Abstract
Purpose
A Regional Technical Commission was set in 2017 by Veneto region (Italy) to provide opinions and recommendations on drug prescriptions and to implement treatment-pathway guidelines for severe asthma. In this observational study, we describe the first structured, integrated, multidisciplinary, patient-centered outpatient clinic for the care of severe-asthma patients in Italy, and characterize patients referring to the center for specialist visits.
Patients and Methods
To characterize patients that accessed the outpatient clinic in 2018, data on demographic characteristics, treatments, severity of asthma, phenotypes, and relevant comorbidities by phenotype were collected. Use of biologic agents and indicators of the performance of the outpatient clinic were described.
Results
A structured multidisciplinary outpatient pathway for taking charge of patients and for administration and monitoring of biological agents was developed. A total of 146 patients accessed the outpatient clinic in 2018: 62.3% had uncontrolled asthma upon admission and 27.4% were already being treated with biologic agents. Among patients with uncontrolled asthma, 66% had severe uncontrolled asthma, 22% had moderate–severe uncontrolled asthma, and 12% had mild–moderate uncontrolled asthma. Main asthma phenotypes in uncontrolled-asthma patients were allergic (58% of patients), eosinophilic (22%), allergic plus eosinophilic (10%) and non-atopic asthma (10%). Among patients affected by severe asthma, 47% had allergic asthma, 28% had eosinophilic asthma, 13% had allergic plus eosinophilic asthma, and 12% had non-atopic asthma. Nasal polyps were more frequent in eosinophilic and allergic plus eosinophilic asthma, while gastro-esophageal reflux disease was more frequent in non-atopic asthma.
Conclusion
This structure of an outpatient clinic for the treatment of severe asthma, with its dedicated pathway and multidisciplinary approach, may allow a stricter control of asthma and optimization of therapies, as well as minimization of drug misuse.
Keywords: asthma, severe, multidisciplinary, outpatient
Introduction
Asthma, a major noncommunicable disease, affected in 2019 262 million persons and is responsible of 461,000 deaths. Asthma affects both children and adults and represents the most frequent chronic disease in children.1,2 It is a chronic inflammatory disorder of the airways caused by an interaction between genetic factors and environmental factors. Asthma is characterized by widespread, variable, and reversible airflow obstruction, airway inflammation, excessive production of mucus, and airway hyper-responsiveness. These features lead to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing.3
Asthma can have several phenotypes4,5 driven by multiple underlying disease processes. A different etiology of inflammation in asthma can be associated with a different response to asthma treatments.6
Asthma presents with varying degrees of severity, ranging from mild, intermittent disease to severe presentations with debilitating (even life-threatening) symptoms. Asthma is considered to be “severe” if it is uncontrolled despite adherence to maximal therapy or worsened with step-down of high-dose treatments.7 Severe asthma affects only a minority of all asthma patients, and prevalence can vary from country-to-country, due largely to variation between clinical and epidemiological definitions.8,9
To be classified as having “severe asthma”, patients treated with the maximum dose of inhalation therapy should be assessed appropriately in a specialist setting to confirm the diagnosis, evaluate comorbidities and their management, as well as to assess the level of adherence to therapy and inference of environmental factors.7,10–12 Severe asthma is estimated to affect about 3–10% of asthma patients: despite such a small percentage of the population, total expenditure accounts for >60% of the total costs attributed to asthma.7,13,14 Most asthma patients can be cared for adequately based on global guidelines (ie, Global Initiative for Asthma (GINA); Global Strategy for Asthma Management and Prevention). Nevertheless, a subpopulation of patients with severe asthma will not be treated adequately with the current standard of care despite the availability of therapies reported as being “highly efficacious” in clinical trials,15,16 and their treatment remains an important unmet need.17
Observational studies carried out in Italy have estimated suboptimal control of asthma to affect ~35% of asthma patients.18–20 Two real-world studies in Italy revealed that the prevalence of severe uncontrolled asthma ranged from 0.025% (Veneto Region, based on a population aged >6 years)20 to 0.04% (based on an adult subpopulation).21 Patients with severe uncontrolled asthma carry a high risk of severe exacerbations and mortality, and are often dependent upon oral corticosteroids (OCs).22
Asthma is frequently associated with several comorbidities and concomitant conditions that may share with asthma a common pathophysiological mechanism, affect the control of the pathology, its phenotype and treatment response. The most frequent co-morbidities are rhinitis, sinusitis, gastroesophageal reflux disease, obstructive sleep apnea, hormonal disorders, and psychopathologies.23,24
Long-term use of OCs is associated, in addition to psychological side-effects, with adverse effects: obesity, diabetes mellitus, osteoporosis, cataracts, hypertension, and adrenal suppression,25,26 Even short-term use of OCs can lead to sleep disturbance, increased risk of infection, bone fracture, and thromboembolism.27,28 Strategies to minimize OC need are, therefore, a high priority.
An outpatient clinic structured with a dedicated pathway and multidisciplinary team for the treatment of severe asthma can provide complete services and allow the optimization of asthma treatment and outcomes. Besides the adequate clinical control of severe asthma, in order to meet patient needs and optimization of management within a landscape of limited resources, a specific pathway for the care of adult patients affected by severe bronchial asthma was implemented in the Local Health Unit “ULSS9 Scaligera” in Veneto, Italy.
Foreseeing the evolution of therapeutic approaches against severe asthma and upcoming marketing authorization of a new class of monoclonal antibodies, in 2016 the Veneto Region highlighted the need for pharmacological management of severe asthma to define (i) therapeutic alternatives according to the literature; (ii) criteria for selecting patients who could benefit from new drug therapies; (iii) indicators for monitoring the appropriateness of prescriptions.
In this observational study, we aimed to describe the first structured, integrated, multidisciplinary, patient-centered outpatient clinic for the clinical management of severe asthma in Italy. We also aimed to characterize severe-asthma patients referred to the outpatient clinic for specialist visits. The purpose of the outpatient clinic was to create an optimized treatment pathway for the entire ULSS (local Health Unit) to meet regional goals. The treatment pathway was approved by the General Manager Health and Social Care Veneto Region and the results of the first year of activity are presented here following.
Materials and Methods
This was a retrospective cohort study based on real-world, patient-level results from the database of asthma outpatient clinics of ULSS 9 Scaligera. We evaluated the organization of the Pulmonology Unit of Bussolengo/Villafranca in Verona Azienda (ULSS 9 Scaligera) and its performance for the first year of activity (2018). The innovative structure of the ambulatory unit and specific pathways for new and consolidated patients are described.
We wished to characterize patients who accessed the outpatient clinic during 2018. Hence, data on demographic characteristics (age, sex), treatments, asthma severity, specific phenotypes according to the assessment of the treating physician (allergic asthma, eosinophilic asthma, non-allergic asthma, mixed phenotype) and relevant comorbidities by phenotype were collected. Moreover, the origin of the patients accessing the outpatient clinic is reported (eg, Emergency Room, Intensive Care Unit).
To describe ambulatory activities in 2018, the healthcare resources analyzed were specialist visits, diagnostic examinations, and drug prescriptions. Patients with uncontrolled asthma accessed the outpatient clinic irrespective of asthma severity, so the overall population and population of patients with severe asthma are described separately. Finally, use of biologic agents and indicators of performance are described.
Three main indicators allowed evaluation of the degree of achievement with respect to the declared objective. Indicator 1 was (number of patients taken in charge/total number of patients evaluated) × 100; the expected value was ≥70%. Indicator 2 was (number of patients treated with biologic agents/total number of patients taken in charge) × 100; the expected value was ≥50%. Indicator 3 was (number of patients with suspension of OC use/number of patients treated with biologic agents) × 100; the expected value was ≥50%.
This manuscript reviewed and describe the center activities in order to present the new organization of the outpatient clinic that was established by a Veneto Regional Law (Law Decree n.54 2018 and Law Decree n.85 2018). All patients accessing the outpatient ambulatory signed the informed consent to the treatment and collection of sanitary routine data as forecasted by the European General Data Protection Regulation (GDPR) (2016/679), Law Decree 196/2003 (Italian legal rules regarding the protection of personal data that specifically includes the management and evaluation of activities of the outpatient clinic and epidemiology and scientific research activities) integrated with Law Decree 10/08/2018 nr 101; only data covered by reported laws were collected and presented in aggregated and anonymous way.
All patients who accessed Pulmonology Unit of Bussolengo/Villafranca in 2018 were evaluated. A formal hypothesis was not specified because this was not an analytical study. General descriptive statistics for continuous numerical variables were the number of observations, the mean, and the standard deviation. For categorical variables, the proportion of patients with a certain event/characteristic are presented.
Results
The outpatient clinic aimed, through a multidisciplinary and integrated approach, to (i) provide the correct diagnosis; (ii) identify the most suitable pharmacological therapy; (iii) undertake specialist monitoring personalized according to the need of each adult patient with severe asthma.
Outpatient Pathways and Organization
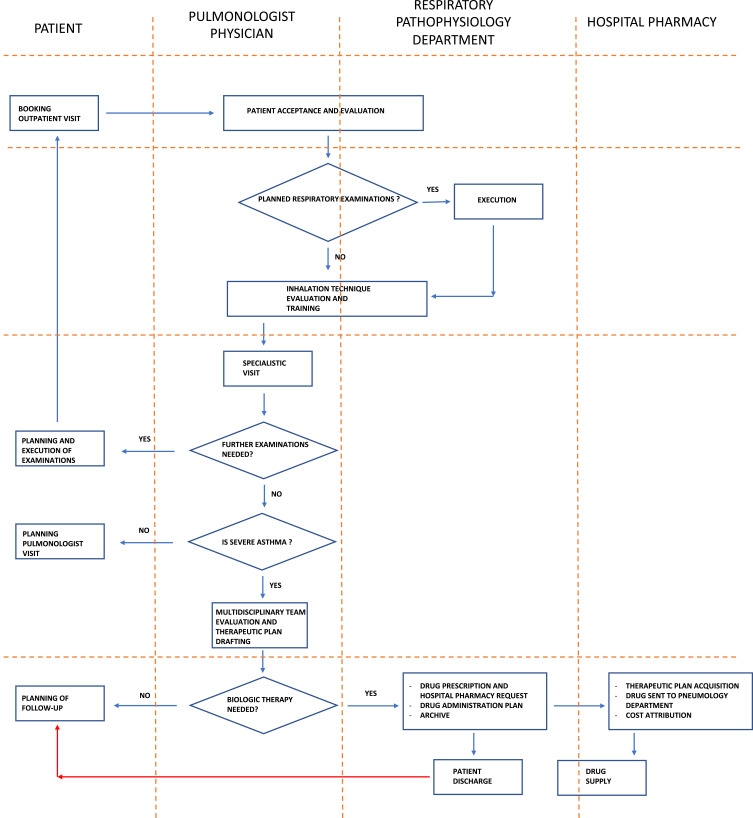
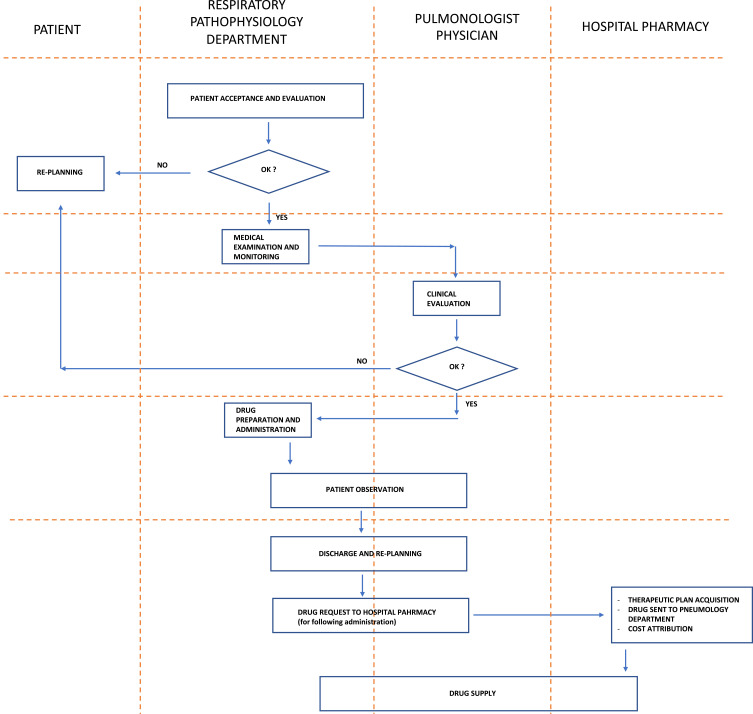
The outpatient clinical pathway for a patient with severe asthma, and the clinical pathway for the administration and monitoring of biological therapies for a patient with severe asthma, are described in Figures 1 and 2, respectively.
Figure 1.
Outpatient clinical pathway for a patient with severe asthma.
Figure 2.
Clinical pathway for the administration and monitoring of biological therapies for a patient with severe asthma.
Organization of the diagnostic, therapeutic, and medical-assistance pathways involved the outpatient clinic of the Pulmonology Unit, Respiratory Pathophysiology Unit, Allergy Unit, as well as the hospital pharmacy, booking center of the outpatient clinic.
To manage the associated diseases, the following departments were involved (if needed): Ear, Nose and Throat; Gastrointestinal; Rheumatology; Laboratory Testing. Outpatient clinics from the Department of Clinical Psychology and dietary services were available on demand to offer additional support to patients.
During 2018, the outpatient clinic of Pulmonology Unit of Bussolengo/Villafranca undertook: 424 visits by pneumologists to control asthma; four first visits by pneumologists; three evaluations of exhaled gas; 393 measurements of simple/global spirometry; 649 administrations of biologic agents (at the moment of starting this outpatient structure only omalizumab and mepolizumab were available and reimbursed by Italian National Health Service (NHS)).
Description of Outpatient Clinic Activities
A total of 146 patients accessed the outpatient clinic in 2018. Among them, 91 (62.3%) had uncontrolled asthma upon admission, 40 (27.4%) were already being treated with biologic agents, and 10 (10.3%) were discharged with a different diagnosis. The average age of patients with uncontrolled asthma was 53.8 ± 16.9 years, and 64.5% were men.
Following GINA definitions, out of the 91 patients with uncontrolled asthma who accessed the outpatient clinic, 60 (66%) were affected by severe uncontrolled asthma, 20 (22%) had moderate–severe uncontrolled asthma, and 11 (12%) had mild–moderate uncontrolled asthma.
Among patients with uncontrolled asthma, 76 (84%) were referred from a first-level outpatient clinic, four (4%) from the intensive care unit (after hospital admission caused by atopic uncontrolled asthma), six (7%) from hospital admission due to respiratory failure, and four (4%) from the Emergency Department; one patient was pregnant.
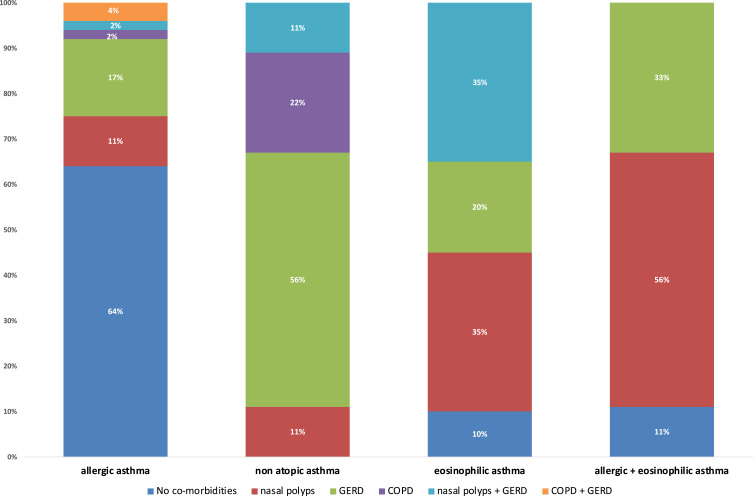
The most frequently reported diagnosis was allergic asthma in 53 (58%) cases (average age, 49 ± 17.5 years), eosinophilic asthma in 20 (22%) patients (58.8 ± 13.7 years), allergic plus eosinophilic asthma in nine (10%) cases (60.8 ± 10.1 years) and non-atopic asthma in nine (10%) patients (69 ± 10.5 years). The prevalence of comorbidities by asthma phenotype is described in Figure 3.
Figure 3.
Prevalence of comorbidities by asthma phenotype.
Abbreviations: GERD, gastro-esophageal reflux disease; COPD, chronic obstructive pulmonary disease, COPD.
Among the 60 patients affected by the “severe” phenotype (61.6% females; average age, 56.2 ± 15.2 years), 28 (47%) had allergic asthma, 17 (28%) had eosinophilic asthma, eight (13%) had allergic plus eosinophilic asthma, and seven (12%) had non-atopic asthma.
Nasal polyps were more frequent in eosinophilic and allergic plus eosinophilic asthma, while gastro-esophageal reflux disease was more frequent in non-atopic asthma.
Overall, 36.6% of cases with severe asthma met the criteria for use of biologic agents: 29% had the allergic phenotype, 53% had the eosinophilic phenotype, and 63% had the allergic plus eosinophilic phenotype. Among patients treated with biologic agents, 86.2% discontinued OC use.
Discussion
Severe uncontrolled asthma carries a substantial burden in terms of severely impaired quality of life and a significant economic impact. A real-world study in Italy recently showed the average cost per patient per year (excluding pharmacological treatments) to be €3701; pharmacological treatments costs range from €138 to >€17,000.29 Moreover, corticosteroids shadow costs, due to OCs-induced morbidity, range from 45% to 30%.30 Maintenance of asthma control is expected to reduce the overall economic impact of asthma as well as the indirect costs associated with decreased productivity of the affected patients.
The innovative structure of the outpatient clinic described here allowed a dedicated treatment pathway for each specific phenotype of severe asthma. It allowed different modalities to be used and scheduling of clinical evaluations compared with that observed in most outpatient clinics in Italy. Particularly, compared with the standard pulmonology outpatient clinics of the Italian NHS, the one presented in this manuscript is dedicated only to patients with suspected severe asthma operating with physicians and nurses already trained in the management of this pathology allowing patients a dedicated access to the structure and reduction of waiting lists.
Within the population that was followed in the first year of the clinic, the allergic phenotype is confirmed to be predominant (58%), and with an apparently lower average age than the other phenotypes. Persistent and significant eosinophilia was found in only 10% of patients suffering of allergic asthma, while 22% of patients affected by non-allergic asthma could be diagnosed as an eosinophilic phenotype. A small proportion of subjects (9/91 patients) were found to be affected by non-allergic asthma and without eosinophils, thus being able to fall into the more generic category of “intrinsic” asthma, characterized by a significantly higher average age of allergic asthmatics. The importance of evaluating the nasal polyposis-asthma association is confirmed which was found to be mainly associated with the eosinophilic phenotype compared to the purely atopic one.
A small percentage of patients (12%) was diagnosed with mild-moderate asthma and the inclusion in the dedicated pathway made it possible to achieve control of asthma symptoms and to return the patient to the first level outpatient clinic.
The assessment of the first year of activity of the clinic showed a substantial adequacy of the referral of patients by the referring specialists; in fact, only 15 patients were discharged from the clinic with another diagnosis and 91 patients had uncontrolled asthma at the time of the first assessment in the clinic.
Most (77/91 patients) of the patients came from first level specialist outpatient clinics. The other patients were referred for evaluation at the outpatient clinic after serious acute events requiring hospitalization in intensive care unit (4 patients, all subjects with uncontrolled atopic bronchial asthma), after an ordinary hospitalization due to respiratory failure (6 patients) and after accessing to emergency department (4 patients). All this confirms, once again, that despite the wide availability of treatments, and the ease of access to treatment in Italy, asthma remains a potentially life-threatening pathology.
For 60 patients (66%) it was possible to conclude the course proposed by the guidelines during the year of observation and to effectively diagnose severe asthma, while 20 subjects (22%) remained under close monitoring with a diagnosis of moderate asthma-severe “difficult to control”
In the organizational reality of first level clinic, waiting lists and booking difficulties would not have allowed the above results to be achieved. Thanks to the project described here, with the use of dedicated medical and nursing staff, it was possible to guarantee access to specific planning for patients in the clinic, to closely monitor subject in times established by the therapeutic changes but also to quickly guarantee re-evaluations if exacerbations occur.
This type of structure permitted correct identification of severe-asthma patients and enabled their evaluation through specific indicators. Once a patient had been diagnosed with severe asthma and the specific phenotype had been identified though characterization, the most appropriate treatment (according to GINA recommendations) was prescribed. Subsequently, patients were re-evaluated periodically for dose adjustments/changes and check on control of comorbidities.
The outpatient clinic also accepted patients with uncontrolled asthma of different severity. It allowed personalization of pharmacological treatment thanks to identification of the asthma phenotype. This strategy avoided inappropriate immediate use of biologic agents, chronic use of high doses of OCs, and allowed optimization of pharmacological treatment. Even for severe-asthma patients, identification of the phenotype and regular follow-up allowed correct decisions on initiation of biologic-agent therapy (as per GINA recommendations) to be made.
Moreover, patients had regular contact with specialist physicians and dedicated nurses. This availability contributed to improvement in compliance with treatment and the possibility of step-down of supportive treatment (eg, OCs). In the future, optimization of use of healthcare resources can be estimated to evaluate the performance of the integrated and multidisciplinary approach of this outpatient clinic.
Conclusion
The new structure of the outpatient clinic ULSS 9 Scaligera for the treatment of severe asthma is based on a dedicated pathway and a multidisciplinary approach. This structure allowed strict control of patients and optimization of pharmacological treatment with the potential of better control of asthma and minimization of drug misuse.
Funding Statement
Financial support for manuscript preparation was provided by AstraZeneca. The latter had no role in the study design, collection/analysis/interpretation of data, writing of the report, or the decision to submit the manuscript for publication.
Disclosure
AR and DPR are consultants for AstraZeneca. ST is speaker for and reports personal fees from Chiesi and GSK, outside the submitted work. CM received fees as a speaker in local, national and international congress by GSK, AstraZeneca, Sanofi, and Novartis. DA reports grants from Aipo Ricerche, Chiesi, and Menarini, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Vos T, Lim SS, Abbafati Cet al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization.WHO factsheets on Asthma. Available from https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed July29, 2021.
- 3.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–486;quiz 487. doi: 10.1016/j.jaci.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 5.Hancox RJ, Cowan DC, Aldridge RE, et al. Asthma phenotypes: consistency of classification using induced sputum. Respirology. 2012;17(3):461–466. doi: 10.1111/j.1440-1843.2011.02113.x [DOI] [PubMed] [Google Scholar]
- 6.Douwes J. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643–648. doi: 10.1136/thorax.57.7.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung KF, Wenzel SE, Brozek JR, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 8.Wenzel S. Severe asthma: epidemiology, pathophysiology and treatment. Mt Sinai J Med. 2003;70:185–190. [PubMed] [Google Scholar]
- 9.Engelkes M, de Ridder MAJ, Svensson E, et al. Multinational, database cohort study to assess severe exacerbation rate in asthma. Respir Med. 2020;165:105919. doi: 10.1016/j.rmed.2020.105919 [DOI] [PubMed] [Google Scholar]
- 10.Bel EH, Sousa A, Fleming L, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the innovative medicine initiative (IMI). Thorax. 2011;66(10):910–917. doi: 10.1136/thx.2010.153643 [DOI] [PubMed] [Google Scholar]
- 11.Chanez P, Wenzel SE, Anderson GP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119(6):1337–1348. doi: 10.1016/j.jaci.2006.11.702 [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma. Difficult-to-treat & severe asthma in adolescent and adult patients. diagnosis and management. A GINA pocket guide for health professionals; 2018. Available from: https://ginasthma.org/wp-content/uploads/2018/11/GINA-SA-FINAL-wms.pdf. Accessed July29, 2021.
- 13.Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 14.Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17:74–80. doi: 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braido F, Brusselle G, Guastalla D, et al. Determinants and impact of suboptimal asthma control in Europe: the international cross-sectional and longitudinal assessment on asthma control (LIAISON) study. Respir Res. 2016;17(1):51. doi: 10.1186/s12931-016-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC [DOI] [PubMed] [Google Scholar]
- 17.Gauthier M, Ray A, Wenzel SE. Evolving concepts of asthma. Am J Respir Crit Care Med. 2015;192(6):660–668. doi: 10.1164/rccm.201504-0763PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allegra L, Cremonesi G, Girbino G, et al; PRISMA (PRospectIve Study on asthMA control) Study Group. Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med. 2012;106:205–214. doi: 10.1016/j.rmed.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 19.Bettoncelli G, Magnoni MS, Fassari C, et al. Il controllo dell’asma in Italia misurato con ACT (Asthma Control Test). Società Italiana di Medicina Generale. 2006;6:10–17. [Google Scholar]
- 20.Vianello A, Caminati M, Andretta M, et al. Prevalence of severe asthma according to the drug regulatory agency perspective: an Italian experience. World Allergy Organ J. 2019;12(4):100032. doi: 10.1016/j.waojou.2019.100032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedrini A, Rossi E, Calabria S, et al. Current management of severe refractory asthma in Italy: analysis of real-world data. Glob Reg Health Technol Assess. 2017;4(1):e216–e220. doi: 10.5301/grhta.5000273 [DOI] [Google Scholar]
- 22.Accordini S, Bugiani M, Arossa W, et al. Poor control increases the economic cost of asthma. A multicentre population-based study. Int Arch Allergy Immunol. 2006;141(2):189–198. doi: 10.1159/000094898 [DOI] [PubMed] [Google Scholar]
- 23.Poddighe D, Brambilla I, Licari A, Marseglia GL. Pediatric rhinosinusitis and asthma. Respir Med. 2018;141:94–99. PMID: 30053979. doi: 10.1016/j.rmed.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 26.Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J. 2017;50(3):1700765. doi: 10.1183/13993003.00765-2017 [DOI] [PubMed] [Google Scholar]
- 27.Waljee AK, Rogers MAM, Paul L, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi: 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global Initiative for Asthma.Difficult-to-treat & severe asthma in adolescent and adult patients. diagnosis and management. A GINA pocket guide for health professionals; 2018. Available from https://ginasthma.org/wp-content/uploads/2018/11/GINA-SA-FINAL-wms.pdf. Accessed July29, 2021.
- 29.Colombo GL, Di Matteo S, Heffler E, et al. Risultati dello studio HERCULES: valutazione economica dell’impiego di risorse sanitarie e descrizione del profilo clinico del paziente con asma grave non controllato in Italia secondo dati di real-world. [Results of the HERCULES study: economic evaluation of healthcare resources use and description of the clinical profile of the patient with severe uncontrolled asthma in Italy according to real-world data]. Clin Econ Italian Articles Outcomes Res. 2020;15:63–78. Italian. [Google Scholar]
- 30.Canonica GW, Colombo GL, Bruno GM, et al. Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the severe asthma network in Italy (SANI) registry. World Allergy Organ J. 2019;12(1):100007. doi: 10.1016/j.waojou.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]