ABSTRACT
Staphylococcus aureus is associated with the development of persistent and severe inflammatory diseases of the upper airways. Yet, S. aureus is also carried asymptomatically in the sinonasal cavity of ∼50% of healthy adults. The causes of this duality and host and microbial factors that tip the balance between S. aureus pathogenesis and commensalism are poorly understood. We have shown that by degrading mucins, anaerobic microbiota support the growth of airway pathogens by liberating metabolites that are otherwise unavailable. Given the widely reported culture-based detection of anaerobes from individuals with chronic rhinosinusitis (CRS), here we tested our hypothesis that CRS microbiota is characterized by a mucin-degrading phenotype that alters S. aureus physiology. Using 16S rRNA gene sequencing, we indeed observed an increased prevalence and abundance of anaerobes in CRS relative to non-CRS controls. PICRUSt2-based functional predictions suggested increased mucin degradation potential among CRS microbiota that was confirmed by direct enrichment culture. Prevotella, Fusobacterium, and Streptococcus comprised a core mucin-degrading community across CRS subjects that generated a nutrient pool that augmented S. aureus growth on mucin as a carbon source. Finally, using transcriptome sequencing (RNA-seq), we observed that S. aureus transcription is profoundly altered in the presence of mucin-derived metabolites, though expression of several key metabolism- and virulence-associated pathways varied between CRS-derived bacterial communities. Together, these data support a model in which S. aureus metabolism and virulence in the upper airways are dependent upon the composition of cocolonizing microbiota and the metabolites they exchange.
KEYWORDS: Staphylococcus aureus, anaerobes, cross-feeding, mucin, sinusitis
INTRODUCTION
Chronic rhinosinusitis (CRS) is a heterogeneous inflammatory disease of the upper airways that affects 2 to 13% of the U.S. population (1). Despite its prevalence, the complex pathogenesis of CRS is poorly understood. The disease has been linked to anatomic variation, immune dysfunction, host genetics, and microbial dysbiosis (2), but the importance of these factors and the order in which they occur remain controversial (3, 4). The consensus is that bacterial infection is associated with CRS pathogenesis; thus, antibiotic therapy remains the standard of care (2). However, given the low efficacy of conventional treatment regimens and rising concerns about multidrug resistance, there is a critical need to understand the precise role(s) of microbiota in CRS and to identify new therapeutic strategies.
Staphylococcus aureus is recognized as a primary pathogen of CRS and is commonly isolated by clinical culture (5, 6). Among these isolates, anywhere from 20 to 60% have been shown to be methicillin resistant (7, 8). Paradoxically, S. aureus also resides, persistently or intermittently, in the sinonasal cavity of up to 50% of adults without complications (9). Individuals colonized by S. aureus tend to retain the same strain over time (10), and many S. aureus infections (CRS and otherwise) are caused by those same strains carried asymptomatically (11). Interestingly, S. aureus prevalence and abundance between CRS and non-CRS controls vary widely between studies (5, 6, 12, 13). These conflicting reports may suggest that host and environmental variables, not bacterial overgrowth, are determinants of the commensal versus pathogenic lifestyle of S. aureus within the upper airways.
S. aureus pathogenesis can be attributed to its repertoire of virulence factors, whose expression is dependent on complex regulatory networks and diverse environmental stimuli (14, 15). Interactions with cocolonizing microbiota can also affect S. aureus pathogenicity, particularly in the upper airways, where S. aureus thrives as part of a complex community. For example, Cutibacterium spp. can inhibit S. aureus growth through fermentation of glycerol into short-chain fatty acids (SCFAs) or decrease virulence via extracellular porphyrins (16, 17). Similarly, Corynebacterium spp. restrict S. aureus pathogenesis through bactericidal activity (18) or by suppressing virulence factor expression (19). Early culture-based studies found an association between facultative and obligate anaerobic bacteria with CRS cases that were refractory to antibiotic therapy (20, 21, 73). However, mechanistic contributions of anaerobes to CRS pathogenesis, either directly or through interactions with S. aureus, have not yet been identified.
We recently demonstrated that commensal microbiota potentiate the growth of airway pathogens through degradation of mucin glycoproteins (22). Specifically, anaerobe-mediated degradation and fermentation of mucin generate amino acids and short-chain fatty acids (SCFAs) that Pseudomonas aeruginosa can then use as nutrient sources. Building on our prior work and reports of hypoxia (23) and increased mucin expression in CRS (24), here we tested the hypothesis that CRS microbiota are characterized by an anaerobic mucin-degrading phenotype. Using 16S rRNA gene sequencing (RNA-seq), we found that S. aureus abundance did not differentiate CRS mucus from that of non-CRS controls, as expected. However, a significant decrease in Actinobacteria (Corynebacterium and Cutibacterium) in CRS was observed that inversely correlated with an increase in Bacteroidetes (Prevotella), and Fusobacteria. We predicted, in silico, that CRS anaerobes comprised mucin-degrading consortia and further demonstrated this activity in vitro using enrichment culturing. We then hypothesized that CRS-associated mucin degraders alter the nutritional landscape of the sinuses and drive a shift in S. aureus physiology from commensalism to pathogenesis. Indeed, using RNA-seq, we discovered that by converting mucins into bioavailable substrates, anaerobic mucin-degrading bacterial consortia differentially modulate gene expression in S. aureus, including several genes associated with central metabolism and virulence. Collectively, this work reveals that microbiota composition and function (i.e., mucin degradation capacity) may have critical implications for the onset, progression, and treatment of S. aureus-associated upper airway disease.
RESULTS
CRS is associated with an anaerobic mucin-degrading phenotype.
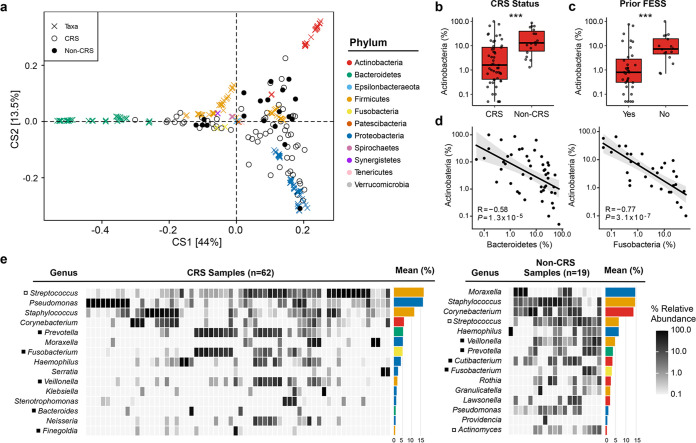
To first test our hypothesis that CRS sinuses harbor a distinct bacterial community composition and increased abundance of anaerobic microbiota, we collected sinus mucus from CRS (n = 62) and non-CRS (n = 19) subjects undergoing functional endoscopic sinus surgery (FESS). The bacterial composition of each sample was described using taxonomy derived from amplicon sequence variants (ASVs) generated from 16S rRNA gene sequences. A comparison of bacterial communities using double principal-coordinate analysis (DPCoA) revealed considerable within-group variation among CRS microbiota (Fig. 1a). With exceptions, non-CRS samples clustered more closely than CRS samples. Forty-four percent of variation was along the first axis, which demonstrates that CRS bacterial communities deviate from non-CRS communities based on the presence/absence of Actinobacteria, Bacteroidetes, and Fusobacteria. Indeed, greater relative abundance of Actinobacteria was associated with non-CRS samples (P < 0.001) (Fig. 1b). Actinobacterial abundance was also higher in CRS subjects with no history of FESS (P = 0.001) (Fig. 1c), suggesting an association with earlier stages of disease. Actinobacteria also exhibited an inverse relationship with the relative abundance of both Bacteroidetes and Fusobacteria (P < 0.001) (Fig. 1d). Second axis variance (13.5%) (Fig. 1a) was primarily driven by Proteobacteria. These analyses demonstrate that while CRS and non-CRS share many of the same bacterial taxa, deviations between groups occur at the phylum level.
FIG 1.
Bacterial community compositions differ between CRS and non-CRS. (a) DPCoA ordination biplot shows dissimilarity in presence and abundance of bacteria in CRS and non-CRS samples. (b) Percentage of abundance of actinobacteria is associated with non-CRS status (Wilcoxon, ***, P < 0.001; CRS, n = 51; non-CRS, n = 18). (c) Among CRS samples, the percentage of abundance of Actinobacteria is associated with no prior FESS treatment (Wilcoxon, ***, P < 0.001; Yes, n = 35; No, n = 16). (d) Spearman’s correlation of percentage of abundance of Actinobacteria negatively correlates with percentages of abundance of both Bacteroidetes and Fusobacteria. (e) Heat map representation of the top 15 genera in each sample group (CRS and non-CRS). Closed boxes (■) represent obligate anaerobic genera, and open boxes (□) denote genera with obligate anaerobic species. Colors throughout the figure match the color key for panel a.
Identification of obligate anaerobes in CRS has previously been hindered by cultivability. Thus, we leveraged the resolution of amplicon sequence variants (ASVs) to provide a detailed characterization of sinus bacterial diversity (Fig. 1e; see Table S1 in the supplemental material). We identified 397 ASVs across all samples, 43% of which were assigned to the species level. Species-level ASV database matches confirmed the high prevalence and abundance of several anaerobic species described in prior culture-based studies (e.g., Prevotella melaninogenica, Fusobacterium nucleatum, Streptococcus pneumoniae), though we also identified additional taxa previously unreported to be associated with CRS (e.g., Fusobacterium periodonticum and Prevotella salivae).
Despite the increased resolution of ASVs over conventional operational taxonomic unit (OTU)-based approaches, classification of many taxa remained restricted to the genus level. This included Staphylococcus, whose abundance did not appreciably differ between CRS (11.7%) and non-CRS (14.7%) samples, supporting our hypothesis that environmental variables, not prevalence or bacterial overgrowth, are determinants of S. aureus pathogenicity. In agreement with the DPCoA (Fig. 1a), non-CRS samples were distinguished by Actinobacteria—Corynebacterium (13.9% in non-CRS versus 6% in CRS), Cutibacterium (3.7% versus 1.1%), and Actinomyces (1.2% versus 0.2%)—consistent with their roles as commensal inhabitants of the upper airways, while attenuating S. aureus virulence (16–19, 25). ASVs classified as Pseudomonas comprised the second most abundant CRS genus, yet were not abundant in non-CRS samples, consistent with the role of Pseudomonas as an airway pathogen. Most notable, however, was that Streptococcus was highly abundant in CRS, at an average relative abundance of 16.9%, compared to 6.8% in non-CRS subjects. Prevotella and Fusobacterium, both obligate anaerobes, were also more abundant in CRS. Of those CRS samples where Staphylococcus was observed, 40% showed copresence of Prevotella, Fusobacterium, and Streptococcus, in contrast to 18% of non-CRS samples (chi-square test, P = 0.205). While differences in relative abundance between subject groups did not reach statistical significance due to the heterogeneity of the sample set, our data suggest that communities consisting of these four genera (Staphylococcus, Prevotella, Fusobacterium, and Streptococcus) are not rare and that the CRS microenvironment favors loss of commensal taxa and growth of facultative and obligate anaerobes.
Given the association of Streptococcus, Fusobacterium, and Prevotella with oral and gut bacterial communities known for metabolizing host-derived glycoproteins (26, 27), we then hypothesized that CRS microbiota would exhibit the functional capacity to degrade mucins, whose expression is increased in CRS (24). To test this hypothesis, we used PICRUSt2 (28) to predict metagenomic content based on 16S rRNA gene sequencing data, which was then summarized by enzyme category (EC) numbers and compared to the Carbohydrate Active Enzyme (CAZy) database (29). Selection of the top CAZy ECs by relative functional abundance revealed that glycoside hydrolases (GHs) and polysaccharide lyases (PLs) were more prevalent in CRS predicted metagenomes (Fig. 2a), including many with previously defined roles in mucin degradation (β-glucuronidase, β-galactosidase, hyaluronate lyase, exo-α-sialidase, and chitinase) (27, 30–32). In contrast, metagenomes predicted from non-CRS samples were characterized by the presence of ECs with glycotransferase (GT) and carbohydrate esterase (CE) functions (Fig. 2b). Mucinase capacity was also clearly present among non-CRS bacterial communities, though our analyses are predictive of CRS microbiota carrying far more potential for cleavage of glycosidic bonds and degradation of mucin oligosaccharides.
FIG 2.
PICRUSt2-predicted metagenomes reveal increased mucin-degrading capacity of CRS microbiota relative to non-CRS. Enyzme classes (ECs) and their corresponding CAZy database classifications (auxiliary activity [AA], carbohydrate esterase [CE], glycoside hydrolases [GH], glycoside transferase [GT], and polysaccharides lyase [PL]) were identified in PICRUSt2-predicted metagenomes. The average relative functional abundances of each CAZy enzyme class contributed by the top 10 taxa in (a) CRS and (b) non-CRS samples are shown. Data are sorted by median relative functional abundance.
To validate our metagenome predictions, and to demonstrate that CRS microbiota retain mucin degradation capacity in vivo, we used an anaerobic (nonrespiratory) enrichment scheme in which an additional set (n = 8) of sinus mucus samples were serially passaged in a defined growth medium containing purified mucin (MUC5AC) as the carbon source (minimal mucin medium [MMM]) (22). Due to limited sample volumes of non-CRS subjects, our analyses were limited to CRS subjects at various stages of disease severity (see Table S2 in the supplemental material). After two passages of 48 h each, bacterial compositions pre- and postenrichment were profiled. Similar to our initial sequencing data (Fig. 1), CRS subjects harbored diverse bacterial communities, often dominated by a single recognized colonizer of the upper airways (Fig. 3a). Postenrichment, cultures were similar between patients, even when the original community memberships differed (Fig. 3c), suggesting a core mucin-degrading bacterial consortium. Indeed, the mean relative abundances of Prevotella (19.5%), Veillonella (19.7%), and Streptococcus (15.3%) all increased across samples (Fig. 3b). Interestingly, the weakly saccharolytic genus Fusobacterium (13.3%) also increased, suggesting that some members of the enriched communities may be supported by interspecies cross-feeding.
FIG 3.
Anaerobic enrichment culturing of sinus mucus reveals a core mucin-degrading community. (a) Relative abundance of ASVs grouped at the genus level for each original sample and enrichment culture. FESS samples for D and F fell below 2,000 sequences but are included here for comparison. (b) Mean relative abundances for the top 20 genera in original sinus mucus samples and enrichment cultures. (c) DPCoA biplot comparing proportional data from original samples and enrichment cultures with associated phyla. (d) FPLC chromatogram of high-molecular-weight mucin proteins in MMM and CFS from 48-h CRS mucin enrichment cultures. Closed boxes (■) represent obligate anaerobic genera, and open boxes (□) denote genera with obligate anaerobic species.
Fast protein liquid chromatography (FPLC) was also used to assess integrity of high-molecular-weight mucins included in the enrichment medium. As predicted, chromatograms show that the peak area derived from each bacterial community was markedly decreased (Fig. 3d), reflecting reduced mucin integrity as a result of enrichment. While the enriched communities represent only a subset of sinus microbiota, these data support our hypothesis that anaerobic bacteria can alter the CRS microenvironment via degradation and fermentation of mucin glycoproteins.
S. aureus growth on mucin is inefficient and respiration dependent.
When Staphylococcus ASVs were present in the original CRS mucus sample (subjects B to F and H), they were notably absent from the enrichment (Fig. 3a and b). This suggests that Staphylococcus spp. are either (i) inhibited by enrichment communities, (ii) unable to efficiently use mucins or degradation by-products as nutrients, or (iii) unable to do so under oxygen limitation, despite fermentative capacity. To test these possibilities, S. aureus USA300 LAC and three CRS-derived clinical isolates (MN222, MN236, and MN239) were first grown aerobically in MMM (Fig. 4a). Growth of all strains was limited on MMM alone, suggesting that the metabolic requirements of S. aureus are not met by intact high-molecular-weight mucins. However, growth rate and yield significantly increased when MMM was supplemented with Casamino Acids, lactate, and glucose, which mimic metabolites generated via mucin degradation and fermentation (Fig. 4a). Growth on these supplements was restricted under fermentative conditions but, with the exception of strain MN222, was partially restored with the addition of sodium nitrate as an electron acceptor (Fig. 4b and c; see Fig. S1 in the supplemental material). From these data, we conclude that while S. aureus does not efficiently use mucins as a nutrient source, in vitro conditions support growth when additional nutrients are supplied, particularly under respiratory conditions.
FIG 4.
S. aureus growth is limited on mucin alone but restored by mucin-derived metabolites. (a) S. aureus LAC and three CRS-derived clinical isolates grown in minimal mucin medium (MMM), amended with 0.5% Casamino Acids (MMMC), 0.5% lactate (MMML), 0.25% glucose (MMMG), or a combination of these (MMMLC and MMMGC). Growth curves were measured under aerobic conditions; error bars represent the mean and standard deviation (SD) for n = 3. (b and c) Growth of LAC measured at OD600 after 24 h of growth under (b) anaerobic conditions and (c) anaerobic conditions with 3 mM nitrate. The data shown are the mean of n = 6 (MMM, MMMC, MMML, and MMMLC) and n = 3 (MMMG and MMMGC) biological replicates. Error bars are SD. Significance relative to MMM was determined by t test with Holm-Bonferroni adjustment. ***, P < 0.001.
Secondary metabolites from mucin degradation support S. aureus growth.
To test whether mucin degradation and fermentation by other CRS microbiota could provide bioavailable nutrients to S. aureus and/or to determine if S. aureus was inhibited by cocolonizing bacteria, strains were cultured on cell-free supernatants (CFSs) derived from enrichment samples (A to H) (Fig. 3a). Under aerobic conditions, S. aureus exhibited equal or improved growth rate and yield in nearly all CFSs, despite having less total carbon in the growth medium (i.e., it was removed by anaerobes during enrichment) (Fig. 5a; see Fig. S2 in the supplemental material). Interestingly, communities A, E, and G, which generated CFSs supporting low S. aureus growth yields, were enriched from samples in which Staphylococcus ASVs were absent or at very low abundance (see Fig. 3a).
FIG 5.
S. aureus growth on cell-free supernatants from CRS mucin-degrading communities. (a) S. aureus LAC aerobic growth on CFS from CRS-derived mucin-degrading communities relative to intact mucin alone (MMM [black]). (b) S. aureus anaerobic growth on CFS with and without sodium nitrate (3 mM). (c) HPLC quantification of mixed-acid fermentation metabolite concentrations (mM) in CFS. x, not detectable; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Fermentative growth yields after 24 h resulted in a similar pattern, signifying the presence of fermentable metabolites (e.g., galactose) derived from anaerobe-mediated degradation of mucin glycans (Fig. 5b). As before, addition of sodium nitrate (3 mM) resulted in increased growth yields under all conditions (Fig. 5c). These data demonstrate that both aerobic growth and anaerobic growth of S. aureus are supported by metabolites liberated or produced by CRS-derived mucin-degrading bacteria.
To identify metabolites exchanged between CRS communities and S. aureus, we performed targeted analyses of CFS using high-performance liquid chromatography (HPLC). Based on recent work demonstrating that mixed-acid fermentation metabolites can both stimulate and inhibit growth of S. aureus in vitro (33–35), we reasoned that SCFAs (acetate, propionate, butyrate, formate, and isovalerate), lactate, succinate, and pyruvate may account for the observed differences in S. aureus growth between enrichments. As expected, fermentative by-products were present, yet highly variable across supernatants. (Succinate and isovalerate were below the detection limit of the instrument.) Levels of acetate, propionate, and butyrate, each known to either be a nonpreferred carbon source or have an inhibitory effect on S. aureus growth, were low among CFSs, supporting higher growth yields. Yet, they were also low in CFS-A, which supported little additional growth over MMM alone. Similarly, formate and pyruvate were detectable in each sample, though could not explain differences in S. aureus growth phenotypes between supernatants. Based on these data, we conclude that additional metabolites resulting from mucin degradation likely influenced S. aureus growth under our in vitro conditions.
Mucin degradation supernatants influence S. aureus transcription.
To gain additional insight into anaerobe-S. aureus metabolite exchange, we used RNA-seq to profile S. aureus gene expression during growth on CFS. CFS samples D, F, and H were selected for their consistent ability to support S. aureus growth under both aerobic and anaerobic conditions (Fig. 5b). Gene expression was compared to growth on MMM supplemented with glucose (MMMG), a preferred carbon source of S. aureus (36).
A grouped MA plot representing differential gene expression in all three supernatants relative to MMMG is shown in Fig. 6a (individual supernatants are shown in Fig. S3 in the supplemental material). Across samples, 93 genes (79 upregulated and 14 downregulated) were differentially expressed (2-fold or greater; P < 0.05). Common to all supernatants was increased expression of genes in the nan locus involved in transport and catabolism of sialic acid (N-acetylneuraminic acid [Neu5Ac]) (37), indicating that sialic acids are liberated by CRS mucin degraders and are a likely source of carbon and nitrogen for S. aureus (Fig. 6a and b). glcC was also highly expressed, which encodes a phosphotransferase system (PTS)-dependent glucose transporter with specificity for ManNAc, a product of bacterial Neu5Ac cleavage. Interestingly, there was increased expression of NAD-dependent formate dehydrogenase (fdh) and formyltetrahydrofolate synthetase (fhs), implying formate metabolism. acsA, involved in conversion of acetate to acetyl coenzyme A (acetyl-CoA) was also significantly increased (Fig. 6b), but not the reverse process (pta and ackA). These data, supported by HPLC analyses (Fig. 5c), demonstrate that mixed-acid fermentation metabolites (formate and acetate) are likely provided exogenously to S. aureus as a result of mucin degradation.
FIG 6.
S. aureus transcription is altered by mucin degradation and is dependent on cocolonizing microbiota. (a) MA plot shows differential expression of genes (79 upregulated and 14 downregulated) in cell-free supernatant conditions from communities D, F, and H compared to MMMG controls. The Wald test was used to assign significance for genes with a fold change (|FC|) of >2, and false-discovery rate (FDR)-adjusted P value of <0.05. (b and c) Transcripts involved in sialic acid degradation and central metabolism were differentially increased in expression under supernatant conditions compared to glucose. (d) Heat map of select genes with significant differences between each cell-free supernatant condition. Significance was determined using the likelihood ratio test, keeping only genes with a log fold change (|LFC|) of >2 and FDR-adjusted P value of <1 × 10−10. The data presented are differences from the mean for each gene calculated from regularized log-transformed counts. All data are representative of three biological replicates per condition. Panel b was modified from reference 33.
Genes associated with glutamate import (gltS) and synthesis via arginine and proline (rocF, rocD, rocA, and putA) were also upregulated (Fig. 6a and c) (38). Increased expression of gudB signifies glutamate conversion to 2-oxoglutarate for entry into the tricarboxylic acid (TCA) cycle. Expression of genes associated with the reductive branch of the TCA cycle and gluconeogenesis (sucA, sucC, sdhA, fumC, mqo1, and pckA) were also increased, indicating that amino acids may be fueling gluconeogenesis. These data also support the importance of glutamate (and its synthesis from proline and arginine) in the absence of preferred carbon sources (e.g., glucose) that are thought to be limiting in the sinonasal cavity (38, 39). Other functional categories of genes consistently expressed across supernatants included peptidoglycan recycling (murQ), fatty acid metabolism (fadA, fadB, fadD, fadE, and fadX), biotin biosynthesis (bioA, bioB, bioD, bioF, bioW, and bioY), and virulence (spa and RS09510, a gene encoding a putative immunoglobulin-blocking virulence protein).
Interestingly, several transcripts were differentially expressed between supernatants. Hierarchical clustering revealed that the S. aureus transcriptome from CFS-H was distinct from those of CFS-D and CFS-F (Fig. 6d). Transcripts associated with amino acid transport and biosynthesis were more abundant in CFS-D/F. Of note was expression of the ilv-leu operon involved in branched-chain amino acid (BCAA) synthesis, accompanied by increased expression of genes encoding ɑ-acetolactate decarboxylase (budA) and ɑ-acetolactate synthase (budB). Genes required for galactose import (lacCDEF) were also upregulated in CFS-D/F, suggestive of its availability in the supernatants. Indeed, lactose/galactose availability via mucin degradation in the airways has been previously suggested (39, 40). Notably, recent work shows that galactose and branched-chain amino acids support a metabolic state adopted by S. aureus to resist antibiotic and nitrosative stresses (39). Genes involved in cysteine-sulfur-methionine homeostasis (ssuABC and metICFH-mdf operons) and peptide transport (opp3DAF) were also seen at higher levels in CFS-D/F.
Finally, virulence-related gene transcription also differentiated supernatant growth conditions. Notably, transcription of the agr operon, RNAIII (hld), staphopain B (sspB), aureolysin (aur), and immunodominant antigen B (isaB) was significantly higher in CFS-H. Conversely, CFS-D/F supernatants yielded transcriptomes that exhibited more of a biofilm-like phenotype, with increased prevalence of transcripts for holin protein (cidA) and exopolysaccharide (icaABC) (41, 42). Taken together, these data demonstrate that, in addition to supporting S. aureus growth, expression of genes associated with metabolism, virulence, and other cellular processes is highly dependent on the specific composition of cocolonizing microbiota and the metabolites they exchange.
DISCUSSION
S. aureus is consistently associated with CRS development and the inefficacy of clinical therapies. However, S. aureus is also present in the upper airways of ∼50% of healthy individuals (8, 9), suggesting that the sinonasal microenvironment and bacterial activities within tip the balance between commensalism and pathogenesis of S. aureus. Here, we demonstrate striking differences in CRS and non-CRS microbiota and implicate mucin as a nutrient source supporting anaerobic bacterial diversity. Moreover, we provide evidence supporting a role for anaerobic mucin degradation in S. aureus metabolism and pathogenicity in chronic airway disease.
Research spanning decades has described the microbiology of CRS by both culture-based and molecular methods. There is general agreement on the prevalence of canonically pathogenic bacteria, including S. aureus. However, surveying bacterial diversity in this niche is subject to bias in the observation of low abundance or fastidious organisms based on sampling methods, cultivability, and representation in databases for taxonomic assignment. The reported prevalence and abundance of anaerobes in CRS, in particular, have therefore varied across studies (43–45). To address this knowledge gap, we leveraged the increased resolution of ASVs to provide a detailed characterization of CRS bacterial diversity. Species-level identification (i) confirmed previous culture-based identification of anaerobes, (ii) revealed as-yet-unreported species associated with CRS, and (iii) showed that anaerobe proliferation may represent a distinct stage in disease progression. While anaerobes (and thus, mucin degradation capacity) also clearly exist in some non-CRS cases, we favor a model of CRS in which mucus drainage is blocked and oxygen tension is reduced, in turn facilitating anaerobe overgrowth and further conditioning of the sinus microenvironment.
Due to their complex structure, mucins, the major macromolecular constituents of mucus, paradoxically provide mucosal barrier protection while serving as a rich source of nutrients for organisms that can degrade them. In the gut and oral cavity, mucins act as important mediators of microbiota development, including community assembly and spatial organization (26, 46, 47). To our knowledge, this work represents the first characterization of mucin-microbe interactions in the context of CRS. Metagenomic prediction showing increased carbohydrate-active enzyme abundance in CRS bacterial communities led us to hypothesize that mucins support anaerobic bacterial taxa in this niche. Indeed, CRS enrichment cultures were dominated by abundant facultative and obligate anaerobic genera observed in our original sequence data—Streptococcus, Veillonella, Prevotella, and Fusobacterium. These data are consistent with our prior work showing that anaerobic mucin degradation supports P. aeruginosa growth in the cystic fibrosis airways (22). However, in our prior study, oral contamination of sputum during collection could not be ruled out, limiting direct evidence for the presence of anaerobes in the lung. By relying on FESS for sampling, oral contamination issues are circumvented, and this lends further evidence to a systemic role of oral-associated microbiota in chronic disease (48).
A critical aspect of S. aureus’ capacity for colonization and pathogenic potential is its ability to adopt metabolic states given the range of environmental conditions encountered in vivo (38, 39, 49, 50). This is particularly true of the sinuses, where reactive oxygen and nitrogen species, inflammation, altered mucociliary flow, and mucus hypersecretion all contribute to a dynamic microenvironment with fluctuating nutrient and electron acceptor availability (51–54). Glucose, in particular, while a preferred carbon source of S. aureus, has been shown to fluctuate dramatically in the nasal cavity, suggesting the availability of other carbon sources (39). Yet, use of mucins by S. aureus as a nutrient source has not yet been described, and here we show that despite their increased expression in CRS, high-molecular-weight mucins do not directly support robust S. aureus growth. In contrast, lactate, amino acids, and glycolytic substrates have all been implicated in S. aureus survival and pathogenicity (36, 50), and their addition to defined mucin medium supported growth of all strains tested under our experimental conditions. Proposed sources of these metabolites in vivo are host and S. aureus processes such as collagen degradation and carbohydrate fermentation (38, 55). However, given their diverse repertoires of glycolytic and proteolytic enzymes, we predicted and confirmed that cocolonizing anaerobes also provide metabolites via mucin degradation and interspecies cross-feeding.
Interestingly, S. aureus was not recovered by enrichment culturing in the absence of an electron acceptor (e.g., NO3− and O2). Furthermore, some enriched communities (e.g., CFS-A, -E, and -G), despite degrading mucins (Fig. 3d), supported low S. aureus growth yields. These data suggest that antagonistic bacterial interactions (e.g., H2O2 production by S. pneumoniae) (56) or specific environmental conditions may restrict mucin-based cross-feeding support of S. aureus growth. Interbacterial interactions governing S. aureus coexistence with mucin-degrading communities, other members of the sinus microbiota, and/or the in vivo environmental parameters that shape these relationships remain to be determined.
In addition to supporting S. aureus growth, mucin degradation altered metabolism, virulence, and other cellular processes at the transcriptional level. RNA-seq data notably inferred sialic acid transport and catabolism, supporting its role as a nutrient source and providing a competitive edge for S. aureus in the host environment (37). Neuraminidase expression by commensal microbiota (e.g., S. pneumoniae) has previously been shown to release NeuAc from host glycans, making it available to other commensals (74). Here, we show that S. aureus can also capitalize on its bioavailability when liberated by CRS microbiota and when glucose is limiting. Current models of S. aureus metabolism in the host support a strong link between metabolite pool, metabolic state, and virulence (14). In support of these models, expression profiles strikingly revealed upregulation of many of the same pathways deemed important for survival and pathogenic potential, such as glutamate synthesis via arginine and proline. Supernatants also impacted virulence-related gene transcription in distinct ways. For example, genes of the agr locus, a master regulatory system controlling expression of multiple virulence factors, were upregulated only when S. aureus was grown in CFS-H. In contrast, CFS-D/F stimulated expression of genes associated with nitrosative stress tolerance, clumping, adhesion, and biofilm formation, which are also implicated in pathogenicity and reduced efficacy of antibiotic therapy (57). We recognize that the three community-derived supernatants tested here likely underestimate the microbiological heterogeneity among the CRS population and make it difficult to generalize across all subjects with S. aureus infection. We also acknowledge that differences in S. aureus growth rates that occurred between spent supernatants may also contribute to the differential gene expression observed across samples. Despite these limitations, our data suggest that individual bacterial species and the metabolites they produce govern S. aureus physiology in unique ways.
In summary, data presented here support a role for mucin degradation in CRS disease. While direct effects of individual taxa on S. aureus pathogenicity and the mechanistic bases of those interactions remain to be determined, this work informs new hypotheses that can be tested using in vitro and in vivo CRS models. Notably, infection experiments using a recently described sinusitis rabbit model (58) and pairwise combinations of single anaerobes with S. aureus will help elucidate the effect of interspecies interactions on the host. Use of animal models will also help assess the potential role of host-mediated mucin degradation, including sialidases and/or inflammatory proteases, which may also contribute to the bioavailable nutrient pool. While both host and bacterial processes undoubtedly play a role in S. aureus pathogenesis, the continued study of mucin degradation in CRS and other chronic airway disease will likely motivate alternative strategies to existing therapies that are generally ineffective.
MATERIALS AND METHODS
Patient recruitment and sample collection.
Eighty-nine participants with a positive diagnosis of CRS undergoing functional endoscopic sinus surgery (FESS) and 23 participants with no history of CRS, but undergoing unrelated sinonasal surgery, were recruited at the University of Minnesota Department of Otolaryngology. Exclusion criteria were diagnosis of cystic fibrosis, granulomatous polyangiitis, sarcoidosis, or Churg-Strauss syndrome. Informed consent was obtained from all subjects. Prior to surgery, each patient completed a sinonasal outcomes test (SNOT-22) (59). Sinus secretions were obtained from a single maxillary sinus under endoscopic visualization by suction into Argyle mucus traps (Cardinal Health, Dublin, OH) and frozen at −80°C. Clinical data were also obtained for each subject (Table S2). Protocols were approved by the UMN Institutional Review Board (no. 1403M49021).
DNA sequencing and analysis.
Genomic DNA was extracted from 300 μl of mucus using DNeasy Powersoil kits (Qiagen, Carlsbad, CA) and submitted to the UMN Genomics Center (UMGC) for 16S rRNA gene library preparation (60). The V4 region was amplified and sequenced using Illumina MiSeq TruSeq 2 × 300 paired-end technology. Reagent control samples were also submitted but were below detection thresholds.
The initial 16S rRNA gene sequence analysis was conducted on 81 CRS and 23 non-CRS samples. Enrichment experiments were carried out with 8 CRS samples for which there are pre- and postenrichment sequence data. All analyses were preceded by the same preprocessing scheme. Sequence analyses, statistical analysis, and data visualizations were performed in R. Cutadapt/2.6 (61) was used to remove primer sequences, with size filtering set to 215 bp at minimum and 285 bp at maximum. DADA2/1.14 (62) was used to trim sequences and filter for quality. DADA2 inferred a parametric error model used to identify and correct sequencing errors. Reads were dereplicated, paired ends merged, and chimeric reads removed using default options. Genus-level taxonomy was assigned using the RDP Bayesian classifier (63) and SILVA-132 taxonomy training set (64). Species-level taxonomy was assigned only if an ASV unambiguously matched a sequence in SILVA-132 or eHOMD databases (65). A phylogenetic tree was approximated by first performing a multiple alignment using DECIPHER/2.14.0 (66). Phangorn/2.5.5 (67) was used to construct a phylogenetic tree. Sequences without classification at the phylum level, phyla with a total feature prevalence of less than 10, or a mean feature prevalence of 1 were removed, as were ASVs with a mean relative abundance below 1 × 10−4. Prior to data visualization and analysis, sequences from the initial data set were subsampled to 2,000 reads, resulting in 62 CRS and 19 non-CRS samples and 397 ASVs, 43%% of which were assigned to the species level. All statistical testing for the 16S rRNA gene sequence analysis was performed in R. A Wilcoxon test was used to test for significant differences in relative abundances of taxa between sample groups. Spearman’s correlation was used to determine the significant associations between the relative abundances of taxa.
PICRUSt2 (28) was used to perform hidden-state prediction of metagenomic content of the subsampled data set, summarized into enzyme classes (ECs). Carbohydrate active ECs were identified through comparison to the Carbohydrate Active Enzyme Database (CAZy) (29) downloaded from the dbCAN2 meta server (68). EC count abundances were normalized to relative abundances of the top 10 contributing ASVs in each subject group (CRS/non-CRS).
Bacterial strains and culture conditions.
Enrichments of mucin-degrading microbial communities were obtained through inoculation of 3 ml of a defined minimal mucin medium (MMM) (21) (see Table S3 in the supplemental material) with 100 μl of FESS-derived mucus. Cultures were incubated at 37°C under anaerobic conditions (5% H2, 5% CO2, 90% N2) in a Coy anaerobic chamber (Coy, Grass Lake, MI) under static conditions for 48 h, subcultured 1:100 into fresh MMM, and incubated for another 48 h. Aliquots for 16S rRNA gene sequencing and 20% glycerol stocks were retrieved from each enrichment culture and stored at −80°C. Cell-free supernatants (CFSs) were generated through inoculation of MMM from glycerol stocks and incubated for 48 h at 37°C under anaerobic conditions. Each culture was then subcultured 1:100 in MMM and incubated for 48 h. Cells were removed by centrifugation at 7,000 × g for 10 min, followed by filtration through a 0.2-μm-pore polyethersulfone (PES) membrane filter. Filtrates were stored at −80°C until use.
S. aureus strain USA300 LAC (69) was maintained on tryptic soy agar (TSA). Prior to all growth experiments, overnight cultures were grown in tryptic soy broth shaking at 220 rpm at 37°C. Cells were washed three times with phosphate-buffered saline (PBS) and diluted to an optical density at 600 nm (OD600) of 0.02 in MMM or CFS. Amendments to MMM included 0.25% glucose (MMMG), 0.5% lactate (MMML), 0.5% Casamino Acids (MMMC), or 0.5% lactate and 0.5% Casamino Acids (MMMLC). Respiratory conditions were induced in anaerobic cultures by addition sodium nitrate (3 mM final concentration). OD600 measurements were obtained using a Synergy H1 plate reader (BioTek, Winooski, VT) with continuous orbital shaking (282 cycles per minute) at 37°C. Growth experiments were performed in triplicate using three biological replicates.
FPLC and HPLC.
Fast protein liquid chromatography (FPLC) was used to evaluate high-molecular-weight mucin integrity. Using an kta Pure fast protein liquid chromatograph (GE Bio-Sciences, Marlborough, MA) at 4°C, 500 μl of MMM or CFS was injected and subject to an isocratic run at a flow rate of 0.4 ml/min for a 1.5-ml column volume (CV) with 50 mM Na2HPO4 (pH 7.2) and 150 mM NaCl on a 15-ml-CV 10/200-mm Tricorn column packed with Sepharose CL-4B beads. Data were collected using Unicorn 7 software (GE Bio-Sciences) and analyzed using a custom R script.
High-performance liquid chromatography (HPLC) was used to measure acetate, butyrate, formate, isovalerate, propionate, lactate, succinate, and pyruvate. Pure standards ranging from 0.1 mM to 10 mM were used. Forty-eight-hour cell-free supernatants (CFS) were filtered using polyethersulfone (PES) protein concentrators (3,000 molecular weight cutoff [MWCO]; Amicon, Millipore Sigma). Eluent was analyzed using a Dionex UltiMate 3000 UHPLC (Thermo Fisher) system equipped with an Acclaim organic acid column (5 μm, 120 Å, 4.0 by 250 mm). A 32-min isocratic instrument method was used consisting of an 8-min equilibration period followed by a 24-min sample run at 1.0 ml/min at 30°C. Sodium sulfate (100 mM, pH 2.6) was the mobile phase. Samples were analyzed at a wavelength of 210 nm. Chromeleon 7 software (Dionex, Sunnyvale, CA, USA) was used to visualize and process data, and Cobra Wizard was used to identify and gate chromatogram peaks of interest.
RNA sequencing.
Total RNA was extracted from 5-ml cultures grown to early stationary phase in CFS or MMMG following a previously described method (70) with modifications. Briefly, cells were pelleted by centrifugation for 5 min at 7,000 × g and snap-frozen in liquid nitrogen before storage at −80°C overnight. RNA was extracted from cell pellets using an RNeasy minikit (Qiagen). DNase I treatment was carried out as part of the RNA Clean and Concentrator kit (Zymo, Irvine, CA). RNA quality (RIN ≥ 9.8) and quantity were assessed using an Agilent Bioanalyzer. Paired-end 75-bp libraries were prepared by UMGC using an Illumina TruSeq stranded RNA workflow with bacterial ribosomal reduction using Ribo-Zero (Illumina, San Diego, CA). Libraries were sequenced using the Illumina NextSeq platform.
Raw fastq files were checked for quality and adapter sequences using FastQC. Sequences were then aligned to the USA300_FPR3757 genome (NCBI RefSeq NC_007793.1) using the Subread aligner, implemented using RSubread (71). Gene counting was performed using both “featureCounts” and RSubread. Sample ordination was performed on regularized log-transformed counts using “rlog” and “pcomp” functions within DESeq2/3.9 (72). “deseq” was used to estimate size factors and carry out variance-stabilizing transformation and statistical testing via the Wald test. Genes with a log2 fold change greater than 1 (set using the lfcThreshold parameter) with a two-tailed Benjamini-Hochberg adjusted P value of <0.05 were considered significant.
Data availability.
Raw 16S rRNA gene sequence and RNA-seq files are available in the NCBI BioProject PRJNA656590. Code and data files are shared at https://github.com/Hunter-Lab-UMN/Lucas_SK_2020.git.
ACKNOWLEDGMENTS
We acknowledge the UMN Genomics Center and Juan Abrahante (UMN Informatics Institute) for sequencing assistance and Sabrina Arif for FPLC expertise.
This work was supported by a National Institute of Dental and Craniofacial T32 Fellowship (T90DE0227232) and a Ruth L. Kirschstein Predoctoral Fellowship (1F31DE027602) awarded to S.K.L., a National Heart, Lung, Blood Institute Research Project Grant (1R01HL136919) to R.C.H., and an Administrative Research Supplement (HL136919-03S1) to A.R.V. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
Contributor Information
Ryan C. Hunter, Email: rchunter@umn.edu.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.Bhattacharyya N, Gilani S. 2018. Prevalence of potential adult chronic rhinosinusitis symptoms in the United States. Otolaryngol Head Neck Surg 159:522–525. 10.1177/0194599818774006. [DOI] [PubMed] [Google Scholar]
- 2.Hoggard M, Mackenzie BW, Jain R, Taylor MW, Biswas K, Douglas RG. 2017. Chronic rhinosinusitis and the evolving understanding of microbial ecology in chronic inflammatory mucosal disease. Clin Microbiol Rev 30:321–348. 10.1128/CMR.00060-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ooi EH, Wormald PJ, Tan LW. 2008. Innate immunity in the paranasal sinuses: a review of nasal host defenses. Am J Rhinol 22:13–19. 10.2500/ajr.2008.22.3127. [DOI] [PubMed] [Google Scholar]
- 4.Ou J, Wang J, Xu Y, Tao ZZ, Kong YG, Chen SM, Shi WD. 2014. Staphylococcus aureus superantigens are associated with chronic rhinosinusitis with nasal polyps: a meta-analysis. Eur Arch Otorhinolaryngol 271:2729–2736. 10.1007/s00405-014-2955-0. [DOI] [PubMed] [Google Scholar]
- 5.Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. 2012. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 122:467–472. 10.1002/lary.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakrishnan VR, Feazel LM, Abrass LJ, Frank DN. 2013. Prevalence and abundance of Staphylococcus aureus in the middle meatus of patients with chronic rhinosinusitis, nasal polyps, and asthma. Int Forum Allergy Rhinol 3:267–271. 10.1002/alr.21101. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya N, Kepnes LJ. 2008. Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol 117:448–452. 10.1177/000348940811700608. [DOI] [PubMed] [Google Scholar]
- 8.Brook I. 2016. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis 35:1059–1068. 10.1007/s10096-016-2640-x. [DOI] [PubMed] [Google Scholar]
- 9.Wertheim HF, Melles DC, Voc MC, van Leeuwen W, van Belkum Verbrugh HA, Nouwen JL. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762. 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 10.Drilling A, Coombs GW, Tan JL, Pearson JC, Boase S, Psaltis A, Speck P, Vreugde S, Wormald PJ. 2014. Cousins, siblings, or copies: the genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Int Forum Allergy Rhinol 4:953–960. 10.1002/alr.21423. [DOI] [PubMed] [Google Scholar]
- 11.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluymans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson MF, Mfuna L, Dowd SE, Wolcott RD, Barbeau J, Poisson M, James G, Desrosiers M. 2010. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg 39:182–187. 10.2310/7070.2009.090060. [DOI] [PubMed] [Google Scholar]
- 13.Boase S, Foreman A, Cleland E, Tan L, Melton-Kreft R, Pant H, Hu FZ, Ehrlich GD, Wormald PJ. 2013. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis 13:210. 10.1186/1471-2334-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenul C, Horswill AR. 2019. Regulation of Staphylococcus aureus virulence. Microbiol Spectr 7. 10.1128/microbiolspec.GPP3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balasubramanian D, Harper L, Shopsin B, Torres VJ. 2017. Staphylococcus aureus pathogenesis in diverse host environments. Pathog Dis 75:ftx005. 10.1093/femspd/ftx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wollenberg MS, Claesen J, Escapa IF, Aldridge KL, Fischbach MA, Lemon KP. 2014. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5:e01286-14. 10.1128/mBio.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Dai A, Huang S, Kuo S, Shu M, Tapia CP, Yu J, Two A, Zhang H, Gallo RL, Huang CM. 2014. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef Microbes 5:161–168. 10.3920/BM2013.0031. [DOI] [PubMed] [Google Scholar]
- 18.Hardy BL, Dickey SW, Plaut RD, Riggins DP, Stibitz S, Otto M, Merrell DS. 2019. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. mBio 10:e02491-18. 10.1128/mBio.02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. 2016. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front Microbiol 7:1230. 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook I, Thompson DH, Frazier EH. 1994. Microbiology and management of chronic maxillary sinusitis. Arch Otolaryngol Head Neck Surg 120:1317–1320. 10.1001/archotol.1994.01880360015003. [DOI] [PubMed] [Google Scholar]
- 21.Finegold SM, Flynn MJ, Rose FV, Jousimies-Somer H, Jakielaszek C, McTeague M, Wexler HM, Berkowitz E, Wynne B. 2002. Bacteriologic findings associated with chronic bacterial maxillary sinusitis in adults. Clin Infect Dis 35:428–433. 10.1086/341899. [DOI] [PubMed] [Google Scholar]
- 22.Flynn JM, Niccum D, Dunitz JM, Hunter RC. 2016. Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathog 12:e1005846. 10.1371/journal.ppat.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsune S, Kono M, Sun D, Ushikai M, Kurono Y. 2003. Hypoxia in paranasal sinuses of patients with chronic sinusitis with or without the complication of nasal allergy. Acta Otolaryngol 123:519–523. 10.1080/0036554021000028113. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Chu HS, Lee JY, Hwang S, Lee SH, Lee HM. 2004. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 130:747–752. 10.1001/archotol.130.6.747. [DOI] [PubMed] [Google Scholar]
- 25.Valour F, Senechal Dupieux Karsenty J, Lustig S, Breton P, Gleizal A, Boussel L, Laurent F, Braun E, Chidiac C. 2014. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist 7:183–197. 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradshaw DJ, Homer KA, Marsh PD, Beighton D. 1994. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140:3407–3412. 10.1099/13500872-140-12-3407. [DOI] [PubMed] [Google Scholar]
- 27.Tailford LE, Crost EH, Kavanaugh D, Juge N. 2015. Mucin glycan foraging in the human gut microbiome. Front Genet 6:81. 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MG. 2020. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38:685–688. 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. 2014. The Carbohydrate-Active Enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495. 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyohara M, Tanigawa K, Chaiwangsri Katayama T, Ashida H, Yamamoto K. 2011. An exo-alpha-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 21:437–447. 10.1093/glycob/cwq175. [DOI] [PubMed] [Google Scholar]
- 31.Terra VS, Homer KA, Rao SG, Andrew PW, Yesilkaya H. 2010. Characterization of novel beta-galactosidase activity that contributes to glycoprotein degradation and virulence in Streptococcus pneumoniae. Infect Immun 78:348–357. 10.1128/IAI.00721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders NN, Eijsink VG, van den Pangaart PS, van Neerven RJ, Simons PJ, De Smedt SC, Demeester J. 2007. Mucolytic activity of bacterial and human chitinases. Biochim Biophys Acta 1770:839–846. 10.1016/j.bbagen.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Rice KC, Nelson JB, Patton TG, Yang SJ, Bayles KW. 2005. Acetic acid induces expression of the Staphylococcus aureus cidABC and lrgAB murein hydrolase regulator operons. J Bacteriol 187:813–821. 10.1128/JB.187.3.813-821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong S, Kim HY, Kim AR, Yun CH, Han SH. 2019. Propionate ameliorates Staphylococcus aureus skin infection by attenuating bacterial growth. Front Microbiol 10:1363. 10.3389/fmicb.2019.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper L, Balasubramanian D, Ohneck EA, Sause WE, Chapman J, Mejia-Sosa B, Lhakhang T, Heguy A, Tsirigos A, Ueberheide B, Boyd JM, Lun DS, Torres VJ. 2018. Staphylococcus aureus responds to the central metabolite pyruvate to regulate virulence. mBio 9:e02272-17. 10.1128/mBio.02272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitko NP, Grosser MR, Khatri D, Lance TR, Richardson AR. 2016. Expanded glucose import capability affords Staphylococcus aureus optimized glycolytic flux during infection. mBio 7:e00296-16. 10.1128/mBio.00296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson ME, King J, Yahr TL, Horswill AR. 2013. Sialic acid catabolism in Staphylococcus aureus. J Bacteriol 195:1779–1788. 10.1128/JB.02294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halsey CR, Lei S, Wax JK, Lehman MK, Nuxoll AS, Steinke L, Sadykov M, Powers R, Fey PD. 2017. Amino acid catabolism in Staphylococcus aureus and the function of carbon catabolite repression. mBio 8:e01434-16. 10.1128/mBio.01434-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho SM, de Jong A, Kloosterman TG, Kuipers OP, Saraiva LM. 2017. The Staphylococcus aureus α-acetolactate synthase ALS confers resistance to nitrosative stress. Front Microbiol 8:1273. 10.3389/fmicb.2017.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchette KA, Shenoy AT, Milner J, Gilley RP, McClure E, Hinojosa CA, Kumar N, Daugherty SC, Tallon LJ, Ott S, King SJ. 2016. Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun 84:2922–2932. 10.1128/IAI.00277-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. 10.1128/IAI.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brook I. 2011. Microbiology of sinusitis. Proc Am Thorac Soc 8:90–100. 10.1513/pats.201006-038RN. [DOI] [PubMed] [Google Scholar]
- 44.Doyle PW, Woodham JD. 1991. Evaluation of the microbiology of chronic ethmoid sinusitis. J Clin Microbiol 29:2396–2400. 10.1128/jcm.29.11.2396-2400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramadan HH. 1995. What is the bacteriology of chronic sinusitis in adults? Am J Otolaryngol 16:303–306. 10.1016/0196-0709(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 46.Sonnenburg JL, Xu J, Liep DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955–1959. 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 47.Johansson ME, Larsson JM, Hansson GC. 2011. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 108:4659–4665. 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius DM. 2019. Association between periodontal pathogens and systemic disease. Biomed J 42:27–35. 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Under C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spahich NA, Vitko NP, Thurlow LR, Temple B, Richardson AR. 2016. Staphylococcus aureus lactate- and malate-quinone oxidoreductases contribute to nitric oxide resistance and virulence. Mol Microbiol 100:759–773. 10.1111/mmi.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deroee AF, Naraghi M, Sontou AF, Ebrahimkhani MR, Dehpour AR. 2009. Nitric oxide metabolites as biomarkers for follow-up after chronic rhinosinusitis surgery. Am J Rhinol Allergy 23:159–161. 10.2500/ajra.2009.23.3289. [DOI] [PubMed] [Google Scholar]
- 52.Jardeleza C, Jones D, Baker L, Miljkovic D, Boase S, Tan NCW, Vreugde S, Tan LW, Wormald PJ. 2013. Gene expression differences in nitric oxide and reactive oxygen species regulation point to an altered innate immune response in chronic rhinosinusitis. Int Forum Allergy Rhinol 3:193–198. 10.1002/alr.21114. [DOI] [PubMed] [Google Scholar]
- 53.Cope EK, Goldberg AN, Pletcher SD, Lynch SV. 2017. Compositionally and functionally distinct sinus microbiota in chronic rhinosinusitis patients have immunological and clinically divergent consequences. Microbiome 5:53. 10.1186/s40168-017-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen NA. 2006. Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol 196:S20–S26. 10.1177/00034894061150s904. [DOI] [PubMed] [Google Scholar]
- 55.Lehman MK, Nuxoll AS, Yamada KJ, Kielian T, Carson SD, Fey PD. 2019. Protease-mediated growth of Staphylococcus aureus on host proteins is opp3 dependent. mBio 10:e02553-18. 10.1128/mBio.02553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. 2006. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol 188:4996–5001. 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho DY, Mackey C, Van Der Pol WJ, Skinner D, Morrow CD, Schoeb TR, Rowe SM, Swords WE, Tearney GJ, Woodworth BA. 2018. Sinus microanatomy and microbiota in a rabbit model of rhinosinusitis. Front Cell Infect Microbiol 7:540. 10.3389/fcimb.2017.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC. 2013. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol 111:246–251. 10.1016/j.anai.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gohl DM, Vangay P, Garbe J, MacLean A, Hauge A, Becker A, Gould TJ, Clayton JB, Johnson TJ, Hunter RC, Knights D, Beckman KB. 2016. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat Biotechnol 34:942–949. 10.1038/nbt.3601. [DOI] [PubMed] [Google Scholar]
- 61.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 62.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. 2012. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. 2018. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems 3:e00187-18. 10.1128/mSystems.00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright E, Yilmaz LS, Noguera DR. 2012. DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl Environ Microbiol 78:717–725. 10.1128/AEM.06516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schliep KP. 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang Z, Busk PK, Xu Y, Yin Y. 2018. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 46:W95–W101. 10.1093/nar/gky418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731–739. 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 70.Carroll RK, Weiss A, Shaw LN. 2016. RNA-sequencing of Staphylococcus aureus messenger RNA. Methods Mol Biol 1373:131–141. 10.1007/7651_2014_192. [DOI] [PubMed] [Google Scholar]
- 71.Liao Y, Smyth GK, Shi W. 2019. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res 47:e47. 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Love M, Anders S, Huber W. 2014. Differential analysis of count data—the DESeq2 package. http://www.bioconductor.org/packages//2.13/bioc/vignettes/DESeq2/inst/doc/DESeq2.pdf.
- 73.Klossek JM, Dubreuil L, Richet H, Richet B, Beutter P. 1998. Bacteriology of chronic purulent secretions in chronic rhinosinusitis. J Laryngol Otol 112:1162–1166. 10.1017/s0022215100142732. [DOI] [PubMed] [Google Scholar]
- 74.Burnaugh AM, Frantz LJ, King SJ. 2008. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J Bacteriol 190:221–230. 10.1128/JB.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00153-21-s0001.pdf, PDF file, 0.9 MB (952.8KB, pdf)
Data Availability Statement
Raw 16S rRNA gene sequence and RNA-seq files are available in the NCBI BioProject PRJNA656590. Code and data files are shared at https://github.com/Hunter-Lab-UMN/Lucas_SK_2020.git.