Abstract
Tamoxifen is an estrogen receptor (ER) ligand with widespread use in clinical and basic research settings. Beyond its application in treating ER-positive cancer, tamoxifen has been co-opted into a powerful approach for temporal-specific genetic alteration. The use of tamoxifen-inducible Cre-recombinase mouse models to examine genetic, molecular, and cellular mechanisms of development and disease is now prevalent in biomedical research. Understanding off-target effects of tamoxifen will inform its use in both clinical and basic research applications. Here, we show that prenatal tamoxifen exposure can cause structural birth defects in the mouse. Administration of a single 200 mg/kg tamoxifen dose to pregnant wildtype C57BL/6J mice at gestational day 9.75 caused cleft palate and limb malformations in the fetuses, including posterior digit duplication, reduction, or fusion. These malformations were highly penetrant and consistent across independent chemical manufacturers. As opposed to 200 mg/kg, a single dose of 50 mg/kg tamoxifen at the same developmental stage did not result in overt structural malformations. Demonstrating that prenatal tamoxifen exposure at a specific time point causes dose-dependent developmental abnormalities, these findings argue for more considerate application of tamoxifen in Cre-inducible systems and further investigation of tamoxifen’s mechanisms of action.
Introduction
Tamoxifen is the oldest synthetic selective estrogen receptor (ER) modulator and is widely used in both clinical and basic research applications. Included in the World Health Organization list of essential medicines, tamoxifen is used to treat individuals with ER-positive breast cancer. Tamoxifen is also increasingly applied in biomedical research as part of powerful genetic recombination systems that leverage a fusion protein of Cre recombinase and a mutated ligand binding domain of the human ER (ERT). Upon tamoxifen binding to the ERT, nuclear translocation of the fusion protein facilitates Cre-mediated excision of loxP-flanked sequences in DNA [1]. Tamoxifen-inducible systems that allow temporally controlled genetic recombination are used for gene deletion, gene overexpression, and lineage tracing. This toolkit is applied to examine genetic, molecular, and cellular mechanisms, including those governing embryonic and postnatal development.
Although tamoxifen-inducible Cre models are widely employed to achieve temporal-specific gene alteration, developmental effects independent of Cre recombination have been suggested in multiple reports [2–10]. Animal model studies have shown that in utero tamoxifen exposure can cause mammary tumors, proliferative uterine lesions, oviduct hyperplasia, and impaired oocyte differentiation in offspring [5–7]. However, some reports also suggest that tamoxifen may have additional impacts on development that are not linked to ER signaling and endocrine disruption. Two recent reports described structural malformations in mouse embryos following maternal tamoxifen treatment [8, 11], while published human case reports document congenital anomalies associated with tamoxifen treatment during pregnancy, including craniofacial and limb defects [9, 10].
Here, we examined the impact of acute prenatal tamoxifen exposure on embryonic development in wildtype C57BL/6J mice. Our findings demonstrate that prenatal tamoxifen exposure causes structural limb and craniofacial malformations in a dose-dependent manner and suggest a previously unrecognized mechanism of action that may have significant implications for its use in clinical and basic research settings.
Results
Tamoxifen-induced malformations
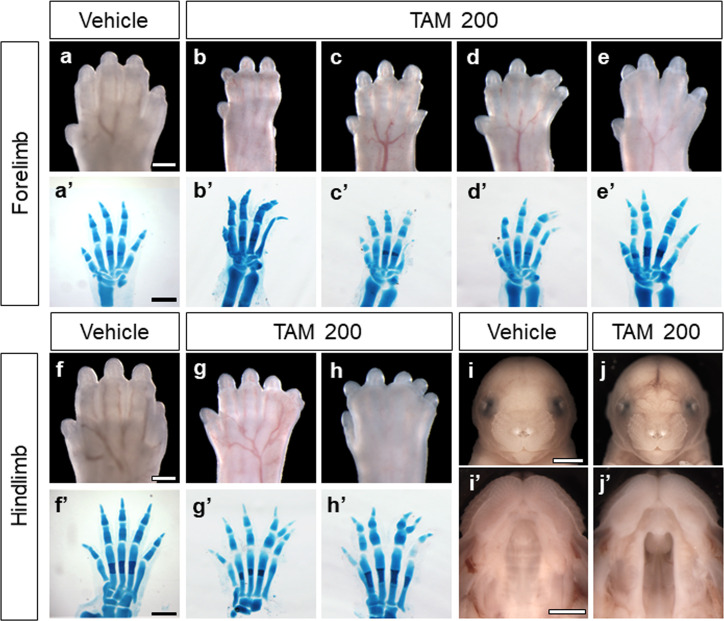
Timed pregnant wildtype C57BL/6J mice were administered a single dose of 50 mg/kg or 200 mg/kg tamoxifen (Sigma) or vehicle alone by IP injection at gestational day (GD)9.75. Dams were euthanized at GD17, and uteri were carefully inspected. The number of live fetuses and resorptions in each litter as well as mean crown-rump length were calculated (Table 1). No statistically significant differences in average number of surviving fetuses, average number of resorptions, or average fetal crown-rump length were detected between treatment groups. No overt structural malformations were observed in either the vehicle-exposed control group (Fig 1A, 1A’, 1F, 1F’, 1I, 1I’) or in the 50 mg/kg tamoxifen-exposed group. In the 200 mg/kg tamoxifen dose group, limb malformations were observed including posterior digit reduction (Fig 1B and 1C), posterior digit duplication with or without fusion (Fig 1D, 1G and 1H), and other distal limb defects (Fig 1E). Bone and cartilage staining of animals with gross limb malformations further revealed abnormalities in the metacarpals and phalanges (Fig 1B’–1E’, 1G’ and 1h’). Inspection of the oral cavity also revealed cleft palate in the group exposed to 200 mg/kg tamoxifen (Fig 1J and 1J’) but not in the group exposed to 50 mg/kg tamoxifen.
Table 1. Descriptors of the wildtype C57BL/6J study population found in Fig 2.
| Treatment (Distributor) | Litters Collected | Live Fetuses (Mean ± SD) | Resorptions (Mean ± SD) | Crown-rump Mean ± SD (mm) |
|---|---|---|---|---|
| Vehicle | 9 | 52 (5.8 ± 1.91) | 14 (1.56 ± 1.61) | 16.63 ± 0.73 |
| TAM 50 mg/kg (Sigma) | 11 | 67 (6.1 ± 2.07) | 13 (1.18 ± 1.40) | 17.03 ± 1.24 |
| TAM 200 mg/kg (Sigma) | 21 | 93 (4.4 ± 2.85) | 61 (2.90 ± 2.89) | 16.80 ± 1.08 |
| TAM 200 mg/kg (Selleckchem) | 3 | 18 (6 ± 3.56) | 1 (0.33 ± 0.47) | 16.25 ± 1.12 |
Timed-pregnant wildtype C57BL/6J mice were administered the indicated doses of tamoxifen (TAM) at gestational day (GD)9.75 and inspected at GD17 for live fetuses and resorptions. One-way ANOVA was used to compare mean numbers of live fetuses, resorptions, and crown-rump lengths between vehicle- and TAM-treated groups. No statistically significant differences were detected across treatment groups. TAM, tamoxifen; SD, standard deviation.
Fig 1. Tamoxifen-induced limb and craniofacial malformations.
Along with representative vehicle controls of forelimb (a), hindlimb (f), and palate (i’), examples of malformations in forelimbs (b-e), hindlimbs (g, h), and palate (j’) of 200 mg/kg tamoxifen-treated animals are shown at GD17. Bone and cartilage staining of tamoxifen-treated animals revealed underlying skeletal abnormalities compared to controls (a’-h’). In the forelimbs of animals with reduction malformations (b, c), dysmorphology in both the metacarpals and phalanges of the 5th digit are apparent. Additionally, the distal-most phalanx of the 4th digit appears to be duplicated in these animals (b’, c’). An animal with a forelimb duplication malformation (d) exhibited a replication of the distal phalanx of the 4th digit (d’). In hindlimbs of animals with duplication malformations (g, h), staining revealed replications of the distal and middle phalanges of the 4th digit (g’, h’). In an animal with a non-reduction/duplication phenotype (e), no overt skeletal abnormalities are apparent (e’). Secondary cleft palate (j’) was also observed in 200 mg/kg tamoxifen-treated animals. TAM, tamoxifen. Scale bars in a, a’, f, and f’: 0.5 mm; Scale bar in i: 2.0 mm; Scale bar in i’: 1.0 mm.
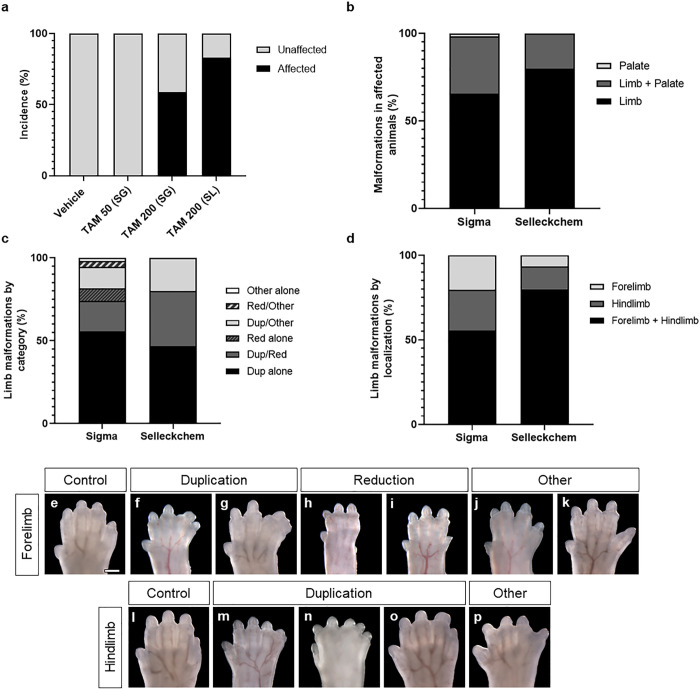
The incidence of tamoxifen-induced structural malformations as visualized by whole mount brightfield imaging is shown in Fig 2. In the 200 mg/kg treatment group, gross structural malformations were observed in 59.14% of fetuses collected (n = 55/93) with affected fetuses identified in 15/21 litters. The majority of affected fetuses exhibited limb malformations (n = 54/93) including apparent posterior digit duplication with or without fusion (n = 48/54), apparent posterior digit reduction (n = 16/54), or other defects (n = 10/54). Limb abnormality patterns within individually affected fetuses were diverse and included both reduction and duplication phenotypes in separate limbs (n = 10/54), duplication and non-reduction/duplication phenotypes in separate limbs (n = 8/54), and reduction and non-reduction/duplication phenotype in separate limbs (n = 2/54). Malformations were present in both hindlimbs and forelimbs (n = 30/54), restricted to forelimbs (n = 11/54), or restricted to hindlimbs (n = 13/54). Cleft palate was observed in 20.43% of fetuses (n = 19/93), with 18 of 19 fetuses with cleft palate also exhibiting limb malformations.
Fig 2. Incidence of tamoxifen-induced limb and craniofacial malformations.
The incidence of malformations occurring in animals treated with tamoxifen or vehicle at GD9.75 are summarized (a-d). The percentage of animals displaying at least one structural malformation in each treatment group is shown (a). Structural malformations appeared in animals treated with 200 mg/kg Sigma or Selleckchem tamoxifen, and these affected animals displayed either limb malformations, craniofacial malformations, or co-occurring limb and craniofacial malformations (b). Except for a single animal exhibiting an isolated secondary cleft palate, all affected animals exhibited limb malformations (b). Therefore, limb abnormalities were further classified according to apparent dysmorphology (c) and limb-specific localization of the abnormality (d). Examples of limb morphology in vehicle controls and tamoxifen-treated animals (e-p). A duplication phenotype was assigned to limbs that exhibited an extra posterior digit with or without cutaneous fusion to adjacent digits in both forelimbs (f, g) and hindlimbs (m, n, o). Posterior digit reduction phenotypes were exclusive to forelimbs and included apparent absence of a 5th digit (h), or a shortening of the 5th digit (i). Non-reduction/duplication phenotypes included apparent hyperplasia of interdigital tissue between the 2nd and 3rd forelimb digits (j) and laterally displaced 5th digits in forelimbs (k) and hindlimbs (p). Scale bar in a: 0.5 mm. TAM, tamoxifen; SG, Sigma; SL, Selleckchem; Dup, duplication; Red, reduction.
To test whether these outcomes were dependent upon chemical manufacturer, a parallel trial was conducted using tamoxifen supplied by an independent manufacturer and distributor (Selleckchem). No statistical differences in average number of surviving fetuses, average number of resorptions, or fetal crown-rump length were detected when comparing the 200 mg/kg Selleckchem tamoxifen group against the 200 mg/kg Sigma tamoxifen group and vehicle-exposed groups (Table 1). In the 200 mg/kg Selleckchem tamoxifen group, malformations were observed in 83.33% of fetuses (n = 15/18), with affected individuals identified in each of the 3 litters. All affected fetuses exhibited limb malformations, including posterior digit duplication with or without fusion (n = 15/15), posterior digit reduction (n = 5/15), or other defects (n = 3/15). All animals that exhibited a posterior digit reduction or other defect additionally exhibited a duplication malformation. In this tamoxifen (Selleckchem) cohort, most affected fetuses exhibited malformations affecting both the fore- and hindlimbs (n = 12/15), while a minority had only forelimb (n = 1/15) or only hindlimb abnormalities (n = 2/15). Cleft palate was 16.67% penetrant in these fetuses (n = 3/18) and always accompanied by limb malformations.
Discussion
The tamoxifen/CreERT genetic recombination system is a powerful and widely used approach to examine molecular and cellular mechanisms of development and disease. However, off-target effects of tamoxifen treatment, including Cre toxicity [12], may confound interpretation of results from the use of these models [13]. Previous studies examining the potential of tamoxifen to affect developmental processes have focused upon outcomes related to endocrine modulation. Here, we demonstrate that in utero high-dose tamoxifen exposure results in developmental abnormalities in the absence of genetic recombination and causing structural malformations in wildtype C57BL/6J mice. Specifically, we found that prenatal tamoxifen exposure at a precise critical period of development results in malformations of the limbs and palate. These findings are consistent with several published reports of prenatal tamoxifen exposure. One clinical case report described limb (“club foot”) and craniofacial (micrognathia and cleft palate) malformations in a child exposed to tamoxifen during the first trimester [14]. Two recent reports noted structural malformations in mice exposed to tamoxifen in utero, including neural tube defects, cleft palate, and eye abnormalities [8, 11]. These observations suggest that tamoxifen has off-target effects that may impact its use in mouse model research and that usage of lower doses may be a strategy to mitigate these off-target effects.
Tamoxifen is used in CreERT transgenic models to trigger temporally specific Cre-recombinase activity in adult mice, neonates, and embryos and fetuses in utero via maternal administration. Across published studies, protocols for tamoxifen administration to pregnant dams in order to induce recombination in embryos vary considerably with respect to preparation, route, dose, duration of exposure, and co-administration with other drugs such as progesterone [15–39]. In this study, we chose a single IP administration of 50 or 200 mg/kg to encompass commonly utilized administration regimens [19, 26, 36–39]. We also tested whether developmental effects caused by tamoxifen were consistent across multiple suppliers of tamoxifen. While a high dose of tamoxifen from both suppliers caused structural malformations, there was a (not statistically significant) trend toward higher resorptions and lower malformation penetrance in the Sigma cohort and lower resorptions and higher malformation penetrance in the Selleckchem cohort. Whether the activity demonstrated here for tamoxifen administration is retained by other tamoxifen manufacturers, administration routes, drug preparations, or administration of tamoxifen metabolites that also activate ERT (e.g., 4-hydroxytamoxifen) is unclear and should be examined.
The observations reported herein follow two recent reports of structural malformations in wildtype mice following prenatal tamoxifen exposure. In one report, repeated tamoxifen exposure targeting slightly later critical periods of development (~GD10.5–13.5) was found to cause cleft palate [11]. In another report, embryos and fetuses from pregnant dams dosed with 10–200 mg/kg tamoxifen between GD5.5 and GD7.5 exhibited abnormal development and malformations, including neural tube defects, cleft palate, and eye abnormalities [8]. In this previous study, 8 out of 8 dams were reported to develop severe intrauterine hemorrhaging, and maternal toxicity was implicated as a causal or contributing factor to the observed developmental outcomes. The observations collected for the study described herein, however, are not consistent with a significant role for maternal toxicity in causing the malformations that followed targeted administration of tamoxifen. In our study cohort of wildtype C57BL/6J mice, a total of 11 dams were treated with 50 mg/kg tamoxifen and 24 dams were treated with 200 mg/kg tamoxifen at GD9.75. Signs of overt maternal toxicity were not observed, and no significant differences were detected in litter size at GD17, number of resorbs, or crown-rump lengths of fetuses (Table 1). Rather than a constellation of developmental outcomes, targeted tamoxifen exposure produced consistent and specific malformations that, to our knowledge, are not associated with maternal toxicity. While a role of maternal toxicity cannot be excluded, the observations reported herein better align with the premise that tamoxifen disrupts embryonic developmental through a currently unknown mechanism.
Tamoxifen’s biological effects are thought to be predominantly mediated through its binding to ERα and ERβ [40, 41]. To our knowledge, neither limb nor palate malformations have been reported to directly result from endocrine disruption and have not been reported in Esr1 (ERα) or Esr2 (ERβ) knockout mice [42, 43]. We performed preliminary experiments to examine the impact of in utero tamoxifen exposure in Esr1-/- and Esr2-/- mice (Jax strains 026176 and 004745, respectively) where timed-pregnant dams were administered 200 mg/kg tamoxifen (Sigma) at GD9.75, and structural malformations in offspring were examined at GD17 as in our wildtype cohort. While the sample size generated was not large enough for statistical analyses of malformation incidence across each genotype, limb malformations were observed in some Esr1-/- and Esr2-/- fetuses (S1 Fig and S1 and S2 Tables). A previous study also reported that mRNA expression of Esr1 and Esr2 was not detected in the limb during the critical period examined here for tamoxifen-induced limb malformations [44]. Together, these findings suggest that the mechanism by which tamoxifen exposure results in structural malformations may be independent of ER signaling, but determining whether ER or G protein coupled ER signaling plays any role in tamoxifen-induced structural malformations will require further investigation.
While thought to act predominantly through ER signaling, tamoxifen has previously been shown to affect processes such as proliferation, apoptosis, and angiogenesis through ER-independent mechanisms [45–54]. Additionally, tamoxifen or its metabolites have been reported to directly bind to off-target receptors such as aryl hydrocarbon receptor [55] and histamine, muscarinic, and dopamine receptors [53]. Previous studies have also reported that tamoxifen potently interferes with cholesterol synthesis in vitro and in vivo and that this activity is ER-independent [56–58]. Several signaling pathways are dependent upon cholesterol biosynthesis, including the Sonic hedgehog (Shh) signaling pathway. Covalent binding of cholesterol produces fully active SHH peptide that initiates signaling in effector cells through the Smoothened protein [59], the activity of which was recently also shown to be dependent upon cholesterol binding [60, 61]. Shh signaling plays multiple roles in development including craniofacial and limb morphogenesis, and disruption of this pathway at the same time point as the one used in this study has been shown to cause cleft palate and limb malformations [62], consistent with those reported herein to result from in utero tamoxifen exposure. This period during development encompasses major tissue patterning events, including limb and orofacial morphogenesis, and these processes may be particularly susceptible to chemical perturbation [63]. Future investigation into the mechanism(s) underlying the biological impacts of tamoxifen should benefit from demonstration herein that in utero tamoxifen exposure at a specific critical period in development results in specific malformations.
Here, we present a previously unreported action of tamoxifen that may have important implications for its use in both clinical and basic research settings. Demonstrating that a high dose of tamoxifen during a specific critical period in development causes structural malformations in the absence of genetic recombination suggests previously undescribed mechanisms for this widely used drug. We focused on structural malformations because they are novel, robust, and highly penetrant, but the importance of understanding whether and how tamoxifen acts through additional mechanisms is likely not limited to developmental contexts, as most developmental signaling pathways play roles in postnatal homeostasis, healing and repair, and disease processes. These findings, along with other reports illustrating off-target effects in utilization of CreERT models, support a more considerate use of these systems and interpretation of generated experimental results.
Methods
Materials
Tamoxifen used in these studies was obtained from two independent chemical distributors. Tamoxifen from MilliporeSigma (Catalog No. T5648, Lot No. WXBC7537V) was manufactured by AstraZeneca and had a supplier-stated purity of 99.00%. In this study, tamoxifen from MilliporeSigma is abbreviated as “Sigma”. Tamoxifen from Selleckchem (Catalog No. S1238, Lot No. S123802) was manufactured by Selleckchem and had a supplier-stated purity of 99.04%. Both vendors reported tamoxifen to have a solubility of 50 mg/ml in DMSO as well as a melting point between 96–98°C.
Timed mouse mating
These studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The protocol was approved by the University of Wisconsin School of Veterinary Medicine Institutional Animal Care and Use Committee (Protocol No. 13–081.0). Wildtype C57BL/6J (Strain No. 00664) mice were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions in disposable, ventilated cages. Rooms were maintained at 22 ± 2°C and 30–70% humidity on a 12-h light, 12-h dark cycle. Mice were fed Irradiated Soy Protein-Free Extruded Rodent Diet (Catalog No. 2920x; Envigo Teklad Global) until day of plug, when dams received Irradiated Teklad Global 19% Protein Extruded Rodent Diet (Catalog No. 2919; Envigo Teklad Global). For timed matings, 1–2 nulliparous female mice were placed with a single male for 1–2 h and subsequently examined for the presence of copulation plugs as previously described [64]. The beginning of the mating period was designated as gestational day (GD)0.
Tamoxifen exposure
Tamoxifen (Sigma or Selleckchem) was dissolved into solution with corn oil (Acros Organics). Aliquots were stored at 4°C and used within 3 weeks of preparation. Pregnant mice were administered an intraperitoneal (IP) injection of either 50 mg/kg tamoxifen, 200 mg/kg tamoxifen, or corn oil vehicle at GD9.75 ± 0.05.
Dissection and imaging
Pregnant females were euthanized by CO2 asphyxiation followed by cervical dislocation at GD17 ± 2 h. Fetuses were placed in phosphate buffered saline on ice for no less than 30 min before dissection. Crown-rump measurements and initial gross phenotyping were conducted before fetal specimens were fixed in 10% formalin for at least 24 h before imaging. Images were taken of whole body, face, and palate using a MicroPublisher 5.0 camera connected to an Olympus SZX-10 stereomicroscope.
Phenotypic assessment
Fetuses were assessed for gross malformations by thorough visual inspection. For each fetus, whole mount brightfield images were captured to document morphology, with a focus on the limbs and craniofacial regions. Limb malformations were grouped into one of three classes of overt distal limb abnormalities: apparent posterior digit duplication with or without fusion, apparent posterior digit reduction, and additional defects that do not exhibit overt digit reduction or duplication (Fig 2E–2P). Craniofacial malformations were characterized by the presence of secondary palate clefting. Animals were classified as affected by displaying at least one of these malformations.
Bone & cartilage staining
Bone and cartilage staining was employed to reveal underlying skeletal abnormalities in animals classified as affected based upon careful scrutiny of light images. Following euthanasia, fetal specimens with overt structural malformations were skinned, eviscerated, and fixed in 95% or 100% ethanol for at least 3 d, then placed overnight in an alcian blue staining solution containing 8 ml of 100% ethanol, 10 ml of 100% glacial acetic acid, and 2 ml of 1% alcian blue in 3% acetic acid. They were then rinsed twice for 1 h and subsequently left overnight in 100% ethanol. Following clearing for 2 h in 1% potassium hydroxide, staining in 0.005% alizarin red in 2% potassium hydroxide for 4 h was performed. Fetuses were then rinsed in 2% potassium hydroxide once quickly, once for 1 h, and then left overnight in 1% potassium hydroxide. Finally, they were transferred to 1:3 solution of glycerol: 2% potassium hydroxide for 8 h and stored and imaged in a 1:1 solution of glycerol: 2% potassium hydroxide.
Statistics
Graphpad Prism 8 was used for all statistical analyses. One-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple comparisons was used for analyses of litter sizes, resorptions, and crown-rump lengths. An alpha value of 0.05 was maintained for determination of significance for all experiments.
Supporting information
Representative examples of distal limb dysmorphology in hindlimbs of 200 mg/kg tamoxifen-treated animals (b, d, f, h) are shown along with vehicle controls (a, c, e, g). TAM 200, tamoxifen 200 mg/kg. Scale bars in a, e: 0.5 mm.
(TIF)
Female Esr1+/- mice were mated with male Esr1+/- mice, and female Esr2+/- mice were mated with either Esr2+/- or Esr2-/- male mice. Timed-pregnant dams were administered 200 mg/kg of tamoxifen at gestational day (GD)9.75. At GD17, dams were inspected for live fetuses and resorptions, and fetuses were measured for crown-rump length and genotyped from tail tissue. TAM, tamoxifen; SD, standard deviation.
(DOCX)
Incidence of limb malformations, cleft palate, or limb malformations or cleft palate are listed for each genotype and treatment group of the Esr1 and Esr2 study populations. TAM, tamoxifen.
(DOCX)
Acknowledgments
The authors are grateful to Eric Sandgren and Linda Schuler for critical discussion, Kristen Uchtmann and William Ricke for sharing reagents, and the University of Wisconsin-Madison Biotron Laboratory and the University of Wisconsin Biomedical Research Model Services Mouse Breeding Core for providing research services.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by The University of Wisconsin Office of Vice Chancellor for Graduate and Research Education Fall Competition Award (https://research.wisc.edu/funding/fall-competition/) to RJL and The NIH Office of the Director NIH Training Grant (T35OD011078) to EAS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24(1):71–80. doi: 10.1006/meth.2001.1159 . [DOI] [PubMed] [Google Scholar]
- 2.GC C, GA B, LA L, PJ S, WL M. Abnormal Reproductive Development in Rats After Neonatally Administered Antiestrogen (Tamoxifen). Biology of reproduction. 1979;21(5). doi: 10.1095/biolreprod21.5.1087 . [DOI] [PubMed] [Google Scholar]
- 3.T I, M H, N T. Occurrence of Genital Tract Abnormalities and Bladder Hernia in Female Mice Exposed Neonatally to Tamoxifen. Toxicology. 1986;42(1). doi: 10.1016/0300-483x(86)90087-9 . [DOI] [PubMed] [Google Scholar]
- 4.GR C, O T, R N, Y N, SJ R. Teratogenic Effects of Clomiphene, Tamoxifen, and Diethylstilbestrol on the Developing Human Female Genital Tract. Human pathology. 1987;18(11). doi: 10.1016/s0046-8177(87)80381-7 . [DOI] [PubMed] [Google Scholar]
- 5.L H-C, E C, I O, DJ L, R C. Maternal Exposure to Tamoxifen During Pregnancy Increases Carcinogen-Induced Mammary Tumorigenesis Among Female Rat Offspring. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6(1). . [PubMed] [Google Scholar]
- 6.L R, JS R, K A. Maternal Tamoxifen Treatment Alters Oocyte Differentiation in the Neonatal Mice: Inhibition of Oocyte Development and Decreased Folliculogenesis. The journal of obstetrics and gynaecology research. 2010;36(2). doi: 10.1111/j.1447-0756.2009.01129.x . [DOI] [PubMed] [Google Scholar]
- 7.BA D, LM A, JM W. Proliferative Lesions of Oviduct and Uterus in CD-1 Mice Exposed Prenatally to Tamoxifen. Carcinogenesis. 1997;18(10). doi: 10.1093/carcin/18.10.2009 . [DOI] [PubMed] [Google Scholar]
- 8.Ved N, Curran A, Ashcroft FM, Sparrow DB. Tamoxifen administration in pregnant mice can be deleterious to both mother and embryo:. https://doiorg/101177/0023677219856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braems G, Denys H, De Wever O, Cocquyt V, Van den Broecke R. Use of Tamoxifen Before and During Pregnancy. Oncologist. 162011. p. 1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewari K, Perinatology Division DoOaG, Women’s Hospital, Memorial Medical Center, Long Beach, California, and Division of Pediatric Urology and Urologic Oncology DoU, University of California, Bonebrake RG, Perinatology Division DoOaG, Women’s Hospital, Memorial Medical Center, Long Beach, California, and Division of Pediatric Urology and Urologic Oncology DoU, University of California, et al. Ambiguous genitalia in infant exposed to tamoxifen in utero. Elsevier, 1997 1997/07/19. Report No.: 1474-547X Contract No.: 9072.
- 11.Xu J, Huang Z, Wang L, Li H, Zhang Y, Shao M, et al. Tamoxifen exposure induces cleft palate in mice. Br J Oral Maxillofac Surg. 2021;59(1):52–7. Epub 2020/07/25. doi: 10.1016/j.bjoms.2020.07.009 . [DOI] [PubMed] [Google Scholar]
- 12.Brash JT, Bolton RL, Rashbrook VS, Denti L, Kubota Y, Ruhrberg C. Tamoxifen-Activated CreERT Impairs Retinal Angiogenesis Independently of Gene Deletion. Circ Res. 2020;127(6):849–50. Epub 2020/07/08. doi: 10.1161/CIRCRESAHA.120.317025 ; PubMed Central PMCID: PMC7447161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wüst RCI, Houtkooper RH, Auwerx J. Confounding factors from inducible systems for spatiotemporal gene expression regulation. J Cell Biol. 2020;219(7). doi: 10.1083/jcb.202003031; PubMed Central PMCID: PMC7337492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger JC, Clericuzio CL. Pierre Robin sequence associated with first trimester fetal tamoxifen exposure. Am J Med Genet A. 2008;146A(16):2141–4. doi: 10.1002/ajmg.a.32432 . [DOI] [PubMed] [Google Scholar]
- 15.PS D, D M, DH R, SK M, AP M. Modification of Gene Activity in Mouse Embryos in Utero by a Tamoxifen-Inducible Form of Cre Recombinase. Current biology: CB. 1998;8(24). doi: 10.1016/s0960-9822(07)00562-3 . [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Wang H, Li J, Yu S, Xu M, Qiu Z, et al. Differential contribution of the two waves of cardiac progenitors and their derivatives to aorta and pulmonary artery. Dev Biol. 2019;450(2):82–9. Epub 2019/04/02. doi: 10.1016/j.ydbio.2019.03.019 . [DOI] [PubMed] [Google Scholar]
- 17.Ellisor D, Zervas M. Tamoxifen dose response and conditional cell marking: is there control? Mol Cell Neurosci. 2010;45(2):132–8. Epub 2010/06/17. doi: 10.1016/j.mcn.2010.06.004 . [DOI] [PubMed] [Google Scholar]
- 18.Weber T, Böhm G, Hermann E, Schütz G, Schönig K, Bartsch D. Inducible gene manipulations in serotonergic neurons. Front Mol Neurosci. 2009;2:24. Epub 2009/11/06. doi: 10.3389/neuro.02.024.2009; PubMed Central PMCID: PMC2779094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnamour G, Soret R, Pilon N. Dhh-expressing Schwann cell precursors contribute to skin and cochlear melanocytes, but not to vestibular melanocytes. Pigment Cell Melanoma Res. 2021;34(3):648–54. Epub 2020/11/03. doi: 10.1111/pcmr.12938 . [DOI] [PubMed] [Google Scholar]
- 20.El Agha E, Al Alam D, Carraro G, MacKenzie B, Goth K, De Langhe SP, et al. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLoS One. 2012;7(6):e38452. Epub 2012/06/13. doi: 10.1371/journal.pone.0038452; PubMed Central PMCID: PMC3374781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savery D, Maniou E, Culshaw LH, Greene NDE, Copp AJ, Galea GL. Refinement of inducible gene deletion in embryos of pregnant mice. Birth Defects Res. 2020;112(2):196–204. Epub 2019/12/03. doi: 10.1002/bdr2.1628 ; PubMed Central PMCID: PMC7003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q, Xu M, Xu J, Xie X, Yang H, Gan L. Gfi1-GCE inducible Cre line for hair cell-specific gene manipulation in mouse inner ear. Genesis. 2019;57(9):e23304. Epub 2019/05/11. doi: 10.1002/dvg.23304. [DOI] [PubMed] [Google Scholar]
- 23.Schneider S, Hotaling N, Campos M, Patnaik SR, Bharti K, May-Simera HL. Generation of an inducible RPE-specific Cre transgenic-mouse line. PLoS One. 2018;13(11):e0207222. Epub 2018/11/15. doi: 10.1371/journal.pone.0207222; PubMed Central PMCID: PMC6237357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Guo Q, Bai X, Scheller A, Kirchhoff F. Early embryonic NG2 glia are exclusively gliogenic and do not generate neurons in the brain. Glia. 2019;67(6):1094–103. Epub 2019/02/06. doi: 10.1002/glia.23590 . [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Bai X, Stopper L, Catalin B, Cartarozzi LP, Scheller A, et al. During Development NG2 Glial Cells of the Spinal Cord are Restricted to the Oligodendrocyte Lineage, but Generate Astrocytes upon Acute Injury. Neuroscience. 2018;385:154–65. Epub 2018/06/18. doi: 10.1016/j.neuroscience.2018.06.015 . [DOI] [PubMed] [Google Scholar]
- 26.Gil-Sanz C, Espinosa A, Fregoso SP, Bluske KK, Cunningham CL, Martinez-Garay I, et al. Lineage Tracing Using Cux2-Cre and Cux2-CreERT2 Mice. Neuron. 2015;86(4):1091–9. doi: 10.1016/j.neuron.2015.04.019 ; PubMed Central PMCID: PMC4455040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan AS, Boylan M, Smith C, Perez-Santamarina E, Kowalska K, Hung IH, et al. Generation and validation of novel conditional flox and inducible Cre alleles targeting fibroblast growth factor 18 (Fgf18). Dev Dyn. 2019;248(9):882–93. Epub 2019/07/22. doi: 10.1002/dvdy.85 ; PubMed Central PMCID: PMC7029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takenoshita M, Takechi M, Vu Hoang T, Furutera T, Akagawa C, Namangkalakul W, et al. Cell lineage- and expression-based inference of the roles of forkhead box transcription factor Foxc2 in craniofacial development. Dev Dyn. 2021. Epub 2021/03/05. doi: 10.1002/dvdy.324. [DOI] [PubMed] [Google Scholar]
- 29.Holl AM. Survival of mature mouse olfactory sensory neurons labeled genetically perinatally. Mol Cell Neurosci. 2018;88:258–69. Epub 2018/02/07. doi: 10.1016/j.mcn.2018.02.005 . [DOI] [PubMed] [Google Scholar]
- 30.Tsai YH, Hill DR, Kumar N, Huang S, Chin AM, Dye BR, et al. LGR4 and LGR5 Function Redundantly During Human Endoderm Differentiation. Cell Mol Gastroenterol Hepatol. 2016;2(5):648-62.e8. Epub 2016/06/23. doi: 10.1016/j.jcmgh.2016.06.002; PubMed Central PMCID: PMC5042889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol Metab. 2017;6(3):236–44. Epub 2017/01/12. doi: 10.1016/j.molmet.2017.01.003 ; PubMed Central PMCID: PMC5323890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrysostomou E, Zhou L, Darcy YL, Graves KA, Doetzlhofer A, Cox BC. The Notch Ligand Jagged1 Is Required for the Formation, Maintenance, and Survival of Hensen’s Cells in the Mouse Cochlea. J Neurosci. 2020;40(49):9401–13. Epub 2020/10/30. doi: 10.1523/JNEUROSCI.1192-20.2020 ; PubMed Central PMCID: PMC7724135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deal KK, Rosebrock JC, Eeds AM, DeKeyser JL, Musser MA, Ireland SJ, et al. Sox10-cre BAC transgenes reveal temporal restriction of mesenchymal cranial neural crest and identify glandular Sox10 expression. Dev Biol. 2021;471:119–37. Epub 2020/12/13. doi: 10.1016/j.ydbio.2020.12.006 ; PubMed Central PMCID: PMC7855809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Corral I, Stanczuk L, Frye M, Ulvmar MH, Diéguez-Hurtado R, Olmeda D, et al. Vegfr3-CreER (T2) mouse, a new genetic tool for targeting the lymphatic system. Angiogenesis. 2016;19(3):433–45. Epub 2016/03/18. doi: 10.1007/s10456-016-9505-x . [DOI] [PubMed] [Google Scholar]
- 35.Lüdtke TH, Rudat C, Kurz J, Häfner R, Greulich F, Wojahn I, et al. Mesothelial mobilization in the developing lung and heart differs in timing, quantity, and pathway dependency. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L767–L83. Epub 2019/01/31. doi: 10.1152/ajplung.00212.2018 . [DOI] [PubMed] [Google Scholar]
- 36.El Shahawy M, Reibring CG, Neben CL, Hallberg K, Marangoni P, Harfe BD, et al. Cell fate specification in the lingual epithelium is controlled by antagonistic activities of Sonic hedgehog and retinoic acid. PLoS Genet. 2017;13(7):e1006914. Epub 2017/07/17. doi: 10.1371/journal.pgen.1006914; PubMed Central PMCID: PMC5536368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rockwell DM, O’Connor AK, Bentley-Ford MR, Haycraft CJ, Croyle MJ, Brewer KM, et al. A transgenic Alx4-CreER mouse to analyze anterior limb and nephric duct development. Dev Dyn. 2021. Epub 2021/03/16. doi: 10.1002/dvdy.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235(9):2603–12. doi: 10.1002/dvdy.20892 . [DOI] [PubMed] [Google Scholar]
- 39.Lizen B, Claus M, Jeannotte L, Rijli FM, Gofflot F. Perinatal induction of Cre recombination with tamoxifen. Transgenic Res. 2015;24(6):1065–77. Epub 2015/09/22. doi: 10.1007/s11248-015-9905-5 . [DOI] [PubMed] [Google Scholar]
- 40.An KC. Selective Estrogen Receptor Modulators. Asian Spine J. 2016;10(4):787–91. doi: 10.4184/asj.2016.10.4.787 ; PubMed Central PMCID: PMC4995266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.M D, CL S. Molecular Mechanisms of Selective Estrogen Receptor Modulator (SERM) Action. The Journal of pharmacology and experimental therapeutics. 2000;295(2). . [PubMed] [Google Scholar]
- 42.Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res. 2012;28(2):71–6. doi: 10.5625/lar.2012.28.2.71 ; PubMed Central PMCID: PMC3389841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.JF C, KS K. Estrogen Receptor Null Mice: What Have We Learned and Where Will They Lead Us? Endocrine reviews. 1999;20(3). doi: 10.1210/edrv.20.3.0370 . [DOI] [PubMed] [Google Scholar]
- 44.Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech Dev. 1999;81(1–2):163–7. doi: 10.1016/s0925-4773(98)00223-8 . [DOI] [PubMed] [Google Scholar]
- 45.Todorova VK, Kaufmann Y, Luo S, Klimberg VS. Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother Pharmacol. 2011;67(2):285–91. Epub 2010/04/11. doi: 10.1007/s00280-010-1316-y . [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa G, Akatsuka K, Nakashima Y, Yokoe Y, Higo N, Shimonaka M. Tamoxifen inhibits the proliferation of non‑melanoma skin cancer cells by increasing intracellular calcium concentration. Int J Oncol. 2018;53(5):2157–66. Epub 2018/08/31. doi: 10.3892/ijo.2018.4548 . [DOI] [PubMed] [Google Scholar]
- 47.Benson JR, Wakefield LM, Baum M, Colletta AA. Synthesis and secretion of transforming growth factor beta isoforms by primary cultures of human breast tumour fibroblasts in vitro and their modulation by tamoxifen. Br J Cancer. 1996;74(3):352–8. doi: 10.1038/bjc.1996.365 ; PubMed Central PMCID: PMC2074642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferlini C, Scambia G, Marone M, Distefano M, Gaggini C, Ferrandina G, et al. Tamoxifen induces oxidative stress and apoptosis in oestrogen receptor-negative human cancer cell lines. Br J Cancer. 1999;79(2):257–63. doi: 10.1038/sj.bjc.6690042 ; PubMed Central PMCID: PMC2362195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142(1):21-4.e7. Epub 2011/10/14. doi: 10.1053/j.gastro.2011.09.050; PubMed Central PMCID: PMC3708546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson KE, Forward JA, Tippy MD, Ceglowski JR, El-Husayni S, Kulenthirarajan R, et al. Tamoxifen Directly Inhibits Platelet Angiogenic Potential and Platelet-Mediated Metastasis. Arterioscler Thromb Vasc Biol. 2017;37(4):664–74. Epub 2017/02/02. doi: 10.1161/ATVBAHA.116.308791 ; PubMed Central PMCID: PMC5364056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackwell KL, Haroon ZA, Shan S, Saito W, Broadwater G, Greenberg CS, et al. Tamoxifen inhibits angiogenesis in estrogen receptor-negative animal models. Clin Cancer Res. 2000;6(11):4359–64. . [PubMed] [Google Scholar]
- 52.Shim JS, Li RJ, Lv J, Head SA, Yang EJ, Liu JO. Inhibition of angiogenesis by selective estrogen receptor modulators through blockade of cholesterol trafficking rather than estrogen receptor antagonism. Cancer Lett. 2015;362(1):106–15. Epub 2015/03/20. doi: 10.1016/j.canlet.2015.03.022 ; PubMed Central PMCID: PMC4469646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flynn M, Heale KA, Alisaraie L. Mechanism of Off-Target Interactions and Toxicity of Tamoxifen and Its Metabolites. Chem Res Toxicol. 2017;30(7):1492–507. Epub 2017/06/14. doi: 10.1021/acs.chemrestox.7b00112 . [DOI] [PubMed] [Google Scholar]
- 54.Manna S, Holz MK. Tamoxifen Action in ER-Negative Breast Cancer. Sign Transduct Insights. 2016;5:1–7. doi: 10.4137/STI.S29901 ; PubMed Central PMCID: PMC4792287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DuSell CD, Nelson ER, Wittmann BM, Fretz JA, Kazmin D, Thomas RS, et al. Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Mol Endocrinol. 2010;24(1):33–46. Epub 2009/11/09. doi: 10.1210/me.2009-0339 ; PubMed Central PMCID: PMC2802893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holleran AL, Lindenthal B, Aldaghlas TA, Kelleher JK. Effect of tamoxifen on cholesterol synthesis in HepG2 cells and cultured rat hepatocytes. Metabolism. 1998;47(12):1504–13. doi: 10.1016/s0026-0495(98)90078-6 . [DOI] [PubMed] [Google Scholar]
- 57.Gylling H, Pyrhönen S, Mäntylä E, Mäenpää H, Kangas L, Miettinen TA. Tamoxifen and toremifene lower serum cholesterol by inhibition of delta 8-cholesterol conversion to lathosterol in women with breast cancer. J Clin Oncol. 1995;13(12):2900–5. doi: 10.1200/JCO.1995.13.12.2900 . [DOI] [PubMed] [Google Scholar]
- 58.Cho SY, Kim JH, Paik YK. Cholesterol biosynthesis from lanosterol: differential inhibition of sterol delta 8-isomerase and other lanosterol-converting enzymes by tamoxifen. Mol Cells. 1998;8(2):233–9. . [PubMed] [Google Scholar]
- 59.Jeong J, McMahon AP. Cholesterol modification of Hedgehog family proteins. J Clin Invest. 2002;110(5):591–6. doi: 10.1172/JCI16506 ; PubMed Central PMCID: PMC151115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang P, Zheng S, Wierbowski BM, Kim Y, Nedelcu D, Aravena L, et al. Structural Basis of Smoothened Activation in Hedgehog Signaling. Cell. 2018;174(2):312-24.e16. Epub 2018/05/24. doi: 10.1016/j.cell.2018.04.029; PubMed Central PMCID: PMC6046275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers BR, Neahring L, Zhang Y, Roberts KJ, Beachy PA. Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc Natl Acad Sci U S A. 2017;114(52):E11141–E50. Epub 2017/12/11. doi: 10.1073/pnas.1717891115 ; PubMed Central PMCID: PMC5748227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heyne GW, Melberg CG, Doroodchi P, Parins KF, Kietzman HW, Everson JL, et al. Definition of critical periods for Hedgehog pathway antagonist-induced holoprosencephaly, cleft lip, and cleft palate. PLoS One. 2015;10(3):e0120517. doi: 10.1371/journal.pone.0120517; PubMed Central PMCID: PMC4368540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shenefelt RE. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972;5(1):103–18. doi: 10.1002/tera.1420050115 . [DOI] [PubMed] [Google Scholar]
- 64.Heyne GW, Plisch EH, Melberg CG, Sandgren EP, Peter JA, Lipinski RJ. A Simple and Reliable Method for Early Pregnancy Detection in Inbred Mice. J Am Assoc Lab Anim Sci. 2015;54(4):368–71. doi: n/a. ; PubMed Central PMCID: PMC4521569. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative examples of distal limb dysmorphology in hindlimbs of 200 mg/kg tamoxifen-treated animals (b, d, f, h) are shown along with vehicle controls (a, c, e, g). TAM 200, tamoxifen 200 mg/kg. Scale bars in a, e: 0.5 mm.
(TIF)
Female Esr1+/- mice were mated with male Esr1+/- mice, and female Esr2+/- mice were mated with either Esr2+/- or Esr2-/- male mice. Timed-pregnant dams were administered 200 mg/kg of tamoxifen at gestational day (GD)9.75. At GD17, dams were inspected for live fetuses and resorptions, and fetuses were measured for crown-rump length and genotyped from tail tissue. TAM, tamoxifen; SD, standard deviation.
(DOCX)
Incidence of limb malformations, cleft palate, or limb malformations or cleft palate are listed for each genotype and treatment group of the Esr1 and Esr2 study populations. TAM, tamoxifen.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.