Abstract
Human Immunodeficiency virus (HIV) virus-like Particles (VLPs) composed of HIVIIIB Gag and HIVBaL gp120/gp41 envelope are a pseudovirus vaccine capable of presenting antigens in their native conformations. To enhance the immunogenicity of the HIV Env antigen, VLPs were coupled to VesiVax Conjugatable Adjuvant Lipid Vesicles (CALV) containing one of four toll-like-receptor (TLR) ligands, each activating a receptor with distinct cellular localization and downstream pathways. C57Bl/6 mice were vaccinated by intranasal prime followed by two sub-cheek boosts and their sera immunoglobulin and neutralizing potency were measured over a duration of 3 months after vaccination. PBS control, VLPs alone, CALV + VLPs, and VLPs complexed with CALV and ligands for TLR2 (PAM3CAG), TLR3 (dsRNA), TLR4 (MPLA), and TLR7/8 (resiquimod) were evaluated based on antibody titer, IgG1 and IgG2c class switching, germinal center formation, T follicular cells and potency of neutralizing antibodies. Consistently, the TLR3 ligand dsRNA complexed to CALV and in combination with VLPs (CALV(dsRNA)+VLPs) induced the strongest response. CALV(dsRNA)+VLPs induced the highest titers against the recombinant vaccine antigens clade B Bal gp120 and pr55 Gag. Additionally, CALV(dsRNA)+VLPs induced cross-clade antibodies, represented by high titers of antibody to clade c 96ZM651 gp120. CALV(dsRNA)+VLPs induced predominantly IgG2c over IgG1, a response associated with T helper type 1 (Th1)-like cytokines. In turn, CALV(dsRNA)+VLP immunized mice generated the most potent neutralizing antibodies against HIV strain MN.3. Finally, at time of sacrifice, a significant increase in germinal center B cells and T follicular cells was detected in mice which received CALV(dsRNA)+VLPs compared to PBS. Our results indicate that CALV(dsRNA) is a superior adjuvant for HIV VLPs in generating a Th1-like immunoglobulin profile, while prolonging lymph node germinal centers, T follicular cells and generating neutralizing antibodies to a highly sensitive tier 1A variant of HIV.
Keywords: Virus-like Particle (VLP), TLR3, HIV, Neutralizing Antibody, Germinal Center, Vaccine
Introduction
1Virus-like particles (VLPs) are replication-incompetent subunit vaccines, which lack a genome, but maintain the original surface antigenic composition of the virus. HIV VLPs are composed of the HIV Gag structural protein and the membrane components of the host cell, which typically includes the expression of the exogenous HIV envelope membrane component gp160, cleaved into functional gp120/gp41 [1,2]. HIV VLPs have previously been shown to act as potent immunogens, capable of activating dendritic cells and macrophages, as well as directly activating B cells [3–5]. Recently, our lab identified intranasal prime, sub-cheek boost as a novel route of vaccine administration, which when coupled with HIV VLPs and a liposomal formulation containing the toll-like-receptor (TLR) 4 ligand monophosphoryl lipid A (MPLA), induced high serum immunoglobulin (IgG) titers and a Th1-like IgG profile [6].
TLR ligands make ideal adjuvants due to their receptor expression on antigen presenting cells (APCs), strong innate immune activation, and lack of host expression [7,8]. Ligands for TLRs 3, 4, 5, 7/8, and 9 have independently been combined with HIV VLPs in previous studies, but no direct comparison has been undertaken [6,9–11]. Additionally, how these adjuvants affect the adaptive immune system, in particular B cell hypermutation and class switching, over an extended period of time is glaringly understudied.
Germinal centers, located in the lymph nodes (LNs) and spleen, are the regions in which B cells undergo antigen specific somatic hypermutation and antibody class switching [12,13]. To maintain the germinal center, germinal center B cells are supported by a subset of T cells known as T follicular (TFH) cells, which through direct cell signaling and cytokine secretion maintain B cell maturation [14–16]. B cell maturation, in particular somatic hypermuation, is critical for the development of B cells capable of secreting high affinity HIV neutralizing antibodies [17,18].
In this study, we evaluated and compared the adjuvant properties among a panel of TLR ligands and assessed their ability to facilitate VLP immunogenicity by producing Th1-like class switching, high sera IgG titers, maintain germinal centers, and TFH cells which contribute to the antibody production against the target antigens. By targeting distinct TLRs in combination with our VLPs, we aimed to deduce how TLR cellular localization and activated downstream pathways could induce the optimal immune response.
Materials and Methods
Animals:
Female C57BL/6 mice from Jackson Labs (Farmington, CT) were purchased and used at 8 weeks of age. All mice were maintained under specific pathogen-free conditions in the animal facilities of Baylor College of Medicine and in accordance with the animal protocol approved by Institutional Animal Care and Use Committee (IACUC).
Western blot:
Western blot was performed as described previously [6]. Envelope protein was detected with primary human monoclonal antibody to V3 of HIV-1 Env (447-52D; NIH AIDS Reagent Program) and secondary anti-human HRP-conjugated antibody (Southern Biotech, Birmingham AL).
Mammalian VLP production:
Production of HIV VLPs was conducted as previously described using XC-18 cells transfected with HIV IIIB gag (tet-on promoter) and HIVBaL env (gift from Dr. Spearman at Emory University) [6]. Densitometric analysis of three independent VLP preparations indicated an average gp120 concentration of 11.2 μg/ml (Supplementary Figure 1A).
Immunization:
VLPs, at a concentration of 8 mg/ml, were mixed at a 1:1 v/v ratio with the indicated VesiVax Conjugatable Adjuvant Lipid Vesicles (CALV) and TLR ligands containing the indicated concentration and dose for each adjuvant (Table 1). For the VLP only group, VLPs were diluted 1:1 v/v in PBS (with Ca2+ and Mg2+). Samples were incubated for 1 hour at RT before inoculation. After anesthetizing the mice, 10 μl of VLPs were applied to the anterior nares of the nasal cavity. The process was repeated 5 times for a total of 200 μg of VLPs in 50 μl of solution. Intranasal prime was administered on day 0, after which, two boosts were delivered sub-cheek on days 14 and 28 (Supplementary Figure 1B). For sub-cheek administration, mice were first anesthetized and then injected with 25 μl of vaccine or PBS into each cheek, for a total volume of 50 μl (200 μg).
Table 1.
Immunization groups.
| Group | TLR Ligand | Target Receptor | Group Name | VLP dose (μg) | Adjuvant Dose (μg) | Adjuvant Concentration (μM) | Adjuvant Concentration (nmol/dose) |
|---|---|---|---|---|---|---|---|
| 1 | PBS | ||||||
| 2 | VLPs | 200 | |||||
| 3 | CALV + VLPs | 200 | |||||
| 4 | PAM3CAG | TLR2 | CALV(PAM3CAG) + VLPs | 200 | 100 | 500 | 25 |
| 5 | dsRNA | TLR3 | CALV(dsRNA) + VLPs | 200 | 100 | 1 | 0.05 |
| 6 | MPLA | TLR4 | CALV(MPLA) + VLPs | 200 | 25 | 300 | 15 |
| 7 | resiquimod (R848) | TLR7/8 | CALV(R848) + VLPs | 200 | 100 | 6000 | 300 |
Tissue collection:
At the indicated time points, blood was collected via submandibular puncture and either set aside to collect sera or PBMCs were isolated by gradient centrifugation with Histopaque 1083 (Sigma-Aldrich, St. Louis, MO). Mice were sacrificed at week 16 by cervical dislocation and sera and LNs were harvested. Blood was drawn by heart puncture. LNs were extracted by sterile dissection and lymphocyte suspensions were prepared by passing the tissue fragments through a 70 μm nylon mesh.
Endpoint Titer ELISA:
To detect Env- and Gag-specific antibodies, microtiter plates were coated with the following recombinant proteins at a concentration of 1 μg/ml: HIV-1BaL gp120 (Env), HIV-196ZM651 gp120, HIV-1BR29 gp140, or HIVIIIB Pr55 Gag (Gag) protein (NIH AIDS Research and Reference Reagent Program). Plates were blocked with PBS containing 5% bovine serum albumin (BSA). Sera were diluted 1:500 in PBS and the indicated serial dilutions were made for each recombinant protein. Plates were incubated overnight, washed, and incubated with anti-mouse IgG (Sigma-Aldrich, St. Louis, MO). After washing, TMB colorimetric substrate solution (Pierce, Rockford, IL) was added. The reaction was stopped with 2 N H2SO4, and optical density was read at 450 nm (reference 570 nm) in a microtiter reader (EL800, Bio-Tek Instruments, Winooski, VT). Endpoint titer was determined by the mean background + 2 times the standard deviation.
Quantitative ELISA:
ELISA plates were coated with a standard curve of the respective isotype and the remaining wells were coated with 2 μg/ml HIV-1BaL gp120. Plates were blocked with PBS containing 5% BSA. Afterwards, sera from the indicated time points were diluted 1:500 and added to each well. Secondary antibodies were IgG1-HRP, IgG2b-HRP, IgG2c-HRP, or IgG3-HRP (Southern Biotech, Birmingham AL). Plates were developed with TMB substrate for 5 minutes after which time 2 N H2SO4 was added to stop the reaction. Optical density was read at 450 nm (reference 570 nm) and sera IgG concentrations were calculated within the standard curve.
Neutralization assay:
Neutralizing antibodies were measured as a function of reductions in luciferase (Luc) reporter gene expression after a single round of infection with molecularly cloned Env-pseudotyped virus in TZM-bl cells as described [19]. Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLUs in cell control wells. Activity against MN.3 and Bal.26 in pooled post-immune samples was considered positive if the ID50 was >3x the signal detected in the pooled pre-immune sample against the same virus. Assay stocks of MN.3, Bal.26 and MLV Env-pseudotyped viruses were prepared by transfection in 293T/17 cells (American Type Culture Collection) and titrated in TZM-bl cells. Additional information on the assay and all supporting protocols may be found at: http://www.hiv.lanl.gov/content/nab-reference-strains/html/home.htm.
Intracellular cytokine staining (ICC):
ICC was performed as previously described [6]. In brief, PBMCs, 1×106 cells/well, were seeded into 96-well plates. Brefeldin A, monensin, and anti-CD107a (clone: 1D4B; BD Biosciences, San José, CA) antibody were added to each well and stimulated with media alone, Env, or Gag peptide pools at 2 μg/ml for 5 hours. Afterwards, cells were stained with anti-CD3e (clone: 500A2; BD Biosciences, San José, CA), anti-CD4 (clone: GK1.5; BD Biosciences, San José, CA), and anti-CD8a (clone: 53-6.7; BD Biosciences, San José, CA), incubated, and fixed with BD Perm/Fix (BD Biosciences, San José, CA). Cells were washed and resupended for cytokine analysis on an LSR-Fortessa (BD Biosciences, San José, CA).
Flow cytometry:
Lymphocytes, 5×105 cells/well, were resuspended in PBS containing 2% BSA, 5 mM EDTA, and 0.03% NaN3 and added to 96-well conical-bottom plates. The TFH cell panel included antibodies against mouse CD3e, CD4, CD8a, CXCR5 (clone: 2G8; BD Biosciences, San José, CA), and PD-1/CD279 (clone: J43; Ebiosciences, San Diego, CA). The germinal B cell panel included antibodies against mouse B220/CD45R (clone: RA3-6B2; BD Biosciences, San José, CA), CD3e, CD95 (clone: Jo2; BD Biosciences, San Diego, CA), and GL-7/Ly-77 (clone: GL7; BD Biosciences, San José, CA). Cells were incubated with the primary antibody, fixed for 15 minutes in BD Perm/Fix, resuspended in BD Perm/Wash, and analyzed on an LSR-Fortessa.
Statistical analysis:
Data from treated and control groups were analyzed and results presented as the arithmetic mean ± standard error mean (SEM). Statistical analyses were done with Student’s unpaired t-test, Kruskal–Wallis and Dunn’s posttest for comparison of nonparametric data, or with 2-Way ANOVA and the Bonferonni post-hoc test for comparison of parametric data between two or more groups. The Mann-Whitney test was used for comparing two nonparametric means. Graphpad Prism was used to calculate statistics (Graphpad Software, Inc., La Jolla, CA). A value of p < 0.05 was considered significant.
Results
VLPs induce robust cellular immune response:
Mice were immunized with one intranasal prime followed by two sub-cheek boosts of VLPs with or without the indicated adjuvants, and then retained for an additional 12 weeks following the final boost to monitor sera immunoglobulin concentrations (Table 1; Supplementary Figure 1B). VLPs consisted of HIVIIIB Gag and HIV-1 BaL gp160, which was cleaved into gp120 and gp41; each mouse received an average of 2.24 μg per immunization of BaL gp120 envelope (Supplementary Figure 1A). Two weeks after the second boost, PBMCs were stimulated with consensus B Env or Gag peptide pools and the membrane localization of CD107a was measured with flow cytometry. Mice vaccinated with VLPs, CALV+VLPs, CALV(PAM3CAG)+VLPs, and CALV(R848)+VLPs had significantly greater membrane CD107a localization when compared to PBS after Env stimulation (Supplementary Figure 2A). In addition, mice vaccinated with CALV(PAM3CAG)+VLPs had significantly greater membrane CD107a localization when compared to PBS after Gag peptide stimulation (Supplementary Figure 2B). No change in CD107a was observed after stimulation of PBMCs from mice immunized with either CALV(dsRNA)+VLPs or CALV(MPLA)+VLPs.
TLR Adjuvants Induce Cross-Clade Antibodies:
After sacrifice, the endpoint sera IgG titers against recombinant HIV-1 Bal gp120, pr55 Gag, 96ZM651 gp120, and BR29 gp140 from 0 (pre-immune), 8, 12, and 16 weeks after beginning immunization was determined for each group. Against HIV-1 gp120 BaL (clade B), CALV(dsRNA)+VLPs consistently exhibited the highest endpoint titer at all time points tested (Figure 1A). At 8 weeks after immunization, CALV(dsRNA)+VLPs (1:16000), CALV(PAM3CAG)+VLPs (1:5750), and CALV(R848)+VLPs (1:5375) had significantly greater endpoint titers compared to PBS (1:562). At 12 weeks after immunization, CALV(dsRNA)+VLPs (1:14500), CALV(PAM3CAG)+VLPs (1:6750), and CALV(MPLA)+VLPs (1:6250) had significantly greater titers when compared to PBS (1:500). Finally, at 16 weeks after the initial immunization, CALV(dsRNA)+VLPs (1:14250) and CALV(R848)+VLPs (1:5500) had significantly greater endpoint titers compared to PBS (1:562). Additionally, CALV(dsRNA)+VLPs had significantly greater endpoint titers at 8, 12 and 16 weeks compared to all other immunization groups.
Figure 1:
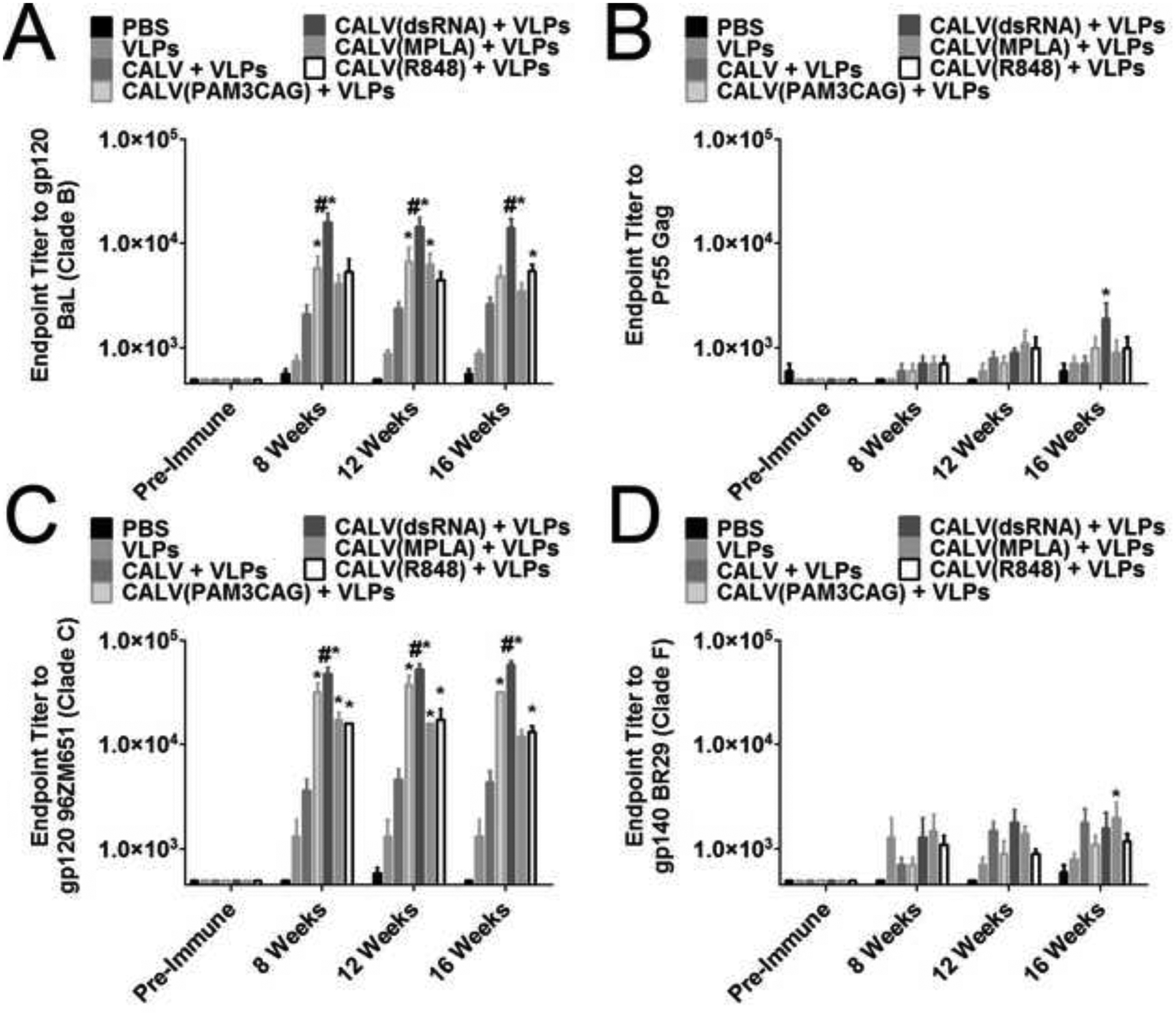
Sera Endpoint Titers against HIV antigens. (A) Endpoint dilution of pooled mouse sera against clade B gp120 BaL Env at the indicated time points (n=8). (B) Endpoint dilution of pooled mouse sera against Pr55 Gag at the indicated time points (n=5). (C) Endpoint dilution of pooled mouse sera against clade C gp120 96ZM651 Env at the indicated time points (n=6). (D) Endpoint dilution of pooled mouse sera against clade F gp140 BR29 Env at the indicated time points (n=5). * indicates p <0.05 by Two-Way ANOVA and Bonferroni posttest when compared to PBS control. # indicates p < 0.05 by Two-Way ANOVA and Bonferroni posttest when compared to all other groups.
To evaluate the immunogenicity of the other component of the VLPs, HIVIIIB Gag, the endpoint titer of mouse sera was calculated against recombinant HIV-1 pr55 Gag, and was consistently less immunogenic than BaL gp120 (Figure 1B). Endpoint titers were measured from pooled sera at 0 (pre-immune), 8, 12, and 16 weeks from each immunization group. Only at 16 weeks were any significant endpoint dilutions detected, with CALV(dsRNA)+VLPs (1:1900) having a significantly greater endpoint titer compared to PBS (1:600).
To determine if the VLPs elicited cross-clade antibody binding, endpoint titers were calculated for HIV-1 96ZM651 gp120 (96Z), a clade c isoform, and BR29 gp140 (BR29), a clade F isoform. Similar to BaL gp120, CALV(dsRNA)+VLPs induced the greatest endpoint titers against 96Z at 8 (1:48000), 12 (1:53333), and 16 (1:58668) weeks, which were significantly greater than PBS, as well as all other immunization groups (Figure 1C). CALV(PAM3CAG)+VLPs, CALV(MPLA)+VLPs, and CALV(R848)+VLPs also had endpoint titers to 96Z that were significantly greater than PBS at 8, 12 and 16 weeks.
Sera from all immunization groups were less reactive to BR29 gp140 compared to the clade B (BaL gp120) and clade C (96ZM651 gp120) isoforms. No group had significant endpoint titers at 8 weeks; however, at 12 weeks CALV(dsRNA)+VLPs endpoint titers of 1:1800 were significantly greater than PBS (Figure 1D). At 16 weeks, CALV(MPLA)+VLPs (1:2000) had significantly greater endpoint titers compared to PBS.
CALV(dsRNA)+VLPs induces strong Th1-like responses:
After establishing that the VLPs, when coupled with the liposomal adjuvants, induced strong sera IgG titers against the target antigens, quantitative ELISAs were employed to calculate class-switching induced by the indicated adjuvants. IgG1, which is associated with Th2-like cytokines, and IgG2c, which is associated with Th1-like cytokines, represent the majority of the sera IgG detected for each group. At 8 weeks after initial immunization, VLPs (11.6 μg/ml), CALV+VLPs (18.2 μg/ml), CALV(PAM3CAG)+VLPs (22.7 μg/ml), CALV(dsRNA)+VLPs (14.2 μg/ml), CALV(MPLA)+VLPs (13.6 μg/ml), and CALV(R848)+VLPs (43.9 μg/ml) had significantly greater Env specific IgG1 concentrations when compared to PBS (Figure 2A). By 12 weeks after initial immunization, CALV+VLPs (13.2 μg/ml), CALV(PAM3CAG)+VLPs (14.6 μg/ml), CALV(dsRNA)+VLPs (11.6 μg/ml), CALV(MPLA)+VLPs (10.1 μg/ml), and CALV(R848)+VLPs (33.2 μg/ml) had significantly greater Env specific IgG1 sera concentrations when compared to PBS; while the VLP group (3.45 μg/ml) had a marked decrease in IgG1 concentration and was not significantly different than PBS. Finally, at time of sacrifice, 16 weeks after the initial immunization, CALV+VLPs (16.2 μg/ml), CALV(PAM3CAG)+VLPs (13.3 μg/ml), CALV(dsRNA)+VLPs (14.1 μg/ml), CALV(MPLA)+VLPs (11.7 μg/ml), and CALV(R848)+VLPs (29.4 μg/ml) maintained significantly greater Env specific sera IgG1 compared to PBS. In addition, mice immunized with CALV(R848)+VLPs had significantly higher concentrations of sera IgG1 when compared to all other groups at 8, 12, and 16 weeks.
Figure 2:
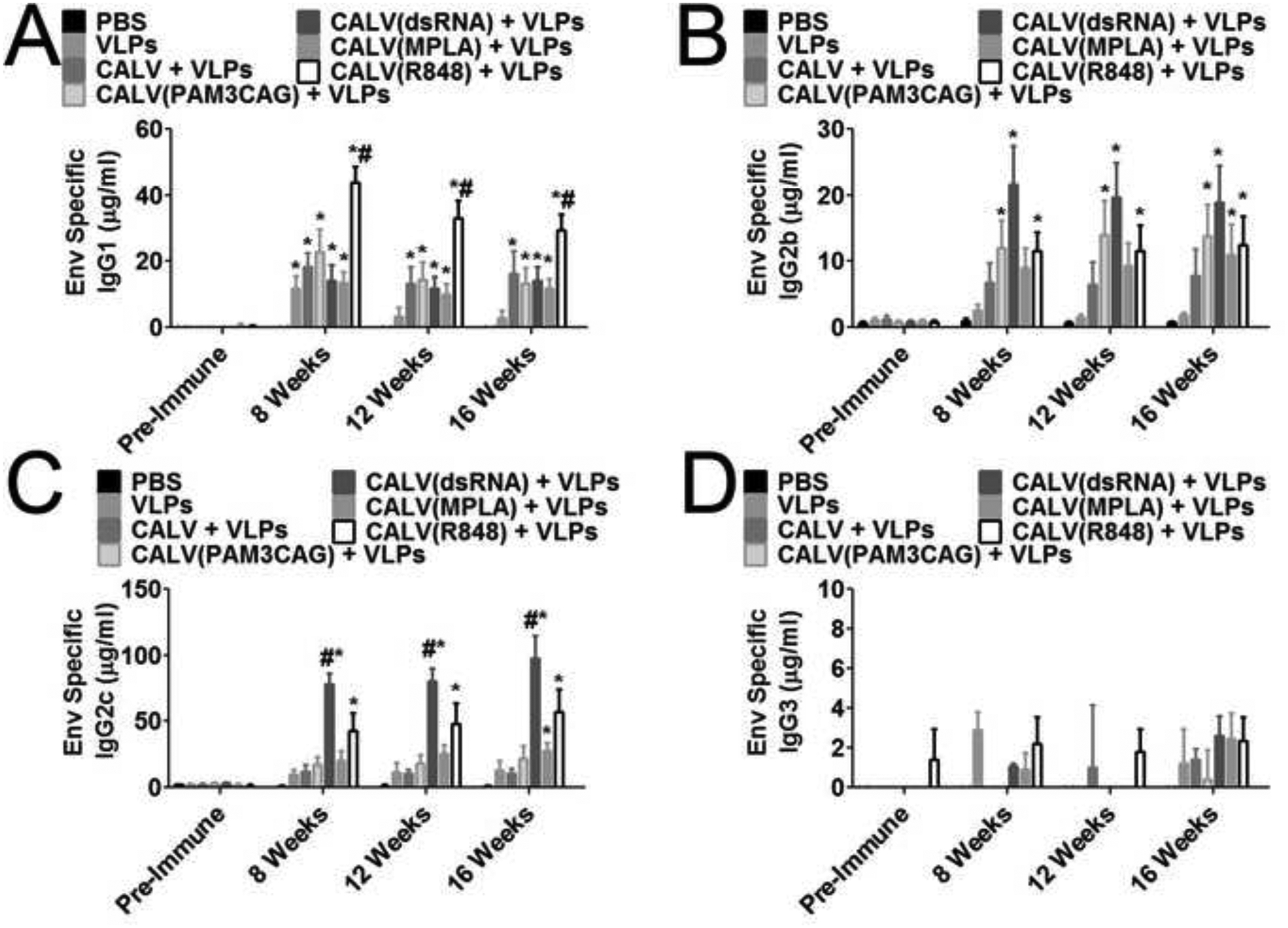
IgG Subtype Quantification against recombinant HIV-1BaL gp120 (Env) (A) Quantitative ELISA of IgG1 Env specific antibodies from individual mouse sera at 0, 8, 12, and 16 weeks after immunization with VLPs and the indicated adjuvants (n = 10). (B) Quantitative ELISA of IgG2b Env specific antibodies from individual mouse sera at 0, 8, 12, and 16 weeks after immunization with VLPs and the indicated adjuvants (n = 10). (C) Quantitative ELISA of IgG2c Env specific antibodies from individual mouse sera at 0, 8, 12, and 16 weeks after immunization with VLPs and the indicated adjuvants (n = 10). (D) Quantitative ELISA of IgG3 Env specific antibodies from pooled mouse sera at 0, 8, 12, and 16 weeks after immunization with VLPs and the indicated adjuvants (n = 5). * indicates p <0.05 by 2-Way ANOVA and Bonferroni posttest when compared to PBS control. # indicates p < 0.05 by 2-Way ANOVA and Bonferroni posttest when compared to all other groups.
CALV(dsRNA)+VLPs induced the highest concentration of IgG2c, which is the complementary isotype to IgG2a (Figure 2C). At 8 weeks after initial immunization, CALV(dsRNA)+VLPs (77.9 μg/ml) and CALV(R848)+VLPs (43.2 μg/ml) had significant sera IgG2c concentrations when compared to PBS. By 12 weeks, IgG2c concentrations were either maintained or increased and CALV(dsRNA)+VLPs (80.1 μg/ml) and CALV(R848)+VLPs (48.1 μg/ml) had significant Env specific sera IgG2c concentrations compared to PBS. Finally, at 16 weeks, CALV(dsRNA)+VLPs (97.3 μg/ml), CALV(MPLA)+VLPs (27.5 μg/ml), and CALV(R848)+VLPSs (57.3 μg/ml) all had significantly greater Env specific sera IgG2c concentrations when compared to PBS. In addition, CALV(dsRNA)+VLPs maintained significantly greater Env specific IgG2c titers compared to all other groups at 8, 12, and 16 weeks.
Finally, we examined the two IgG subtypes associated with TGF-β, IgG2b and IgG3. At 8 weeks after initial immunization, CALV(PAM3CAG)+VLPs (12.0 μg/ml), CALV(dsRNA)+VLPs (21.6 μg/ml), and CALV(R848)+VLPs (11.5 μg/ml) had significantly greater IgG2b Env specific sera concentrations when compared to PBS (Figure 2B). These concentrations remained relatively steady between time points. By 12 weeks after immunization, CALV(PAM3CAG)+VLPs (14.0 μg/ml), CALV(dsRNA)+VLPs (19.7 μg/ml), and CALV(R848)+VLPs (11.6 μg/ml) had maintained significantly greater sera Env specific IgG2b concentrations compared to PBS. Finally, at 16 weeks after the initial immunization, CALV(PAM3CAG)+VLPs (13.9 μg/ml), CALV(dsRNA)+VLPs (18.9 μg/ml), CALV(MPLA)+VLPs (11.0 μg/ml), and CALV(R848)+VLPs (12.5 μg/ml) had significantly greater Env specific sera IgG2b when compared to PBS. IgG3 concentrations were low in all immunization groups; therefore, samples were pooled to better detect Env specific sera IgG3 (Figure 2D). No significant IgG3 titers were detected when compared to PBS at 8, 12, or 16 weeks.
CALV(dsRNA)+VLPs induces neutralizing antibody titers against HIV MN.3:
Neutralizing antibody titers were measured with pooled serum from each experimental group from serum taken at 0 (pre-immune), 8, 12, and 16 weeks after immunization (Table 2). VLPs, CALV+VLPs, CALV(dsRNA)+VLPs, CALV(MPLA)+VLPs, and CALV(R848)+VLPs induced significant neutralizing titers at 8 and 16 weeks after immunization against the clade B MN.3 HIV strain. Surprisingly, a modest decline in neutralizing titers occurred at week 12, where only VLPs and CALV(dsRNA)+VLPs produced significant titers above PBS. CALV(dsRNA)+VLPs consistently generated the highest ID50 against MN.3 compared to all other groups. No positive activity above the background in pre-immune serum or in the PBS control group was detected against clade B BaL.26.
Table 2.
Neutralization ID50 titers.
| Strain | MN.3 | BaL.26 | |
|---|---|---|---|
| Clade | Clade B (Tier 1A) | Clade B (Tier 1B) | |
| Sample ID | Bleed Week | ID50 in TZM-bl Cells | |
| PBS | 0 | 31 | <20 |
| 8 | 32 | <20 | |
| 12 | 76 | 82 | |
| 16 | 38 | <20 | |
| VLPs | 0 | 36 | 29 |
| 8 | 634 | <20 | |
| 12 | 348 | 86 | |
| 16 | 951 | 27 | |
| CALV + VLPs | 0 | <20 | <20 |
| 8 | 387 | <20 | |
| 12 | 113 | 59 | |
| 16 | 481 | <20 | |
| CALV(PAM3CAG) + VLPs | 0 | <20 | <20 |
| 8 | 50 | <20 | |
| 12 | 127 | 97 | |
| 16 | 93 | <20 | |
| CALV(dsRNA) + VLPs | 0 | <20 | <20 |
| 8 | 1366 | <20 | |
| 12 | 625 | 85 | |
| 16 | 1765 | 22 | |
| CALV(MPLA) + VLPs | 0 | <20 | <20 |
| 8 | 370 | <20 | |
| 12 | 224 | 83 | |
| 16 | 601 | <20 | |
| CALV(R848) + VLPs | 0 | 27 | 29 |
| 8 | 311 | <20 | |
| 12 | 186 | 78 | |
| 16 | 431 | <20 | |
Note: Bolded values are considered positive for neutralizing antibody activity in the post-immune sample based on the criterion of >3X the observed background against the pre-immune serum and >3x the background activity of the PBS control group serum against the same virus.
CALV(dsRNA)+VLPs induces long-lasting germinal centers:
After sacrifice, LNs were analyzed for the presence of germinal centers by calculating the percentage of germinal center B cells (CD3−, B220+, GL-7+, CD95+) and TFH cells (CD3+, CD8−, CD4+, CXCR5+, PD-1+). A significant increase in germinal center B cells was detected in CALV(dsRNA)+VLPs (4.7%) group when compared to PBS (1.6%) (Figure 3A, 3B). To determine the relationship between neutralizing ID50 titers and germinal center B cells at 16 weeks, we calculated the Pearson correlation coefficient by linear regression (Figure 3C). Linear regression yielded a statistically significant r value of 0.93 (p=0.0023). Finally, the percentage of TFH cells cells were calculated in LNs from PBS and CALV(dsRNA)+VLPs mice (Figure 4A, 4B). A significant increase in TFH cells was also observed in the CALV(dsRNA)+VLPs group (2.2%) when compared to PBS (1.1 %).
Figure 3:
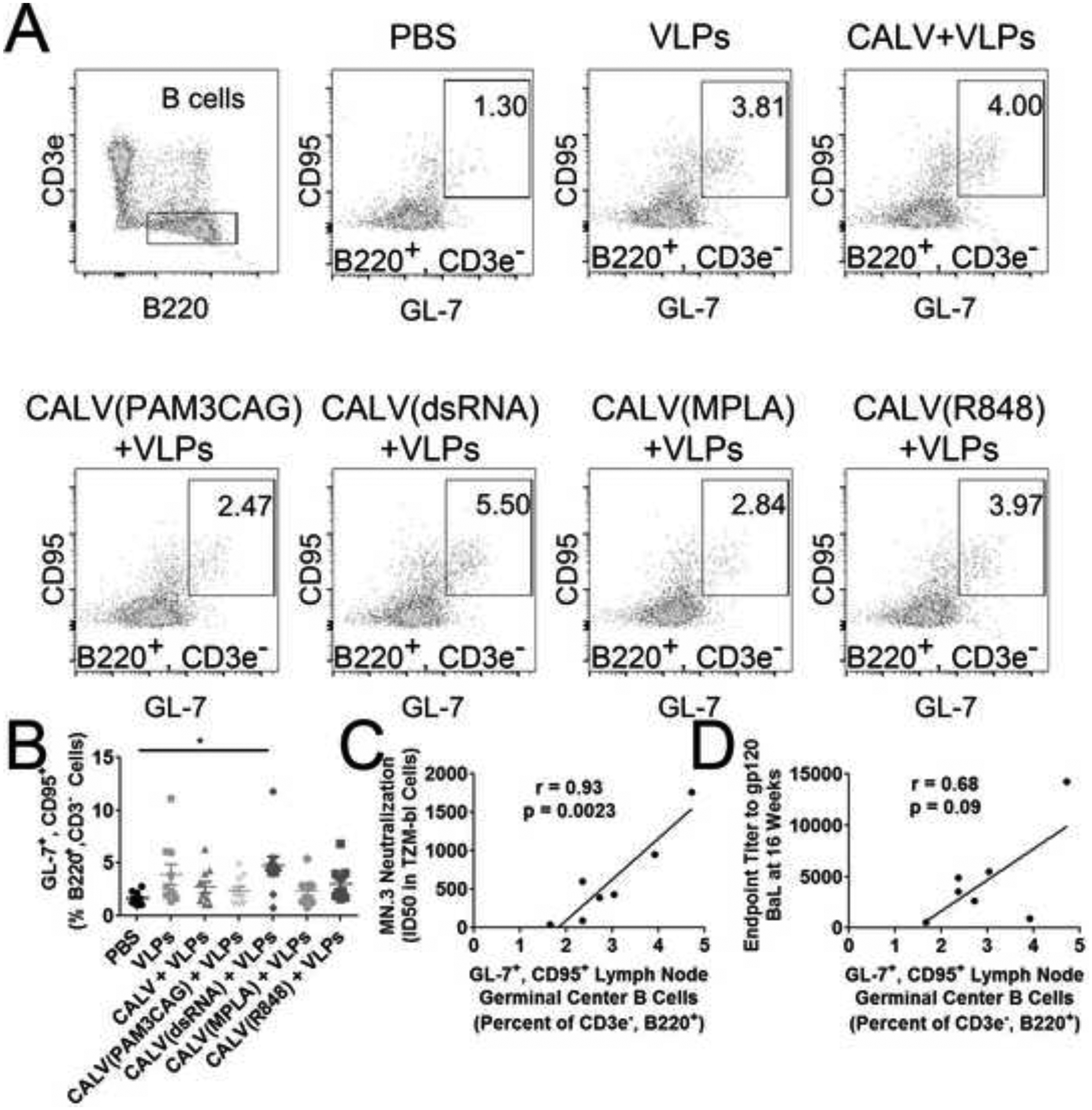
Lymph Node Germinal Center B Cells (A) Representative flow cytometry dot plots indicate gating for B cells (CD3−, B220+) and germinal center B Cells (CD95+, GL-7+) from each immunization group. (B) LN germinal center B cells in the indicated immunization groups as a percentage of total B cells (n=10). (C) Linear regression of mean percent lymph node germinal center B cells and MN.3 ID50 value at 16 weeks. (D) Linear regression of mean percent lymph node germinal center B cells and BaL gp120 endpoint IgG titer at 16 weeks. * indicates p < 0.05 by Kruskal–Wallis test and Dunn’s posttest when compared to PBS control.
Figure 4:
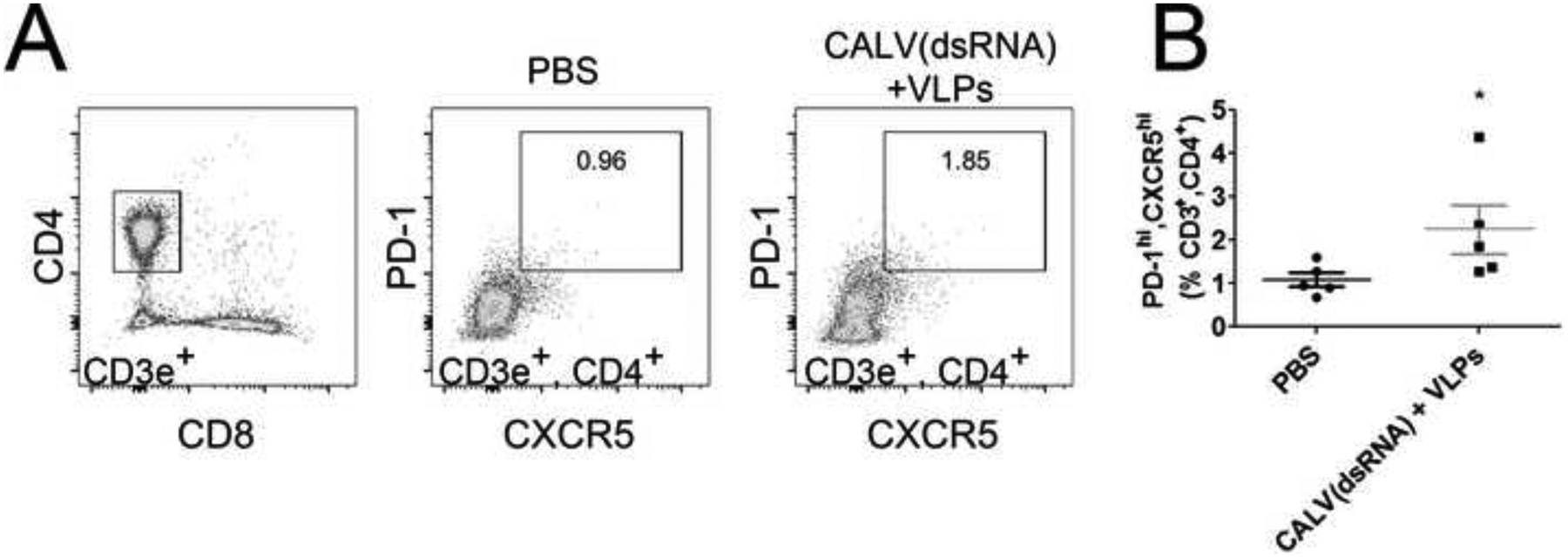
Lymph Node TFH Cells (A) Representative flow cytometry dot plots with gating for T cells (CD3+, CD4+, CD8−) and TFH cells (PD-1+, CXCR5+). (B) LN TFH cells in the indicated immunization groups as a percentage of total T cells (n=5). * indicates p < 0.05 by two-tailed Mann-Whitney U test when compared to PBS control.
Discussion
The aim of this study was to investigate a panel of TLR ligands as adjuvants for our HIV VLPs, assessed by longitudinal measurements of serum antibodies and germinal center B cells. By measuring serum antibody titers for an additional 3 months after the last immunization, we were able to better determine which adjuvants could induce long-lasting Th1-like IgG class-switching, as well as observe sustained germinal center activity.
By measuring serum IgG titers over a 3 month period after immunization, we observed changes in sera IgG subtype sera concentration, detecting a decline of Env specific IgG1, but no change in IgG2b or IgG2c over the same period. IgG2b, IgG2c (IgG2a homolog), and IgG3 are associated with the optimal antiviral response and are triggered by Th1 cytokines, in particular IFN-γ [20]. Additionally, TGF-β induces IgG2b class switching, while suppressing IgG3 [21]. Adjuvants were selected in order to observe the combined effect of VLPs and activation of four different TLRs, with dissimilar localization and signaling pathways: 1) PAM3CAG binds TLR2 located on the cell surface and activates MyD88 and TIRAP pathways; 2) dsRNA binds TLR3 located in the endosomal compartment and activates the TRIF pathway; 3) MPLA binds TLR4 located on the cell surface and activates the TRIF pathway; 4) R848 binds TLR7/8 located in the endosomal compartment and activates MyD88 [22–26]. As expected, different adjuvants induced either an IgG1 or an IgG2c dominant sera titer, which at 8 weeks after beginning the immunizations, all groups had more IgG1 than IgG2c except for VLPs conjugated to CALV(dsRNA) or CALV(MPLA). However, between 8 and 16 weeks we observed a decline of Env specific IgG1 in every group except CALV+VLPs. Therefore, by 16 weeks, Env specific IgG2c was the dominant subtype of all the immunization groups except CALV+VLPs.
We hypothesized that IgG end-point titers or IgG subtype concentration would predict the potency of the neutralizing antibodies generated by our vaccine; instead, the percentage of germinal center B cells in the LNs at time of sacrifice directly correlated with the potency of the neutralizing antibodies as measured by their ID50 against HIV MN.3. Previous research has demonstrated that germinal center B cells and TFH cells correlate with persistent antigen, while circulating TFH cells have been shown to correlate with broadly neutralizing antibody responses against tier 2 circulating strains of HIV [27,28]. However, no direct correlation between neutralizing antibodies and germinal center B cells has been reported. In this regard, our results might be explained by the fact that we measured neutralization using a highly sensitive tier 1A virus. Additional studies are needed in larger animals to determine whether our immunogens are capable of generating neutralizing antibodies against tier 2 strains of HIV.
In conclusion, among the adjuvants tested here with our VLP immunogen, CALV(dsRNA) proved superior for generating high serum antibody titers, robust IgG2c class switching in C57Bl/6 mice, and the highest percentage of Env-specific LN germinal center B cells, which were maintained throughout the duration of the study. Additionally, CALV(dsRNA)+VLP immunized mice had long-lasting germinal centers and neutralizing antibodies against HIV MN.3. Finally, we observed a strong correlation between germinal centers at time of sacrifice to neutralizing potency, but not between antibody titers and neutralizing potency.
Supplementary Material
Highlights:
VLPs in combination with TLR3 Adjuvant induce a strong humoral response
VLPs in combination with TLR3 adjuvant induced most potent neutralizing antibodies
Correlation between germinal centers and potency of neutralizing antibodies
Acknowledgements
We would like to thank Dr. Paul Spearman at Emory University for the VLP producing cell lines. VA merit Grants 1I01BX001474-01A1 (PI: Yao), NIH SBIR R43AI104073 (PI: Fujii). Phoebe Lewis received IMSD support R25GM56929. Neutralization assays were funded by NIAID-NIH under contract # HHSN27201100016C (to DCM). This project was supported by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (P30 AI036211, P30 CA125123, and S10 RR024574) and the expert assistance of Joel M. Sederstrom. This publication was made possible with help from the Baylor-UT Houston Center for AIDS Research (CFAR), an NIH funded program (AI036211). The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-196ZM651 gp120, Cat # 12066 BR29 gp140, HIV-1BaL gp120, HIV-1IIIB pr55 Gag, HIV-1 Consensus Subtype B Env Peptide Set, and HIV-1 Con B Gag Peptide Set.
Footnotes
Conflict of Interest: Sam On Ho, Thai Do, Su Ming Chiang and Gary Fujii are all employees of the Molecular Express, Inc., and there is a competing interest. The other authors have no conflicts of interest to report.
Human Immunodeficiency virus (HIV), Virus Like Particle (VLP), sub-cheek (SC), immunoglobulin (IgG), toll like receptor (TLR), antigen presenting cell (APC), T follicular (TFH), T helper type (Th), Lymph nodes (LN), Conjugatable Adjuvant Lipid Vesicles (CALV), phosphate buffered saline (PBS), Monophosphoryl Lipid A (MPLA), resiquimod (R848), double stranded RNA (dsRNA), HIV-1BaL gp120, (Env), Myeloid differentiation primary response gene 88 (MyD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), Toll-Interleukin 1 Receptor (TIR) Domain Containing Adaptor Protein (TIRAP)
References:
- [1].Wagner R, Flie??bach H, Wanner G, Motz M, Niedrig M, Deby G, et al. Studies on processing, particle formation, and immunogenicity of the HIV-1 gag gene product: a possible component of a HIV vaccine. Arch Virol 1992;127:117–37. doi: 10.1007/BF01309579. [DOI] [PubMed] [Google Scholar]
- [2].Doan LX, Li M, Chen C, Yao Q. Virus-like particles as HIV-1 vaccines. Rev Med Virol 2005;15:75–88. doi: 10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- [3].Buonaguro L, Tornesello ML, Tagliamonte M, Gallo RC, Wang LX, Kamin-Lewis R, et al. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol 2006;80:9134–43. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sailaja G, Skountzou I, Quan F-S, Compans RW, Kang S-M. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 2007;362:331–41. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang S, Cubas R, Li M, Chen C, Yao Q. Virus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cells. Mol Immunol 2009;46:1988–2001. doi: 10.1016/j.molimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Poteet E, Lewis P, Li F, Zhang S, Gu J, Chen C, et al. A Novel Prime and Boost Regimen of HIV Virus-Like Particles with TLR4 Adjuvant MPLA Induces Th1 Oriented Immune Responses against HIV. PLoS One 2015;10:e0136862. doi: 10.1371/journal.pone.0136862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta - Mol Cell Res 2002;1589:1–13. doi: 10.1016/S0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- [8].Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine 2011;29:3341–55. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wille-Reece U, Flynn BJ, Loré K, Koup R a, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Visciano ML, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J Transl Med 2012;10:4. doi: 10.1186/1479-5876-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adjuvant M, Vassilieva EV, Wang B, Vzorov AN. Enhanced Mucosal Immune Responses to HIV Virus-Like Particles Containing a Membrane-Anchored Adjuvant 2011. doi: 10.1128/mBio.00328-10.Editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB J 1994;8:832–40. [DOI] [PubMed] [Google Scholar]
- [13].Berek C, Berger a., Apel M. Maturation of the immune response in germinal centers. Cell 1991;67:1121–9. doi: 10.1016/0092-8674(91)90289-B. [DOI] [PubMed] [Google Scholar]
- [14].Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T Follicular Helper Cells Express a Distinctive Transcriptional Profile, Reflecting Their Role as Non-Th1/Th2 Effector Cells That Provide Help for B Cells. J Immunol 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- [15].Cells HT, Bowen MB, Butch AW, Parvin CA, Levine A, Nahm MH. Germinal Center T Cells Are Distinct 1991;75:67–75. [DOI] [PubMed] [Google Scholar]
- [16].Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol 2007;179:5099–108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- [17].Zhou T, Georgiev I, Wu X, Yang Z, Dai K, Finzi A, et al. Structural Basis for Broad and Potent. Science (80-) 2010;329:811. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TYK, et al. Sequence and Structural Convergence 2011;333:1633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- [20].Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- [21].Deenick EK, Hasbold J, Hodgkin PD. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J Immunol 1999;163:4707–14. [PubMed] [Google Scholar]
- [22].Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- [23].Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- [24].Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- [25].Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 2002;169:6668–72. [DOI] [PubMed] [Google Scholar]
- [26].Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 1999;11:115–22. [DOI] [PubMed] [Google Scholar]
- [27].Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- [28].Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013;39:758–69. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


