Abstract
Aims
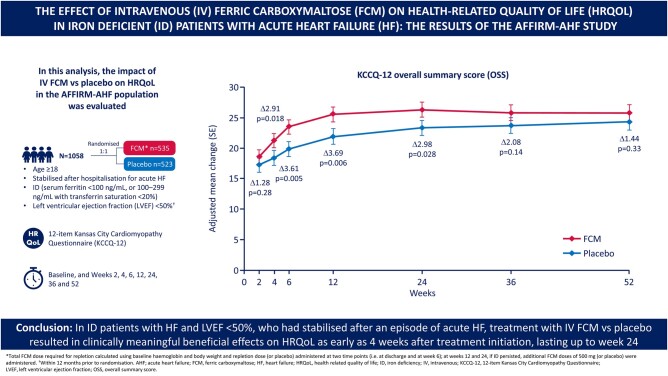
Patients with heart failure (HF) and iron deficiency experience poor health-related quality of life (HRQoL). We evaluated the impact of intravenous (IV) ferric carboxymaltose (FCM) vs. placebo on HRQoL for the AFFIRM-AHF population.
Methods and results
The baseline 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12), which was completed for 1058 (535 and 523) patients in the FCM and placebo groups, respectively, was administered prior to randomization and at Weeks 2, 4, 6, 12, 24, 36, and 52. The baseline KCCQ-12 overall summary score (OSS) mean ± standard error was 38.7 ± 0.9 (FCM group) and 37.1 ± 0.8 (placebo group); corresponding values for the clinical summary score (CSS) were 40.9 ± 0.9 and 40.1 ± 0.9. At Week 2, changes in OSS and CSS were similar for FCM and placebo. From Week 4 to Week 24, patients assigned to FCM had significantly greater improvements in OSS and CSS scores vs. placebo [adjusted mean difference (95% confidence interval, CI) at Week 4: 2.9 (0.5–5.3, P = 0.018) for OSS and 2.8 (0.3–5.3, P = 0.029) for CSS; adjusted mean difference (95% CI) at Week 24: 3.0 (0.3–5.6, P = 0.028) for OSS and 2.9 (0.2–5.6, P = 0.035) for CSS]. At Week 52, the treatment effect had attenuated but remained in favour of FCM.
Conclusion
In iron-deficient patients with HF and left ventricular ejection fraction <50% who had stabilized after an episode of acute HF, treatment with IV FCM, compared with placebo, results in clinically meaningful beneficial effects on HRQoL as early as 4 weeks after treatment initiation, lasting up to Week 24.
Keywords: Heart failure, Acute heart failure, Iron deficiency, Intravenous ferric carboxymaltose therapy, Health-related quality of life, Randomized clinical trial
Graphical Abstract
See page 3021 for the editorial comment on this article (doi:10.1093/eurheartj/ehab365)
Introduction
Heart failure (HF) is a debilitating condition associated with considerable morbidity, premature mortality, and substantial use of healthcare resources.1–4 In particular, HF patients experience a high burden of symptoms and physical and social limitations, all of which negatively impact upon their quality of life.3 , 5 , 6 According to the European Society of Cardiology (ESC) guidelines on management of HF, an improvement in health status (symptoms, function, and quality of life) is one of the major therapeutic goals in the management of these patients.3 This view has also been acknowledged and endorsed by regulatory authorities and by the patients themselves.7–9
Iron deficiency (ID) negatively impacts upon symptom burden, exercise capacity, and quality of life in HF patients.10–12 Randomized controlled trials have demonstrated that intravenous (IV) ferric carboxymaltose (FCM) alleviates symptoms, and improves exercise capacity and quality of life in ambulatory iron-deficient patients with chronic HF and left ventricular ejection fraction (LVEF) ≤45%.13–15
The AFFIRM-AHF trial, which was a randomized, double-blind, placebo-controlled trial, demonstrated that administration of IV FCM in iron-deficient patients who had stabilized after an acute HF episode reduced the risk of recurrent HF hospitalizations.16 The effect of IV FCM on health-related quality of life (HRQoL) in this high-risk population has not been previously investigated—the latter was one of the predefined other secondary outcomes in the AFFIRM-AHF trial. In this analysis, we evaluated the effect of IV FCM, compared with placebo, administered just prior to discharge in patients with acute HF and ID on the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12) overall summary score (OSS) and clinical summary score (CSS) up to 52 weeks after randomization.
Methods
Study design and population
The rationale and design of the AFFIRM-AHF trial has been previously published.17 Briefly, the AFFIRM-AHF trial was a double-blind, placebo-controlled trial in which eligible subjects were 18 years or older and hospitalized with clinical signs, symptoms, and biomarkers consistent with acute HF. During the index hospitalization, patients had to have received at least 40 mg of IV furosemide (or equivalent) and an LVEF <50% within 12 months prior to randomization. In addition, patients had to be iron-deficient, defined as serum ferritin <100 ng/mL, or between 100 and 299 ng/mL with transferrin saturation <20%.16 , 17 Iron status was assessed on the basis of serum ferritin and transferrin saturation, with measurement allowed at any time during the index hospitalization.
Prior to discharge, eligible patients were randomly (1:1) assigned to receive either IV FCM or placebo. The total FCM dose required for repletion was calculated using baseline haemoglobin and body weight, and the repletion dose was administered at two time points (i.e. at discharge and Week 6). The first and subsequently administered doses were up to 1000 mg FCM (or placebo). At Weeks 12 and 24, if ID persisted, additional FCM doses of 500 mg (or placebo) were administered.16 , 17
The protocol was approved by the institutional review board at each participating centre. Written informed consent was obtained from all patients before any study-related procedures were performed. The first and the last authors (E.A.J. and P.P.) had full access to the data, and took responsibility for its integrity and analysis.
Assessment of HRQoL in the whole trial cohort using the KCCQ-12 was prospectively planned and was specified among other outcomes in the statistical analysis plan (SAP). The primary composite outcome of AFFIRM-AHF was recurrent HF hospitalizations and cardiovascular death, and there were five clinical secondary outcomes.
Health status outcome measures
The KCCQ-12 was used to evaluate the HF-specific health status.18 The KCCQ-12 is a self-administered, disease-specific instrument for measuring HF-specific health status, regardless of HF aetiology. It is a 12-item questionnaire that quantifies physical function, symptoms (frequency, severity and recent change), social function, self-efficacy and knowledge, and quality of life.6 , 18 , 19
To simplify the clinical interpretation, all scores and subscores are represented on a scale from 0 to 100, in which lower scores represent comparatively more severe symptoms and/or limitations, and a score of 100 indicates no symptoms, no limitations, and excellent quality of life.18 , 19 The KCCQ-12 tool is used to estimate the OSS, which includes pooled information on symptoms, physical and social functioning and perception of quality of life, whereas the CSS includes pooled information reflecting mainly symptoms and physical and social functioning of an examined patient. In addition to two summary scores, four domains can be derived in order to separately describe physical limitation, symptom frequency, quality of life, and social limitation.
The baseline KCCQ-12 was administered just prior to randomization during the index hospitalization. Patients completed the paper-based version of the questionnaire, and validated translations were used in countries where English was not the mother tongue. During follow-up, the KCCQ-12 was completed by patients at Weeks 2, 4, 6, 12, 24, 36, and 52. Participants were placed in a quiet environment and requested to complete the KCCQ-12 prior to any other assessment or procedure being performed at the visit concerned. For visits conducted by telephone (i.e. visits at Weeks 2, 4, and 36 post-randomization), participants were requested to complete the KCCQ-12 just prior to the call and to return the completed questionnaire at the next scheduled outpatient visit.
Statistical analyses
For each visit as described above, the KCCQ OSS and KCCQ CSS were calculated. The actual values and change from baseline in these two summary scores were descriptively summarized at each visit. The treatment difference in KCCQ-12 scores (one model for each summary score) at Weeks 2, 4, 6, 12, 24, 36, and 52 were analysed by comparing the model-adjusted means of the respective visits based on a repeated-measures model adjusted for corresponding baseline KCCQ-12 value, sex, age at randomization (<70 years/≥70 years), HF aetiology at randomization (ischaemic/non-ischaemic/unknown), HF duration (newly diagnosed at index hospitalization/known documented HF prior to index hospitalization), country, time, treatment, and treatment-by-time interaction using an unstructured covariance matrix to model the within-subject variability. Similar analyses were carried out using unadjusted models.
Taking into consideration the COVID-19 pandemic, which interfered with the progress of the trial and which could potentially have affected the subjective assessment of quality of life, COVID-19 sensitivity analyses were performed using adjusted and unadjusted models. In these analyses, all KCCQ-12 assessments occurring after the date when the first COVID-19 case was diagnosed in each country were deleted and were considered as missing values without any imputation.
In order to evaluate the consistency of the treatment effect, differences in adjusted mean changes in the KCCQ-12 OSS and CSS from baseline to Week 24 with IV FCM compared with placebo were assessed in 22 pre-specified subgroups.
The pre-specified analyses of the KCCQ-12 described in the SAP did not include any imputations for death. An additional sensitivity analysis with imputed values accounting for patient mortality was performed, in which KCCQ-12 values for patients who were dead at the time of the scheduled assessment were assigned 0 points (worst health status).
Responder analyses were performed, examining the proportion of patients with a deterioration or an improvement in KCCQ-12 during subsequent study visits. We used thresholds that had been established as clinically meaningful for KCCQ for patients with stable chronic HF.19 , 20 The number and percentage of subjects with respective improvements of ≥5, ≥8, ≥10, ≥20, and ≥30 points and deterioration of ≥5 points in KCCQ-12 OSS and CSS scores were assessed for the FCM and placebo groups. Odds ratios to estimate differences between the study groups (FCM vs. placebo) and their corresponding 95% confidence intervals (CIs) and two-sided P-values were estimated from logistic regression models.
Missing data were not imputed. The number of missing values were reported with the identification of missing values due to death. A P-value of <0.05 was considered statistically significant and no adjustments for multiple testing were carried out.
Results
Baseline characteristics
Among the 1108 patients included in the modified intention-to-treat AFFIRM-AHF analysis, a baseline KCCQ-12 was completed for 1058 (95%) patients (535 and 523 in the FCM and placebo groups, respectively). The baseline characteristics of the patients who completed the baseline KCCQ-12 were comparable between the two study groups (Table 1).
Table 1.
Characteristics of patients at baselinea
| Ferric carboxymaltose (N = 535) | Placebo (N = 523) | |
|---|---|---|
| Age (years) | 71.0 ± 10.85 | 70.9 ± 11.3 |
| Sex | ||
| Male | 298 (55.7) | 283 (54.1) |
| Female | 237 (44.3) | 240 (45.9) |
| Race | ||
| White | 509 (95.1) | 499 (95.4) |
| Other | 26 (4.9) | 24 (4.6) |
| Comorbidities | ||
| Previous myocardial infarction | 220 (41.1) | 206 (39.4) |
| Previous stroke | 51 (9.5) | 63 (12.0) |
| Previous coronary revascularization | 187 (35.0) | 197 (37.7) |
| Hypertension | 449 (83.9) | 448 (85.7) |
| Atrial fibrillation | 303 (56.6) | 286 (54.7) |
| Diabetes mellitus | 222 (41.5) | 228 (43.6) |
| Dyslipidaemia | 287 (53.6) | 275 (52.6) |
| Chronic kidney disease | 211 (39.4) | 215 (41.1) |
| Smoking (current) | 54 (10.1) | 48 (9.2) |
| Smoking (former) | 154 (28.8) | 144 (27.5) |
| Body mass index (kg/m2) | 28.2 ± 5.7 | 28.1 ± 5.7 |
| NYHA functional class | ||
| I | 14 (2.6) | 8 (1.5) |
| II | 242 (45.3) | 229 (44.0) |
| III | 263 (49.3) | 263 (50.6) |
| IV | 15 (2.8) | 20 (3.8) |
| Left ventricular ejection fraction (%)b | 32.8 ± 9.6 | 32.8 ± 9.9 |
| Left ventricular ejection fractionb | ||
| <25% | 99 (18.5) | 113 (21.6) |
| 25–39% | 272 (50.8) | 234 (44.8) |
| 40–49% | 164 (30.7) | 175 (33.5) |
| Ischaemic aetiology of HF | 255 (47.7) | 246 (47.0) |
| Device therapy | ||
| Implantable cardioverter-defibrillator | 64 (12.0) | 60 (11.5) |
| Cardiac resynchronization therapy | 31 (5.8) | 30 (5.7) |
| Heart failure history | ||
| Newly diagnosed at index hospitalization | 144 (26.9) | 153 (29.3) |
| Hospitalization for heart failure in previous 12 months | 142 (36.3) | 145 (39.2) |
| Pharmacotherapy | ||
| Angiotensin-converting enzyme inhibitor | 297 (55.5) | 282 (53.9) |
| Angiotensin II receptor blocker | 123 (23.0) | 103 (19.7) |
| Angiotensin receptor-neprilysin inhibitor | 27 (5.0) | 27 (5.2) |
| Mineralocorticoid receptor antagonist | 344 (64.3) | 346 (66.2) |
| Beta-blocker | 392 (73.3) | 397 (75.9) |
| Digitalis glycosides | 110 (20.6) | 109 (20.8) |
| Loop diuretic | 532 (99.4) | 522 (99.8) |
| KCCQ-12 | ||
| Overall summary score, mean (±SE) | 38.1 (±0.9) | 37.1 (±0.8) |
| Clinical summary score, mean (±SE) | 40.9 (±0.9) | 40.1 (±0.9) |
| Laboratory test results | ||
| Median NT-proBNP (Q1, Q3) (pg/mL) | 4657 (2724, 8060) | 4654 (2758, 8780) |
| Median BNP (Q1, Q3) (pg/mL) | 1076 (820, 1715) | 1170 (797, 1964) |
| Haemoglobin (g/dL) | 12.2 ± 1.6 | 12.15 ± 1.6 |
| Anaemia | ||
| Adult males (Hb <13 g/dL) | 171 (32) | 172 (32.9) |
| Adult females, non-pregnant (Hb <12 g/dL) | 108 (20.2) | 124 (23.7) |
| Ferritin (ng/mL) | 84.3 ± 63.0 | 87.65 ± 67.5 |
| Ferritin <100 ng/mL | 390 (73.0) | 362 (69.2) |
| Transferrin saturation (%) | 15.2 ± 8.4 | 14.3 ± 7.6 |
| Transferrin saturation <20% | 439 (82.7) | 444 (85.4) |
| eGFR (mL/min/1.73 m2) | 55.7 ± 21.3 | 56.0 ± 23.1 |
| Phosphorus | ||
| 2.5–4.4 mg/dL | 442 (87.0) | 408 (81.6) |
| ≥4.5 mg/dL | 46 (9.1) | 80 (16.0) |
Data are mean ± standard deviation or n (%) unless otherwise indicated. Percentages might not add to 100% because of rounding.
BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; KCCQ-12, 12-item Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
With a baseline KCCQ-12.
Left ventricular ejection fraction was measured within a maximum of 12 months before randomization.
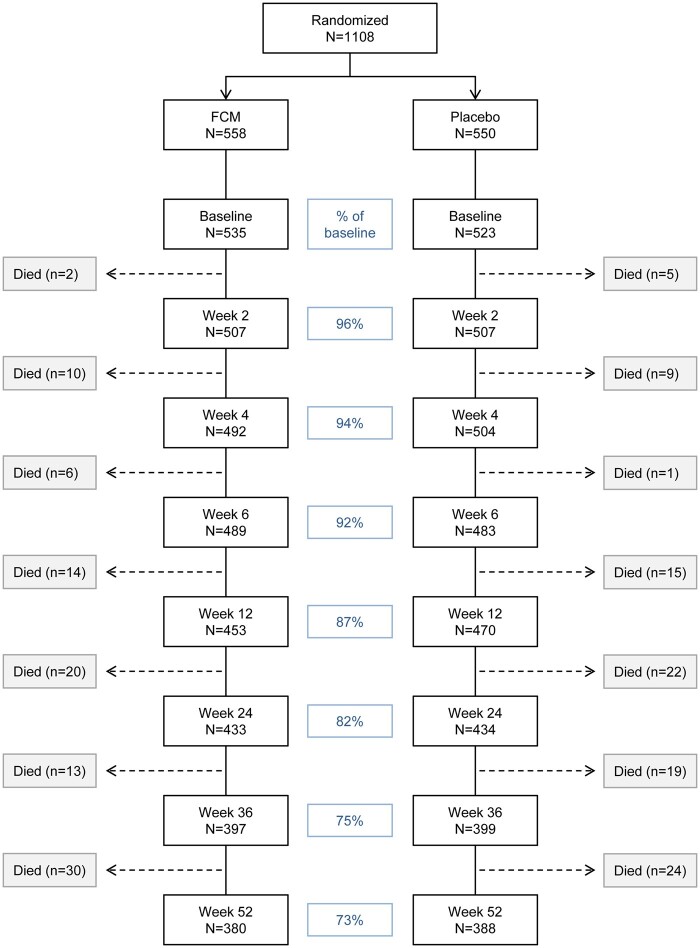
Overall, the KCCQ-12 completion rate decreased from 96% at Week 2 to 73% at Week 52 (Figure 1). The proportion of patients who did not complete the questionnaire during follow-up were similar in both the FCM and placebo groups.
Figure 1.
Proportion of patients with available HRQoL data through Week 52. Weeks shown are relative to randomization date. FCM, ferric carboxymaltose; HRQoL, health-related quality of life.
The KCCQ-12 OSS and CSS scores were similar and markedly impaired at baseline for both study groups. The mean (± standard error) KCCQ-12 OSS scores in the FCM and placebo groups were 38.1 ± 0.9 points and 37.1 ± 0.8 points, respectively. The mean KCCQ-12 CSS scores in the FCM and placebo groups were 40.9 ± 0.9 points and 40.1 ± 0.9 points, respectively.
During the course of the trial, 5 patients in the FCM arm (0.9%) and 13 patients in the placebo arm (2.4%) received open-label IV iron preparations beyond the study treatment (see Supplementary material online, Table S1 for details).
Changes in KCCQ scores
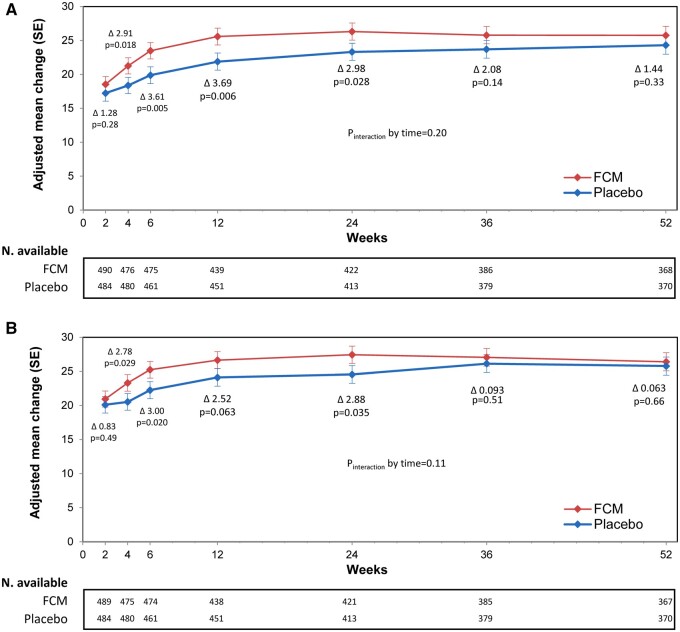
The mean adjusted changes from baseline in the KCCQ-12 OSS for both study groups are presented in Figure 2A. In both the FCM and placebo groups, the mean KCCQ-12 OSS score improved at 2 weeks post-discharge (by +18.5 ± 1.2 points and +17.2 ± 1.2 points in the FCM and placebo groups, respectively). The difference in OSS score change between FCM and placebo was not statistically significant (P = 0.277). As of Week 4 and up to Week 24 (i.e. end of the treatment period), the difference in OSS score was statistically significant in favour of FCM, with a mean change of +2.9 (95% CI 0.5–5.3, P = 0.018) and +3.0 (95% CI 0.3–5.6, P = 0.028) at Weeks 4 and 24, respectively. These results were also consistent across the KCCQ-12 CSS (Figure 2B). At Week 2 post-discharge, changes in CSS were similar between FCM and placebo (+20.94 ± 1.18 and +20.10 ± 1.21, respectively). The CSS mean change in favour of FCM at Week 4 and Week 24 was +2.8 (95% CI 0.3–5.3, P = 0.029) and +2.9 (95% CI 0.2–5.6, P = 0.035), respectively. At Week 52, the treatment effect was still present but in an attenuated manner [differences in adjusted mean changes for OSS and CSS at Week 52 were +1.44 (95% CI –1.45 to +4.33) and +0.63 (95% CI –2.21 to +3.47), respectively].
Figure 2.
KCCQ-12—overall summary score and clinical summary score mean change—full analysis set (adjusted model). Effects of ferric carboxymaltose, compared with placebo, on mean overall summary score (A) and clinical summary score (B). FCM, ferric carboxymaltose; KCCQ, Kansas City Cardiomyopathy Questionnaire; SE, standard error.
The COVID-19 sensitivity analyses (which excluded data on quality of life obtained after the outbreak of the COVID-19 pandemic) using the adjusted model confirmed that the pattern of changes in the KCCQ-12 OSS and CSS and differences between the two treatment groups were similar to the results obtained for the complete follow-up (Supplementary material online, Figure S1A and B).
The pattern of KCCQ-12 score changes and differences between the two treatment groups in analyses with unadjusted models were in agreement with those demonstrated with adjusted models, both for the overall study population (Supplementary material online, Figure S2) and for the COVID-19 sensitivity analysis population (Supplementary material online, Figure S1C and D).
The sensitivity analysis that incorporated imputed values to account for death of patients into the model (see Methods section for details) also showed a pattern of changes in the KCCQ-12 OSS and CSS and differences between study arms that were similar to that for the main results.
The effects of IV FCM, in comparison with placebo, on the KCCQ-12 OSS and CSS scores were assessed in 22 pre-specified subgroups (Supplementary material online, Figure S3).
Responder analyses
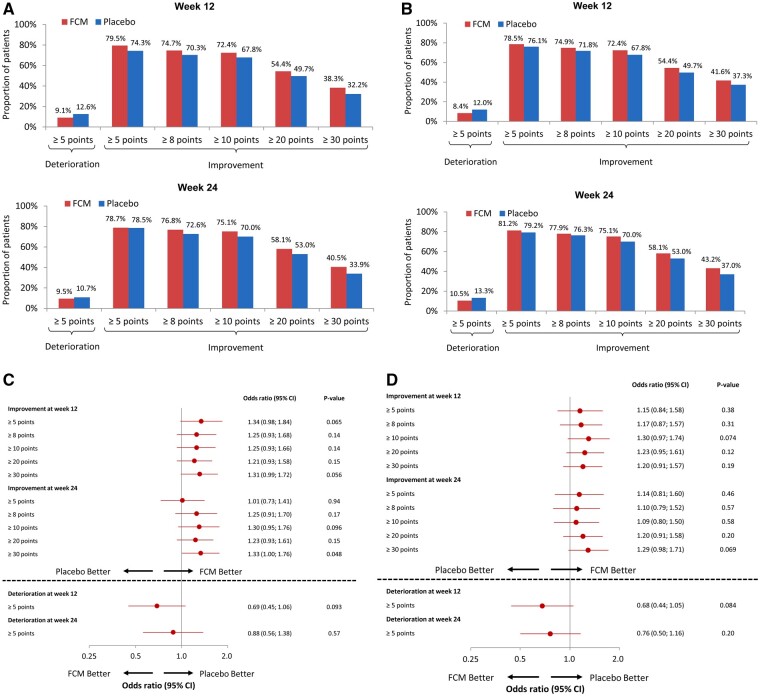
In the responder analyses, numerically fewer patients treated with FCM had a clinically meaningful deterioration (≥5-point decline in the KCCQ-12 OSS), and a greater proportion of patients had a clinically meaningful improvement in the KCCQ-12 OSS at Weeks 12 and 24, compared with the placebo group, although these results did not reach statistical significance (Figure 3A and C). An analogous pattern of responder analyses was seen for the KCCQ-12 CSS in the FCM group, compared with the placebo group (Figure 3B and D).
Figure 3.
Responder analyses of clinically meaningful changes in the KCCQ-12 OSS and CSS at 12 and 24 months, comparing FCM with placebo. Responder analyses of clinically meaningful changes in KCCQ-12 OSS (A and C) and KCCQ-12 CSS (B and D) at Weeks 12 and 24 after randomization. %, proportion of patients; CSS, clinical summary score; FCM, ferric carboxymaltose; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, overall summary score.
Discussion
In this pre-specified analysis of the AFFIRM-AHF trial, we observed that patients who had stabilized after an episode of acute HF and who had concomitant ID had severely impaired HRQoL at baseline, and after discharge experienced an improvement in health status during follow-up. Compared with placebo, patients treated with IV FCM had significantly greater improvements in health status starting at Week 4, and continuing up to Week 24, with a subsequent attenuation of treatment benefit by Week 52 (Graphical abstract).
Improving symptoms, function and quality of life is an important standalone target of therapy for patients with HF.19 Previous analyses have identified IV iron as being one of a very limited number of HF treatments that is able to confer improvements in HRQoL.5 Collectively, data on the impact of various treatments on health status have become an integral part of evaluating therapies and improving care for this high-risk patient population. Only a few interventions have demonstrated benefits in terms of health status in patients with chronic HF with reduced ejection fraction: these include dapagliflozin,21 empagliflozin,22 sacubitril/valsartan,23 exercise training,24 self-management interventions with or without remote monitoring,25 , 26 and IV FCM.13–15 Importantly, the modest effects of these therapies (a net effect of +1.5–3.0 points of the KCCQ, at maximum) have been demonstrated in three- to four-fold larger studies and under more stable clinical conditions, which cannot be extrapolated to patients recovering from acute HF. Beyond pharmacotherapies with demonstrated benefits on quality of life in patients with HF, the beneficial effects of cardiac resynchronization therapy (CRT) merit attention. Although the heterogeneity in clinical response to CRT (including improvement in clinical status and quality of life) is commonly acknowledged, selected patients (‘good responders’) benefit from a clinically meaningful improvement in quality of life, as demonstrated by an increase in KCCQ score exceeding 10 points.27 , 28
In other recently reported trials (EVEREST18 and SOLOIST-WHF29) patients with a recent episode of acute HF also demonstrated a markedly impaired HRQoL. These observations justify the particular need to consider this poor quality of life seen in patients directly after an episode of acute HF as an important therapeutic target.
The 23-item KCCQ is an instrument for measuring HRQoL in patients with HF and has excellent psychometric properties, but its major limitation for its broader use in clinical practice is its length, so it requires several minutes for patients to complete.18 , 19 Therefore, we used a shorter and simpler measure (the KCCQ-12 derived and validated from the 23-item KCCQ), which allows the capture of symptom frequency, physical and social limitations, and quality of life impairment as a result of HF, as well as an OSS. The KCCQ-12 has been demonstrated to have high correlations with the original 23-item tool and high test–retest reliability, as well as comparable prognostic significance and interpretation of clinically important differences, compared with the 23-item KCCQ.18 , 19
In the AFFIRM-AHF study, we demonstrated the favourable effects of IV FCM treatment on KCCQ-12 OSS and CSS scores, which were statistically significant and clinically relevant between Weeks 4 and 24. It has been established that a two to three-point mean improvement in the KCCQ score translates into a relevant increase in subjective patient wellbeing.19 , 20 In the AFFIRM-AHF trial, the beneficial effect of IV FCM treatment, compared to placebo, on the KCCQ-12 OSS and CSS scores was persistent up to Week 24, which was the end of the treatment phase. This suggests that treatment with FCM in these acutely ill patients positively impacts HRQoL, and the positive effect of FCM appears to correspond with the time points of IV FCM administration. Indeed, a diminishing proportion of patients with fully repleted iron status following treatment cessation may have contributed to the reduced quality of life benefit seen at Weeks 36 and 52. FCM was given at baseline and Week 6 in the vast majority of patients (i.e. 80% of patients in the FCM arm). In the FCM arm, only 20% required further administration of the drug at Weeks 12 and/or 24. In the placebo arm, approximately 50% of patients received the assigned therapy at either Week 12 and/or Week 24. The discontinuation of therapy (regardless of whether a placebo or an active drug was administered) could have had an impact on the subjective perception of quality of life by the patients.
We have demonstrated a significant increase in KCCQ-12 OSS and CSS scores as early as Week 2, which was evident in both FCM and placebo arms. The ‘spontaneous improvement’ in the placebo arm reached +17.2 ± 1.2 points and +20.1 ± 1.2 points for, respectively, the KCCQ-12 OSS and CSS, and was even more pronounced in the FCM group. Comparable patterns and magnitudes of changes in the KCCQ-12 OSS and CSS scores in patients having recently undergone hospitalization for acute HF have already been reported in the EVEREST trial (e.g. a change of +21.8 ± 21.3 points in KCCQ-12 OSS after 1 week in the placebo arm)18 and in the SOLOIST-WHF trial (an increase of 13.6 points in KCCQ-12 OSS at Month 4 in the placebo arm).29 Taking into consideration the important effect of ‘spontaneous’ improvement seen in the placebo group for the KCCQ-12 OSS and CSS scores, which is probably associated with the intensification of HF treatment during the index hospitalization, the traditional KCCQ-12 thresholds for responder analyses derived and validated for chronic settings are less meaningful and make the methodological approach much more challenging in the context of a recent episode of acute HF. Therefore, it is not surprising that the proportion of patients treated with FCM vs. placebo that experienced deterioration in health status was consistently numerically lower, and the proportion of patients with clinically meaningful improvements established for chronic settings was numerically greater, although none of these differences reached statistical significance. The reported responder analyses for the KCCQ-12 OSS and CSS scores using the cut-off values validated for stable clinical settings are therefore not meaningful in a post-acute HF patient population, where the overwhelming majority of patients experience substantial improvements regardless of treatment. Additionally, it is important to distinguish between the clinically relevant difference in average KCCQ scores when compared between study groups (analysed collectively) and the clinically relevant change in KCCQ score for individual subjects. It should be emphasized that a difference of ≥2‒3 points in average KCCQ scores compared between study groups has been shown as clinically relevant in several trials in patients with HF,21–24 in contrast to greater increases in KCCQ scores considered to be clinically relevant for individuals with HF in stable clinical settings.
Study limitations
It is difficult to compare the changes we observed in the KCCQ-12 OSS and CSS scores with other data. Available evidence on the patterns of change in the KCCQ is limited mainly to assessments performed in stable ambulatory patients with HF. At the time when the trial was planned, there was no detailed information on changes in HRQoL after hospitalization due to circulatory decompensation reported regularly during the 12-month follow-up. Recently, the KCCQ-12 data were reported in the SOLOIST-WHF trial, but the follow-up assessment was limited to a single time point.29 Taking into consideration the magnitude of dynamic changes seen in our trial after an episode of acute HF, being in contrast to patterns seen in stable cohorts, any conclusion about the clinical significance of reported changes in the KCCQ-12 in post-acute settings on the basis of standards developed in chronic settings needs to be considered with caution.
The primary analysis of AFFIRM-AHF showed a beneficial effect of IV FCM vs. placebo in the reduction in recurrent HF hospitalization in patients who were stabilized after an episode of acute HF. Our analysis provides evidence that these patients also benefit from an improvement in HRQoL. There is undoubtedly a relationship between HF hospitalizations and a subjective perception of quality of life in patients with HF, and the distinction between these two effects of any applied therapy is difficult. One may argue that the reported benefits in quality of life are just a reflection of fewer hospitalizations for HF. However, it needs to be emphasized that in the AFFIRM-AHF trial, all follow-up KCCQ-12 assessments were performed during ambulatory visits—hence the data for patients who were hospitalized at that time of scheduled assessments were missing and, as per the protocol, no data imputation was applied. Therefore, we could conclude that the changes in quality of life reported in this paper are independent of any direct influence of recent HF hospitalizations that patients could experience. There is no doubt that if the KCCQ assessments had been performed at the time of hospitalization and had been imputed in the model, the gradients in quality of life benefits would have been much more prominent.
In iron-deficient patients with HF and an LVEF <50% who had stabilized after an episode of acute HF, treatment with IV FCM, compared with placebo, results in clinically meaningful beneficial effects on quality of life as early as 4 weeks after treatment initiation that last up to Week 24.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data that support the findings of this study are available from the corresponding author, E.A.J., upon reasonable request.
Supplementary Material
Acknowledgements
We are grateful to the patients, their families, and the investigators for their participation in this study; Dr Teba Haboubi, Dr Emanuele Noseda, and the respective study teams for study monitoring and management; and Dr Bridget-Anne Kirwan, Ms Caroline Gombault, Mr Robin Wegmüller (SOCAR Research), and Ms Helen Sims (AXON Communications) who provided editorial assistance with the preparation of the tables and figures, funded by Vifor Pharma.
Funding
The AFFIRM-AHF trial was funded by Vifor Pharma.
Conflict of interest: E.A.J. has received research grants and personal fees from Vifor Pharma (co-PI of the AFFIRM trial); personal fees from Bayer, Novartis, Abbott, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Berlin Chemie, Cardiac Dimensions, Fresenius, and Gedeon Richter. M.K. reports grants from Astra Zeneca, Boehringer Ingelheim; personal fees from Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi and Vifor Pharma. J.B. has received personal fees from Vifor Pharma, Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and V-Wave Limited (consultant). S.D.A. has received research grants and personal fees from Vifor Int and Abbott Vascular (IIT/Trial steering committee work), personal fees from Bayer, Boehringer Ingelheim and Impulse Dynamics (Trial steering committee work), Novartis, Cardiac Dimensions and Occlutech (Adivsory committee work), Servier (Registry Steering Committee). T.McD. has received personal fees from Vifor Pharma (Honoraria for Presentations at Symposia). J.D. has received research grants from Vifor Pharma. G.F. has received personal fees from Servier (Lecture and Registry Commitee member), personal fees from Novartis (Lecture fees and Trial/Registry Committee member), personal fees from Boehringer Ingelheim (Lecture and Trial Committee member). A.K. has received personal fees from Vifor Pharma (consultancy, lecture, lead investigator, participation in scientific meetings), lecture and consulting fees from Novartis, Bayer and CTS companies. F.A.M. has received personal fees from Vifor Pharma (Steering Committee Member), personal fees from AstraZeneca and Novartis (Executive Committee Member). M.Me. has received personal fees from Vifor Pharma (Executive Committee member), personal fees from Amgen (Executive Committee member and National PI), personal fees from Astra-Zeneca, Abbott vascular, Bayer (participation in Advisory Boards), personal fees from Servier (participation in Advisory Boards and speeches at sponsored symposia), Edwards Therapeutics (speeches at sponsored symposia), Actelion (DMC Member), LivaNova (Executive Committee member), Windtree therapeutics (Executive Committee member and Advisory Board). D.M. has received personal fees from Vifor Pharma (investigator and Steering Committee member). J.C.N. has received research grants and personal fees from Vifor Pharma (NLI/SC member for AFFIRM), research grants from AstraZeneca (NLI), Bayer (PI Compass), Esperion and CLS Behring (NLI/SC member), Dalcor (NLI), Janssen (NLI/SC member), Novartis (PI, consultant), Novo Nordisk (PI), Sanofi (NLI, PI, Advisory Board); personal fees from AMGEN (consultant), Bayer, Daiichi-Sankyo (speaker), Novartis, Sanofi, Servier (speaker, Advisory Board). M.O. has received personal fees from Vifor Pharma (speaker). A.P. has received research grants from Vifor Pharma (NLI for AFFIRM-AHF), Amgen (NLI), research grants and personal fees from Bayer and AstraZeneca (honoraria, lectures). D.A.P-F. has received research grants from Roche Diagnostics, AstraZeneca and Pfizer; personal fees from Vifor Pharma (Advisory Board), Novartis, Servier, AstraZeneca, Pfizer (Advisory Board, speaker), and Abbot (speaker). F.R. reports personal fees from Vifor for his role in the Clinical Event Adjudication Committee for Vifor in 2016, during the conduct of the study; F.R. has not received personal payments by pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent in activities, such as participation in and steering committee member of clinical trials, were made directly to the University of Zurich); the Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research-, educational-, and/or travel grants from Abbott, Amgen, Astra Zeneca, Bayer, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, V-Wave, Vascular Medical, Vifor, Wissens Plus, and ZOLL; the research and educational grants do not impact F.R.’s personal remuneration. P.v.d.M. has received research grants and personal fees from Vifor Pharma (Executive Committee, speaker); research grants from AstraZeneca, Ionis, Pfizer, and Corvidia; and personal fees from Novartis and Servier (Advisory Board). B.L. has received research grants and personal fees from MSD (honoraria); and personal fees from Vifor Pharma (services rendered). J.C.C. has received research grants and personal fees from Vifor Pharma (Adjudication Committee, Advisory Board and conferences). S.v.H. has received personal fees from Vifor Pharma, Bayer, Boehringer Ingelheim, BRAHMS/ThermoFisher, Grünenthal, Helsinn, Hexal, Novartis, Pharmacosmos, RespiCardia, Roche, and Servier. A.C.S. has received research grants and personal fees from Menarini (Boards); and personal fees from Vifor, AstraZeneca, Merck and Bayer (boards, studies and meetings), We Health, Leo, Boehringer Ingelheim, Sanofi, Abbott (boards). N.D. has received research grants and personal fees from Amgen, AstraZeneca, Bayer, BMS, and Sanofi; and personal fees from Vifor Pharma, Boehringer Ingelheim, Eli-Lilly, MSD, Novo-Nordisk, Intercept, Pfizer, Servier, and UCB. W.D. has received research grants and personal fees from Vifor Pharma; research grants from ZS Pharma; and personal fees from Pfizer, Boehringer Ingelheim, Sphingotec, Bayer, and Medtronic. T.F. has received personal fees from Vifor Pharma (statistical consultancies), Novartis, Bayer, Janssen, Roche, Boehringer Ingelheim, Daiichi-Sankyo, Galapagos, Penumbra, Parexel, BiosenseWebster, CSL Behring, Fresenius Kabi, Coherex Medical, and LivaNova (consultancies). V.F. and F.D. have received personal fees from Vifor Pharma (Vifor Pharma employee). S.P. has received personal fees from Vifor Pharma (consultancy). P.P. has received research grants and personal fees from Vifor Pharma (PI of AFFIRM-AHF; participation in clinical trials); personal fees from Amgen, Bayer, Novartis, Abbott Vascular, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Berlin Chemie, Cibiem, BMS, Impulse Dynamics (participation in clinical trials). All other authors declare no competing interests (B.A.K., M.D., I.K., H.K., D.S., H.S., H.D., and M.Mo.).
Contributor Information
Ewa A Jankowska, Department of Heart Diseases, Wrocław Medical University, Borowska 213, 50-556 Wroclaw, Poland; Center for Heart Diseases, University Hospital in Wrocław, Borowska 213, 50-556 Wroclaw, Poland.
Bridget-Anne Kirwan, Department of Clinical Research, SOCAR Research SA, Chemin de Chantemerle 18, 1260 Nyon, Switzerland; London School of Hygiene and Tropical Medicine, University College London, Keppel St, Bloomsbury, London WC1E 7HT, UK.
Mikhail Kosiborod, Saint Luke’s Mid America Heart Institute and University of Missouri-Kansas City, 4401 Wornhall Rd, Kansas City, MO 64111, USA.
Javed Butler, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216, USA.
Stefan D Anker, Charité, Campus Virchow-Klinikum, Augustenburger Platz 1, 13353 Berlin, Germany.
Theresa McDonagh, King’s College Hospital, Denmark Hill, Brixton, London SE5 9RS, UK; King’s College London, Strand, London WC2R 2LS, UK.
Maria Dorobantu, Cardiology Department, Emergency Hospital of Bucharest, Calea Floreasca 8, Bucharest 014461, Romania.
Jarosław Drozdz, Department Cardiology, Medical University of Lodz, al. Tadeusza Kościuszki 4, 90-149 Lodz, Poland.
Gerasimos Filippatos, Department of Cardiology, Heart Failure Unit, National and Kapodistrian University of Athens, School of Medicine, Athens University Hospital Attiko, Athens 157 72, Greece.
Andre Keren, Assuta Hashalom, Assuta Hospitals, HaBarzel St 20, Tel Aviv-Yafo, Israel.
Irakli Khintibidze, Aleksandre Aladashvili Clinic, LLC, 103 Uznadze St., Tbilisi, Georgia.
Hans Kragten, Maastricht University Medical Center, P. Debyelaan 25, 6229 Maastricht, Netherlands.
Felipe A Martinez, Universidad Nacional de Córdoba, International Society of Cardiovascular Pharmacotherapy, Av. Haya de la Torre s/n, Argentina.
Marco Metra, Department of Cardiology, University and Civil Hospital, Piazzale Spedali Civilli, 1, 25123 Brescia, Italy.
Davor Milicic, University Hospital Center Zagreb, Kišpatićeva ul. 12, 10000 Zagreb, Croatia.
José C Nicolau, Instituto do Coracao (InCor), Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Av. Dr. Enéas Carvalho de Aguiar, 44 - Cerqueira César, Sao Paulo-SP, 05403-900, Brazil.
Marcus Ohlsson, Department of Internal Medicine, Skane University Hospital Malmo, Carl-Bertil Laurells gata 9, 214 28 Malmo, Sweden.
Alexander Parkhomenko, The M.D. Strazhesko Institute of Cardiology, Narodnoho Opolchennya St, 5, Kyiv 03680, Ukraine.
Domingo A Pascual-Figal, Cardiology Department, Hospital Virgen de la Arrixaca, University of Murcia, Ctra. Madrid-Cartagena, s/n, 30120 El Palmar, Murcia, Spain.
Frank Ruschitzka, UniversitätsSpietal Zürich, Klinik für Kardiologie, Rämistrasse 100, 8006 Zürich, Switzerland.
David Sim, National Heart Center, Clinical Translational and Research Office, 5 Hospital Dr, Singapore 169609.
Hadi Skouri, American University of Beirut, Medical Center Beirut, Maamari Street - Hamra, 1107 2020 Beirut, Lebanon.
Peter van der Meer, Department of Cardiology, University Medical Center Groningen, Hanzeplein 1, 9713 Groningen, The Netherlands.
Basil S Lewis, Lady Davies Carmel Medical Center, Clinical Cardiovascular Research Institute, 21 Ehud Street, Haifa, Haifa District, Israel.
Josep Comin-Colet, Department of Cardiology, University Hospital Bellvitge and IDIBELL, University of Barcelona, Gran Via de l’Hospitalet, 199 08908, Hospitalet de Llobregat, Barcelona, Spain.
Stephan von Haehling, Department of Cardiology and Pneumology, University Medical Center Göttingen, Robert-Koch-Straße 40, 37075 Göttingen, Germany; German Center for Cardiovascular Research (DZHK), partner site Göttingen, 37099 Göttingen, Germany.
Alain Cohen-Solal, Hospital Lariboisière, INSERM, 2 Rue Ambroise Paré, 75010 Paris, France.
Nicolas Danchin, European Hospital Georges Pompidou, 20 Rue Leblanc, 75015 Paris, France.
Wolfram Doehner, BCRT—Berlin Institute of Health Center for Regenerative Therapies, Föhrer Str. 15, 13353; Department of Cardiology (Virchow Campus), Charité- Universitätsmedizin Berlin, Augustenburger Pl. 1, 13353; and German Centre for Cardiovascular Research (DZHK), Partner Site Berlin, Potsdamer Straße 58, 10785 Berlin, Germany.
Henry J Dargie, Robertson Center for Biostatistics, University of Glasgow, Boyd Orr Building University Avenue, Glasgow G12 8QQ, UK.
Michael Motro, Sheba Medical Center, Tel-Aviv University, Sackler School of Medicine, 6997801 Tel Aviv, Israel.
Tim Friede, German Center for Cardiovascular Research (DZHK), partner site Göttingen, 37099 Göttingen, Germany; Department of Medical Statistics, University Medical Center Göttingen, Robert-Koch-Straße 40, 37075 Göttingen, Germany.
Vincent Fabien, Vifor Pharma Ltd, Flughofstrasse 61, P.O. Box 8152, Glattbrugg, Switzerland.
Fabio Dorigotti, Vifor Pharma Ltd, Flughofstrasse 61, P.O. Box 8152, Glattbrugg, Switzerland.
Stuart Pocock, London School of Hygiene and Tropical Medicine, University College London, Keppel St, Bloomsbury, London WC1E 7HT, UK.
Piotr Ponikowski, Department of Heart Diseases, Wrocław Medical University, Borowska 213, 50-556 Wroclaw, Poland; Center for Heart Diseases, University Hospital in Wrocław, Borowska 213, 50-556 Wroclaw, Poland.
References
- 1. Maggioni AP, Anker SD, Dahlström U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L; Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 2. Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J 2008;155:200–207. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Korves C, Eldar-Lissai A, McHale J, Lafeuille MH, Hwa Ong S, Sheng Duh M. Resource utilization and costs following hospitalization of patients with chronic heart failure in the US. J Med Econ 2012;15:925–937. [DOI] [PubMed] [Google Scholar]
- 5. von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, Herrmann-Lingen C, Garfias Macedo T, Koziolek M, Noutsias M, Schulze PC, Wachter R, Hasenfuß G, Laufs U. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail 2021;23:92–113. [DOI] [PubMed] [Google Scholar]
- 6. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 7. Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016–1024. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. EMA Regulatory Science to 2025: Strategic Reflection. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ema-regulatory-science-2025-strategic-reflection_en.pdf (22 January 2021).
- 9.Food and Drug Administration. Patient-Focused Drug Development Glossary. https://www.fda.gov/drugs/development-approval-process-drugs/patient-focused-drug-development-glossary (22 January 2021).
- 10. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, Polonski L, Filippatos G, Anker SD, Ponikowski P. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 11. Alcaide-Aldeano A, Garay A, Alcoberro L, Jiménez-Marrero S, Yun S, Tajes M, García-Romero E, Díez-López C, González-Costello J, Mateus-Porta G, Cainzos-Achirica M, Enjuanes C, Comín-Colet J, Moliner P. Iron deficiency: impact on functional capacity and quality of life in heart failure with preserved ejection fraction. J Clin Med 2020;9:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comín-Colet J. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014;174:268–275. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 15. Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J 2013;34:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ponikowski P, Kirwan B-A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual-Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin-Colet J, von Haehling S, Cohen-Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA; AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396:1895–1904. [DOI] [PubMed] [Google Scholar]
- 17. Ponikowski P, Kirwan B‐A, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, Kragten H, Martinez FA, McDonagh T, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, Meer P, Jankowska EA. Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur J Heart Fail 2019;21:1651–1658. [DOI] [PubMed] [Google Scholar]
- 18. Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:2379–2390. [DOI] [PubMed] [Google Scholar]
- 20. Butler J, Khan MS, Mori C, Filippatos GS, Ponikowski P, Comin-Colet J, Roubert B, Spertus JA, Anker SD. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2020;22:999–1005. [DOI] [PubMed] [Google Scholar]
- 21. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF Trial. Circulation 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Piña IL, Lam CSP, Ponikowski P, Sattar N, Verma S, Brueckmann M, Jamal W, Vedin O, Peil B, Zeller C, Zannad F, Packer M; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J 2021;42:1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail 2017;10:e003430. [DOI] [PubMed] [Google Scholar]
- 24. Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP, Action Investigators HF. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Q, Chen C, Zhang J, Ye Y, Fan X. Effects of self-management interventions on heart failure: systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud 2020;110:103689. [DOI] [PubMed] [Google Scholar]
- 26. Krishnaswami A, Beavers C, Dorsch MP, Dodson JA, Masterson Creber R, Kitsiou S, Goyal P, Maurer MS, Wenger NK, Croy DS, Alexander KP, Batsis JA, Turakhia MP, Forman DE, Bernacki GM, Kirkpatrick JN, Orr NM, Peterson ED, Rich MW, Freeman AM, Bhavnani SP; Innovations, Cardiovascular Team and the Geriatric Cardiology Councils, American College of Cardiology. Gerotechnology for older adults with cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol 2020;76:2650–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Veazie PJ, Noyes K, Li Q, Hall WJ, Buttaccio A, Thevenet-Morrison K, Moss AJ. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol 2012;60:1940–1944. [DOI] [PubMed] [Google Scholar]
- 28. Chan PS, Khumri T, Chung ES, Ghio S, Reid KJ, Gerritse B, Nallamothu BK, Spertus JA. Echocardiographic dyssynchrony and health status outcomes from cardiac resynchronization therapy: insights from the PROSPECT trial. JACC Cardiovasc Imaging 2010;3:451–460. [DOI] [PubMed] [Google Scholar]
- 29. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, E.A.J., upon reasonable request.