ABSTRACT
Short-chain fatty acids (SCFAs) are the main metabolites produced by the gut microbiota via the fermentation of complex carbohydrates and fibers. Evidence suggests that SCFAs play a role in the control of infections through direct action both on microorganisms and on host signaling. This review summarizes the main microbicidal effects of SCFAs and discusses studies highlighting the effect of SCFAs in the virulence and viability of microorganisms. We also describe the diverse and complex modes of action of the SCFAs on the immune system in the face of infections with a specific focus on bacterial and viral respiratory infections. A growing body of evidence suggests that SCFAs protect against lung infections. Finally, we present potential strategies that may be leveraged to exploit the biological properties of SCFAs for increasing effectiveness and optimizing patient benefits.
KEYWORDS: bacteria, gut, respiratory infection, short-chain fatty acid, virus
INTRODUCTION
Short-chain fatty acids (SCFAs), namely, acetate (C2), propionate (C3), and butyrate (C4), are the main metabolites produced by the gut microbiota (in a 60:20:20 proportion, respectively). These compounds are derived from the anaerobic fermentation of nondigestible polysaccharides, such as resistant starches and dietary fibers. The SCFA concentration in the intestine can be as high as 10 to 100 mM, and the SCFAs exert many physiological functions (1–4). For example, butyrate and (to a lesser extent) the other two SCFAs are major energy sources for colonocytes and act as key factors in intestinal epithelial cell growth and function. SCFAs regulate inflammatory responses, and SCFA supplementation can reduce the severity of intestinal disorders such as colitis (5, 6). It is important to note that the effects of microbiota-derived SCFAs are not limited to the intestinal compartment. In fact, SCFAs can cross into the blood and act at distal sites, such as the lungs (7). Many studies have reported that SCFAs protect against infections. Various modes of action have been reported in this context, ranging from a direct effect on the growth and/or virulence of microorganisms to an indirect effect on the host immune system.
Infections.
Despite the advent and widespread use of vaccines, antibiotics, antiviral drugs, and antifungal drugs, microbial infections still constitute a major public health issue, exemplified by the recent coronavirus disease 19 (COVID-19). In 2019, a report from World Health Organization stated that 26% of deaths worldwide are caused by communicable diseases. With 3 million deaths per year, lower respiratory tract infections constitute the fourth leading cause of death overall and the deadliest communicable disease. Nowadays, there is an increasing number of infections caused by multiresistant microorganisms that can mutate and spread easily. Given the impact of infections on mortality and morbidity rates worldwide, it is extremely important to study these diseases. As mentioned above, we lack effective means of preventing and treating many infections. The rise in antibiotic resistance, the large number of bacterial serotypes, and viral shift and drift are major concerns for physicians and researchers. Furthermore, the general therapeutic time frame to treat patients with antivirals and antibiotics is short. If the treatment is not administered early enough, an exacerbated inflammatory response will lead to tissue damage, loss of function, and greater morbidity. Thus, we need to find alternative approaches that boost host defenses and keep inflammation under control.
The SCFAs’ anti-infective modes of action.
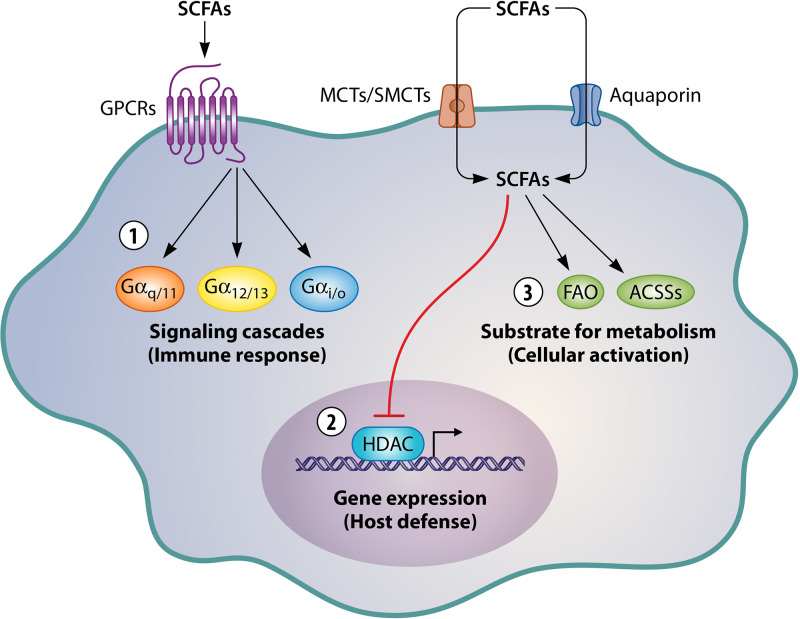
The ability of SCFAs to protect against infections has long been known; it has been extensively studied since the 1930, when SCFAs in sweat were first described as having potential fungicidal and bactericidal activities (8, 9). Thanks to scientific and technological progress, SCFAs can now be detected in high concentrations in the gut, where the compounds have several essential roles. The SCFA concentrations in the gut are directly related to the respective compositions of the diet and the microbiota. Many studies have shown that a high SCFA concentration in the gut is correlated with resistance to infections (10–12). SCFAs can exert effects on infections in two ways. First, they can act directly on the pathogen’s growth or virulence (13, 14). Second, they can act indirectly by modulating the host’s defenses (i.e., its immune or epithelial cells) through three different mechanisms (Fig. 1). The first mechanism to be discovered (and the best studied to date) is direct binding of SCFAs to free fatty acid receptor 2 (FFAR2) and FFAR3 and to the hydroxycarboxylic acid receptor, which are also referred to as G protein-coupled receptors 43, 41, and 109A, respectively. SCFA binding causes the receptor to couple with various G protein alpha subunit classes (Gαi/o, Gαq/11, and Gα12/13), which in turn activate signaling cascades and modulate the host’s defensive response. Furthermore, the receptors can form homodimers or heterodimers—an action that further amplifies the range of responses (15, 16). SCFAs can also enter cells via transporters (such as the monocarboxylate transporter, the sodium monocarboxylate transporter, and aquaporin) or by passive diffusion across the cell membrane (17, 18). Once inside the cell, SCFAs can exert a second action, namely inhibition of histone deacetylase (HDAC), which opens up the chromatin, upregulates gene expression, and thus regulates the host’s defenses (19). The third mechanism is due to cellular metabolism of the SCFAs, which can culminate in cell activation (20). The SCFAs either enter the fatty acid β-oxidation or are directly converted to acetyl-coenzyme A (CoA) by acetyl-coenzyme A synthetase (5, 6). Hence, SCFAs have a broad range of mechanisms of action and targets. It is therefore important to (i) gain a better understanding of how SCFAs boost host defenses and (ii) determine whether these compounds can be used to treat infections (e.g., in combination with antibiotics or immunomodulators) in general and lung infections in particular.
FIG 1.
Modes of action of SCFAs. SCFAs can activate G protein-coupled receptors (no. 1) and/or enter cells, where they inhibit HDAC (no. 2) or influence metabolic pathways (no. 3). MCT, monocarboxylate transporter; SMCT, sodium-dependent monocarboxylate transporter; GPCR, G-protein-coupled receptor; HDAC, histone deacetylase.
THE ROLE OF SCFAs DURING INFECTION
Direct effects of SCFAs on microorganisms.
(i) Effects on microbial growth. The potential of SCFAs for direct microbicidal activity was already being investigated in the 1930s. At that time, researchers found that sweat had antimicrobial activity and that this was due to the presence of SCFAs (21). During the same period, the antifungal activity of SCFAs was also described (9). Since that time, the microbicidal activity of SCFAs has been extensively investigated; these compounds are now known to kill or to slow the growth of Enterococcus faecalis (22), Pseudomonas aeruginosa (23), Mycobacterium tuberculosis (24), Cryptococcus neoformans (14), and several other bacteria (10, 25–32). However, few of these studies reported on the mechanism by which the bacterial growth was abrogated or impaired. It has been shown that propionate can diffuse across the cell membrane of Gram-negative bacteria (e.g., Salmonella enterica subsp. enterica serovar Typhimurium); the resulting change in cytoplasmic pH causes an acid stress response and ultimately inhibits bacterial growth (10). Another research group reported that acetate’s bactericidal effect on Shigella flexneri was due to an impairment of glucose metabolism (31). The growth of Escherichia coli growth in acid medium was also inhibited by SCFAs, and especially by acetate (33). Due to intracellular accumulation of acid anions, acetate affected the E. coli cell’s anion balance and contributed to growth inhibition (27). It was subsequently reported that acetate could also reduce methionine levels, increase the concentration of methionine’s toxic intermediate (homocysteine), and thus cause a reduction in the cytoplasmic pH in E. coli (26). Despite metabolic differences that depend on the SCFA and the microorganism in question, all SCFAs have to enter the cell if they are to inhibit growth. To do so, the extracellular pH must be low. The toxicity of SCFAs at low pH is due to uncoupling. At a low pH, greater amounts of undissociated (i.e., lipid-soluble) SCFAs can diffuse across the cell membrane. Once in the alkaline cytoplasm, the SCFAs dissociate and thus raise the intracellular concentrations of SCFA anions and protons. The resulting perturbation of the cell’s anion balance changes the osmotic balance, reduces the intracellular pH, dissipates the proton motive force, and thus compromises cell metabolism. Gram-positive bacteria are usually more resistant than Gram-negative bacteria to SCFAs, because they are more tolerant to a low intracellular pH. Thus, the influx of undissociated SCFAs is not as great in Gram-positive bacteria as it is in Gram-negative bacteria. Furthermore, Gram-positive bacteria have higher concentrations of potassium ions, which pair with the SCFA anions (27, 34). Thus, the antimicrobial activity of SCFAs requires an acid pH and high SCFA concentrations.
(ii) Effects on microbial virulence. Along with a direct effect on growth, SCFAs are also potent modulators of virulence factors. As mentioned above, SCFAs can decrease the intracellular pH in Salmonella, which then slows the flagellar motor and reduces bacterial motility (35). Furthermore, SCFAs can reduce Salmonella biofilm formation by impairing the extracellular production of polysaccharides and cell-cell communication (i.e., antiquorum activity) (35, 36). More specifically, it was shown that butyrate can downregulate the expression of pathogenicity island genes in Salmonella strains and impair bacterial invasion and translocation from the intestine to the bloodstream (37–39). Thus, SCFAs reduce Salmonella virulence and might attenuate the severity of infections in various ways. In contrast to their action in Salmonella, SCFAs upregulate virulence genes in Borrelia burgdorferi. Lin and colleagues suggested that increased expression of RpoS, OspC, and DbpA proteins resulted in greater sensing of the bacteria by the adaptive immune system and thus in enhanced bacterial clearance (13). Butyrate was shown to also suppress Listeria monocytogenes virulence factors and compromise the bacterium’s ability to evade the phagosome. Accordingly, macrophages efficiently eliminated butyrate-treated L. monocytogenes (40). Similarly, butyrate inhibited biofilm formation by C. neoformans, Candida albicans, and Trichosporon spp. (14, 25) and reduced filamentation in C. albicans and capsule formation in C. neoformans (14). Taken as a whole, these mechanistic data suggest that SCFAs modulate virulence factors in various species of bacteria and yeasts and thus enhance the host’s recognition and elimination of pathogenic microorganisms. It has also been reported that variations in SCFA concentrations in the avian intestinal tract modulate colonization by commensal bacteria such as Campylobacter jejuni; higher SCFA concentrations in the lower intestinal tract activate acetogenesis-dependent genes, which allow bacteria to adhere and colonize the region (41). A similar effect is also seen on enterohemorrhagic Escherichia coli (EHEC) strains. Tobe and colleagues showed that high concentrations of SCFAs (mostly acetate) activate flagellar genes and other genes related to the motility of EHEC strains. Given the known intestinal concentrations of SCFAs, bacteria might migrate to the distal ileum, where butyrate concentrations are higher; next, genes related to bacterial adherence might be activated (42, 43). Collectively, SCFAs mainly limit bacterial and fungal virulence by altering gene expression. To date, direct effects of SCFAs on viruses or parasites have not been reported. Although the above-mentioned (mostly in vitro) studies provide information on the effects of SCFAs on microorganism virulence and may explain the beneficial effects on infections, it is extremely important to validate these observations effects in vivo.
Preclinical and clinical studies of the effects of SCFAs on infections.
SCFAs are reportedly beneficial in several infective settings. This section focuses on preclinical and clinical studies of nonrespiratory infections. Thanks to their antibacterial and antifungal properties, SCFAs have been used since 1947 to successfully treat “external” infections, such as conjunctivitis (8), dermatomycosis (44), and vulvovaginitis (45). More recently, SCFAs have been studied in animal models of colitis, necrotic enteritis, and septic arthritis, among other diseases (Table 1). Most of these studies looked at bacterial infections. However, a few studies showed that SCFAs can also have an effect on fungal, viral, and parasitic infections.
TABLE 1.
Effects of SCFAs on infection
| Disease | Pathogen(s) | Host(s) | SCFA type | Effect of SCFAs | Reference(s) |
|---|---|---|---|---|---|
| Eye infection | Staphylococcus aureus, hemolytic streptococci, and others | Humans and rabbits | Propionate | Relieved symptoms, improved healing, and reverted corneal ulcers | 8 |
| Vulvovaginitis | Candida spp. | Humans | Propionate | Relieved symptoms | 45 |
| Dermatomycosis | Tinea spp. | Humans | Propionate | Relieved symptoms; led to cure and no recurrence | 44 |
| Infected wounds | Pseudomonas aeruginosa | Humans | Acetate | Accelerated healing and pathogen clearance | 46 |
| Skin infection | MRSA | Humans | Propionate | Reduced inflammation, abscess size, and bacterial loads | 29 |
| Colitis | Shigella flexneri | Rabbits | SCFA | Improved clinical symptoms; reduced inflammation and bacterial loads | 11, 47 |
| Humans | Butyrate | Reduced inflammation and increased LL-37 production | 48 | ||
| Intestinal infections | Salmonella enterica subsp. enterica serovar Typhimurium | Mice | Propionate | Limited pathogen growth and impaired the infection | 10 |
| Enteritis | Citrobacter rodentium | Mice | Butyrate | Reduced inflammation and improved epithelial repair and bacterial clearance | 49 |
| Intestinal infection | Clostridioides difficile | Mice | Butyrate | Reduced inflammation and improved epithelial repair | 50 |
| Mice | Acetate | Increased neutrophil recruitment, epithelial repair, and bacterial clearance | 51 | ||
| Necrotic enteritis | Clostridium perfringens | Broilers | Butyrate | Reduced intestinal lesions and increased weight gain | 53 |
| Hemolytic uremic syndrome | Escherichiacoli O157 | Piglets | Butyrate | Improved clinical symptoms; reduced inflammation and bacterial loads | 52 |
| Intestinal infection | Trichinella spiralis | Mice | SCFA | Reduced invasion and number of parasites; reduced histopathological changes | 54 |
| Subcutaneous infection | Aggregatibacter actinomycetemcomitans | Mice | SCFA | Increased bacterial loads; impaired phagocytosis and killing by neutrophils | 55 |
| Arthritis | Chikungunya virus | Mice | Butyrate | Increased edema | 56 |
Propionate’s antibiotic properties, lack of side effects, and broad spectrum of action against bacteria and fungi meant that it was used to treat human eye infections as early as the 1940s. Theodore and colleagues showed that propionate prevents eye infections and helps corneal ulcers to heal without causing opacity (8). Propionate was also used to treat vulvovaginitis caused by Candida spp., with symptom relief and elimination of the pathogen in almost all treated patients (45). Recently, acetic acid was used in a clinical trial of wounds infected by P. aeruginosa; the treatment shortened the healing time and eliminated the pathogen (46). Likewise, propionate ameliorated skin infections caused by methicillin-resistant Staphylococcus aureus by reducing cytokine production, bacterial loads, and abscess size (29).
Along with their antibacterial effects, SCFAs can also help the host’s defense mechanisms to combat infections. Protective effects of this type have been described in several models of intestinal infections. Colonic infusion of SCFAs in a rabbit model of acute S. flexneri-driven colitis was shown to attenuate clinical symptoms (such as blood in the feces and mucosal congestion) and reduce cellular infiltration and bacterial counts in the colon (11, 47). The latter study was followed by clinical trial in shigellosis. Butyrate treatment was associated with a lower level of inflammation in the rectum and greater expression of antimicrobial peptides (48). Treatment with butyrate has also shown beneficial effects in murine models of intestinal Citrobacter rodentium and Clostridioides difficile infections. Relative to untreated mice, the treated animals gained more weight, had less intestinal inflammation, and upregulated the expression of genes related to pathogen clearance and epithelial repair (49–51).
The beneficial effects of SCFAs are not restricted to humans and rodents. Local treatment with butyrate ameliorated necrotic enteritis in broilers infected with Eimeria maxima and then Clostridium perfringens and showed a protective effect in hemolytic uremic syndrome caused by E. coli O157 in piglets (52, 53). Under both conditions, butyrate relieved clinical symptoms and reduced intestinal inflammation. In the piglet study, a lower bacterial load and greater production of host defense peptides were observed. In addition to their effects on bacterial and fungal infections, SCFAs also have a beneficial effect in the context of parasitic infections. Mista and colleagues reported that in a murine model of Trichinella spiralis infection, animals treated with SCFAs had a lower number of parasites in the intestine, fewer histopathological changes, and less extensive parasite invasion (54).
However, a few studies have evidenced a detrimental effect of SCFAs. For instance, SCFA treatment increased the load of Aggregatibacter actinomycetemcomitans in a murine subcutaneous chamber model (55). In the context of Chikungunya virus infection in a murine model, butyrate increased edema and altered endothelial barrier repair in the paw (56).
In summary, the administration of SCFAs in humans and other animals is generally beneficial in the context of infection. The predominant effect of SCFAs is a decrease in the pathogen load and the level of inflammation. This is the result of a stronger immune response in which the clearance mechanisms are more effective and drive a mild inflammatory response. The above-mentioned data illustrate the therapeutic potential of SCFAs.
Mechanisms of action of the SCFAs.
As described above, SCFAs have antimicrobial effects and other beneficial effects. It has been demonstrated that the activity of SCFAs depends on the host cell, the host tissue, and the infectious agent. The vast majority of these compounds’ actions are exerted through epithelial and immune cells, culminating in beneficial effects on the host and detrimental effects on the pathogen. It has already been mentioned that, in vitro, SCFAs reduced cell colonization, translocation, and invasion by C. jejuni, EHEC, and Salmonella Typhimurium. Cellular invasion by bacteria is a major event in enteritis in humans, and thus enteritis might be a promising target for future treatments with SCFAs (57–59). Internalization of bacterial bovine mammary epithelial cells is an important step in the development of mastitis. Two research groups showed that butyrate and acetate inhibited S. aureus internalization and induced the expression of antimicrobial peptides by mammary epithelial cells. Butyrate and acetate inhibited bacterial internalization in different ways, as follows: with butyrate, the inhibition resulted from greater histone acetylation; with acetate, the inhibition involved a reduction in nuclear factor κB activation (60, 61). As seen with mastitis, butyrate and acetate also ameliorate C. difficile infections via different mechanisms. Butyrate treatment was associated with less intestinal inflammation and a tighter epithelial barrier via activation of hypoxia-inducible factor 1 alpha in intestinal epithelial cells (50). In contrast, acetate acted through FFAR2 by accelerating neutrophil recruitment and inflammasome activation, and thus interleukin (IL)-1β production. Furthermore, acetate enhanced the production of interleukin 22 (IL-22), known to induce antimicrobial and epithelial repair mechanisms by innate lymphoid cells (51). In some contexts, different SCFAs can have the same effect. In a study of S. aureus, butyrate and propionate were both found to inhibit nuclear factor κB activation and inducible nitric oxide synthase expression by murine macrophages and thus decrease the level of inflammation (62). In contrast, the production of proinflammatory cytokines did not change in murine macrophages treated with butyrate and challenged with S. enterica, adherent-invasive E. coli, S. aureus, or C. rodentium; however, increased antimicrobial peptide expression was observed (19, 63, 64). Schulthess and colleagues reported that macrophages differentiated in the presence of butyrate showed greater antimicrobial activity against S. enterica, adherent-invasive E. coli, S. aureus, and C. rodentium as a result of HDAC3 inhibition (64). This inhibition reduced glycolysis, increased the activity of autophagy protein microtubule-associated protein 1 light chain 3 alpha, and potentialized antimicrobial peptide production. Butyrate has a direct effect on lung pulmonary cells by imprinting an antibacterial program in macrophages during their differentiation from monocytes (64). The latter findings are in line with a report by Trompette and colleagues, in which butyrate impacted the differentiation of bone marrow progenitors and influenced monocyte/macrophage functions (65). In another study, butyrate increased the phagocytosis and killing of C. albicans and C. neoformans by raising nitric oxide production (14). In contrast, butyrate was shown to reduce the neutrophils’ production of reactive oxygen species and thus the ability to kill C. albicans (66). Although many researchers have described the SCFAs’ effects on infection, mechanistic data are scarce. At present, the best-characterized mechanisms are HDAC3 inhibition (culminating in greater production of antimicrobial peptides) and FFAR activation (with nuclear factor κB inhibition and downregulation of inflammation).
SCFAs IN RESPIRATORY INFECTIONS
The first evidence of gut-lung cross talk came from studies showing a correlation between perturbation of the gut microbiota and airway disease. The dysbiosis caused by antibiotic administration or chronic disease was associated with greater susceptibility to allergic diseases and lung infections (67–69). Accordingly, germfree mice are more susceptible to lung diseases than are conventional mice harboring commensal bacteria (70–72). This finding indicates that factors produced by the gut microbiota have a remote effect on the lung’s immune status. In this setting, SCFAs are potential drug candidates. Patients with intestinal disorders have lower levels of SCFAs and are more susceptible to lung diseases (73). As mentioned above, SCFAs can be microbicidal and can modulate the immune response. It is well known that SCFAs produced in the gut can pass into the circulation and disseminate to distal organs (7). However, there is no consensus about the SCFAs’ source, concentrations, and direct effects on the lungs. Most of the research data suggest that SCFAs are produced by the gut microbiota and can diffuse into the blood, and only a few studies have suggested that lung microbiota can produce SCFAs. Trompette and colleagues believed that lung-resident bacteria did not contribute to SCFA production because (i) their substrates are lacking, and (ii) SCFAs could not be detected in the lungs. SCFAs produced by gut microbiota might therefore have systemic effects—especially on progenitor cells in the bone marrow or on circulating immune cells—that subsequently act on the lung compartment (74). In contrast, Segal and colleagues’ study of people living with HIV found that the SCFA concentration was 370 times higher in the lungs than that in the blood (75). The researchers suggested that anaerobic microorganisms in the lungs can form multicellular complexes in a biofilm, which might enable them to survive and produce SCFAs through fermentation. Thus, it is likely that SCFAs are not solely produced by the gut microbiota. Furthermore, there is no consensus on the SCFA level in the lung compartment; depending on the study, it ranges from 0 to 3 μM/g of lung tissue in mice and from 30 to 10,000 μM in bronchoalveolar lavage or sputum from humans (23, 72, 74–76). Regardless of the source and lung concentration of SCFAs, these compounds have a broad range of immunomodulatory functions and have often been studied in the context of lung infection (Fig. 2).
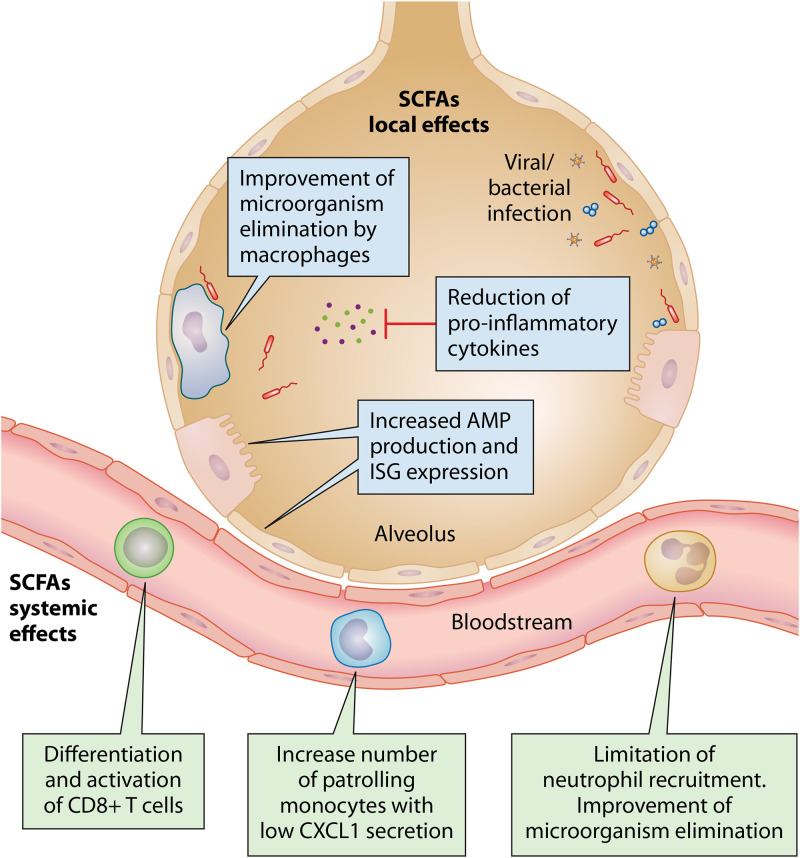
FIG 2.
Main effects of SCFAs on lung infections. SCFAs can modulate the systemic and/or local immune response; this increases pathogen clearance and decreases the tissue damage caused by exacerbated inflammation.
Bacterial infections.
At present, there is no consensus on the impact of SCFAs on bacterial infections of the respiratory tract. However, a growing body of evidence suggests that some SCFAs are involved in the host’s defense against these infections. Butyrate and phenylbutyrate (PBA; a butyrate derivative used to treat hyperammonemia in patients with urea cycle disorders) have been evaluated in the context of tuberculosis (77). PBA had already been shown to increase the production of antimicrobial peptides by lung epithelial cells (78). Thus, PBA has been combined with vitamin D3, a well-known regulator of the immune response that boosts antimicrobial peptide production and has already been used to treat tuberculosis (79). This combination was first administered orally to healthy people. PBA and vitamin D3 had synergistic effects on the macrophages’ and lymphocytes’ production of the antimicrobial peptide LL-37. Furthermore, the PBA-vitamin D3 combination was associated with the enhanced killing of M. tuberculosis by macrophages (79). In the same context, another study showed that the major antibacterial effect seen was due to PBA, which directly inhibited M. tuberculosis growth in vitro and boosted macrophage-mediated bacterial killing (24). In phase 2 clinical trials, the treatment of patients infected with M. tuberculosis using PBA and vitamin D3 led to better clearance of the infection, less inflammation, and symptom relief (77, 80, 81). Despite the presence of a clear improvement in treated patients, the PBA-vitamin D3 combination is no longer being studied in the clinic. In a murine model of pneumonia, it was demonstrated that acetate treatment reduced Klebsiella pneumoniae proliferation and lung inflammation by acting through FFAR2 (82). In this setting, acetate increased the phagocytosis and killing of bacteria by alveolar macrophages and neutrophils. These data are in line with our recent observation in which acetate treatment accentuated the ability of macrophages to kill S. pneumoniae in vitro and ex vivo (68). In the context of prior influenza infection, the drop in acetate production by the gut microbiota could also be overcome by treatment with acetate or a highly selective FFAR2 agonist, resulting in protection against secondary S. pneumoniae infection. In another study, however, propionate treatment failed to protect against S. pneumoniae and K. pneumoniae (83). Thus, the SCFAs appear to differ in their efficacy against respiratory bacterial infections. Accordingly, Tian and colleagues demonstrated that the dysbiosis caused by antibiotic treatment resulted in a higher propionate concentration and ultimately worsened S. aureus pneumonia (84). In contrast, low-dose propionate supplementation did not affect the S. aureus burden. These results show that altering the SCFA balance can influence the lung’s immune and inflammatory responses. Indeed, the pulmonary dysbiosis caused by antiretroviral therapy in people with HIV led to anaerobic bacteria overgrowth and high concentrations of SCFAs (especially butyrate and propionate) in the lungs (75). Patients with higher concentrations of SCFAs in the lungs were more likely to develop tuberculosis. This increase in susceptibility was linked to a lower CD4+ T-cell count and a higher regulatory T-cell count in the lungs. Butyrate also reduced the in vitro production of gamma interferon (IFN-γ) and IL-17A by the patients’ macrophages and lymphocytes. These observations testify to an impairment of the host’s defenses against M. tuberculosis. Together, pulmonary and intestinal dysbioses directly influence SCFA concentrations and heighten the host’s susceptibility to infection. Furthermore, supplementation with acetate and (perhaps) with butyrate in healthy individuals might contribute to resistance against respiratory bacterial infections. In contrast, propionate either accentuates or has no impact on respiratory infections. It is therefore important to evaluate concomitant comorbidities that might alter the treatment outcome; for example, the response to M. tuberculosis is improved by SCFAs in healthy patients but is impaired by SCFAs in people with HIV.
Fungal infections.
Although fungal lung infections are rare in healthy people, they can be life-threatening for immunocompromised individuals such as cancer patients receiving chemotherapy, people with HIV, and transplant recipients taking immunosuppressants (85). Hence, fungal infections in immunosuppressed patients are a major public health concern. To the best of our knowledge, the possible effect of SCFAs on respiratory fungal infection has not been studied. Nevertheless, it is known that dysbiosis can impair antifungal pulmonary immunity and that SCFAs can inhibit yeast growth and improve the killing ability of macrophages in vitro (14, 86). More research will be needed to delineate the potential value of the SCFAs in combating fungal infections.
Viral infections.
A growing body of evidence indicates that low SCFA levels in humans are correlated with susceptibility to viral infections. In allogeneic hematopoietic stem cell transplant recipients, antibiotic treatment, chemotherapy, and radiotherapy modified the composition of the intestinal microbiota (87). This disturbance led to low or even null concentrations of butyrate in the feces and was correlated with greater susceptibility to respiratory infections caused by rhinovirus, adenovirus, respiratory syncytial virus (RSV), coronavirus, and other viruses (87). Experiments in animal models have demonstrated an overall beneficial effect of SCFAs in the context of respiratory viral infection. It was reported that RSV infection was ameliorated by the administration of acetate (12, 88). In these studies, acetate mediated the expression of interferon-stimulated genes (ISGs) in the lungs in general and in alveolar macrophages in particular, resulting in a lower viral load and a lower level of lung inflammation. Although the potential effect of SCFAs was not evaluated in this setting, gut microbiota depletion by antibiotic treatment reduces ISG expression by stromal pulmonary cells and heightens susceptibility to influenza A virus infection (89). Another study showed that butyrate supplementation in mice boosted host defenses against influenza (65). Butyrate affected bone marrow hematopoiesis by increasing (via FFAR3) the number of patrolling monocytes, which then became alternatively activated macrophages. The latter produced lower concentrations of chemokine (C-X-C motif) ligand 1, which limited the neutrophil recruitment and tissue damage caused by inflammation. Furthermore, butyrate enhanced the metabolic activity of naive T cells and led to effector cell differentiation and activation. CD8+ T-cell activation (via FFAR3 and fatty acid β-oxidative metabolism) was associated with better effector function against influenza virus (65). Last, it was recently demonstrated that SCFAs ameliorate equine herpesvirus infection. This virus replicates primarily in the respiratory epithelium and disseminates through the body via a leukocyte-associated viremia. Treatment with SCFAs did not affect viral replication in the respiratory epithelium but did reduce lateral dissemination. Moreover, SCFAs inhibited the endothelial cells’ expression of adhesion molecules; this decreased the transmission of viruses from infected immune cells to endothelial cells and thus limited viral dissemination (90). Taken as a whole, these data (i) highlight the beneficial effects of the SCFAs acetate and butyrate on respiratory viral infection and (ii) underline the compounds’ complex, broad range of actions.
In view of the beneficial effects of SCFAs on viral infection, it might be worth studying their potential effects on the prevention and/or treatment of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This respiratory disease is characterized by dysregulated cytokine production, barrier leakage, and organ dysfunction. As in influenza A virus infection (91–96), SARS-CoV-2 infection also leads to gut dysbiosis in humans and in animal models (92, 97–102). Interestingly, influenza A virus and SARS-CoV-2 infections both lead to a drop in SCFA production by the gut microbiota (68, 102). As mentioned above, SCFAs can enhance the antiviral response by promoting ISG expression and T-cell effector functions. Thus, SCFAs might also reduce SARS-CoV-2 viral loads, as seen in the setting of RSV infection (12, 88). Furthermore, SCFAs are able to reduce inflammation (notably in the context of infection) and reinforce the epithelial barrier (50, 65, 73, 82). Hence, there is an urgent need to test the potential beneficial effects of SCFAs in animal models of COVID-19 and, if the tests are successful, in humans.
STRATEGIES FOR MODULATING SCFA LEVELS
SCFAs are produced by the gut microbiota via the fermentation of nondigestible fibers. The insufficient consumption of fiber-rich fruits and vegetables in Western countries shapes the composition and function of the gut microbiota and is likely to impair host defenses (3, 103, 104). At present, there are three main ways of manipulating (increasing, in most cases) the concentration of SCFAs in the body, namely, probiotics, prebiotics, and a combination of the two. Probiotics are live microorganisms that provide health benefits when ingested. Prebiotics are nutrients (typically fibers) that can be degraded by the microbiota. Both probiotics and prebiotics can modulate the growth and metabolic activity of the microbiota (105, 106).
Preclinical models have shown that probiotics, prebiotics, or a combination of both, promote protection against respiratory infections, although mechanisms have not been characterized in details (107, 108). Most probiotic organisms are lactobacilli with an intrinsic ability to stimulate the growth of SCFA-producing gut bacteria (109). Probiotics such as Lactobacillus gasseri and Lactobacillus rhamnosus can protect mice against influenza and RSV infections (106, 107, 110–113). Likewise, bacterial respiratory infections were also shown to be ameliorated by the administration of probiotics (114–116). Studies in mice have shown that prebiotics improve resistance to RSV and influenza infections (12, 65). The administration of a fiber-rich diet increased the levels of SCFAs in the gut and improved the antiviral immune responses. Similar, the ingestion of pectin fiber improved the outcome of P. aeruginosa pneumonia in a murine model (117). This improvement was correlated with greater SCFA production and modulation of the immune response.
Probiotics and prebiotics have been studied in many different contexts and seem to be beneficial for combating respiratory infections in humans (118). A meta-analysis of a large number of clinical trials in children showed that probiotic administration decreased the incidence of respiratory tract infections (119). Studies have also shown that administration of prebiotics for children in early life protects them from respiratory infections (120, 121). The combination of probiotics and prebiotics is also a good strategy. A recent meta-analysis of studies in healthy people evidenced a relationship between administration of a probiotic-prebiotic combination and a reduction in the frequency of respiratory tract infections (122). This approach is also under investigation for COVID-19. Ongoing clinical trials with probiotics administration are evaluating viral loads, symptom durations, and viral transmission in patients and in health care professionals (ClinicalTrials.gov registration no. NCT04621071, NCT04666116, and NCT04666116).
Conclusions.
Due to the great impact of respiratory infections on morbidity and mortality worldwide, it is essential to understand the corresponding susceptibility mechanisms and to develop new therapies. The current research evidence suggests that SCFAs reduce the incidence and severity of infections. However, the modes of action of the SCFAs are very complex, and their effects can vary dramatically according to the conditions. A better understanding of the biological properties of SCFAs is a prerequisite for the development of appropriate pharmacological approaches based on (for example) FFAR agonism. In the meantime, the use of prebiotics and probiotics that modulate local and systemic SCFA concentrations appears to be promising.
ACKNOWLEDGMENTS
We acknowledge support from our funding agencies, the Institut National de la Santé et de la Recherche Médicale, the Centre national de la Recherche Scientifique, the University of Lille, the Pasteur Institute of Lille, the région des Hauts-de-France, state of Minais Gerais/FAPEMIG (Franco-Brazilian call 2014-2015, FLUMICROBIOT), and l’Agence Nationale de la Recherche (ANR-17-CE15-0020-01, ACROBAT, and ANR APP Flash COVID19 [AM-CoV-Path]). M.G.M. and V.S. received salary support (Ph.D. fellowships) from Lille University (M.G.M. and V.S.) and from the Fondation pour la Recherche Médicale (V.S.). F.T. received salary support by the CNRS.
M.G.M., V.S., and F.T. wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
François Trottein, Email: francois.trottein@pasteur-lille.fr.
Andreas J. Bäumler, University of California, Davis
REFERENCES
- 1.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. 2009. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. 2014. The role of short-chain fatty acids in health and disease, p 91–119. In Alt F (ed), Advances in immunology, vol 121. Academic Press, Cambridge, MA. [DOI] [PubMed] [Google Scholar]
- 3.Thorburn AN, Macia L, Mackay CR. 2014. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 40:833–842. 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Huffnagle GB. 2014. Increase in dietary fiber dampens allergic responses in the lung. Nat Med 20:120–121. 10.1038/nm.3472. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Wu W, Liu Z, Cong Y. 2017. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52:1–8. 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JK, Mckenzie C, Mari E, Macia L, Mackay CR. 2017. Metabolite-sensing G protein-coupled receptors—facilitators of diet-related immune regulation. Annu Rev Immunol 35:371–402. 10.1146/annurev-immunol-051116-052235. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, MacFarlane GT. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227. 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theodore FH. 1950. Use of sodium propionate in external infections of the eyes. JAMA 143:226–228. 10.1001/jama.1950.02910380010004. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman C, Schweitzer TR, Dalby G. 1939. Fungistatic properties of the fatty acids and possible biochemical significance. J Food Science 4:539–545. 10.1111/j.1365-2621.1939.tb17151.x. [DOI] [Google Scholar]
- 10.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, Bouley DM, Vilches-Moure JG, Smith M, Sonnenburg JL, Bhatt AS, Huang KC, Monack D. 2018. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24:296–307.e7. 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B. 2006. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A 103:9178–9183. 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, dos Santos AÁ, Dias GBM, Vargas JE, Puga R, Mayer FQ, Maito F, Zárate-Bladés CR, Ajami NJ, Sant’Ana MR, Candreva T, Rodrigues HG, Schmiele M, Silva Clerici MTP, Proença-Modena JL, Vieira AT, Mackay CR, Mansur D, Caballero MT, Marzec J, Li J, Wang X, Bell D, Polack FP, Kleeberger SR, Stein RT, Vinolo MAR, de Souza APD. 2019. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun 10:3273. 10.1038/s41467-019-11152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YH, Chen Y, Smith TC, Karna SLR, Seshu J. 2018. Short-chain fatty acids alter metabolic and virulence attributes of Borrelia burgdorferi. Infect Immun 86:e00217-18. 10.1128/IAI.00217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LN, Lopes LCL, Cordero RJB, Nosanchuk JD. 2011. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J Antimicrob Chemother 66:2573–2580. 10.1093/jac/dkr358. [DOI] [PubMed] [Google Scholar]
- 15.Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT. 2018. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 8:1065–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang Z, Xiong D, Wu M, Ding JL. 2018. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J 32:289–303. 10.1096/fj.201700252RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moschen I, Bröer A, Galić S, Lang F, Bröer S. 2012. Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT). Neurochem Res 37:2562–2568. 10.1007/s11064-012-0857-3. [DOI] [PubMed] [Google Scholar]
- 18.Ferro S, Azevedo-Silva J, Casal M, Côrte-Real M, Baltazar F, Preto A. 2016. Characterization of acetate transport in colorectal cancer cells and potential therapeutic implications. Oncotarget 7:70639–70653. 10.18632/oncotarget.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunkara LT, Jiang W, Zhang G. 2012. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS One 7:e49558. 10.1371/journal.pone.0049558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, Krzyzaniak MA, King CG, Burgener AV, Fischer M, Develioglu L, Belle R, Recher M, Bonilla WV, Macpherson AJ, Hapfelmeier S, Jones RG, Hess C. 2016. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44:1312–1324. 10.1016/j.immuni.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Peck SM, Rosenfeld H, Leifer W, Bierman W. 1939. Role of sweat as a fungicide: with special reference to the use of constituents of sweat in the therapy of fungous infections. Arch Derm Syphilol 39:126–148. 10.1001/archderm.1939.01480190129012. [DOI] [Google Scholar]
- 22.Jeong S, Lee Y, Yun CH, Park OJ, Han SH. 2019. Propionate, together with triple antibiotics, inhibits the growth of enterococci. J Microbiol 57:1019–1024. 10.1007/s12275-019-9434-7. [DOI] [PubMed] [Google Scholar]
- 23.Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TMS, Palaniyar N, Grasemann H. 2015. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 46:1033–1045. 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- 24.Coussens AK, Wilkinson RJ, Martineau AR. 2015. Phenylbutyrate is bacteriostatic against Mycobacterium tuberculosis and regulates the macrophage response to infection, synergistically with 25-hydroxy-vitamin D3. PLoS Pathog 11:e1005007. 10.1371/journal.ppat.1005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Cordeiro RA, Aguiar ALR, Pereira VS, Pereira LMG, Portela FVM, Brilhante RSN, de Camargo ZP, Sidrim JJC, de Castelo-Branco DSCM, Rocha MFG. 2019. Sodium butyrate inhibits planktonic cells and biofilms of Trichosporon spp. Microb Pathog 130:219–225. 10.1016/j.micpath.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Roe AJ, O’Byrne C, McLaggan D, Booth IR. 2002. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology 148:2215–2222. 10.1099/00221287-148-7-2215. [DOI] [PubMed] [Google Scholar]
- 27.Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR. 1998. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180:767–772. 10.1128/JB.180.4.767-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CB, Alimova Y, Myers TM, Ebersole JL. 2011. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 56:650–654. 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong S, Kim HY, Kim AR, Yun CH, Han SH. 2019. Propionate ameliorates Staphylococcus aureus skin infection by attenuating bacterial growth. Front Microbiol 10:1363–1363. 10.3389/fmicb.2019.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guinan J, Wang S, Hazbun TR, Yadav H, Thangamani S. 2019. Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci Rep 9:8872. 10.1038/s41598-019-45467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baskett RC, Hentges DJ. 1973. Shigella flexneri inhibition by acetic acid. Infect Immun 8:91–97. 10.1128/iai.8.1.91-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Deun K, Haesebrouck F, Van Immerseel F, Ducatelle R, Pasmans F. 2008. Short chain fatty acids and lactate as feed additives to control Campylobacter jejuni infections in broilers. Avian Pathol 37:379–383. 10.1080/03079450802216603. [DOI] [PubMed] [Google Scholar]
- 33.Prohászka L. 1980. Antibacterial effect of volatile fatty acids in enteric E. coli‐infections of rabbits. Zentralbl Für Veterinärmed B 27:631–639. 10.1111/j.1439-0450.1980.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 34.Russell JB, Diez-Gonzalez F. 1998. The effects of fermentation acids on bacterial growth. Adv Microb Physiol 39:228–234. [DOI] [PubMed] [Google Scholar]
- 35.Lamas A, Regal P, Vázquez B, Cepeda A, Franco CM. 2019. Short chain fatty acids commonly produced by gut microbiota influence Salmonella enterica motility, biofilm formation, and gene expression. Antibiotics 8:265. 10.3390/antibiotics8040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amrutha B, Sundar K, Shetty PH. 2017. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb Pathog 111:156–162. 10.1016/j.micpath.2017.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Boyen F, Haesebrouck F, Vanparys A, Volf J, Mahu M, Van Immerseel F, Rychlik I, Dewulf J, Ducatelle R, Pasmans F. 2008. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet Microbiol 132:319–327. 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Hautefort I, Thompson A, Hinton JC, Van Immerseel F. 2006. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol 72:946–949. 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawhon SD, Maurer R, Suyemoto M, Altier C. 2002. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46:1451–1464. 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, O’Riordan MXD. 2012. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J Bacteriol 194:5274–5284. 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luethy PM, Huynh S, Ribardo DA, Winter SE, Parker CT, Hendrixson DR. 2017. Microbiota-derived short-chain fatty acids modulate expression of Campylobacter jejuni determinants required for commensalism and virulence. mBio 8:e00407-17. 10.1128/mBio.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobe T, Nakanishi N, Sugimoto N. 2011. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect Immun 79:1016–1024. 10.1128/IAI.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi N, Tashiro K, Kuhara S, Hayashi T, Sugimoto N, Tobe T. 2009. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology 155:521–530. 10.1099/mic.0.023499-0. [DOI] [PubMed] [Google Scholar]
- 44.Peck SM, Russ WR. 1947. Propionate-caprylate mixtures in the treatment of dermatomycoses, with a review of fatty acid therapy in general. Arch Derm Syphilol 56:601–613. 10.1001/archderm.1947.01520110047007. [DOI] [PubMed] [Google Scholar]
- 45.Alter RL, Jones CP, Carter B. 1947. The treatment of mycotic vulvovaginitis with propionate vaginal jelly. Am J Obstet Gynecol 53:241–244. 10.1016/0002-9378(47)90338-4. [DOI] [PubMed] [Google Scholar]
- 46.Madhusudhan VL. 2016. Efficacy of 1% acetic acid in the treatment of chronic wounds infected with Pseudomonas aeruginosa: prospective randomised controlled clinical trial. Int Wound J 13:1129–1136. 10.1111/iwj.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabbani GH, John Albert M, Hamidur Rahman ASM, Moyenul Isalm M, Nasirul Islam KM, Alam K. 1999. Short‐chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J Infect Dis 179:390–397. 10.1086/314584. [DOI] [PubMed] [Google Scholar]
- 48.Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman ASM, Rekha RS, Andersson J, Gudmundsson GH, Cravioto A, Agerberth B. 2012. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis 12:111. 10.1186/1471-2334-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiminez JA, Uwiera TC, Abbott DW, Uwiera RRE, Inglis GD. 2017. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. mSphere 2:e00243-17. 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fachi JL, Felipe J, de S, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales e Souza ÉL, dos Santos Martins F, Guima SES, Thomas AM, Setubal JC, Magalhães YT, Forti FL, Candreva T, Rodrigues HG, de Jesus MB, Consonni SR, Farias A, dos S, Varga-Weisz P, Vinolo MAR. 2019. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep 27:750–761.e7. 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 51.Fachi JL, Sécca C, Rodrigues PB, de Mato FCP, Di Luccia B, de Felipe JS, Pral LP, Rungue M, de Rocha VM, Sato FT, Sampaio U, Clerici MTPS, Rodrigues HG, Câmara NOS, Consonni SR, Vieira AT, Oliveira SC, Mackay CR, Layden BT, Bortoluci KR, Colonna M, Vinolo MAR. 2020. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J Exp Med 217:jem.20190489. 10.1084/jem.20190489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong H, Guo B, Gan Z, Song D, Lu Z, Yi H, Wu Y, Wang Y, Du H. 2016. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep 6:27070. 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu JD, Lumpkins B, Mathis G, Williams SM, Fowler J. 2019. Evaluation of encapsulated sodium butyrate with varying releasing times on growth performance and necrotic enteritis mitigation in broilers. Poult Sci 98:3240–3245. 10.3382/ps/pez049. [DOI] [PubMed] [Google Scholar]
- 54.Mista D, Piekarska J, Houszka M, Zawadzki W, Gorczykowski M. 2010. The influence of orally administered short chain fatty acids on intestinal histopathological changes and intensity of Trichinella spiralis infection in mice. Vet Med (Praha) 55:264–274. 10.17221/2992-VETMED. [DOI] [Google Scholar]
- 55.Corrêa RO, Vieira A, Sernaglia EM, Lancellotti M, Vieira AT, Avila-Campos MJ, Rodrigues HG, Vinolo MAR. 2017. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell Microbiol 19:e12720. 10.1111/cmi.12720. [DOI] [PubMed] [Google Scholar]
- 56.Prow NA, Hirata TDC, Tang B, Larcher T, Mukhopadhyay P, Alves TL, Le TT, Gardner J, Poo YS, Nakayama E, Lutzky VP, Nakaya HI, Suhrbier A. 2019. Exacerbation of Chikungunya virus rheumatic immunopathology by a high fiber diet and butyrate. Front Immunol 10:2736. 10.3389/fimmu.2019.02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cobbold RN, Desmarchelier PM. 2004. In vitro studies on the colonization of bovine colonic mucosa by Shiga-toxigenic Escherichia coli (STEC). Epidemiol Infect 132:87–94. 10.1017/s0950268803001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durant JA, Lowry VK, Nisbet DJ, Stanker LH, Corrier DE, Ricke SC. 1999. Short-chain fatty acids affect cell-association and invasion of HEp-2 cells by Salmonella Typhimurium. J Environ Sci Health B 34:1083–1099. 10.1080/03601239909373246. [DOI] [PubMed] [Google Scholar]
- 59.van Deun K, Pasmans F, Van Immerseel F, Ducatelle R, Haesebrouck F. 2008. Butyrate protects Caco-2 cells from Campylobacter jejuni invasion and translocation. Br J Nutr 100:480–484. 10.1017/S0007114508921693. [DOI] [PubMed] [Google Scholar]
- 60.Wei Z, Xiao C, Guo C, Zhang X, Wang Y, Wang J, Yang Z, Fu Y. 2017. Sodium acetate inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-κB activation. Microb Pathog 107:116–121. 10.1016/j.micpath.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 61.Ochoa-Zarzosa A, Villarreal-Fernández E, Cano-Camacho H, López-Meza JE. 2009. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb Pathog 47:1–7. 10.1016/j.micpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Park JW, Kim HY, Kim MG, Jeong S, Yun C-H, Han SH. 2019. Short-chain fatty acids inhibit staphylococcal lipoprotein-induced nitric oxide production in murine macrophages. Immune Netw 19:e9. 10.4110/in.2019.19.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, Lamont S, Lillehoj HS, Beker A, Teeter RG, Zhang G. 2011. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One 6:e27225. 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulthess J, Pandey S, Capitani M. 2019. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50:432–445.e7. 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. 2018. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48:992–1005.e8. 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 66.Vinolo MAR, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. 2009. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct 27:48–55. 10.1002/cbf.1533. [DOI] [PubMed] [Google Scholar]
- 67.Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, Reynolds LA, Hacker L, Mohr J, Finlay BB, Zaph C, McNagny KM, Mohn WW. 2018. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol 11:785–795. 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 68.Sencio V, Barthelemy A, Tavares LP, Machado MG, Soulard D, Cuinat C, Queiroz-Junior CM, Noordine ML, Salomé-Desnoulez S, Deryuter L, Foligné B, Wahl C, Frisch B, Vieira AT, Paget C, Milligan G, Ulven T, Wolowczuk I, Faveeuw C, Le Goffic R, Thomas M, Ferreira S, Teixeira MM, Trottein F. 2020. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep 30:2934–2947.e6. 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Yazar A, Atis S, Konca K, Pata C, Akbay E, Calikoglu M, Hafta A. 2001. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am J Gastroenterol 96:1511–1516. 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 70.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci 108:5354–5359. 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. 2012. Transient TLR Activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol 188:1411–1420. 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 72.Lewis G, Wang B, Shafiei Jahani P, Hurrell BP, Banie H, Aleman Muench GR, Maazi H, Helou DG, Howard E, Galle-Treger L, Lo R, Santosh S, Baltus A, Bongers G, San-Mateo L, Gilliland FD, Rehan VK, Soroosh P, Akbari O. 2019. Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front Immunol 10:2051. 10.3389/fimmu.2019.02051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dang AT, Marsland BJ. 2019. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol 12:843–850. 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 74.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 75.Segal LN, Clemente JC, Li Y, Ruan C, Cao J, Danckers M, Morris A, Tapyrik S, Wu BG, Diaz P, Calligaro G, Dawson R, van Zyl-Smit RN, Dheda K, Rom WN, Weiden MD. 2017. Anaerobic bacterial fermentation products increase tuberculosis risk in antiretroviral-drug-treated HIV patients. Cell Host Microbe 21:530–537.e4. 10.1016/j.chom.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirković B, Murray MA, Lavelle GM, Molloy K, Azim AA, Gunaratnam C, Healy F, Slattery D, McNally P, Hatch J, Wolfgang M, Tunney MM, Muhlebach MS, Devery R, Greene CM, McElvaney NG. 2015. The role of short-chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway. Am J Respir Crit Care Med 192:1314–1324. 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bekele A, Gebreselassie N, Ashenafi S, Kassa E, Aseffa G, Amogne W, Getachew M, Aseffa A, Worku A, Raqib R, Agerberth B, Hammar U, Bergman P, Aderaye G, Andersson J, Brighenti S. 2018. Daily adjunctive therapy with vitamin D 3 and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: a randomized controlled trial in Ethiopia. J Intern Med 284:292–306. 10.1111/joim.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinmann J, Halldórsson S, Agerberth B, Gudmundsson GH. 2009. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother 53:5127–5133. 10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mily A, Rekha RS, Kamal SMM, Akhtar E, Sarker P, Rahim Z, Gudmundsson GH, Agerberth B, Raqib R. 2013. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med 13:23. 10.1186/1471-2466-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mily A, Rekha RS, Kamal SMM, Arifuzzaman ASM, Rahim Z, Khan L, Haq MA, Zaman K, Bergman P, Brighenti S, Gudmundsson GH, Agerberth B, Raqib R. 2015. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS One 10:e0138340. 10.1371/journal.pone.0138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rekha RS, Mily A, Sultana T, Haq A, Ahmed S, Mostafa Kamal SM, van Schadewijk A, Hiemstra PS, Gudmundsson GH, Agerberth B, Raqib R. 2018. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D3 as host directed therapy. BMC Infect Dis 18:303. 10.1186/s12879-018-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galvão I, Tavares LP, Côrrea RO, Fachi JL, Rocha VMVM, Rungue MM, Garcia CC, Cassali G, Ferreira CM, Martins FS, Oliveira SC, Mackay CR, Teixeira MM, Vinolo MAR, Vieira ATAT, Corrêa RO, Fachi JL, Rocha VMVM, Rungue MM, Garcia CC, Cassali G, Ferreira CM, Martins FS, Oliveira SC, Mackay CR, Teixeira MM, Vinolo MAR, Vieira ATAT. 2018. The metabolic sensor GPR43 receptor plays a role in the control of Klebsiella pneumoniae infection in the lung. Front Immunol 9:142. 10.3389/fimmu.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ciarlo E, Heinonen T, Herderschee J, Fenwick C, Mombelli M, Le Roy D, Roger T. 2016. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep 6:37944. 10.1038/srep37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian X, Hellman J, Horswill AR, Crosby HA, Francis KP, Prakash A. 2019. Elevated gut microbiome-derived propionate levels are associated with reduced sterile lung inflammation and bacterial immunity in mice. Front Microbiol 10:159. 10.3389/fmicb.2019.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Lu G, Meng G. 2019. Pathogenic fungal infection in the lung. Front Immunol 10:1524. 10.3389/fimmu.2019.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAleer JP, Nguyen NLH, Chen K, Kumar P, Ricks DM, Binnie M, Armentrout RA, Pociask DA, Hein A, Yu A, Vikram A, Bibby K, Umesaki Y, Rivera A, Sheppard D, Ouyang W, Hooper LV, Kolls JK. 2016. Pulmonary Th17 antifungal immunity is regulated by the gut microbiome. J Immunol 197:97–107. 10.4049/jimmunol.1502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haak BW, Littmann ER, Chaubard JL, Pickard AJ, Fontana E, Adhi F, Gyaltshen Y, Ling L, Morjaria SM, Peled JU, Van Den Brink MR, Geyer AI, Cross JR, Pamer EG, Taur Y. 2018. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 131:2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji J-J, Sun Q-M, Nie D-Y, Wang Q, Zhang H, Qin F-F, Wang Q-S, Lu S-F, Pang G-M, Lu Z-G. 2021. Probiotics protect against RSV infection by modulating the microbiota-alveolar-macrophage axis. Acta Pharmacol Sin 10.1038/s41401-020-00573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradley KC, Finsterbusch K, Schnepf D, Fuchs SY, Staeheli P, Wack A, Crotta S, Llorian M, Davidson S. 2019. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep 28:245–256. 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 90.Poelaert KCK, Van Cleemput J, Laval K, Descamps S, Favoreel HW, Nauwynck HJ. 2019. Beyond gut instinct: metabolic short-chain fatty acids moderate the pathogenesis of alphaherpesviruses. Front Microbiol 10:723. 10.3389/fmicb.2019.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W, Cheng G. 2016. Influenza virus affects intestinal microbiota and secondary Salmonella infection in the gut through type I interferons. PLoS Pathog 12:e1005572. 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. 2020. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis 71:2669–2678. 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin N, Zheng B, Yao J, Guo L, Zuo J, Wu L, Zhou J, Liu L, Guo J, Ni S, Li A, Zhu Y, Liang W, Xiao Y, Ehrlich SD, Li L. 2015. Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci Rep 5:14771. 10.1038/srep14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. 2014. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 211:2397–2410. 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. 2018. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 6:9. 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q, Hu J, Feng J-W, Hu X-T, Wang T, Gong W-X, Huang K, Guo Y-X, Zou Z, Lin X, Zhou R, Yuan Y-Q, Zhang A-D, Wei H, Cao G, Liu C, Chen L-L, Jin M-L. 2020. Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol 21:99. 10.1186/s13059-020-02007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gou W, Fu Y, Yue L, Chen GD, Cai X, Shuai M, Xu F, Yi X, Chen H, Zhu Y, Xiao ML, Jiang Z, Miao Z, Xiao C, Shen B, Wu X, Zhao H, Ling W, Wang J, Chen YM, Guo T, Zheng JS. 2020. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv 10.1101/2020.04.22.20076091. [DOI] [Google Scholar]
- 98.Yu L, Tong Y, Shen G, Fu A, Lai Y, Zhou X, Yuan Y, Wang Y, Pan Y, Yu Z, Li Y, Liu T, Jiang H. 2020. Immunodepletion with hypoxemia: a potential high risk subtype of coronavirus disease 2019. medRxiv 10.1101/2020.03.03.20030650. [DOI] [Google Scholar]
- 99.Zuo T, Zhan H, Zhang F, Liu Q, Tso EYK, Lui GCY, Chen N, Li A, Lu W, Chan FKL, Chan PKS, Ng SC. 2020. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology 159:1302–1310.e5. 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuo T, Liu Q, Zhang F, Lui GCY, Tso EYK, Yeoh YK, Chen Z, Boon SS, Chan FKL, Chan PKS, Ng SC. 2021. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 70:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeoh YK, Zuo T, Lui GCY, Zhang F, Liu Q, Li AYL, Chung ACK, Cheung CP, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chow KM, Ng SSS, Li TCM, Ng RWY, Yip TCF, Wong GLH, Chan FKL, Wong CK, Chan PKS, Ng SC. 2021. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–699. 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sokol H, Contreras V, Maisonnasse P, Desmons A, Delache B, Sencio V, Machelart A, Brisebarre A, Humbert L, Deryuter L, Gauliard E, Heumel S, Rainteau D, Dereuddre-Bosquet N, Menu E, Ho Tsong Fang R, Lamaziere A, Brot L, Wahl C, Oeuvray C, Rolhion N, Van Der Werf S, Ferreira S, Le Grand R, Trottein F. 2021. SARS-CoV-2 infection in nonhuman primates alters the composition and functional activity of the gut microbiota. Gut Microbes 13:1–19. 10.1080/19490976.2021.1893113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Statovci D, Aguilera M, MacSharry J, Melgar S. 2017. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 8:838. 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. 2016. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep 6:19032. 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. 2015. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734. 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 106.Song JA, Kim HJ, Hong SK, Lee DH, Lee SW, Song CS, Kim KT, Choi IS, Lee JB, Park SY. 2016. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J Microbiol Immunol Infect 49:16–23. 10.1016/j.jmii.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 107.Shinde T, Hansbro PM, Sohal SS, Dingle P, Eri R, Stanley R. 2020. Microbiota modulating nutritional approaches to countering the effects of viral respiratory infections including SARS-CoV-2 through promoting metabolic and immune fitness with probiotics and plant bioactives. Microorganisms 8:921. 10.3390/microorganisms8060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lehtoranta L, Pitkäranta A, Korpela R. 2014. Probiotics in respiratory virus infections. Eur J Clin Microbiol Infect Dis 33:1289–1302. 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, Kitzman DW, Becton T, Read R, Yadav H. 2018. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep 8:12649. 10.1038/s41598-018-30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, Le Goffic R, Courau S, Molimard P, Chatel JM, Langella P, Bermúdez-Humarán LG. 2013. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol 79:1491–1499. 10.1128/AEM.03075-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eguchi K, Fujitani N, Nakagawa H, Miyazaki T. 2019. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci Rep 9:4812. 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, Yasui H, Kiso Y. 2010. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol 10:1101–1106. 10.1016/j.intimp.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 113.Harata G, He F, Hiruta N, Kawase M, Kubota A, Hiramatsu M, Yausi H. 2010. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Lett Appl Microbiol 50:597–602. 10.1111/j.1472-765X.2010.02844.x. [DOI] [PubMed] [Google Scholar]
- 114.Shoaib A, Xin L, Xin Y. 2019. Oral administration of Lactobacillus acidophilus alleviates exacerbations in Pseudomonas aeruginosa and Staphylococcus aureus pulmonary infections. Pak J Pharm Sci 32:1621–1630. [PubMed] [Google Scholar]
- 115.Alvarez S, Herrero C, Bru E, Perdigon G. 2001. Effect of Lactobacillus casei and yogurt administration on prevention of Pseudomonas aeruginosa infection in young mice. J Food Prot 64:1768–1774. 10.4315/0362-028x-64.11.1768. [DOI] [PubMed] [Google Scholar]
- 116.Vieira AT, Rocha VM, Tavares L, Garcia CC, Teixeira MM, Oliveira SC, Cassali GD, Gamba C, Martins FS, Nicoli JR. 2016. Control of Klebsiella pneumoniae pulmonary infection and immunomodulation by oral treatment with the commensal probiotic Bifidobacterium longum 51A. Microbes Infect 18:180–189. 10.1016/j.micinf.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 117.Bernard H, Desseyn J-L, Gottrand F, Stahl B, Bartke N, Husson M-O. 2015. Pectin- derived acidic oligosaccharides improve the outcome of Pseudomonas aeruginosa lung infection in C57BL/6 mice. PLoS One 10:e0139686. 10.1371/journal.pone.0139686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. 2014. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized, placebo-controlled trial. J Allergy Clin Immunol 133:405–413. 10.1016/j.jaci.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Y, Zhang Y, Ho W, Yu G, Zhang T. 2016. Probiotics for prevention and treatment of respiratory tract infections in children. Medicine (Baltimore) 95:e4509. 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. 2008. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 138:1091–1095. 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 121.Shahramian I, Kalvandi G, Javaherizadeh H, Khalili M, Noori NM, Delaramnasab M, Bazi A. 2018. The effects of prebiotic supplementation on weight gain, diarrhoea, constipation, fever and respiratory tract infections in the first year of life. J Paediatr Child Health 54:875–880. 10.1111/jpc.13906. [DOI] [PubMed] [Google Scholar]
- 122.Chan CKY, Tao J, Chan OS, Li H-B, Pang H. 2020. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 11:979–988. 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]