Abstract
Overlapping spectrum of mutated genes affected in myelodysplastic syndrome (MDS) and primary acute myeloid leukemia suggest common pathogenic mechanisms. However, the frequencies of specific mutations are significantly different between them, which implies they might determine specific disease phenotype. For instance, there are overrepresentations of mutations in RNA splicing factors or epigenetic regulators in MDS. We provide an overview of recent advances in our understanding of the biology of MDS and related disorders. Our focus is how mutations of in splicing factors or epigenetic regulators identified in MDS patients demonstrate phenotypes in knockin/knockout mouse models. For instance, mutant Srsf2 mice could alter Srsf2’s normal sequence-specific RNA binding activity. It exhibited changing in the recognition of specific exonic splicing enhancer motifs to drive recurrent missplicing of Ezh2, which reduces Ezh2 expression by promoting nonsense-mediated decay. Consistent with this, SRSF2 mutations are mutually exclusive with EZH2 loss-of-function mutations in MDS patients. We also review how gene editing technology identified unique associations between pathogenic mechanisms and targeted therapy using lenalidomide, including: (i) how haploinsufficiency of the genes located in the commonly deleted region in del(5q) MDS patients promotes MDS; (ii) how lenalidomide causes selective elimination of del(5q) MDS cells; and (iii) why del(5q) MDS patients become resistant to lenalidomide. Thus, this review describes our current understanding of the mechanistic and biological effects of mutations in spliceosome and epigenetic regulators by comparing wild-type normal to mutant function as well as a brief overview of the recent progresses in MDS biology.
Introduction
Myelodysplastic syndromes (MDSs) are characterized by pancytopenia, bone marrow (BM) hyperplasia, dysplasia, and progression to secondary acute myeloid leukemia (AML) [1, 2]. Next-generation sequencing (NGS) has revealed a landscape of genetic alterations including coding exons and copy number alterations [3–5]. This review presents an overview of recent advances in the biology of MDS and related disorders, including other MDS/myeloproliferative neoplasms (MPN), largely focusing on the mechanism of somatically mutated genes that determine MDS phenotypes. It summarizes functional evaluation of each driver mutation including studies that mimic patient-specific mutations using conditional knockin (KI) mice.
Overview of somatic mutations
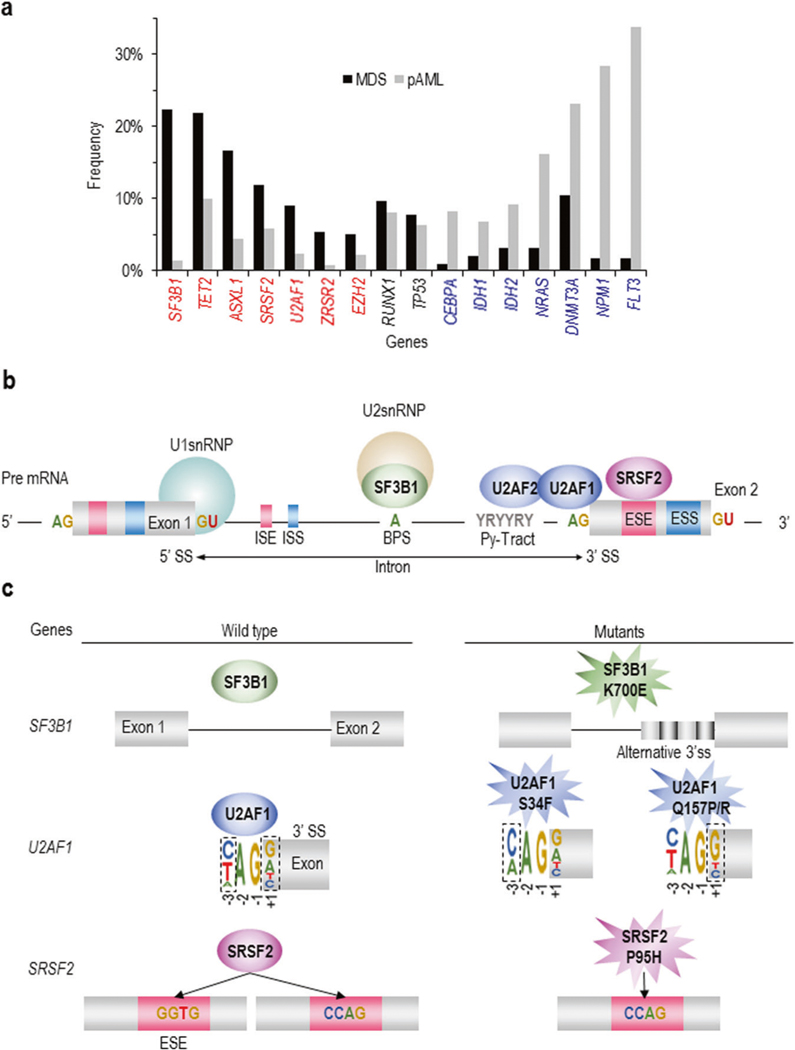
By combining SNP array karyotyping and high-throughput capillary sequencing, it was demonstrated that TET2 [6], EZH2 [7], TP53 [8], CBL [9], and RUNX1 [10] were frequently mutated in MDS. NGS including whole-exome sequencing revealed that MDS patients carry a median of nine somatic mutations in the entire coding region, including driver mutations that advance clonal selection and passenger mutations (random mutations) that do not promote disease [11]. Focusing on the most frequently mutated pathways, 65% of MDS patients harbored mutations in RNA splicing (SF3B1, SRSF2, U2AF1, ZRSR2) [12], followed by 47% harboring mutations in DNA methylation genes (DNMT3A, IDH1/2, TET2) [13–15] and 28% in histone modification genes (ASXL1,BCOR, EZH2) [5, 7]. Mutational targets are largely similar between MDS and primary AML, suggesting common pathogenesis in different neoplasms. However, the frequencies of these mutations are significantly different between MDS and primary AML; there is an overrepresentation of mutations in splicing factors (SFs) and epigenetic regulators in MDS, while mutations in receptor tyrosine kinases (e.g., FLT3), RAS pathway genes, and CEBPA and IDH1/2 mutations are more common in AML (Fig. 1a) [16]. In this review we focus on the biology of splicing factor and epigenetic regulatory genes.
Fig. 1.
Genetic mutations determining MDS phenotypes and their functional mechanisms. a Frequency of somatic mutations between MDS vs. primary AML (pAML). Genes significantly mutated in MDS and pAML are depicted in red and blue letters, respectively. The frequencies of mutations in RUNX1 and TP53 are approximately the same in both diseases. b RNA splicing and the role of those proteins frequently mutated in MDS. Within the intron of the pre-mRNA, the U2 snRNP complex containing SF3B1 binds the branch point site adenosine (A). U2AF2 binds to the polypyrimidine tract sequence, and U2AF1 binds the 3′ splice site the AG dinucleotides; SRSF2 binds to the exonic splice enhancer (ESE) sequence within the exonic sequence. c Current understanding of the mechanistic effect of mutations in SF3B1, U2AF1, and SRSF2 on RNA splicing. Panels left and right depict wild type and mutant function, respectively. SF3B1 mutants have been shown to promote the use of 3′ alternative splice sites (SSs) that are 10–30 base pairs upstream of the canonical 3′ SS. U2AF1 mutants repress 3′ SS recognition based on the identity of the nucleotide at the −3 or +1 sites that flank the AG of the 3′ SS. This occurs in an allele-specific manner in which U2AF1 S34 mutations drive alterations at the −3 position (with a C/A ≫ T preference), and mutations at Q157 drive alterations at the +1 position (with a G ≫ A preference). SRSF2 mutations affecting Pro95 alter the recognition of exons containing specific ESE sequences. Wild-type SRSF2 normally binds to and promotes splicing of C- and G-rich ESEs equally well, while mutant SRSF2 has skewed preference for C-rich ESEs
Splicing mechanisms
RNA splicing is a highly coordinated and essential process carried out by major and minor spliceosomes to remove noncoding regions (e.g., introns) of the pre-mRNA before protein translation [17]. Trans-acting SFs, such as serine-arginine-rich proteins and heterogeneous nuclear RNPs, bind to the regulatory elements located in the exons and introns to enhance or repress the splicing activity and contribute to alternative splicing (Fig. 1b) [18]. The regulatory elements include both intronic and exonic splicing enhancers and silencers. Many intron-containing mRNAs are exported from the nucleus and subject to degradation by nonsense-mediated-decay (NMD) due to premature termination codons contained in the retained intron [19]. The major spliceosome consists of five small nuclear ribonucleoprotein complexes (snRNPs), U1, U2, U4, U5, and U6.
Knowledge of the transcription factors directing cellular lineage commitment and posttranscriptional modifications altering protein function contributes for our current understanding of normal hematopoiesis and aberrancies in hematopoietic differentiation resulting in leukemian [20]. It is estimated that >90% of multiexon human genes undergo alternative splicing to generate proteome diversity [18, 20], yet relatively little is known about the regulation and functional role of alternative splicing in normal hematopoiesis.
Mutations in genes encoding spliceosomal proteins such as SF3B1, SRSF2, U2AF1, and ZRSR2 are the most frequent mutations in patients with MDS [3]. These mutations occur in a mutually exclusive manner with one another and at highly conserved domains in SF3B1, SRSF2, and U2AF1 that are known to confer an alteration in mRNA splicing. These genes were distributed on the U2 snRNP complex where they play an important role in the recognition of 3′ splice sites (SSs). Each of these mutations is associated with distinct clinical subtypes of MDS and their own unique correlation with other driver mutations. In this review, we describe functional aspects of genes, which are significantly mutated more in MDS than primary AML and focus on murine model recapitulating point mutations identified in MDS patients.
SF3B1
SF3B1 is a core member of the U2 snRNP complex, which is responsible for recognizing the branch point, a degenerate sequence motif usually located 21–34 base pairs of the adenosine (A) base within the intron and facilitates the binding of the U2 snRNP on pre-mRNA (Fig. 1b) [19]. The U2 snRNP is directed to the 3′ SS by short, conserved pre-mRNA sequences, including the branch point sequence upstream of the 3′ SS), the Py tract, and the AG (adenosine–guanine) dinucleotide at the intron–exon junction.
Mutations in SF3B1, located in chromosome 2q33, were identified in MDS, chronic lymphocytic leukemia, and other solid cancers [21]. They largely cluster within the C-terminal HEAT domains (residues 622–781), with the most commonly mutated amino-acid residue being 700. It was reported that SF3B1 hot spot mutations promote use of cryptic 3′ SSs [22]. SF3B1 mutations occur in >80% of MDS with ring sideroblasts (RSs) and predict for a favorable outcome. Because of this strong association, the 2016 WHO classification described that the presence of SF3B1 mutations could be helpful for the diagnosis of MDS-RSs with as little as 5% of RS. SF3B1 mutations are mutually exclusive not only with other SF mutations, but also with other mutations/CNAs, except for DNMT3A, TET2, JAK2, and del(5q) [5].
Mutated-SF3B1 promotes alternative 3′-SS usage in ABCB7, a heme transporter mutated in congenital sideroblastic anemia, and other genes involved in mitochondrial iron trafficking, and leads to their premature truncation and reduced gene expression in MDS patients [23]. This leads to abnormal deposition of iron (in the shape of rings) in the mitochondria of erythroblasts. Other putative targets of mutant SFs implicated in MDS pathogenesis include MAP3K7, NF1, and PDS5 [24, 25]. A recent study highlighted that the majority of cryptic AGs generated in SF3B1-mutant cells were located upstream of the canonical 3′ SSs and induced frameshifts, potentially causing many of the resulting transcripts to be degraded by NMD [26]. Research on the functional implications of SF3B1 mutations in cancer thus far has identified that expression of heterozygous SF3B1 mutations impart a characteristic change in splicing, namely the use of aberrant 3′ SSs and cryptic AG dinucleotides 10–30 bp upstream of the canonical AG dinucleotide [26, 27]. A conditional KI mouse model to study the consequences of the most common SF3B1 mutation has been generated [25, 28]. Sf3b1K700E/+ mice developed macrocytic anemia associated with a terminal block in erythroid maturation. However, the mice did not exhibit RS, the phenotypic hallmark of MDS-RS (Table 1) [25]. RNA sequencing of myeloid progenitors from Sf3b1K700E/+ mice and littermate controls demonstrated an increased usage of aberrant 3′ SSs, as noted in prior studies of SF3B1 mutations across other cancers and also in MDS patient’s samples (Fig. 1c).
Table 1.
Clinical and biological characteristics of mutations in MDS
| Biologic process | Genes | Type of mutation | Frequency | Effect on outcome in MDS patients | Normal biological function | Mutant biologic impact | Features of mouse model | Targeted downstream genes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Model studied | White blood cells | Red blood cells | Platelets | HSCs and progenitors | HSC functions | MDS phenotype in mice | Period to onset | Other myeloid features | Competitive transplantations | ||||||||
| Splicing | SF3B1 | Missense, clustering the C-terminal HEAT domains (residues 622–781), with the most commonly mutated residue being K700 | 20–25%, (60–75% in RS-MDS) | Strong association with RS. Improved OS of SF3B1-mutant RARS patients compared with their wild-type counterparts. | Recognizes branching points of intron and adjusts 3′ splice site sp icing patterns | Increases aberrant 3′ splice site selection | Sf3b1K700E/+ Inducible knockin (Mx1-Cre) endogenous Sf3b1 locus (Obeng et al.) | No change | Macrocytic anemia | No change | LT-HSC↑ GMP↓ | Decreased reconstitution in vivo | Dysplastic erythroid precursors without RS | 64 weeks | No elevated blast, no leukemia | Impaired | ABCB7, PDS5A, PPP2R5A, MAP3K7 |
| SRSF2 | Missense, clustering P95 | 8–12% | Mutations appear to cluster in MDS patients with RAEB or CMML and were found to be predictors for worsened OS | Recognizes ESE elements within pre-mRNA to promote exon recognition | Alters cassette exon splicing based on ESE sequence | Srsf2P95H/+X Inducible knockin (Mx1-Cre) endogenous Srsf2 locus (Kim et al.) | Leukopenia | Macrocytic anemia | No change | LT-HSC↑ | Decreased reconstitution in vivo | Trilineage dysplasia | 18 weeks | No leukemia (in up to 70 weeks) | Impaired | EZH2, CASP8 | |
| U2AF1 | Missense, clustering S34 and Q157 | 7–10% | Co-occurrs with ASXL1 mutations and were found to be predictors for worsened OS | Binds to the AG nucleotides near the 3′ splice cite in the intronic sequence | Alters 3′ splice acceptor sequence preference | U2af1S34f/+ Inducible knockin of S34F mutants U2AF1 (Mx1-Cre) Col1a1 locus (Shirai et al.) | Leukopenia | No change | No change | LT-HSC↑ | Decreased reconstitution in vivo | No evidence of bone marrow dysplasia | 4 weeks | No leukemia (in up to 52 weeks) | Impaired | H2AFY, EZH2, GNAS | |
| ZRSR2 | Nonsense, frameshift | 2–6% | Located on X chromosome and interacts with other prespliceosome components including the U2AF1/2 heterodimer and SRSF2 | Required for efficient splicing of both the major (U2) and the minor (U12) class of introns | Produces aberrant retention of U12-type introns | No mouse models | WDR41, FRA10AC1 | ||||||||||
| Chromatin modifications | EZH2 | Nonsense, frameshift | 3–7% | Co-occurring RUNX1 and STAG2 mutations. EZH2 mutations in MDS patients are associated with significantly worse prognosis, which is not due to AML transformation | Increases H3K27me3 as catalytic components of PRC2 | Reduces H3K27me3 | Ezh2Δ/Δ Inducible konckout (Cre- ERT)(Muto et al.) | Leukopenia | Anemia | Elevated | LT-HSC↑ MEP↑ | N.A. | Myeloid dysplasia such as hyposegmentation | 40 weeks | MDS/MPN like disease | N.A. | HMGA, PBX3, LMO1 |
| ASXL1 | Nonsense, frameshift in C-terminal (exon 12) | 15–20% | Co-occurring RUNX1 or STAG2 mutations. The mutation of ASXL1 predicted poor outcome in terms of response and OS after HMAs | Binds to EZH2 and increases globally H3K4me3 and H3K27me3 in specifically HOXA gene lesions. Removes H2AK119Ub due to cooperative BAP1 | Reduces global H3K4me3 and H3K27me3 in HOX genes and H2AK119Ub | Asxl1E635RfsX15/+ conditional knockin (Vav- Cre) Rosa26 locus (Nagase et al.) | No change | Reduction in RBC | Elevated | LT-HSC↓ MEP↑ | Decreased reconstitution in vivo | No evidence of bone marrow dysplasia | 70 weeks | No leukemia (in up to 78 weeks) | Impaired | HOXA5/7/9, IRF8, BAP1 | |
| DNA methylation | TET2 | Nonsense, frameshift, and missense in DSBH domain | 20–25% | TET2 mutations do not appear to affect OS | Catalyzes conversion of 5- mc to 5-hmc | Tet2−/− conditional knockout (Li et al.) | Leukocytosis (monocytosis) | Reduction in RBC | No change | LT-HSC↓ GMP↑ | Increased reconstitution in vitro colony formation capacity | Monocytosis, MDS/MPN features | 16 weeks | TET2 −/− (33%), TET2 +/− (8%) died within 1 year because of CMML-like diseases | Impaired | Concurrent depletion of EZH2 and TET2, or ASXL1 and TET2 establishes more advanced MDS phenotypes | |
| DNMT3A | Missense, clustering exon 23 with the most commonly mutated R882 residues | 8–12% | DNMT3A mutations do not appear to affect OS | Catalyzes conversion of 5- cytosine to 5-mc | Reduces 5-mc | Dnmt3aR882H/+ Inducible knockin (Mx1-Cre) endogenous Dnmt3a locus (Guryanova et al.) | No change | No change | No change | LT-HSC↑ | Increased reconstitution in vitro colony formation capacity | No evidence of bone marrow dysplasia | 40 weeks | No leukemia | N.A. | Cooperated with FLT3- ITD and NPM1 mutations to induce AML in vivo | |
| IDH1/2 | Missense, clustering R132 (IDH1), R140, and R172 (IDH2) | 1–4% (IDH1) 2–5% (IDH2) | Mutations in IDH1R132 and the analogous IDH2R172 mutations are possibly associated with adverse OS in MDS. | Catalyzes the oxidative decarboxylation of isocitrate to α-KG and carbon dioxide | Produces 2– HG, which serves as a competitive inhibitor of α– KG–dependent enzymes, including the TET family of enzymes and the Jumonji family of histone lysine demethylases | Idh2R140Q/+ Inducible knockin Rosa26 locus (Kats et al.) | No change | No change | No change | LT-HSC↑ | Block of erythroid differentiation | No evidence of bone marrow dysplasia | 28 weeks | No leukemia | No differences | IDH2 and FLT3-ITD cooperate in leukemia initiation | |
RS-MDS ring sideroblasts-myelodysplastic syndromes, RARS refractory-anemia with RS, OS Overall survival, N.A. not available, H3K4me3 histone 3 at lysing 4 trimethylation, H3K27me3 histone 3 at lysing 27 trimethylation, H2AK119Ub ubiquitination at lysine 119 of histone H2A, DSBH double-stranded β-helix, MML chronic myelomonocytic leukemia, LT-HSC long-term hematopoietic stem cell, GMP granulocyte myeloid progenitor, AG adenine Guanine dinucleotides, ESE exonic splice enhancer, 2- HG 2-hydroxyglutarate, α-KG alpha-Ketoglutaric acid
There was minimal overlap in misspliced events between human MDS and murine samples in part due to lack of conservation of intronic DNA sequences between the two species. For example, although ABCB7 is misspliced in human SF3B1K700E mutant cells, this was not seen in Sf3b1K700E/+ mice because of the distinct intronic sequence at this region of Abcb7 [20, 25]. Despite differences in splicing events between mouse and human cells expressing K700E mutation, Sf3b1K700E/+ mice clearly develop some key phenotypic hallmarks of human MDS. This discrepancy suggests a possible effect of the mutations on conserved functions of SF3B1 that are unrelated to splicing. As noted earlier, each mutated splicing factor appears to be associated with its own spectrum of coexisting and mutually exclusive mutations [5]. They also demonstrated that loss of Tet2, which plays a key role in active DNA demethylation through the conversion from 5-methylcytosine to 5-hydroxymethylcytosine [6], cooperates with Sf3b1K700E to exacerbate the macrocytic anemia and expansion of long-term hematopoietic stem cells compared to sole mutations [29].
SRSF2
SRSF2 normally recognizes exonic splice enhancer elements (ESEs) within the pre-mRNA with a consensus motif of SSNG (where S represents Cytosine or Guanine and N any nucleotide) to promote exon recognition (Fig. 1b) [30]. In the wild-type state, SRSF2 binds ESEs with motifs of CCNG and GGNG equally well to promote inclusion of the exons bearing these motifs [19].
SRSF2 mutations occur in 20–30% of MDS and ~50% of chronic myelomonocytic leukemia (CMML) patients [5, 31]. Most are heterozygous missense mutations especially of proline (Pro) 95. SRSF2 mutations are associated with a worse prognosis and higher rates of progression to leukemia. TET2 and ASXL1 mutations are the most frequently co-occurring mutations.
Several studies provided independent and consistent data showing that mutations in SRSF2 alter the protein’s role in RNA splicing in a manner completely distinct from the loss of function [32–34]. Wild-type mice transplanted with BM cells harboring the Srsf2P95H/+ mutation developed significant anemia and leukopenia at 18 weeks posttransplant (Table 1) [32]. In addition to the observed bicytopenia, Srsf2P95H/+ mice displayed macrocytic erythrocytes, accompanied by normocellular BM with dysplasia in the erythroid and myeloid lineages, mimicking features of human MDS. Moreover, Srsf2P95H/+ mice showed increased Lineage−Sca1 + cKit + (LSK) cells, increased early apoptosis and an increased proportion of cells in the S-phase of the cell cycle. The increase in hematopoietic stem and progenitor cells (HSPCs) and progenitor cells in conjunction with peripheral cytopenia is suggestive of impaired differentiation. Flow cytometry showed that the observed peripheral leukopenia was predominantly due to reduced B-lymphopoiesis. Srsf2P95H mice also had reduced early erythroid progenitors with reduced pre-MegE and pre-colony-forming units [32]. Of note, the nontransplanted Srsf2P95H mice did not develop overt MDS phenotypes or AML even after more than one year of monitoring with the primary phenotypes reported present only in the context of transplant studies.
More specifically, cells expressing mutant SRSF2 are enriched for CCNG ESE motifs in cassette exons with increased inclusion [32, 33]. SRSF2 mutations affecting P95 alter the recognition of exons containing specific ESE sequences. Wild-type SRSF2 normally binds to and promotes splicing of C- and G-rich ESEs equally well (GGTG or CCAG), while mutant SRSF2 has skewed preference for C-rich ESEs (CCAG) (Fig. 1c) [32, 33].
Mutant SRSF2 promotes missplicing and degradation of EZH2 [20]. Missplicing of Ezh2 leads to reduced Ezh2 protein expression via nonsense-mediated decay, and in turn, contributes to impaired hematopoietic differentiation. Consistent with SRSF2 mutations promoting a disabling splicing change in EZH2; EZH2 loss-of-function mutations are common in MDS [7]. In an analysis of >1800 MDS patients where EZH2 and SRSF2 were both sequenced, EZH2 loss-of-function mutations were mutually exclusive with SRSF2 mutations [5, 32]. Finally, restoration of normally spliced EZH2 expression by cDNA rescue partially rescued the hematopoietic defects of Srsf2 mutant HSPCs [32]. This links SRSF2 mutations to aberrant epigenetic regulation via insufficient EZH2 expression.
U2AF1
U2AF1 is one of the components which binds the AG dinucleotide in 3′ SSs of boundary introns and exons (Fig. 1b) [18, 20]. U2AF1 preferentially recognizes the core RNA sequence motif yAG|r (y = pyrimidine and r = purine), which matches the genomic consensus 3′ SS and intron/exon boundary that crosslinks with U2AF1. Our understanding of U2AF1 mutational mechanisms for RNA interactions is incomplete. U2AF1 mutations are highly specific—they uniformly affect the S34 and Q157 residues within the first and second CCCH zinc fingers of the protein—making comprehensive studies of all mutant alleles feasible. MDS patients with these mutations have poor prognosis [5, 12, 31].
Recent studies reported generation of a doxycycline-inducible transgenic mouse model with the most commonly identified U2AF1 S34F mutation (Table 1) [35, 36]. Peripheral blood (PB) leukopenia was observed in the U2af1S34F/+ mice after one month of doxycycline treatment, and appears to be related to B-lymphopenia and monocytopenia based on flow cytometry [35]. Leucopenia persisted up to 12 months, with white cell counts recovering to levels similar to that of controls following withdrawal of doxycycline treatment, suggesting a relationship between expression of mutant U2AF1 and the phenotype seen. Strikingly, U2af1S34F/+ mice showed increased proportions of HSPC in the bone marrow, particularly in the multipotent progenitors and common myeloid progenitor (CMP) compartments.
Several studies using unbiased genomic analysis of isogenic human and murine cells expressing ectopic mutant or wild-type U2AF1 have now revealed that U2AF1 mutations alter splice site recognition [20, 35, 36]. Mutations in U2AF1 consistently modify 3′ SS preference in a sequence-specific manner based on the identity of the nucleotide surrounding the AG dinucleotide that forms the 3′ SSs’ core consensus motif [37, 38]. Specifically, expression of U2AF1 S34F/Y promotes recognition of the 3′ SS bearing a C or A immediately preceding the AG and represses those bearing a T at this position (Fig. 1c) [35]. Overall suggesting that U2AF1 mutations are not loss of function hits.
Interestingly, analysis of the effects of Q157P/R mutations revealed that these mutations also affect 3′ SS recognition but in a distinct manner. Q157P/R mutations promoted recognition of the 3′ SS bearing a G immediately following (rather than preceding) the AG and repressed those bearing an A at this position (Fig. 1c) [20, 39]. These data suggest that S34F and Q157 mutations have different effects, even though both of them alter the same loci; such as −3 and +1 cryptic 3′ splice cite motifs.
In contrast to the sequence motif seen in non-dysregulated control junctions, exons skipped more frequently by mutant U2AF1 (S34F) relative to U2AF1 (WT) were enriched for uracil (indicated by a T) in the −3 position relative to the AG dinucleotide. We observed a similar pattern in alternative 3′ SS usage; there is an enrichment of T in the −3 position of canonical 3′ SSs whose alternative 3′ SS was used more frequently in mutant U2AF1(S34F)/rtTA samples relative to U2AF1(WT)/rtTA controls. This pattern is the same as seen in AML patient samples with U2AF1 mutations and in primary human CD34 + cells expressing U2AF1(S34F) or U2AF1(WT), and similar to previously reported data in human samples [37, 38].
U2AF1 S34 mutations affected H2AFY splicing by altering transcript isoforms. Although this splicing event has been validated in several reports, the downstream effects on macroH2A1 isoform expression and distribution and consequent biological effects of altered macroH2A1.1/macroH2A1.2 ratios have not been studied in the context of hematopoiesis [20, 40]. H2AFY contains two mutually exclusive exons, generating the two isoforms macroH2A1.1 and macroH2A1.2 of macroH2A1. The functions of these two isoforms differ, with important functional and prognostic implications in other forms of cancer [40, 41]. Many factors are involved in correct SS recognition in complex with U2AF1, including U2AF2, hnRNPA1, and DEK, among others [20]. Thus, future efforts are needed to determine whether S34 and Q157 mutations in U2AF1 alter the extensive protein–protein interaction networks across the exons and the introns that are necessary for spliceosome assembly.
ZRSR2
ZRSR2 (also known as URP) is located on the X chromosome (Xp22.1) and encodes for a splicing factor involved in recognition of 3′ intron SSs [3]. It interacts with other components of the prespliceosome assembly including the U2AF2/U2AF1 heterodimer and SRSF2 [42]. ZRSR2 is a component of the U12 snRNP and contacts the 3′ SSs of U12-type introns [43]. U12-type introns are a rare class of introns that are removed by a dedicated, U12-dependent “minor” spliceosome that is distinct from the U2-dependent “major” spliceosome [44]. U12-type introns comprise only ∼0.5% of all human introns [45]. Intriguingly, a subset of U12-type introns is spliced inefficiently relative to their U2-type counterparts. This inefficient splicing can result in nuclear retention of mRNAs containing unspliced U12-type introns, possibly followed by nuclear decay, resulting in a regulatory mechanism analogous to that observed by the study in erythropoiesis [20].
Nonsense, splice-site and frame-shift mutations in ZRSR2 frequently occur in males, suggesting a loss of function [3]. Mutations in ZRSR2 are more prevalent in MDS subtypes without RSs and in CMML [31]. They are associated with an elevated percentage of BM blasts and a higher rate of progression to AML. The link between ZRSR2 deficiency and MDS has not been explored mechanistically.
A paucity of studies evaluated the cellular and functional consequences of the loss of ZRSR2 in cell lines and patient samples [20, 46]. These studies showed that ZRSR2 plays a pivotal role in splicing of U12-type introns, while the U2-dependent splicing is largely unaffected. RNA sequencing analysis of TF-1 cells with RNAi-mediated downregulation of ZRSR2 as well as ZRSR2MT and ZRSR2WT primary MDS patient samples revealed that intron retention was enriched in ZRSR2 mutant or knockdown samples relative to ZRSR2 wild-type and normal BM. Categorization of the introns affected as U2- or U12-type revealed that U12-type introns were preferentially affected by ZRSR2 loss.
The epigenetic nature of MDS
Before genome-wide epigenetic studies were feasible, aberrant silencing of tumor suppressor and DNA damage-repair genes through hypermethylation of promoter-associated CpG islands was described in MDS [47]. Whereas MDS is particularly resistant to conventional chemotherapy, treatment with hypomethylating agents (HMAs) such as 5-azacytidine (AZA) and 5-aza-2′-deoxycytidine (decitabine) improve hematopoiesis, increase overall survival (OS) and delay the onset of AML progression [48]. Although HMAs cause broad hypomethylation of DNA by inhibition of DNA methyl-transerases, catalyzing cytosine to methyl-cytosine, specific target molecules for treating MDS cells are not revealed yet. HMA effect is transient with responses maintained for 6–24 months [49]. Survival for refractory/relapsed MDS patients is extremely short [50].
Recent genome-wide and candidate-gene discovery efforts have identified a series of novel somatic genetic alterations in patients with myeloid malignancies with relevance to pathogenesis, prognostication, and/or therapy [5]. Notably, these include mutations in genes with known or putative roles in the epigenetic regulation of gene transcription. Epigenetic alterations are involved in the establishment of gene expression profiles associated with cancers, and the accumulation of genetic and epigenetic alterations promote tumorigenesis [51]. In this review, we focus on two epigenetic mechanisms; (i) Histone-modifying enzymes and (ii) DNA methylation.
Histone-modifying enzymes
Polycomb group proteins were originally identified in Drosophila as regulators of body segmentation through the repression of homeotic genes and were subsequently identified in mammals [52]. PcG proteins function in the maintenance of gene silencing via posttranslational modifications of histones and chromatin compaction [53]. In mammals, there are two major complexes of PcG: Polycomb repressive complex 1 (PRC1) and 2 (PRC2), which modify monoubiquitination at lysine 119 of histone H2A (H2AK119ub1) and mono-, di-, and trimethylation at lysine 27 of histone H3 (H3K27me1/me2/me3), respectively [54–56]. In the canonical pathway, PRC2 initiates gene silencing by catalyzing H3K27me3 modification. PRC2 consists of EED, SUZ12, and the PRC2 enzymatic component, enhancer of zeste homolog 2 (EZH2). PRC2-mediated H3K27me3 plays critical roles in the stage-specific repression of developmental regulator genes, which frequently exhibit bivalency with an active histone modification, H3K4me3, during cellular differentiation. PRC1 is then recruited to the target regions by binding to H3K27me3 through the CBX component of PRC1, and catalyzes the monoubiquitination of H2AK119 to maintain gene silencing [57, 58]. However, recent findings have shown that PRC1 can be recruited to target genes independently of H3K27me3 [59, 60]. The biological function of canonical PRC1 and PRC2 has been characterized in detail in ES and hematopoietic stem cells (HSCs) [61].
EZH2
Comprehensive genome sequencing studies identified somatic mutations of EZH2 in MDS [62]. They are widely distributed throughout the coding region, but the majority of them are nonsense or frameshift mutations, suggesting loss-of-function mechanisms. EZH2 is located at chromosome 7q36.1 which is frequently deleted in MDS, suggesting that loss of EZH2 has a role in MDS pathogenesis [7]. Loss-of-function mutations in EZH2 have been identified in patients with MDS (3–13%), MPN (3–13%), and MDS/MPN overlap disorders (8–15.6%); all clonal myeloid disorders originating from HSCs [63]. Other components of PRC2, EED, and SUZ12 appear to be mutated in a manner similar to EZH2, although at lower frequencies.
On the other hand, EZH2 mutations were also identified in malignant lymphomas such as diffuse large B-cell lymphoma, the majority are missense mutations with a hot spot of heterozygous Y641 mutations in the histone methyltransferase Su(var)3–9, E(z), and Trithorax (SET) domain. EZH2 is the catalytic member of the PRC2 [64]; however, EZH2 alone has very weak histone-methylating activity. Other members of the PRC2 complex; EED, SUZ12, AEBP2, and RbAp48 are required for full activity. Expression of EZH2Y641F/N mutants in cells with EZH2WT resulted in an increase of H3K27me3 levels in vivo through increase in trimethylation activity of the enzyme. Functional analysis revealed that these mutations could increase EZH2 function and are perhaps gain-of-function in the pathogenesis of malignant lymphoma [65].
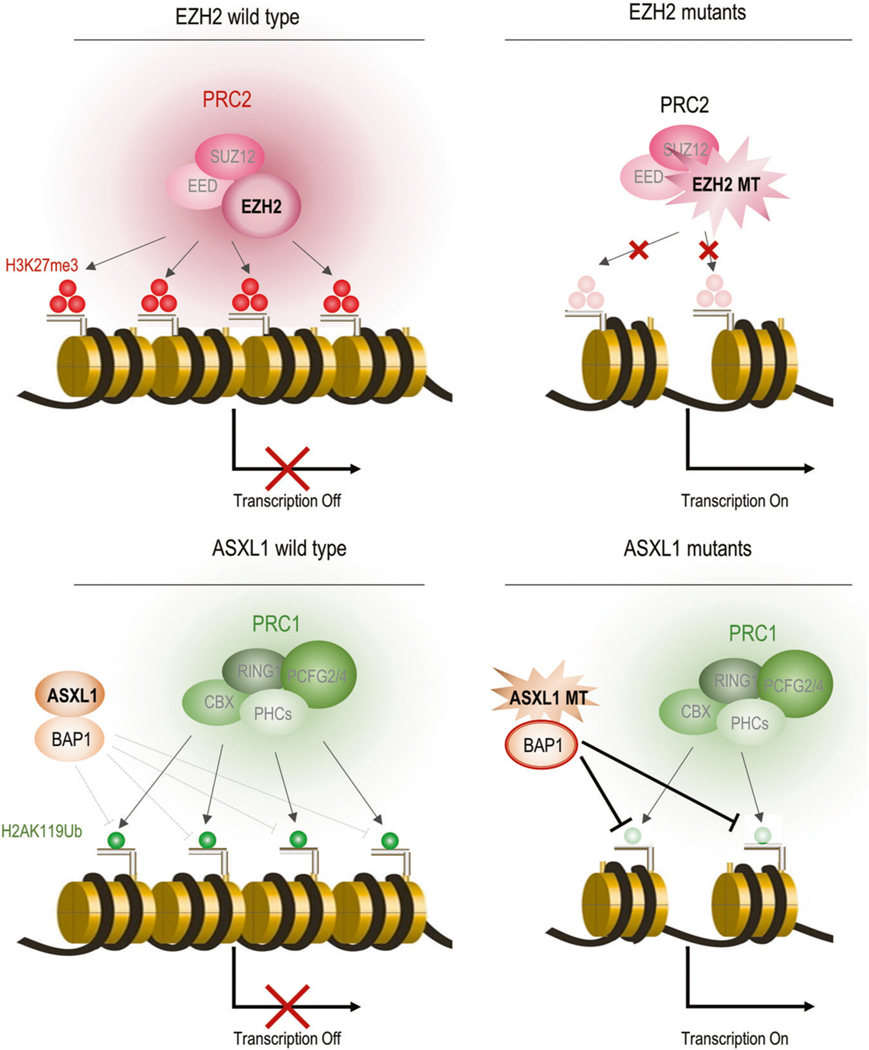
Several groups have examined the impact of loss-of-function mutations of EZH2 in the pathogenesis of MDS by using conditional EZH2 knockout mice [66, 67]. These mutations reduce global levels of H3K27me3 and have been shown to increase expression levels of EZH2 target genes including potential oncogenes in tumor cells in patients and murine models, which indicate that EZH2 has a tumor suppressor function (Fig. 3a). Hematopoietic cell-specific deletion of Ezh2 resulted in the development of myeloid malignancies including MDS, MDS/MPN, and T-ALL, but not AML, after a long latency [68].
Fig. 3.
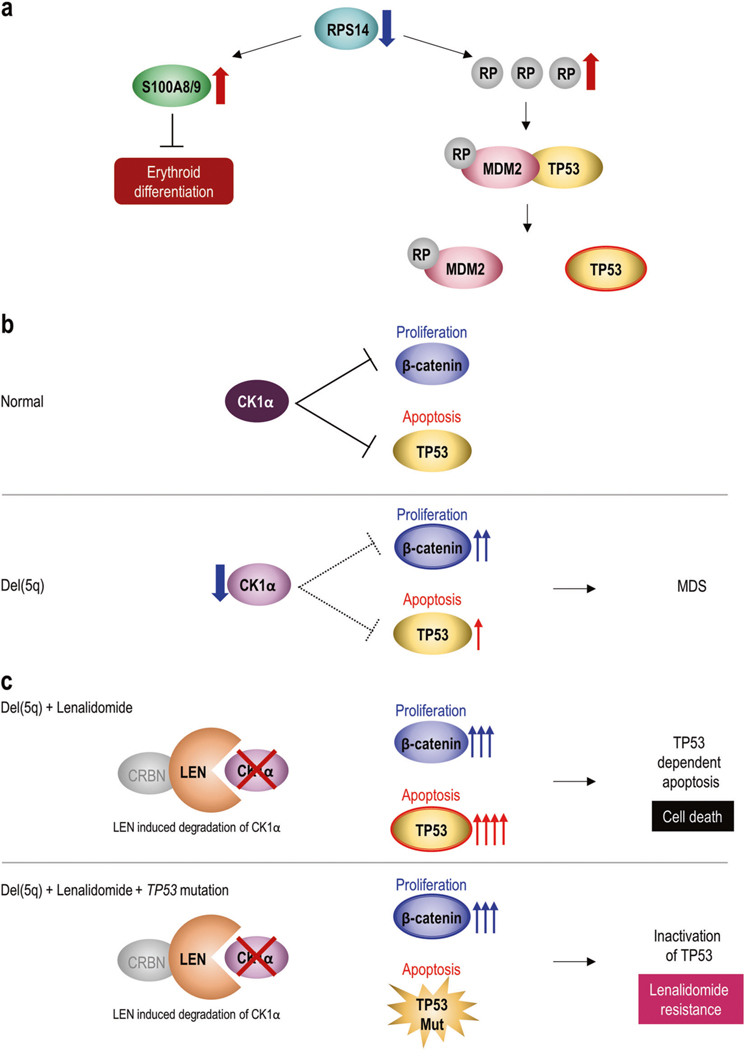
Functional implications of del(5q) and mechanism of lenalidomide resistance. a Effects of RPS14 haploinsufficiency. Decreasing RPS14 expression enhances other ribosomal proteins (RPs) such as RPL11 or RPS19, which sequester MDM2, an E3 ubiquitin ligase that also negatively regulates TP53. Elevated TP53 activation leads to enhanced TP53-dependent apoptosis of erythroid progenitors. Haploinsufficiency of RPS14 also induces high expression of S100a8 and S100a9, which are involved in innate immune signaling, leading to a block in erythroid differentiation. b Effects of CSNK1A1 haploinsufficiency. CK1α, which is encoded by CSNK1A1 gene, negatively regulates both cell proliferation due to WNT/β-catenin signaling and apoptosis due to TP53. It fine-tunes the balance between cell proliferation and apoptosis in normal hematopoiesis. Decreased expression of CK1α due to del(5q) weakens inhibition of cell proliferation and apoptosis. It enhances proliferation by decreasing inhibition of β-catenin more than activation of apoptosis through de-repressed TP53, resulting in MDS. c Lenalidomide decreases expression of CK1α by binding to cereblon (CRBN), a Cul4-based E3 ubiquitin ligase component, leading to ubiquitination and degradation. Normal cells treated with lenalidomide can survive because 50% of CK1α expression remains. On the other hand, in del(5q) MDS cells, CK1α is already at 50% due to haploinsufficiency, so lenalidomide treatment is lethal to cells by completely losing CK1α. There would be increased cell death through more apoptosis due to TP53 activation than proliferation due to β–catenin activation (Upper panel). However, if del(5q) MDS cells acquire TP53 loss-of-function mutations, they would become resistant to lenalidomide because complete loss of CK1α could not induce TP53-dependent apoptosis (Lower panel)
Deletion of Ezh2 causes myeloid dysplasia in mice (Table 1) [66]. PB from Ezh2 conditional homozygous knockout mice at 3 months showed reduced white blood cell counts due to lymphopenia, increased platelet counts, and mild but significant anemia. Cytological analysis of Ezh2 knockout mice PB revealed morphological abnormalities in myeloid cells, such as delayed maturation of neutrophils, hyposegmented neutrophils consistent with a pseudo Pelger–Huët anomaly, hypersegmented neutrophils, and dysplasia of monocytes. BM analysis at 3 months after deletion of Ezh2 revealed that all mutant mice had a greater proportion of LSK cells and CMPs and/or MEPs.
In patients, EZH2 mutations frequently occur with loss-of-function mutations in TET2 and RUNX1 [5, 31]. An Ezh2 deficiency in combination with a Tet2 hypomorph (Tet2KD/KD) in mice accelerates the transformation of HSCs and induces MDS and MDS/MPN [69]. These findings indicate that EZH2 fine-tunes the commitment and differentiation of HSCs, and EZH2 insufficiency promotes the transformation of HSCs to MDS and MPN stem cells.
ASXL1
Mammals have three ASXL genes (ASXL1, ASXL2, and ASXL3) which are the homologs of Dorosophila Additional sex combs (Asx) [70]. Asx, the Drosophila melanogaster homolog of mammalian ASXL1–3, is required both to maintain repression and to activate expression of Hox genes. ASXL genes are thought to mediate the balance between polycomb and trithorax functions [71], despite their lack of enzymatic activity. ASXL1 wild-type protein can support H3K4 methylation through OGT [72], H2AK119 ubiquitination through PRC1, and H3K27 methylation in specific loci such as the posterior HOXA genes through PRC2 [67, 73].
ASXL1 is frequently mutated in MDS as well as in clonal hematopoiesis of indeterminate potential (CHIP) [74]. The human ASXL1 gene is located on chromosome 20q11. Mutations in ASXL1 most commonly occur as frameshift and nonsense mutations in the last exon before the C-terminal plant homeofinger domain, suggesting that mutant mRNA escapes from nonsense-mediated decay and that most ASXL1 mutations generate stable truncated proteins [70].
Similar to EZH2 mutations, mutations in ASXL1 have been found to be associated with worsened OS among patients with MDS independent of other clinical parameters; age, cytogenetics, and number of cytopenias. Comparing other somatic mutations, ASXL1 mutations were independent predictors of a poorer prognosis in a multivariate analysis [62].
Asxl1 knockout mice exhibit impaired hematopoiesis and development of MDS-like disease through decreased H3K4me3 and H3K27me3 in specific loci such as posterior Hoxa genes due to loss of interactions with the PRC2 complex [75]. These findings indicate that wild-type ASXL1 plays crucial roles as a tumor suppressor in hematopoiesis and suggest loss-of-function features of ASXL1 mutations. However, the role of the physiological expression of Asxl1-MT in histone modifications is not well understood. Conditional ASXL1 mutant KI mice which mimic the human E635RfsX15 mutants induced a modest MDS-like phenotype (Table 1) [76]. Rosa26 locus mutant Asxl1 KI (Asxl1-MT KI) mice resulted in a MDS-like phenotype characterized by myeloid skewing, age-dependent anemia, thrombocytosis, and morphological dysplasia. Expression of ASXL1-MT reduced the number of HSCs and stemness similarly to other MDS mouse models, and maintained HSC survival in competitive repopulation assays. ASXL1-MT KI mice also displayed substantial reduction in H3K4me3 and H2AK119ub. Unlike Asxl1 knockout mice, global levels of H3K27me3 were not significantly changed in Asxl1-MT KI mice. However, the level of H3K27me3 at Hoxa gene loci was decreased and the expression of Hoxa was upregulated in Asxl1-MT KI mice [73]. H3K4me3 reduction was observed especially at promoter loci of the genes associated with erythroid differentiation such as Sox6, Id3, Tjp1, and Hba [72]. Of note, overexpression of Id3 ameliorated erythroid clonogenicity in BM cells derived from Asxl1-MT KI mice. A study reported generation of Asxl1G643fs mutant KI mice and reported that mutant KI mice alone did not develop hematological malignancies within 18 months follow-up [77]. On the contrary, another study also generated Asxl1G643fs mutant KI mice and reported that a portion of the mice eventually developed MDS/MPN-like disease after a long latency, about 18–24 months [78]. These findings indicate that expression of the mutant ASXL1 results in altering histone modifications and defects in hematopoiesis promoting myeloid transformation.
It was recently reported that the mutant ASXL1 altered histone modification by unique mechanisms [72, 73]. ASXL1 WT could induce H2AK119 ubiquitination (Ub) through negatively regulating BAP1 which removes ubiquitination of H2AK119 as a deubiquitinase (Fig. 2b). BAP1 is rarely mutated in myeloid malignancies [79]. The mutant ASXL1 enhanced the catalytic function of BAP1 and profoundly reduced the global level of H2AK119Ub by strengthening deubiquitnation. The mutant ASXL1/BAP1 complex inhibits the differentiation of HSPCs toward multilineage except for immature monocytes, and promoted myeloid leukemogenesis induced by RUNX1-ETO, an oncogenic fusion protein frequently coexisting with ASXL1 mutations [72]. Mechanistically, this complex directly binds to promoter loci of HOXA genes and IRF8, and decreases H2AK119 at these loci, resulting in dysregulation of posterior HOXA genes and IRF8. HOXA gene expression is essential for leukemic transformation and IRF8 plays a critical role in monopoiesis.
Fig. 2.
Histone modification pathways affected by somatic mutations in MDS. a EZH2 mutants’ mechanisms. EZH2 works as a methylase component of polycomb complex 2 (PRC2) and promotes trimethylation of histone H3 at lysine 27 (H3K27me3). It leads to structurally tightened chromatin and negatively regulates transcription of genes. Mutant EZH2 loses its methylase activity and decreases H3K27me3. Transcription factors could easily access DNA and accelerate genetic expression due to loosening histone proteins. b ASXL1 mutants’ mechanisms. ASXL1 promotes ubiquitination of histone H2A at Lysine 119 (H2AK119Ub) through PRC1 by stabilizing BAP1, which negatively regulates H2AK119Ub. It leads to structurally tightened chromatin and negatively regulates transcription of genes. Mutant ASXL1 could not regulate BAP1 so BAP1 is constitutionally activated. This results in decreases in H2AK119U via an enhanced ability to remove H2AK119Ub. Transcription factors can then easily access DNA and accelerate genetic expression due to the loosening of histones
The DNA methylation machinery
DNA methylation occurs exclusively at the 5′ position of cytosine nucleotides in the context of a CpG dinucleotide [16]. CpG islands are major regulatory units and around 50% of them are in gene promoter regions, while another 25% lie in gene bodies, often serving as alternative promoters [80]. The addition of the methyl group is catalyzed by the DNMT family of proteins, DNMT3A, DNMT3B, and DNMT1 [14]. DNMT3A is a member of the mammalian family of DNA methyltransferases that add a methyl group to cytosine enzymatically in CpG dinucleotides. DNMT3A and its homolog, DNMT3B are responsible for initiating de novo DNA methylation. Silencing of a DNA repair gene by hypermethylation may be a very early step in progression to cancer [81]. In contrast, Ten-Eleven-Translocation (TET) proteins TET1, TET2, and TET3 have resulted in the identification of additional active mechanisms of DNA methylation [82]. TET proteins are alpha-ketoglutarate (α-KG) and Fe2+-dependent oxygenases that are capable of modifying 5′-methyl-cytosine (5mc) to generate 5′-hydroxmethylcytosine (5-hmc) whose function is not fully established. Ultimately, they are oxidized further to forms that are removed and replaced by cytosines by base excision repair. The α-KG required for this reaction can be produced by Isocitrate Dehydrogenase 1 (IDH1) and IDH2 from isocitrate in the cytoplasm and mitochondria, respectively. Under hypoxic conditions, IDH2 carries out the reverse reaction of carboxylating aKG to form isocitrate. When mutated, IDH1 and IDH2 instead convert α-KG to an oncometabolite, 2-hydroxyglutarate (2-HG), which in turn inhibits many oxidation reactions that require α-KG as a substrate, including TET2 and a number of histone demethylases including jumonji domain-containing proteins, such as KDM6A (UTX), KDM4A,B and KDM2 [83, 84].
DNMT3A
DNMT3A is recurrently mutated in de novo AML. Almost half of all DNMT3A mutations occur at a single hot spot, arginine 882, which is in the methyltransferase domain [14]. DNMT3AR882 mutations are the most prevalent somatic mutations observed in individuals with CHIP [74]. AML patients with DNMT3AR882 mutations have an inferior outcome when treated with standard-dose daunorubicin-based induction chemotherapy [14]. DNMT3A mutations are less frequent in MDS (10%) than in primary AML (23%) (Fig. 1a) [16]. Biochemical studies have suggested that DNMT3AR882 can function as a dominant-negative mutation with respect to methyltransferase activity; however, the role of these mutations in leukemia pathogenesis and in the response to antileukemic therapies has not been elucidated. A recent study reported a generation of a mouse model that conditionally expresses Dnmt3aR878H (mouse homolog to human DNMT3AR882H) from the endogenous locus [85]. Mice expressing Dnmt3aR878H in the absence of other disease alleles did not develop leukemia (Table 1), but were instead characterized by the accumulation of LSK stem cell–enriched cells and by an increased percentage of circulating c-Kit-positive progenitor cells, consistent with HSPC expansion.
TET2
Mutations in TET2, located on chromosome 4q24, were identified in MDS (up to 30%) and other myeloid malignancies. They are more frequent in MDS/MPN overlap syndromes (~50%). Copy number alterations including deletion, and copy number neutral loss-of heterozygosity, also occurred simultaneously in many cases. Resulting losses of TET2 function led to reductions in the amount of 5-hmC, and this has been demonstrated in samples of patients with myeloid malignancies, suggesting that TET2 acts as a tumor suppressor gene [86].
Recently, a study described the phenotype of mice hypomorphic for TET2 as a mouse model of CMML and MDS [66, 87]. In this study, TET2 gene trap mice (Tet2KD/KD) were engineered to express ~20% of the TET2 mRNA of WT mice. Tet2KD/KD mice developed overt features of myeloid malignancy after about 11 months. Whilst the majority of mice had features of CMML, 3 out of 13 mice developed MDS with pancytopenia, granulocytic dysplasia, and increased erythroid apoptosis. Thus, alterations in TET2 expression resulted in two distinct phenotypes of CMML and MDS, after a considerable latency. Recurrent somatic TET2 mutations have been identified in normal, elderly individuals with acquired CHIP without overt clinical manifestations [74].
IDH1/2
Isocitrate dehydrogenase 1 (IDH1) and IDH2 are metabolic enzymes that decarboxylate isocitrate to αKG whilst producing NADPH from NADP+, and importantly, also perform the reverse reaction under hypoxic conditions; Missense mutations in IDH1/2 occur at specific conserved residues (R132 in IDH1; R140 and R172 in IDH2) and are always heterozygous [5]. They reported the characterization of conditional KI mice in which the most common IDH1 mutation, IDH1 (R132H), is inserted into the endogenous murine Idh1 locus and is expressed in all hematopoietic cells (Vav-KI mice) or specifically in cells of the myeloid lineage (LysM-KI mice) [88]. These mutants showed increased numbers of early hematopoietic progenitors and develop splenomegaly and anemia with extra medullary hematopoiesis, suggesting a dysfunctional bone marrow niche (Table 1).
Del(5q) and lenalidomide
Deletion of the long arm of chromosome 5, del (5q), is one of the most common cytogenetic abnormalities found in MDS, accounting for approximately 10–15% of MDS cases [89]. The 5q- syndrome is characterized by del(5q) being the sole cytogenetic abnormality. The 5q- syndrome was registered in the 2008 World Health Organization classification as a distinct entity because of unique clinical features such as female predominance, severe macrocytic anemia, normal or elevated platelet counts with hypolobulated micro-megakaryocytes, and a low rate of progression to AML [90]. Lenalidomide, an immunomodulatory drug with efficacy in multiple myeloma (MM), also induces impressive responses specifically in MDS patients with del(5q) [91].
There are two distinct commonly deleted regions (CDR) in 5q-. The more distal CDR is mapped to a 1.5 megabase region between 5q31 and 5q33 [92]. Molecular studies have shown allelic haploinsufficiency for several genes within the critical 5q deletion, including RPS14, CSNK1A1, APC, DDX41, and mir-145/146a, of which, RPS14, CSNK1A1, and mir-145 are included in the CDR [16].
The biological function of del(5q) has been extensively studied, revealing key pathogenic drivers of del (5q) including (i) enhanced apoptosis due to P53 activation, (ii) activation of cell proliferation due to Wnt/β-catenin signaling, and (iii) innate immune activation. RPS14 encodes a small subunit of ribosomal proteins (RPs). Small hair pin RNAs targeting RPS14 cause a severe block in erythroid differentiation, whereas forced overexpression of RPS14 in cells from MDS patients with del(5q) rescues erythropoiesis. Ribosomal dysfunction is sensed by the P53 pathway, leading to cell cycle arrest and apoptosis (Fig. 3a). Haploinsufficiency of RPS14 induces other RPs and ribosomal components, including RPS19 and RPL11. MDM2 inhibits TP53. RPs decrease MDM2, thus activating TP53 and enhancing apoptosis. It has also been reported that S100 calcium-binding proteins S100a8 and S100a9, which are involved in innate immune signaling, were more highly expressed in conditional RPS14 inactivated mice [93]. Addition of recombinant S100a8 was enough to induce defective erythroid differentiation in wild-type erythroid cells. Given the data, RPS14 haploinsufficiency in del(5q) MDS leads to activation of the innate immune system and induction of S100a8-S100a9 expression, resulting in erythroid differentiation defects.
CSNK1A1 encodes a casein kinase 1α (Ck1α), which is a serine-threonine kinase that fine-tunes the balance between cell proliferation through WNT/β-catenin signaling and apoptosis mediated by TP53 (Fig. 3b). Haploinsufficiency of CSNK1A1 enhances proliferation by decreasing inhibition of β-catenin more than decreasing inhibition of apoptosis through de-repressed TP53, resulting in MDS [94]. This functional mechanism is unique to the pathogenesis of del (5q) and has been associated with how lenalidomide is effective against 5q- cells. Cells tolerated 50% expression of CK1α, but complete loss of CK1α was not tolerated and induced cell death due to cell cycle arrest [89]. Hence, partial degradation of CK1α in wild-type cells is better tolerated than in del(5q) MDS cells. The direct protein target of lenalidomide is cereblon (CRBN), a component of Cul4-based E3 ubiquitin ligase. Lenalidomide modulates CRBN function, leading to ubiquitination and degradation of specific protein substrates. Lenalidomide may also bind CK1α, Ikaros (IKZF1) and Aiolos (IKZF3), which are targetable molecules in the pathogenesis of MM, and degrades them through ubiquitination, ultimately resulting in deceased protein levels [95].
Therefore, lenalidomide causes selective elimination of del(5q) MDS cells through complete loss of CK1α, which induces more apoptosis due to TP53 activation than proliferation due to β–catenin activation (Fig. 3c) [95]. Interestingly, del(5q) MDS patients often face lenalidomide resistance which is associated with TP53 alterations. Identifying the pharmacological effects of lenalidomide for del (5q) patients sheds light on the mechanisms of this resistance. Degradation of CK1α leads to activation of TP53 and consequent cell cycle arrest and apoptosis [95]. In a mouse model, inactivation of TP53 led to lenalidomide resistance [96]. Indeed, TP53 mutations have been linked to lenalidomide resistance in del(5q) MDS patients [97]. Hence, if del(5q) MDS cells acquire deletions or inactivating mutations of TP53, they would become resistant to lenalidomide due to complete loss of CK1α and the inability to induce TP53-dependent apoptosis and cell death. Clinical sequencing is gradually becoming more available, so genetic assessment of TP53 mutations could be beneficial in lenalidomide treatment decisions.
Conclusion
Mutations affecting RNA splicing and epigenetic modifier pathways are the most common genetic alterations in MDS, which contribute specifically to the pathogenesis of MDS rather than primary AML. Many insights have already been made about the global effects of these mutations on RNA splicing and the epigenome, and initial murine models of several of these mutations have been described [16]. Further rigorous assessment of the mechanistic effects of the mutations at both genome-wide and locus-specific levels will be critical for future efforts to therapeutically manipulate splicing in spliceosome mutant malignancies. Advantages of these studies were that each single genetic mutation in mouse models demonstrated alterations of splicing or epigenetic mechanisms which were identified in human MDS patients.
Several controversies and limitations still exist. First, the majority of MDS patients have multiple somatic mutations which could drive clonal evolution, suggesting that accumulated genetic mutations play an important role in the pathogenesis of MDS. Although a handful of pairs of genetic mutations were evaluated in mouse models, functional mechanisms in combinations of them have not yet been elucidated. Given the time it takes to generate KI/knockout mice for multiple genes, developing a more efficient experimental method such as multiplex CRISPR/Cas9-based genome editing will be needed to move the field forward.
In addition, around 5% of del(5q) patients had mutations of CSNK1A1 [98]. Given that 50% expression of CK1α promoted clonal advantage, however, complete loss of CK1α could synthetic lethal, how simultaneous mutations and deletion of CSNK1A1 could be pathogenic in del(5q) patients are still unclear. Moreover, MDS patients had similar clinical features including cytopenias or bone marrow dysplasia with no regard to mutations in RNA splicing or epigenetic nature even though their functional roles differ. To shed light on these problems, it was hypothesized that driver mutations were the activator of common underlying mechanisms involved in the MDS phenotype. Identification of these key mediators would reveal fundamental insights into MDS pathogenesis and present novel opportunities for therapeutic intervention beyond specific mutations for MDS [99].
Finally, new FDA-approved drugs for MDS are needed. Currently, only azacitidine and decitabine are available for high-risk MDS and only lenalidomide is available for del 5q) MDS. Understanding the landscape of MDS genomics needs to be used to identify new target molecules. At the same time, novel and efficient immunotherapy options such as CAR-T or PD-1/PD-L1 inhibitors developed in the last 5 years for other hematologic tumors, need to be further explored in clinical trials of MDS patients.
Acknowledgements
This work was supported by US National Institute of Health (NIH) grants R35 HL135795, R01HL123904, R01 HL118281, R01 HL128425, R01 HL132071, a grant from Edward P. Evans Foundation (Maciejewski) and a grant JSPS Overseas Research Fellow (Nagata).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. [DOI] [PubMed] [Google Scholar]
- 2.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–51. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–9. [DOI] [PubMed] [Google Scholar]
- 4.Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. New Engl J Med. 2011;365:1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tefferi A, Lim KH, Levine R. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;361:1117. [DOI] [PubMed] [Google Scholar]
- 7.Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tönnissen ER, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–7. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto K, Hirano N, Toyoshima H, Chiba S, Mano H, Takaku F, et al. Mutations of the p53 gene in myelodysplastic syndrome (MDS) and MDS-derived leukemia. Blood. 1993;81:3022–6. [PubMed] [Google Scholar]
- 9.Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–8. [DOI] [PubMed] [Google Scholar]
- 10.Osato M. Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene. 2004;23:4284–96. [DOI] [PubMed] [Google Scholar]
- 11.Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graubert TA, Shen D, Ding L, Okeyo-Owuor T, Lunn CL, Shao J, et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2011;44:53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delhommeau F, Dupont S, Valle VD, James C, Trannoy S, Massé A, et al. Mutation in TET2 in myeloid cancers. New Engl J Med. 2009;360:2289–301. [DOI] [PubMed] [Google Scholar]
- 14.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. New Engl J Med. 2010;363:2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa S. Genetics of MDS. Blood. 2019;133:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–18. [DOI] [PubMed] [Google Scholar]
- 18.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu XD, Ares M. Context-dependent control of alternative splicing by RNA-binding proteins. Nat Rev Genet. 2014;15:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue D, Bradley RK, Abdel-Wahab O. Spliceosomal gene mutations in myelodysplasia: molecular links to clonal abnormalities of hematopoiesis. Genes Dev. 2016;30:989–1001. 05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malcovati L, Papaemmanuil E, Bowen DT, Boultwood J, Della Porta MG, Pascutto C, et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118:6239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolatshad H, Pellagatti A, Fernandez-Mercado M, Yip BH, Malcovati L, Attwood M, et al. Disruption of SF3B1 results in deregulated expression and splicing of key genes and pathways in myelodysplastic syndrome hematopoietic stem and progenitor cells. Leukemia. 2015;29:1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolatshad H, Pellagatti A, Liberante FG, Llorian M, Repapi E, Steeples V, et al. Cryptic splicing events in the iron transporter ABCB7 and other key target genes in SF3B1-mutant myelodysplastic syndromes. Leukemia. 2016;30:2322–31. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsafadi S, Houy A, Battistella A, Popova T, Wassef M, Henry E, et al. Cancer-associated SF3B1 mutations affect alternative splicing by promoting alternative branchpoint usage. Nat Commun. 2016;7:10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obeng EA, Chappell RJ, Seiler M, Chen MC, Campagna DR, Schmidt PJ, et al. Physiologic expression of Sf3b1(K700E) causes impaired erythropoiesis, aberrant splicing, and sensitivity to therapeutic spliceosome modulation. Cancer Cell. 2016;30:404–17. 09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darman RB, Seiler M, Agrawal AA, Lim KH, Peng S, Aird D, et al. Cancer-associated SF3B1 hotspot mutations induce cryptic 3′ splice site selection through use of a different branch point. Cell Rep. 2015;13:1033–45. [DOI] [PubMed] [Google Scholar]
- 27.DeBoever C, Ghia EM, Shepard PJ, Rassenti L, Barrett CL, Jepsen K, et al. Transcriptome sequencing reveals potential mechanism of cryptic 3′ splice site selection in SF3B1-mutated cancers. PLoS Comput Biol. 2015;11:e1004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mupo A, Seiler M, Sathiaseelan V, Pance A, Yang Y, Agrawal AA, et al. Hemopoietic-specific Sf3b1-K700E knock-in mice display the splicing defect seen in human MDS but develop anemia without ring sideroblasts. Leukemia. 2017;31:720–7. 03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue D, Abdel-Wahab O. Modeling SF3B1 mutations in cancer: advances, challenges, and opportunities. Cancer Cell. 2016;30:371–3. 09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daubner GM, Cléry A, Jayne S, Stevenin J, Allain FH. A syn-anti conformational difference allows SRSF2 to recognize guanines and cytosines equally well. EMBO J. 2012;31:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122: 3616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E, Ilagan JO, Liang Y, Daubner GM, Lee SC, Ramakrishnan A, et al. SRSF2 mutations contribute to myelodysplasia by mutant-specific effects on exon recognition. Cancer Cell. 2015;27:617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kon A, Yamazaki S, Nannya Y, Kataoka K, Ota Y, Nakagawa MM, et al. Physiological Srsf2 P95H expression causes impaired hematopoietic stem cell functions and aberrant RNA splicing in mice. Blood. 2018;131:621–35. 02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smeets MF, Tan SY, Xu JJ, Anande G, Unnikrishnan A, Chalk AM, et al. initiates myeloid bias and myelodysplastic/myeloproliferative syndrome from hemopoietic stem cells. Blood. 2018;132:608–21. [DOI] [PubMed] [Google Scholar]
- 35.Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J, et al. Mutant U2AF1 expression alters hematopoiesis and Pre-mRNA splicing in vivo. Cancer Cell. 2015;27:631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fei DL, Zhen T, Durham B, Ferrarone J, Zhang T, Garrett L, et al. Impaired hematopoiesis and leukemia development in mice with a conditional knock-in allele of a mutant splicing factor gene. Proc Natl Acad Sci USA. 2018;115:E10437–46. 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Przychodzen B, Jerez A, Guinta K, Sekeres MA, Padgett R, Maciejewski JP, et al. Patterns of missplicing due to somatic U2AF1 mutations in myeloid neoplasms. Blood. 2013;122:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okeyo-Owuor T, White BS, Chatrikhi R, Mohan DR, Kim S, Griffith M, et al. U2AF1 mutations alter sequence specificity of pre-mRNA binding and splicing. Leukemia. 2015;29:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilagan JO, Ramakrishnan A, Hayes B, Murphy ME, Zebari AS, Bradley P, et al. U2AF1 mutations alter splice site recognition in hematological malignancies. Genome Res. 2015;25:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Ruiz PD, Novikov L, Casill AD, Park JW, Gamble MJ. MacroH2A1.1 and PARP-1 cooperate to regulate transcription by promoting CBP-mediated H2B acetylation. Nat Struct Mol Biol. 2014;21:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novikov L, Park JW, Chen H, Klerman H, Jalloh AS, Gamble MJ. QKI-mediated alternative splicing of the histone variant MacroH2A1 regulates cancer cell proliferation. Mol Cell Biol. 2011;31:4244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Lieu YK, Ali AM, Penson A, Reggio KS, Rabadan R, et al. Disease-associated mutation in SRSF2 misregulates splicing by altering RNA-binding affinities. Proc Natl Acad Sci USA. 2015;112:E4726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen H, Zheng X, Luecke S, Green MR. The U2AF35-related protein Urp contacts the 3′ splice site to promote U12-type intron splicing and the second step of U2-type intron splicing. Genes Dev. 2010;24:2389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niemelä EH, Frilander MJ. Regulation of gene expression through inefficient splicing of U12-type introns. RNA Biol. 2014;11: 1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alioto TS. U12DB: a database of orthologous U12-type spliceosomal introns. Nucleic Acids Res. 2007;35(Jan):D110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madan V, Kanojia D, Li J, Okamoto R, Sato-Otsubo A, Kohlmann A, et al. Aberrant splicing of U12-type introns is the hallmark of ZRSR2 mutant myelodysplastic syndrome. Nat Commun. 2015;6:6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20: 2429–40. [DOI] [PubMed] [Google Scholar]
- 49.Navada SC, Steinmann J, Lübbert M, Silverman LR. Clinical development of demethylating agents in hematology. J Clin Invest. 2014;124:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santini V. How I treat MDS after hypomethylating agent failure. Blood. 2019;133:521–9. [DOI] [PubMed] [Google Scholar]
- 51.Herceg Z, Hainaut P. Genetic and epigenetic alterations as biomarkers for cancer detection, diagnosis and prognosis. Mol Oncol. 2007;1:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45. [DOI] [PubMed] [Google Scholar]
- 53.Comet I, Riising EM, Leblanc B, Helin K. Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat Rev Cancer. 2016;16:803–10. 12 [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–8. [DOI] [PubMed] [Google Scholar]
- 55.Laugesen A, Højfeldt JW, Helin K. Molecular Mechanisms Directing PRC2 Recruitment and H3K27 Methylation. Mol Cell. 2019;74:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298:1039–43. [DOI] [PubMed] [Google Scholar]
- 57.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53. [DOI] [PubMed] [Google Scholar]
- 58.Francis NJ, Kingston RE, Woodcock CL. Chromatin compaction by a polycomb group protein complex. Science. 2004;306:1574–7. [DOI] [PubMed] [Google Scholar]
- 59.Tavares L, Dimitrova E, Oxley D, Webster J, Poot R, Demmers J, et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell. 2012;148:664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol Cell. 2013;49:808–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoyama K, Oshima M, Koide S, Suzuki E, Mochizuki-Kashio M, Kato Y, et al. Ezh1 targets bivalent genes to maintain self-renewing stem cells in Ezh2-insufficient myelodysplastic syndrome. iScience. 2018;9:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. New Engl J Med. 2011;364: 2496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sashida G, Harada H, Matsui H, Oshima M, Yui M, Harada Y, et al. Ezh2 loss promotes development of myelodysplastic syndrome but attenuates its predisposition to leukaemic transformation. Nat Commun. 2014;5:4177. [DOI] [PubMed] [Google Scholar]
- 64.Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12. [DOI] [PubMed] [Google Scholar]
- 66.Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, et al. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J Exp Med. 2013;210:2627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sashida G, Oshima M, Iwama A Deregulated polycomb functions in myeloproliferative neoplasms. Int J Hematol. 2019;110:170–8. [DOI] [PubMed] [Google Scholar]
- 68.Mochizuki-Kashio M, Aoyama K, Sashida G, Oshima M, Tomioka T, Muto T, et al. Ezh2 loss in hematopoietic stem cells predisposes mice to develop heterogeneous malignancies in an Ezh1-dependent manner. Blood. 2015;126:1172–83. [DOI] [PubMed] [Google Scholar]
- 69.Hasegawa N, Oshima M, Sashida G, Matsui H, Koide S, SarayaA, et al. Impact of combinatorial dysfunctions of Tet2 and Ezh2 on the epigenome in the pathogenesis of myelodysplastic syndrome. Leukemia. 2017;31:861–71. 04 [DOI] [PubMed] [Google Scholar]
- 70.Inoue D, Kitaura J, Togami K, Nishimura K, Enomoto Y, Uchida T, et al. Myelodysplastic syndromes are induced by histone methylation–altering ASXL1 mutations. J Clin Invest. 2013;123: 4627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park UH, Yoon SK, Park T, Kim EJ, Um SJ. Additional sex comb-like (ASXL) proteins 1 and 2 play opposite roles in adipogenesis via reciprocal regulation of peroxisome proliferator-activated receptor {gamma}. J Biol Chem. 2011;286:1354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asada S, Kitamura T Aberrant histone modifications induced by mutant ASXL1 in myeloid neoplasms. Int J Hematol. 2019;110:179–86. [DOI] [PubMed] [Google Scholar]
- 73.Inoue D, Fujino T, Kitamura T. ASXL1 as a critical regulator of epigenetic marks and therapeutic potential of mutated cells. Oncotarget. 2018;9:35203–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagase R, Inoue D, Pastore A, Fujino T, Hou HA, Yamasaki N, et al. Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J Exp Med. 2018;215:1729–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu YC, Chiu YC, Lin CC, Kuo YY, Hou HA, Tzeng YS, et al. The distinct biological implications of Asxl1 mutation and its roles in leukemogenesis revealed by a knock-in mouse model. J Hematol Oncol. 2017;10:139. 07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uni M, Masamoto Y, Sato T, Kamikubo Y, Arai S, Hara E, et al. Modeling ASXL1 mutation revealed impaired hematopoiesis caused by derepression of p16Ink4a through aberrant PRC1-mediated histone modification. Leukemia. 2019;33:191–204. [DOI] [PubMed] [Google Scholar]
- 79.Asada S, Goyama S, Inoue D, Shikata S, Takeda R, Fukushima T, et al. Mutant ASXL1 cooperates with BAP1 to promote myeloid leukaemogenesis. Nat Commun. 2018;9:2733. 07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. [DOI] [PubMed] [Google Scholar]
- 82.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–6. [DOI] [PubMed] [Google Scholar]
- 84.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–95. [DOI] [PubMed] [Google Scholar]
- 85.Guryanova OA, Shank K, Spitzer B, Luciani L, Koche RP, Garrett-Bakelman FE, et al. DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat Med. 2016;22:1488–95. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiba S. Dysregulation of TET2 in hematologic malignancies. Int J Hematol. 2017;105:17–22. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kats LM, Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P, et al. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell. 2014;14: 329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.List A, Ebert BL, Fenaux P. A decade of progress in myelodysplastic syndrome with chromosome 5q deletion. Leukemia. 2018;32:1493–9. 07 [DOI] [PubMed] [Google Scholar]
- 90.Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux JL, Sokal G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251:437–8. [DOI] [PubMed] [Google Scholar]
- 91.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. New Engl J Med. 2006;355:1456–65. [DOI] [PubMed] [Google Scholar]
- 92.Ribezzo F, Snoeren IAM, Ziegler S, Stoelben J, Olofsen PA, Henic A, et al. Rps14, Csnk1a1 and miRNA145/miRNA146a deficiency cooperate in the clinical phenotype and activation of the innate immune system in the 5q- syndrome. Leukemia 2019;33:1759–72. [DOI] [PubMed] [Google Scholar]
- 93.Schneider RK, Schenone M, Ferreira MV, Kramann R, Joyce CE, Hartigan C, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med. 2016;22:288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boultwood J, Pellagatti A, Cattan H, Lawrie CH, Giagounidis A, Malcovati L, et al. Gene expression profiling of CD34+ cells in patients with the 5q- syndrome. Br J Haematol. 2007;139:578–89. [DOI] [PubMed] [Google Scholar]
- 95.Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015;126:2366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caceres G, McGraw K, Yip BH, Pellagatti A, Johnson J, Zhang L, et al. TP53 suppression promotes erythropoiesis in del(5q) MDS, suggesting a targeted therapeutic strategy in lenalidomide-resistant patients. Proc Natl Acad Sci USA. 2013;110:16127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011;29:1971–9. [DOI] [PubMed] [Google Scholar]
- 98.Bello E, Pellagatti A, Shaw J, Mecucci C, Kušec R, Killick S, et al. CSNK1A1 mutations and gene expression analysis in myelodysplastic syndromes with del(5q). Br J Haematol. 2015; 171:210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hayashi Y, Zhang Y, Yokota A, Yan X, Liu J, Choi K, et al. Pathobiological pseudohypoxia as a putative mechanism underlying myelodysplastic syndromes. Cancer Disco. 2018;8: 1438–57. [DOI] [PMC free article] [PubMed] [Google Scholar]