Abstract
Aims
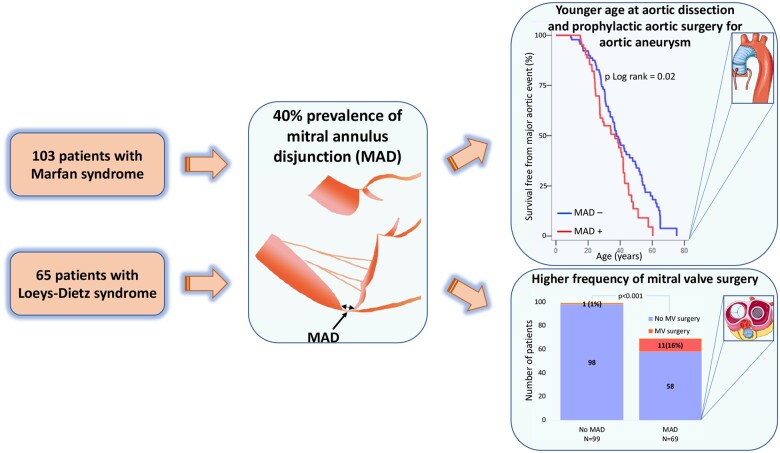
We aimed to assess the prevalence of mitral annulus disjunction (MAD) and to explore the association with aortic disease and mitral valve surgery in patients with Marfan syndrome (MFS) and Loeys–Dietz syndrome (LDS).
Methods and results
We included consecutive MFS patients fulfilling Revised Ghent Criteria and LDS patients fulfilling Loeys–Dietz Revised Nosology. MAD was identified by echocardiography and was quantified as the longitudinal distance from the ventricular myocardium to the hinge point of the posterior mitral leaflet. Aortic events were defined as aortic dissection or prophylactic aortic surgery. We recorded the need of mitral valve surgery including mitral valve repair or replacement. We included 168 patients (103 with MFS and 65 with LDS). The prevalence of MAD was 41%. MAD was present in all age groups. Aortic events occurred in 112 (67%) patients (27 with dissections and 85 with prophylactic surgical interventions). Patients with MAD were younger at aortic event than those without MAD (log rank = 0.02) Patients with aortic events had greater MAD distance in posterolateral wall [8 (7–10) mm vs. 7 (6–8) mm, P = 0.04]. Mitral events occurred more frequently in patients with MAD (P < 0.001).
Conclusion
MAD was highly prevalent in patients with MFS and LDS. MAD was a marker of severe disease including aortic events at younger age and need of mitral valve surgery. Screening patients with MFS an LDS for MAD may provide prognostic information and may be relevant in planning surgical intervention. Detection of MAD in patients with MFS and LDS may infer closer clinical follow-up from younger age.
Keywords: mitral annulus disjunction, Marfan, Loeys–Dietz, aortic syndrome, mitral valve prolapse, mitral valve disease
Graphical Abstract
From Smart Servier Medical Art with permission (https://creativecommons.org/licenses/by/3.0/legalcode)
Introduction
Marfan syndrome (MFS) and Loeys–Dietz syndrome (LDS) are connective tissue diseases carrying a high risk of mortality related to aortic pathology.1 Management of these patients is mainly focused on prevention of aortic dissection and timing of aortic surgery.2 Mitral valve involvement [myxomatous degeneration, mitral valve prolapse (MVP), mitral regurgitation] is frequent in these patients occurring in 60–75% of them and is a considerable source of morbidity.3,4 Mitral annulus disjunction (MAD) was described 30 years ago5 and was since then closely associated with prolapse of the mitral valve.6–8 Identification of MAD by echocardiography is important given its association with ventricular arrhythmias (VAs).9 The prevalence of MAD in patients with MFS and LDS has not yet been described.
We aimed to assess the prevalence of MAD in consecutive patients with MFS and LDS and to explore the association with aortic disease and mitral valve surgery. We hypothesized that MAD is highly prevalent in patients with MFS and LDS and is associated with worse prognosis.
Methods
Study population
From 2003 to 2018, we prospectively recruited patients who presented with aortic syndromes or aortic aneurysms and with suspected connective tissue disease and their family members identified through cascade genetic screening and included them in the database of the Department of Cardiothoracic Surgery, Oslo University Hospital, Norway.
For this study, we retrospectively identified patients diagnosed with MFS and LDS in the surgical database. MFS was diagnosed according to Revised Ghent Criteria10 and LDS was diagnosed in the presence of a disease-causing genetic mutation for LDS with either arterial aneurysm or dissection or with a family history of documented LDS as described by Loeys–Dietz Revised Nosology.4 Patients with associated cardiac comorbidities were excluded. Paediatric patients were defined as those <16 years old.
The study complied with the Declaration of Helsinki was approved by the Institutional Review Board and informed consent was waived (PVO 19/11168). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Surgical and arrhythmic events
We defined aortic events (AoE) as aortic dissection (Stanford Type A or B) and prophylactic aortic surgery indicated due to aortic aneurysms. We recorded aortic root dilatation Z-score at prophylactic surgery on the ascending aorta in all patients. Ascending aortic aneurysm and thoracoabdominal descending aortic aneurysm surgery was performed according to recommendations.4,11,12
We defined mitral events as isolated mitral valve surgery before or after aortic surgery and mitral valve surgery performed combined with aortic surgery.
In a subset of patients, we recorded presence of ventricular premature contractions (VPCs) documented by implantable cardiac device or by symptoms-triggered 24 h electrocardiogram (ECG) monitoring. VAs were defined as >10 VPC/h, complex VPC (ventricular bigeminy, trigeminy, and couplets) or non-sustained ventricular tachycardia (≥3 consecutive VPC).13
Follow-up was performed on clinical indication. We retrieved data regarding surgical events, death occurrence, and cause of death from patients’ electronical medical files.
Genetic testing
Genetic testing was performed in all patients and included FBN1 from 2003. From 2005, genetic testing was extended to include also TGFBR1 and TGFBR2. From 2013, genetic screening was performed by high-throughput sequencing analysis of a targeted panel of 33 genes associated with MFS and related conditions, including the aforementioned genes in addition to SMAD3 and TGFB2.
Echocardiography
We assessed first available and analysable transthoracic echocardiography at our centre (GE Vivid7, E9 or E95). An echocardiographic examination was available before mitral surgery in all patients with mitral events.
For patients ≥16years, we measured the aortic root at sinuses of Valsalva in diastole from leading edge to leading edge,14 while for patients <16 years the aortic root was measured at sinuses of Valsalva in systole from inner edge to inner edge according to guidelines.15 Aortic root dilatation Z-score was calculated using the online calculator available on Marfan Foundation website (https://www.marfan.org/dx/zscore) (Retrieved: 10/12/2019) in all patients except from patients with type A dissection and with the index echocardiogram after prophylactic surgery on ascending aorta.
MVP was defined as superior displacement ≥2 mm of any part of the mitral leaflet beyond the mitral annulus16 in parasternal or apical long-axis view. Mitral regurgitation was graded according to guidelines16 and reported if moderate or severe. MAD was assessed in parasternal and apical long-axis views and defined as an end-systolic distance ≥1 mm from the left atrium-mitral leaflet junction to the top of left ventricular (LV) myocardium (Figure 1). Posterolateral MAD distance was measured at end-systole from left atrial wall-mitral valve leaflet junction to the top of the LV posterolateral wall in parasternal long-axis view and anterolateral MAD distance was measured at end-systole from left atrial wall-mitral valve leaflet junction to the top of the LV anterolateral wall in apical four-chamber view.9 Longitudinal MAD distance was indexed to body surface area (BSA). Mitral annulus diameter was measured in systole and diastole in parasternal long-axis view and indexed to BSA. Mitral annulus dilatation was defined as a maximum antero-posterior mitral annulus diameter >35 mm throughout the cardiac cycle in the adult population only.17 Intra- and interobserver variability for posterolateral MAD distance and mitral annulus diameter was assessed reanalysing 10 random echocardiographic studies by two independent experienced sonographers (M.C. and E.S.) (Supplementary data online, Table S1).
Figure 1.
Echocardiogram of two patients with MFS and two patients with LDS with MAD with and without MVP. End-systolic and mid-diastolic frames of mitral valve in parasternal long-axis view. Orange arrows indicate longitudinal MAD distance; yellow dotted lines demarcate the mitral annulus; and yellow solid lines are prolapse depth where present. Patient A is a 10-year-old patient with MFS, MAD, bileaflet MVP, and mild mitral regurgitation (see Supplementary data online, Video S1). Patient B is a 16-year-old patient with MFS, MAD, systolic curling motion of the LV basal posterolateral wall, no MVP, discrete billowing of mitral leaflets, and mild mitral regurgitation (see Supplementary data online, Video S2). Patient C is a 16-year-old patient with SMAD3 mutation positive LDS, MAD, bileaflet MVP, and mild mitral regurgitation (see Supplementary data online, Video S3). Patient D is a 33-year-old patient with TGFBR2 mutation positive LDS, MAD, typical systolic hyperkinetic curling motion of the LV basal posterolateral wall, no MVP, and mild mitral regurgitation (see Supplementary data online, Video S4). LDS, Loeys–Dietz syndrome; LV, left ventricle; MAD, mitral annulus disjunction; MFS, Marfan syndrome; MVP, mitral valve prolapse.
LV volumes and ejection fraction were assessed according to current recommendations.18 LV volumes were indexed to BSA. Systolic myocardial velocities and diastolic function parameters were assessed according to guidelines.19
All echocardiographic analyses were performed blinded to clinical data.
Statistic methods
Continuous data were presented as average with standard deviation or median with interquartile range (IQR) and categorical data as numbers (percentages). Continuous variables were compared using Student’s t-test or the Mann–Whitney U test and categorical data using χ2 or Fisher’s exact test, as appropriate. Kaplan–Meier analysis was used to illustrate age difference at aortic and mitral events in patients with and without MAD. Pearson correlation was used to assess the linear relationship between longitudinal MAD distance and age or mitral annulus diameter. We assessed association between MAD and ventricular dimensions adjusted for the presence of mitral regurgitation and between MAD and peak systolic mitral annular velocities and E/e′ ratio adjusted for age and presence of mitral regurgitation using linear regression. Intra- and interobserver variability was assessed using intraclass correlation coefficient (ICC). P-values were two-sided and values <0.05 were considered significant. Statistical analysis was performed using SPSS v.25 (SPSS, Inc, Chicago, IL, USA) and Stata/SE 16.1 (StataCorp LLC, TX, USA).
Results
Study population
We identified 224 patients with MFS and LDS fulfilling diagnostic criteria. Patients with neonatal MFS and with associated comorbidities were excluded. We included 168 patients with available and analysable echocardiographic studies (Figure 2) [103 (61%) MFS and 65 (39%) LDS]. Among the 103 MFS patients, 97 (94%) were FBN1 mutation positive. All 65 LDS patients were positive for one of the LDS associated mutations (TGFBR1, TGFBR2, SMAD3, TGFB2). Among the 65 patients with LDS, 55 (85%) had an arterial dilatation or dissection, and 10 (15%) had a family history of documented LDS as an additional criterion to the presence of a genetic mutation.
Figure 2.
Flow chart of study methodology and distribution of MAD in patients with MFS and LDS. Patients with MFS and LDS were retrospectively identified in the surgical database (n = 257). Patients with MFS not fulfilling Revised Ghent Criteria (n = 32) as well as patients with neonatal MFS (n = 2) were excluded. LDS patients not fulfilling Loeys–Dietz Revised Nosology (n = 1) were excluded. Another two patients were excluded due to associated comorbidities (one with coronary artery disease and one with tachycardia induced cardiomyopathy with heart failure) and 52 patients were excluded due to unavailable echocardiography or inappropriate image quality. LDS, Loeys–Dietz syndrome; MAD, mitral annulus disjunction; MFS, Marfan syndrome.
At first examination patients with MFS were younger than LDS patients (Table 1).
Table 1.
Characteristics of patients with MFS and LDS
| Total (n = 168) | MFS (n = 103) | LDS (n = 65) | P-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age at first exam ination (years) | 24 ± 17 | 20 ± 14 | 32 ± 20 | <0.001 |
| Male sex, n (%) | 90 (54) | 56 (54) | 34 (52) | 0.79 |
| Family members, n (%) | 90 (54) | 44 (43) | 46 (71) | <0.001 |
| Echocardiographic parameters | ||||
| Age at echocardiography (years) | 33 ± 17 | 31 ± 15 | 36 ± 19 | 0.04 |
| Aortic root dilatation Z-score (n = 131, pre-event echocardiographies) | 4.1 ± 2.6 | 4.6 ± 2.6 | 3.4 ± 2.5 | 0.009 |
| MAD, n (%) | 69 (41) | 47 (46) | 22 (34) | 0.13 |
| Posterolateral MAD distance (mm) | 8 ± 3 | 9 ± 3 | 7 ± 3 | 0.05 |
| Posterolateral MAD distance indexed (mm) | 4.6 ± 1.7 | 4.9 ± 1.9 | 3.9 ± 1.2 | 0.03 |
| Anterolateral MAD distance (mm) | 7 ± 3 | 7 ± 2 | 6 ± 4 | 0.16 |
| Anterolateral MAD distance indexed (mm) | 4.0 ± 2.1 | 4.2 ± 2.1 | 3.3 ± 2.1 | 0.22 |
| MVP, n (%) | 52 (31) | 41 (40) | 11 (17) | 0.002 |
| Mitral regurgitation, n (%) | 30 (18) | 22 (21) | 8 (13) | 0.15 |
| Diastolic mitral annulus diameter indexed (mm/m2) | 18 ± 5 | 18 ± 4 | 18 ± 5 | 0.98 |
| Systolic mitral annulus diameter indexed (mm/m2) | 17 ± 5 | 18 ± 5 | 16 ± 5 | 0.06 |
| Dilated mitral annulus (n = 136, adult patients only), n (%) | 46 (27) | 35 (34) | 11 (17) | 0.005 |
| LVEDVi (mL/m2) | 57 ± 19 | 59 ± 17 | 54 ± 21 | 0.10 |
| LVESVi (mL/m2) | 23 ± 9 | 24 ± 9 | 21 ± 9 | 0.05 |
| EF (%) | 60 ± 5 | 60 ± 5 | 62 ± 5 | 0.03 |
| Outcome data | ||||
| AoE, n (%) | 112 (67) | 66 (64) | 46 (71) | 0.37 |
| Aortic dissection, n (%) | 27 (16) | 15 (15) | 12 (18) | 0.50 |
| Prophylactic aortic surgery, n (%) | 85 (51) | 51 (50) | 34 (52) | 0.72 |
| Age at AoE (years) | 35 ± 14 | 33 ± 13 | 39 ± 16 | 0.01 |
| Aortic root dilatation Z-score at prophylactic surgery on ascending aorta (n = 81) | 6.6 ± 3.2 | 7.6 ± 3.4 | 5.0 ± 2.3 | <0.001 |
| Mitral events, n (%) | 12 (7) | 7 (7) | 5 (8) | 0.81 |
| Age at mitral event (years) | 37 ± 15 | 31 ± 15 | 45 ± 11 | 0.12 |
Values are average ± SD or frequencies(%). P-values are calculated by Student’s t test or chi-square, as appropriate. Bold values denote statistical significance.
AoE, aortic events; EF, ejection fraction; LDS, Loeys–Dietz syndrome; LVEDVi, left ventricular end-diastolic volume indexed for body surface area; LVESVi, left ventricular end-systolic volume indexed for body surface area; MAD, mitral annulus disjunction; MFS, Marfan syndrome; MVP, mitral valve prolapse.
Prevalence of mitral annulus disjunction
MAD was present in 69 (41%) patients. Prevalence of MAD was not different in patients with MFS compared to patients with LDS [47 (46%) vs. 22 (34%), P = 0.13], nor when adjusted for age [adjusted odds ratio 0.7 95% confidence interval (CI) (0.3–1.3), P = 0.22], while MVP was more prevalent in MFS patients [41 (40%) vs. 11 (17%), P = 0.002] (Table 1).
Association of mitral annulus disjunction with aortic events and mitral events
AoE occurred in 112 (67%) patients after 5.3 (IQR 1.0–12.6) years of follow-up. Of these, 20 were type A and 7 were type B aortic dissections, and 85 were prophylactic aortic surgical interventions [63 (74%) for aortic root dilatation fulfilling indication for surgery, 14 (16%) for aortic root dilatation fulfilling indication for surgery due to associated risk factors, 4 (5%) for ascending aorta dilatation with mitral valve disease as the primary surgical indication, and 4 (5%) for thoracoabdominal and descending aortic aneurysm].
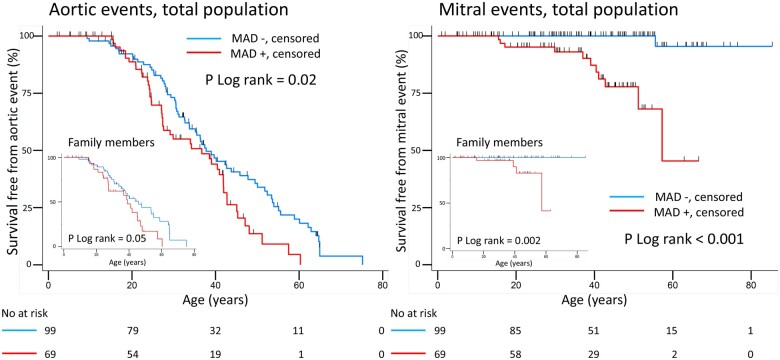
AoE (dissections and prophylactic aortic surgical interventions) occurred both in the presence and in the absence of MAD (Tables 2 and 3). However, importantly, patients with MAD were younger at the time of AoE than those without MAD (Table 3, Figure 3, Graphical abstract). Patients with AoE had greater posterolateral (Figure 4) and anterolateral MAD distance [7 (6–8) mm vs. 6 (4–7) mm, P = 0.04]. Aortic root dilatation Z-score did not differ between patients with and without MAD, neither when assessed by echocardiography [missing in 37 patients due to aortic dissection (n = 21), or prophylactic aortic surgery before index echocardiography (n = 16)], nor when assessed at prophylactic surgery on the ascending aorta (Table 3).
Table 2.
Comparison of patients with and without AoE
| No AoE (n = 56) | AoE (n = 112) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Patients with MFS, n (%) | 37 (66) | 66 (59) | 0.37 |
| Age at first examination (years) | 16 ± 17 | 29 ± 16 | <0.001 |
| Male sex, n (%) | 18 (32) | 72 (64) | <0.001 |
| Family members, n (%) | 37 (66) | 53 (47) | 0.02 |
| Echocardiographic parameters | |||
| Age at echocardiography (years) | 23 ± 16 | 38 ± 15 | <0.001 |
| MAD, n (%) | 24 (43) | 45 (40) | 0.74 |
| Posterolateral MAD distance (mm) | 7 ± 3 | 8 ± 3 | 0.10 |
| Posterolateral MAD distance indexed (mm/m2) | 5.1 ± 2.3 | 4.3 ± 1.2 | 0.07 |
| Anterolateral MAD distance (mm) | 6 ± 2 | 7 ± 3 | 0.12 |
| Anterolateral MAD distance indexed (mm/m2) | 4.5 ± 2.9 | 3.6 ± 1.5 | 0.16 |
| MVP, n (%) | 15 (27) | 37 (33) | 0.41 |
| Mitral regurgitation, n (%) | 4 (7) | 26 (23) | 0.01 |
| Diastolic mitral annulus diameter indexed (mm/m2) | 19 ± 7 | 17 ± 3 | 0.01 |
| Systolic mitral annulus diameter indexed (mm/m2) | 18 ± 7 | 16 ± 3 | 0.01 |
| Dilated mitral annulus (n = 136, adult patients only), n (%) | 5 (9) | 41 (37) | 0.006 |
Values are average ± SD or frequencies (%). P-values are calculated by Student’s t test or chi-square as appropriate. Bold values denote statistical significance.
AoE, aortic events; MAD, mitral annulus disjunction; MFS, Marfan syndrome; MVP, mitral valve prolapse.
Table 3.
Comparison between patients with and without MAD
| No MAD (n = 99) | MAD (n = 69) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Patients with MFS, n (%) | 56 (58) | 47 (67) | 0.22 |
| Age at first examination (years) | 27 ± 19 | 21 ± 14 | 0.02 |
| Male sex, n (%) | 51 (52) | 39 (57) | 0.52 |
| Family members, n (%) | 56 (56) | 34 (49) | 0.35 |
| Echocardiographic parameters | |||
| Age at echocardiography (years) | 35 ± 18 | 30 ± 15 | 0.05 |
| Aortic root dilatation Z-score (n = 131, pre-event echocardiographies) | 4.1 ± 2.9 | 4.2 ± 2.1 | 0.86 |
| MVP, n (%) | 8 (8) | 44 (64) | <0.001 |
| Mitral regurgitation, n (%) | 6 (6) | 24 (35) | <0.001 |
| Diastolic mitral annulus diameter indexed (mm/m2) | 17 ± 4 | 19 ± 5 | 0.08 |
| Systolic mitral annulus diameter indexed (mm/m2) | 16 ± 4 | 19 ± 6 | <0.001 |
| Dilated mitral annulus (n = 136, adult patients only), n (%) | 22 (22) | 24 (35) | 0.06 |
| LVEDVi (mL/m2) | 55 ± 16 | 61 ± 21 | 0.02 |
| LVESVi (ml/m2) | 21 ± 8 | 25 ± 10 | 0.02 |
| EF (%) | 61 ± 6 | 60 ± 5 | 0.20 |
| Septal S (cm/s) | 7 ± 2 | 8 ± 2 | 0.01 |
| Lateral S (cm/s) | 9 ± 2 | 11 ± 4 | <0.001 |
| E/A ratio | 1.6 ± 0.6 | 1.5 ± 0.5 | 0.56 |
| E/e′ ratio | 7.7 ± 2.6 | 6.5 ± 2.1 | 0.03 |
| Septal e′ (cm/s) | 8 ± 3 | 9 ± 2 | 0.06 |
| Lateral e′ (cm/s) | 10 ± 4 | 11 ± 3 | 0.30 |
| Tricuspid regurgitation velocity (m/s) | 2.2 ± 0.3 | 2.2 ± 0.3 | 0.53 |
| Left atrium volume indexed (mL/m2) | 28 ± 10 | 30 ± 11 | 0.18 |
| Diastolic dysfunction, n (%) | 5 (5) | 2 (3) | 0.91 |
| Outcome data | |||
| Aortic root dilatation Z-score at prophylactic aortic surgery (n = 81) | 7.0 ± 3.8 | 6.1 ± 2.3 | 0.23 |
| AoE, n (%) | 67 (68) | 45 (65) | 0.74 |
| Aortic dissection, n (%) | 19 (19) | 8 (12) | 0.19 |
| Prophylactic aortic surgery, n (%) | 48 (48) | 37 (54) | 0.51 |
| Age at AoE (years) | 37 ± 15 | 32 ± 12 | 0.04 |
| Mitral events, n (%) | 1 (1) | 11 (16) | <0.001 |
| Age at mitral event (years) | 56 | 35 ± 14 | 0.20 |
| Age at mitro-aortic event (years) | 37 ± 15 | 32 ± 11 | 0.03 |
| Arrhythmic outcome | |||
| Documented ventricular arrhythmias (n = 17), n (%) | 1 (1) | 6 (9) | 0.30 |
Values are average ± SD or frequencies (%). P-values are calculated by Student’s t test, chi-square, as appropriate. Bold values denote statistical significance.
AoE, aortic events; EF, ejection fraction; LVEDVi, left ventricular end-diastolic volume indexed for body surface area; LVESVi, left ventricular end-systolic volume indexed for body surface area; MAD, mitral annulus disjunction; MFS, Marfan syndrome; MVP, mitral valve prolapse.
Figure 3.
Kaplan–Meier analysis of estimated survival free of AoE and mitral events in patients with Marfan and Loeys–Dietz syndromes. Red curve represents patients with mitral annulus disjunction and blue curve patients without MAD. Vertical lines show multiple hash marks for multiple censoring at the same time. Left panels: patients with MAD had aortic events at younger age also in the subgroup of family members only (lower left smaller panel). AoE included acute aortic dissections and prophylactic aortic surgical interventions Right panels: mitral valve interventions were more frequent in patients with MAD, also in the subgroup of family members (lower left smaller panel). AoE, aortic events; MAD, mitral annulus disjunction.
Figure 4.
Display of longitudinal MAD distance in posterolateral wall in patients with and without AoE, MVP and mitral regurgitation (A) and scatter plot graph of correlation between longitudinal MAD distance in posterolateral wall and systolic mitral annulus dilatation (B). Box plots represent longitudinal MAD distance in posterolateral wall in patients with Marfan and Loeys–Dietz syndromes with and without AoE (left plot), with and without MVP (middle plot), and with and without mitral regurgitation (right plot) (A). Blue line shows that longitudinal MAD distance in posterolateral wall was greater with larger systolic mitral annulus diameter (B). P-values are calculated by Mann–Whitney U test (A) and Pearson correlation (B), respectively. AoE, aortic events; MAD, mitral annulus disjunction; MR, mitral regurgitation; MVP, mitral valve prolapse.
Mitral events occurred in 12 (7%) patients of which 11 (92%) had MAD (P < 0.001) (Table 3). Importantly, mitral events occurred more frequently in patients with MAD (Figure 3, Graphical abstract). Mitral valve surgery was performed before (n = 2) or after (n = 2) aortic surgery and was combined with aortic surgery in eight patients (four with mitral valve disease as primary surgical indication).
Death occurred in five patients (three cardiac deaths, age 49 ± 7 years, four with LDS, and one with MFS) and two of these had MAD.
Distribution of mitral annulus disjunction according to age
Longitudinal MAD distance, indexed by BSA, decreased with age at examination in the whole study population (posterolateral wall r = −0.27, P = 0.03; anterolateral wall, r = −0.38, P = 0.006). Separate analysis in adult patients showed no correlation between age at examination and posterolateral (MFS r = −0.18 P = 0.31; LDS r = 0.35 P = 0.13) and anterolateral MAD distance (MFS r = −0.13 P = 0.52; LDS r = 0.22 P = 0.54).
Intra- and interobserver variability for posterolateral MAD distance by ICC was 0.95 and 0.97, respectively (Supplementary data online, Table S1).
Association between mitral annulus disjunction, cardiac size and function, mitral valve disease and ventricular arrhythmias
MAD was associated with larger LV dimensions, greater systolic mitral annulus diameter, and mitral regurgitation (Table 3), but differences in LV dimensions were no longer evident after adjustment for mitral regurgitation [B 1.30, 95% CI (−4.42 to 7.03), P = 0.65]. MAD was associated with higher lateral and septal peak systolic mitral annular velocity (Table 3), and only with higher lateral S velocity also when adjusted for age and mitral regurgitation [B 2.32, 95% CI (0.93–3.71), P = 0.001].
Diastolic parameters did not differ between patients with MFS and LDS (data not shown). MAD patients had lower E/e′ ratio than those without (Table 3). However, there was no association between MAD and E/e′ ratio when adjusted for age and mitral regurgitation [B −1.07 95% CI (−2.20 to 0.05), P = 0.06].
Patients with MVP and those with mitral regurgitation had greater posterolateral (Figure 4) and anterolateral MAD distance [7 (6–8) mm vs. 5 (4–6) mm, P = 0.02 and 8 (7–10) mm vs. 6 (4–6) mm, P < 0.001] and systolic mitral annulus diameter correlated with posterolateral (Figure 4) and anterolateral MAD distance (r = 0.57, P < 0.001).
Only 17 patients had an implantable cardiac device (n = 5) or underwent a symptoms-triggered 24 h ECG monitoring during follow-up (n = 12). VAs were documented in seven patients of which 6 (86%) had MAD (P = 0.30) (Table 3).
Discussion
This is the first study reporting the prevalence of MAD in patients with MFS and LDS. MAD was present in more than 40% of patients and was associated with MVP, suggesting MAD as a frequent part of the mitral apparatus pathology in these patients. AoE were frequent and, importantly, occurred at younger age in patients with MAD indicating MAD as part of a more severe disease phenotype.
High prevalence of mitral annulus disjunction in Marfan and Loeys–Dietz syndromes
We found a prevalence of MAD as high as 41% among patients with MFS and LDS. The spectrum of mitral valve involvement encountered in patients with MFS and LDS ranges from discrete mitral valve billowing with no or trivial mitral regurgitation to severe myxomatous degeneration of mitral valve with prolapse and significant mitral regurgitation. Importantly, the presence of MAD has never been described before in this group of patients.
It is well known that patients with MFS and LDS have high prevalence of MVP supporting the theory that deficiency of connective tissue of the valvular leaflets might predispose to MVP and this was supported by our study showing a 31% prevalence of MVP. Previous studies from Hutchins et al.5 have reported a high prevalence of MAD in patients with prolapsed mitral valves and our study showed, for the first time, the finding of very frequent MAD in patients with MFS and LDS. We speculate that deficiency of connective tissue might not only predispose to prolapsed mitral valve but also to an abnormal fibrous annulus including MAD, and which is associated with a more severe phenotype including deficiency of aortic tissue with AoE at younger age.
Earlier aortic events in patients with mitral annulus disjunction
Progressive aortic root dilatation is highly prevalent in patients with MFS and LDS20 and subsequent ascending aortic dissection is the most feared cardiovascular complication. Importantly, we found that AoE occurred at younger age in the presence of MAD. Previous studies have shown that MFS patients with MVP have larger aortic diameters,3 indicating a relation between aortic dimensions and mitral valve disease. Our findings extended this information showing that MAD and MVP might be part of a more severe disease phenotype with younger age at first examination and with earlier AoE. Furthermore, longitudinal MAD distance was greater in patients with AoE, and further studies should explore if quantification of MAD can be useful in grading disease severity.
Distribution of mitral annulus disjunction according to age
We found MAD in all age groups of patients and no evidence for progression of longitudinal MAD distance with age. Previous studies have reported extra-aortic cardiac features like MVP and mitral regurgitation in early infancy and childhood in MFS.21 In autopsied hearts, isolated MAD has been observed in specimens of younger patients, while MAD along with MVP was seen in older hearts suggesting that MAD might be a variant of the atrioventricular junction that could predispose to valvular tissue degeneration by prolonged abnormal stretching of the myocardium during systole5 and indicating an age-related process. Our study did not include longitudinal echocardiographic data and studies from infancy to adulthood are required to explore if MAD is congenital and stationary or may progress over time.
Mitral annulus disjunction, mitral valve involvement, and mitral surgery
Although mitral valve surgery is less common than aortic disease in these patients, mitral valve disease remains an important cause of morbidity in patients with MFS and LDS.3 Interestingly, MAD was diagnosed in almost all patients requiring mitral valve surgery suggesting a high need of surgical mitral intervention in MFS and LDS patients with MAD.
The presence of MAD was associated with MVP and mitral regurgitation as well as with larger mitral annulus diameters. These findings indicate MAD as an important part of the mitral valve involvement in patients with MFS and LDS. Furthermore, greater longitudinal MAD distance was associated with MVP, mitral regurgitation and larger mitral annulus, supporting the role of a more extended MAD in the degeneration of the mitral valve as suggested before.5,7
MAD was associated with higher peak systolic myocardial velocities in the lateral wall in line with previous reports,22 supporting the typical curling movement of the lateral wall as a hallmark of MAD. Patients with MAD had lower E/e′ ratio, but not when adjusted for age and mitral regurgitation, indicating that MAD itself had no obvious impact on diastolic function in our young population.
Mitral annulus disjunction and arrhythmic outcome
Previous studies have recognized significant VPC and arrhythmic deaths in patients with MFS23 which were supposed to be induced by LV dilatation and volume overload.13,23
Our study was retrospective and not designed to evaluate VA and only 10% had heart rhythm monitoring. Of these, however, more than 40% had documented VA and 86% of patients with arrhythmias had MAD. Future studies should perform heart rhythm monitoring in MFS and LDS patients and specifically investigate presence of MAD.
Clinical implications
MAD was highly prevalent in patients with MFS and LDS. The presence of MAD was associated with AoE at younger age. Additionally, MAD was present in almost all patients requiring mitral valve surgery.
We propose that screening for MAD could be of interest in patients with MFS and LDS since MAD was associated with a more severe phenotype requiring surgical procedures at younger age.
The high prevalence of MAD in patients with MFS or LDS also suggests that the finding of MAD in a patient with aortic dilation may lead the clinician to suspect MFS or LDS in patients yet undiagnosed with connective tissue disease.
Limitations
We performed a retrospective cohort study with the inherent limitations to this design. Patients were recruited at the cardiothoracic surgery department of a single tertiary centre, resulting in a referral bias leading to overestimation of incidence of events compared to a community-derived population.
The number of observations and in particular heart rhythm monitoring was relatively low, which may have had an impact on the robustness of the statistical analyses.
Lack of recommendations in the definition of MVP in presence of MAD and exclusion of milder forms of mitral valve involvement like billowing may have resulted in differences in MVP prevalence compared to other studies.
Conclusion
MAD was highly prevalent in patients with MFS and LDS. Importantly, MAD was part of a severe disease phenotype with AoE at younger age and need for mitral valve surgery. Screening patients with MFS and LDS for MAD may provide prognostic information, lead to closer monitoring and may also be relevant in planning surgical intervention.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
This study was performed at Department of Cardiology and Department of Thoracic Surgery, Oslo University Hospital, Norway and was supported by the South-Eastern Norway Regional Health Authority (18/00183-124). M.C. was supported by Fredriksens fond.
Conflict of interest: The authors do not have any conflicts of interest.
Supplementary Material
References
- 1.Kuijpers JM, Mulder BJ.. Aortopathies in adult congenital heart disease and genetic aortopathy syndromes: management strategies and indications for surgery. Heart 2017;103:952–66. [DOI] [PubMed] [Google Scholar]
- 2.Meester JAN, Verstraeten A, Schepers D, Alaerts M, Van Laer L, Loeys BL.. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann Cardiothorac Surg 2017;6:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taub CC, Stoler JM, Perez-Sanz T, Chu J, Isselbacher EM, Picard MH. et al. Mitral valve prolapse in Marfan syndrome: an old topic revisited. Echocardiography 2009;26:357–64. [DOI] [PubMed] [Google Scholar]
- 4.MacCarrick G, Black JH, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL. et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med 2014;16:576–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins GM, Moore GW, Skoog DK.. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–40. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb AE, David TE, Lad VS, Bobiarski J, Armstrong S, Maganti M.. Mitral valve repair for advanced myxomatous degeneration with posterior displacement of the mitral annulus. J Thorac Cardiovasc Surg 2008;136:1503–9. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson MJ, Bitkover CY, Omran AS, David TE, Ivanov J, Ali MJ. et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr 2005;18:1014–22. [DOI] [PubMed] [Google Scholar]
- 8.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A. et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. [DOI] [PubMed] [Google Scholar]
- 9.Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F. et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–9. [DOI] [PubMed] [Google Scholar]
- 10.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB. et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476–85. [DOI] [PubMed] [Google Scholar]
- 11.Milewicz DM, Dietz HC, Miller DC.. Treatment of aortic disease in patients with Marfan syndrome. Circulation 2005;111:e150–7. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H. et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926. [DOI] [PubMed] [Google Scholar]
- 13.Mah DY, Sleeper LA, Crosson JE, Czosek RJ, Love BA, McCrindle BW. et al. Frequency of ventricular arrhythmias and other rhythm abnormalities in children and young adults with the Marfan syndrome. Am J Cardiol 2018;122:1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devereux RB, de Simone G, Arnett DK, Best LG, Boerwinkle E, Howard BV. et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol 2012;110:1189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colan SD. Normal echocardiographic values for cardiovascular structures. In: Wyman W, Lai LLM, Meryl Cohen S, Geva Tal eds. Echocardiography in Pediatric and Congenital Heart Disease, From Fetus to Adult. 2nd Edition, Wiley-Blackwell: NJ; USA, 2016. p883–901. [Google Scholar]
- 16.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA. et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 17.Caldarera I, Van Herwerden LA, Taams MA, Bos E, Roelandt JR.. Multiplane transoesophageal echocardiography and morphology of regurgitant mitral valves in surgical repair. Eur Heart J 1995;16:999–1006. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 20.Attias D, Stheneur C, Roy C, Collod-Béroud G, Detaint D, Faivre L. et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation 2009;120:2541–9. [DOI] [PubMed] [Google Scholar]
- 21.Faivre L, Masurel-Paulet A, Collod-Beroud G, Callewaert BL, Child AH, Stheneur C. et al. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics 2009;123:391–8. [DOI] [PubMed] [Google Scholar]
- 22.Muthukumar L, Rahman F, Jan MF, Shaikh A, Kalvin L, Dhala A. et al. The Pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging 2017;10:1078–80. [DOI] [PubMed] [Google Scholar]
- 23.Yetman AT, Bornemeier RA, McCrindle BW.. Long-term outcome in patients with Marfan syndrome: is aortic dissection the only cause of sudden death? J Am Coll Cardiol 2003;41:329–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.