Abstract
Background
Human immune deficiency virus (HIV) remains one of the leading causes of infectious disease mortality and morbidity in Sub-Saharan Africa. Although remarkable progress has been made in prevention and treatment of HIV, there is a higher rate of loss to follow-up in HIV-infected children than in adults, once they enter care.
Objective
To determine the incidence and identify predictors of loss to follow-up among HIV-infected children on anti-retroviral treatment in Sidama Zone, Ethiopia.
Methods
A retrospective cohort study was done among children that were enrolled in ART care in Sidama Zone from September 2014 to August 2018. A total of 143 eligible children were included in this study. A structured checklist was used to extract data from patients’ medical records such as patient intake forms, electronic database, and registers. Data were entered, cleaned, coded, and analyzed by STATA version 12. Cox proportional hazards models were fitted to investigate predictors of loss to follow-up.
Results
Of the 143 participants, 76 (53.15%) were female children with a median age of 7 years and interquartile range of 4–9. The incidence rate was 5 per 100 person-years and the cumulative incidence 12.59%. The median follow-up time was 2.46 years and the total time at risk was 356.06 person-years. Furthermore, 55.56% and 72.22% of those lost to follow-up were within the first and the second years of follow-up, respectively. In multivariable Cox proportional model, only the TB status of the children was significantly associated with loss to follow-up with hazard ratio 3.348 [1.174831, 9.543494] and p-value of 0.024.
Conclusion
In this study, TB status of children was the significant determinant of loss to follow-up. However, the overall retention was 87.4% and a substantially higher proportion of loss was observed within the first and second years of follow-up.
Keywords: lost to follow-up, attrition, HIV-infected children, ART, Sidama zone
Introduction
The Human Immune Deficiency Virus (HIV) remains one of the leading causes of infectious disease morbidity in the world. While investments in HIV response have achieved promising results, by the end of 2020, 37.6 million people were living with HIV, of which 1.6 million were newly infected.1 Additionally, 1.7 million children were living with HIV around the world in 2020.2 The majority of these cases are in Africa, where acquired immune deficiency syndrome (AIDS) remains the leading cause of death among adolescents.1
The Joint United Nations Program on HIV and AIDS (UNAIDS) 90-90-90 target called on countries to reach the following goals, 90% of people living with HIV diagnosed, 90% of diagnosed people on antiretroviral treatment, 90% of people on treatment with a fully suppressed viral load by 2020.2,32 With the ambition of ending the HIV epidemic, this fast-track approach sets these targets at 95-95-95 by the year 2030.2 However, as of 2019, only 67% of the people that knew their HIV status (81%) were on anti-retroviral treatment (ART).2 These outcomes were even lower in HIV-infected children in all three categories. In 2019, less than fifty percent of HIV-infected children that were younger than 15 years were receiving ART.2,3 The UNAIDS targets cannot be met if children are not retained in care. Thus, it is crucial to understand the determinants and risks of ”lost to follow-up” (LTFU) in ART-receiving children. Preventative measures should also be taken accordingly since treatment interruption results in significant increase of viral load as well as ART drug resistance.2–4,32
LTFU is particularly a problem for developing countries. Previous studies have shown that, even though both children and adults on ART are LTFU, it is more common in children.1–7,9 In Asia and Africa it has been shown that at 18 months after initiation of ART, 12.3% are LTFU.26 Seventy percent of people living with HIV reside in Sub-Saharan Africa where HIV remains the leading cause of death among adults, women of child-bearing age, and children.2,4,9,13 Ethiopia is a country with a low generalized HIV epidemic with significant heterogeneity among regions and population groups. In Ethiopia there is a declining national HIV prevalence of 1.1% and 0.33/1000 incidence.2,9,18 Moreover, the urban population is more affected than rural areas while females are twice as affected as the male population.2,18 ART care and treatment have been expanded at regional, zonal, woreda, and kebele levels through targeted social mobilization and active community participation to prevent and control the spread of the epidemic. Despite this remarkable progress, several adults and children discontinue ART at different time points.
In Ethiopia, HIV-infected children stop coming back for ART for different reasons. Studies conducted in Adama and Northern Ethiopia demonstrated that rates of LTFU in ART-receiving children were 18% and 9.81%, respectively.10,15,30 According to these studies, CD4 count, or level of disease advancement was a factor that can contribute to LTFU of children on ART care.15
To achieve the 95-95-95 targets for children, HIV-infected children must be identified, linked to care, initiated on ART, retained in care, and finally achieve sustained virologic suppression.14,32 High levels of adherence to ART and retention in treatment programs are required for successful virologic suppression and treatment outcomes.32 Accordingly, our study explored the possible predictors of LTFU in HIV-infected children in Sidama zone, which will be important in order to apply a retention mechanism and optimal outcomes among HIV-infected children.
Conceptual Framework
This conceptual framework was adapted from previous similar literature.8–12,15,16,17,20,29 Thus, the relationship between dependent and independent variables can be conceptualized as shown in Figure 1.
Figure 1.
A conceptual framework for determinants of LTFU among HIV-infected children in ART care in Sidama Zone from September 2014 to August 2018.
Objective
To determine the incidence and predictors of LTFU among HIV-infected children that were receiving ART care in Sidama Zone from September 2014 to August 2018.
Methods and Materials
Study Site and Period
This study was conducted in Sidama Zone Government Hospital from September 2014 to August 2018. Sidama Zone is located in Southern Ethiopia. HIV prevalence for the region is 0.9% while the national prevalence is 1.5%.2,18 Sidama Zone is the largest zone in the region covering 6972.1 square kilometers with a total population of 3,019,442. The zone has 21 woredas (districts). The capital of the zone is Hawassa city, and it is located 275 kilometers to the south of Addis Ababa (the capital city of the Ethiopia). In the zone, there are four governmental hospitals and 107 public health centers.19
Study Design
Retrospective cohort study design was used by reviewing children’s intake, follow-up, and routine data forms from both paper-based and electronic database system. CD4 count ≤ 350 (cells/mm3) was considered as exposed group and CD4 count >350 (cells/mm3) non-exposed group based on a similar study conducted in Adama.15
Study Population
The study population was HIV-infected children (age <15 years) registered for antiretroviral therapy from September 2014 to August 2018.
Eligibility Criteria
Children that registered for ART care between September 2014 to August 2018 were eligible for this study. However, those who did not have at least one follow-up visit after the initiation of ART or those who had incomplete baseline data due to transferring to treatment centers or lost records were not included.
Study Variables
In this study, the dependent variable was time to lost to follow-up (LTFU) and LTFU is defined as not seen since ≥ 1 month based on the national ART treatment guideline and WHO patient monitoring guideline for HIV care and ART.20,21
The explanatory variables were socio-demographic variables including the children’s age, sex, family size as well as caregivers’ marital status, religion, and employment status. Moreover, clinical variables such as the children’s baseline TB status, CD4 count, and clinical stage were considered as explanatory variables.
Sample Size Calculation
143 children (age <15) that were enrolled for ART treatment in Sidama Zone from September 2014 to August 2018 were included in this study.
Sampling Procedure
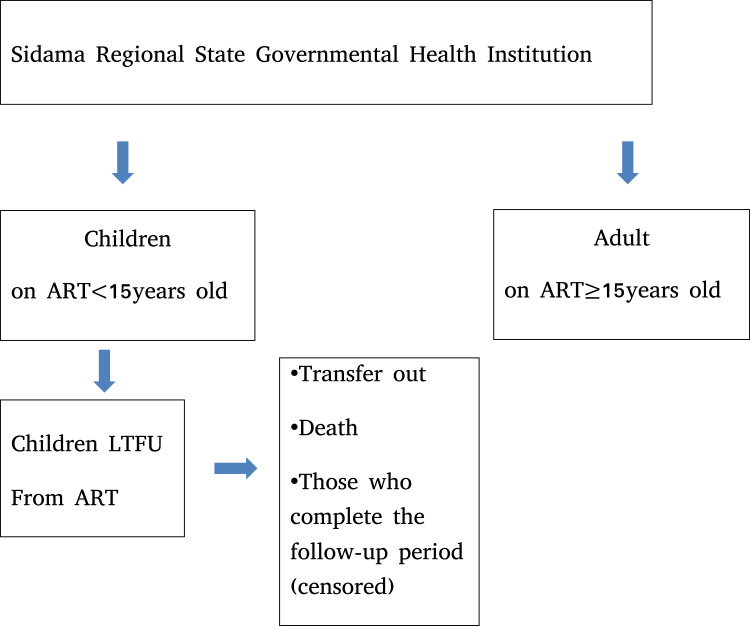
In this study the study population and the sample size were equal (Figure 2).
Figure 2.
Study participant selection procedure for determinants of LTFU in children followed-up on ART care in Sidama regional state from September 2014 to August 30 2018.
Data Collection Tools and Techniques
Structured data extraction checklist was used to capture relevant variables for the study. The tool was developed by consulting the standardized HIV/AIDS care intake forms, individual patient records, and other relevant literature.8–11,20,21 Seven nurses working at ART clinics were recruited for data collection. The principal investigators provided two-day training for data collectors on the objective of the study and the data collection tool. The data collection was closely supervised, and accuracy of the data was cross-checked and managed accordingly daily by two supervisors and the principal investigators.
Data Quality Control
The collection of data was closely supervised by principal investigators. The data quality was maintained through selection and training of data collectors. Both the electronic database and patient intake forms were used as a source of data and inconsistencies were checked and only the appropriate patient intake form was considered for this study.
Data Management
The data were entered, cleaned, and coded by STATA version 12 software package and it was used for all statistical analysis. Event was LTFU and it was coded as 1 and censorship was considered for death, transfer out and follow-up period completion and was coded as 0. Time to event was calculated by subtracting the date of ART started (to) from the date the event occurred (te) and some of the continuous variables like age and CD4 count were categorized depending on need for interpretation.
Data Analysis
Descriptive statistics such as frequency and percentages were calculated for categorical variables and survival analysis was carried out to calculate the incidence of LTFU. Kaplan-Meier retention estimates and the Log rank test were used to estimate retention across the levels of categorical variables. However, univariate Cox-proportional hazard regression was fitted for the continuous variables. Based on Hosmer & Lemeshow recommendation, significant variables (at p1≤0.25) during univariate analysis were identified and selected for multivariable regression, then, by using Collett model selection method, four step process was used.22 Backward selection to eliminate non-significant variables at the level of p2=0.10 was used as the first (1) step, then, those variables that were non-significant from step (1) were considered using forward selection, with significance level p3=0.10. Finally, any that were significant at p3 would be added, then stepwise regression with significance level p4=0.05 was used. Multivariable Cox proportional hazards model was fitted to investigate predictors of LTFU. Proportionality assumptions were checked by using -log-log plot graphically and fitting the model with time-varying covariates and global tests (using Schoenfeld residuals) were used to test for validity of the proportional hazard assumption. Then, multi-collinearity test was done by using variance inflation factor (VIF) and tolerance. The interactions between predictor variables were also checked. The goodness of fit for the model was checked by using Cox-Snell residuals and both the Akaike and Bayesian information criteria (AIC/BIC) were used to assess model parsimony. For detecting the outlier and influential case, deviance residual and delta-beta estimates were used. The statistical significance was tested using Wald statistics, with results p<0.05 considered statistically significant. The strength of association was measured and presented as hazard ratio (HR) with 95% confidence intervals (95% CIs).
Results
Table 1 shows the baseline socio-demographic characteristics of the HIV-infected children and their caregivers included in this study. 76 (53.15%) of the participants were female children with a median age of 7 years and interquartile range of 4−9. The median number of family members where the children resided was 4, as 96.5% of the children were living in a family size of 4. 77% of the children’s caregivers were married, while 15% were widowed. Additionally, the majority of the caregivers were protestants (86.7%) (Table 1).
Table 1.
Baseline Socio-Demographic Characteristics of Children That Were Enrolled in ART Care in Sidama Zone from September 2014 to August 2018
| Characteristics | Number (%) | |
|---|---|---|
| Sex | Male | 67(46.85) |
| Female | 76(53.15) | |
| Age | ≤7 | 95(66.43) |
| >7 | 48(33.57) | |
| Number of family members where the child lives | Family size <4 | 5(3.5) |
| Family size > 4 | 138(96.5) | |
| Caregivers’ related variables | ||
| Employment Status | Employed | 30(20.98) |
| Not employed | 113(79.02) | |
| Marital Status | Never married | 5(3.50) |
| Married | 111(77.62) | |
| Divorced/separated | 5(3.50) | |
| Widowed | 22(15.38) | |
| Religion | Orthodox | 16(11.19) |
| Muslim | 4(2.8) | |
| Protestant | 123(86.01) | |
Baseline Clinical Characteristics of Children
Based on the WHO clinical staging system for HIV/AIDS, 56.64% of the children were stage I while 27.97% of them were stage II (Table 2). At baseline, the median CD4 count was 701 cells/ mm3. Stage III and IV are considered as the advanced stages of the disease, and they accounted for 15.39% of our participants (Table 2).
Table 2.
Baseline Clinical Characteristics of Children That Were Enrolled in ART Care in Sidama Zone from September 2014 to August 2018
| Characteristics | Number (%) | |
|---|---|---|
| WHO clinical stage | I | 81(56.64) |
| II | 40(27.97) | |
| III | 20(13.99) | |
| IV | 2(1.40) | |
| CD4 count (cells/mm3) | ≤701 | 124(86.71) |
| >701 | 19(13.29) | |
| TB Status | TB positive | 19(13.29) |
| TB negative | 124(86.71) | |
| Functional Status | Walking | 96(67.13) |
| Ambulating | 43(30.07) | |
| Bedridden | 4(2.80) | |
Incidence of Lost to Follow-Up
The incidence rate was 5 per 100 person-years and the cumulative incidence rate was 12.59%. The median follow-up time was 2.46 years while the total time at risk was 356.06 person-years. Furthermore, 55.56% and 72.22% of those lost were within the first and the second years, respectively, of follow-up (Table 3).
Table 3.
Incidence of Lost to Follow-Up Time Interval for Children That Were Enrolled in ART Care in Sidama Zone from September 2014 to August 2018
| Interval | Total | Lost to Follow-Up | Censored | Retention Probability | Error | [95% Conf. Int.] |
|---|---|---|---|---|---|---|
| 0–1 | 143 | 10 | 31 | 0.9216 | 0.0238 | [0.8591 0.9570] |
| 1–2 | 102 | 3 | 17 | 0.8920 | 0.0285 | [0.8206 0.9361] |
| 2—3 | 82 | 2 | 19 | 0.8674 | 0.0326 | [0.7876 0.9187] |
| 3–4 | 61 | 1 | 16 | 0.8510 | 0.0359 | [0.7640 0.9079] |
| 4–5 | 44 | 1 | 37 | 0.8177 | 0.0475 | [0.7015 0.8920] |
| 5 | 6 | 1 | 5 | 0.5840 | 0.2003 | [0.1530 0.8572] |
Comparison Groups
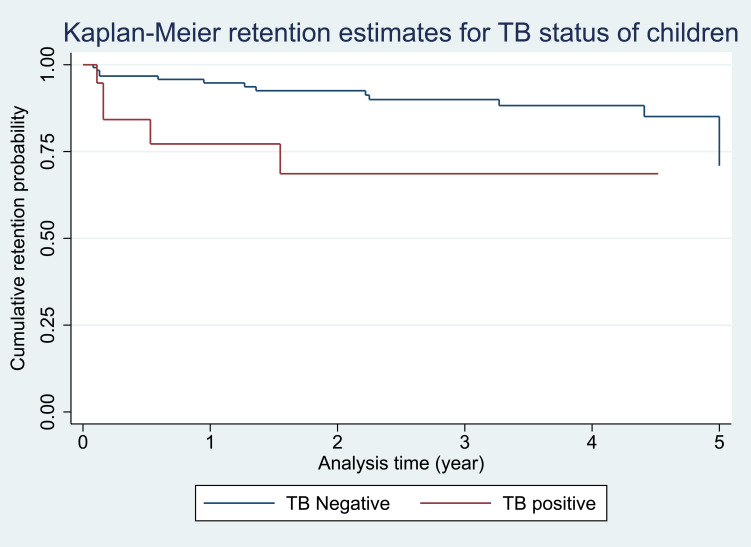
Test of equality across strata to explore whether or not to include the predictor in the model was checked by Log rank test (Chi-squared test). If the predictor test had a p ≤ 0.25 it was considered for model building (Table 4). The Kaplan-Meier retention estimate graphically showed the retention difference between groups and if the difference was significant, the graphs were separated as this indicated an insight to consider the variable in the model and if there was no difference between groups the graphs were close to each other and crossing (Figure 3).
Table 4.
Log Rank Test for Equality of Lost to Follow-Up Between Different Groups of Children Followed-Up in Sidama Zone from September 2014 to August 2018
| Variable | Log Rank Test (Chi2Test) | Degrees of Freedom (DF) | p-value |
|---|---|---|---|
| Age Dummy | 0.90 | 1 | 0.3431 |
| Sex | 1.97 | 1 | 0.1601 |
| WHO clinical stage | 1.18 | 3 | 0.7589 |
| CD4 count Dummy | 0.82 | 1 | 0.3665 |
| Functional status | 9.51 | 2 | 0.0086 |
| TB status | 1.97 | 1 | 0.1602 |
| Marital status | 3.45 | 3 | 0.3267 |
Figure 3.
Kaplan-Meier retention estimates for the variable TB status of children followed-up in ART care in Sidama Zone from September 2014 to August 2018.
Predictors of Lost to Follow-Up
In the univariate analysis, for variables with fewer cases we merged them based on their relationships and we rerun the data with these merged variables. For instance, according to the WHO clinical staging system for HIV/AIDS, stage I and stage II can be considered as early stages of the disease (121 cases after merging) and WHO clinical stage III and stage IV can be considered as advanced stages of the disease (22 cases after merging). However, even after merging, the clinical stages were not candidate variables for multiple Cox regression model.
TB status, sex, and functional status of the HIV-infected children were candidate variables for multivariable Cox proportional hazard model. However, in the multivariable Cox proportional model, we found that only TB status of the child was significantly associated with lost to follow-up (Table 5) with hazard ratio of 3.348 [1.174831, 9.543494] and p-value of 0.024.
Table 5.
Univarate and Multivariable Analysis of Predictors of LTFU for Children Followed-Up in Sidama Zone from September 2014 to August 2018
| Characteristics | Multivariable HR and 95% CI | p-value | |
|---|---|---|---|
| TB status | TB Postive | 3.348 [1.174831 9.543494] | 0.024 |
| TB Negative | |||
| Sex | Male | [0.1865302 1.350691] | 0.172 |
| Female | |||
| Functional status | Walking | 0.391 [0.1191284 1.285411] | 0.172 |
| Ambulating | |||
| Bedridden | |||
Discussion
Several studies have demonstrated that LTFU poses a major threat to the success of ART programs throughout the world. A discontinuation of ART care, in children and adults, is associated with a rapid increase in viral load, in development and transmission of the drug resistant virus variants as well as compromised immune function that puts the patients at risk of other opportunistic infections.3,7,23,24,32 Thus, understanding the predictors associated with LTFU is crucial for the development of targeted interventions to improve patient retention.27,28 Accordingly, our study sought to determine the incidence rate and predictors of LTFU among HIV-infected children in Sidama zone, Ethiopia. In this study, the incidence rate was estimated to be 5 per 100 person-years and the cumulative incidence, 12.59%. The median follow-up time was 2.46 years and the total time at risk was 356.06 person-years.
The attrition rate we calculated is higher than that of similar studies conducted in Gondar (6.2%), Addis Ababa (8.3%), Asia (4.1%), and South Africa (9.0%).4,26,30,31 This is in contrast to reports from Adama (18%), East Africa (14%), and West Africa (21.8%).15,25,26 Regardless of the lower cumulative incidence rate compared to these previous studies, our study shows that a significant number of children were still LTFU from care.
Other studies have also indicated that LTFU is associated with TB/HIV co-infection in both adults and children. Consistently, our findings showed that TB/HIV co-infected children were more likely to be lost compared to those who had no TB/HIV co-infection with hazard ratio of 3.348 [1.174831 9.543494] and p-value of 0.024.30,31,33,34 In addition, we found that 55.56% and 72.22% were lost within the first and the second years of follow-up, respectively. Correspondingly, other studies in different parts of the world have shown that the highest proportion of LTFU is, indeed, observed within the first (14%) and second (28%) years of ART care.15,25,25
Our study explored the characteristics of HIV-infected children and their care givers in an attempt to identify predictors of LTFU in the Sidama zone. However, we also had several limitations as the method we used was a retrospective cohort study. As a result, some important variables such as the children’s nutritional status were not included. Since the patient intake form did not specify whether the children had edema or if their height was measured in standing or recumbent position, we were not able to assess the children’s nutritional status in relation to LTFU.
Conclusion
In this study, we found that TB status of the HIV-infected children was a significant determinant of LTFU in the Sidama zone. Also, higher proportions of loss were observed within the first and second years of follow-up. Our finding is further supported by similar studies conducted in other parts of Ethiopia such as Adama, Tigray, and Benishangulgumz, which showed TB status of HIV-infected children and adults is a significant predictor of LTFU.15,30,31,33,34 Thus, our study calls for a new policy supporting different retention mechanism for children and adults in ART with and without TB infection, especially during the first and second years of ART care follow-up.
Acknowledgments
We would like to thank the Hawassa College of Health Science for funding this research and we also appreciate the Sidama-Zone Health office and other ART clinics for their cooperation.
Funding Statement
Hawassa College of Health Science provided the fund to this research.
Abbreviations
AIDS, Acquired Immune Deficiency Virus; ART, Anti-Retroviral Treatment; ARV, Antiretroviral; BIC, Bayesian Information Criteria; CD4, Cluster of differentiation 4; CI, confidence interval; CPT, Cotrimoxazole Prophylaxis; EDHS, Ethiopian Demographic Health Survey; GOE, The Government of Ethiopia; Hgb, Hemoglobin; HIV, Human Immune Deficiency Virus; HR, hazard ratio; INH, Isoniazid; IQR, interquartile range; LTFU, Lost To Follow-up; NGO, Non-Governmental Organization; NTD, Neglected Tropical Disease; PCP, Pneumocystis Carinii Pneumonia; PLHIV, People Living with Human Immune Deficiency Virus; PMTCT, Prevention of Mother to Child Transmission of HIV; SD, standard deviation; SDG, Sustainable Development Goal; TB, Tuberculosis; UNAIDS, Joint United Nations program on HIV and AIDS and WHO, World Health Organization.
Data Sharing Statement
The datasets used are available from the corresponding author and thus, it will be provided based on reasonable request.
Ethical Consideration of the Study
Ethical approval was obtained from the Institutional Review Board of Hawassa college of Health Science with ref number H/H/S/C/05/15/272/1 and also a support letter sought from Sidama Zone office to all ART treatment centers with ref number H/1/1514/1. Confidentiality of patients’ data was ensured by using non-personal identifiers such as patients’ medical registration number and the unique ART number to distinguish study subjects during data collection. Written consent was not feasible for records’ review in our setting. However, this study was done in accordance with the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.World Health Organization. UNAIDS/WHO 2021 preliminary epidemiological estimates: world health organization. 2021. [Google Scholar]
- 2.UNAIDS; 2020. UNAIDS data 2020. Available from: https://www.unaids.org/en/resources/documents/2020/unaids-data.
- 3.Onubogu CU, Ugochukwu EF. A 17 year experience of attrition from care among HIV infected children in Nnewi South-East Nigeria. BMC Infect Dis. 2021;21(1):021–06099. doi: 10.1186/s12879-021-06099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanoni BC, Phungula T, Zanoni HM, France H, Feeney ME. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6(7):e22706. doi: 10.1371/journal.pone.0022706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zurcher K, Mooser A, Anderegg N, et al. Outcomes of HIV-positive Patients Lost to Follow-up in African Treatment Programs. Trop Med Int Health. 2017;19(10):12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies MA, May M, Bolton-Moore C, et al. Prognosis of children with HIV-1 infection starting antiretroviral therapy in Southern Africa: a collaborative analysis of treatment programs. Pediatr Infect Dis J. 2014;33(6):608–616. doi: 10.1097/INF.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;1:63–69. doi: 10.1111/j.1365-3156.2010.02507.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones M, Stander M, van Zyl M, Cameron D. Recall of lost-to-follow-up pre-antiretroviral therapy patients in the Eastern Cape: effect of mentoring on patient care. S Afr Med J. 2012;102(9):768–769. doi: 10.7196/SAMJ.5957 [DOI] [PubMed] [Google Scholar]
- 9.Kellerman SE, Ahmed S, Feeley-Summerl T, et al. Beyond prevention of mother-to-child transmission: keeping HIV-exposed and HIV-positive children healthy and alive. Aids. 2013;27(2):107. doi: 10.1097/QAD.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucciardini R, Fragola V, Abegaz T, et al. Retention in Care of Adult HIV Patients Initiating Antiretroviral Therapy in Tigray, Ethiopia: a Prospective Observational Cohort Study. PLoS One. 2015;10(9):e0136117. doi: 10.1371/journal.pone.0136117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelmson S, Reepalu A, Balcha TT, Jarso G, Bjorkman P. Retention in care among HIV-positive patients initiating second-line antiretroviral therapy: a retrospective study from an Ethiopian public hospital clinic. Glob Health Action. 2016;9:29943. doi: 10.3402/gha.v9.29943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernays S, Jarrett P, Kranzer K, Ferrand RA. Children growing up with HIV infection: the responsibility of success. Lancet. 2014;383(9925):1355–1357. doi: 10.1016/S0140-6736(13)62328-4 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Accelerating progress on HIV, tuberculosis, malaria, hepatitis and neglected tropical diseases.A new agenda for 2016–2030: World Health Organization. 2015. [Google Scholar]
- 14.Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the s-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):320. doi: 10.1186/s12879-018-3214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagstromer O, Lundstedt L, Balcha TT, Bjorkman P. Decentralised paediatric HIV care in Ethiopia: a comparison between outcomes of patients managed in health centres and in a hospital clinic. Glob Health Action. 2013;6(22274):22274. doi: 10.3402/gha.v6i0.22274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abuogi LL, Smith C, McFarland EJ. Retention of HIV-Infected Children in the First 12 Months of Anti-Retroviral Therapy and Predictors of Attrition in Resource Limited Settings: a Systematic Review. PLoS One. 2016;11(6):e0156506. doi: 10.1371/journal.pone.0156506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coker M, Etiebet MA, Chang H, et al. Socio-Demographic and Adherence Factors Associated with Viral Load Suppression in HIV-Infected Adults Initiating Therapy in Northern Nigeria: a Randomized Controlled Trial of a Peer Support Intervention. Curr HIV Res. 2015;13(4):279–285. doi: 10.2174/1570162X13666150407143838 [DOI] [PubMed] [Google Scholar]
- 18.Ethiopia Demographic and Health Survey E. 2016.
- 19.Dulla D, Daka D, Wakgari N. Knowledge about cervical cancer screening and its practice among female health care workers in southern Ethiopia: a cross-sectional study. Int J Womens Health. 2017;9:365–372. doi: 10.2147/IJWH.S132202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Patient Monitoring Guidelines for Hiv Care and Antiretroviral Therapy (ART). World Health Organization; 2006. 32. [Google Scholar]
- 21.Ababa A. Federal Democratic Republic of Ethiopia Ministry of Health F. HIV Care/ART Follow-Up Form. Ethiopia: Postnatal Care; 2003. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Survival Analysis. Wiley; 1999. [Google Scholar]
- 23.Billong SC, Fokam J, Penda CI, et al. Predictors of poor retention on antiretroviral therapy as a major HIV drug resistance early warning indicator in Cameroon: results from a nationwide systematic random sampling. BMC Infect Dis. 2016;16(1):678. doi: 10.1186/s12879-016-1991-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brits H, Joubert G. Outcomes of children with advanced HIV initiated on antiretroviral therapy in a South African hospice. Int J Palliat Nurs. 2015;21(6):281–286. doi: 10.12968/ijpn.2015.21.6.281 [DOI] [PubMed] [Google Scholar]
- 25.Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health. 2011;11(519):1471–2458. doi: 10.1186/1471-2458-11-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr. 2013;62(2):208–219. doi: 10.1097/QAI.0b013e31827b70bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardura-Garcia C, Feldacker C, Tweya H, et al. Implementation and Operational Research: early Tracing of Children Lost to Follow-Up From Antiretroviral Treatment: true Outcomes and Future Risks. J Acquir Immune Defic Syndr. 2015;70(5):772. doi: 10.1097/QAI.0000000000000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auld AF, Alfredo C, Macassa E, et al. Temporal Trends in Patient Characteristics and Outcomes Among Children Enrolled in Mozambique’s National Antiretroviral Therapy Program. Pediatr Infect Dis J. 2015;34(8):741. doi: 10.1097/INF.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berheto TM, Haile DB, Mohammed S. Predictors of Loss to follow-up in Patients Living with HIV/AIDS after Initiation of Antiretroviral Therapy. N Am J Med Sci. 2014;6(9):453–459. doi: 10.4103/1947-2714.141636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisiha Kassa S, Zemene Worku W, Atalell KA, Agegnehu CD. Incidence of Loss to Follow-Up and Its Predictors Among Children with HIV on Antiretroviral Therapy at the University of Gondar Comprehensive Specialized Referral Hospital: a Retrospective Data Analysis. HIV AIDS. 2020;12:525–533. doi: 10.2147/HIV.S269580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biru M, Hallström I, Lundqvist P, Jerene D. Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: a prospective cohort study. PLoS One. 2018;13(2):e0189777. doi: 10.1371/journal.pone.0189777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endebu T, Deksisa A, Moges T, Kisi T, Ensermu T. Incidence of Virological Failure and Associated Factors among Adult HIV-Positive Patients on First Line Antiretroviral Therapy Regimen, Central Ethiopia, International Journal of HIV/AIDS Prevention. Educ Behav Sci. 2018;4(2):44–51. doi: 10.11648/j.ijhpebs.20180402.13 [DOI] [Google Scholar]
- 33.Dessalegn M, Tsadik M, Lemma H. Predictors of lost to follow up to antiretroviral therapy in primary public hospital of Wukro, Tigray, Ethiopia: a case control study. J AIDS HIV Res. 2015;7(1):1–9. doi: 10.5897/JAHR2014.0315 [DOI] [Google Scholar]
- 34.Degavi G. Influence of Lost to Follow Up from Antiretroviral Therapy Among Retroviral Infected Patients at Tuberculosis Centers in Public Hospitals of Benishangul-Gumuz, Ethiopia. HIV AIDS. 2021;13:315–327. doi: 10.2147/HIV.S306257 [DOI] [PMC free article] [PubMed] [Google Scholar]