ABSTRACT
Zebrafish (Danio rerio) is an attractive model organism to use for an array of scientific studies, including host-microbe interactions. Zebrafish contain a core (i.e., consistently detected) intestinal microbiome consisting primarily of Proteobacteria. Furthermore, this core intestinal microbiome is plastic and can be significantly altered due to external factors. Zebrafish are particularly useful for the study of aquatic microbes that can colonize vertebrate hosts, including Vibrio cholerae. As an intestinal pathogen, V. cholerae must colonize the intestine of an exposed host for pathogenicity to occur. Members of the resident intestinal microbial community likely must be reduced or eliminated by V. cholerae for colonization, and subsequent disease, to occur. Many studies have explored a variety of aspects of the pathogenic effects of V. cholerae on zebrafish and other model organisms but few have researched how a V. cholerae infection changes the resident intestinal microbiome. In this study, 16S rRNA gene sequencing was used to examine how five genetically diverse V. cholerae strains alter the intestinal microbiome following an infection. We found that V. cholerae colonization induced significant changes in the zebrafish intestinal microbiome. Notably, changes in the microbial profile were significantly different from each other, based on the particular strain of V. cholerae used to infect zebrafish hosts. We conclude that V. cholerae significantly modulates the zebrafish intestinal microbiota to enable colonization and that specific microbes that are targeted depend on the V. cholerae genotype.
KEYWORDS: Vibrio cholerae, cholera, microbiome, zebrafish
INTRODUCTION
Vibrio cholerae is a Gram-negative aquatic bacterium belonging to the Gammaproteobacteria class and is the causative agent for the disease cholera, along with various other diarrheal illnesses (1–3). We are currently experiencing the seventh cholera pandemic, which is devastating the developing world (3). In 2016, the World Health Organization (WHO) reported 132,121 cholera cases and 2,420 deaths worldwide, with outbreaks affecting several countries. The real number of cases per year, however, is more likely between 1.4 and 4.0 million, with 21,000 to 143,000 resulting deaths (4). Furthermore, the number of cholera cases has been increasing steadily since 2005 and is likely to continue to climb. This rise in cases is largely due to environmental factors, such as pollution, which is elevating iron levels in aquatic environments, and climate change, which is warming the oceans. The increased ocean temperature is, in turn, leading to higher Vibrio populations (particularly in the summer months) (5–8). In addition to a higher rate of Vibrio infections (notably V. cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus), cases are now appearing in areas where they are not routinely seen, such as Europe and North America (5, 7, 9–13).
There are approximately 200 different serogroups of V. cholerae, and the majority of the V. cholerae bacteria found in the environment are classified as non-O1/O139 strains. These non-O1/O139 strains constitute the third most prevalent type of detected Vibrio bacteria (Vibrio alginolyticus, which can be pathogenic to humans, is generally the most detected Vibrio sp. in the environment) (6, 14, 15). Non-O1/O139 V. cholerae are also genetically diverse, both with respect to each other and from their O1/O139 pandemic counterparts. In contrast, most O1/O139 V. cholerae strains are quite similar genetically (16–18). Only O1/O139 strains have been shown to cause cholera pandemics due to their possession of crucial virulence factors, of which the most notable are cholera toxin (CT) and toxin coregulated pilus (TCP) (19–21). Along with these two virulence factors, nearly all V. cholerae strains (both O1/O139 and non-O1/O139 biotypes) possess a number of other virulence factors, such as hemolysins, additional toxins (e.g., RTX, cholix, and MakA), adhesin proteins, and various secretion systems, including the type one, type two, type three, and type six secretion systems (T1SS, T2SS, T3SS, and T6SS, respectively) (22–32).
Although different animal models have been used to study the pathogenicity of V. cholerae, all of them have their limitations. Most mammalian models require extensive physiological modification in order to be viable models, and some require surgical modification (ileal loop models) or elimination of the resident intestinal microbiome (33–35). Furthermore, the cycle of disease can differ significantly from that which is observed in a human. For example, in mouse models, while CT can be fatal to the animal, mice do not experience the diarrhea that humans experience, which is a hallmark of the cholera disease (33). Another confounding factor is that V. cholerae did not evolve to colonize mice or rabbits, and thus, it cannot successfully compete with the intestinal microbiota to establish a colonization niche. To overcome these limitations of mammalian models, the zebrafish (Danio rerio) has been used to study the entire V. cholerae life cycle (30, 31, 36, 37). Zebrafish are a tropical freshwater fish native to southern Asia and have been utilized for a wider range of studies examining host-microbe interactions and many other aspects of biology in recent years. This is in large part due to the high level of similarity between the zebrafish and human immune systems (38).
The zebrafish is an extremely useful model for studying V. cholerae, as it is environmentally relevant; recapitulates the cholera disease model; does not need to be surgically modified; and perhaps most importantly, it has an intact and fully developed intestinal microbiome that does not need to be altered to facilitate V. cholerae colonization (30, 36, 39, 40). For zebrafish specifically, the intestinal microbiome has been shown to play major roles in fatty acid absorption into the intestinal and extraintestinal tissues and in establishing normal homeostatic levels of neutrophils within the intestine (41, 42). Studies have also shown that the composition of the zebrafish intestinal microbiome varies across development, with zebrafish developing a more diverse intestinal microbiome as they age (43). The zebrafish intestinal microbiome can also be shifted by external factors, such as diet and broad rearing environments (38, 43).
Studies have demonstrated in various animal models that incoming bacteria can clear commensals in the intestine during colonization (44, 45). Another study examining V. cholerae in juvenile zebrafish with a monospecies artificial microbiome found that the type six secretion system (T6SS) is able to expel bacteria within the zebrafish intestine by modulating the intestine itself, causing it to increase its movements and flush out the resident bacterium, which in this case was Aeromonas veronii, a common zebrafish gut commensal. In a normal and healthy zebrafish (adult or juvenile), the intestine has a diverse microbial community, which must be considered when studying the interaction between V. cholerae and the host intestinal microbiome in an infection (38).
V. cholerae (both O1/O139 and non-O1/O139) is able to colonize zebrafish intestines rapidly, persists for a prolonged period of time, and can replicate prodigiously in the presence of a mature, complex microbiome, inducing fish diarrhea (30, 36). Here, we examined the effects that five V. cholerae strains with differing genetic backgrounds have on the zebrafish intestinal microbiome and the tank water microbial community. The goal of the study was to determine if these communities are significantly modulated in different ways depending on the V. cholerae genotype introduced into the system. We used three environmental strains (254-93, AM-19226, and V52), along with two O1 El Tor strains (E7946 and N16961). Strains 254-93 (O144 serogroup), AM-19226 (O39 serogroup), and V52 (O37 serogroup), as non-O1/O139 serogroups of V. cholerae, lack the two most prominent V. cholerae virulence factors CT and TCP. However, they contain a T6SS and both forms of RTX toxin, while AM-19226 also has a T3SS (24, 46–48). It is also possible that all of these strains have the ability to produce cholix toxin (this is likely the case with AM-19226 in particular, as the toxin has been detected in multiple strains of V. cholerae serogroups O39), as well as many of the other accessory toxins associated with V. cholerae (26, 49). E7946 and N16961 can produce CT, TCP, both forms of RTX toxin, T6SS, proteases, and adhesins. Additionally, given the genetic similarity between E7946 (which lacks cholix toxin) and N16961, it is quite probable E7946 lacks this additional virulence factor (49). Our results indicate that these diverse V. cholerae strains induce differential changes to the zebrafish intestinal microbiome and the composition of intestinal metabolites.
RESULTS
Diverse V. cholerae strains can colonize zebrafish intestines, persist in the aquatic environment, and induce diarrhea.
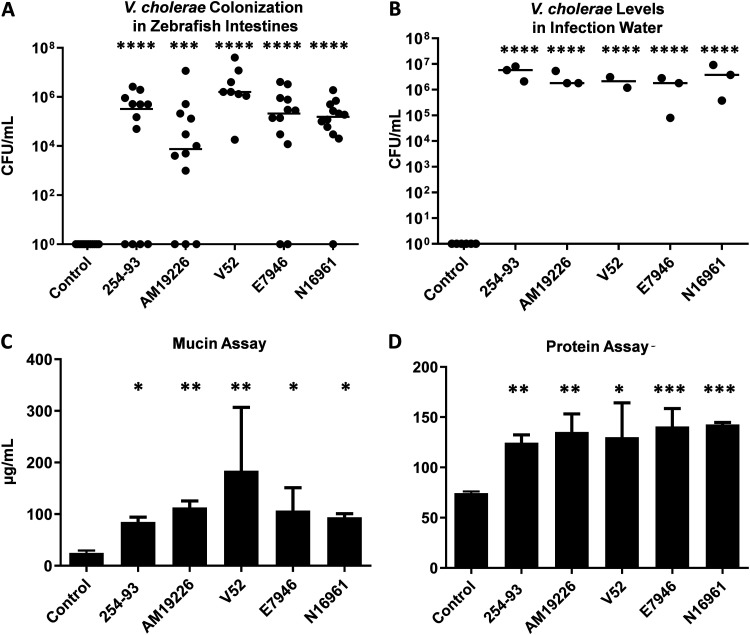
Prior studies indicated that both classical and El Tor V. cholerae could colonize zebrafish and induce diarrhea (30, 36, 50). However, limited non-O1/O139 strains had been examined. Fish were exposed to each of the V. cholerae strains for 24 h, and all strains utilized in this study were capable of colonizing the zebrafish intestines and persisting in autoclaved infection water (Fig. 1A and B).
FIG 1.
CFU and mucin levels following V. cholerae infections in a zebrafish host. CFU/ml levels of V. cholerae in the zebrafish intestines (A) and in the infection water 24 hours postinfection (B). The data include the results from 2 to 8 individual experiments. Fish, n = 8 to 16; water, n = 2 to 6. A total of 50-ml water samples were spun down and resuspended in 1 ml of PBS and then analyzed for mucin concentrations using Schiff’s reagent (C) and Bradford protein assay reagent (D). n = 2 to 6. Statistical tests were performed with Dunnett's multiple-comparison test (A and B) and Tukey’s multiple-comparison test (C and D) on log-transformed data.*, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005; ****, P ≤ 0.0001.
While all strains of V. cholerae tested were able to colonize the zebrafish intestine, the question remained as to whether they were capable of causing diarrhea following gut colonization. Previous work determined several metrics for fish diarrhea, including optical density at 600 nm (OD600), excreted protein, excreted V. cholerae counts, and excreted mucin (30, 50). Mucin secretion is the most sensitive and reproducible assay, so it was primarily used as a proxy for diarrhea in this study. All wild-type strains of V. cholerae tested here induced a significant increase in mucin secretion 24 hours postinfection (Fig. 1C). A Bradford protein assay was also utilized as a secondary means of testing for excreted proteins in the infection water and the same trend was observed, with significantly higher levels of protein detected in all of the zebrafish populations infected with wild-type V. cholerae than in the uninfected control fish (Fig. 1D). No significant differences were detected when comparing the excreted mucin and protein levels from the zebrafish infected with different V. cholerae strains (Tukey’s multiple-comparison test).
V. cholerae infections cause significant changes to the zebrafish intestinal microbiome.
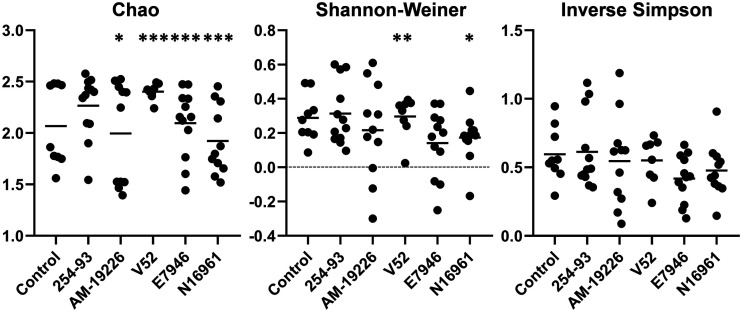
Having established that all tested strains of V. cholerae are capable of colonizing the zebrafish intestine, we next investigated if these infections resulted in significant changes to the intestinal microbiome. 16S rRNA gene sequencing was used to sample the intestinal microbiome of both infected and uninfected zebrafish. Beginning with the α-diversity of the zebrafish intestinal microbiome, multiple groups of zebrafish showed significant differences in the overall richness (Chao index) of their intestinal communities following infection with V. cholerae. Specifically, strains AM-19226 and N16961 induced significant decreases in overall diversity, while strains E7946 and V52 induced significant increases in zebrafish intestinal microbiome richness following infection (Fig. 2). Utilizing the Shannon-Weiner index, fish infected with V. cholerae strain V52 displayed a significant increase in heterogeneity, while fish infected with N16961 experienced a significant decrease in heterogeneity compared with the control fish. No other significant differences were observed when comparing the three other groups of infected zebrafish to the control group utilizing the Shannon-Weiner index; no significant differences were observed in the inverse Simpson index (Fig. 2).
FIG 2.
α-Diversity of the zebrafish intestinal microbiome following V. cholerae infections in a zebrafish host. α-Diversity of the zebrafish intestinal microbiome following a 24-hour V. cholerae infection presented as scatterplots. Statistical tests were performed using generalized linear modeling (multiple comparisons of mean values, with Tukey contrasts). Fish numbers: control, n = 9; 254-93, n = 12; AM-19226, n = 11; V52, n = 8; E7946, n = 12; N16961, n = 11. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005.
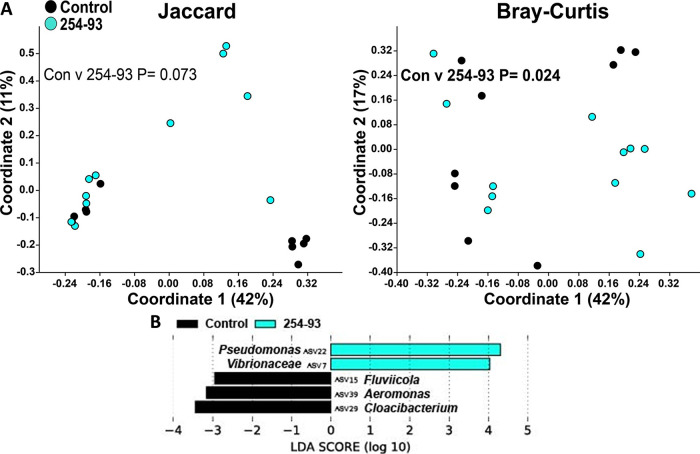
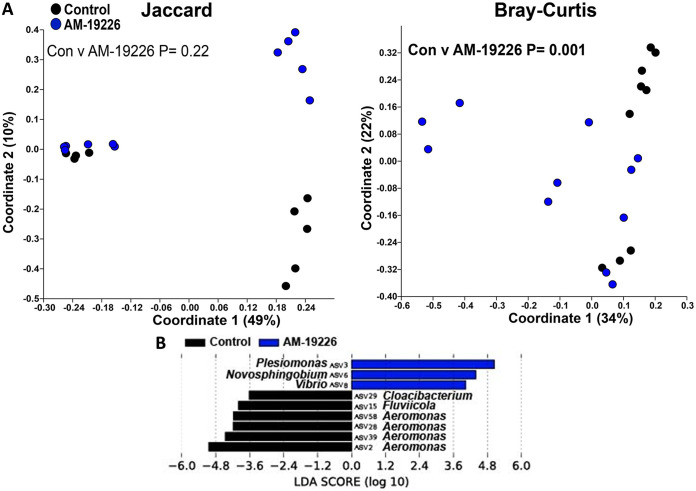
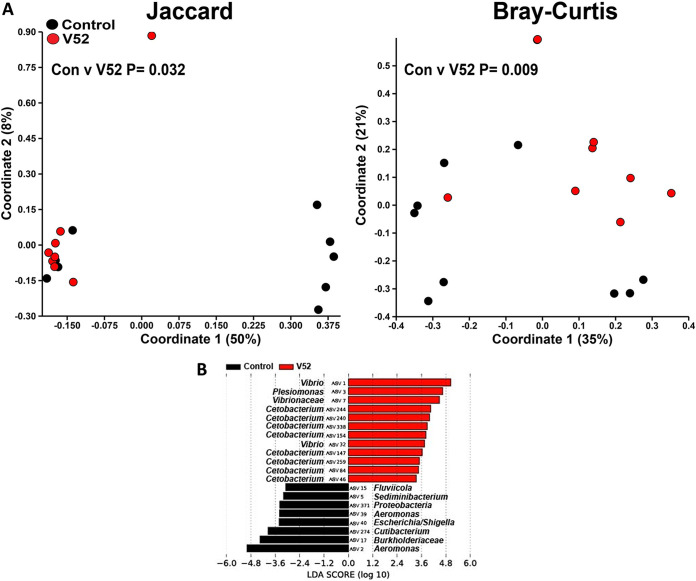
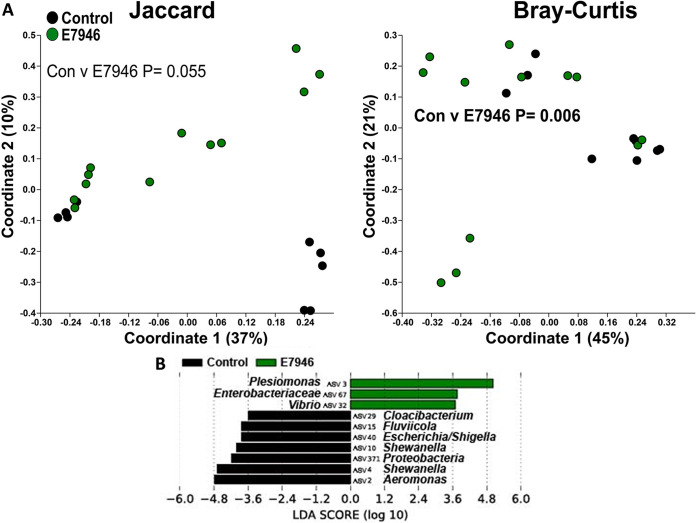
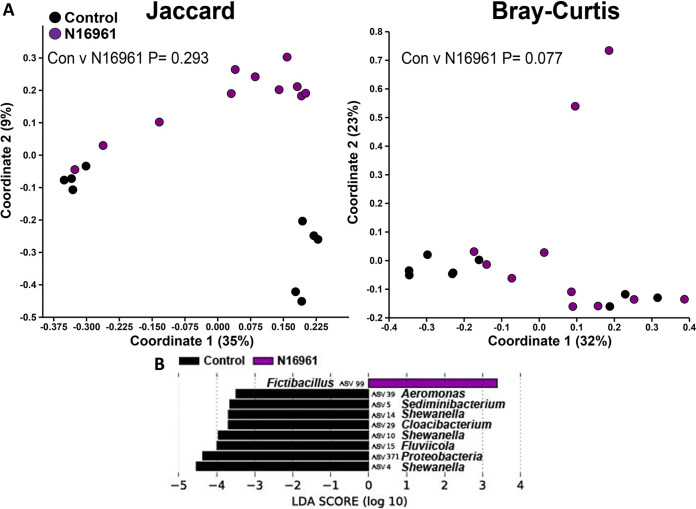
Next, we proceeded with the β-diversity analysis of the zebrafish microbiome. Infections with V. cholerae strains 254-93, AM-19226, V52, and E7946 all caused significant changes to the structure of the zebrafish intestinal microbiome, while strain V52 also caused significant changes to the composition of the zebrafish intestinal microbiome (Fig. 3A to 7A). In terms of which amplicon sequence variants (ASVs) changed pre- to postinfection, the linear discriminant analysis effect size (LEfSe) analyses revealed some general trends, namely, that Fluviicola, Aeromonas, and Cloacibacterium ASVs were consistently higher in most of the uninfected control fish than in V. cholerae-infected fish (Fig. 3B to 7B). The post-V. cholerae infection ASV profile of the zebrafish intestinal microbiome differed depending on which strain of bacteria was used for infection. The LEfSe analyses comparing the control fish to those infected with different V. cholerae strains indicated significant increases in Pseudomonas sp. and Vibrionaceae for fish infected with 254-93 (Fig. 3B); increased Plesiomonas, Novosphingobium, and Vibrio sp. in fish infected with AM-19226 (Fig. 4B); increased Vibrio, Cetobacterium, and Plesiomonas sp. in fish infected with V52 (Fig. 5B); increased Plesiomonas sp., Vibrio sp., and Enterobacteriaceae in E7946 infected fish (Fig. 6B); and increased Fictibacillus sp. in fish infected with N16961 (Fig. 7B).
FIG 3.
β-Diversity of the zebrafish intestinal microbiome following V. cholerae 254-93 infection in a zebrafish host. (A) β-Diversity of the zebrafish intestinal microbiome following a 24-h V. cholerae 254-93 infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. Statistical tests were one-way PERMANOVAs run using Adonis with the strata function controlling for collection date. (B) Linear discriminant analysis effect size (LEfSe) figures displaying which ASVs experienced significant shifts following the V. cholerae infection (again, controlling for sample collection date). All of the ASVs that had an LDA score greater than 2.5 are presented. Fish numbers: control, n = 9; 254-93, n = 12.
FIG 4.
β-Diversity of the zebrafish intestinal microbiome following V. cholerae AM-19226 infection in a zebrafish host. (A) β-Diversity of the zebrafish intestinal microbiome following a 24-h V. cholerae AM-19226 infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. Statistical tests were one-way PERMANOVAs run using Adonis with the strata function controlling for collection date. (B) Linear discriminant analysis effect size (LEfSe) figures displaying which ASVs experienced significant shifts following the V. cholerae infection (again, controlling for sample collection date). All of the ASVs that had an LDA score greater than 2.5 are presented. Fish numbers: control, n = 9; AM-19226, n = 11.
FIG 5.
β-Diversity of the zebrafish intestinal microbiome following V. cholerae V52 infection in a zebrafish host. (A) β-Diversity of the zebrafish intestinal microbiome following a 24-h V. cholerae V52 infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. Statistical tests were one-way PERMANOVAs run using Adonis with the strata function controlling for collection date. (B) Linear discriminant analysis effect size (LEfSe) figures displaying which ASVs experienced significant shifts following the V. cholerae infection (again, controlling for sample collection date). All of the ASVs that had an LDA score greater than 2.5 are presented. Fish numbers: control, n = 9; V52, n = 8.
FIG 6.
β-Diversity of the zebrafish intestinal microbiome following V. cholerae E7946 infection in a zebrafish host. (A) β-Diversity of the zebrafish intestinal microbiome following a 24-h V. cholerae E7946 infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. Statistical tests were one-way PERMANOVAs run using Adonis with the strata function controlling for collection date. (B) Linear discriminant analysis effect size (LEfSe) figures displaying which ASVs experienced significant shifts following the V. cholerae infection (again, controlling for sample collection date). All of the ASVs that had an LDA score greater than 2.5 are presented. Fish numbers: control, n = 9; E7946, n = 12.
FIG 7.
β-Diversity of the zebrafish intestinal microbiome following V. cholerae N16961 infection in a zebrafish host. (A) β-Diversity of the zebrafish intestinal microbiome following a 24-h V. cholerae N16961 infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. Statistical tests were one-way PERMANOVAs run using Adonis with the strata function controlling for collection date. (B) Linear discriminant analysis effect size (LEfSe) figures displaying which ASVs experienced significant shifts following the V. cholerae infection (again, controlling for sample collection date). All of the ASVs that had an LDA score greater than 2.5 are presented. Fish numbers: control, n = 9; N16961, n = 11.
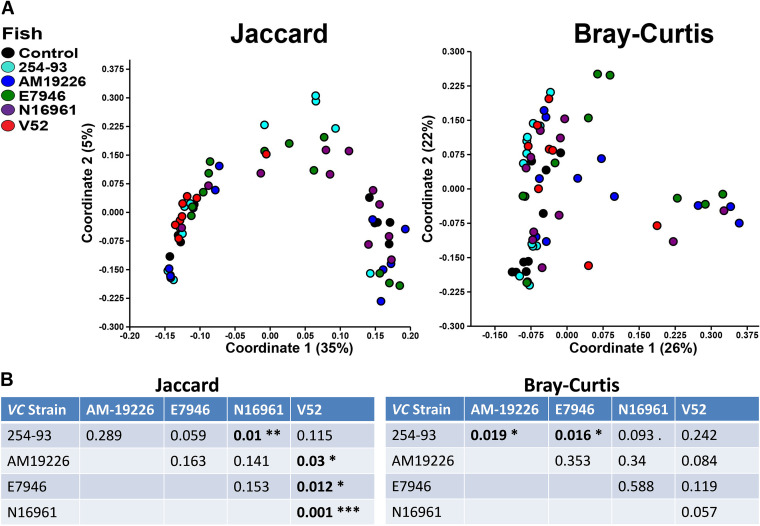
Since most of the V. cholerae infections caused significant changes to the zebrafish microbiome, we next compared all of the zebrafish postinfection intestinal microbiomes to one another to determine if infection with different V. cholerae strains resulted in significantly different postinfection intestinal microbiomes. Fish infected with strain V52 displayed the largest number of significant compositional differences compared with zebrafish infected with other V. cholerae strains postinfection, with significant differences detected compared with AM-19226, E7946, and N16961 (Fig. 8A). Strain 254-93 displayed significant structural differences compared with AM-19226 and E7946, along with significant compositional differences from strain N16961 (Fig. 8A). Not surprisingly, the largest number of differences in ASVs was observed when comparing the environmental strains of V. cholerae (254-93, AM-19226, and V52) to the El Tor O1 strains (E7946 and N16961). Compared with the El Tor strains, the intestinal microbiomes of zebrafish infected with both 254-93 and V52 displayed significantly higher levels of Shewanella and Cetobacterium sp., while the El Tor strains resulted in higher levels of Brevinema, Brucella, and Bradyrhizobium sp. in most instances (see Fig. S1A and B in the supplemental material). Comparisons between the environmental strains were less distinct but still revealed significant differences. Compared with zebrafish infected with 254-93, the intestinal microbiomes of fish infected with AM-19226 had higher relative abundances of Plesiomonas sp., Vibrio sp., Novosphingobium sp., and Enterobacteriaceae and lower relative abundances of Aeromonas sp. and Vibrionaceae. Compared with zebrafish infected with V52, the intestinal microbiomes of zebrafish infected with AM-19226 had higher relative abundances of Pseudomonas, Brevinema, and Novosphingobium sp. and lower relative abundances of Aquabacterium and Cloacibacterium sp. (Fig. S1A and B).
FIG 8.
Comparison of zebrafish intestinal microbiome β-diversities following V. cholerae infections. (A) β-Diversities of the zebrafish intestinal microbiome following a 24-hour V. cholerae infection expressed as PCoA plots based on Jaccard and Bray-Curtis dissimilarity indices. (B) P values indicating statistical differences of the PCoA plots shown in A. Significant differences are indicated by bold type. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005; Fish numbers: control, n = 9; 254-93, n = 12; AM-19226, n = 11; V52, n = 8; E7946, n = 12; N16961, n = 11.
Fish infected with V. cholerae strains V52 and E7946 display increased bacterial abundances in the intestine.
To determine whether V. cholerae infection changed the overall bacterial load of the fish intestine, quantitative PCR (qPCR) was performed on the DNA extracted from the zebrafish intestinal homogenates. The intestines of fish infected with V52 and E7946 had a higher overall bacterial load than those of uninfected control fish (Fig. 9). This observation, together with the above observations of changes to only some members of the intestinal community, strongly suggests that V. cholerae does not completely disrupt the intestinal microbiota but instead selectively targets, competes, and/or forms a symbiotic relationship with select components of the zebrafish intestinal microbiota.
FIG 9.
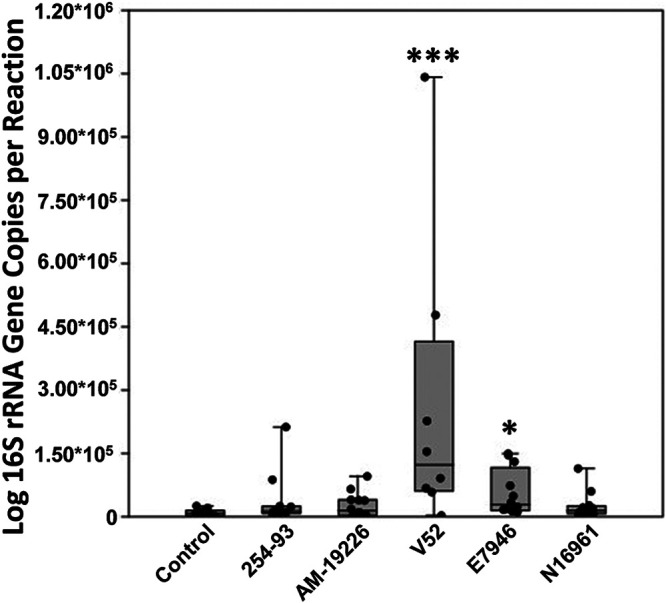
qPCR data of the bacterial load in each zebrafish intestine. Similarities in bacterial load, as assessed by 16S rRNA gene real-time quantitative PCR (qPCR) between zebrafish intestinal homogenates are displayed with accompanying sample list. Statistical tests were performed with Bonferroni’s correction on log-transformed data. *, P ≤ 0.05; ***, P ≤ 0.0005. Fish numbers: control, n = 10; 254-93, n = 12; AM-19226, n = 12; V52, n = 8; E7946, n = 12; N16961, n =12.
V. cholerae infection results in significant changes to the metabolomics profile of the zebrafish intestine.
It was clear from the 16S rRNA gene analysis that V. cholerae infection affects the composition and structure of the zebrafish intestinal microbiota and that the effect varies somewhat depending on the V. cholerae genetic background. Alteration of intestinal metabolites can also have an impact on the composition of the microbiota, disturbing complex relationships between different microbes (50–52). To investigate whether V. cholerae infection perturbed the intestinal metabolome, untargeted metabolomics analysis was performed. The metabolomics profiles in the intestines of uninfected control versus infected zebrafish and among the zebrafish infected with different V. cholerae strains were determined. All of the V. cholerae infections resulted in significant differences to select compounds in the zebrafish intestines. The intestines of control fish generally contained higher levels of metabolites such as spermidine, phosphate, citric acid, citramalic acid, and fucose, than the intestines of infected fish (see Fig. S2A to E in the supplemental material). For the V. cholerae-infected zebrafish, those infected with the environmental strains (254-93, AM-19226, and V52) generally displayed higher levels of glycerol, cortisol, 13-HODE, and cafestol (Fig. S2A to E). For the different infection groups, zebrafish infected with 254-93 had higher levels of 2-nonenoic acid, adenine, l-histidine, and shikimic acid (Fig. S2A); zebrafish infected with AM-19226 had higher levels of traumatin, guanosine, jasmonic acid, and traumatic acid (Fig. S2B); and zebrafish infected with V52 displayed significantly increased amino acid levels, including valine, proline, serine, and lysine (Fig. S2C) than those of the control fish. For the El Tor bacteria, E7946-infected fish displayed higher levels of metabolites such as piperidine, glycerol, serine, uracil, valine, thymine, leucine, arginine, malic acid, camosine, and melatonin (Fig. S2D); while fish infected with N16961 displayed significantly higher levels of metabolites such as thymine, adenine, guanine, traumatin, 13-HOTE, cafestol, shikimic acid, jasmonic acid, traumatic acid, and cortisol (Fig. S2E) when each group was compared with the control population.
Comparisons of the metabolomics profiles between strains were also performed and revealed significant differences in metabolites depending on the V. cholerae strain used to infect the zebrafish. To summarize some of the findings, first beginning with the 254-93 comparisons, compared with AM-19226, fish infected with 254-93 displayed significantly higher levels of metabolites, including spermidine, fucose, tyrosine, threonylleucine, and tretinoin; while fish infected with AM-19226 displayed higher levels of adenine and estrone glucuronide (Fig. S2A). Against V52, fish infected with 254-93 displayed significantly higher levels of fucose, tyrosine, catechol, and shikimic acid; while V52-infected fish had higher levels of piperidine, indole, leucine, and daucic acid (Fig. S2B). Against E7946, fish infected with 254-93 had higher levels of succinic acid, benzoic acid, citramalic acid, tyrosine, and oleic acid; while fish infected with E7946 had higher levels of piperidine, pipecolic acid, tyramine, lysine, guanosine, and cyclic AMP (Fig. S2C). Compared with N16961, 254-93 fish had higher levels of ornithine, tyrosine, phosphate, catechol, and citrulline; while fish infected with N16961 had higher levels of guanine, traumatic acid, and thymine (Fig. S2D).
Next, we summarize the AM-19226 comparisons. Compared to V52, fish infected with AM-19226 displayed higher levels of spermidine, traumatin, pimelic acid, and glycylproline; while fish infected with V52 displayed elevated levels of piperidine, indole, leucine, valine, and lysine (Fig. S2E). Against E7946, AM-19226-infected fish had higher levels of phosphate, cyclohexylamine, succinic acid, adenine, and benzoic acid; while fish infected with E7946 displayed higher levels of tyramine, lysine, fucose, pantothenic acid, cyclic AMP, and tretinoin (Fig. S2F). Against N16961, AM-19226-infected fish had higher levels of benzoic acid, spermidine, citrulline, and phenylpyruvic acid; and N16961-infected fish had higher levels of tyramine and deoxyadenosine (Fig. S2G). For V52, compared with E7946, V52-infected fish had higher levels of alanine, piperidine, valine, and adenine; while E7946-infected fish had higher levels of tyramine, fucose, and taurine (Fig. S2H). Compared with N16961, higher levels of propionic acid, piperidine, valine, proline, and uridine were observed in fish infected with V52; while higher levels of creatine, fucose, azelaic acid, and 2-nonenoic acid were observed in fish infected with N16961 (Fig. S2I). Finally, a comparison of E7946- to N16961-infected fish resulted in higher levels of glycerol, spermidine, camosine, and aspartame in E7946-infected fish; while fish infected with N16961 had higher levels of tyrosine, isobutyric acid, hexanal, propionic acid, and phosphate (Fig. S2J). No significant correlations were found between the 15 most detected ASVs and the 15 most prominent metabolites (Mantel test; correlation, R = 0.03485; P = 0.2801). Quantities of all metabolites and statistical analyses are included as supplemental excel files (ESI+ and ESI− untargeted metabolomics).
Addition of V. cholerae to tank water results in minimal changes to the aquatic microbiomes.
In addition to examining how various strains of V. cholerae affected the zebrafish intestinal microbiome, we also examined how these same V. cholerae strains affected the microbial profiles of the aquatic environments that the fish inhabit. While all strains of V. cholerae were able to persist in the normal zebrafish tank water ecosystems (see Fig. S3 in the supplemental material) and some significant differences were observed in the α-diversity of the water microbiome following exposure, with the addition of strains 254-93, V52, E7946, and N16961, all resulting in decreased heterogeneity to the tank water (inverse Simpson; see Fig. S4 in the supplemental material), the effects of Vibrio strains on the composition and structure of the aquatic microbiome were insignificant or modest in most instances (see Fig. S5A to E in the supplemental material). A comparison of the tank water microbiomes exposed to different V. cholerae strains did however reveal that strain AM-19226 was significantly different structurally from all other V. cholerae strains used in this study, consistently displaying higher levels of a select Vibrio ASV (ASV 8) than any other strain (see Fig. S6 in the supplemental material). Jaccard and Bray-Curtis dissimilarity plots displaying all data points are also included to illustrate the overall diversity of the fish and water microbial profiles (see Fig. S7 in the supplemental material).
DISCUSSION
Multiple strains of V. cholerae, both environmental and El Tor, are able to maintain viability in the aquatic environment and are capable of colonizing the zebrafish intestine. Additionally, these strains, including those that do not produce CT or TCP, induce diarrhea in the fish. However, colonization by the genetically different strains had significantly different effects upon the zebrafish intestinal microbiome. Strains AM-19226 and N16961 induced significant decreases in overall intestinal microbiome richness (Chao index), while strains E7946 and V52 increased richness. As predicted, the most detected ASV in terms of relative abundance was Vibrio. This finding was expected, as V. cholerae was used to infect the zebrafish and because the zebrafish intestine harbors a large number of Proteobacteria, particularly members of the Vibrionaceae family. Previous studies from our laboratory of uninfected fish found high levels of Vibrionaceae as well, making changes in their ASV levels after V. cholerae infection unsurprising (38, 53). Fish infected with V. cholerae strain V52 displayed an increase in microbiome heterogeneity (Shannon-Weiner index), while fish infected with N16961 experienced a decrease in heterogeneity. With respect to β-diversity, four of the V. cholerae strains in this study caused significant structural changes to the zebrafish microbiome and one caused significant compositional changes. Therefore, V. cholerae infection alters the proportions of some, but not all, components of the microbiome. A comparison of the postinfection zebrafish microbiomes revealed there were significant differences in the structures and compositions of the intestinal microbial community based on the infecting V. cholerae strain. It is unclear which genetic differences are responsible for these disparities in impact on the microbiome among V. cholerae strains. In the case of the El Tor strains, generations of cultivation in the laboratory may be responsible for some of the observed differences. However, differing virulence factors among the strains is likely a more salient factor. While an exhaustive comparison of the different virulence factors each strain possesses is currently unavailable, some of the differences between them are known. Specifically, the El Tor strains produce both CT and TCP but appear to lack cholix toxin, which all of the environmental strains likely possess. While studies from our lab have not shown CT and TCP to have a large role in zebrafish colonization, the O1/O139 strains still produce both of these virulence factors (36). Dedication of metabolic resources to making CT and TCP may also account for some of the differences observed in colonization, as CT- and TCP-producing strains may not utilize their other virulence factors that have a larger effect on the zebrafish intestine to the same degree as their non-O1/O139 counterparts. Additionally, strain AM-19226 is likely the only strain of these five that produces a T3SS. It is also possible that, while of all of these strains contain and can utilize a T6SS, given the variability in effector distribution, the regulation and lethality of the T6SS could differ greatly by biotype (54, 55).
The differences in virulence factors among the strains are also likely responsible for the variation observed in bacterial diversity following infection. As stated, AM-19226 has a T3SS, which is highly toxic to eukaryotic cells (56, 57). The properties of this system and its role in AM-19226 colonization could be responsible for the decrease in bacterial diversity in the gut via disruption of the symbiotic relationship between fish intestinal epithelial cells and normal flora. For N16961, while it does not have a T3SS (48), virulence factors unique to this particular strain could be causing a similar stress within the zebrafish intestines, resulting in altered conditions that do not favor a certain population of the microbial community, thus causing their death and clearance (due to diarrhea) from the intestinal microbiome. On the other hand, V52 and E7946 could be inducing conditions that allow for new bacteria entering the gut to remain there, at least temporarily, while the infection is occurring, resulting in a significantly increased Chao index.
Significant microbial differences were observed when comparing the post-254-93 infection zebrafish microbiome with all of the wild-type V. cholerae strains except V52, while V52 was significantly different compositionally from all the wild-type strains except 254-93. These differences suggest that different strains of V. cholerae target different components of the resident microbiome and that this selective elimination may be necessary in order to successfully colonize the host intestine. It is also worth pointing out that we have noticed a trend wherein zebrafish infected with non-O1/O139 strains have higher relative abundances of Cetobacterium ASVs. These results were observed when comparing the postinfection zebrafish intestinal profiles of 254-93 against E7946 and N16961, AM-19226 against both E7946 and N16961 (data not shown), and V52 against both E7946 and N16961. This is an intriguing finding as El Tor strains (particularly these two O1 strains) are highly similar genetically, whereas the non-O1/O139 strains are genetically diverse (18, 58, 59), and it suggests that Cetobacterium sp. needs to be eliminated in order for these O1 strains to colonize the zebrafish intestine or that these El Tor V. cholerae strains modulate the intestine in a way that is detrimental to Cetobacterium sp., while the environmental strains do not. A number of Gram-negative bacteria, such as Aeromonas, Plesiomonas, Cetobacterium, Vibrionaceae, and Shewanella sp., are organisms commonly found in aquatic environments and/or associated with aquatic animals. The finding that most of these types of bacteria experienced the largest changes in relative abundance following a V. cholerae infection is expected, as they are major components of the fish microbiome. Only one V. cholerae strain, namely, N16961, was not found to induce significant changes to β-diversity of the zebrafish intestinal microbiome and instead was nearly significant (P = 0.077). The reason for this result could be related to the lower inoculum (7 × 107) used for one of the three experiments on this group of fish.
qPCR analysis of the bacterial load within the zebrafish intestines displayed an even bacterial distribution in three of the five different V. cholerae strains, while strains V52 and E7946 had an elevated bacterial load. This finding indicates that V. cholerae infection does not cause a total collapse of the microbiome or even a significant reduction in the number of microbes present. For these strains, it suggests that V. cholerae selectively competes with some members of the intestinal microbiome, whereas others are unaffected and some even potentially benefit from the infection. In the cases of V52 and E7946 however, the exposure of the zebrafish intestine to these two infecting and rapidly multiplying V. cholerae strains could be the cause of the differences in metabolites that were observed following infection.
Untargeted metabolomics analysis of the zebrafish intestines following V. cholerae infection revealed numerous metabolites that were modulated as a result of infection. In general, V. cholerae infection resulted in increased levels of glycerol, cortisol, and nucleic acids in the intestine, while control fish had higher levels of spermidine, fucose, and myristoleic acid. The finding that levels of spermidine are higher in the control fish is not surprising as that compound has been shown to interfere with V. cholerae biofilm formation, which plays a crucial, but still enigmatic, role in colonization and disease (60, 61). The other two compounds commonly found in higher quantities in the uninfected zebrafish intestines, namely, fucose and myristoleic acid, may serve as potential carbon sources for the infecting V. cholerae, helping them to colonize and initiate infection in the zebrafish host. Additionally, the decreased presence of fucose may be a sign of intestinal distress as the compound is often found on N-linked glycans on cellular surfaces (62). For the infected fish, the presence of cortisol and nucleic acids is understandable, as cortisol is a stress hormone, while the increase in nucleic acids could be due to the presence of the T6SS and cell-to-cell killing.
When comparing the metabolomics profiles of zebrafish infected with different V. cholerae strains, minimal differences were observed when comparing the two environmental strains AM-19226 and 254-93 to one another, while numerous differences in metabolites were observed when comparing most of the other groups of infected zebrafish to one another. Zebrafish microbiomes infected with the environmental strains typically expressed higher levels of propionic acid, a short-chain saturated fatty acid, which along with other short-chain fatty acids seems to have some inhibitory effects on V. cholerae colonization (63), suggesting that the environmental strains may have a higher tolerance to these types of compounds than their El Tor counterparts. Additionally, zebrafish infected with the environmental strains 254-93 and AM-19226 typically had higher levels of spermidine and fucose than all other examined V. cholerae strains used for infection. Considering that these two compounds were generally found to be in lower abundance in V52-infected fish, this could help explain why such an increased bacterial load was detected in the zebrafish intestines following infection with that strain. In the case of E7946, fish infected with this strain of V. cholerae displayed a decreased level of propionic acid, which as discussed above has inhibitory properties toward V. cholerae. Interestingly, fish infected with E7946 or V52 (V52 especially) displayed higher levels of the compound piperidine than nearly all other groups of infected fish. This is a surprising finding as piperidine has displayed highly potent inhibitory effects on V. cholerae (64). The metabolic differences observed in the zebrafish infected with different strains of V. cholerae again suggest that strain-specific modulations are occurring and necessary for successful colonization in the zebrafish intestinal tract. When evaluating these results, however, it must also be considered which of these metabolites are accessible to V. cholerae in the lumen (whether through food fed to the fish or produced by resident bacteria) and which are contained within zebrafish intestinal cells. Short-chain fatty acids, such as propionic acid, and polyamines, such as spermidine, are available in the intestinal lumen, and previous work has shown the formation of biofilm “clump”-like structures in the zebrafish intestines during infection, suggesting that polyamine compounds could play a major role in the infectious cycle of V. cholerae (36, 63, 65). Other metabolites detected in our analysis such as myristoleic acid (an omega-5 fatty acid) and aspartame are likely to have come from external sources, such as food used to feed the zebrafish.
While numerous significant changes were observed in the microbiome of the zebrafish intestine following V. cholerae exposure, such was not the case with the tank water exposed to V. cholerae. None of the strains utilized in this study induced significant changes to the structure of the tank water microbial community. Although we did not see many significant differences in the tank water microbial community compared with the control samples, there were some significant differences noted in the postexposure tank water microbial communities when comparing the different V. cholerae strains. Most of these significant differences were observed in the environmental strain AM-19226. In all of the comparisons with other strains, tank water exposed to strain AM-19226 consistently had higher levels of Vibrio ASV 8, while all the other bacterial populations it was compared to had higher levels of Vibrio ASV 1, which could be encompassing most or all of the select V. cholerae added to the water.
This study and our other study examining the role of the V. cholerae T6SS in zebrafish colonization are the first to our knowledge to examine how various strains of V. cholerae affect the zebrafish intestinal microbiome and the microbial community of the water that houses them. The specific interactions occurring at the microbial level remain to be elucidated. Additionally, our study examined only one time point (24 hours postinfection); studies with a greater emphasis on a temporal relationship may help to determine if specific microbes and/or metabolites are consistently repressed through the duration of the V. cholerae infection and begin to recover as the disease symptoms are alleviated. Furthermore, differences in V. cholerae infectious dose, which was also not a variable in this study, could be a contributing factor to the variation observed in the zebrafish intestinal microbiome. Future studies will aim to answer these questions, and with increased knowledge of the factors necessary for V. cholerae intestinal colonization, the production of a probiotic and/or preventive prophylactic could go a long way in helping to protect individuals in vulnerable regions from future instances of cholera.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Bacterial strains used in this study are listed in Table 1. All bacteria were cultured at 37°C in LB broth with streptomycin (100 μg/ml).
TABLE 1.
Bacterial strains
| Bacteria | Serogroup |
|---|---|
| V. cholerae 254-93 | O144 |
| V. cholerae AM-19226 | O39 |
| V. cholerae V52 | O37 |
| V. cholerae E7946 | O1 El Tor |
| V. cholerae N16961 | O1 El Tor |
Zebrafish.
Adult, wild-type AB zebrafish were used in all experiments. The fish were housed in an Aquaneering aquatic housing system, with the tank water filtered by reverse osmosis and maintained at pH 7.0 to 7.5. Tank water was conditioned with Instant Ocean salt (Aquarium Systems, Mentor, OH) to a conductivity of 400 to 550 μS. The fish were fasted for at least 12 h prior to each experiment. All zebrafish were euthanized in 100 ml of 320 μg/ml Tricaine-S (tricaine methanesulfonate; MS-222; Western Chemical, Ferndale, WA) for a minimum of 25 min. All animal protocols were approved by the Wayne State University IACUC.
Inoculation of zebrafish and tank water via immersion.
Bacterial cultures were incubated with aeration in LB broth at 37°C for 16 to 18 h. Cells were subsequently washed once and then diluted to the correct concentration in sterile 1× phosphate-buffered saline (PBS). Bacterial cell densities ranged from 107 to 109 (254-93, 1.6 × 108 to 1.3 × 109; AM-19226, 6.6 × 108 to 4 × 109; V52, 5 × 108 to 2 × 109; E7946, 1.2 × 108 to 3.5 × 109; N16961, 7 × 107 to 1.3 × 109) per beaker. Four zebrafish per group were placed into a 400-ml beaker with a perforated lid containing 200 ml of sterile infection water (autoclaved tank water). A total of 1 ml of bacterial inoculum was then added to the beaker with fish. The control groups consisted of fish that were exposed to only 1 ml of 1× PBS. Each beaker was placed into a glass-front incubator set at 28°C for the duration of the experiment (24 hours). For the tank water immersions, 500 ml of tank water was pulled off the zebrafish housing system and placed into a 1-liter flask. V. cholerae was added to the flask at a cell density ranging from 107 to 109 (254-93, 1.05 × 108 to 4 × 108; AM-19226, 1.55 × 109 to 4 × 109; V52, 1.5 × 108 to 2 × 109; E7946, 6 × 107 to 31.3 × 109; N16961, 8 × 107 to 1.75 × 109) per beaker. The flasks were then incubated in a water bath at 28°C and 100 rpm for 24 hours.
Intestinal colonization assessment.
After the specified time point, fish were euthanized as described above. The entire intestinal tract of each fish was aseptically excised; placed into homogenization tubes (2.0-ml screw-cap tubes; Sarstedt, Numbrecht, Germany), with 1.5 g of 1.0-mm glass beads (BioSpec Products, Inc., Bartlesville, OK) and 1 ml of 1× PBS; and held on ice. Homogenization tubes were loaded into a Mini-Beadbeater-24 (BioSpec Products, Inc.) and shaken at maximum speed for two 1-min cycles, with the samples being incubated for 1 min on ice after both the first and last cycles. Intestinal homogenates from each fish were diluted and plated for enumeration on LB agar plates that contained 100 μg/ml streptomycin and incubated overnight at 37°C.
Processing fish infection water.
A total of 50 ml of fish infection water was extracted, in duplicate, and put into two 50-ml conical tubes. Tubes were centrifuged at 10,700 × g for 10 min at 4°C, and the supernatant was decanted, being careful not to disturb the pellet. Each pellet was resuspended in 1 ml of 1× PBS. One of the 50-ml conical tubes was used for bacterial enumeration and microbial analysis, while the other was used for mucin and protein quantification.
Processing tank water.
A total of 50 ml of fish infection water was extracted, in duplicate, and put into two 50-ml conical tubes. Tubes were centrifuged at 10,700 × g for 10 min at 4°C, and the supernatant was decanted, being careful not to disturb the pellet. Each pellet was resuspended in 500 μl of 1× PBS, then combined, and plated for enumeration and microbial analysis.
DNA extraction.
For the zebrafish, 500 μl of the obtained intestinal homogenate was harvested for DNA extraction using a DNeasy Powersoil kit (Qiagen, Germantown, MD) following the manufacturer’s instructions. For the tank water samples, 500 μl of the final 1 ml of sample was utilized for processing and DNA extraction, again using a DNeasy Powersoil kit following the manufacturer’s instructions. The cutoff for low DNA recovery yield for samples was 1 ng/μl. Background technical controls (i.e., blank DNA extraction kits) did not yield detectable 16S rRNA gene PCR products.
DNA sequencing.
DNA isolated from individual fish and water samples was used to generate 16S rRNA gene libraries. Illumina MiSeq sequencing was performed at Michigan State University using methods described previously (66–68). The V4 region of the bacterial 16S rRNA gene was targeted for sequencing using the primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). All raw 16S rRNA gene sequence data were processed using the Divisive Amplicon Denoising Algorithm (DADA2, v1.12) in R software (v3.5.1), (https://www.R-project.org) (69–71). DADA2 is a model-based approach for correcting amplicon errors without constructing operational taxonomic units. This method analyzed the 16S rRNA gene amplicon sequence variants (ASVs), defined by 100% sequence similarity, based on the online MiSeq protocol (https://benjjneb.github.io/dada2/tutorial.html) with minor modifications. These modifications included allowing truncation lengths of 250 bp and 150 bp and a maximum number of expected errors of 2 bp and 7 bp for forward and reverse reads, respectively. Sample inference allowed for pooling of samples, which in turn allowed for increased power to detect rare variants. Additionally, samples in the resulting sequence table were pooled prior to removal of chimeric sequences. Sequences were then classified using the silva_nr_v132_train_set database with a minimum bootstrap value of 80% (69, 70). Three fish samples were ultimately excluded from analysis for insufficient sequence yield (one from the control group, one from the AM-19226-infected group, and one from N16961-infected group). The remaining samples (63 fish and 24 tank water) were subsampled to the number of sequences in the least represented sample (4,771 sequences), and a taxonomy was determined for each of the ASVs (n = 1037; with 131 ASVs being singletons). Good’s coverage values averaged 99.0% ± 0.57% (± standard deviation) and 99.6% ± 0.15% for the fish intestine and tank water samples, respectively, indicating that there was thorough sample coverage in this study. Taxonomic designations, raw ASV count data, and Good’s coverage values for each sample are provided as supplemental data.
α-Diversity analysis (i.e., diversity within a microbiome).
The Chao1 index (here referred to as Chao) was utilized to estimate how many different bacterial ASVs were present in a given sample (i.e., richness). The nonparametric Shannon-Wiener and inverse Simpson indices were calculated to indicate both the richness and evenness of samples (i.e., heterogeneity) (72, 73). Due to a batch effect that arose from collecting the fish over the course of several months, generalized linear modeling (GLM) was performed on log-transformed zebrafish intestinal data to evaluate variation in α-diversity using the car, nlme, lme4, and multcomp packages in R (74–77). For the water samples, no GLM was necessary, as the samples were collected only a few days apart and no significant differences were found when comparing the water control samples. The values of the indices were log-transformed before analysis with or without GLM to normalize the data as the residuals need to be normally distributed if tests requiring normality are to be used (78). The GLM output included post hoc comparisons evaluated by multiple comparisons of mean values, with Tukey contrasts.
β-Diversity analysis (i.e., diversity between two or more microbiomes).
Microbiome composition (i.e., membership) and structure (i.e., membership plus members’ relative abundances) were characterized using Jaccard and Bray-Curtis ecological similarity indices, respectively. Principal-coordinate analysis (PCoA) plots were used to visualize variation in microbiomes among samples, and the effects of V. cholerae infections on microbiome composition and structure were evaluated using permutation multivariate analysis of variance (PERMANOVA or nonparametric multivariate analysis of variance [NPMANOVA]) (73, 79–81). The adonis function in the Vegan package of R (http://cc.oulu.fi/∼jarioksa/softhelp/vegan/html/adonis.html) (82) was used to control for fish batch effect (i.e., by the strata command), specifically to account for the collection date differences in the fish intestinal samples. Raw P values are presented for all multivariate permutation tests (999 permutations). No Bonferroni corrections were applied for β-diversity analyses, as they can be overly conservative when used with permutation tests (83). Linear discriminant analysis effect size (LEfSe) analyses were conducted to identify the amplicon sequence variants (ASVs) that were differentially relatively abundant among the sample types (84).These LEfSe analyses were controlled for batch effect using the ID (i.e., collection time) parameter. Within the LEfSe figures, all the ASVs that had a linear discriminant analysis (LDA) score of >2.5 were presented.
Microtiter PAS assay.
This assay was performed as described in Mitchell et al. (30) to quantify the amount of secreted mucin, a biomarker for diarrhea, in the infection water. A total of 1 ml of a 50% (wt/vol) periodic acid (Sigma-Aldrich, St. Louis, MO) stock solution was made which can be stored protected from light at 4°C for up to 1 week. A 96-well plate (Costar 3361; Corning, NY) was loaded with 100 μl/well of the blank (1× PBS), mucin standards (see below), and samples in triplicate. A volume of 50 μl/well of fresh 0.1% periodic acid solution (10 μl of the 50% periodic acid stock added to 5 ml of 7% acetic acid, used immediately after making) was added and mixed by pipetting. The plate was covered in plastic wrap and incubated at 37°C for 1 to 1.5 h. After incubation, the plate was cooled to room temperature (∼5 min) before adding 100 μl/well Schiff’s reagent (84655; Sigma-Aldrich) and mixing with a pipette. The plate was again covered in plastic wrap and placed on a rocker or shaker for 15 to 40 min or until sufficient color developed. Absorbance was read at 560 nm using a plate reader (Tecan SpectraFluor plus; Mannedorf, Switzerland).
Mucin standards.
Mucin standards were made by suspending the appropriate amount of mucin from the porcine stomach, as follows: type III (M1778; Sigma-Aldrich, St. Louis, MO) in sodium acetate buffer (pH 5.5; 100 mM sodium acetate and 5 mM EDTA [pH 5.5], with glacial acetic acid), at 400 μg/ml, 300 μg/ml, 200 μg/ml, 150 μg/ml, 100 μg/ml, 75 μg/ml, 50 μg/ml, 25 μg/ml, and 10 μg/ml. The mixtures were stored at 4°C.
Protein assay.
One milliliter of the Pierce 660-nm protein assay reagent (Thermo Fisher Scientific, Waltham, MA) was combined with 67 μl of each protein standard or sample, mixed, and incubated at room temperature for 5 minutes. Absorbance was read at 660 nm on a spectrophotometer (Thermo Fisher Scientific) using double-distilled water (ddH2O) as a blank.
Protein standards.
The following bovine serum albumin (BSA) standards were made from an 1,800 μg/ml BSA stock solution: 1,800 μg/ml, 1,000 μg/ml, 750 μg/ml, 500 μg/ml, 250 μg/ml, 125 μg/ml, 50 μg/ml, and 25 μg/ml. The solutions were stored at 4°C.
qPCR.
Total bacterial DNA abundance within samples was measured via amplification of the V1-V2 region of the 16S rRNA gene by the protocol of Dickson et al. (85) with minor modifications. These modifications included the use of a degenerative forward primer (27f-CM [5′-AGA GTT TGA TCM TGG CTC AG-3′]) and a degenerate probe containing locked nucleic acids (+) (BSR65/17 [5′-6-carboxyfluorescein (56FAM)-TAA + YA + C ATG + CA +A GT + C GA-black hole quencher 1 [BHQ1]-3′]). Each 20-μl reaction mixture contained 0.6 μM 27f-CM primer, 0.6 μM 357R primer (5′-CTG CTG CCT YCC GTA G-3′), 0.25 μM BSR65/17 probe, 10.0 μl of 2 TaqMan environmental master mix 2.0 (Life Technologies, Carlsbad, CA), and 3.0 μl of either purified DNA (diluted to 80 ng/μl when possible), elution buffer, or nuclease-free water. The total bacterial DNA qPCR was performed using the following conditions: 95°C for 10 min, followed by 45 cycles consisting of 94°C for 30 s, 50°C for 30 s, and 72°C for 30 s. Duplicate reactions were run for all samples. Raw amplification data were normalized to the ROX passive reference dye and analyzed using the online platform Thermo Fisher Cloud: Standard Curve (SR) 3.3.0-SR2-build15 with automatic threshold and baseline settings. Quantification cycle (Cq) values were calculated for samples based on the mean number of cycles required for normalized fluorescence to exponentially increase. After plotting a regression of log E. coli 16S rRNA gene copy number and Cq value for standard curves included in each qPCR run, 16S rRNA gene copy number in zebrafish samples was calculated according to Gallup (51) using the equation Xo = EAMPb −Cq, where EAMP is the exponential amplification value for the qPCR assay, calculated as EAMP = 10−1/m and b and m are the intercept and slope of the regression, respectively (52, 70).
Metabolomics.
Untargeted metabolomics was performed by Creative Proteomics. Briefly, zebrafish intestinal homogenates in 1× PBS were thawed, and 100-μl aliquots were extracted with 300 μl of 80% methanol. All samples were then kept at −40°C for 1 h. After that step, samples were vortexed for 30 s and centrifuged at 12,000 rpm and 4°C for 15 min. Finally, 200 μl of the supernatant and 5 μl of DL-o-chlorophenylalanine (100 μg/ml) were transferred to a vial for liquid chromatography-mass spectrometry (LC-MS) analysis. Quality-control (QC) samples were used to evaluate the methodology. The same amount of extract was obtained from each sample and mixed as QC samples. The QC sample was prepared using the same sample preparation procedure. Ultraperformance liquid chromatography–time of flight mass spectrometry (UPLC-TOF-MS) separation was performed by an Ultimate 3000LC system combined with a Q Exactive MS instrument (Thermo Scientific) and screened with electrospray ionization mass spectrometry (ESI-MS) (targeted MS/MS mode). The LC system is comprised of an Acquity UPLC HSS T3 column (100 by 2.1 mm, 1.8 μm) with the Ultimate 3000LC system. The mobile phase is composed of solvent A (0.05% formic acid-water) and solvent B (acetonitrile) with a gradient elution (0 to 1 min, 5% B;1 to 12 min, 5 to 95% B;12 to 13.5 min, 95% B;13.5 to 13.6 min, 95 to 5% B;13.6 to 16.0 min, 5% B). The flow rate of the mobile phase is 0.3 ml·min−1. The column temperature is maintained at 40°C, and the sample manager temperature is set at 4°C. Mass spectrometry parameters in positive ion mode (ESI+; where the analyte is sprayed at low pH to encourage positive ion formation, generally detecting protonated and/or alkali adduct analyte molecules) and negative ion mode (ESI−; where the analysis is carried out well above a molecules isoelectric point to deprotonate the molecule) are listed as follows: ESI+: heater temp, 300°C; sheath gas flow rate, 45 arb; aux gas flow rate, 15 arb; sweep gas flow rate, 1 arb; spray voltage, 3.0 kV; capillary temp, 350°C; S-Lens radio frequency (RF) level, 30%; and ESI−: heater temp, 300°C; sheath gas flow rate, 45 arb; aux gas flow rate, 15 arb; sweep gas flow rate, 1 arb; spray voltage, 3.2 kV; capillary temp, 350°C; S-Lens RF level, 60%. The full list of detected metabolites (both ESI+ and ESI–) is included in supplemental files.
Statistical analysis.
Each experiment was performed a minimum of two times on separate occasions, unless otherwise specified in the figure legends. One-way and two-way analyses of variance (ANOVAs) with Tukey’s multiple-comparison test, Dunnett’s multiple-comparison test against a control, Student’s t test, Mantel test, or Bonferroni’s correction were conducted as described in the figure legends. Analyses were performed using GraphPad Prism 7.0, past v4.02; Excel; and R software.
Data availability.
The data that support the findings of this study are available from the corresponding author (J.H.W.) by email, jwithey@med.wayne.edu, upon reasonable request.
ACKNOWLEDGMENTS
We are grateful to members of the Withey and Theis labs for helpful discussions and feedback while preparing the manuscript.
This work was supported by Public Health Service grant R01AI127390 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Supplemental material is available online only.
Contributor Information
Jeffrey H. Withey, Email: jwithey@med.wayne.edu.
Manuela Raffatellu, University of California San Diego School of Medicine.
REFERENCES
- 1.WHO. 2020. Cholera. WHO, Geneva, Switzerland. https://www.who.int/gho/epidemic_diseases/cholera/en/. Accessed 20 February 2020. [Google Scholar]
- 2.Zhang P, Li F, Liang W, Li J, Kan B, Wang D. 2014. The seventh pandemic Vibrio cholerae O1 El Tor isolate in China has undergone genetic shifts. J Clin Microbiol 52:964–967. 10.1128/JCM.03121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhury G, Joshi S, Bhattacharya S, Sekar U, Birajdar B, Bhattacharyya A, Shinoda S, Ramamurthy T. 2016. Extraintestinal infections caused by non-toxigenic Vibrio cholerae non-O1/non-O139. Front Microbiol 7:144. 10.3389/fmicb.2016.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2020. Number of reported cholera cases. WHO, Geneva, Switzerland. https://www.who.int/gho/epidemic_diseases/cholera/cases_text/en/. Accessed 26 February 2020. [Google Scholar]
- 5.CDC. 2020. Cholera - Vibrio cholerae infection. WHO, Geneva, Switzerland. https://www.cdc.gov/cholera/index.html. Accessed 20 February 2020. [Google Scholar]
- 6.CDC. 2014. Non-O1 and non-O139 Vibrio cholerae infections, on CDC. WHO, Geneva, Switzerland. https://www.cdc.gov/cholera/non-01-0139-infections.html. Accessed 27 February 2020. [Google Scholar]
- 7.Vezzulli L, Grande C, Reid PC, Helaouet P, Edwards M, Hofle MG, Brettar I, Colwell RR, Pruzzo C. 2016. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci U S A 113:E5062–E5071. 10.1073/pnas.1609157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M, Isaäcson M. 1999. The effect of iron on the toxigenicity of Vibrio cholerae. Am J Trop Med Hyg 60:392–396. 10.4269/ajtmh.1999.60.392. [DOI] [PubMed] [Google Scholar]
- 9.Asadgol Z, Mohammadi H, Kermani M, Badirzadeh A, Gholami M. 2019. The effect of climate change on cholera disease: the road ahead using artificial neural network. PLoS One 14:e0224813. 10.1371/journal.pone.0224813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J. 2017. Non-cholera vibrios: the microbial barometer of climate change. Trends Microbiol 25:76–84. 10.1016/j.tim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury FR, Nur Z, Hassan N, von Seidlein L, Dunachie S. 2017. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann Clin Microbiol Antimicrob 16:10. 10.1186/s12941-017-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeb R, Tufford D, Scott GI, Moore JG, Dow K. 2018. Impact of climate change on Vibrio vulnificus abundance and exposure risk. Estuaries Coast 41:2289–2303. 10.1007/s12237-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhling BA, Jacobs J, Stock CA, Gaitan CF, Saba VS. 2017. Projections of the future occurrence, distribution, and seasonality of three Vibrio species in the Chesapeake Bay under a high-emission climate change scenario. GeoHealth 1:278–296. 10.1002/2017GH000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momba M, Azab El-Liethy M, Pruden A, Ashbolt N, Miller J. 2019. Vibrio cholerae and cholera biotypes. InRose JB, Jiménez-Cisneros B, (eds) Water and sanitation for the 21st century: health and microbiological aspects of excreta and wastewater management (Global Water Pathogen Project). Michigan State University, East Lansing, MI. [. 10.14321/waterpathogens.28.]. [DOI] [Google Scholar]
- 15.Fu K, Li J, Wang Y, Liu J, Yan H, Shi L, Zhou L. 2016. An innovative method for rapid identification and detection of Vibrio alginolyticus in different infection models. Front Microbiol 7:651. 10.3389/fmicb.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siriphap A, Leekitcharoenphon P, Kaas RS, Theethakaew C, Aarestrup FM, Sutheinkul O, Hendriksen RS. 2017. Characterization and genetic variation of Vibrio cholerae isolated from clinical and environmental sources in Thailand. PLoS One 12:e0169324. 10.1371/journal.pone.0169324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang B, Yan M, Cui Z, Ye X, Diao B, Ren Y, Gao S, Zhang L, Kan B. 2007. Genetic diversity of toxigenic and nontoxigenic Vibrio cholerae serogroups O1 and O139 revealed by array-based comparative genomic hybridization. J Bacteriol 189:4837–4849. 10.1128/JB.01959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faruque SM, Chowdhury N, Kamruzzaman M, Dziejman M, Rahman MH, Sack DA, Nair GB, Mekalanos JJ. 2004. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc Natl Acad Sci U S A 101:2123–2128. 10.1073/pnas.0308485100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyhan S, Tischler AD, Camilli A, Yildiz FH. 2006. Differences in gene expression between the classical and El Tor biotypes of Vibrio cholerae O1. Infect Immun 74:3633–3642. 10.1128/IAI.01750-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murley YM, Behari J, Griffin R, Calderwood SB. 2000. Classical and El Tor biotypes of Vibrio cholerae differ in timing of transcription of tcpPH during growth in inducing conditions. Infect Immun 68:3010–3014. 10.1128/iai.68.5.3010-3014.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matson JS, Withey JH, DiRita VJ. 2007. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun 75:5542–5549. 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyata ST, Kitaoka M, Wieteska L, Frech C, Chen N, Pukatzki S. 2010. The Vibrio cholerae Type VI secretion system: evaluating its role in the human disease cholera. Front Microbiol 1:117. 10.3389/fmicb.2010.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, Bilecen K, Yildiz F, McCardell B. 2010. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One 5:e11558. 10.1371/journal.pone.0011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linhartová I, Bumba L, Mašín J, Basler M, Osička R, Kamanová J, Procházková K, Adkins I, Hejnová-Holubová J, Sadílková L, Morová J, Šebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 34:1076–1112. 10.1111/j.1574-6976.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordero CL, Kudryashov DS, Reisler E, Satchell KJ. 2006. The Actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin. J Biol Chem 281:32366–32374. 10.1074/jbc.M605275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz K, Hammerl JA, Gollner C, Strauch E. 2019. Environmental and clinical strains of Vibrio cholerae non-O1, non-O139 from Germany possess similar virulence gene profiles. Front Microbiol 10:733. 10.3389/fmicb.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dongre M, Singh B, Aung KM, Larsson P, Miftakhova R, Persson K, Askarian F, Johannessen M, von Hofsten J, Persson JL, Erhardt M, Tuck S, Uhlin BE, Wai SN. 2018. Flagella-mediated secretion of a novel Vibrio cholerae cytotoxin affecting both vertebrate and invertebrate hosts. Commun Biol 1:59. 10.1038/s42003-018-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hounmanou YMG, Leekitcharoenphon P, Kudirkiene E, Mdegela RH, Hendriksen RS, Olsen JE, Dalsgaard A. 2019. Genomic insights into Vibrio cholerae O1 responsible for cholera epidemics in Tanzania between 1993 and 2017. PLoS Negl Trop Dis 13:e0007934. 10.1371/journal.pntd.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick R, Ghosal A, Das B, Koley H, Saha DR, Ganguly S, Nandy RK, Bhadra RK, Chatterjee NS. 2008. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun 76:4968–4977. 10.1128/IAI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell KC, Breen P, Britton S, Neely MN, Withey JH. 2017. Quantifying Vibrio cholerae enterotoxicity in a zebrafish infection model. Appl Environ Microbiol 83. 10.1128/AEM.00783-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, Hammer BK, Parthasarathy R. 2018. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc Natl Acad Sci U S A 115:E3779–E3787. 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann V, Kostiuk B, Unterweger D, Diaz-Satizabal L, Ogg S, Pukatzki S. 2015. Bile salts modulate the mucin-activated type VI secretion system of pandemic Vibrio cholerae. PLoS Negl Trop Dis 9:e0004031. 10.1371/journal.pntd.0004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawasvirojwong S, Srimanote P, Chatsudthipong V, Muanprasat C. 2013. An adult mouse model of Vibrio cholerae-induced diarrhea for studying pathogenesis and potential therapy of cholera. PLoS Negl Trop Dis 7:e2293. 10.1371/journal.pntd.0002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matson JS. 2018. Infant mouse model of Vibrio cholerae infection and colonization. Methods Mol Biol 1839:147–152. 10.1007/978-1-4939-8685-9_13. [DOI] [PubMed] [Google Scholar]
- 35.Nygren E, Li BL, Holmgren J, Attridge SR. 2009. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect Immun 77:3475–3484. 10.1128/IAI.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. 2014. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80:1710–1717. 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halpern M, Izhaki I. 2017. Fish as hosts of Vibrio cholerae. Front Microbiol 8:282. 10.3389/fmicb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breen P, Winters AD, Nag D, Ahmad MM, Theis KR, Withey JH. 2019. Internal versus external pressures: effect of housing systems on the zebrafish microbiome. Zebrafish 16:388–400. 10.1089/zeb.2018.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nag D, Breen P, Raychaudhuri S, Withey JH. 2018. Glucose metabolism by Escherichia coli inhibits Vibrio cholerae intestinal colonization of zebrafish. Infect Immun 86:e00486-18. 10.1128/IAI.00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senderovich Y, Izhaki I, Halpern M. 2010. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607. 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archie EA, Theis KR. 2011. Animal behaviour meets microbial ecology. Anim Behav 82:425–436. 10.1016/j.anbehav.2011.05.029. [DOI] [Google Scholar]
- 42.Sekirov I, Russell SL, Antunes LC, Finlay BB. 2010. Gut microbiota in health and disease. Physiol Rev 90:859–904. 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 43.Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJ. 2016. The composition of the zebrafish intestinal microbial community varies across development. ISME J 10:644–654. 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wexler AG, Bao Y, Whitney JC, Bobay LM, Xavier JB, Schofield WB, Barry NA, Russell AB, Tran BQ, Goo YA, Goodlett DR, Ochman H, Mougous JD, Goodman AL. 2016. Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc Natl Acad Sci U S A 113:3639–3644. 10.1073/pnas.1525637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatzidaki-Livanis M, Geva-Zatorsky N, Comstock LE. 2016. Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc Natl Acad Sci U S A 113:3627–3632. 10.1073/pnas.1522510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller KA, Chaand M, Gregoire S, Yoshida T, Beck LA, Ivanov AI, Dziejman M. 2016. Characterization of V. cholerae T3SS-dependent cytotoxicity in cultured intestinal epithelial cells. Cell Microbiol 18:1857–1870. 10.1111/cmi.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linhartova I, Osicka R, Bumba L, Masin J, Sebo P. 2015. RTX toxins: a review, p 1–29. In Gopalakrishnakone P, Stiles B, Alape-Girón A, Dubreuil J, Mandal M (eds), Microbial toxins. Toxinology. Springer, Dordrecht, the Netherlands. 10.1007/978-94-007-6725-6_13-1. [DOI] [Google Scholar]
- 48.Dziejman M, Serruto D, Tam VC, Sturtevant D, Diraphat P, Faruque SM, Rahman MH, Heidelberg JF, Decker J, Li L, Montgomery KT, Grills G, Kucherlapati R, Mekalanos JJ. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci U S A 102:3465–3470. 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purdy AE, Balch D, Lizarraga-Partida ML, Islam MS, Martinez-Urtaza J, Huq A, Colwell RR, Bartlett DH. 2010. Diversity and distribution of cholix toxin, a novel ADP-ribosylating factor from Vibrio cholerae. Environ Microbiol Rep 2:198–207. 10.1111/j.1758-2229.2010.00139.x. [DOI] [PubMed] [Google Scholar]
- 50.Nag D, Mitchell K, Breen P, Withey JH. 2018. Quantifying Vibrio cholerae colonization and diarrhea in the adult zebrafish model. J Vis Exp 57767. 10.3791/57767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallup J. 2001. qPCR inhibition and amplification of difficult templates. InPCR troubleshooting and optimization: the essential guide. Caister Academic, Norfolk, UK. [Google Scholar]
- 52.Theis KR, Romero R, Greenberg JM, Winters AD, Garcia-Flores V, Motomura K, Ahmad MM, Galaz J, Arenas-Hernandez M, Gomez-Lopez N. 2020. No consistent evidence for microbiota in murine placental and fetal tissues. mSphere 5:e00933-19. 10.1128/mSphere.00933-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. 2011. Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, Provenzano D, Pukatzki S. 2014. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 5:3549. 10.1038/ncomms4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cianfanelli FR, Monlezun L, Coulthurst SJ. 2016. Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol 24:51–62. 10.1016/j.tim.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, Waldor MK, Mekalanos JJ. 2011. Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae. mBio 2:e00106-11. 10.1128/mBio.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaand M, Miller KA, Sofia MK, Schlesener C, Weaver JW, Sood V, Dziejman M. 2015. Type 3 secretion system island encoded proteins required for colonization by non-O1/non-O139 serogroup Vibrio cholerae. Infect Immun 83:2862–2869. 10.1128/IAI.03020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Shea YA, Reen FJ, Quirke AM, Boyd EF. 2004. Evolutionary genetic analysis of the emergence of epidemic Vibrio cholerae isolates on the basis of comparative nucleotide sequence analysis and multilocus virulence gene profiles. J Clin Microbiol 42:4657–4671. 10.1128/JCM.42.10.4657-4671.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faruque SM, Sack DA, Sack RB, Colwell RR, Takeda Y, Nair GB. 2003. Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci U S A 100:1304–1309. 10.1073/pnas.0337468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGinnis MW, Parker ZM, Walter NE, Rutkovsky AC, Cartaya-Marin C, Karatan E. 2009. Spermidine regulates Vibrio cholerae biofilm formation via transport and signaling pathways. FEMS Microbiol Lett 299:166–174. 10.1111/j.1574-6968.2009.01744.x. [DOI] [PubMed] [Google Scholar]
- 61.Sobe RC, Bond WG, Wotanis CK, Zayner JP, Burriss MA, Fernandez N, Bruger EL, Waters CM, Neufeld HS, Karatan E. 2017. Spermine inhibits Vibrio cholerae biofilm formation through the NspS-MbaA polyamine signaling system. J Biol Chem 292:17025–17036. 10.1074/jbc.M117.801068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker DJ, Lowe JB. 2003. Fucose: biosynthesis and biological function in mammals. Glycobiology 13:41R–53R. 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 63.You JS, Yong J-H, Kim GH, Moon S, Nam KT, Ryu JH, Yoon MY, Yoon SS. 2019. Commensal-derived metabolites govern Vibrio cholerae pathogenesis in host intestine. Microbiome 7:132. 10.1186/s40168-019-0746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acosta-Smith E, Leon-Sicairos N, Tiwari S, Flores-Villasenor H, Canizalez-Roman A, Kumavath R, Ghosh P, Azevedo V, Barh D. 2019. Piper betel compounds piperidine, eugenyl acetate, and chlorogenic acid are broad-spectrum anti-vibrio compounds that are also effective on MDR strains of the pathogen. Pathogens 8:64. 10.3390/pathogens8020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tofalo R, Cocchi S, Suzzi G. 2019. Polyamines and gut microbiota. Front Nutr 6:16. 10.3389/fnut.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whittaker DJ, Gerlach NM, Slowinski SP, Corcoran KP, Winters AD, Soini HA, Novotny MV, Ketterson ED, Theis KR. 2016. Social environment has a primary influence on the microbial and odor profiles of a chemically signaling songbird. Front Ecol Evol 4:90. 10.3389/fevo.2016.00090. [DOI] [Google Scholar]
- 67.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angoa-Pérez M, Zagorac B, Anneken JH, Briggs DI, Winters AD, Greenberg JM, Ahmad M, Theis KR, Kuhn DM. 2020. Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis. Sci Rep 10:8949. 10.1038/s41598-020-65972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theis KR, Romero R, Winters AD, Jobe AH, Gomez-Lopez N. 2020. Lack of evidence for microbiota in the placental and fetal tissues of rhesus macaques. mSphere 5:e00210-20. 10.1128/mSphere.00210-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao A, Shen T. 2003. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ Ecol Stat 10:429–443. 10.1023/A:1026096204727. [DOI] [Google Scholar]
- 73.Magurran AE. 2003. Measuring biological diversity, 1st edition.Wiley-Blackwell, New Jersey, USA. [Google Scholar]
- 74.Fox J, Weisberg S, Price B, Adler D, Bates D, Baud-Bovy G, Bolker B, Ellison S. 2018. CRAN- Package “car” - R Project. https://cran.r-project.org/web/packages/car/car.pdf.
- 75.Pinheiro J, Bates D, DebRoy S, Sarkar D, Authors E, Heisterkamp S, Willigen BV. 2018. CRAN - Package “nlme” - R Project. https://cran.r-project.org/web/packages/nlme/nlme.pdf.
- 76.Bates D, Maechler M, Bolker B, Walker S, Singmann RHBC, Dai B, Scheipl F, Grothendieck G. 2019. CRAN - Package “lme4” - R Package. https://cran.r-project.org/web/packages/lme4/lme4.pdf.
- 77.Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S. 2019. CRAN - Package “multcomp” - R Project. https://cran.r-project.org/web/packages/multcomp/multcomp.pdf.
- 78.Kéry M, Hatfield JS. 2003. Normality of raw data in general linear models The most widespread myth in statistics. Bull Ecol Soc Amer 84:92–94. 10.1890/0012-9623(2003)84[92:NORDIG]2.0.CO;2. [DOI] [Google Scholar]
- 79.Clarke KR. 1993. Non‐parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- 80.Ramette A. 2007. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160. 10.1111/j.1574-6941.2007.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson MJ. 2001. A new method for non‐parametric multivariate analysis of variance. Austral Ecology 26:32–46. 10.1111/j.1442-9993.2001.01070.pp.x. [DOI] [Google Scholar]
- 82.Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P, O'Hara RB, Simpson GL, Solymos PM, Stevens HH, Szoecs E, Wagner H. 2019. CRAN - Package vegan - R Project. https://CRAN.R-project.org/package=vegan.
- 83.Hammer Ø. 2019. PAST (PAleontological STatistics) reference manual. https://folk.uio.no/ohammer/past/past3manual.pdf.
- 84.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. 2014. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One 9:e97214. 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download IAI.00157-21-s0001.pdf, PDF file, 10.8 MB (10.8MB, pdf)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (J.H.W.) by email, jwithey@med.wayne.edu, upon reasonable request.