Abstract
Background:
Tumor vascular endothelial growth factor (VEGF) is a key angiogenic factor and may have an impact on tumor progression and response to chemotherapy. The insulin-like growth factor (IGF) system is related to cell proliferation and tumor growth. However, there is limited available data regarding the clinical and prognostic significance of VEGF or IGF-1 in advanced gastric cancer. The aim of this study was to evaluate the prognostic significance of serum VEGF and IGF-1 levels in advanced gastric cancer patients who were treated with oxaliplatin/5-fluorouracil (FOLFOX).
Methods:
The study population consisted of 100 advanced gastric cancer patients (median age 56 years). Patients were treated with oxaliplatin 85 mg/m2 as a 2-hour infusion on day 1 plus leucovorin 20 mg/m2 over 10 min, followed by a 5-fluorouracil (5-FU) bolus 400 mg/m2 and 22 h of continuous infusion of 600 mg/m2 on days 1–2. Treatment was repeated in 2-week intervals. The levels of serum VEGF and IGF-1 were measured using enzyme-linked immunoassays.
Results:
There was a significant correlation between the serum level of VEGF and Lauren’s classification (p = 0.030) and previous operations (p = 0.010). IGF-1 was associated with the number of metastases (p = 0.012). The median level of serum VEGF was decreased after FOLFOX chemotherapy (p = 0.034). However, none of the measured serum markers were significantly correlated with response. In univariate analysis, overall survival (p < 0.001) was significantly shorter in patients with high serum levels of VEGF. Multivariate analysis revealed that VEGF was an independent factor for overall survival (HR 2.221; 95% CI 1.377–3.583, p = 0.001). Furthermore, IGF-1 had no significant influence on the clinical outcome.
Conclusion:
A high level of serum VEGF is an independent prognostic factor in patients with advanced gastric cancer treated with chemotherapy. This may help to identify the patients who are more sensitive to the FOLFOX regimen.
Keywords: Vascular endothelial growth factor, Insulin-like growth factor-1, FOLFOX, Gastric carcinoma
Introduction
Gastric cancer remains a significant health problem despite its declining incidence in the West. It is the fourth most common cancer worldwide, accounting for 8.6% of all new cancer diagnoses in 2002 [1]. Although the incidence of stomach cancer among Koreans has decreased over the past 2 decades, gastric cancer is the most common carcinoma in men and the third most common type of cancer in women, and it remains the leading cause of death due to cancer in Korea [2].
Most newly diagnosed gastric cancer patients present with regional or distant metastatic disease where the 5-year overall survival (OS) is dismal and is generally accepted as being less than 10% [3]. To date, a median survival beyond 12 months has not been achieved in any randomized study with combination chemotherapy [4]. 5-Fluorouracil (5-FU) remains the main chemotherapeutic agent for the treatment of gastric cancer, and combination chemotherapy with 5-FU has shown improvements in clinical outcomes. 5-FU in combination with cisplatin has demonstrated an effective clinical outcome; however, toxicities are considerable [4]. Oxaliplatin, another platinum-based agent, has a more favorable tolerability profile than cisplatin. The oxaliplatin/5-FU combination or oxaliplatin/capcitabine (FOLFOX or XELOX) has proven to be an effective first- or second-line treatment for advanced gastric cancer [5–7]. Increasing emphasis on the need for improved techniques for the prediction of treatment response and survival may facilitate the tailoring of chemotherapy and risk-related therapy, resulting in significantly better survival.
Vascular endothelial growth factor (VEGF) is a well-known pro-angiogenic growth factor, and its stimulation under hypoxic conditions plays a critical role in promoting the survival of malignant cells, in local tumor growth and invasion, and in the development of metastases [8]. To date, several important roles of VEGF in the progression of human gastric cancer have been reported. There are conflicting reports about role of the VEGF family. While VEGF-A is a poor prognostic factor in gastric cancer, the role of VEGF-C and VEGF-D is still controversial [9]. The expression of VEGF-A is correlated with tumor vascularity [10], and a significant increase in the frequency of hepatic metastases among patients with VEGF-positive tumors has been reported [11–13]. The expression of VEGF-A has also been correlated with a poor outcome and is considered to be an independent prognostic factor in gastric cancer patients [10, 12].
However, evaluation of tumor expression of VEGF depends on the availability of resected surgical specimens or biopsy material, and there is considerable observer-related variability when using semiquantitative techniques such as immunohistochemical staining. In addition, intratumoral heterogeneity may also be a confounding factor. It has been suggested that measurement of serum VEGF concentrations could be a method that is less observer dependent for quantifying angiogenesis and that such levels could act as a surrogate marker of tumor angiogenesis [14]. There is little information on the prognostic value of plasma or serum VEGF levels in the field of gastric cancer. Recent studies have correlated VEGF expression in serum with tumor vascularity, and demonstrated that high serum VEGF levels can predict a poor prognosis in cancer patients [15, 16]. Two reports have described the significance of VEGF levels in advanced gastric cancer during chemotherapy [17, 18]. However, only small numbers of patients were enrolled into the reported studies, and the results remain contentious.
The insulin-like growth factor (IGF) family is composed of 2 peptide ligands (IGF-1 and IGF-2), 2 cell surface receptors (IGF-1R and IGF-2R), and at least 6 specific IGF binding proteins (IGFBP-1 to IGFBP-6) [19]. The signaling pathway involving IGF-1 plays an important role in cell growth and differentiation [20, 21]. Additionally, IGF-1 affects tumor cell proliferation via the RAS-RAF-mitogen-activated protein (MAP) kinase signaling pathway and also has antiapoptotic effects mediated by the phosphatidylinositol-3 kinase/AKT pathway, which ultimately activates downstream transcription factors that regulate the gene expression of proliferative, differentiation, and antiapoptotic factors [20, 21]. It has also been suggested that IGF-1 may contribute towards the pathogenesis of cancers through its likely role in neo-plastic transformation and angiogenesis via increased production of VEGF, and in tumor growth [21].
The increased concentrations of IGF in the serum may correlate with the presence or the risk of developing carcinoma of the prostate, breast, intestine, and lung [22]. Lung cancer secretes ectopically high levels of IGF and IGFBP, which suggests the usefulness of the measurement of these substances in early diagnostics, tumor type identification, clinical staging, and response to treatment monitoring [23]. High serum levels of IGF-1, IGF-2, and IGFBP-3 have been reported to be associated with a good prognosis in patients with non-small cell lung cancer [24]. Several studies have attempted to assess the association between serum levels of IGFs and IGFBPs and stomach cancer [25, 26]. However, there are no available reports about serum levels of IGF-1 and chemotherapy response in advanced gastric cancer patients.
Therefore, we conducted this study to evaluate the association of pretreatment levels of serum VEGF and IGF-1 with the clinical outcome of advanced gastric cancer patients who were treated with FOLFOX chemotherapy.
Methods
Study Population
All of the patients in this study had histologically confirmed adenocarcinoma of the stomach. These patients were treated with FOLFOX chemotherapy as a first-line treatment. All of the patients had an Eastern Cooperative Oncology Group performance status ≤2 and adequate bone marrow and renal function and were aged over 18 years. Exclusion criteria included the presence of central nervous system metastases, serious or un-controlled concurrent medical illness, and a history of other malignancies. Written informed consent was obtained from each patient before entry into the study. The institutional review board of Dong-A University Hospital approved the use of all patients’ material.
Treatment Protocols and Dose Modification
On day 1, oxaliplatin (85 mg/m2) was administered by intravenous (i.v.) infusion in 500 ml of normal saline or dextrose over 2 h. On days 1 and 2, leucovorin (20 mg/m2) was administered as an i.v. bolus, immediately followed by 5-FU (400 mg/m2) given as a 10-min i.v. bolus, followed by 5-FU (600 mg/m2) as a continuous 22-hour infusion with a light shield. Dose modifications of oxaliplatin or 5-FU were made for hematologic, gastrointestinal, or neurologic toxic effects based on the most severe grade of toxicity that had occurred during the previous cycle. Treatment was delayed for up to 2 weeks in cases of persistence of symptomatic toxicity, or if the absolute number of neutrophils was <1,500/µl or the platelet count was <100,000/µl. The 5-FU dose was reduced by 25% for subsequent courses after the occurrence of grade 3 diarrhea, stomatitis, or dermatitis as per the National Cancer Institute Common Toxicity Criteria (NCI-CTC). The dose of oxaliplatin was reduced by 25% in subsequent cycles if there were persistent paresthesias between cycles or paresthesias with functional impairment lasting >7 days. Treatment was continued until there were signs of disease progression, development of unacceptable toxic effects, or the patient refused further treatment.
Follow-Up Evaluation and Assessment of Response
Before each treatment course, a physical examination, routine hematology, biochemistry, and chest X-ray were carried out. Computed tomography scans to define the extent of the disease and the responses were carried out after 4 cycles of chemotherapy or sooner if there was evidence of any clinical deterioration. Patients were assessed before starting each 2-week cycle using the NCI-CTC, except in the case of neurotoxicity. For the neurotoxicity, an oxaliplatin-specific 3-grade scale was used: grade 1, paresthesias or dysesthesias of short duration, but resolving before the next dosing; grade 2, paresthesias persisting between doses (2 weeks), and grade 3, paresthesias interfering with function.
Responses were evaluated using RECIST criteria. Complete response was defined as the disappearance of all evidence of disease and the normalization of tumor markers for at least 2 weeks. Partial response was defined as a ≥30% reduction in uni-dimensional tumor measurements, without the appearance of any new lesions or the progression of any existing lesion. Progressive disease (PD) was defined as any of the following: a 20% increase in the sum of the products of all of the measurable lesions, appearance of any new lesion, or reappearance of any lesion that had previously disappeared. Stable disease was defined as a tumor response not fulfilling the criteria for complete response, partial response, or progressive disease.
Measurements of Serum Levels of VEGF and IGF-1
A blood sample was drawn from each participant through venipuncture before chemotherapy and after 3 cycles of treatment. The blood samples were each centrifuged for 10 min at 3,000 rpm at −4°C. The serum was subsequently removed and stored at −80°C until the conduction of biochemical analysis. Serum IGF-1 and VEGF enzyme-linked immunosorbent assays (ELISAs) were completed as per the manufacturer’s protocols (R&D Systems, Minneapolis, Minn., USA). Briefly, serum samples were thawed on wet ice 3 h prior to assay. IGF-1 serum samples were pretreated with an acidic solution to promote dissociation of IGF-1 from abundant IGF-1 binding proteins and stabilized in buffer and preservatives. Samples were plated in a 96-well format in duplicate, after which conjugated IGF-1 or VEGF-1/horseradish peroxidase polyclonal secondary antibody, respectively, was added. Substrate solution (hydrogen peroxide/tetramethylbenzidine) was then administered for 30 min, after which the reaction was quenched with sulfuric acid. Plates were read at an absorbance of 450 nm on a Victor 3 plate reader (PerkinElmer, Boston, Mass., USA). Extrapolated absorbance was analyzed using Masterplex Readerfit ELISA software (Hitachi, Waltham, Mass., USA) and concentration was determined following a 4 Parameter Logistic curve fit as per the manufacturer’s recommendation. Measurements were made by a single investigator blinded to the patients’ clinicopathological data.
Statistical Analyses
Serum levels of VEGF and IGF-1 were expressed as means ± SD. Associations between patients’ clinicopathologic features and levels of serum VEGF and IGF-1 were assessed by Mann-Whitney and Kruskal-Wallis tests for continuous variables and by Fisher’s exact test or the χ2 test for categorical variables. In addition, Spearman’s rank correlation coefficients were computed for VEGF and IGF-1. The time to progression (TTP) and OS were calculated from the date of initiation of therapy to the date of disease progression and death, respectively. Patients who were alive at the last follow-up were censored at that time. The association of each marker with survival was analyzed using Kaplan-Meier plots and a log-rank test, and its associated 95% CI was calculated. Multivariate analyses were carried out using the Cox proportional hazards model. All of the tests were two-sided, and p < 0.05 was considered statistically significant. Analyses were done using SPSS version 14.0 (SPSS, Chicago, Ill., USA).
Results
Patient Characteristics
From March 2007 to August 2010, one hundred patients were enrolled into this study. The median follow-up time was 14.9 months (range 1.0–47.9). Demographic details about the patients included in the study are shown in table 1. The patients consisted of 68 men and 32 women, with a median age of 56 years (range 24–74). Fifty patients underwent curative operation [stage I, n = 6; stage II, n = 13; stage III, n = 20; stage IV (M0), n = 11], and a palliative resection was done in 20 stage IV patients. Forty-two patients received 5-FU-based adjuvant chemotherapy.
Table 1.
Patients’ characteristics
| n | % | |
|---|---|---|
| Age | ||
| <60 years | 58 | 58.0 |
| ≥60 years | 42 | 42.0 |
| Gender | ||
| Male | 68 | 68.0 |
| Female | 32 | 32.0 |
| Previous operation | ||
| + | 70 | 70.0 |
| − | 30 | 30.0 |
| Initial TNM stage | ||
| I | 6 | 6.0 |
| II | 13 | 13.0 |
| III | 20 | 20.0 |
| IV | 61 | 61.0 |
| Lauren’s classification | ||
| Diffuse | 25 | 25.0 |
| Intestinal | 16 | 16.0 |
| Mixed | 45 | 45.0 |
| Unknown | 14 | 14.0 |
| Adjuvant chemotherapy | ||
| + | 42 | 42.0 |
| − | 58 | 58.0 |
| Carcinoembryonic antigen | ||
| <5 ng/ml | 67 | 67.0 |
| ≥5 ng/ml | 29 | 29.0 |
| Number of metastases | ||
| 1 | 57 | 57.0 |
| 2 | 26 | 26.0 |
| ≥3 | 17 | 17.0 |
| ECOG performance status | ||
| 0–1 | 100 | 100 |
ECOG = Eastern Cooperative Oncology Group.
Association of Pretreatment Levels of Serum VEGF, and IGF-1, with Patient Clinicopathologic Features
The median serum level of VEGF and IGF-1 was 398.6 pg/ml (range 50.0–1,647.0) and 29.0 ng/ml (range 2.0–125.0), respectively. We examined the association of patient clinicopathologic features including gender, age, Lauren’s classification, carcinoembryonic antigen (CEA), and number of metastases with the pretreatment serum levels of VEGF and IGF-1 (table 2). The mean level of serum VEGF was higher in the patients with no previous operation compared to those who underwent operation (601.1 ± 395.6 vs. 429.2 ± 281.3 pg/ml, p = 0.010). Patients with a diffused type of gastric cancer showed lower VEGF levels than other types (p = 0.030). The IGF-1 level was associated with the number of metastases (p = 0.012). No significant correlation was observed between VEGF and IGF-1 (r = −0.006, p = 0.949).
Table 2.
Association of serum VEGF and IGF-1 levels with patients’ characteristics
| VEGF, pg/ml |
IGF-1, ng/ml |
|||
|---|---|---|---|---|
| median ± SD | p | median ± SD | p | |
| Age | ||||
| <60 years | 459.0 ± 362.8 | 0.198 | 36.6 ± 30.2 | 0.893 |
| ≥60 years | 510.7 ± 272.7 | 39.2 ± 32.1 | ||
| Gender | ||||
| Male | 461.8 ± 313.1 | 0.572 | 37.6 ± 30.9 | 0.894 |
| Female | 521.0 ± 358.1 | 37.9 ± 31.5 | ||
| Previous operation | ||||
| + | 429.2 ± 281.3 | 0.010 | 34.9 ± 28.4 | 0.174 |
| − | 601.1 ± 395.6 | 44.2 ± 35.8 | ||
| Lauren’s classification | ||||
| Diffuse | 369.1 ± 215.2 | 0.030 | 31.0 ± 29.9 | 0.520 |
| Intestinal | 435.3 ± 224.2 | 28.7 ± 27.8 | ||
| Mixed | 519.4 ± 426.0 | 36.5 ± 25.8 | ||
| Unknown | 546.8 ± 364.1 | 44.9 ± 33.0 | ||
| Adjuvant chemotherapy | ||||
| + | 450.4 ± 308.0 | 0.177 | 28.7 ± 26.7 | 0.113 |
| − | 502.7 ± 341.9 | 44.2 ± 32.3 | ||
| Carcinoembryonic antigen | ||||
| <5 ng/ml | 464.0 ± 322.3 | 0.501 | 37.7 ± 32.0 | 0.326 |
| ≥5 ng/ml | 512.6 ± 331.3 | 35.1 ± 26.9 | ||
| Number of metastases | ||||
| 1 | 469.1 ± 353.4 | 0.537 | 46.7 ± 32.4 | 0.012 |
| 2 | 459.0 ± 262.1 | 27.6 ± 26.5 | ||
| ≥3 | 553.0 ± 335.4 | 22.6 ± 21.0 | ||
Association of Pretreatment Serum Levels of VEGF and IGF-1 with Tumor Response
The overall chemotherapy response rate for treatment was 36.0% (36 of 100 cases). Only number of metastases was related to the response to chemotherapy (p = 0.007). Other parameters, such as age, sex, previous operation, and CEA level, were not significantly correlated with clinical response to chemotherapy. We analyzed the association of pretreatment serum levels of VEGF and IGF-1 with tumor response to FOLFOX chemotherapy. None of the pretreatment serum markers measured was significantly correlated with response (fig. 1a, b). The median serum level of VEGF was slightly higher in the responder group than in the nonresponder group (543.6 ± 362.5 vs. 450.4 ± 304.9 pg/ml, p = 0.114), whereas the IGF-1 level was not different between responder and nonresponder groups (33.5 ± 30.3 vs. 40.0 ± 31.3 ng/ml, p = 0.508). We collected serum after chemotherapy from 31 patients and evaluated changes in VEGF serum level after treatment. A decrease in the median serum level of VEGF was observed after FOLFOX chemotherapy (204.3 ± 27.2 vs. 187.7 ± 33.0 pg/ml, p = 0.034). In addition, the median serum level of VEGF was decreased in patients with tumor response, but the differences were not statistically significant (195.3 ± 23.3 vs. 177.0 ± 21.5 pg/ml, p = 0.071). No statistically significant difference in mean IGF-1 level was shown both before and after FOLFOX chemotherapy (p = 0.866).
Fig. 1.
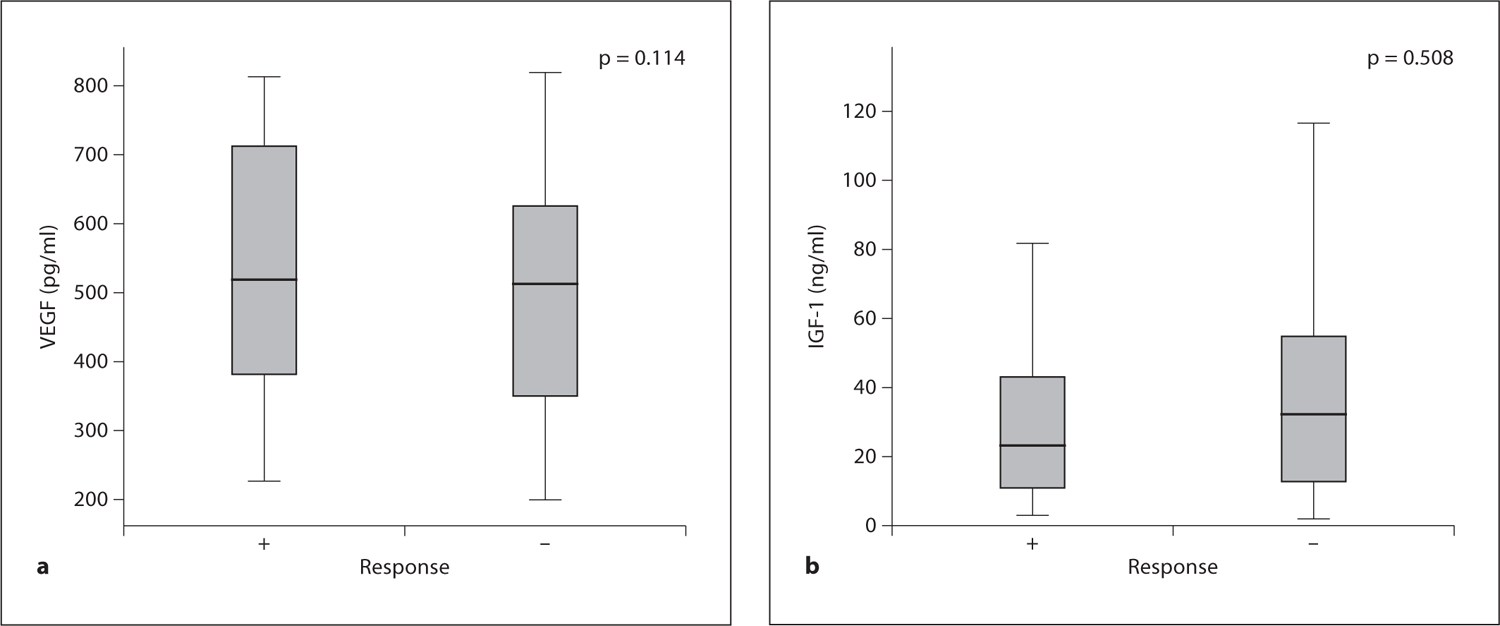
Association between pretreatment serum levels of VEGF (a) and IGF-1 (b) and tumor response to chemotherapy. The p value was calculated using the Mann-Whitney test.
Association of Pretreatment Serum Levels of VEGF and IGF-1 with Survival
The median TTP was 4.8 months (95% CI 24.2–5.3), and the median OS was 15.1 months (95% CI 11.3–18.2). Table 3 shows the association of patients’ clinicopathologic features with TTP and OS in the 100 patients analyzed. Among the evaluated clinical parameters, gender, previous operation, Lauren’s classification, and CEA were not correlated with either TTP or OS. Patient’s age and response to chemotherapy were found to be related to both TTP (p = 0.024 and p = 0.011, respectively) and OS (p = 0.010 and p = 0.049, respectively).
Table 3.
Prognostic factors in univariate analysis
| TTP months | p | OS months | p | |
|---|---|---|---|---|
| Age | ||||
| <60 years | 5.2 | 0.024 | 17.3 | 0.010 |
| ≥60 years | 4.6 | 10.7 | ||
| Gender | ||||
| Male | 4.8 | 0.895 | 14.4 | 0.679 |
| Female | 4.9 | 15.8 | ||
| Previous operation | ||||
| + | 4.8 | 0.962 | 15.8 | 0.147 |
| − | 4.8 | 11.5 | ||
| Lauren’s classification | ||||
| Diffuse | 5.2 | 0.239 | 12.3 | 1.154 |
| Intestinal | 6.4 | 15.8 | ||
| Mixed | 3.9 | 13.3 | ||
| Unknown | 7.1 | 15.0 | ||
| Adjuvant chemotherapy | ||||
| + | 4.9 | 0.908 | 15.1 | 0.887 |
| − | 4.8 | 14.4 | ||
| Carcinoembryonic antigen | ||||
| <5 ng/ml | 5.1 | 0.942 | 15.8 | 0.250 |
| ≥5 ng/ml | 4.4 | 11.5 | ||
| Number of metastases | ||||
| 1 | 5.9 | 0.052 | 14.4 | 0.067 |
| 2 | 4.6 | 15.0 | ||
| ≥3 | 4.0 | 10.1 | ||
| Response | ||||
| + | 6.2 | 0.011 | 17.3 | 0.049 |
| − | 3.7 | 11.6 | ||
| IGF-1 | ||||
| <29.0 ng/ml | 4.8 | 0.455 | 13.1 | 0.403 |
| ≥29.0 ng/ml | 4.8 | 15.8 | ||
| VEGF | ||||
| <398.6 pg/ml | 5.2 | 0.083 | 18.7 | <0.001 |
| ≥398.6 pg/ml | 4.1 | 11.5 |
For the biological markers analyzed, high serum levels were defined as being greater than the median value. Comparison between two groups was made by log-rank analysis. The TTP values for patients with VEGF levels in excess of 398.6 pg/ml were lower than those in patients with VEGF values ≤398.6 pg/ml (4.1 vs. 5.2 months, p = 0.083). The median OS was significantly longer for patients who had low levels of VEGF when compared with patients who had high levels of VEGF, and statistical significance (18.7 vs. 11.5 months, p < 0.001) was noted in the difference achieved. The cumulative TTP and OS survival curves of patients grouped according to serum VEGF level are shown in figures 2a and 3a, respectively. The serum level of IGF-1 was not significantly correlated with TTP (4.8 vs. 4.8 months, p = 0.455; fig. 2b) or OS (13.1 vs. 15.8 months, p = 0.403; fig. 3b) in patients treated with FOLFOX chemotherapy.
Fig. 2.
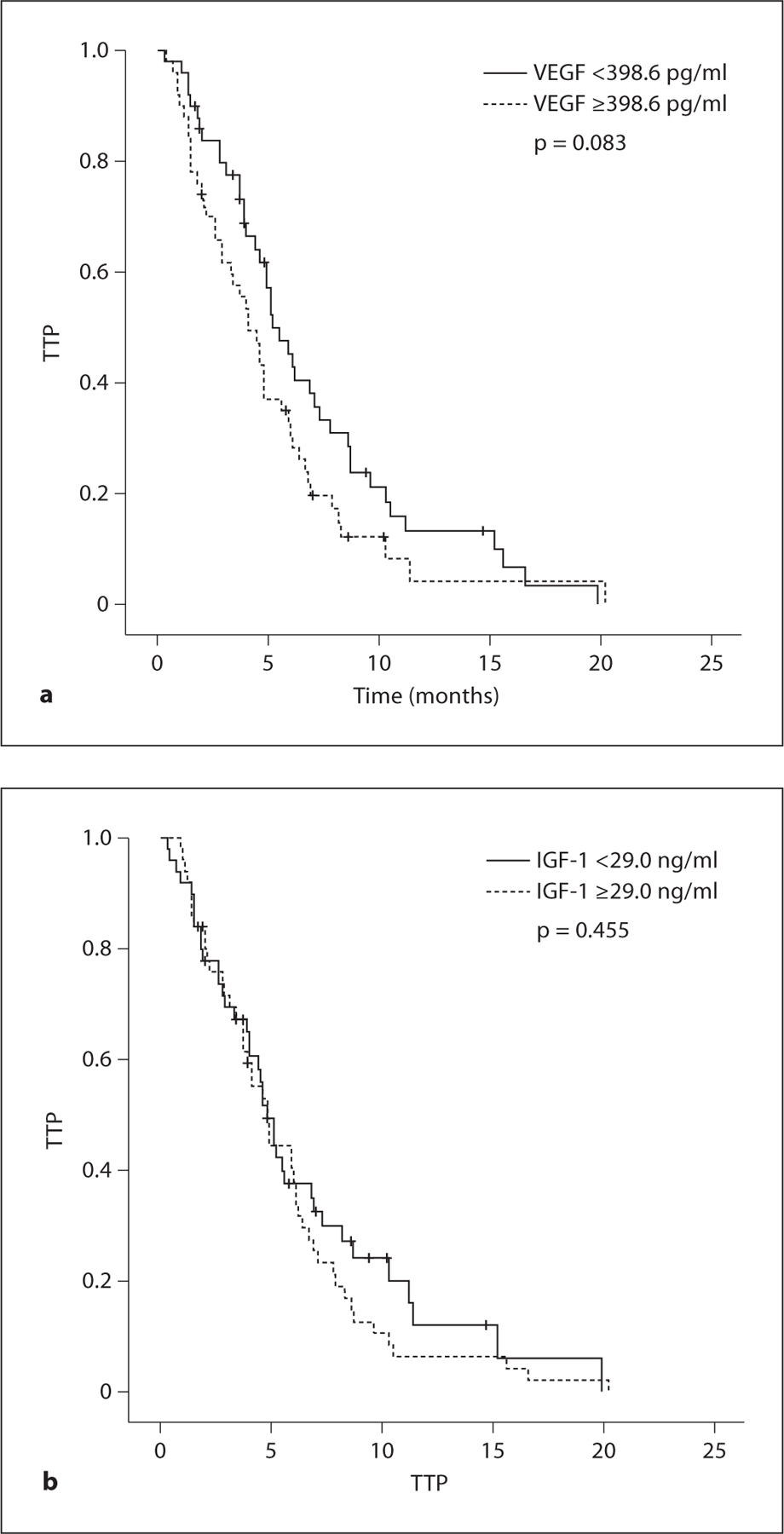
TTP curve according to pretreatment serum levels of VEGF (a) and IGF-1 (b).
Fig. 3.
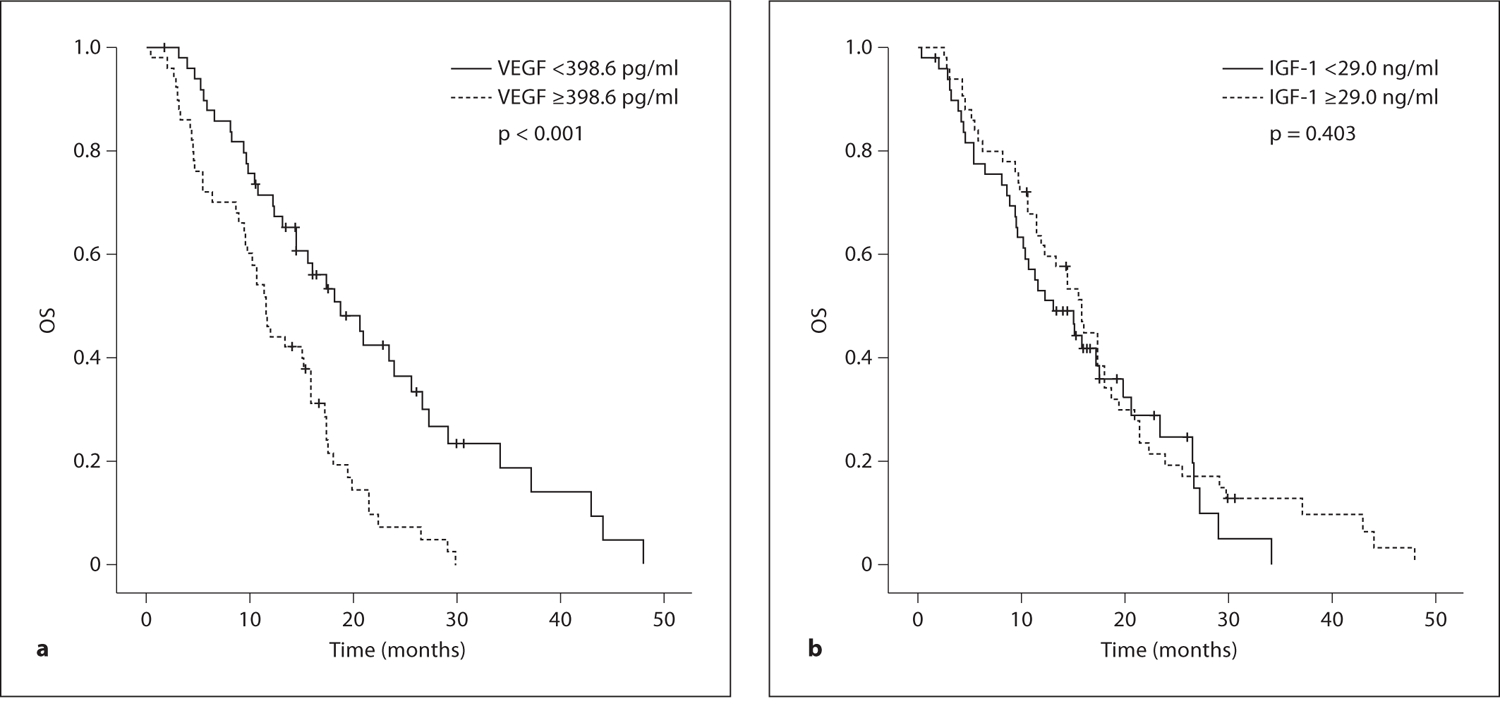
OS curve according to pretreatment serum levels of VEGF (a) and IGF-1 (b).
Factors that had statistical significance in the univariate models were included in the multivariate model. The final multivariable analysis is shown in table 4. In the multivariable analysis, response to chemotherapy (HR 2.000; 95% CI 1.254–3.190, p = 0.004), and number of metastases (HR 1.594; 95% CI 1.194–2.129, p = 0.002) remained as independent prognostic factors for TTP. Serum level of VEGF was the only significant independent prognostic factor which had an impact on OS (HR 2.221; 95% CI 1.377–3.583, p = 0.001).
Table 4.
Multivariate analysis
| TTP |
OS |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.550 | 0.965–2.489 | 0.070 | 1.354 | 0.841–2.180 | 0.212 |
| Response | 2.000 | 1.254–3.190 | 0.004 | 1.512 | 0.934–2.449 | 0.092 |
| Number of metastases | 1.594 | 1.194–2.129 | 0.002 | 1.108 | 0.836–1.469 | 0.477 |
| VEGF | 1.265 | 0.811–1.975 | 0.300 | 2.221 | 1.377–3.583 | 0.001 |
Discussion
The FOLFOX regimen is used as an effective palliative treatment for gastric cancer [5, 6] . We previously reported on the effectiveness of oxaliplatin with biweekly low doses of leucovorin and bolus/continuous infusion of 5-FU (modified FOLFOX 4) as a first-line therapy in advanced gastric cancer patients and found a response rate of 50.0%, a median TTP of 7.7 months, and a median OS time of 11.2 months [5]. We reported that immunohistochemical staining for ERCC1 may be useful in the prediction of clinical outcomes in advanced gastric cancer patients treated with modified FOLFOX4 [27]. It was also shown that the GSTM1-positive genotype evidenced a significantly better TTP in cases of advanced gastric cancer being treated with FOLFOX [28].
Even though this study had limitations that include the lack of a control group, the aim of our study was to evaluate the clinical significance of serum levels of VEGF and IGF-1 in patients with advanced gastric cancer being treated with FOLFOX. The serum assay using ELISA can be frequently and easily performed because it is a noninvasive method in terms of obtaining serum samples in contrast to surgically obtained tissue materials, which might make it useful in monitoring the course of disease or response to treatment.
The prognostic impact of serum VEGF levels in gastric cancer patients has been evaluated in a few studies. Patients with advanced stage and metastases of gastric cancer have higher serum VEGF levels than those with a localized tumor [11, 13, 15–18]. Most studies regarding VEGF have tried to predict the surgical outcome. One study reported a significant association between preoperative serum VEGF levels and OS, with VEGF being an independent prognostic factor in multivariable analysis [16]. That analysis, however, included 42 patients with radically resected gastric cancer and 16 with unresectable tumors undergoing palliative bypass surgery. A large study also showed that high preoperative VEFG concentrations were associated with reduced OS with respect to patients with gastric carcinoma who had lower VEGF serum values, and suggested a biologically relevant role for serum VEGF concentration in patients with gastric cancer [15]. Another study revealed the correlation between serum VEGF per platelet count with shorter progression-free survival and OS [18].
With respect to medically treated gastric cancer patients, Kitamura et al. [17] reported a decrease in the serum VEGF concentration after partial response by chemotherapy; the patients who had disease progression after chemotherapy showed an increase in VEGF levels [17]. We analyzed the association between pretreatment serum levels of VEGF and tumor response to FOLFOX chemotherapy. The pretreatment serum marker measured was not significantly correlated with response (p = 0.114). In 31 patients, serum VEGF was sequentially examined between pre- and postchemotherapy. A decrease in the median serum level of VEGF was observed after FOLFOX chemotherapy (p = 0.034). The median serum level of VEGF was slightly decreased in patients with tumor response, but the differences were not statistically significant (p = 0.071). Furthermore, we found a significant association between the preoperative serum VEGF level and OS, with a serum level of VEGF acting as an independent prognostic factor in multivariate analysis (HR 2.221; 95% CI 1.377–3.583, p = 0.001).
The interaction between the IGF/IGF-IR axis and the VEGF/VEGFR system in cancer has been reported. It has been demonstrated that both the expression of VEGF-A and vessel density in colon tumors depend on the levels of serum IGF-1 [29], and that IGF-1 induces the expression of VEGF-A, which can promote the progression of cancer by regulating neovascularization [30]. Autocrine activation of the IGF-IR axis also significantly affects VEGF-A expression and angiogenesis in human pancreatic cancer [31]. IGF-1R is involved in angiogenesis and lymphagiogenesis through the modulation of VEGF ligand expression in the gastric cancer cell line [32].
Since there are only few preliminary data in the available literature, the significance of IGF in gastric cancer remains undefined. A relevant study reported that there were no differences between IGF-1, IGF-2, and IGFBP-3 levels in stomach cancer cases and matched controls [25]. Another study in Korea examined the change in serum IGF-1 and IGF-2 levels in 20 stomach cancer cases after surgery using blood samples obtained within 10 days before and once after surgery [26]. The serum concentrations of IGF-1 and IGF-2 were significantly lower after surgery, but both pre- and postoperative serum concentrations were still higher than the ones obtained from age- and sex-matched controls. Recently, there was a report stating that genetic polymorphism of IGF-1 may have a substantial effect on the recurrence of cancer in gastric cancer patients who have undergone curative resection [33].
Several studies have provided information on the serum levels of IGF and chemotherapy responses in lung cancer [24, 34]. Izycki et al. [34] evaluated the influence of chemotherapy on the serum levels of IGF-1 and IGF-2 in patients with advanced non-small cell lung cancer and found no significant differences in IGFs levels both before and after chemotherapy [34]. Another study showed that the median plasma levels of IGF-1, IGF-2, and IGFBP-3 were slightly increased in patients with a partial response to chemotherapy, though the changes were not statistically significant [24]. We evaluated the serum levels of IGF-1 and their association with the prognosis in patients with advanced gastric cancer who underwent FOLFOX chemotherapy. However, we did not demonstrate any statistical significance of IGF-1 in clinical outcomes. The median levels of serum IGF-1 were not different between responders and nonresponders (p = 0.508), and the serum level of IGF-1 was not significantly correlated with TTP (p = 0.455) or OS (p = 0.403).
Conclusion
We were able to find a correlation between clinical outcome and pretreatment levels of serum VEGF. We suggest that levels of serum VEGF may be useful in prediction of the OS of advanced gastric cancer patients who were treated with FOFLOX. However, because of the small groups of patients enrolled into this study, further large collaborative studies are necessary to confirm our results.
Acknowledgements
This paper was supported by the Dong-A University Research Fund.
Footnotes
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P: Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, Lee JS: Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat 2011;43:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE: Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–2909. [DOI] [PubMed] [Google Scholar]
- 4.Pasini F, Fraccon AP, G De Mazoni G: The role of chemotherapy in metastatic gastric cancer. Anticancer Res 2011;31:3543–3554. [PubMed] [Google Scholar]
- 5.Oh SY, Kwon HC, Seo BG, Kim SH, Kim JS, Kim HJ: A phase II study of oxaliplatin with low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) as first line therapy for patients with advanced gastric cancer. Acta Oncol 2007;46:336–341. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Hong J, Sym SJ, Park SH, Park J, Cho EK, Lee JH, Shin DB: Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX-4) combination chemotherapy as a salvage treatment in advanced gastric cancer. Cancer Res Treat 2010;42:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang XJ, Zhang L, Qiu F, Yu F, Zhan ZY, Feng M, Yan J, Zhao JG, Xiong JP: A phase II study of capecitabine plus oxaliplatin as first-line chemotherapy in elderly patients with advanced gastric cancer. Chemotherapy 2012;58:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25. [DOI] [PubMed] [Google Scholar]
- 9.Eroglu A: Serum levels of vascular endothelial growth factor in gastric cancer patients. J Surg Oncol 2011;104:222. [DOI] [PubMed] [Google Scholar]
- 10.Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T: Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol 1997;15:826–832. [DOI] [PubMed] [Google Scholar]
- 11.Peng L, Zhan P, Zhou Y, Fang W, Zhao P, Zheng Y, Xu N: Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep 2012;39:9473–9484. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M: Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858–863. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Li T, Wu Y, He L, Zhang L, Shi T, Yi Z, Liu M, Pang X: Prognostic significance of vascular endothelial growth factor expression in gastric carcinoma: a meta-analysis. J Cancer Res Clin Oncol 2011;137:1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon RT, Fan ST, Wong J: Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 2001;19:1207–1225. [DOI] [PubMed] [Google Scholar]
- 15.Vidal O, Metges JP, Elizalde I, Valentini M, Volant A, Molina R, Castells A, Pera M: High preoperative serum vascular endothelial growth factor levels predict poor clinical outcome after curative resection of gastric cancer. Br J Surg 2009;96:1443–1451. [DOI] [PubMed] [Google Scholar]
- 16.Karayiannakis AJ, Syrigos KN, Zbar A, Baibas N, Polychronidis A, Simopoulos C, Karatzas G: Clinical significance of preoperative serum vascular endothelial growth factor levels in patients with colorectal cancer and the effect of tumor surgery. Surgery 2002;131:548–555. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura M, Toi M, Arai K, Iwasaki Y, Suzuki H, Matsuo K: Concentrations of vascular endothelial growth factor in the sera of gastric cancer patients. Oncol Rep 1998;5: 1419–1424. [DOI] [PubMed] [Google Scholar]
- 18.Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, Kim YH, Kim JS: Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol 2010;40:1147–1153. [DOI] [PubMed] [Google Scholar]
- 19.Le Roith D: Seminars in medicine of the Beth Israel Deaconess Medical Center: insulin-like growth factors. N Engl J Med 1997;336: 633–640. [DOI] [PubMed] [Google Scholar]
- 20.Samani AA, Yakar S, LeRoith D, Brodt P: The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007;28:20–47. [DOI] [PubMed] [Google Scholar]
- 21.Pollak M: The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–169. [DOI] [PubMed] [Google Scholar]
- 22.LeRoith D, Roberts CT Jr: The insulin-like growth factor system and cancer. Cancer Lett 2003;195:127–137. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Kim SJ, Lee YC: Serum insulin-like growth factor (IGF)-I and IGF-binding proteins in lung cancer patients. J Korean Med Sci 1999;14:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han JY, Choi BG, Choi JY, Lee SY, Ju SY: The prognostic significance of pretreatment plasma levels of insulin-like growth factor (IGF)-1, IGF-2, and IGF binding protein-3 in patients with advanced non-small cell lung cancer. Lung Cancer 2006;54:227–234. [DOI] [PubMed] [Google Scholar]
- 25.Pham TM, Fujino Y, Kikuchi S, Tamakoshi A, Yatsuya H, Matsuda S, Yoshimura T: A nested case-control study of stomach cancer and serum insulin-like growth factor (IGF)-1, IGF-2 and IGF-binding protein (IGFBP)-3. Eur J Cancer 2007;43:1611–1616. [DOI] [PubMed] [Google Scholar]
- 26.Lee DY, Yang DH, Kang CW, Kim SJ, Joo CU, Cho SC, Kim JS: Serum insulin-like growth factors (IGFs) and IGF binding protein (IGFBP)-3 in patients with gastric cancer: IGFBP-3 protease activity induced by surgery. J Korean Med Sci 1997;12:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, Kim HJ: Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol 2007;18: 504–509. [DOI] [PubMed] [Google Scholar]
- 28.Seo BG, Kwon HC, Oh SY, Lee S, Kim SG, Kim SH, Han H, Kim HJ: Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep 2009;22:127–136. [PubMed] [Google Scholar]
- 29.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D: Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res 2002;62:1030–1035. [PubMed] [Google Scholar]
- 30.Akagi Y, Liu W, Zebrowski B, Xie K, Ellis LM: Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res 1998;58:4008–4014. [PubMed] [Google Scholar]
- 31.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, Evans DB, Semenza GL, Ellis LM: Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol 2003;163:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Adachi Y, Yamamoto H, Min Y, Ohashi H, Ii M, Arimura Y, Endo T, Lee CT, Carbone DP, Imai K, Shinomura Y: Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer 2011; 117: 3135–3147. [DOI] [PubMed] [Google Scholar]
- 33.Shitara K, Ito S, Misawa K, Ito Y, Ito H, Hosono S, Watanabe M, Tajima K, Tanaka H, Muro K, Matsuo K: Genetic polymorphism of IGF-I predicts recurrence in patients with gastric cancer who have undergone curative gastrectomy. Ann Oncol 2012;23:659–664. [DOI] [PubMed] [Google Scholar]
- 34.Izycki T, Chyczewska E, Naumnik W, Talalaj J, Panek B, Ossolinska M: Serum levels of IGF-I and IGF-II in patients with lung cancer during chemotherapy. Exp Oncol 2004;26: 316–319. [PubMed] [Google Scholar]


