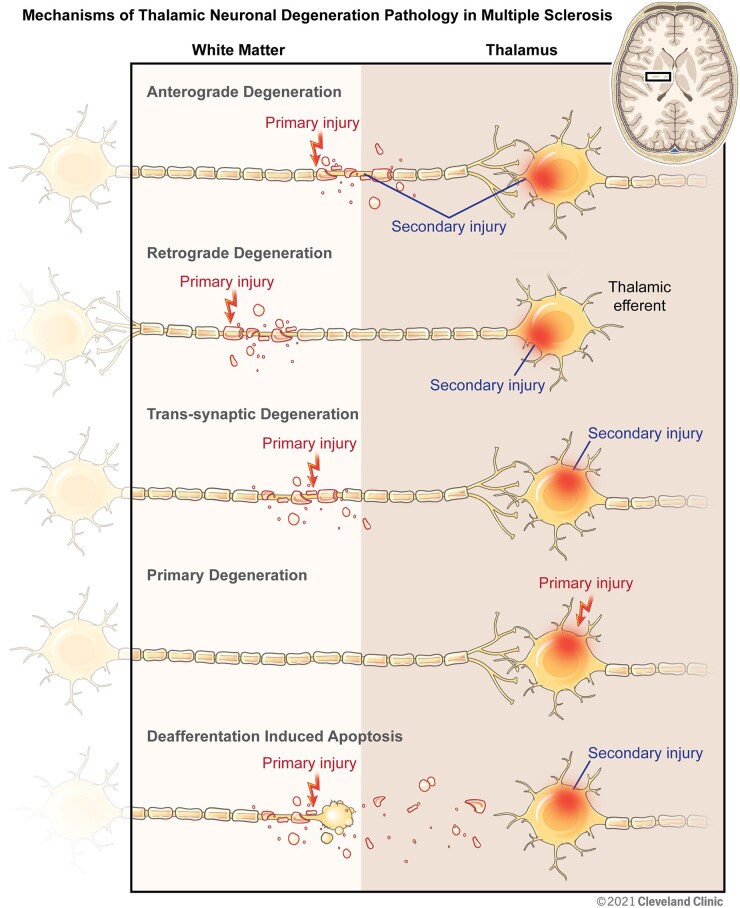
DGM volume loss occurs early in multiple sclerosis and precedes measurable whole brain volume loss.8,19,39–41 The histopathological changes observed in non-lesional DGM may help explain this early susceptibility. Neuronal density in normal appearing DGM is reduced by as much as 33%17 compared to controls and could be explained by several mechanisms. Retrograde and anterograde degeneration due to the focal axonal transection that occurs within white matter lesions are probably major mechanisms of DGM neuronal loss.42–46 Trans-synaptic degeneration, which has been well-documented with hippocampal demyelination but has also been described in the visual system (which runs through the thalamus),47,48 may also play a role. Finally, deafferentation-induced neuronal loss, as described in the cortex, may also occur in DGM.49 The cascade of white matter pathology resulting in axonal, neuronal, and synaptic changes suggests that early neurodegeneration in DGM may be a secondary phenomenon; however, a primary mechanism cannot be excluded (Fig. 2).
Figure 2.
Mechanisms of thalamic neuronal degeneration pathology in multiple sclerosis. The figure shows representations of afferent/efferent neuronal cell bodies and axons in the thalamus and outflow/inflow tracts. Under ‘Anterograde Degeneration’, a focal white matter lesion in a thalamic afferent with secondary anterograde degeneration in the axon is depicted. Under ‘Retrograde Degeneration’, a focal white matter lesion in a thalamic efferent is depicted. Under ‘Trans-synaptic Degeneration’, secondary injury in the neuron as a result of degeneration of the axon with which it forms a synapse is demonstrated. Under ‘Primary Neurodegeneration’, neuronal injury independent of a direct connection to an axon is illustrated. Under ‘Deafferentation Induced Apoptosis’ we illustrate loss of neurons resulting as a consequence of axonal transection. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography ©2020. All Rights Reserved.
Iron accumulation is a proposed mechanism for DGM injury. DGM contains high iron content, which increases with age in controls.50 In multiple sclerosis DGM, iron accumulation has been demonstrated better in vivo using iron-sensitive MRI sequences51 than on pathology.16 Iron accumulation may lead to oxidative stress, cellular damage, and neuronal injury.52 Iron release from injured oligodendrocytes results in higher turnover to microglia, which may be heightened in DGM structures and may drive more chronic inflammatory responses.43 Susceptibility measures from the thalamus are associated with disability53 and may differentiate relapsing remitting multiple sclerosis from primary progressive multiple sclerosis.54 Increased iron concentration may be the result of atrophy in the setting of a fixed iron content.55 In thalamus, some studies have shown increased T2*-weighted signal, which may represent loss of iron27,56 but may also be explained by loss of myelin or inflammation. Overall, the exact role of iron in thalamus is not clear and quantitative susceptibility mapping (QSM) may help resolve these questions. QSM has already been explored in DGM in multiple sclerosis.43,57,58
Future directions
Larger and more detailed pathologic studies are needed to elucidate the exact cellular and subcellular substrates of DGM tissue loss in multiple sclerosis. Single cell genomics to further characterize DGM cell types may shed further light on changes secondary to afferent/efferent tracts with lesions or selective vulnerability of various thalamic subregions.
Measurement of deep grey matter injury
MRI acquisition
Recent advances in pulse sequences, increased magnetic field strength, and post-processing algorithms have improved the accuracy of MRI-based DGM volume measurements.59 Several MRI contrasts can be used to identify DGM structures (Table 2), including T1-weighted imaging,60 T2-weighted imaging,62 diffusion-weighted imaging,70 magnetization transfer imaging,71,73 R2* relaxation, and T2*-weighted imaging/QSM.11,56–58 Gadolinium accumulation may preferentially affect DGM structures74,75 and may affect analysis of pixel intensities on T1/T2-weighted images and values of T2*, R2*, quantitative susceptibility mapping.
Table 2.
MRI contrasts in DGM
| Contrast | Utility | Acquisition comments |
|---|---|---|
| T1-weighted10,60,61 | Anatomical identification of boundaries and segmentation for volumetric assessments; potentially useful for lesion identification | High resolution images can be obtained with relatively short acquisition time; substantial experience in multicentre applications; thalamus may be difficult to segment due to high myelin content |
| T2-weighted62 or T2-FLAIR |
Identification of focal demyelinating lesions Intrinsic changes in T2 signal, some sensitivity to iron |
Quick acquisition; substantial experience in multicentre applications |
| T1 relaxation63,64 | Quantitative measure, can differentiate different DGM structures and subregions | Requires multiple acquisitions hence multicentre applications have been limited, but newer single-sequences approaches (e.g. MP2RAGE) may alleviate these drawbacks |
| T2 relaxation64,65 | Quantitative measure, has some sensitivity to iron content in DGM, short component is particularly sensitive to myelin | Time consuming, with limited success in multicentre applications |
| T2*11,56,66,67 | Sensitive to iron and myelin content and blood vessels in DGM, also ideal to identify boundaries of iron-containing deep grey matter structures | High resolution can be attained with relatively quick acquisition times; multicentre application is relatively straightforward; less useful at 1.5 T |
| Double inversion recovery68 | Sensitive to detection of DGM lesions | Feasible, and may also allow identification of few DGM lesions but sensitivity unknown |
| Quantitative susceptibility mapping69 | Sensitive to iron, myelin and blood vessels; can differentiate diamagnetic and paramagnetic changes | Feasible, but post-processing required; less useful at 1.5 T |
| Diffusion weighted imaging70 | Useful sensitive to microstructural | Typically lower resolution than other sequences; acquisition times are long for more complex biophysical models, which are not routinely available on commercial scanners; limited success in multicentre studies |
| Magnetization transfer imaging71,72 | Sensitive to deep grey matter demyelination and useful for thalamic segmentation | Feasible clinical acquisition times; needs careful normalization for multicentre application |
MRI analysis
DGM segmentation methods can be either atlas-based, algorithm-based, learning-based, or hybrid.76 Atlas-based approaches generated using histology or MRI have been the most widely used and include the creation of anatomical labels, which are non-linearly warped to new data.77 Atlases from several MR contrasts with specific sensitivity to different DGM structures are available. Atlas-based packages using probabilistic algorithms for segmentation of DGM structures are widely used and include FMRIB’s Integrated Registration and Segmentation Tool (FIRST),78 FreeSurfer,79 and statistical parametric mapping (SPM).80 These are primarily based on T1-weighted images, but can be improved by multiple contrasts and MR modalities used simultaneously.58 Segmentation of lesional multiple sclerosis tissue is an important step to avoid misclassification of gray matter.81 Integrated probabilistic methods with simultaneous lesion masking are available.82
Several comparisons of FSL-FIRST, SPM, and FreeSurfer have been conducted. FSL-FIRST shows the highest correlations with cognitive measures.83 A recent systematic review compared several automated segmentation techniques76 in multiple sclerosis and showed that learning-based approaches achieved the highest DGM segmentation accuracy. Multi-atlas approaches and use of additional MRI contrasts (QSM, T2*, R2*) improved results. These techniques can be used in learning-based approaches for training datasets to establish improved measures of ground truth.
Segmentation of thalamic subregions has been achieved using ultra-high field MRI in combination with multicontrast atlases. Data thus far suggest that certain subregions may show greater volume loss, including the antero-ventral, pulvinar, and habenular regions.61 Using probabilistic connectivity at 3 T, differences in thalamic subregions have been demonstrated between multiple sclerosis and neuromyelitis optica,84 with subregion 5 (premotor connection) mean diffusivity being the best discriminator. Selective vulnerability of thalamic subregions may relate to lesion location, susceptibility of specific pathways, and neuronal factors. Lesions in projecting/receiving tracts may be preferentially affected,46 as well as neurons located closer to the ventricular surface.85
Machine learning approaches
Machine learning may offer several distinct advantages over current analysis and processing techniques. Machine learning and deep learning can incorporate several MRI contrasts and measures to more efficiently explore tissue composition, structure, and function. When presented with labelled training data, machine learning algorithms can identify complex patterns from large datasets with a high number of variables, make generalizations from these learned patterns, rank the importance of variables, and then use this information to make predictions on new data.
From an image-processing standpoint, deep learning may improve the accuracy of image segmentation in multiple sclerosis,86,87 and importantly, can reduce image heterogeneity across studies. Deep learning methods can normalize image intensity, resolution, and contrast across time points and across subjects.88 Supervised machine learning methods using random forest classification of volume, thickness, surface area of cortical grey matter regions, and volumes of DGM nuclei, distinguished relapsing remitting multiple sclerosis from neuromyelitis optica with an accuracy of 74%.89 Further development of supervised machine learning and unsupervised deep learning approaches may improve analysis of DGM structures to identify optimal regions of interest.
Future directions
Significant progress has been made in DGM segmentation. Use of multimodal approaches, multi-atlas approaches, subregional approaches, and machine learning may significantly improve DGM segmentation and its utility. Pulse sequences that generate quantitative data should take into consideration the local contrast generated for each specific structure and the resolution needed. Creation of data repositories and imaging toolboxes as shared resources will advance discovery. To enhance real-world utility, segmentation would ideally use pulse sequences that are widely available or postprocessing algorithms that are robust to different input sequences. Finally, the long-term goal of these measures is clinical translation, which requires multicentre validation and determination of clinical meaningfulness.
Clinical relevance of deep grey matter injury
Thalamus
Thalamic volume loss is observed on MRI in the earliest identifiable phases of multiple sclerosis, including pediatric multiple sclerosis,90,91 clinically isolated syndrome (CIS),40,41 and radiologically isolated syndrome,19 whereas whole brain volume and total grey matter volume may still be preserved. Thalamic volume declines consistently as a function of disease7 and, correlates with clinical end points, including cognition,6,7,92,93 and provides feasible sample sizes as a primary end point for clinical trials.7 Thalamic volume was shown to be modifiable in several recent randomized, placebo-controlled trials (see below). Given its high sensitivity in early disease and correlation with cognition, thalamic volume may be particularly useful in studies targeting cognition, or younger patients early in the disease.
Impact of normal ageing on deep grey matter measurements
Limited data exist regarding the impact of normal ageing on grey matter structures in multiple sclerosis. Whole and regional brain volumes can follow a linear or non-linear trajectory of decline,94–96 suggesting that the contribution of normal ageing to brain atrophy in multiple sclerosis may not be constant.
In a recent analysis, normal ageing was shown to contribute to whole brain and thalamic atrophy, whereas it did not in the putamen and caudate.20 Most atrophy observed in whole brain and thalamus in early adulthood was multiple sclerosis-related, and by age 60, most of the atrophy was primarily attributable to normal ageing. The lack of ageing effect in the caudate and putamen suggests DGM atrophy may differ across structures, an area of high interest for future studies.
Deep grey matter as an outcome in clinical trials
While the effects of disease-modifying therapies on whole brain volume in multiple sclerosis97 have been extensively described, effects of disease-modifying therapies on DGM structures are only beginning to be investigated. Thalamic volume loss was reduced by ozanimod and is the first example where thalamic volume was reported in the primary analysis of a phase 3 study in multiple sclerosis.98,99 Thalamic volume loss was slower in laquinomod,100 fingolimod,101 and ibudilast102 treated patients compared to placebo. In a multivariate retrospective analysis of a small, non-randomized cohort, natalizumab and rituximab were associated with a slower rate of thalamic and putaminal atrophy compared to interferon-beta and glatiramer acetate, whereas whole-brain volume decline did not differ between the two groups.103 Exploring the differential effect of treatments on DGM may provide insight into specific pathways of injury related to these structures101; however, the feasibility of advanced MRI sequences must be considered for trial application (Table 2). It is likely that DGM MRI end points will be incorporated in future clinical trials. Tailoring the MRI end point to the patient population or clinical end point being studied could reduce sample sizes and improve trial efficiency.
Incorporating deep grey matter measurements into clinical practice
Incorporating brain volumetrics, including DGM, into clinical practice has proven challenging for several reasons, which have been reviewed.104 Developing image-processing algorithms that are robust to image heterogeneity would address many barriers. A gold standard for DGM measurements is needed, and the variability in each structure needs to be known and accounted for. Differences in acquisition, gradient distortion, intrascanner variability, movement, and scanner upgrades have been cited as sources of variability for brain volume.105 Whether DGM measures are susceptible to these same confounders as well as the effects of pseudoatrophy (initial apparent brain volume loss associated with anti-inflammatory effect),106,107 in measures of total versus individual DGM structures, are outstanding questions. Statistical techniques to translate DGM measurements into clinically relevant outcomes at the individual level are needed, and will need to account for age, treatment status, and measurement error at a minimum.
Hippocampus
Although not considered a DGM region, many studies examining DGM include the hippocampus in the analysis and classification of DGM structures,108 and also because imaging methods sensitive to DGM damage are used to assess the hippocampus. The hippocampus is composed of several subregions, each of which has a distinct function and susceptibility to pathology.109 It plays an important role in memory, regulation of mood, and emotional response.110 Histopathological studies show extensive involvement of the hippocampus in multiple sclerosis, including widespread demyelination, neuronal loss, and synaptic loss.29–32 Using MRI, total hippocampal volume loss has been associated with cognitive impairment in multiple sclerosis.111 Atrophy in the CA1 subregion is associated with deficits in verbal memory in early relapsing remitting multiple sclerosis,21 and atrophy in the CA3 subregion is associated with depression.112 CA4 subregion/dentate gyrus may be the earliest subfield with volume loss compared to healthy controls, and that CA4/dentate volume predicts atrophy in the CA1 subregion 1 year later,113 suggesting a ‘dynamic spread’ of pathology.
Total hippocampal volume, or CA1 and/or CA3 subregional volumes, could be useful to study cognitive dysfunction and depression in multiple sclerosis, which lack sensitive and specific MRI correlates. Moreover, because of the potential for structural and functional plasticity within the hippocampal network,114 the hippocampus may be an interesting target for cognitive rehabilitation and neuroprotection interventions. The effects of exercise115 and cognitive rehabilitation116 on the hippocampus have been studied in several populations, including multiple sclerosis. Exercise interventions preserve or even increase hippocampal volume or activity, potentially leading to improvements in memory.116,117
Because of low MR contrast with surrounding tissues, the hippocampus remains a technically challenging region to segment, particularly at the subregion level. Multiple automated segmentation algorithms exist, with variable levels of agreement with manual segmentation.118 Areas of interest for future hippocampal work in multiple sclerosis include improving longitudinal volume measurements, validating automated subregion techniques, pursuing hippocampal connectivity studies, and exploring the potential to preserve or reverse hippocampal damage, memory loss, and mood impairments using rehabilitative strategies.
Deep grey matter atrophy to understand disease biology better
Most of the current literature in multiple sclerosis has examined whole-brain and DGM volumes as predictors of clinical worsening. However, brain atrophy represents irreversible tissue loss, which is precisely what disease-modifying therapies aim to prevent. Future studies may use atrophy as the outcome rather than the predictor, to study biologic mechanisms of volume loss. The DGM is particularly well-suited for such studies because of the breadth of multiple sclerosis pathology present, mixed white and GM structures, early disease impact, and the highly connected nature of several regions. Different structures may provide insights into specific pathogenic mechanisms. For example, the mechanism of thalamic atrophy likely differs from that of hippocampal or basal ganglia atrophy, with different contributions from white matter lesions, intrinsic DGM lesions, iron deposition, oxidative stress, primary degeneration, etc., within each structure.10,11,18,45,46 A long-term goal is to understand the contribution of mechanisms of injury within each structure, leading to more specific targets using imaging endpoints for specific biological pathways.
Conclusions
DGM injury in multiple sclerosis is common and results from a combination of focal lesions and changes in normal-appearing DGM, as well as upstream/downstream injury secondary to distant lesions. Significant improvement in DGM segmentation has resulted in ample evidence demonstrating the clinical relevance of DGM in multiple sclerosis. Improvements in acquisition, segmentation (including subregions), and analysis methods promise to refine DGM measures further (DGM lesional volume, thalamic volume, caudate volume, hippocampal volume) and enable future use in clinical trials, potentially even as primary outcome for phase 2 studies focusing on neuroprotection. DGM clinical diagnostic tools and biomarkers of therapeutic response will be accelerated by shared resources including pulse sequences, imaging datasets, and analysis tools.
Acknowledgements
The authors acknowledge Jennifer Brydges and Emily Viles from AMPed for meeting organization and the American Committee for Research and Treatment in MS for use of space for the consensus meeting. The authors also acknowledge Amanda Mendelsohn for assistance with creation and editing of figures.
Funding
No direct funding supported this work. The authors thank the Race to Erase MS Foundation for support of NAIMS and meeting logistics.
Competing interests
D.O. reports research support from the National Institutes of Health, National Multiple Sclerosis Society, Patient Centered Outcomes Research Institute, Race to Erase MS Foundation, Genentech, Genzyme, and Novartis. Consulting fees from Biogen Idec, Genentech/Roche, Genzyme, Novartis, and Merck. K.R.M. reports research support from the National MS Society and National Institute of Health. D.L.A. reports personal compensation for serving as a Consultant for Alexion, Biogen, Celgene, Frequency Therapeutics, GENeuro, Genentech, Merck/EMD Serono, Novartis, Roche, and Sanofi. D.L.A. has an ownership interest in NeuroRx. M.G.D reports research support from the National Institutes of Health, National Multiple Sclerosis Society, Novartis, Merck, and Bristol Myers Squibb. Consulting fees from Novartis, Bristol Myers Squibb, and Keystone Heart. S.A.G reports research support from the National Institutes of Health, Genentech, Genzyme, and Mallinckrodt. Consulting fees from Biogen Idec. D.N.G. reports no competing interests related to this work; research support from the National Institutes of Health grants R01EB023281, R01NS105820, and R01NS112161. D.M.H. reports research support from the National Institutes of Health, National Multiple Sclerosis Society, Genentech, and EMD-Serono. Consulting fees from Biogen, Genentech, EMD-Serono, and Sanofi-Genzyme. Royalties and writing fees from UpToDate Inc. and the American College of Physicians. R.G.H. reports consulting fees from Roche/Genentech, Atara, Sanofi/Genzyme, Celgene/Bristol Myers Squibb, MEDDAY, QIA Consulting and grants from Roche/Genentech, MEDDAY, and Atara. D.K.B.L. has received research funding from the Canadian Institute of Health Research and Multiple Sclerosis Society of Canada. He is Emeritus Director of the UBC MS/MRI Research Group which has been contracted to perform central analysis of MRI scans for therapeutic trials with Roche and Sanofi-Genzyme. The UBC MS/MRI Research Group has also received grant support for investigator-initiated studies from Sanofi-Genzyme, Novartis and Roche. He has served on the PML-MS Steering Committee for Biogen. He has given lectures, supported by non-restricted education grants from Academy of Health Care Learning, Biogen, Consortium of MS Centers and Sanofi-Genzyme. C.M. has received funding from Sanofi-Genzyme, EMD Serono, Genentech, the MS National Society, the National Institute of Health and the Department of Defense outside the scope of this work. W.M. reports current research funding from the Multiple Sclerosis Society of Canada. At the time of this conference he was a member of the Medical Advisory Committee of the Multiple Sclerosis Society of Canada. In the past, he has received a grant in aid of research from Berlex Canada, has been a consultant for Schering, and received an honorarium from Teva for teaching. S.N. reports research funding from the Canadian Institutes of Health Research, the Myelin Repair Foundation and Immunotec; consulting fees from Genentech; part-time employment with NeuroRx Research. J.O. reports esearch support from the MS Society of Canada, Brain Canada, National MS Society, Biogen-Idec, Roche, EMD-Serono. Consulting fees from Biogen-Idec, EMD-Serono, Sanofi-Genzyme, Roche, Novartis, BMS, and Alexion. D.P. has received consulting fees from Biogen, Roche, Sanofi Genzyme, Novartis, and EMD Serono. A.R. has received research funding from the Canadian Institutes of Health Research and the National Multiple Sclerosis Society. W.D.R. reports research support from the National Institutes of Health, Conrad N. Hilton Foundation, Paul G. Allen Family Foundation, Race to Erase MS Foundation, and Revalesio, Inc. N.L.S. reports research support from the National MS Society, Patient Centered Outcomes Research Institute, and the National Institute of Health. R.T. reports grants from Multiple Sclerosis Society of Canada, Natural Sciences and Engineering Research Council of Canada, Mitacs, Praxis Spinal Cord Institute, and UBC Data Science Institute. D.S.R is supported by the Intramural Research Program of NINDS, NIH. C.J.A. has received consulting fees from Guerbet LLC, Genentech, Biogen Idec, Novartis, Sanofi Genzyme, EMD Serono, and Alexion Pharmaceuticals, outside the submitted work. All other authors report no competing interests.
Glossary
- DGM =
deep grey matter
- MR =
magnetic resonance
References
- 1.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L.. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–285. [DOI] [PubMed] [Google Scholar]
- 2.Kornek B, Lassmann H.. Axonal pathology in multiple sclerosis. A historical note. Brain Pathol. 1999;9(4):651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutzelnigg A, Lassmann H.. Cortical lesions and brain atrophy in MS. J Neurologic Sci. 2005;233(1-2):55–59. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi R, Czarnecki D, Shaikh ZA.. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport. 2000;11(6):1153–1158. [DOI] [PubMed] [Google Scholar]
- 5.Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM.. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002;52(5):650–653. [DOI] [PubMed] [Google Scholar]
- 6.Houtchens MK, Benedict RHB, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69(12):1213–1223. [DOI] [PubMed] [Google Scholar]
- 7.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018;83(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zivadinov R, Havrdova E, Bergsland N, et al. Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology. 2013;268(3):831–841. [DOI] [PubMed] [Google Scholar]
- 9.Shiee N, Bazin PL, Zackowski KM, et al. Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One. 2012;7(5):e37049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison DM, Oh J, Roy S, et al. Thalamic lesions in multiple sclerosis by 7T MRI: Clinical implications and relationship to cortical pathology. Mult Scler. 2015;21(9):1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehndiratta A, Treaba CA, Barletta V, et al. Characterization of thalamic lesions and their correlates in multiple sclerosis by ultra-high-field MRI. Mult Scler J. 2021;27(5):674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: A consensus approach. Mult Scler. 2008;14(9):1157–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M.. Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;70(3):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy H, Narayanan S, Arnoutelis R, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123(11): 2314–2320. [DOI] [PubMed] [Google Scholar]
- 15.Sarchielli P, Presciutti O, Tarducci R, et al. Localized (1)H magnetic resonance spectroscopy in mainly cortical gray matter of patients with multiple sclerosis. J Neurol. 2002;249(7):902–910. [DOI] [PubMed] [Google Scholar]
- 16.Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014;85(12):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vercellino M, Masera S, Lorenzatti M, et al. Demyelination, inflammation, and neurodegeneration in multiple sclerosis deep gray matter. J Neuropathol Exp Neurol. 2009;68(5):489–502. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan KR, Nakamura K, Cohen JA, Trapp BD, Ontaneda D.. Intrinsic and extrinsic mechanisms of thalamic pathology in multiple sclerosis. Ann Neurol. 2020;88(1):81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azevedo CJ, Overton E, Khadka S, et al. Early CNS neurodegeneration in radiologically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azevedo CJ, Cen SY, Jaberzadeh A, Zheng L, Hauser SL, Pelletier D.. Contribution of normal aging to brain atrophy in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(Pt 4):1134–1141. [DOI] [PubMed] [Google Scholar]
- 22.Harrison DM, Roy S, Oh J, et al. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 2015;72(9):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggieri S, Petracca M, Miller A, et al. Association of deep gray matter damage with cortical and spinal cord degeneration in primary progressive multiple sclerosis. JAMA Neurol. 2015;72(12):1466. [DOI] [PubMed] [Google Scholar]
- 24.Roosendaal SD, Moraal B, Pouwels PJW, et al. Accumulation of cortical lesions in MS: Relation with cognitive impairment. Mult Scler. 2009;15(6):708–714. [DOI] [PubMed] [Google Scholar]
- 25.Hopp K, Popescu BFG, McCrea RPE, et al. Brain iron detected by SWI high pass filtered phase calibrated with synchrotron X-ray fluorescence. J Magn Reson Imaging. 2010;31(6): 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Radaideh AM, Wharton SJ, Lim S-Y, et al. Increased iron accumulation occurs in the earliest stages of demyelinating disease: An ultra-high field susceptibility mapping study in Clinically Isolated Syndrome. Mult Scler. 2013;19(7):896–903. [DOI] [PubMed] [Google Scholar]
- 27.Khalil M, Langkammer C, Pichler A, et al. Dynamics of brain iron levels in multiple sclerosis: A longitudinal 3T MRI study. Neurology. 2015;84(24):2396–2402. [DOI] [PubMed] [Google Scholar]
- 28.Huitinga I, De Groot CJ, Van der Valk P, Kamphorst W, Tilders FJ, Swaab DF.. Hypothalamic lesions in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60(12):1208–1218. [DOI] [PubMed] [Google Scholar]
- 29.Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P.. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64(12):1101–1107. [DOI] [PubMed] [Google Scholar]
- 30.Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66(9):819–827. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R.. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19(2):238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutta R, Chang A, Doud MK, et al. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69(3):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dal Bianco A, Bradl M, Frischer J, Kutzelnigg A, Jellinger K, Lassmann H.. Multiple sclerosis and Alzheimer’s disease. Ann Neurol. 2008;63(2):174–183. [DOI] [PubMed] [Google Scholar]
- 34.Peterson JW, Bo L, Mork S, Chang A, Trapp BD.. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389–400. [DOI] [PubMed] [Google Scholar]
- 35.Kipp M, Wagenknecht N, Beyer C, Samer S, Wuerfel J, Nikoubashman O.. Thalamus pathology in multiple sclerosis: From biology to clinical application. Cell Mol Life Sci. 2015;72(6):1127–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology. 2013;80(2):210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmore CP, Donaldson I, Bö L, et al. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: A comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80(2):182–187. [DOI] [PubMed] [Google Scholar]
- 38.Adams CWM, Abdulla YH, Torres EM, Poston RN.. Periventricular lesions in multiple sclerosis: Their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol. 1987;13(2):141–152. [DOI] [PubMed] [Google Scholar]
- 39.Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain. 2018;141(6):1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steckova T, Hlustik P, Sladkova V, Odstrcil F, Mares J, Kanovsky P.. Thalamic atrophy and cognitive impairment in clinically isolated syndrome and multiple sclerosis. J Neurol Sci. 2014;342(1-2):62–68. [DOI] [PubMed] [Google Scholar]
- 41.Henry RG, Shieh M, Okuda DT, Evangelista A, Gorno-Tempini ML, Pelletier D.. Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry. 2008;79(11):1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikić I, Merkler D, Sorbara C, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17(4):495–499. [DOI] [PubMed] [Google Scholar]
- 43.Schweser F, Raffaini Duarte Martins AL, Hagemeier J, et al. Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: A proposed mechanistic relationship between inflammation and oligodendrocyte vitality. Neuroimage. 2018;167:438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadopoulou A, Gaetano L, Pfister A, et al. Damage of the lateral geniculate nucleus in MS. Neurology. 2019;92(19):e2240–e2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolasinski J, Stagg CJ, Chance SA, et al. A combined post-mortem magnetic resonance imaging and quantitative histological study of multiple sclerosis pathology. Brain. 2012;135(Pt 10):2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry RG, Shieh M, Amirbekian B, Chung S, Okuda DT, Pelletier D.. Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci. 2009;282(1-2):61–66. [DOI] [PubMed] [Google Scholar]
- 47.Reich DS, Smith SA, Gordon-Lipkin EM, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66(8):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Louzi O, Button J, Newsome SD, Calabresi PA, Saidha S.. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: A case series. Mult Scler. 2017;23(7):1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koliatsos VE, Dawson TM, Kecojevic A, Zhou Y, Wang YF, Huang KX.. Cortical interneurons become activated by deafferentation and instruct the apoptosis of pyramidal neurons. Proc Natl Acad Sci U S A. 2004;101(39):14264–14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallgren B, Sourander P.. The effect of age on the non‐haemin iron in the human brain. J Neurochem. 1958;3(1):41–51. [DOI] [PubMed] [Google Scholar]
- 51.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64(6):707–713. [DOI] [PubMed] [Google Scholar]
- 52.Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR.. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–873. [DOI] [PubMed] [Google Scholar]
- 53.Zivadinov R, Tavazzi E, Bergsland N, et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology. 2018;289(2):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgetova A, Dusek P, Vaneckova M, et al. Thalamic iron differentiates primary-progressive and relapsing-remitting multiple sclerosis. Am J Neuroradiol. 2017;38(6):1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernández‐Torres E, Wiggermann V, Machan L, et al. Increased mean R2 in the deep gray matter of multiple sclerosis patients: Have we been measuring atrophy? J Magn Reson Imaging. 2019;50(1):201–208. [DOI] [PubMed] [Google Scholar]
- 56.Louapre C, Govindarajan ST, Giannì C, et al. Heterogeneous pathological processes account for thalamic degeneration in multiple sclerosis: Insights from 7 T imaging. Mult Scler J. 2018;24(11):1433–1444. [DOI] [PubMed] [Google Scholar]
- 57.Cobzas D, Sun H, Walsh AJ, Lebel RM, Blevins G, Wilman AH.. Subcortical gray matter segmentation and voxel-based analysis using transverse relaxation and quantitative susceptibility mapping with application to multiple sclerosis. J Magn Reson Imaging. 2015;42(6):1601–1610. [DOI] [PubMed] [Google Scholar]
- 58.Feng X, Deistung A, Dwyer MG, et al. An improved FSL-FIRST pipeline for subcortical gray matter segmentation to study abnormal brain anatomy using quantitative susceptibility mapping (QSM). Magn Reson Imaging. 2017;39:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller SS, Gerdes JS, Mohammadi S, et al. Volume estimation of the thalamus using freesurfer and stereology: Consistency between methods. Neuroinformatics. 2012;10(4):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rocca MA, Comi G, Filippi M.. The role of T1-weighted derived measures of neurodegeneration for assessing disability progression in multiple sclerosis. Front Neurol. 2017;8:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Planche V, Su JH, Mournet S, et al. White-matter-nulled MPRAGE at 7T reveals thalamic lesions and atrophy of specific thalamic nuclei in multiple sclerosis. Mult Scler J. 2020;26(8):987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bakshi R, Benedict RH, Bermel RA, et al. T2 hypointensity in the deep gray matter of patients with multiple sclerosis: A quantitative magnetic resonance imaging study. Arch Neurol. 2002;59(1):62–68. [DOI] [PubMed] [Google Scholar]
- 63.Steenwijk MD, Vrenken H, Jonkman LE, et al. High-resolution T1-relaxation time mapping displays subtle, clinically relevant, gray matter damage in long-standing multiple sclerosis. Mult Scler. 2016;22(10):1279–1288. [DOI] [PubMed] [Google Scholar]
- 64.Traynor CR, Barker GJ, Crum WR, Williams SCR, Richardson MP.. Segmentation of the thalamus in MRI based on T1 and T2. Neuroimage. 2011;56(3):939–950. [DOI] [PubMed] [Google Scholar]
- 65.Hasan KM, Walimuni IS, Abid H, et al. Multimodal quantitative magnetic resonance imaging of thalamic development and aging across the human lifespan: Implications to neurodegeneration in multiple sclerosis. J Neurosci. 2011;31(46):16826–16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khalil M, Langkammer C, Ropele S, et al. Determinants of brain iron in multiple sclerosis: A quantitative 3T MRI study. Neurology. 2011;77(18):1691–1697. [DOI] [PubMed] [Google Scholar]
- 67.Yarnykh VL, Krutenkova EP, Aitmagambetova G, et al. Iron-insensitive quantitative assessment of subcortical gray matter demyelination in multiple sclerosis using the macromolecular proton fraction. Am J Neuroradiol. 2018;39(4):618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van De Pavert SHP, Muhlert N, Sethi V, et al. DIR-visible grey matter lesions and atrophy in multiple sclerosis: Partners in crime? J Neurol Neurosurg Psychiatry. 2016;87(5):461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujiwara E, Kmech JA, Cobzas D, et al. Cognitive implications of deep gray matter iron in multiple sclerosis. Am J Neuroradiol. 2017;38(5):942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV.. Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiol Aging. 2010;31(3):482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helms G, Draganski B, Frackowiak R, Ashburner J, Weiskopf N.. Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. Neuroimage. 2009;47(1):194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallik S, Muhlert N, Samson RS, et al. Regional patterns of grey matter atrophy and magnetisation transfer ratio abnormalities in multiple sclerosis clinical subgroups: A voxel-based analysis study. Mult Scler J. 2015;21(4):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gringel T, Schulz-Schaeffer W, Elolf E, Frölich A, Dechent P, Helms G.. Optimized high-resolution mapping of magnetization transfer (MT) at 3 Tesla for direct visualization of substructures of the human thalamus in clinically feasible measurement time. J Magn Reson Imaging. 2009;29(6):1285–1292. [DOI] [PubMed] [Google Scholar]
- 74.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D.. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadoliniumbased contrast material. Radiology. 2014;270(3):834–841. [DOI] [PubMed] [Google Scholar]
- 75.McDonald RJ, Levine D, Weinreb J, et al. Gadolinium retention: A research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289(2):517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pagnozzi AM, Fripp J, Rose SE.. Quantifying deep grey matter atrophy using automated segmentation approaches: A systematic review of structural MRI studies. Neuroimage. 2019;201:116018. [DOI] [PubMed] [Google Scholar]
- 77.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 78.Patenaude B, Smith SM, Kennedy DN, Jenkinson M.. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fischl B.FreeSurfer. Neuroimage. 2012;62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashburner J, Friston KJ.. Unified segmentation. Neuroimage. 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 81.González-Villà S, Oliver A, Huo Y, Lladó X, Landman BA.. Brain structure segmentation in the presence of multiple sclerosis lesions. NeuroImage Clin. 2019;22:101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cerri S, Hoopes A, Greve D, Mühlau M, Van Leemput K. A Longitudinal Method for Simultaneous Whole-Brain and Lesion Segmentation in Multiple Sclerosis. In: Kia SM, Mohy-ud-Din H, Abdulkadir A, et al, eds. Machine Learning in Clinical Neuroimaging and Radiogenomics in Neuro-oncology. MLCN 2020, RNO-AI 2020. Lecture Notes in Computer Science; vol 12449. Springer, Cham; 2020:119–128.
- 83.Popescu V, Schoonheim MM, Versteeg A, et al. Grey matter atrophy in multiple sclerosis: Clinical interpretation depends on choice of analysis method. PLoS One. 2016;11(1):e0143942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y, Duan Y, Huang J, et al. Multimodal quantitative MR imaging of the thalamus in multiple sclerosis and neuromyelitis optica1. Radiology. 2015;277(3):784–792. [DOI] [PubMed] [Google Scholar]
- 85.Fadda G, Brown RA, Magliozzi R, et al. ; Canadian Pediatric Demyelinating Disease Network. A surface-in gradient of thalamic damage evolves in pediatric multiple sclerosis. Ann Neurol. 2019;85(3):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sweeney EM, Vogelstein JT, Cuzzocreo JL, et al. A comparison of supervised machine learning algorithms and feature vectors for MS lesion segmentation using multimodal structural MRI. PLoS One. 2014;9(4):e95753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brosch T, Tang LYW, Yoo Y, Li DKB, Traboulsee A, Tam R.. Deep 3D convolutional encoder networks with shortcuts for multiscale feature integration applied to multiple sclerosis lesion segmentation. IEEE Trans Med Imaging. 2016;35(5):1229–1239. [DOI] [PubMed] [Google Scholar]
- 88.Dewey BE, Zhao C, Reinhold JC, et al. DeepHarmony: A deep learning approach to contrast harmonization across scanner changes. Magn Reson Imaging. 2019;64:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eshaghi A, Wottschel V, Cortese R, et al. Gray matter MRI differentiates neuromyelitis optica from multiple sclerosis using random forest. Neurology. 2016;87(23):2463–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aubert-Broche B, Fonov V, Ghassemi R, et al. Regional brain atrophy in children with multiple sclerosis. Neuroimage. 2011;58(2):409–415. [DOI] [PubMed] [Google Scholar]
- 91.Mesaros S, Rocca MA, Absinta M, et al. Evidence of thalamic gray matter loss in pediatric multiple sclerosis. Neurology. 2008;70(13 Part 2):1107–1112. [DOI] [PubMed] [Google Scholar]
- 92.Zivadinov R, Bergsland N, Dolezal O, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. Am J Neuroradiol. 2013;34(10):1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eshaghi A, Prados F, Brownlee WJ, et al. ; on behalf of the MAGNIMS study group. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fjell AM, Walhovd KB, Westlye LT, et al. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage. 2010;50(4):1376–1383. [DOI] [PubMed] [Google Scholar]
- 95.Schippling S, Ostwaldt AC, Suppa P, et al. Global and regional annual brain volume loss rates in physiological aging. J Neurol. 2017;264(3):520–528. [DOI] [PubMed] [Google Scholar]
- 96.Coupé P, Catheline G, Lanuza E, Manjón JV.; Alzheimer's Disease Neuroimaging Initiative. Towards a unified analysis of brain maturation and aging across the entire lifespan: A MRI analysis. Hum Brain Mapp. 2017;38(11):5501–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): A multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–1020. [DOI] [PubMed] [Google Scholar]
- 99.Cohen JA, Comi G, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): A multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021–1033. [DOI] [PubMed] [Google Scholar]
- 100.Filippini G, Del Giovane C, Clerico M, et al. Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis. Cochrane Database Syst Rev. 2017;4:CD012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaetano L, Häring DA, Radue EW, et al. Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurology. 2018;90(15):e1324–e1332. [DOI] [PubMed] [Google Scholar]
- 102.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials. 2016;50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sotirchos ES, Gonzalez-Caldito N, Dewey BE, et al. Effect of disease-modifying therapies on subcortical gray matter atrophy in multiple sclerosis. Mult Scler J. 2020;26(3):312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azevedo CJ, Pelletier D.. Whole-brain atrophy: Ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol. 2016;29(3):237–242. [DOI] [PubMed] [Google Scholar]
- 105.Sastre-Garriga J, Pareto D, Battaglini M, et al. ; MAGNIMS study group. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dwyer MG, Zivadinov R, Tao Y, et al. Immunological and short-term brain volume changes in relapsing forms of multiple sclerosis treated with interferon beta-1a subcutaneously three times weekly: an open-label two-arm trial. BMC Neurol. 2015;15:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fisher E, Nakamura K, Lee JC, You X, Sperling B, Rudick RA.. Effect of intramuscular interferon beta-1a on gray matter atrophy in relapsing-’remitting multiple sclerosis: a retrospective analysis. Mult Scler. 2016;22(5):668–676. [DOI] [PubMed] [Google Scholar]
- 108.Narvacan K, Treit S, Camicioli R, Martin W, Beaulieu C.. Evolution of deep gray matter volume across the human lifespan. Hum Brain Mapp. 2017;38(8):3771–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nunn J, Hodges H.. Cognitive deficits induced by global cerebral ischaemia: Relationship to brain damage and reversal by transplants. Behav Brain Res. 1994;65(1):1–31. [DOI] [PubMed] [Google Scholar]
- 110.McDonald AJ, Mott DD.. Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. J Neurosci Res. 2017;95(3):797–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Damjanovic D, Valsasina P, Rocca MA, et al. Hippocampal and deep gray matter nuclei atrophy is relevant for explaining cognitive impairment in MS: A multicenter study. Am J Neuroradiol. 2017;38(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gold SM, Kern KC, , O’Connor MF, et al. Smaller cornu ammonis 2-3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms.Biol Psychiatry. 2010;68(6):553–559. [DOI] [PMC free article] [PubMed]
- 113.Planche V, Koubiyr I, Romero JE, et al. Regional hippocampal vulnerability in early multiple sclerosis: Dynamic pathological spreading from dentate gyrus to CA1. Hum Brain Mapp. 2018;39(4):1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rocca MA, Barkhof F, De Luca J, et al. The hippocampus in multiple sclerosis. Lancet Neurol. 2018;17(10):918–926. [DOI] [PubMed] [Google Scholar]
- 115.Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage. 2018;166:230–238. [DOI] [PubMed] [Google Scholar]
- 116.Hampstead BM, Stringer AY, Stilla RF, Giddens M, Sathian K.. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus. 2012;22(8):1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dill V, Franco AR, Pinho MS.. Automated methods for hippocampus segmentation: The evolution and a review of the state of the art. Neuroinformatics. 2015;13(2):133–150. [DOI] [PubMed] [Google Scholar]