Abstract
Backgound:
Vascular smooth muscle cells (VSMCs) transdifferentiated ectopically trigger vascular calcifications, contributing to clinical cardiovascular disease in the aging population. AnxA5 and TNAP play a crucial role in (patho)physiological mineralization.
Methods:
We performed affinity studies between DPPC and 9:1 DPPC:DPPS-proteoliposomes carrying AnxA5 and/or TNAP and different types of collagen matrix: type I, II, I+III and native collagenous extracellular matrix (ECM) produced from VSMCs with or without differentiation, to simulate ectopic calcification conditions.
Results:
AnxA5-proteoliposomes had the highest affinity for collagens, specially for type II. TNAP-proteoliposomes bound poorly and the simultaneous presence of TNAP in the AnxA5-proteoliposomes disturbed interactions between AnxA5 and collagen. DPPC AnxA5-proteoliposomes affinities for ECM from transdifferentiating cells went up 2-fold compared to that from native VSMCs. The affinities of DPPC:DPPS-proteoliposomes were high for ECM from VSMCs with or without differentiation, underscoring a synergistic effect between AnxA5 and DPPS. Co-localization studies uncovered binding of proteoliposomes harboring AnxA5 or TNAP+AnxA5 to various regions of the ECM, not limited to type II collagen.
Conclusion:
AnxA5-proteoliposomes showed the highest affinities for type II collagen, deposited during chondrocyte mineralization in joint cartilage. TNAP in the lipid/protein microenvironment disturbs interactions between AnxA5 and collagen. These findings support the hypothesis that TNAP is cleaved from the MVs membrane just before ECM binding, such facilitating MV anchoring to ECM via AnxA5 interaction.
General Significance:
Proteoliposomes as MV biomimetics are useful in the understanding of mechanisms that regulate the mineralization process and may be essential for the development of novel therapeutic strategies to prevent or inhibit ectopic mineralization.
Keywords: Matrix vesicles, proteoliposomes, phosphatidylserine, Annexin A5, Alkaline phosphatase, collagen
1. Introduction
Biomineralization is a complex, multifactorial process, which results in the deposition of mineral crystals along collagen fibrils in the extracellular matrix (ECM) of various tissues, such as bones, teeth, and certain areas of cartilage [1]. This process is initiated inside chondrocyte- and osteoblast-derived matrix vesicles (MVs), structures ranging from 100 to 300 nm in diameter, which serve as initial sites to the formation of crystalline hydroxyapatite (HA) mineral [2–7]. In these sites, initially amorphous mineral complexes (nucleation) are deposited [1,8–9]. In a second step, MV membranes break down and expose preformed HA to the extracellular fluid, thus allowing for propagation of HA deposition onto the collagenous ECM. This process is orchestrated by the balanced action of promoters and inhibitors of calcification including many non-collagenous matrix proteins. Inorganic pyrophosphate (PPi) suppresses HA crystal formation and propagation, and acts as a potent calcification inhibitor in biologic fluids [10].
Ectopic mineralization occurs in many soft tissues, including articular cartilage, and smooth muscle cells of various tissues, most prominently in the kidney, ligaments, and tendons, leading to vascular stiffening and hypertension, risk factors of cardiovascular mortality [11]. Hence, ectopic vascular calcifications increasingly cause clinical cardiovascular disease in the aging population. Vascular smooth muscle cells (VSMCs) mediate vessel calcification as a result of their transdifferentiation into an osteoblast/ chondrocyte-like phenotype, recapitulating processes that initiate mineralization during normal bone formation, including the generation of MVs, which serve as a nidus for Ca2+-phosphate deposition in the vessel wall.
HA generation and its deposition are accomplished by several proteins, involved in Ca2+ and phosphate (Pi) homeostasis, amongst them tissue-nonspecific alkaline phosphatase (TNAP), an enzyme attached to the outer membrane of MVs, and Annexin A5 (AnxA5), a Ca2+ and lipid-binding pore-forming protein, which can form Ca2+-ion channels within vesicular membranes. During skeletal and dental mineralization, TNAP acts as a pyrophosphatase hydrolyzing PPi, itself a potent mineralization inhibitor, controlling deposition and growth of HA crystals [6, 12–13]. AnxA5 forms a predominant cluster of α-helices that define a hydrophilic pore through the center of the protein. This pore serves as a selective Ca2+-channel in the MV membrane. More than any other protein studied in biomineralization so far, AnxA5 greatly accelerates the nucleational activity of the acidic phospholipid-Ca2+-Pi complexes present in the nucleational core, that triggers de novo mineral formation in MVs [14].
AnxA5 interacts with collagen and this interaction regulates the mineralization of growth plate chondrocytes. Increased secretion of type II and X collagen by the chondrocyte in the presence of ascorbate promotes enhanced interactions between these collagen fibers and AnxA5, stimulating Ca2+-influx, and, ultimately (as a consequence of increased [Ca2+]i) augmented TNAP expression, cell activity and mineralization [15–16]. Overexpression of AnxA5, which binds to the cytoplasmic domain of β5 integrin and protein kinase Cα leads to a cascade of molecular events resulting in apoptotic events in hypertrophic growth plate chondrocytes [17]. The precise role of various MV-associated proteins in this process is incompletely understood, including the role of AnxA5 and TNAP in MVs binding to collagenous ECM, respectively nucleation and mineral propagation.
Here, proteoliposomes were used with only AnxA5, TNAP or both combined, as key components on their surface, as a simplified MV mimetic system. Two different lipids have been used to compound the proteoliposomes, dipalmitoylphosphatidylcholine (DPPC) as a neutral lipid and dipalmitoylphosphatidylserine (DPPS) anionic lipid. Such proteoliposomes were used in previously studies [18] and were presently applied to quantitatively study interactions between AnxA5, TNAP, lipids (DPPC, DPPS) and types I, II and I+III collagens, in mineralization initialization.
2. Methodology
2.1. Materials
All the aqueous solutions were prepared with Millipore DirectQ ultra pure apyrogenic water. Bovine serum albumin (BSA), trichloroacetic acid (TCA), tris-hydroxymethyl-amino-methane (Tris), N-(2-hydroxyethyl)piperazine-N’-ethanesulfonic acid (HEPES), 2-amino-2-methyl-propan-1-ol (AMPOL), sodium dodecylsulfate (SDS), p-nitrophenyl phosphate disodium salt (pNPP), dexamethasone, β-glycerophosphate, polyoxyethylene-9-lauryl ether (polidocanol), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine (DPPS), Rhodamine 6G, collagens type I (from calf skin), type II (from bovine nasal septum), types I+III (from horse tendon) and collagenase were obtained from Sigma-Aldrich (St Louis, MO, USA). Sodium phosphate, magnesium chloride, acetic acid and chloroform were acquired from Merck (São Paulo, SP, Brazil). Plastic culture flasks (75 cm2) were purchased from Corning (Cambridge, MA, USA). 96 well plates, α-MEM, fetal bovine serum, ascorbic acid, gentamicin, penicillin-streptomycin and fungizone were supplied by Gibco-Life Technologies (Grand Island, NY, USA). Collagen II monoclonal antibody (2B1.5, MA5-12789) and Goat anti-mouse Alexa Fluor 488 (A11001) were purchased from Invitrogen (Massachusetts, USA). Analytical grade reagents were used without further purification.
2.2. Expression of human Annexin A5 and human Alkaline phosphatase
AnxA5 was expressed in E. coli with a histidine tag to facilitate its purification, as previously described [18–19]. Human TNAP was expressed as previously described [20] and used immediately after detergent removal to avoid aggregation. Preparation of a membrane fraction rich in TNAP and its solubilization were performed as previously described [21–22].
2.3. Construction of liposomes
The lipids DPPC and DPPS were used in the preparation of the DPPC-liposomes and 9:1 DPPC:DPPS-liposomes (molar ratio) as previously described [18]. Lipids were dissolved in chloroform and dried under nitrogen flow. The resulting lipid film was kept under vacuum overnight and suspended in the stock buffer (50 mM Tris-HCl buffer, pH 7.5, containing 2 mM MgCl2), to obtain a final lipid solution of 1.5 mg/mL. The mixture was then incubated for 1 h at 70 °C, above the critical phase transition temperature of the lipid, and vortexed at 10 min intervals. Large unilamellar vesicles (LUVs) were prepared by submitting the suspension to extrusion through 100 nm polycarbonate membranes in a LiposoFast extrusion system (Liposofast, Sigma-Aldrich). Liposomes also were labeled with 0.2 mol % of fluorescent Rhodamine 6G, to facilitate detection by microscopy and flow cytometry and to allow time-lapse observations via confocal microscopy.
2.4. Construction of proteoliposomes
TNAP (0.02 mg/mL) and AnxA5 (0.2 mg/mL) were incorporated into DPPC-liposomes and 9:1 DPPC:DPPS-liposomes (molar ratio) by direct insertion in the stock buffer, resulting in a 1:15,000 and 1:100 (protein:lipid ratio) respectively. The mixture was incubated overnight at 25 °C to avoid possible thermal inactivation. Then, the mixture was ultracentrifuged at 100,000×g during 1 h, at 4 °C. The pellet containing proteoliposomes was resuspended in an appropriate volume of the same buffer. Protein concentrations were estimated in the presence of 2% (w/v) SDS and BSA was used as standard [23].
2.5. Collagen matrix coating
Different sources of collagen (type I, type II, and types I + III) were used in order to produce the collagen matrix coating. First, the collagen was dissolved in 50 mM acetic acid at 1.0 mg/mL and placed under stirring for at least 1 h. After that, the collagen solution was diluted to a final concentration of 125 μg/mL in 50 mM acetic acid. 50 μL of collagen solution and 200 μL of stock buffer were then added to a 96 well plate, per each well, corresponding to 6.25 μg collagen per well. To achieve maximal refolding during coating, these pepsin-solubilized collagens were renatured in a neutral buffer during the coating process. The plate was kept covered, overnight at 4 °C. The next day, the plate was emptied and blocked with stock buffer containing 1% BSA for blocking non-occupied sites (250 μL/well) for 1 h at room temperature. The plate was washed 3 times with stock buffer without BSA and used as such to perform binding assays between proteoliposomes and collagens.
2.6. Analysis of binding
Several proteoliposome compositions were selected to investigate the interaction with collagen fibers, making use of vesicles, fluorescently labeled with Rhodamine 6G. Plates treated with collagen matrix coating were used in the assay. Proteoliposomes (0.6 mg/mL total lipid) with different lipid and protein composition were incubated with the plate (from 0 to 600 μg/mL total lipid) under gentile stirring, in the dark, for 2 h at 25 °C. The sample was removed, the plate was washed with stock buffer and the binding measurements were performed in an “IN Cell 2000” Analyzer (GE Healthcare Life Sciences).
2.7. Isolation of Vascular Smooth Muscle Cells (VSMCs)
VSMCs were isolated from human umbilical cords, via two different methodologies.
First, umbilical cords were washed in PBS to remove excess blood and the parts damaged by clamping were cut off. Blood clots were pushed out and 5 mm was cut off from each end. A cannula was placed inside the vein/arteries, attached to the cord with thread and rinsed with PBS. Another cannula was placed on the other end, which was then injected with 0.2 % collagenase via a syringe. The cannulas were closed and collagenase was left inside the vein for 10 min. at 37 °C. The umbilical cord was massaged, the cannula was opened and rinsed with culture medium in order to remove the cells. The medium was centrifuged at 1,000 g for 10 min. The supernatant was removed and the pellet containing endothelial and smooth muscle cells was resuspended with culture medium and in 6 well plates previously coated with 1% gelatin, for expansion. Cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 and 95% air, and the medium renewed every third day. The procedure was then repeated, but now for 20 min. of incubation with collagenase, to obtain vascular smooth muscles cells.
In a second approach, umbilical veins/arteries were cut in very small slices, directly cultured in the same conditions, with medium in 6 well plates previously coated with 1% gelatin, and outgrowth cells were selected.
For both methodologies, cells were cultured using DMEM, supplemented with 10% fetal bovine serum, 50 μg/mL ascorbic acid and 1% Penicillin-Streptomycin [24].
2.8. Production of native collagen from VSMCs
Native collagen fibers were produced in 96 well plates during culturing VSMCs. The cells were cultured (in quintuplicate) at a density of 1 × 104 cells/cm2 using DMEM, supplemented with 10% fetal bovine serum, 50 μg/mL ascorbic acid and 1% Penicillin-Streptomycin, for 2 weeks up to apparent confluence [24]. This culture produces an extracellular matrix, containing fibrillar type I and III collagens [25–26]. At confluency, the cells were gently removed by a treatment for 2 min. with 1 M NH3 in water and the extracellular matrix was rinsed with PBS, to remove ammonia. The resulting three-dimensional collagen-rich matrix was investigated via phase contrast microscopy.
2.9. Transdifferentiation of VSMCs into osteoblast/chondrocyte-like phenotype
Once the culture of VSMCs produces mainly fibrillar type I and III collagens [25–26], these cells were also cultured (in quintuplicate) in the presence of several Pi concentrations (0, 2.5 and 5 mM of NaH2PO4, in 50 mM HEPES buffer, pH 7.4) and ascorbic acid concentrations (0, 10 and 50 μg/mL), in order to trigger their transdifferentiation into an osteoblast/chondrocyte-like phenotype. The cells were cultured at a density of 1 × 104 cells/cm2 using DMEM, supplemented with 10% fetal bovine serum and 1% Penicillin-Streptomycin to confluency, for up to 2 weeks. Morphological changes of the cells were followed by optical microscopy.
2.10. Co-localization Analysis of binding
Co-localization between proteoliposomes and native collagen matrix were studied by fluorescence microscopy (Pendragon AxioVert 200M Microscope and/or IN Cell 2000 Analyzer GE Healthcare Life Sciences). To this end, proteoliposomes (0.6 mg/mL) were labeled with 0.2 mol% Rhodamine 6G and empty liposomes (equally labeled and without proteins) composed of DPPC or 9:1 DPPC:DPPS (molar ratio) were used as negative vesicle controls. Binding was analyzed in plates containing an ECM-coating comprising native collagen from VSMCs differentiated into an osteoblasts-like phenotype. Proteoliposomes (150 μL) were incubated (450 μg/mL lipid concentration) under gentile stirring for 2 h at 25 °C in the dark. The supernatant was removed and bound proteoliposomes were fixed with 1% paraformaldehyde in PBS (200 uL per well) for 10 min. The plate was then washed twice with stock buffer and incubated with a specific antibody against type II collagen for 30 min., at 37 °C, in the dark under gentile stirring (2 ug/mL collagen II monoclonal antibody 2B1.5, MA5-12789, Invitrogen, Massachusetts, USA) for detection of areas specifically containing native collagen fibers type II. The plate was washed twice with stock buffer and treated with secondary antibody (10 ug/mL, Goat anti-mouse Alexa Fluor 488 (A11001), Invitrogen, Massachusetts, USA) for 30 min., at 37 °C, in the dark under gentile stirring. The plate was washed again twice with stock buffer and analysis of binding were performed via image analysis in the IN Cell 2000 Analyzer. The overlap of red rhodamine fluorescence (proteoliposomes) bound to native collagen fibers type II (green fluorescence) was analyzed as yellow staining, reflecting co-localization. Because proteoliposomes binding interfered with subsequent collagen II detection, collagen II staining was also done via separate fluoresecence microscopy (Pendragon AxioVert 200M Microscope). Specific areas, rich in collagen II were detected via incubation of the wells containing transdifferentiated matrix with the type II collagen antibody for 30 min., at 37 °C, in the dark under gentile stirring (2 ug/mL, followed by treatment with the secondary antibody (10 ug/mL, Goat anti-mouse Alexa Fluor 488 (A11001).
2.11. PCR measurements
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) and cDNA was synthetized following the manufacturer’s instructions (Promega Corporation). Gene expression for SPP1 and COL2A1 was measured using IDT PrimeTime qPCR primers Hs.PT.58.19252426 (Forward primer: 5’-ttcaactcctcgctttccat-3’ Reverse primer: 5’-ccccacagtagacacatatgatg-3’) and Hs.PT.58.19672697 (Forward primer: 5’-attccatctgttccagggttac-3’ Reverse primer: 5’-ctggagccaagggtgaa-3’), respectively, combined with SYBR green intercalating dye from Life Technologies. Gene expression data were normalized against GAPDH expression calculated using in-house designed primers (Forward primer: 5’-ctcagacaccatggggaag-3’ and Reverse primer: 5’-acggtgccatggaatttgcc-3’). The expression of human αSM was also measured via mRNA expression measurements (Forward primer: 5’-tggtgtgtgacaatggctct-3’ Reverse primer: 5’-cttttccatgtcgtcccagt-3’).
2.12. Statistical analysis
All quantitative measurements were carried out 5 times and values are reported as mean ± standard error of the mean (SEM). Groups were compared with the one-way ANOVA or a two-tailed Student’s t-test (*p-value of < 0.05; **p-value of < 0.01 was considered significant).
3. Results and Discussion
3.1. Characteristics of DPPC and 9:1 DPPC:DPPS Proteoliposomes
The lipid microenvironment affects insertion and activity of the proteins reconstituted into model vesicles [18,27]. Protein incorporation was, therefore, measured in both DPPC and 9:1 DPPC:DPPS proteoliposomes (Table 1). Protein quantification revealed that both proteins were incorporated into both types of proteoliposomes, irrespective of the presence of DPPS (Table 1).
Table 1: Concentration of protein reconstituted into liposomes composed of DPPC or 9:1 DPPC:DPPS (molar ratio).
The protein and lipid concentration were determined as described in Materials and Methods. Proteoliposomes contained AnxA5 or TNAP, or both, as indicated.
| Proteoliposomes | [Protein] incorporated (μg protein/mg lipid) | ||
|---|---|---|---|
| Lipid specificity | Protein content | ||
| AnxA5 | TNAP | ||
| DPPC | + | − | 29.0 ± 2.3 |
| − | + | 30.8 ± 3.3 | |
| + | + | 40.2 ± 4.2 | |
| 9:1 DPPC:DPPS | + | − | 30.7 ± 3.5 |
| − | + | 30.0 ± 2.3 | |
| + | + | 38.5 ± 3.2 | |
Treatment with phosphatidylinositol-specific phospholipase C (PIPLC), which cleaves and releases TNAP from the proteoliposomes, and protein quantification revealed that in those proteoliposomes that contained both proteins, TNAP and AnxA5 represented 25% and 75%, respectively of the total protein incorporated into the proteoliposomes, regardless of the presence of DPPS [18]. This distribution is in agreement with earlier findings that TNAP and AnxA5 influence each other’s incorporation, as well as the kinetic behavior of TNAP [18].
3.2. Interaction of MVs biomimetics for collagens matrix
3.2.1. Affinity of Proteoliposomes harboring AnxA5 for type II Collagen
Collagen is the major structural protein in animals, comprising up to 30% of total protein weight [28]. AnxA5 was originally identified as a collagen-binding protein and also called anchorin CII. This protein was isolated from chondrocyte membranes by affinity chromatography on native type II collagen. When AnxA5 is reconstituted in liposomes, its binding to native collagen type II is stable at physiological ionic strength [15]. The binding of AnxA5, conditioned in either DPPC or 9:1 DPPC:DPPS proteoliposomes with this collagen matrix was investigated for increasing amounts of protein (contained within proteoliposomes).
Fluorescence microscopic images illustrate that the AnxA5-proteoliposome interaction on the collagen fibers is dose-dependent for both lipid compositions DPPC (Fig. S1) and 9:1 DPPC:DPPS (Fig. S2). Figures S1-A and S2-A show absent fluorescence for the collagen II, whereas Figure S1-E illustrates the DPPC-proteoliposomes binding to the collagen fibers in detail. Proteoliposomes binding occurred to collagen fibers specifically, i.e. proteoliposome fluorescence was only found on the collagen II fibers, as confirmed via visible light microscopy (not shown).
DPPC-proteoliposomes harboring AnxA5 showed a maximum of 32% binding to type II collagen after 2 hours of incubation, at 0.9 μg of AnxA5 (protein content per well in deposited proteoliposomes) with apparently decreasing affinity at higher concentrations (Figure 1).
Figure 1.
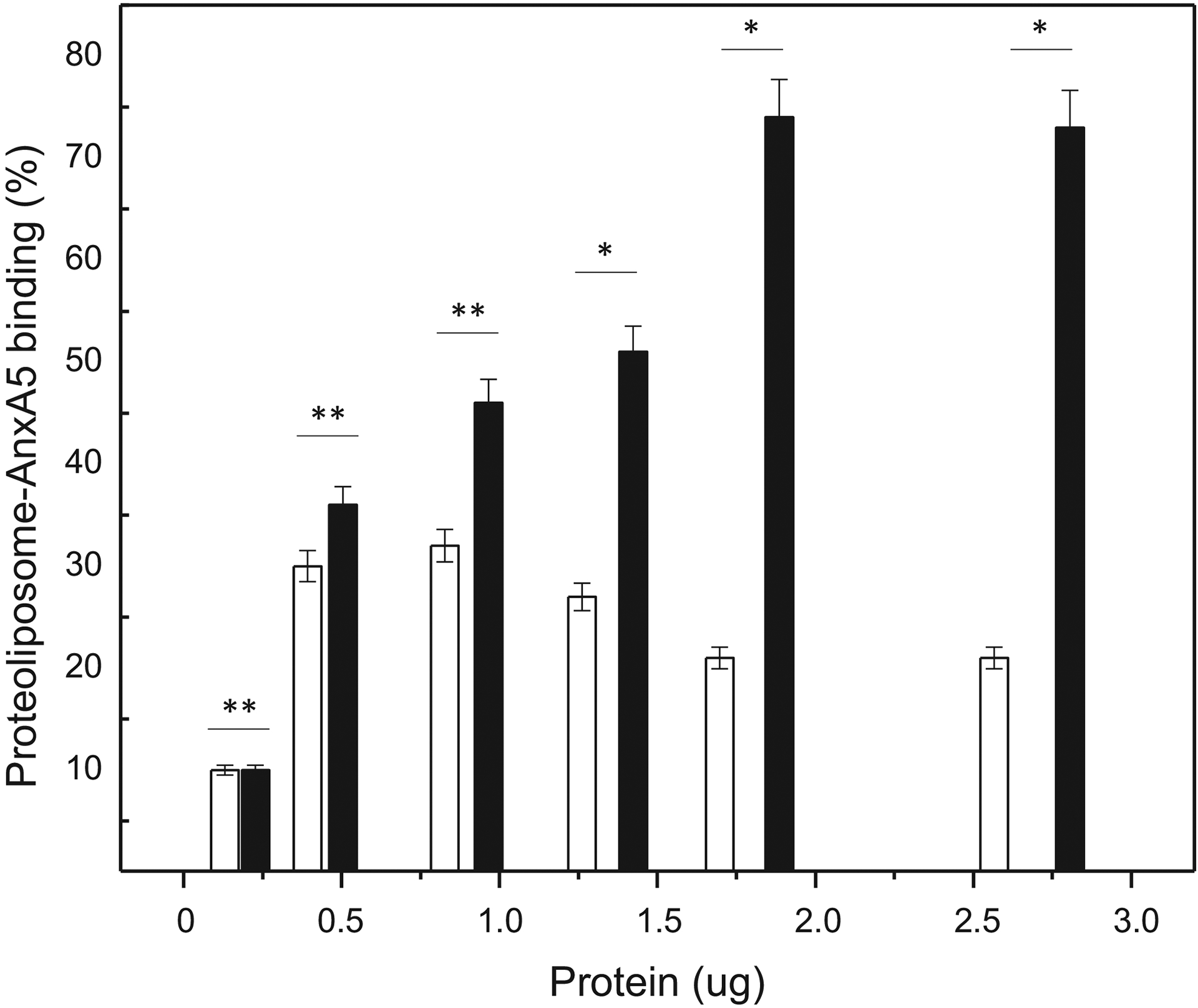
Effect of protein amount and lipid composition on binding of AnxA5 containing proteoliposomes to microtiter plates coated with type II collagen: DPPC-proteoliposomes (open bars) and 9:1 DPPC:DPPS-proteoliposomes (filled bars). Binding is expressed as a percentage of the protein adhered, as a function of the dose applied per well (indicated on the x-axis).
When conditioned in DPPC:DPPS-proteoliposomes, the affinity of AnxA5 for coated collagen II appeared to be comparable at low concentrations of proteoliposomes, but binding only saturated at 2 μg of AnxA5, in agreement with the importance of negative charges in the interaction between vesicles and collagen matrix, thus reaching 74% binding for 1.8 μg of AnxA5 (protein content into proteoliposomes) (Figure 1).
It is known, indeed, that AnxA5 interacts better with DPPC:DPPS than with DPPC liposomes [18]. Presently, we also find that proteoliposomes harboring AnxA5 interact noticeably better with collagen II, when AnxA5 is conditioned in DPPS containing proteoliposomes (around 2.3 times more), compared to exclusive DPPC proteoliposomes (Figure 1).
Considering the average molecular mass of fibrillary collagen of 300 kDa [29] and the molecular mass of monomeric AnxA5 of 35 kDa [14], it is possible to calculate the molar ratio of AnxA5/collagen II. The higher AnxA5 binding in 9:1 DPPC:DPPS proteoliposomes (74%) computes to 2 mols of AnxA5 bound per mol of coated collagen. For the DPPC-proteoliposomes, the maximum binding was 32%, corresponding to 0.5 mol of AnxA5 bound per mol of coated collagen.
This indicates that the lipid composition affects the affinity of AnxA5 for collagen. DPPS both improves AnxA5 incorporation into MV’s biomimetics and favors binding of proteoliposomes to collagen, helping to concentrate other useful proteins present in MV’s to participate in the calcification process.
3.2.2. Affinity of Proteoliposomes harboring TNAP for type II Collagen
Irrespective of its conditioning in DPPC or 9:1 DPPC:DPPS proteoliposomes, TNAP interacted more weakly with coated collagen II (Figure 2), although with different affinities, compared to the binding of AnxA5 (Figure 1), not exceeding 20% binding in any of the two lipid compositions tested. At low concentrations, when TNAP was conditioned in 9:1 DPPC:DPPS proteoliposomes, it reached a plateau, already below 0.5 μg/ml TNAP/well. Conditioned in DPPC proteoliposomes, TNAP reached its (weaker) plateau at 1.5 μg/ml. Unlike for AnxA5, the presence of DPPS did not significantly boost the affinity of TNAP for collagen II.
Figure 2.
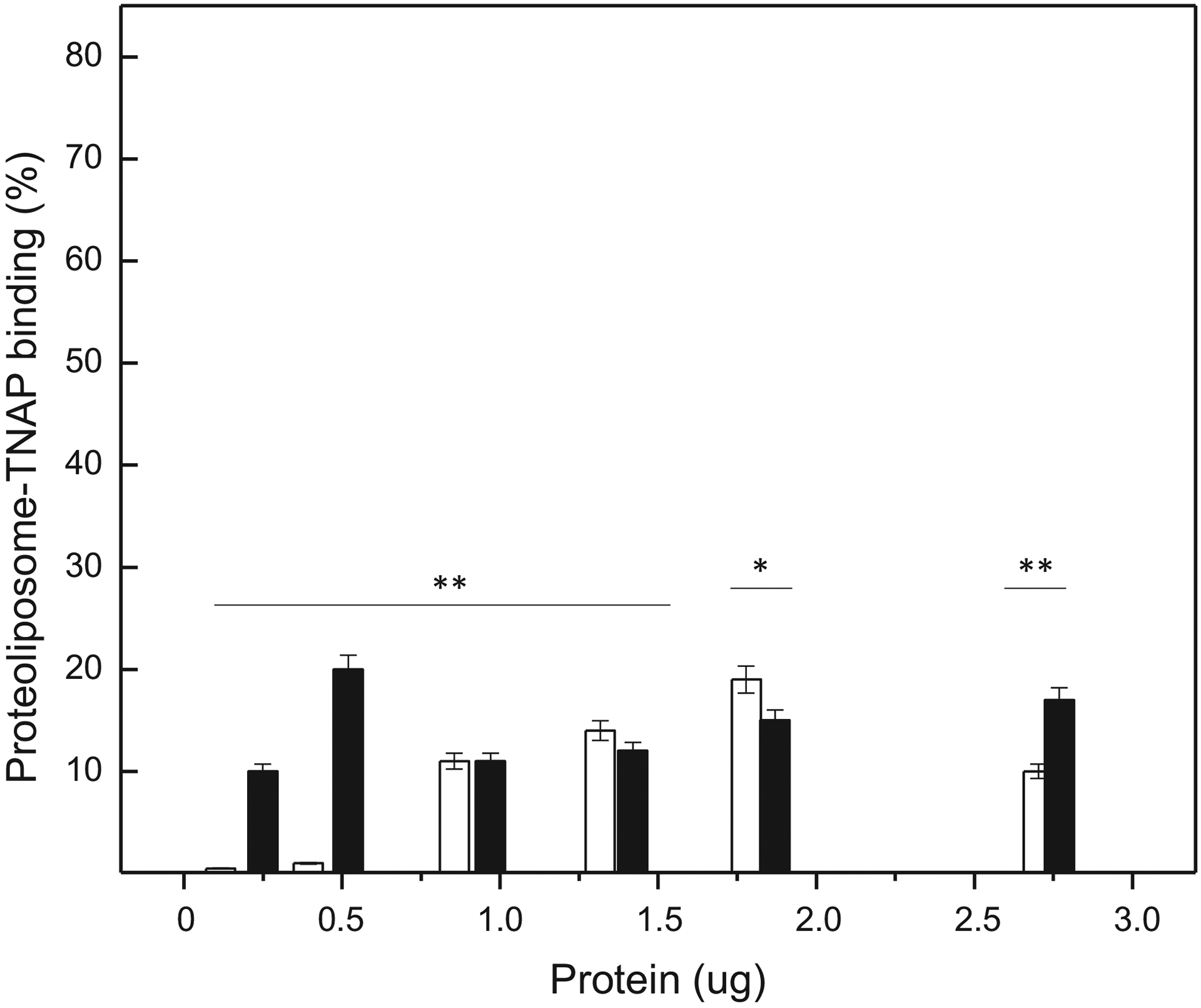
Effect of protein amount and lipid composition on binding of TNAP containing proteoliposomes to microtiter plates coated with type II collagen: DPPC-proteoliposomes (open bars) and 9:1 DPPC:DPPS-proteoliposomes (filled bars). Binding is expressed as a percentage of the protein adhered, as a function of the dose applied per well (indicated on the x-axis).
3.2.3. Affinity of Proteoliposomes harboring TNAP+AnxA5 for type II Collagen
For proteoliposomes simultaneously harboring TNAP+AnxA5, up to 30% total protein binding was found for 9:1 DPPC:DPPS proteoliposomes, for incubations with 3.5 μg of proteoliposome protein (Figure 3).
Figure 3.
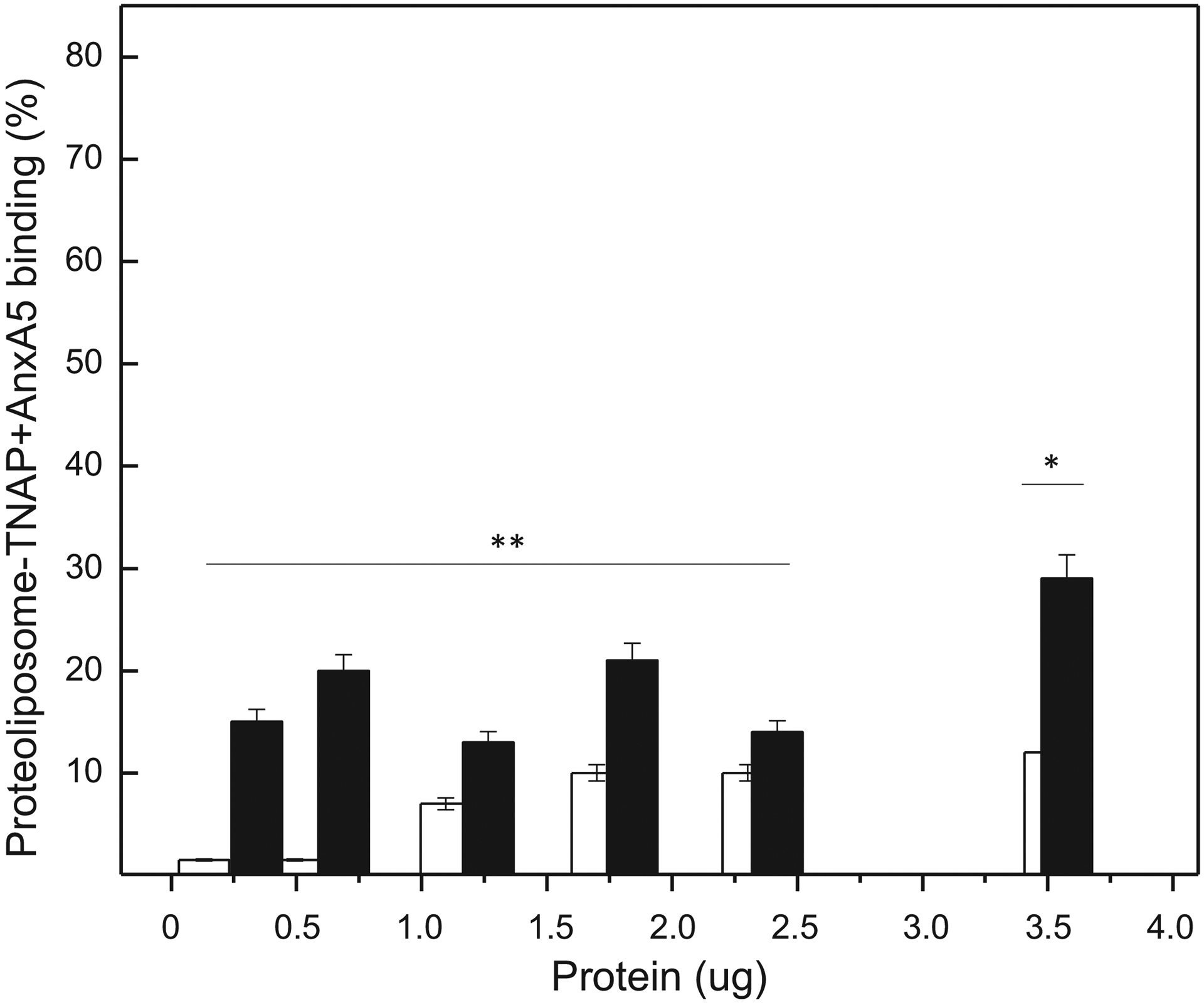
Effect of protein amount and lipid composition on binding of TNAP+AnxA5 containing proteoliposomes to microtiter plates coated with collagen type II: DPPC-proteoliposomes (open bars) and 9:1 DPPC:DPPS-proteoliposomes (filled bars). Binding is expressed as a percentage of the protein adhered, as a function of the dose applied per well (indicated on the x-axis).
With both proteins co-localized in the same DPPC proteoliposome, the affinity overall was weak and binding never exceeded 12% (for 3.6 μg of proteins in proteoliposomes) (Figure 3). Yet again, at low protein concentration (around 0.5 μg) the 9:1 DPPC:DPPS proteoliposomes context favored protein binding over the DPPC proteoliposomes context.
These findings illustrate that AnxA5 has a higher affinity for type II collagen fibers than TNAP. Surprisingly, the simultaneous presence of both proteins in the same vesicle, strongly reduced the binding strength of AnxA5. Even for 9:1 DPPC:DPPS proteoliposomes harboring both proteins, binding is fairly weak (Figure 3). This may be related to the microdomain structures formed when both proteins are present in the same vesicle, as shown previously [30]. In AFM in moderate/ soft tapping mode, images of the 9:1 DPPC:DPPS proteoliposomes surface suggest that, when AnxA5 and TNAP are simultaneously present within a lipid membrane, they organize themselves in more complex geometries, suggestive of mutual interactions between both proteins in the lipid membrane [30]. In addition, the presence of AnxA5 significantly affected the kinetic parameters of TNAP, when both are present in proteoliposomes, increasing the specificity for PPi hydrolysis, when compared to ATP and ADP hydrolysis [18]. These findings illustrate how the presence of TNAP in the lipid/protein microenvironment can disturb the interaction between AnxA5 and collagen matrix, probably as a result of steric effects.
3.2.4. Interaction between proteoliposomes and different Collagen Fibers
The principal structural elements of the extracellular matrix (ECM) are collagens, which play a predominant role in maintaining the biological and structural integrity of various tissues and organs, including bone, skin, muscle, tendon, blood vessels, and cartilage [28]. Depending on the tissue and its function, these fibers are highly oriented in a single direction and they present layers of oriented fibers angled to each other, or they are distributed randomly [31]. For example, it has been shown in cartilage that type II and X collagens are bound to the outer surfaces of MVs and may serve as a bridge for crystal propagation outside into the extravesicular matrix [31]. Whereas type II and X collagen are the main collagens that interact with MVs in cartilage, the majority of arterial collagens are type I and III [25–26]. Furthermore, type I collagen expression is upregulated in human atherosclerotic plaques and during medial calcification [26,32–33].
Individual collagen molecules are composed of three left-handed helical polypeptide chains forming a right-handed triple-helical structure that is stabilized by hydrogen bonds, showing to be approximately 300 nm long and 1.5 nm in diameter [31,34–36]. These differences in orientation and structure provide specific tissues with the appropriate mechanical properties [31].
To study the impact of the collagen structure and function, we have analyzed different types of collagen (I, II and I+III types) in the binding assays, using collagen matrixes coated with the concentration (6.25 μg/well), similar to the amount used in Figure 1–3. To achieve maximal refolding during coating, these pepsin-solubilized collagens were renatured in a neutral buffer during the coating process. Those experiments were performed using proteoliposomes harboring AnxA5 and TNAP+AnxA5, because these showed the highest relative affinity for type II collagen (Figure 1, 3). Although proteoliposome-AnxA5 binding had the highest affinity for type II collagen fibers, for both lipid compositions, a weaker but measurable affinity was noted for renatured type I collagen (Figure 4A). The opposite effect was observed for proteoliposomes harboring TNAP+AnxA5 (Figure 4B), their binding to collagen I even being weaker, when conditioned in 9:1 DPPC:DPPS proteoliposomes. Likewise, the binding of TNAP+AnxA5 conditioned in 9:1 DPPC:DPPS proteoliposomes to mixtures of collagen I+III was very weak, compared to the DPPC proteoliposomes conditioning. These findings confirm that collagen II has higher affinities for AnxA5 than collagens I and III, but also evidence that affinities for collagen I and III are non-negligible, dependent on the lipid context.
Figure 4. Effect of lipid composition (90 μg of total lipid incubated) on proteoliposome binding to different collagens.
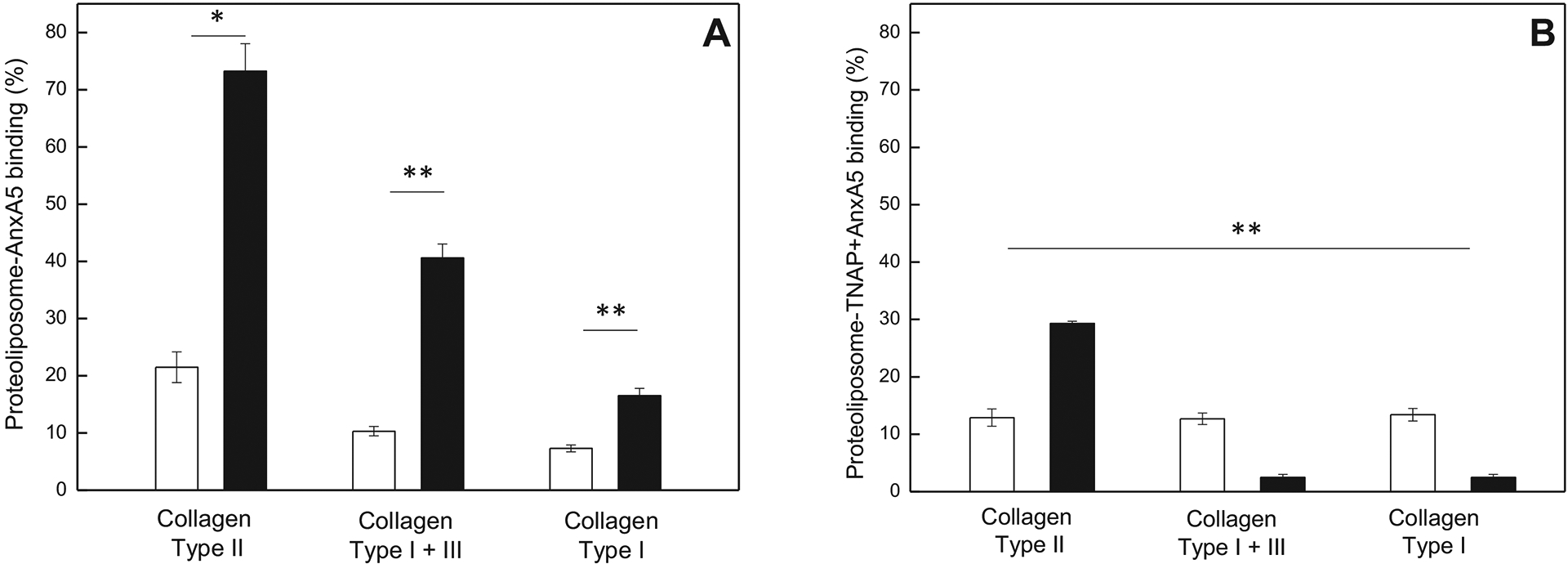
(A) AnxA5 proteoliposomes containing DPPC (2.6 μg of total protein incubated) (open bars) or 9:1 DPPC:DPPS (2.7 μg of total protein incubated) (filled bars); (B) TNAP+AnxA5 proteoliposomes containing DPPC (3.6 μg of total protein incubated) (open bars) or 9:1 DPPC:DPPS (3.5 μg of total protein incubated) (filled bars).
3.3. Production of native collagen matrix by native primary cultures and transdifferentiated VSMCs.
Vascular calcification can lead to cardiovascular morbidity and mortality. Recent evidence has shown that the pathogenesis of vascular calcification is complex and involves transformation of VSMCs to osteoblast/chondrocyte-like cells that express RUNX2, produce matrix vesicles and apoptotic bodies that increase nanocrystal deposition and can trigger ectopic manifestations of bone mineralization, among other phenomena [24,38–39]. Expression studies have confirmed that VSMCs give rise to osteochondrogenic-like cells in calcified blood vessels, as opposed to circulating cells [40]. It is hypothesized that vascular calcification in chronic kidney disease can be regarded as the result of premature aging of VSMC [39]. For this reason, we also produced native collagen matrix from transdifferentiating VSMCs in culture, in order to assess the impact of this process on interactions with the different proteoliposomes.
The majority of arterial collagen produced is type I and III [25–26]. Yet, more specifically in the case of chronic kidney disease associated with hyperphosphatemia, it was shown that the treatment of VSMCs in culture with Pi concentrations comparable to those found in hemodialysis patients (> 1.4 mM) could trigger mineral deposition in cultures and induce expression of the osteochondrogenic markers osteocalcin and Runx2, alongside induction of collagen II [24,40–41]. For this reason, we studied the occurrence of VSMC-transdifferentiation into an osteoblast/chondrocyte-like phenotype by high levels of Pi and ascorbic acid, to produce a relevant matrix enriched in native type II collagen. Hence, Pi (from 0 to 10 mM) was added to VSMC cell cultures (Figure 3S-A). Concentrations > 3 mM Pi appeared to be toxic for VSMCs, slowing down cellular proliferation rate.
Correspondingly, analysis by qRT-PCR measuring the relative expression of the smooth muscle marker αSM and the osteochondrogenic markers SPP1 (osteopontin mRNA) and type II collagen (COLA21) after Pi treatment vs. that of GAPDH showed a mixed picture. Whereas ascorbic acid (50 μg/mL) raised the expression of SPP1 in all conditions tested, it was maximal when combined with phosphate at 5 mM. COLA21 was induced in most conditions, although we found no consistent rise in the presence of 50 μg/ml ascorbic acid. The expression of αSM was induced in the majority of cases, except when ascorbic acid was combined with Pi 5 mM. Since actively dividing VSMCs hardly express αSM, this is compatible with a return to the contractile, quiescent state, when cellular proliferation slows down in the presence of ascorbic acid and Pi. At the highest degree of transdifferantiation (50 μg/mL ascorbic acid + 5 mM Pi) cells have lost the vascular phenotype and don’t trigger αSM any longer (Figure 3S-A). Collectively, these data are indicative of a progressive and slow VSMC differentiation into an osteoblast/chondrocyte-like phenotype (Figure S3-B, C). However, when cultured for 14 days with increasing concentrations of ascorbic acid and phosphate, Pi retarded cell growth and modified cell morphology from a bundle- (Figure S3-B) to needle-like phenotype (Figure S3-C). This may explain why the total content of collagen II stayed low in the most severely affected conditions, despite clear transdifferentiation. Nonetheless, Alizarin red staining confirmed that the transdifferentiation led to mineralization competent cells (Figure 5A–B) and cells depositing native collagen, some of it being collagen II (Figure S3-F, G, H).
Figure 5.
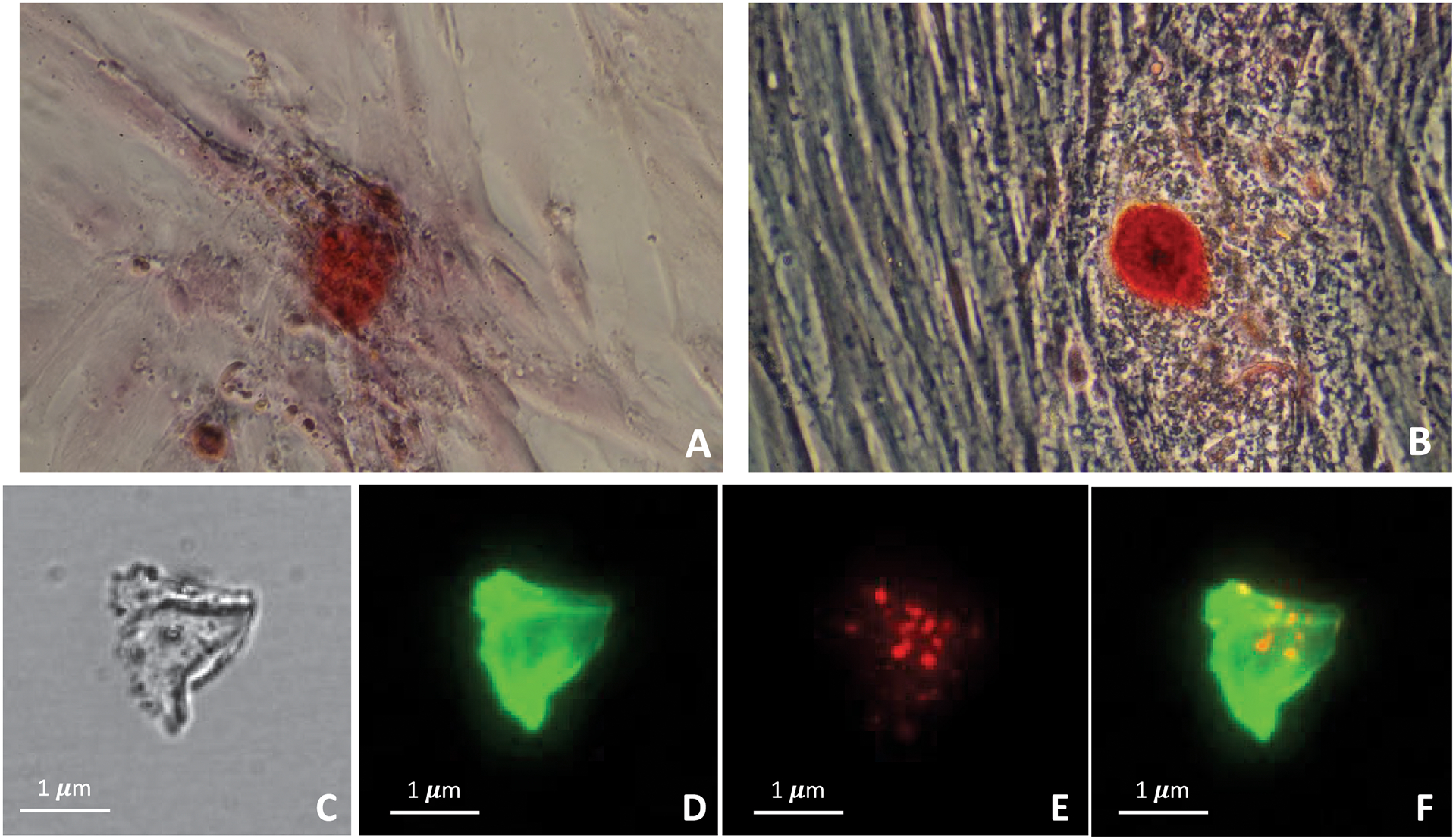
Alizarin red staining of VSMCs differentiated in chondrocyte/osteoblast phenotype under different conditions for 14 days: (A) 10 μg/mL ascorbic acid and 5 mM Na2PO4 and (B) 50 μg/mL ascorbic acid and 5 mM Na2PO4 (magnification factor 200x). Colocalization analysis by immunofluorescent staining between proteoliposomes and type II collagen matrix produced from culture of osteo/chondrocytic cells: (C) white light image of native collagen matrix; (D) type II collagen detected by anti-collagen type II antibody (green fluorescence); (E) Bound AnxA5 9:1 DPPC:DPPS proteoliposomes (1.87 μg of protein content) labeled with Rhodamine to type II collagen matrix (red fluorescence); (F) Co-localization of bound proteoliposomes with type II collagen (yellow).
3.4. Proteoliposomes interaction with native collagen produced by transdifferentiated VSMCs.
Since immunofluorescent staining had identified the presence of collagen II in the native ECM deposited by transdifferentiating VSMCs (Fig. S3- F, G, H), we performed co-localization experiments, using 9:1 DPPC:DPPS proteoliposomes harboring AnxA5, which show the highest affinity for type II collagen as shown in Figure 1. Thus, Figure 5C shows a white light image of the collagen matrix and Figure 5D the immunofluorescent detection of collagen type II (green fluorescence). The AnxA5 9:1 DPPC:DPPS proteoliposomes binding (red fluorescence) is shown in Figure 5E as a collection of individual vesicles, whereas Figure 5F confirms co-localization of proteoliposomes and type II collagen (yellow fluorescence).
Additional co-localization studies (data not shown) with DPPC proteoliposomes harboring AnxA5, DPPC and 9:1 DPPC:DPPS proteoliposomes harboring TNAP+AnxA5 (red fluorescence) to native collagen were performed. These show that proteoliposomes binding occurs both to collagen II and other parts of the native matrix (Figure S3- I and J), but exclusively to the collagen matrix, in agreement with the presence also of collagen I and III in such matrices, deposited early (and later) during the transdifferentiation process, over an interval of 14 days.
To estimate the contribution of different collagens to matrix binding we, therefore, compared proteoliposomes binding to the collagen matrix of native VSMCs and that of the 14-day-old transdifferentiated cells. The affinity of DPPC-proteoliposomes harboring AnxA5 or TNAP+AnxA5 for native collagen produced from native VSMC cultures was very low (less than 10% binding, Table 2), with affinities comparable to those for type I and type “I + III” collagens (Figure 4-A, B, Table 2). However, the transdifferentiation of VSMCs resulted in an ~2 fold increase in proteoliposomes binding (Table 2), although not reaching 20%, as found for the binding of AnxA5 DPPC-proteoliposomes to collagen II (Table 2). These findings indicate that the percentage of collagen II increases in the ECM during transdifferentiation, where it incorporates alongside collagen I and III.
Table 2:
Effect of collagen type and matrix composition on binding of proteoliposomes harboring AnxA5 and TNAP+AnxA5. A total of 90 μg lipid was deposited per well, both for DPPC and 9:1 DPPC:DPPS proteoliposomes.
| Proteoliposomes | Different collagens sources (binding %) | ||||||
|---|---|---|---|---|---|---|---|
| Lipid content | Protein content | ||||||
| AnxA5 | TNAP | Type II | Type I + III | TypeI | Native from VSMCs | Native from osteoblast/chondrocyte-like cells | |
| DPPC | + | − | 21.5 ± 2.7 | 10.3 ± 0.8 | 7.3 ± 0.6 | 6 ± 0.8 | 13 ± 0.6 |
| + | + | 12.9 ± 1.5 | 12.7 ± 1.0 | 13.4 ± 1.1 | 8 ± 0.9 | 17 ± 0.9 | |
| 9:1 DPPC:DPPS | + | − | 73.2 ± 4.8 | 40.6 ± 2.4 | 16.5 ± 1.3 | 95 ± 2.3 | 81 ± 4.0 |
| + | + | 29.3 ± 0.4 | 1.3 ± 0.5 | 0.8 ± 0.5 | 73 ± 2.4 | 29 ± 1.5 | |
The affinities of 9:1 DPPC:DPPS proteoliposomes harboring AnxA5 for native collagen from VSMCs with or without differentiation were very high (Table 2). After differentiation, lower affinities were observed to values similar to those found for the binding study to collagen II (Table 2). Especially with 9:1 DPPC:DPPS proteoliposomes, the presence of both proteins in the same vesicle strongly reduces the impact of AnxA5 on the binding to various collagens resulting in a low affinity for collagen I and III (Figure 4). Therefore, the drop in binding from 73 to 29% upon transdifferentiation reflects a drop of the impact of collagen I and III, in favor of binding to collagen II, in further agreement with the interaction profile of 9:1 DPPC:DPPS-proteoliposomes containing both TNAP and AnxA5 (Figure 3). TNAP anchoring in the proteoliposomes together with AnxA5 probably influences the charge distribution of the proteoliposomes, modifying the density of negative charges in the surface of proteoliposomes decreasing their ability to collagen binding, compared to pure AnxA5 proteoliposomes.
In a physiological context, it seems that TNAP is not a positive catalyst of the binding processes studies here, and that is rather involved in the nucleation and propagation of the minerals of apatite, whereas AnxA5 with its considerable higher affinity for collagen, would target MVs to specific collagen-rich areas in the ECM. This process depends on the collagen density and the type of collagen. It’s worth to note that the role of TNAP in controlling the PPi/Pi ratio and thus controlling the mineralization is well stablished. However, whether TNAP performs its function solely bound to the MVs membrane or also after being cleaved is still not clear and this issue deserves attention.
4. Conclusion
In conclusion, this work focused on the relation between AnxA5, collagens and TNAP in liposomes and investigated how lipid and protein compositions regulate the interactions between vesicles and matrix collagens.
AnxA5 in proteoliposomes targets them to collagen fibers with high affinity and proteoliposomes harboring TNAP alone binding poorly to collagen matrices. The highest affinities were found for collagen matrices containing type II collagen, supportive of a role for AnxA5 during chondrocyte mineralization in joint cartilage.
The proteoliposomes harboring AnxA5 interact considerably better with collagen in liposomes containing DPPS, compared to vesicles composed of DPPC exclusively. Thus, the lipid DPPS plays a very important role both for improving AnxA5 incorporation into liposomes and for favoring binding of proteoliposomes to collagens.
Native collagen coatings were produced by culturing VSMCs in native and transdifferentiating conditions, in an effort to gradually mimic ectopic calcification conditions in cell culture conditions. Co-localization studies uncovered the binding of proteoliposomes to various regions in a deposited native collagen-rich matrix, including binding to deposited type II collagen.
Binding affinities of DPPC proteoliposomes for native matrix from osteo/chondrocyte cells went up two-fold compared to matrix produced by VSMCs. The affinities of 9:1 DPPC:DPPS proteoliposomes were high for native matrix from VSMCs with or without differentiation, these numbers bringing out the synergic effect of AnxA5 function with the negative charges of the DPPS lipid.
In all, TNAP in the lipid/protein microenvironment disturbs interactions between AnxA5 and collagen, probably as a consequence of steric effects, modifying the negative charge density of the proteoliposomes surface, thus diminishing binding to collagen. From these findings, the possibility arises that TNAP might be cleaved from the MVs membrane just before ECM binding, thus allowing MVs anchoring to ECM via AnxA5 interaction to collagen, during the physiological mineralization process. This hypothesis will be further tested in future work.
Taken together, understanding those factors and mechanisms that regulate the mineralization process are essential for the development of novel therapeutic strategies to prevent or inhibit ectopic mineralization, especially of vascular smooth muscle cells.
Supplementary Material
Figure S1: Analysis of binding between DPPC Proteoliposomes harboring AnxA5 and type II collagen matrix by fluorescence microscopy (magnification factor 100x). The vesicles were labeled with Rhodamine 6G (0.2 mol%) and analyses were performed using IN Cell 2000 Analyzer: (A) Collagen coating alone; (B) AnxA5-proteoliposome (0.87 μg protein content); (C) AnxA5-proteoliposome (2.6 μg protein content); (D) AnxA5-proteoliposome (3.5 μg protein content) incubated with collagen and (E) zoom of selected area from image D.
Figure S2: Analysis of binding between 9:1 DPPC:DPPS Proteoliposomes (molar ratio) harboring A5 and type II collagen coating by fluorescence microscopy (magnification factor 100x). The vesicles were labeled with Rhodamine 6G (0.2 mol%) and the analysis were performed using IN Cell 2000 Analyzer: (A) Collagen coating alone; (B) AnxA5-proteoliposome (0.92 μg protein content); (C) AnxA5-proteoliposome (1.8 μg protein content); (D) AnxA5-proteoliposome (3.6 μg protein content) incubated with collagen and (E) zoom of selected area from image D.
Figure S3. (A) PCR results showing the expression regulation for SPP1 (empty bars), COL2A1(striped bars) and α-SM (filled bars), illustrating the progressive differentiation process of VSMCs in an osteoblasts/chondrocyte-like phenotype, after treatment with different concentrations of NaH2PO4 and ascorbic acid. Sample 1: 10 μg/ml ascorbic acid; Sample 2: 10 μg/ml ascorbic acid + 2.5 mM NaH2PO4; Sample 3: 10 μg/ml ascorbic acid + 5.0 mM NaH2PO4; Sample 4: 50 μg/ml ascorbic acid; Sample 5: 50 μg/ml ascorbic acid + 2.5 mM NaH2PO4; Sample 6: 50 μg/ml ascorbic acid + 5.0 mM NaH2PO4. (B) Phase contrast microscopy images of VSMCs culture and (C) VSMCs culture with ascorbic acid (50 μg/mL) and phosphate (5 mM) (magnification factor 100x). (D) Native collagen produced by VSMCs culture in the presence of ascorbic acid (50 μg/mL) and phosphate (5 mM) magnification factor 50x and (E) 400x. (F, G, H) Immunofluorescence microscopy images (Pendragon AxioVert 200M Microscope) from different areas in the same well plate, showing native collagen type II (green fluorescence) produced during VSMCs differentiation in osteoblast/chondrocyte-like cells in the presence of 50 μg/ml ascorbic acid + 5.0 mM NaH2PO4, as described in item 2.10 of Material and Methods. (I) White light image of nature collagen matrix after proteoliposomes binding and (J) Fluorescent light microscopy (IN Cell 2000 Analyzer) illustrating proteoliposome binding (red fluorescence) to deposited collagen matrix exclusively. These pictures show binding of 9:1 DPPC:DPPS-proteoliposomes harboring AnxA5+TNAP (0.6 mg/mL lipid concentration and 0,2 mol% Rhodamine 6G) to native matrix produced by differentiated osteoblast/chondrocyte cells.
Acknowledgements
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2014/00371-1, 2015/06814-5, 2016/21236-6, 2017/08892-9), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (Finance Code 001, #88887.320304/2019-00), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (304021/2017-2) for the financial support given to our laboratory. MB received CAPES grant. PC and APR also acknowledges CNPq for research fellowships. This work was also supported in part by grant DE12889 from the National Institutes of Health (USA).
Abbreviations
- ECM
collagenous extracellular matrix
- MVs
matrix vesicles
- HA
hydroxyapatite
- PPi
Inorganic pyrophosphate
- Pi
Inorganic phosphate
- VSMCs
vascular smooth muscle cells
- TNAP
alkaline phosphatase
- AnxA5
annexin A5
- DPPC
dipalmitoylphosphatidylcholine
- DPPS
dipalmitoylphosphatidylserine
Footnotes
Conflict of interest
All authors report no conflicts of interest.
5. References
- [1].Kirsch T (2012), Biomineralization: an active or passive process? Connect Tissue Res. 53(6):438–445. [DOI] [PubMed] [Google Scholar]
- [2].Wuthier RE; Chin JE; Hole JE; Register TC; Laura YH and Ishikawa Y (1985), Isolation and characterization of calcium-accumulating matrix vesicles from chondrocytes of chicken epiphyseal growth plate cartilage in primary culture. J. Biol. Chem 260:15972–15979. [PubMed] [Google Scholar]
- [3].Anderson HC (2003), Matrix vesicles and calcification. Curr. Rheumatol. Rep 5:222–226. [DOI] [PubMed] [Google Scholar]
- [4].Balcerzak M; Hamade E; Zhang L; et al. , Pikula S; Azzar G; Radisson J; Bandorowicz-Pikula J and Buchet R (2003), The roles of annexins and alkaline phosphatase in mineralization process. Acta Biochim. Pol 50:1019–1038. [PubMed] [Google Scholar]
- [5].Thouverey C; Strzelecka-Kiliszek A; Belcerzak M; Buchet R; Pikula S (2009), Matrix vesicles originate from apical membrane microvilli of mineralizing osteoblast-like Saos-2 cells. J Cel. Biochem 106:127–138. [DOI] [PubMed] [Google Scholar]
- [6].Bolean M; Simão AMS; Barioni MB; Favarin BZ; Sebinelli HG; Veschi EA; Janku TAB; Bottini M; Hoylaerts MF; Itri R; Millán JL; Ciancaglini P (2017), Biophysical aspects of biomineralization. Biophys Rev. 9:747–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bottini M; Mebarek S; Anderson KL; Strzelecka-Kiliszek A; Bozycki L; Simão AMS; Bolean M; Ciancaglini P; Pikula JB; Pikula S; Magne D; Volkmann N; Hanein D; Millán JL and Buchet R (2018), Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochem. Biophys. Acta 1862:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wuthier RE and Lipscomb GF (2011), Matrix vesicles: structure, composition, formation and function in calcification. Front. Biosci. (Landmark Ed.) 17:2812–2902. [DOI] [PubMed] [Google Scholar]
- [9].Golub EE (2011), Biomineralization and matrix vesicles in biology and pathology. Semin Immunopathol. 33(5):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Millán JL (2013), The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int 93:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leopold JA (2015), Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 25(4):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Millán JL (2006), In: Mammalian Alkaline Phosphatase: From Biology to Applications in Medicine and Biotechnology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. [Google Scholar]
- [13].Hoylaerts MF; Van Kerckhoven S; Kiffer-Moreira T; Sheen C; Narisawa S and Millán JL (2015), Functional significance of calcium binding to tissue-nonspecific alkaline phosphatase. PLoS One. 16;10(3):e0119874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huber R; Romisch J and Pâques EP (1990), The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 9(12):3867–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].von der Mark K and Mollenhauer J; (1997), Annexin V interactions with collagen. Cell Mol Life Sci. 53(6):539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim HJ and Kirsch T (2008), Collagen/annexin V interactions regulate chondrocyte mineralization. J Biol Chem. 18; 283(16):10310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang W and Kirsch T (2006), Annexin V/beta5 integrin interactions regulate apoptosis of growth plate chondrocytes. J Biol Chem. 13;281(41):30848–56. [DOI] [PubMed] [Google Scholar]
- [18].Bolean M; Simão AMS.; Kiffer-Moreira T; Hoylaerts MF; Millán JL; Itri R and Ciancaglini P (2015) Proteoliposomes with the ability to transport Ca2+ into the vesicles and hydrolyze phosphosubstrates on their surface. Arch. Biochem. Biophys 584:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Logue SE; Elgendy M and Martin SJ (2009), Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat. Protoc 4, 1383–1395. [DOI] [PubMed] [Google Scholar]
- [20].Simão AM; Yadav MC; Narisawa S; Bolean M; Pizauro JM; Hoylaerts MF; Ciancaglini P and Millán JL (2010), Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem. 5;285(10):7598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bolean M; Simão AMS; Favarin BZ; Millán JL and Ciancaglini P (2010), The effect of cholesterol on the reconstitution of alkaline phosphatase into liposomes. Biophys. Chem 152: 74–9. [DOI] [PubMed] [Google Scholar]
- [22].Bolean M; Simão AMS; Favarin BZ; Millán JL and Ciancaglini P (2011), Thermodynamic properties and characterization of proteoliposomes rich in microdomains carrying alkaline phosphatase. Biophys Chem. 158: 111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hartree EF (1972), Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal. Biochem 48(2):422e427. [DOI] [PubMed] [Google Scholar]
- [24].Hortells L; Sosa C; Millán Á and Sorribas V (2015), Critical Parameters of the In Vitro Method of Vascular Smooth Muscle Cell Calcification. PLoS One. 10(11):e0141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mayne R (1986), Collagenous proteins of blood vessels. Arteriosclerosis 6: 585–593. [DOI] [PubMed] [Google Scholar]
- [26].Chen NX; O’Neill KD; Chen X and Moe SM (2008), Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 23(11):1798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Favarin BZ; Andrade MAR; Bolean M; Simão AMS; Ramos AP; Hoylaerts MF; Millán JL; Ciancaglini P (2017), Effect of the presence of cholesterol in the interfacial microenvironment on the modulation of the alkaline phosphatase activity during in vitro mineralization. Colloids Surf B Biointerfaces. 155:466–476. [DOI] [PubMed] [Google Scholar]
- [28].Kolácná L; Bakesová J; Varga F; Kostáková E; Plánka L; Necas A; Lukás D; Amler E and Pelouch V (2007), Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res. 2007;56 Suppl 1:S51–60. [DOI] [PubMed] [Google Scholar]
- [29].Viidik A, Vuust J - “Biology of Collagen”, London, Academic Press, v. 1 (1980). [Google Scholar]
- [30].Bolean M; Borin IA; Simão AMS; Bottini M; Bagatolli LA; Hoylaerts MF; Millán JL; Ciancaglini P (2017), Topographic analysis by atomic force microscopy of proteoliposomes matrix vesicle mimetics harboring TNAP and AnxA5. Biochim Biophys Acta. 1859(10):1911–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tenboll A; Darvish B; Hou W; Duwez AS; Dixon SJ; Goldberg HA; Grohe B and Mittler S (2010), Controlled deposition of highly oriented type I collagen mimicking in vivo collagen structures. Langmuir. 26(14):12165–72. [DOI] [PubMed] [Google Scholar]
- [32].Moe SM; O’Neill KD; Duan D; Ahmed S; Chen NX; Leapman SB; Fineberg N and Kopecky K (2002), Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int 61: 638–647. [DOI] [PubMed] [Google Scholar]
- [33].Rekhter MD; Zhang K; Narayanan AS; Phan S; Schork MA and Gordon D (1993), Type I collagen gene expression in human atherosclerosis. Localization to specific plaque regions. Am J Pathol 143(6):1634–1648. [PMC free article] [PubMed] [Google Scholar]
- [34].Nimni ME (1988) Ed. Collagen Vol. I Biochemistry; CRC Press: Boca Raton. [Google Scholar]
- [35].Hulmes DJ (2002), Building collagen molecules, fibrils, and suprafibrillar structures. Struct. Biol 137(1–2):2–10. [DOI] [PubMed] [Google Scholar]
- [36].Hulmes DJ (1992), The collagen superfamily:diverse structures and assemblies. Essays Biochem. 27:49–67. [PubMed] [Google Scholar]
- [37].Huster D; Arnold K and Gawrisch K (2000), Strength of Ca2+ binding to retinal lipid membranes: consequences for lipid organization. Biophysical J. 78(6):3011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Reynolds JL; Joannides AJ; Skepper JN; McNair R; Schurgers LJ; Proudfoot D; Jahnen-Dechent W; Weissberg PL and Shanahan CM (2004), Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 15(11):2857–67. [DOI] [PubMed] [Google Scholar]
- [39].Chen NX and Moe SM (2015), Pathophysiology of Vascular Calcification. Curr Osteoporos Rep. 13(6):372–80. [DOI] [PubMed] [Google Scholar]
- [40].Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM (2009), Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 104(6):733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jono S; McKee MD; Murry CE; Shioi A; Nishizawa Y; Mori K; Morii H and Giachelli CM (2000), Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 87(7):E10–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Analysis of binding between DPPC Proteoliposomes harboring AnxA5 and type II collagen matrix by fluorescence microscopy (magnification factor 100x). The vesicles were labeled with Rhodamine 6G (0.2 mol%) and analyses were performed using IN Cell 2000 Analyzer: (A) Collagen coating alone; (B) AnxA5-proteoliposome (0.87 μg protein content); (C) AnxA5-proteoliposome (2.6 μg protein content); (D) AnxA5-proteoliposome (3.5 μg protein content) incubated with collagen and (E) zoom of selected area from image D.
Figure S2: Analysis of binding between 9:1 DPPC:DPPS Proteoliposomes (molar ratio) harboring A5 and type II collagen coating by fluorescence microscopy (magnification factor 100x). The vesicles were labeled with Rhodamine 6G (0.2 mol%) and the analysis were performed using IN Cell 2000 Analyzer: (A) Collagen coating alone; (B) AnxA5-proteoliposome (0.92 μg protein content); (C) AnxA5-proteoliposome (1.8 μg protein content); (D) AnxA5-proteoliposome (3.6 μg protein content) incubated with collagen and (E) zoom of selected area from image D.
Figure S3. (A) PCR results showing the expression regulation for SPP1 (empty bars), COL2A1(striped bars) and α-SM (filled bars), illustrating the progressive differentiation process of VSMCs in an osteoblasts/chondrocyte-like phenotype, after treatment with different concentrations of NaH2PO4 and ascorbic acid. Sample 1: 10 μg/ml ascorbic acid; Sample 2: 10 μg/ml ascorbic acid + 2.5 mM NaH2PO4; Sample 3: 10 μg/ml ascorbic acid + 5.0 mM NaH2PO4; Sample 4: 50 μg/ml ascorbic acid; Sample 5: 50 μg/ml ascorbic acid + 2.5 mM NaH2PO4; Sample 6: 50 μg/ml ascorbic acid + 5.0 mM NaH2PO4. (B) Phase contrast microscopy images of VSMCs culture and (C) VSMCs culture with ascorbic acid (50 μg/mL) and phosphate (5 mM) (magnification factor 100x). (D) Native collagen produced by VSMCs culture in the presence of ascorbic acid (50 μg/mL) and phosphate (5 mM) magnification factor 50x and (E) 400x. (F, G, H) Immunofluorescence microscopy images (Pendragon AxioVert 200M Microscope) from different areas in the same well plate, showing native collagen type II (green fluorescence) produced during VSMCs differentiation in osteoblast/chondrocyte-like cells in the presence of 50 μg/ml ascorbic acid + 5.0 mM NaH2PO4, as described in item 2.10 of Material and Methods. (I) White light image of nature collagen matrix after proteoliposomes binding and (J) Fluorescent light microscopy (IN Cell 2000 Analyzer) illustrating proteoliposome binding (red fluorescence) to deposited collagen matrix exclusively. These pictures show binding of 9:1 DPPC:DPPS-proteoliposomes harboring AnxA5+TNAP (0.6 mg/mL lipid concentration and 0,2 mol% Rhodamine 6G) to native matrix produced by differentiated osteoblast/chondrocyte cells.


