Abstract
Aims
Rosuvastatin (10 mg per day) compared with placebo reduced major adverse cardiovascular (CV) events by 24% in 12 705 participants at intermediate CV risk after 5.6 years. There was no benefit of blood pressure (BP) lowering treatment in the overall group, but a reduction in events in the third of participants with elevated systolic BP. After cessation of all the trial medications, we examined whether the benefits observed during the active treatment phase were sustained, enhanced, or attenuated.
Methods and results
After the randomized treatment period (5.6 years), participants were invited to participate in 3.1 further years of observation (total 8.7 years). The first co-primary outcome for the entire length of follow-up was the composite of myocardial infarction, stroke, or CV death [major adverse cardiovascular event (MACE)-1], and the second was MACE-1 plus resuscitated cardiac arrest, heart failure, or coronary revascularization (MACE-2). In total, 9326 (78%) of 11 994 surviving Heart Outcomes Prevention Evaluation (HOPE)-3 subjects consented to participate in extended follow-up. During 3.1 years of post-trial observation (total follow-up of 8.7 years), participants originally randomized to rosuvastatin compared with placebo had a 20% additional reduction in MACE-1 [95% confidence interval (CI), 0.64–0.99] and a 17% additional reduction in MACE-2 (95% CI 0.68–1.01). Therefore, over the 8.7 years of follow-up, there was a 21% reduction in MACE-1 (95% CI 0.69–0.90, P = 0.005) and 21% reduction in MACE-2 (95% CI 0.69–0.89, P = 0.002). There was no benefit of BP lowering in the overall study either during the active or post-trial observation period, however, a 24% reduction in MACE-1 was observed over 8.7 years.
Conclusion
The CV benefits of rosuvastatin, and BP lowering in those with elevated systolic BP, compared with placebo continue to accrue for at least 3 years after cessation of randomized treatment in individuals without cardiovascular disease indicating a legacy effect.
Trial Registration Number
Keywords: Primary prevention, Cardiovascular disease, Statins
Graphical Abstract
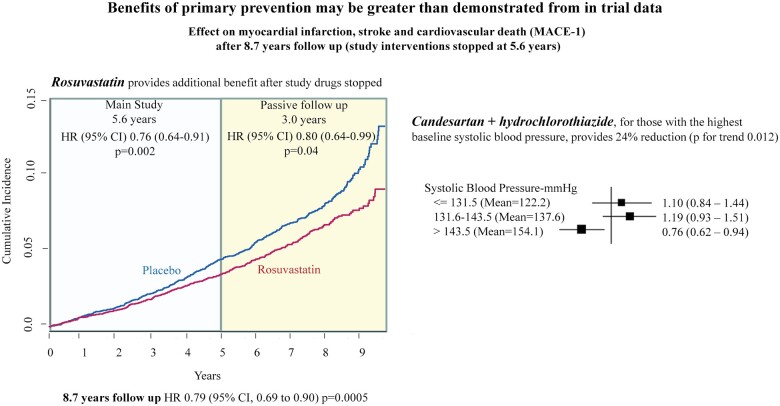
Benefits of primary prevention may be greater than demonstrated from in trial data.
See page 3024 for the editorial comment on this article (doi:10.1093/eurheartj/ehab208)
Introduction
The majority of cardiovascular (CV) events occur in people with no clinical evidence of CV disease (CVD).1 The Heart Outcomes Prevention Evaluation (HOPE)-3 study was designed to determine if a reduction in low-density lipoprotein cholesterol (LDL-C) or blood pressure (BP), either alone or in combination, would reduce CV events in those at intermediate risk with no prior overt clinical CV events.2 After 5.6 years of treatment, rosuvastatin 10 mg daily compared with placebo reduced major adverse CV events (MACE, including myocardial infarction, stroke, or death from CV causes) by 24%.3 Despite reducing systolic BP by 6 mmHg, the combination of candesartan (16 mg daily) and hydrochlorothiazide (12.5 mg daily) compared with placebo did not reduce MACE significantly in the overall trial population. However, a significant reduction in CVD was reported in participants in the upper third of baseline BP [systolic BP >143 mmHg (mean 154 mmHg)].4 Importantly, HOPE-3 included a substantial proportion of non-Caucasians among whom prior data on preventive therapies are limited.
It is possible that the results observed during the period of active treatment may not capture the longer-term effects of treatments.5 Both statins and BP lowering may cause structural changes in the vasculature, such as alterations of plaque morphology and composition that may lead to continued or enhanced benefits during further observation.6–8 We hypothesized that even after cessation of active study treatment, the benefits of statins, and of BP lowering in those with elevated BP, would be preserved or enhanced for some years, and that late benefits from BP lowering might also emerge.
Documenting the long-term effects of preventive strategies is both of public health and clinical importance for patients, prescribers, funders, and policymakers when making decisions about use of medications. Whilst it is theoretically desirable to conduct randomized trials of prevention strategies over prolonged periods to determine the ‘full’ effects of treatments, this might take 10–15 years of intervention, and such prolonged trials are impractical. Instead, documenting events after the cessation of active treatments may provide some useful indicators of whether late benefits from treatments exist. In the HOPE-3 trial, we reported a significant reduction in CVD with rosuvastatin during the active phase of 5.6 years, whereas the benefits of BP lowering were confined to those with elevated BP at baseline. Subsequently, in this passive extended follow-up of HOPE-3 participants, where participants previously receiving study drug were no longer doing so, we aimed to determine whether (i) the benefits observed with rosuvastatin during the active treatment period were sustained, enhanced, or attenuated and (ii) delayed benefits would emerge in the group randomized to BP lowering compared with placebo.
Methods
The methods of the main trial have previously been reported in detail.2 Briefly, men aged ≥55 years and women aged ≥65 years were enrolled if they had one CV risk factor; women who were between 60 and 65 years were eligible if they had two risk factors. Risk factors included elevated waist-to-hip ratio (≥0.85 in women, ≥0.90 in men), current smoking, impaired fasting glucose, impaired glucose tolerance, or diabetes requiring only diet control, estimated glomerular filtration rate between 45 and 60 mL/min/1.73 m2, or a family history of premature heart disease in first-degree relatives (<65 years in women or <55 years in men). Participants were excluded if in the opinion of the treating physician, they needed or had contraindications to study medications or had previous CVD.
Eligible participants who provided consent entered a single-blind run-in phase where they received rosuvastatin 10 mg daily and candesartan 16 mg plus hydrochlorothiazide 12.5 mg as a combination pill. If adherent to study drugs, participants were centrally randomized to the active phase to receive rosuvastatin 10 mg daily or placebo and candesartan 16 mg/hydrochlorothiazide 12.5 mg daily or placebo. They were seen 6 weeks later and then every 6 months. Data were collected on adherence, adverse events and outcomes, and participants had their BP and heart rate measured annually. Counselling on diet and other lifestyle behaviours were provided at every visit. At the end of the active phase, all blinded study medications were stopped, and participants were asked to follow-up with their usual care physician who were free to determine appropriate treatment for each individual. Those who were alive at the end of the active intervention phase, and consented to long-term follow-up, were included irrespective of their lipid or BP levels or whether or not they had a MACE during the active phase of the trial. Participants were contacted annually and data on CV events, mortality, and medications were recorded using the same processes and definitions as those used in the active phase (Supplementary material online, Figure S1).
The HOPE-3 study was conducted in 228 centres in 21 countries. Each participant provided written informed consent for the active phase of the study and, if alive at the end of the active phase, they were asked to provide consent for passive follow-up. Ethics and regulatory approvals were obtained to conduct the study at each site and in each country. During the passive follow-up, use of lipid- and BP-lowering medications was at the discretion of each participant’s physician(s) responsible for his/her clinical care.
There were two co-primary study outcomes in both the active and passive follow-up phases: myocardial infarction, stroke, or death due to CV causes (MACE-1), and MACE-1 plus resuscitated cardiac arrest, heart failure, or coronary revascularization (MACE-2). Secondary outcomes for the BP-lowering arm were MACE-2 plus angina with objective evidence of ischaemia, and stroke. Because rosuvastatin had the largest effects on coronary ischaemic events during the active treatment phase of the study, as a post hoc analysis for the passive follow-up, we explored the effects on these events (fatal or non-fatal myocardial infarction, resuscitated cardiac arrest, angina, and revascularization). Pre-specified subgroups were defined by sex, age, LDL-C, baseline BP, and ethnicity. Participants were asked about the occurrence of study outcomes at each visit during the active and passive follow-up phases. We also recorded use of key medications during follow-up (statins and BP-lowering agents). If patients reported that outcomes occurred, investigators were requested to provide supporting documentation but events were not adjudicated during the passive follow-up.
Data were censored at the time of death or last available follow-up and were analysed according to the original allocations for each treatment (intention to treat). Descriptive statistics were used to present baseline characteristics [means and standard deviations, medians and interquartile ranges (IQR)]. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated using Cox proportional hazards models stratified according to the other treatment. Outcomes from randomization to the end of the passive follow-up period were included after censoring participants who did not experience the event of interest at their last contact date or if a non-CV death had occurred. Proportional hazards for first events were calculated separately for the active and passive phase (i.e. events during the passive phase were considered first events regardless of whether a previous event occurred during the active phase). The HR for total follow-up included only first events over the entire follow-up period. Hazard ratios for recurrent events were analysed using a proportional means model.9 Incidence of outcomes over time was calculated using Kaplan–Meier analysis. To determine whether there were any biases introduced by selective participation in the passive follow-up phase, a sensitivity analysis was performed using data from centres where at least 80% of the eligible participants continued in the passive follow-up phase. A cumulative incidence function analysis10 was also done to determine the impact of non-CV deaths as competing risks on the MACE-1 outcome.
The Population Health Research Institute, McMaster University, co-ordinated all aspects of study conduct. The funders had no role in the design, conduct, analysis, or reporting of the results.
Results
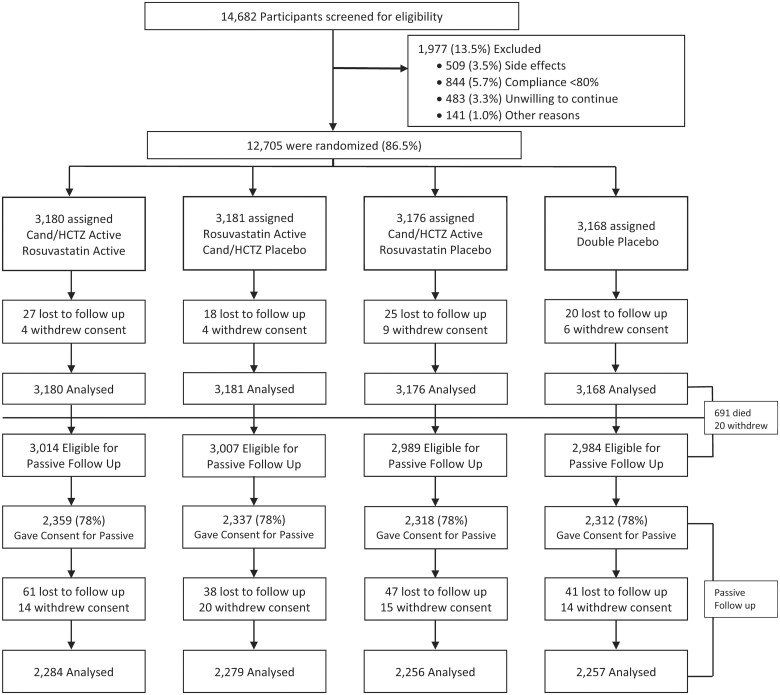
Recruitment into HOPE-3 began in May 2007 and was completed in November 2010. Participants continued on active treatment until December 2015 (median 5.6 years of follow-up) at which point passive follow-up began and continued until December 2018 for a combined median length of follow-up of 8.7 years (IQR 8.1–9.3 years). Of the original 228 centres, 166 (with a total of 9326 participants) agreed to participate in the passive follow-up. Of these 166 centres, 149 centres recruited at least 80% of eligible participants for the passive follow-up phase. Of 12 705 participants randomized, 11 994 were eligible for passive follow-up, 9326 (78%) consented to participate, and 97% were followed for 3 years (Figure 1).
Figure 1.
Participant disposition in HOPE-3. Cand, candesartan; HCTZ, hydrochlorothiazide.
Baseline characteristics were similar between those who agreed to continue in passive follow-up and the overall population (Supplementary material online, Table S1). There were no additional BP or lipid measurements after the end of active follow-up, as visits were completed by telephone. One year after completing the active phase of the study, 37% of participants were prescribed a statin (36% of those randomized to rosuvastatin and 38% of those randomized to placebo), and 25% were taking two or more BP-lowering drugs, which was similar in the randomized groups. During the extended follow-up period, statin use stayed the same over 3 years, while use of two or more BP lowering drugs increased to 30% by the 3rd year of follow-up (with no differences between randomized groups).
Passive follow-up over 3.1 years: rosuvastatin vs. placebo
During the passive follow-up phase, patients originally randomized to rosuvastatin compared with placebo had a further 20% reduction in risk of MACE-1 [146 events (3.1%) in the rosuvastatin group vs. 181 events (3.9%) in the placebo group; HR 0.80, 95% CI 0.64–0.99], with a similar trend towards benefit for MACE-2 [173 (3.7%) in the rosuvastatin group vs. 207 (4.5%) in the placebo group; HR 0.83, 95% CI 0.68–1.01]. There was a 19% reduction in MACE-2 plus angina [177 events (3.8%) in those originally randomized to rosuvastatin vs. 215 events (4.6%) in those originally randomized to placebo; HR 0.81, 95% CI 0.67–0.99] and a 46% reduction in coronary ischaemic events [28 events (0.6%) in those on rosuvastatin vs. 51 events (1.1%) in those on placebo; HR 0.54, 95% CI 0.34–0.86] (Table 1).
Table 1.
Participants with outcomes during active, passive, and entire follow-up
| Active intervention period (5.6 years) |
Passive follow-up (additional 3.1 years) |
Total follow-up (8.7 years) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosuvastatin vs. placebo |
||||||||||||||
| Rosuva (n = 6361) | Plac (n = 6344) | HR (95% CI) | P-value | Rosuva (n = 4696) | Plac (n = 4630) | HR (95% CI) | P-value | Rosuva (n = 6361) | Rate per 100 py | Plac (n = 6344) | Rate per 100 py | HR (95% CI) | P-value | |
| MACE-1 | 235 (3.7%) | 304 (4.8%) | 0.76 (0.64–0.91) | 0.002 | 146 (3.1%) | 181 (3.9%) | 0.80 (0.64–0.99) | 0.042 | 378 (5.9%) | 0.77 | 472 (7.4%) | 0.98 | 0.79 (0.69–0.90) | 0.0005 |
| MACE-2 | 277 (4.4%) | 363 (5.7%) | 0.75 (0.64–0.88) | 0.0004 | 173 (3.7%) | 207 (4.5%) | 0.83 (0.68–1.01) | 0.065 | 440 (6.9%) | 0.90 | 550 (8.7%) | 1.15 | 0.79 (0.69–0.89) | 0.0002 |
| MACE-2 + angina | 306 (4.8%) | 393 (6.2%) | 0.77 (0.66–0.89) | 0.0006 | 177 (3.8%) | 215 (4.6%) | 0.81 (0.67–0.99) | 0.043 | 471 (7.4%) | 0.97 | 584 (9.2%) | 1.22 | 0.79 (0.70–0.89) | 0.0002 |
| Coronary ischaemic eventsa ,b | 113 (1.8%) | 148 (2.3%) | 0.76 (0.59–0.97) | 0.025 | 28 (0.6%) | 51 (1.1%) | 0.54 (0.34–0.86) | 0.01 | 139 (2.2%) | 0.28 | 191 (3.0%) | 0.40 | 0.72 (0.58–0.90) | 0.003 |
| CV death | 154 (2.4%) | 171 (2.7%) | 0.89 (0.72–1.11) | 0.306 | 115 (2.5%) | 128 (2.8%) | 0.89 (0.69–1.15) | 0.377 | 269 (4.7%) | 0.54 | 299 (4.7%) | 0.61 | 0.89 (0.76–1.05) | 0.171 |
|
BP-lowering therapyc vs. placebo
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP (n = 6356) | Plac (n = 6349) | HR (95% CI) | P-value | BP (n = 4677) | Plac (n = 4649) | HR (95% CI) | P-value | BP (n = 6356) | Rate per 100 py | Plac (n = 6349) | Rate per 100 py | HR (95% CI) | P-value | |
| MACE-1 | 260 (4.1%) | 279 (4.4%) | 0.93 (0.79–1.10) | 0.40 | 169 (3.6%) | 158 (3.4%) | 1.07 (0.86–1.33) | 0.52 | 419 (6.6%) | 0.86 | 431 (6.8%) | 0.89 | 0.97 (0.85–1.11) | 0.67 |
| MACE-2 | 312 (4.9%) | 328 (5.2%) | 0.95 (0.81–1.11) | 0.51 | 196 (4.2%) | 184 (4.0%) | 1.07 (0.88–1.31) | 0.51 | 492 (7.7%) | 1.02 | 498 (7.8%) | 1.03 | 0.99 (0.87–1.12) | 0.84 |
| MACE-2 + angina | 335 (5.3%) | 364 (5.7%) | 0.92 (0.79–1.06) | 0.26 | 203 (4.3%) | 189 (4.1%) | 1.08 (0.89–1.32) | 0.45 | 522 (8.2%) | 1.08 | 533 (8.4%) | 1.11 | 0.98 (0.87–1.10) | 0.72 |
| Stroke | 75 (1.2%) | 94 (1.5%) | 0.80 (0.59–1.08) | 0.14 | 44 (0.9%) | 34 (0.7%) | 1.30 (0.83–2.03) | 0.26 | 116 (1.8%) | 0.24 | 128 (2.0%) | 0.91 | 0.91 (0.70–1.16) | 0.44 |
| Coronary ischaemic eventsa , b | 120 (1.9%) | 141 (2.2%) | 0.85 (0.67–1.08) | 0.19 | 36 (0.8%) | 43 (0.9%) | 0.84 (0.54–1.31) | 0.43 | 154 (2.4%) | 0.32 | 176 (2.8%) | 0.36 | 0.87 (0.70–1.09) | 0.23 |
|
Combination therapyc vs. double placebo
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combo (n = 3180) | Dbl Plac (n = 3168) | HR (95% CI) | P-value | Combo (n = 2539) | Dbl Plac (n = 2312) | HR (95% CI) | P-value | Combo (n = 3180) | Rate per 100 py | Dbl Plac (n = 3168) | Rate per 100 py | HR (95% CI) | P-value | |
| MACE-1 | 113 (3.6%) | 157 (5.0%) | 0.71 (0.56–0.90) | 0.005 | 70 (3.0%) | 82 (3.5%) | 0.85 (0.62–1.17) | 0.32 | 181 (5.7%) | 0.74 | 234 (7.4%) | 0.97 | 0.76 (0.63–0.92) | 0.006 |
| MACE-2 | 136 (4.3%) | 187 (5.9%) | 0.72 (0.57–0.89) | 0.003 | 83 (3.5%) | 94 (4.0%) | 0.88 (0.65–1.18) | 0.39 | 214 (6.7%) | 0.88 | 272 (8.6%) | 1.13 | 0.77 (0.65–0.92 | 0.005 |
| MACE-2 + angina | 147 (4.6%) | 205 (6.5%) | 0.71 (0.57–0.87) | 0.001 | 86 (3.6%) | 98 (4.2%) | 0.87 (0.65–1.17) | 0.36 | 228 (7.2%) | 0.94 | 290 (9.2%) | 1.21 | 0.77 (0.65–0.92 | 0.003 |
| Coronary ischaemic eventsa ,b | 52 (1.6%) | 80 (2.5%) | 0.64 (0.45–0.91) | 0.013 | 13 (0.6%) | 28 (1.2%) | 0.46 (0.24–0.89) | 0.02 | 65 (2.0%) | 0.27 | 102 (3.2%) | 0.42 | 0.63 (0.46–0.86) | 0.004 |
BP, blood pressure; CI, confidence interval; CV, cardiovascular; Dbl Plac, double placebo; HR, hazard ratio; MACE-1, myocardial infarction, stroke or cardiovascular death; MACE-2, myocardial infarction, stroke, cardiovascular death, revascularization, resuscitated cardiac arrest or heart failure; Plac, placebo; py, person-years; Rosuva, rosuvastatin.
Coronary ischaemic events: fatal or non-fatal myocardial infarction, resuscitated cardiac arrest, angina, and revascularization.
Post hoc outcome.
BP-lowering therapy: candesartan + hydrochlorothiazide.
Combintion therapy: candesartan/hydrochlorothiazide + rosuvastatin.
Passive follow-up over 3.1 years: blood pressure lowering vs. placebo
During the passive follow-up phase, patients originally randomized to BP lowering compared with placebo did not demonstrate a significant reduction in any of the primary or secondary outcomes (Table 1).
Passive follow-up over 3.1 years: blood pressure lowering + rosuvastatin vs. double placebo
During the passive follow-up phase, those originally randomized to the combination of rosuvastatin and BP lowering compared with those randomized to double placebo had a 15% reduction in MACE-1, which was similar to the benefits from rosuvastatin alone. However, given that this was based on fewer individuals, this difference was not statistically significant [70 events (3.0%) in those on combination therapy vs. 82 events (3.5%) in those on placebo; HR 0.85, 95% CI 0.62–1.17, P = 0.32]. Similar results were seen for MACE-2 and MACE-2 plus angina, while there was a significant reduction (54%) in coronary ischaemic events (Table 1).
Complete follow-up over 8.7 years: rosuvastatin vs. placebo
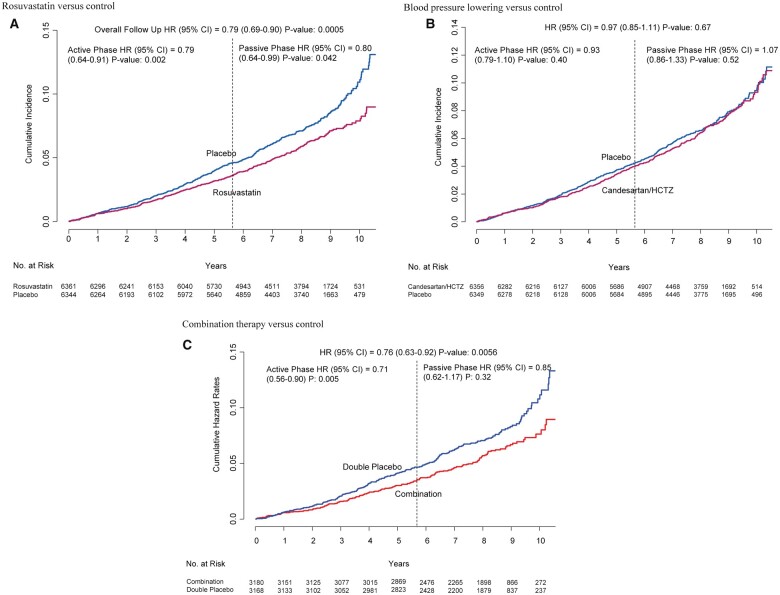
During 8.7 years of follow-up (5.6 years of active intervention plus 3.1 years of passive follow-up), patients originally randomized to rosuvastatin compared with placebo had a 21% reduction in MACE-1 [378 (5.9%) in those on rosuvastatin vs. 472 (7.4%) in those on placebo; HR 0.79, 95% CI 0.69–0.90, P = 0.0005] (Figure 2A). Similar results were seen for the MACE-2, MACE-2 plus angina, and coronary ischaemic events (Table 1), and for recurrent events for MACE-1 (HR 0.77, 95% CI 0.67–0.89, P = 0.0003).
Figure 2.
Cumulative incidence of MACE-1 according to treatment arm for the entire length of follow-up (8.7 years). (A) Rosuvastatin vs. control. (B) Blood pressure lowering vs. control. (C) Combination therapy vs. control. CI, confidence interval; HR, hazard ratio.
Complete follow-up over 8.7 years: blood pressure lowering vs. placebo
During the entire length of follow-up, patients originally randomized to BP lowering compared with placebo did not have a significant reduction in MACE-1 [419 (6.6%) events in those on BP lowering vs. 431 (6.8%) in those on placebo; HR 0.97, 95% CI 0.85–1.11, P = 0.67] (Figure 2B). There was also no effect on MACE-2, MACE-2 plus angina, or coronary ischaemic outcomes (Table 1). A recurrent event analyses for all components of MACE-1 also indicated little benefit (HR 0.95, 95% CI 0.83–1.10, P = 0.67).
Complete follow-up over 8.7 years: blood pressure lowering + rosuvastatin vs. double placebo
Among patients originally randomized to both BP lowering and rosuvastatin compared with double placebo, there was a 24% reduction in MACE-1 [181 (5.7%) vs. 234 (7.4%); HR 0.76, 95% CI 0.63–0.92, P = 0.006] (Figure 2C). Similar results were seen for MACE-2 and MACE-2 plus angina, and for recurrent events for MACE-1 (HR 0.73, 95% CI 0.60–0.90, P = 0.002). There was also a 37% reduction in coronary ischaemic events (2.0% vs. 3.2%; HR 0.63, 95% CI 0.46–0.86, P = 0.004) (Table 1).
Similar results for all comparisons were seen in sensitivity analyses restricted to those centres who followed 80% or more of their participants in the passive phase (Supplementary material online, Table S2) as well as using a competing risk analysis for non-CV deaths.
Subgroup and landmark analyses
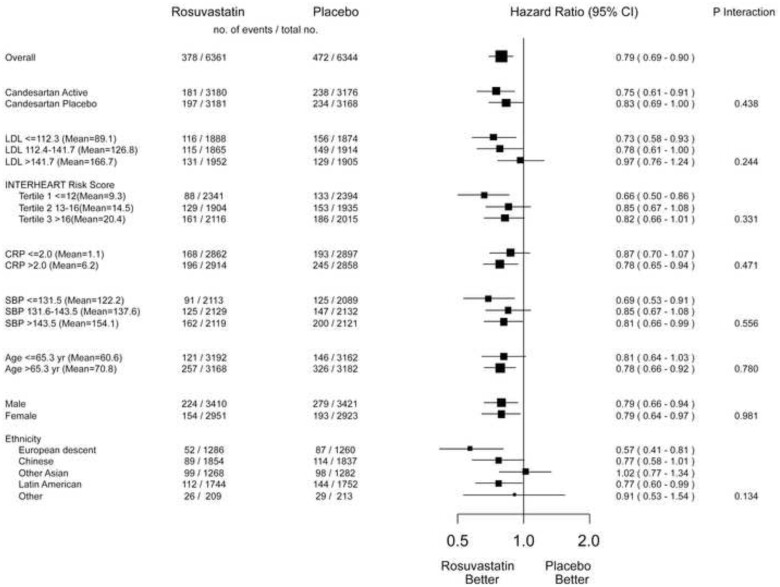
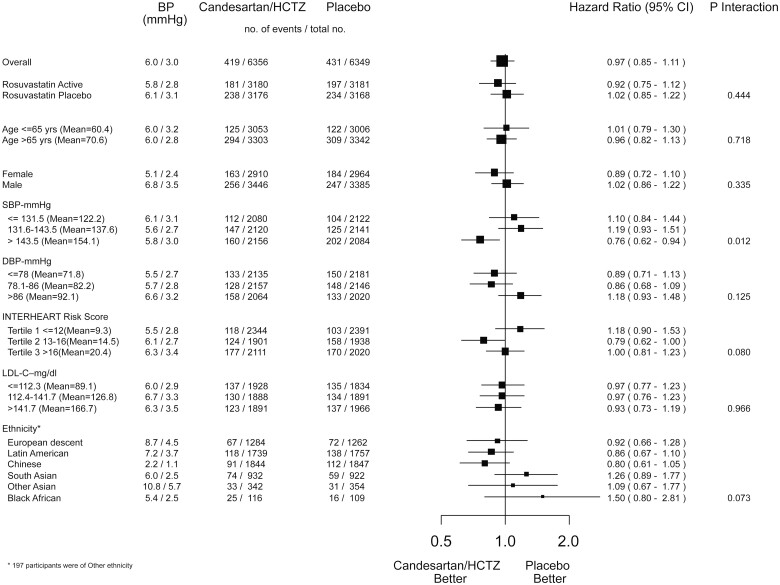
After 8.7 years, the benefits of rosuvastatin were consistent across pre-specified subgroups regardless of baseline LDL-C level, CV risk, age, ethnicity, and sex (Figure 3). Blood pressure lowering benefitted those with the highest third of baseline systolic BP (>143 mmHg) during the 8.7 years of passive follow-up.
Figure 3.
Effect of rosuvastatin on MACE-1 in key subgroups (8.7 years of follow-up). CI, confidence interval; CRP, C-reactive protein; LDL, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
During the active intervention phase, there was a 24% risk reduction in MACE-1 (95% CI 0.62–0.96) for those with the highest baseline systolic BP, whereas no benefit was observed in those in the lower two tertiles of baseline systolic BP (≤143 mmHg) (P for interaction = 0.009). During the passive follow-up, there was a 17% non-significant reduction in MACE-1 for those with the highest baseline systolic BP and no benefit for those in the lower tertiles (Table 2). Over 8.7 years, there was a 24% reduction in MACE-1 for those with the highest baseline systolic BP (95% CI 0.62–0.94) and no benefit for those in the lower tertiles of baseline systolic BP (Table 2, Figure 4). For the combination therapy compared with double placebo, those with the highest third of baseline BP on double active therapy had a 39% lower risk of MACE-1 during passive follow-up (HR 0.61, 95% CI 0.45–0.82) and a 24% risk reduction during 8.7 years of follow-up (95% CI 0.62–0.96, P for trend = 0.249).
Table 2.
MACE-1 outcome by tertiles of baseline systolic blood pressure
| Active intervention period (5.6 years) |
Passive follow-up (additional 3.1 years) |
Total follow-up (8.7 years) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BP-lowering therapya vs. placebo
|
||||||||||||
| BP (n = 6356) | Plac (n = 6349) | HR (95% CI) | P for trend | BP (n = 4677) | Plac (n = 4649) | HR (95% CI) | P for trend | BP (n = 6356) | Plac (n = 6349) | HR (95% CI) | P for trend | |
| 1st Tertile: ≤131.5 mmHg Mean (SD) 122.2 (7.5) | 90 (4.3%) | 74 (3.5%) | 1.25 (0.92–1.70) | 0.009 | 47 (3.0%) | 42 (2.7%) | 1.17 (0.77–1.77) | 0.163 | 112 (5.4%) | 104 (4.9%) | 1.10 (0.84–1.44) | 0.012 |
| 2nd Tertile: 131.6–143.5 mmHg Mean (SD) 137.6 (3.3) | 99 (4.7%) | 98 (4.6%) | 1.02 (0.77–1.34) | 61 (3.9%) | 45 (2.9%) | 1.36 (0.93–2.00) | 147 (6.9%) | 125 (5.8%) | 1.19 (0.93–1.51) | |||
| 3rd Tertile: >143.5 mmHg Mean (SD) 154.1 (8.9) | 123 (5.7%) | 156 (7.5%) | 0.76 (0.62–0.96) | 61 (3.9%) | 71 (4.7%) | 0.83 (0.59–1.17) | 160 (7.4%) | 202 (9.7%) | 0.76 (0.62–0.94) | |||
|
Combination therapyb vs. placebo
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combo (n = 3180) | Dbl Plac (n = 3168) | HR (95% CI) | P for trend | BP (n = 2359) | Plac (n = 2312) | HR (95% CI) | P for trend | BP (n = 3180) | Plac (n = 3168) | HR (95% CI) | P for trend | |
| 1st Tertile: ≤131.5 mmHg Mean (SD) 122.2 (7.5) | 24 (2.3%) | 33 (3.1%) | 0.71 (0.42–1.21) | 0.386 | 20 (2.4%) | 23 (3.6%) | 0.89 (0.49–1.62) | 0.297 | 43 (4.1%) | 56 (5.2%) | 1.25 (0.92–1.70) | 0.249 |
| 2nd Tertile: 131.6–143.5 mmHg Mean (SD) 137.6 (3.3) | 44 (4.2%) | 50 (4.7%) | 0.89 (0.59–1.33) | 26 (3.3%) | 21 (2.7%) | 1.25 (0.70–2.22) | 70 (6.7%) | 70 (6.6%) | 1.02 (0.77–1.34) | |||
| 3rd Tertile: >143.5 mmHg Mean (SD) 154.1 (8.9) | 45 (4.2%) | 74 (7.1%) | 0.59 (0.40–0.85) | 24 (3.2%) | 38 (5.2%) | 0.61 (0.37–1.02) | 68 (6.4%) | 108 (10.4%) | 0.76 (0.62–0.96) | |||
BP, blood pressure; CI, confidence interval; Dbl Plac, double placebo; HR, hazard ratio; MACE-1, myocardial infarction, stroke or cardiovascular death; Plac, placebo; SD, standard deviation.
BP-lowering therapy: candesartan + hydrochlorothiazide.
Combination therapy: BP lowering + rosuvastatin.
Figure 4.
Effect of blood pressure (BP) lowering on MACE-1 in key subgroups (8.7 years of follow-up). CI, confidence interval; CRP, C-reactive protein; DBP, diastolic blood pressure; HCTZ, hydrochlorothiazide; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Landmark analyses, using 3-year intervals, demonstrated that those taking rosuvastatin had a 16% reduction in MACE-1 in the first 3 years (95% CI 0.65–1.08), a 23% risk reduction in years 3–6 (95% CI 0.62–0.97) and a 24% reduction beyond 6 years (95% CI 0.61–0.95). For those on combination therapy, there was a 22%, 28%, and 21% reduction over the same time intervals (Supplementary material online, Table S3).
Discussion
During an additional 3.1 years of passive follow-up of HOPE-3 study participants without CVD but at intermediate risk for CV events, there were further reductions in CVD for those who had been originally allocated to receive rosuvastatin during the active phase. During the passive follow-up phase, the use of statins was similar (37%) in those originally allocated to receive rosuvastatin or placebo. These results indicate a sustained and perhaps enhanced benefit (legacy effect) that lasts for at least 3 years after the first 5.6 years of active therapy (Graphical abstract). These findings are consistent with the known effects of statin-based LDL-C lowering on the structure of the arterial wall and plaque morphology and composition that may explain a lasting effect even after treatment is stopped.11 , 12 By lowering serum LDL-C, statins may reduce the size of the lipid core leading to plaque stabilization, and can also induce plaque regression or stabilization. Statins have also been reported to improve vascular remodelling.13 The full effects of statins on plaque stabilization and regression are dependent on the magnitude of LDL-C lowering and duration of intervention. Previous reports suggest that the clinical benefits of statins take at least 6–12 months to become evident.14 , 15 Our results, demonstrating further benefits of rosuvastatin during 3.1 years of passive follow-up in those previously treated for 5.6 years, suggest that the clinical benefits may be due to plaque stabilization, regression, and vascular remodelling and may continue for several years after the cessation of statin therapy. However, our data should not be interpreted that statins should be discontinued after 5 or 6 years of treatment, but instead indicate that sustained and enhanced benefits occur even after stopping statin therapy. It is quite possible that had we been able to continue active treatment vs. control, the net benefits observed after 9 or 10 years may have been even larger.
Longer-term observation also reveals larger absolute risk reduction among those allocated to receive rosuvastatin. The absolute risk reduction for MACE-1 was 1.1% and for MACE-2 was 1.3% during the in-trial period, and this increased to 1.5% and 1.8% for the entire duration of follow-up of over 8 years. This is relevant for informing physicians and guidelines about the absolute long-term benefits of statin therapy in CV prevention.
Our results are similar to the sustained benefits of statin therapy on MACE and CV mortality described in the West of Scotland Coronary Prevention Study (WOSCOPS) 20-year follow-up and on mortality in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA) Legacy Study (Table 3). WOSCOPS demonstrated that 40 mg daily of pravastatin reduced MACE by 25% (95% CI 0.62–0.91, P = 0.004) during the 4.8-year trial. After an additional 15 years of post-trial observational follow-up, there was a further 20% reduction in MACE (95% CI 0.71–0.90, P < 0.001).16 The ASCOT-LLA trial was stopped after 3.3 years because of a clear benefit of atorvastatin (10 mg) on myocardial infarction or CV death (HR 0.64, 95% CI 0.50–0.83, P = 0.0005). At that time, there was a non-significant 15% reduction in CV death (P = 0.51). There was a 14% further non-significant reduction in CV death during the 12 years of additional follow-up (only mortality data were collected during passive follow-up), and over the total 15.7 years of follow-up, there was a 15% significant reduction in CV death (95% CI 0.72–0.99, P = 0.0395).17 Both WOSCOPS and ASCOT-LLA enrolled participants with elevated LDL-C and so our study adds new information regarding the sustained benefits of statins in those with average LDL-C levels (Table 3). The only other trial of statin therapy in a primary prevention population with long-term passive follow-up, the ALLHAT-LLA Long-Term Follow-up, did not demonstrate an effect during either the in-trial or post-trial periods.18 This result was deemed to be due to a modest difference in LDL-C (0.5 mmol/L) between the active and placebo groups. Although we did not observe a significant effect of rosuvastatin on CV death, there was a non-significant trend with a 15% relative risk reduction both during the active intervention and the passive follow-up phases, which is directionally similar to the WOSCOPS and ASCOT-LLA results and proportionate to the smaller difference in LDL-C. Our results for MACE were also similar to those seen in WOSCOPS after 20 years, although there are important differences between the populations studied. The WOSCOPS long-term observation studied 5529 Scottish men aged 45–64 years with very high mean LDL-C of 4.9 mmol/L (190 mg/dL) or greater, whereas in HOPE-3, mean LDL-C at baseline was 3.3 mmol/L (127.8 mg/dL), which is much lower and would be considered to be an ‘average’ level. Individuals with such average levels of LDL-C are typically not targeted for statin therapy in primary prevention settings by most physicians and primary prevention guidelines. Furthermore, HOPE-3 included a higher proportion of women (46% of the study population) than previous trials (WOSCOPS enrolled only men and only 13% of the ASCOT-LLA participants were women). By including a large proportion (80%) of participants who were not from Western countries, our study is one of few providing new information in those with lower LDL-C, in women and in non-European populations. This makes our results more widely applicable.
Table 3.
Summary of primary prevention randomized controlled trials of low-density lipoprotein cholesterol lowering with post-trial passive follow-up on cardiovascular disease
| Study | Intervention/participants | Mean follow-up period (RCT/total follow-up [RCT + passive]) | Method of data collection in passive follow-up | Mean baseline LDL-C/% women/results of RCT phase | Results during extended passive follow-up period | Results during total follow-up period |
|---|---|---|---|---|---|---|
| WOSCOPS 20-year follow-up16 | Pravastatin 40 mg vs placebo in high-risk primary prevention participants | 4.8/20 years | National mortality and hospital discharge data—NHS Scotland (CV events and death) | 4.9 mmol/L; 0 women; 25% reduction in MACE (95% CI 0.62–0.91, P = 0.004); 16% reduction in CV death (95% CI 0.54-1.30, P = 0.434) | 20% reduction in MACE (95% CI 0.71–0.90, P < 0.001); 17% reduction in CV death (95% CI 0.71–0.98, P = 0.024) | 21% reduction in MACE (95% CI 0.71–0.88, P < 0.001); 17% reduction in CV death (95% CI 0.71–0.96, P = 0.015) |
| ASCOT-LLA Legacy Study17 | Atorvastatin 10 mg vs. placebo in participants with hypertension | 3.3/15.7 years | UK National Death Registry | 3.5 mmol/L; 13% women; 15% reduction in CV death (95% CI 0.52–1.38, P = 0.5128) | 14% reduction in CV death (95% CI 0.74–1.02, P = 0.079) | 15% reduction in CV death (95% CI 0.72–0.99, P = 0.0395) |
| ALLHAT-LLT Long-Term Follow-up18 | Pravastatin 40 mg vs. placebo in participants with hypertension | 4.8/8.8 years | US National Death Index and Social Security Administration (death only) | 3.78 mmol/L; 49% women; no reduction in CV death (HR 0.96, 95% CI 0.83–1.13, P = 0.65) | No reduction in CV death (HR 0.91, 95% CI 0.79–1.04, P = 0.17) | No reduction in CV death (HR 0.89, 95% CI 0.76–1.03, P = 0.11) |
| HOPE-3 | Rosuvastatin 10 mg vs. placebo in primary prevention participants at intermediate risk | 5.6/3.1 years | Telephone follow-up | 3.31 mmol/L; 46% women; 24% reduction in MACE (95% CI 0.64–0.91, P = 0.0019) | 20% reduction in MACE (95% CI 0.64–0.99, P = 0.042) | 21% reduction in MACE (95% CI 0.69–0.90, P = 0.0042) |
CI, confidence interval; CV, cardiovascular; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular event; RCT, randomized controlled trial.
Our study tested the effects of BP lowering in a general population opposed to the reduction in BP among those with hypertension. While there were no differences in the number of events between those who were randomized to receive BP-lowering therapy vs. controls in the overall trial population, pre-specified subgroup analyses during the active phase indicated significant benefits in those in the highest third of baseline systolic BP (>143.5 mmHg) with little benefit in the middle third, and perhaps a trend towards excess events in the lowest third. During the passive follow-up phase, the pattern of results was generally similar to that observed in the active phase; there were fewer events for those with highest systolic BP who had been randomized to BP lowering compared with those randomized to placebo, but the differences were not statistically significant. Consequently, over the entire 8.7 years of follow-up, those in the highest tertile of systolic BP allocated to BP-lowering medication had a 24% reduction in MACE-1 (95% CI 0.62–0.94) with little benefit in those with lower levels of systolic BP (P for trend 0.012). The lack of benefit from BP lowering in those in the lower two-thirds of BP distribution, both in the active and passive phases of the trial, is noteworthy. This suggests that the approach to BP lowering should continue to focus on reducing elevated BP to guideline-recommended targets.19 The benefits for those with the highest BP were seen over 8.7 years even though two-thirds of the passive follow-up participants were on at least one BP-lowering medication and 30% were on two or more BP-lowering medications. Sustained benefit of BP treatment, even after cessation of BP-lowering medication, has previously been reported in some, but not all antihypertensive trials6 , 17 but the mechanisms of benefit are less well understood than for lipid lowering.6 There are some data from animals and humans suggesting that BP-lowering medications (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, and beta-blockers) increase plaque stability and decrease plaque volume.20
We were surprised that only 37% of the HOPE-3 participants received statin therapy after the end of the active phase, despite demonstration of clear benefits. Sites were provided with summaries of the study results and physicians were asked to share this information with all their participants. Study participants were discharged to the care of their personal physicians and a letter was sent to these physicians informing them of the results of the active study. The low uptake of statins in our trial population after demonstration of clear benefits suggests that demonstrating the benefits of prevention strategies and informing participants and their physicians of the results is by itself inadequate to change clinical practice. Instead, additional strategies to overcome health-care barriers after considering local health systems barriers, and perhaps with the assistance of non-physician health workers, as was done in the HOPE-4 study,21 may be needed to change practice.
The foundations for prevention of CVD are lifestyle modification, including smoking cessation, moderate alcohol intake, healthy diet, and regular exercise. However, these strategies are not fully implemented, and even when implemented, do not completely eliminate the risk of CVD. Therefore, added interventions with drugs are often required. In HOPE-3, all participants received advice on lifestyle modification at every visit. Against this background we have demonstrated that in those at intermediate CV risk, long-term treatment with rosuvastatin (in addition to advice on diet, activity, and smoking cessation) reduces CV events. However, BP lowering appears to be beneficial only in those with elevated BP. The combination of these treatments will reduce CV events to a greater extent in those with elevated BP. These results are in line with current guidelines for use of statins and BP-lowering medications in primary prevention for those at intermediate risk.22 , 23 These results are also consistent with the report of the PolyIran study in which a polypill (enalapril 5 mg, hydrochlorothiazide 12.5 mg, atorvastatin 20 mg, aspirin 81 mg) compared with placebo reduced CVD by 34% (95% CI 0.55–0.80), despite only a 1.4 mmHg reduction in systolic BP and a 0.51 mmol/L (19.54 mg/dL) reduction in LDL-C over 5 years.24 Similar results have also been reported by the recent International Polycap Study 3 (TIPS-3) trial where those randomized to receive a polypill (40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril) had a 5.8 mmHg reduction in systolic BP and a 0.42 mmol/L reduction in LDL-C. This was associated with a reduction in the composite of CV death, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, or arterial revascularization by 21% (95% CI 0.63–1.00).25 After taking non-adherence into account, there was a 30% risk reduction in CVD, which was further enhanced with the addition of aspirin (40% relative risk reduction). Importantly, HOPE-3, PolyIran and TIPS-3 provide evidence of effectiveness in non-European populations.
Our study has a few potential limitations. First, we were able to follow only about 80% of those eligible at the end of the active phase of the study. However, there was no evidence of selection biases, as the majority of those not followed were not followed because centres withdrew entirely. Furthermore, baseline characteristics of those followed were similar to those of the overall trial population during the active phase. In addition, sensitivity analyses that excluded data from centres who included <80% of randomized patients yielded similar results. Second, our conclusions regarding the benefits of BP lowering are based on a subgroup analysis of patients with highest baseline BP. However, the benefits observed in patients with elevated BP are consistent with the results of several previous BP-lowering trials.26 Finally, although BP or LDL-C measurement at the end of the passive phase was not available, there is no reason to believe that in the absence of differences in the rates of statin or BP medication use in the active vs. placebo groups that differences in BP or lipid levels would occur.
Conclusions
Following the end of randomized statin treatment, in patients without prior CVD and at intermediate risk of CV events who were treated with rosuvastatin for a median of 5.6 years, the benefits of treatment continue to accrue for at least several years after it is discontinued. Similar results were observed for BP-lowering treatment in those with elevated BP, but not for those with systolic BP <140 mmHg. These data underscore the importance of early treatment of risk factors and suggest that the benefits of primary prevention may be underestimated in primary prevention trials that do not follow patients beyond the period of active intervention.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data underlying this article may be shared for select projects deemed to be priority projects upon request to the corresponding author, and reimbursement of costs for provision of such data.
Funding
Funding was provided by the Canadian Institutes of Health Research and AstraZeneca, who also provided study drug. Additional funding was provided by the Population Health Research Institute through the Hamilton Health Sciences Research Institute.
Conflict of interest: Dr J.B. reports research support from the Canadian Institutes of Health Research, AstraZeneca, and Bayer AG, support from Bayer for participation in advisory boards and adjudication. Dr E.M.L. reports research support with payments to institution from Astra Zeneca, Amgen, Bayer, Boehringer Ingelheim, Sanofi, and Novartis and personal consulting honoraria from Amgen, Bayer, Sanofi, Novartis, Novo Nordisk, The Medicines Company, and Resvirrologix. Dr J.Z. reports study funding to institution from the Population Health Research Institute, Boehringer Ingelheim, Bayer, Sanofi, and Bristol-Myers Squibb; personal speaker fees from Boehringer Ingelheim, Bayer, Sanofi, Bristol-Myers Squibb, Pfizer, Astra Zeneca, and Novartis; support to attend steering and investigator meetings from the Population Health Research Institute and free support for study drugs used in clinical trials from Boehringer Ingelheim, Bayer, Sanofi, and Bristol-Myers Squibb. Dr P.P. reports grants (institution), consulting fees (institution), and travel fees to attend steering and investigator meetings from the Population Health Research Institute. Dr G.D. reports support for the adjudication committee from the Population Health Research Institute; presentations for Bayer and Eli Lilly; support for travel from the Population Health Research Institute, Eli Lilly, and Bayer and participation on a data safety monitoring board/advisory board for the Montreal Heart Institute (no remuneration). Dr A.A. reports personal fees from Novartis and MSD. Dr K.K. reports travel costs to attend steering and investigator meetings from the Population Health Research Institute. Dr C.H. reports grants/contracts to institution for adjudication work from Astra Zeneca; payment for adjudication of endpoints from Astra Zeneca, and participation on advisory boards for Astra Zeneca, Coala Life, Novo Nordisk, Boehringer Ingelheim, and Bayer. Dr P.J. reports grants/consulting fees for activities related to national study co-ordination from the Population Health Research Institute and travel costs to attend steering and investigator meetings from the Population Health Research Institute. Dr K.K. reports grants/contracts to institution from Astra Zeneca, Novartis, Novo Nordisk, sanofi-aventis, Lilly, Servier, Pfizer, Boehringer Ingelheim, and Merck Sharp & Dohme and personal fees from Amgen, Astra Zeneca, Bayer, NAPP, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Berlin-Chemie AG/Menarini Group, Boehringer Ingelheim, sanofi-aventis, and Servier. Dr W.D.T. reports research grant to institution from Astra Zeneca and Canadian Institutes of Health Research (Grant IR2-91038). Dr J.V. reports travel fees for steering and investigator meetings from the Population Health Research Institute and grants for role in study activities from the Population Health Research Institute. Dr P.J. reports institution study grant support from Bayer and Cadila Pharmaceuticals and travel support for lecture from Bayer. Dr L.A.L. reports grants to institution from Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Kowa, Novartis, Novo Nordisk, Sanofi, and the Medicines Company; honoraria for lectures from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, HSL, Janssen, Merck, Novo Nordisk, Sanofi, and Servier and participation on the advisory board for Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion, HSL, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, and Servier. Dr S.Y. reports grant for the study, honoraria, and travel for lectures from Astra Zeneca. Ms H.J., Drs L.L., P.L.-J., D.X., R.D., A.D., L.S.P., A.P., K.K., K.S., J.G.P., B.S.L., K.Y., and C.M.R. report no conflicts of interest.
Supplementary Material
Contributor Information
Jackie Bosch, The Population Health Research Institute, Hamilton Health Sciences, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada; The School of Rehabilitation Science, McMaster University, IAHS, Room 403, 1400 Main St. West, Hamilton, ON L8S 1C7, Canada.
Eva M Lonn, The Population Health Research Institute, Hamilton Health Sciences, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada; The Department of Medicine, 1200 Main St. West, McMaster University, Hamilton, ON L8N 3Z5, Canada.
Hyejung Jung, The Population Health Research Institute, Hamilton Health Sciences, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada.
Jun Zhu, Fu Wai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 9 Dongdan 3rd Alley, Dong Dan, Dongcheng, Beijing.
Lisheng Liu, Fu Wai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 9 Dongdan 3rd Alley, Dong Dan, Dongcheng, Beijing.
Patricio Lopez-Jaramillo, Instituto Masira, Facultad de Salud, Universidad de Santander, Calle 70 No 55-210, Bucaramanga, Colombia.
Prem Pais, St. John’s Research Institute, 100 Feet Rd, John Nagar, Koramangala, Bangalore, Karnataka 560034, India.
Denis Xavier, St. John’s Research Institute, 100 Feet Rd, John Nagar, Koramangala, Bangalore, Karnataka 560034, India; St. John’s Medical College, Sarjarpur Road, Bangalore, Karnataka 560034, India.
Rafael Diaz, Instituto Cardiovascular de Rosario, DSR, Bv. Oroño 440, S2000 Rosario, Santa Fe, Argentina.
Gilles Dagenais, Institut Universitaire de Cardiologie et Pneumologie de Québec, Université Laval, 2725 Ch Ste-Foy, Québec, QC G1V 4G5, Canada.
Antonio Dans, College of Medicine, University of the Philippines, Pedro Gil Street, Taft Ave, Ermita, Manila, 1000 Metro Manila, Philippines.
Alvaro Avezum, Dante Pazzanese Institute of Cardiology and Sao Paulo University, Av. Dr. Dante Pazzanese, 500 – Vila Mariana, São Paulo – SP, 04012-909, Brazil.
Leopoldo S Piegas, HCor-Hospital do Coração, Des. Eliseu Guilherme, 147 – Paraíso, São Paulo – SP, 04004-030, Brazil.
Alexander Parkhomenko, Institute of Cardiology, Narodnoho Opolchennya St, 5, Kiev 03680, Ukraine.
Kati Keltai, Hungarian Institute of Cardiology, Semmelweis University, Budapest, Hungary.
Matyas Keltai, Hungarian Institute of Cardiology, Semmelweis University, Budapest, Hungary.
Karen Sliwa, Department of Medicine, Hatter Institute for Cardiovascular Research, University of Cape Town, Soweto Cardiovascular Research Group, 4th, 5th and 6th Floor, Chris Barnard Building Faculty of Health Sciences, Private Bag X3 7935, Cape Town, South Africa.
Claus Held, The Uppsala Clinical Research Centre and Institute for Medical Sciences, Cardiology, Uppsala University, Uppsala Academic Hospital, Dag Hammarskjölds Väg 21, 752 37 Uppsala, Sweden.
Ronald J G Peters, The Department of Cardiology, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, Netherlands.
Basil S Lewis, Lady Davis Carmel Medical Center, Ruth and Bruce Rappaport School of Medicine, Technion–Israel Institute of Technology, Efron St 1, Haifa, Israel.
Petr Jansky, University Hospital Motol, V Úvalu 84, 150 06 Praha 5, Czechia.
Khalid Yusoff, Universiti Teknologi Majlis Amansh Rakyat, Jalan Ilmu 1/1, 40450 Shah Alam, Selangor, Malaysia; University College Sedaya International University, UCSI Heights, 1, Jalan Puncak Menara Gading, Taman Connaught, 56000 Cheras, Wilayah Persekutuan Kuala Lumpur, Malaysia.
Kamlesh Khunti, Leicester Diabetes Centre, Gwendolen Rd, Leicester LE5 4PW, UK.
William D Toff, Department of Cardiovascular Sciences, University of Leicester, University Rd, Leicester LE1 7RH, UK; UK and National Institute for Health Research, Leicester Biomedical Research Centre, Glenfield Hospital, Groby Road, Leicester LE3 9QP, UK.
Christopher M Reid, School of Public Health and Preventive Medicine, Monash University, 553 St. Kilda Rd., Melbourne, VIC 3004, Australia; The School of Public Health, Curtin University, Kent St, Bentley Perth, WA 6102, Australia.
John Varigos, School of Public Health and Preventive Medicine, Monash University, 553 St. Kilda Rd., Melbourne, VIC 3004, Australia.
Philip Joseph, The Population Health Research Institute, Hamilton Health Sciences, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada; The School of Rehabilitation Science, McMaster University, IAHS, Room 403, 1400 Main St. West, Hamilton, ON L8S 1C7, Canada.
Lawrence A Leiter, Li Ka Shing Knowledge Institute and Keenan Research Centre for Biomedical Science, St. Michael’s Hospital, University of Toronto, 209 Victoria St, Toronto, ON M5B 1T8, Canada.
Salim Yusuf, The Population Health Research Institute, Hamilton Health Sciences, 237 Barton Street East, Hamilton, Ontario L8L 2X2, Canada; The School of Rehabilitation Science, McMaster University, IAHS, Room 403, 1400 Main St. West, Hamilton, ON L8S 1C7, Canada.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2. Lonn E, Bosch J, Pogue J, Avezum A, Chazova I, Dans A, Diaz R, Fodor GJ, Held C, Jansky P, Keltai M, Keltai K, Kunti K, Kim JH, Leiter L, Lewis B, Liu L, Lopez-Jaramillo P, Pais P, Parkhomenko A, Peters RJ, Piegas LS, Reid CM, Sliwa K, Toff WD, Varigos J, Xavier D, Yusoff K, Zhu J, Dagenais G, Yusuf S, HOPE-3 Investigators. Novel approaches in primary cardiovascular disease prevention: the HOPE-3 trial rationale, design, and participants' baseline characteristics. Can J Cardiol 2016;32:311–318. [DOI] [PubMed] [Google Scholar]
- 3. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, Lopez-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E, HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 4. Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, Avezum A, Piegas LS, Keltai K, Keltai M, Chazova I, Peters RJ, Held C, Yusoff K, Lewis BS, Jansky P, Parkhomenko A, Khunti K, Toff WD, Reid CM, Varigos J, Leiter LA, Molina DI, McKelvie R, Pogue J, Wilkinson J, Jung H, Dagenais G, Yusuf S, HOPE-3 Investigators. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2009–2020. [DOI] [PubMed] [Google Scholar]
- 5. Khunti K, Kosiborod M, Ray KK. Legacy benefits of blood glucose, blood pressure and lipid control in individuals with diabetes and cardiovascular disease: time to overcome multifactorial therapeutic inertia? Diabetes Obes Metab 2018;20:1337–1341. [DOI] [PubMed] [Google Scholar]
- 6. Hirakawa Y, Arima H, Rodgers A, Woodward M, Chalmers J. Cumulative in-trial and post-trial effects of blood pressure and lipid lowering: systematic review and meta-analysis. J Hypertens 2017;35:905–913. [DOI] [PubMed] [Google Scholar]
- 7. Nayak A, Hayen A, Zhu L, McGeechan K, Glasziou P, Irwig L, Doust J, Gregory G, Bell K. Legacy effects of statins on cardiovascular and all-cause mortality: a meta-analysis. BMJ Open 2018;8:e020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lv HL, Jin DM, Liu M, Liu YM, Wang JF, Geng DF. Long-term efficacy and safety of statin treatment beyond six years: a meta-analysis of randomized controlled trials with extended follow-up. Pharmacol Res 2014;81:64–73. [DOI] [PubMed] [Google Scholar]
- 9. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stata Soc Series B Stat Methodol 2000;62:711–730. [Google Scholar]
- 10. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 11. Bittencourt MS, Cerci RJ. Statin effects on atherosclerotic plaques: regression or healing? BMC Med 2015;13:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, Rysz J, Toth PP, Muntner P, Mosteoru S, García-García HM, Hovingh GK, Kastelein JJP, Serruys PW, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med 2015;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kostapanos MS, Milionis HJ, Elisaf MS. An overview of the extra-lipid effects of rosuvastatin. J Cardiovasc Pharmacol Ther 2008;13:157–174. [DOI] [PubMed] [Google Scholar]
- 14. Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif J-C, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM; ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556–1565. [DOI] [PubMed] [Google Scholar]
- 15. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJP, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 16. Vallejo-Vaz AJ, Robertson M, Catapano AL, Watts GF, Kastelein JJ, Packard CJ, Ford I, Ray KK. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above: analyses from the WOSCOPS (West of Scotland Coronary Prevention Study) 5-year randomized trial and 20-year observational follow-up. Circulation 2017;136:1878–1891. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Mackay J, Whitehouse A, Godec T, Collier T, Pocock S, Poulter N, Sever P. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet 2018;392:1127–1137. [DOI] [PubMed] [Google Scholar]
- 18. Margolis KL, Davis BR, Baimbridge C, Ciocon JO, Cuyjet AB, Dart RA, Einhorn PT, Ford CE, Gordon D, Hartney TJ, Julian Haywood L, Holtzman J, Mathis DE, Oparil S, Probstfield JL, Simpson LM, Stokes JD, Wiegmann TB, Williamson JD, ALLHAT Collaborative Research Group. Long-term follow-up of moderately hypercholesterolemic hypertensive patients following randomization to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). J Clin Hypertens (Greenwich) 2013;15:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 20. Takata K, Imaizumi S, Zhang B, Miura S, Saku K. Stabilization of high-risk plaques. Cardiovasc Diagn Ther 2016;6:304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwalm J-D, McCready T, Lopez-Jaramillo P, Yusoff K, Attaran A, Lamelas P, Camacho PA, Majid F, Bangdiwala SI, Thabane L, Islam S, McKee M, Yusuf S. A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet 2019;394:1231–1242. [DOI] [PubMed] [Google Scholar]
- 22. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O, ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 23. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 24. Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, Ostovaneh MR, Nateghi A, Majed M, Navabakhsh B, Merat S, Pourshams A, Nalini M, Malekzadeh F, Sadeghi M, Mohammadifard N, Sarrafzadegan N, Naemi-Tabiei M, Fazel A, Brennan P, Etemadi A, Boffetta P, Thomas N, Marshall T, Cheng KK, Malekzadeh R. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet 2019;394:672–683. [DOI] [PubMed] [Google Scholar]
- 25. Yusuf S, Joseph P, Dans A, Gao P, Teo K, Xavier D, Lopez-Jaramillo P, Yusoff K, Santoso A, Gamra H, Talukder S, Christou C, Girish P, Yeates K, Xavier F, Dagenais G, Rocha C, McCready T, Tyrwhitt J, Bosch J, Pais P, International Polycap Study 3 Investigators. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med 2021;384:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article may be shared for select projects deemed to be priority projects upon request to the corresponding author, and reimbursement of costs for provision of such data.