Summary:
T-cell large granular lymphocyte leukaemia (T-LGLL) is a chronic clonal lymphoproliferative disorder of cytotoxic T lymphocytes (CTL), which commonly occurs in older patients and is often associated with autoimmune diseases. Among 246 patients with T-LGLL seen at our institution over the last 10 years, we encountered 15 cases following solid organ or haematopoietic stem cell transplantation. Here, we studied the clinical characterization of these cases and compared them to de novo T-LGLL. This experience, represented a clear picture of the intricate nature of the disease manifestation and the complexities of several immune mechanisms triggering the clonal expansion.
Keywords: LGLL, solid organ transplantation, bone marrow transplantation
T-cell large granular lymphocytic leukaemia (T-LGLL) is a chronic clonal lymphoproliferative syndrome of cytotoxic T lymphocytes (CTL). T-LGLL occurs most commonly in older patients and is often associated with autoimmune diseases such as rheumatoid arthritis (RA), B cell dyscrasias, non-hematologic cancers and immunodeficiency (e.g., hypogammaglobulinemia). While there is a great deal of overlap with reactive (physiologic) CTL responses, some cases are autonomous due to the acquisition of STAT3 mutations (STAT3MT) (Andersson et al, 2013). Similarly, the clinical spectrum of manifestations ranges from self-limited polyclonal expansion to oligo/monoclonal lymphocytosis or even to an overt LGL leukaemia (LGLL) with cytopenia as a paraneoplastic clinical presentation. The resemblance of LGLL manifestations to reactive immune processes leads to frequent diagnostic dilemmas; LGLL looks like and behaves like CTL during viral infections, for instance during Epstein Barr virus (EBV) associated infectious mononucleosis.
Exuberant CTL responses (presumed origin of LGLL) can be envisioned in autoimmune processes, strong immune reactions due to alloantigen stimulation in graft rejection or graft-vs-host disease (GvHD) or over-compensatory CTL reactions to infectious agents in the setting of humoral immunodeficiency [e.g., hypogammaglobulinemia, common variable immunodeficiency (CVID)] (Viny et al, 2008). Previously, we and others described clonally skewed CTL responses and oligoclonal immunodominant T cell clonotypes following allogeneic haematopoietic stem cell transplantation (HSCT) (O’Keefe et al, 2004 & O’Keefe et al, 2007 & Li et al, 2015). Such skewed CTL responses, are reminiscent of T-LGLL we repeatedly encountered in allotransplant recipients. Additionally, our meta-analysis of case reports and series (1994-2014) has identified 62 cases (solid organ transplantation: SOT, n=22; HSCT–associated T-LGL/LGLL, n=40) seen in 10 different institutions (Table S1). However, early historical reports while clinically insightful, were not all uniformly diagnosed with some of the cases likely corresponding to oligoclonality skewed reactive CTL responses rather than real T-LGLL. For instance, in one series, diagnostic confirmation was available in only 5/13 cases (Nann-Rutti et al, 2012). It is likely that only a fraction of the reported cases represented a true “WHO-defined T-LGLL” manifestation (Swerdlow et al, 2016). In our cohort, all cases were stringently and uniformly diagnosed when 3 out of 4 following criteria were fulfilled, including: 1) LGL count >500/μL in blood for more than 6 months; 2) presence of abnormal CTLs expressing CD3, CD8 and CD57 by flow cytometry; 3) preferential usage of a TCR Vβ family by flow cytometry; 4) TCR gene rearrangement by PCR. Moreover, targeted deep sequencing for STAT3MT (Koskela et al, 2012; Sanikommu et al, 2018) was performed as well as bone marrow biopsies to exclude other conditions. Here, we report an analysis of 15 cases of overt T-LGLL following SOT (n=8) and HSCT (n=7; Table 1). Overall, these cases constituted 6% of a total of 246 adult patients with T-LGLL seen in our clinic over the last 10 years. While this series is not the first report of the post-transplantation T-LGLL, it is the first to aim to comprehensively study “WHO-defined T-LGLL” in both SOT and HSCT simultaneously and to compare them to a control group of de novo T-LGLL (cases with no prior history of SOT or HSCT; n=231).
Table 1.
Type of transplantation and individual characteristics of post-transplantation T-LGLL patients
| Case | Age*/Gender | Antecedent diagnosis |
Type of transplant |
Immunosuppressive therapy |
Organ rejection |
T-cell chimerism |
GvHD | Time since transplant (y) |
|---|---|---|---|---|---|---|---|---|
| 1 | 53/M | DLBCL | autologous SCT | − | NA | − | 7 | |
| 2 | 59/M | ESRD/cardiomyopathy | kidney/heart | CsA, MMF | + | GF | − | 8/25 |
| 3 | 38/M | cardiomyopathy | heart | Gc | − | NA* | − | 4 |
| 4 | 31/F | ESRD | kidney | ATG, Tac, Gc, MMF | − | NA* | − | 3.6 |
| 5 | 16/M | aplastic anemia | allogeneic SCT | CsA, Gc | + | GF | − | 0.3 |
| 6 | 55/M | CLL/SLL | autologous SCT | − | NA | − | 2 | |
| 7 | 57/M | HCV cirrhosis | liver | CsA, Gc | − | NA* | − | 26 |
| 8 | 58/M | mantle cell lymphoma | autologous SCT | − | NA | − | 0.2 | |
| 9 | 43/M | lupus nephritis | kidney | Tac, Gc, CP | + | GF | − | 13 |
| 10 | 60/F | PSC | liver | Tac | − | NA* | − | 13 |
| 11 | 57/F | ESRD | kidney | Tac, MMF, Gc | − | NA* | − | 7 |
| 12 | 65/M | HBV cirrhosis | liver | − | − | NA* | − | 27 |
| 13 | 39/F | CML | UCBT | − | − | C | − | 0.5 |
| 14 | 57/F | ALL | allogeneic SCT | CsA, MMF | − | C | + | 4 |
| 15 | 36/F | ALL | allogeneic SCT | CsA, MMF | − | C | + | 3.7 |
At time of T-LGLL diagnosis
Abbreviations: M, male; F, female; DLBCL, diffuse large B-cell lymphoma; ESRD, end-stage renal disease, CLL/SLL, chronic lymphocytic leukaemia/small lymphocytic leukaemia; HCV, hepatitis C virus; PSC, primary sclerosing cholangitis; HBV, hepatitis B virus; CML, chronic myelogenous leukaemia; ALL, acute lymphoblastic leukaemia; SCT, stem cell transplant; UCBT, umbilical cord blood transplant; CsA, cyclosporine A; MMF, mycophenolate mofetil; Gc, glucocorticoids; ATG, anti-thymocyte globulin; Tac, tacrolimus; CP, cyclophosphamide; GvHD, graft versus host disease; y, years; +, present; −, absent; NA, not applicable; NA*, not available; GF, primary graft failure, C, complete/full donor chimerism achieved.
In our cohort, diagnosis was made 0.2-27 years post-transplantation (median: 4 years) suggesting a diverse etiology of T-LGLL. Signs of rejection were observed in 20% (n=3/15) and GvHD in 13% (n=2/15) of the patients (Table 1). Interestingly, T-LGLL evolved in 10 patients (67%; 10/15) despite concurrent immunosuppressive therapy (acquired immunodeficiency) including cyclosporine (n=5), tacrolimus (n=4), mycophenolate mofetil (n=5), cyclophosphamide (n=1), anti-thymocyte globulin (n=1), and corticosteroids (n=6). Pre-existing immunodeficiency including hypogammaglobulinemia, was present in 3 cases with 2 patients having diagnosis of CVID. Post-transplantation, 27% of cases presented with neutropenia (absolute neutrophil count <1.5 x109/L; n=4), 33% with thrombocytopenia (platelet count <150 x109/L; n=5) and 25% with anemia (hemoglobin <10 g/dL; n=3) (Table S2). Lymphadenopathy and splenomegaly were seen in 13% (n=2) and 33% (n=5) of the patients, respectively. Other associated conditions observed were monoclonal gammopathy of undetermined significance (MGUS, 20%; n=3) and RA (7%; n=1). At the time of T-LGLL diagnosis, relative lymphocytosis (15-91%), T lymphocytosis (49-99%) and elevated absolute LGL counts (>500 /μL; in 93% of cases) were also seen. TCR Vβ analysis identified clonal expansion of ≥1 of the Vβ proteins in 60% (n=9) of the cases; the remaining 40% (n=6) of the cases showed either a clonal process involving a Vβ protein not tested in the panel (20%; n=3) or no obvious expansion (20%; n=3). TCR gene re-arrangements were present in all tested patients (100%; 14/14). Conventional cytogenetic showed 89% normal karyotype (n=11, tested individuals 13/15). Somatic STAT3MT were identified in 15% of patients (n=2, sequenced individuals 13/15). Bone marrow evaluations in all the patients ruled out other differential etiologies for cytopenia including: myelodysplastic syndromes, aplastic anemia, pure red cell aplasia or others. All patients were tested for EBV and CMV DNA by PCR in addition to serologies of their specific IgM and IgG antibodies; 60% of cases (n=9) were seropositive for EBV when tested at different time points after transplant. Similarly, 53% (n=8) were seropositive for CMV, of which, 5 were positive post-transplantation only and 3 pre-/post-transplantation. In terms of treatment, indications included neutropenia or mixed cytopenia. Therapies, initial and overall response rates among these patients were summarized in Table S3.
As compared to de novo T-LGLL, post-transplantation T-LGLL patients were significantly younger (median age: 55 vs. 65 years; P=0.0002; Table 2), consistent with a younger age of patients undergoing transplantation. Post-transplantation T-LGLL was also characterized by significantly higher absolute LGL count (median: 4.5 vs. 8.5 k/μ, P=0.0005), reduced neutropenia but similar incidence of thrombocytopenia (27 vs. 43%, P=0.3 and 33 vs. 35%, P=1, respectively) and significantly lower odds of anemia (20% vs. 55%; P=0.01) likely due to a higher specialized medical care after transplantation, while de novo cases may represent a more symptomatic tip of the iceberg in a clinical continuum of T-LGLL. To that end, a more objective marker (e.g., STAT3MT and cytogenetic abnormalities) did not differ between the groups suggesting a similar pathophysiologic mechanism.
Table 2.
Baseline and clinical features of de novo vs. post-transplantation T- LGLL cases
| Variables | de novo T-LGLL | PT T-LGLL | P-value |
|---|---|---|---|
| Total (n) | 231 | 15 | |
| Age, median (range) in years | 65 (18-89) | 55 (16-65) | 0.0002 A |
| ≥ 60 y, n(%) | 159 (69) | 2 (13) | <0.0001 B |
| Gender | |||
| M/F | 53/47 | 60/40 | 0.6B |
| Conventional cytogenetic † | |||
| Abnormal, n(%) | 8 (3) | 2 (15) | 0.07B |
| LGL count, median (range) in k/μL | 1.3 (0.01-970) | 4.5 (0.1-12.4) | 0.0005 A |
| TCR-R†, n(%) | 140 (96) | 14 (100) | 1B |
| STAT3MT†, n(%) | 87 (39) | 2 (15) | 0.1B |
| Splenomegaly | 60 (26) | 5 (33) | 0.5B |
| Hematological features * | |||
| Neutropenia, n(%) | 99 (43) | 4 (27) | 0.3B |
| Anemia, n(%) | 127 (55) | 3 (20) | 0.01 B |
| Thrombocytopenia, n(%) | 80 (35) | 5 (33) | 1B |
Some data were unable to assess
Neutropenia, absolute neutrophil count <1.5 x 109/L; Anemia, hemoglobin <10 g/dL; Thrombocytopenia, platelet count <150 x 109/L.
Abbreviations: T-LGLL, T-cell large granular lymphocytic leukaemia; PT T-LGLL, post-transplant T-cell large granular lymphocytic leukaemia; TCR-R, T-cell receptor gene rearrangement; STAT3MT, STAT3 mutant; M, male; F, female; y, years; n, number.
Wilcoxon test, 2-sided
Fisher’s exact test
Significantly different parameters are in bold text.
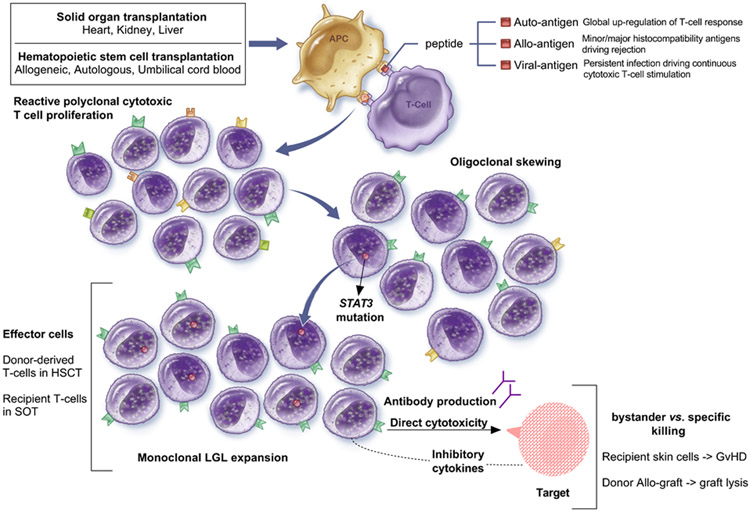
T-LGLL likely arises in the context of a polyclonal CTL response, which upon chronic stimulation undergoes conversion to more oligo- and then monoclonal rearrangements. Several mechanisms of clonal expansion post-transplantation may be responsible, including active viral infections, latent oncogenic viral reactivation, graft allo-antigenic stimulation or reactivation of pre-existing autoimmune process (Fig.1). In general, albeit oligoclonal skewing has been described after transplantation (Gorochov et al, 1994; Mohty et al, 2002; Sabnani et al, 2006; Qiu et al, 2017), the vast majority of patients with this complication are not known to develop T-LGLL. Theoretically, T-LGLL may originate from pre-transplantation autoimmunity of the donor in HSCT or recipient in SOT, which has been augmented by a heightened level of immune responsiveness due to allo-stimulation. While autoimmune conditions were present in 50% of SOT recipient (n=4/8, including RA, systemic lupus erythematosus), this type of past medical history is not available for HSCT graft donors. Previously, we have described that inherited or acquired hypogammaglobulinemia is associated with T-LGLL (Viny et al, 2008). Indeed, some of our patients had low immunoglobulin levels. However, hypogammaglobulinemia may be a mere marker of immunodeficiency in transplantation recipients due to immunosuppression, GvHD-associated immunosuppression or chronic disease. Overt EBV (post-transplant lymphoproliferative disorder) and CMV reactivation have been diagnosed in only 27% (4/15) of the patients, but similar undiagnosed infection may be present in an immunosuppressed host. According to this theory, inability to mount antibody or cellular response could result in persistent, unresolved infection and thus chronic CTL stimulation. Such an immunosuppressive state has been described in typical T-LGLL in the form of hypogammaglobulinemia, prior history of cancers, lymphoma, or co-existence with B-cell dyscrasias and may also be present following autologous HSCT. Others have encountered T-LGLL in auto-HSCT (8 cases in 3 studies), and in our series in 3 patients. However, it is difficult to establish a causative relationship in this cohort given that these patients also had history of lymphoma (diffuse large B-cell, mantle cell lymphoma, chronic lymphocytic leukaemia/small lymphocytic leukaemia).
Figure 1. Development of large granular lymphocytic leukaemia after transplantation.
Several mechanisms have been postulated to explain T-cell large granular lymphocytes clonal proliferation after solid organ and haematopoietic stem cell transplantation, as summarized. (Abbreviations: APC, antigen presenting cell; HSCT, haematopoietic stem cell transplantation; SOT, solid organ transplantation; LGL, LGLL, large granular lymphocytes; GvHD, graft versus host disease).
In sum, our report highlights the intricate nature of T-LGLL evolution following SOT and HSCT and points out the complexities of several postulated mechanisms. While the origin of LGLL in these patients is difficult to identify, it would be interesting to find the risk of transmission through the donated transplantation graft versus evolution from the recipient cells itself following the procedure. Our cohort does not have sufficient information from the donor available, making this difficult to answer. Undoubtedly, a comprehensive review of the medical history of the patients and scrutiny of their pre vs. post-transplantation baseline, clinical and molecular characteristics is warranted to better understand this phenomenon. Routine long-term blood analysis for LGL should be considered in immunosuppressed/post-transplantation patients, regardless of viral infection, to monitor T-LGLL evolution.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL118281, R01HL123904, R01HL132071, and R35HL135795.
Footnotes
Conflicts-of-Interests Disclosures
The authors have nothing to disclose
References
- Andersson EI, et al. , Novel somatic mutations in large granular lymphocytic leukemia affecting the STAT-pathway and T-cell activation. (2013) Blood Cancer J, 3, e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorochov G, et al. , Oligoclonal expansion of CD8+ CD57+ T cells with restricted T-cell receptor beta chain variability after bone marrow transplantation. (1994) Blood, 83, 587–595. [PubMed] [Google Scholar]
- Koskela HL, et al. , Somatic STAT3 mutations in large granular lymphocytic leukaemia. (2012) New England Journal of Medicine. 366, 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y and Xu L, Evaluation of TCR repertoire diversity in patients after hematopoietic stem cell transplantation. (2015) Stem Cell Investigation, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M, et al. , Features of large granular lymphocytes (LGL) expansion following allogeneic stem cell transplantation: a long-term analysis. (2002) Leukemia, 16, 2129–2133. [DOI] [PubMed] [Google Scholar]
- Nann-Rutti S, et al. , Large granular lymphocyte expansion after allogeneic hematopoietic stem cell transplant is associated with a cytomegalovirus reactivation and shows an indolent outcome. (2012) Biol Blood Marrow Transplant, 18, 1765–1770. [DOI] [PubMed] [Google Scholar]
- O’Keefe C,L, et al. , Molecular TCR diagnostics can be used to identify shared clonotypes after allogeneic hematopoietic stem cell transplantation. (2004) Experimental Hematology, 32, 1010–1022. [DOI] [PubMed] [Google Scholar]
- O’Keefe CL, et al. , Molecular analysis of alloreactive CTL post-hemopoietic stem cell transplantation. (2007). Journal of Immunology, 179, 2013–2022. [DOI] [PubMed] [Google Scholar]
- Qiu ZY, et al. Large granular lymphocytosis after transplantation. (2017) Oncotarget, 8, 81697–81708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnani I, et al. , Clonal T-large granular lymphocyte proliferation in solid organ transplant recipients. (2006) Transplant Proceedings, 38, 3437–3440. [DOI] [PubMed] [Google Scholar]
- Sanikommu SR, et al. , Clinical features and treatment outcomes in large granular lymphocytic leukemia (LGLL). (2018) Leukemia & Lymphoma, 59, 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, et al. , The 2016 revision of the World Health Organization classification of lymphoid neoplasms. (2016) Blood, 127, 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viny AD, et al. , Chronic B-cell dyscrasias are an important clinical feature of T-LGL leukemia. (2008) Leukemia & Lymphoma, 49, 932–938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.