Abstract
Purpose
The majority of individuals with clinically significant obstructive sleep apnoea (OSA) are undiagnosed and untreated. A simple screening tool may support risk stratification, identification, and appropriate management of at-risk patients. Therefore, this systematic review and meta-analysis evaluated and compared the accuracy and clinical utility of existing screening questionnaires for identifying OSA in different clinical cohorts.
Methods
We conducted a systematic review and meta-analysis of observational studies assessing the diagnostic value of OSA screening questionnaires. We identified prospective studies, validated against polysomnography, and published to December 2020 from online databases. To pool the results, we used random effects bivariate binomial meta-analysis.
Results
We included 38 studies across three clinical cohorts in the meta-analysis. In the sleep clinic cohort, the Berlin questionnaire’s pooled sensitivity for apnoea-hypopnoea index (AHI) ≥ 5, ≥ 15, and ≥ 30 was 85%, 84%, and 89%, and pooled specificity was 43%, 30%, and 33%, respectively. The STOP questionnaire’s pooled sensitivity for AHI ≥ 5, ≥ 15, and ≥ 30 was 90%, 90%, and 95%, and pooled specificity was 31%, 29%, and 21%. The pooled sensitivity of the STOP-Bang questionnaire for AHI ≥ 5, ≥ 15, and ≥ 30 was 92%, 95%, and 96%, and pooled specificity was 35%, 27%, and 28%. In the surgical cohort (AHI ≥ 15), the Berlin and STOP-Bang questionnaires’ pooled sensitivity were 76% and 90% and pooled specificity 47% and 27%.
Conclusion
Among the identified questionnaires, the STOP-Bang questionnaire had the highest sensitivity to detect OSA but lacked specificity. Subgroup analysis considering other at-risk populations was not possible. Our observations are limited by the low certainty level in available data.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11325-021-02450-9.
Keywords: Obstructive sleep apnoea, Diagnostic accuracy, Screening questionnaires, Systematic review, Meta-analysis
Introduction
With an estimated 425 million individuals affected worldwide, clinically important obstructive sleep apnoea (OSA) poses a global public health problem [1]. Characterised by upper airway collapse, exaggerated negative intrathoracic pressure, oxidative stress, and systemic inflammation, OSA is associated with significant cardiovascular and metabolic complications, including hypertension, stroke, heart failure, and diabetes [2–7].
Despite the high prevalence and associated sequelae, most individuals with OSA remain undiagnosed, posing a significant risk to the individual patient and health care systems as complications develop [1, 8–10]. Barriers to the diagnosis and treatment of OSA are multifaceted and include geographical variation and inequity in the availability of sleep services and access to polysomnography (PSG), often limited by cost and long waiting times [11].
To support risk stratification and appropriate referrals in individuals at-risk, a simple and reliable screening tool may help triage patients at risk of OSA, for consideration of referral to specialist services for appropriate management [12–14]. Clinical prediction formulae have been developed but are limited by complexity and the requirement for a computer or mathematical calculations [15]. In contrast, OSA screening questionnaires are less complicated and may be a viable alternative to clinical prediction formulae in specific settings.
To date, there have been four systematic reviews exploring the accuracy of OSA screening tools in adults [12, 16–18]. One of the first systematic reviews and meta-analyses to explore the accuracy of screening tools for OSA identified four screening questionnaires; however, due to heterogeneity pertaining to the questionnaire, OSA definition, and threshold, these were not meta-analysed [16]. Ramachandran [17] reported that clinical prediction models performed better than the eight questionnaires studied to predict OSA in pre-operative cohorts. Abrishami [12] focused on a ‘sleep disorder’ cohort and a cohort ‘without a history of sleep disorders’. It was concluded that questionnaires were useful for early detection of OSA, especially in the surgical population. Despite finding it difficult to draw a definite conclusion about questionnaire accuracy, the STOP and STOP-Bang questionnaires were recommended for screening in a surgical population [12]. Recently, Chui [18] compared the diagnostic accuracy of the Berlin, STOP-Bang, STOP, and Epworth Sleepiness Scale. In line with Abrishami [12], they reported the STOP-Bang to have the highest sensitivity in both the sleep clinic and surgical populations.
Since the publication of these systematic reviews, new OSA screening questionnaires have emerged, further validation studies conducted, and different clinical settings and patient cohorts considered. As test performance often varies across clinical cohorts, it is recommended that tools are evaluated in clinically relevant cohorts [19]. Hence, the objective of this systematic review and meta-analysis was to evaluate the accuracy and clinical utility of existing questionnaires, when used alone, as screening tools for the identification of OSA in adults in different clinical cohorts.
Methods
The protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42018104018) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [20].
Types of studies
We included observational studies that met the following eligibility criteria:
Inclusion criteria: (1) prospective studies measuring the diagnostic value of screening questionnaires for OSA; (2) studies in adults (> 18 years of age); (3) studies in which the accuracy of the questionnaire was validated by level one or two PSG; (4) OSA was defined as apnoea-hypopnoea index (AHI) or Respiratory Disturbance Index (RDI) > 5; (5) data allowed for construction of 2 × 2 contingency tables; (6) publication in English, Spanish, or Portuguese.
Exclusion criteria: (1) studies measuring the diagnostic value of clinical scales, scores, and prediction equations as screening tools for OSA; (2) conference proceedings, reviews, or case reports; (3) insufficient data for analysis after several attempts to contact the author; (4) studies in children (< 18 years of age); (5) level three and four portable studies were used as the reference standard; (6) studies conducted in in-patient settings; (7) publication language is other than English, Spanish, or Portuguese.
Index test: the test under evaluation was only OSA screening questionnaires (self-reported or clinician completed).
Reference standard: the reference standard was a level one or two PSG.
Target conditions: the target condition was OSA, defined as AHI or RDI.
AHI/RDI ≥ 5—diagnostic cut-off for OSA
AHI/RDI ≥ 15—diagnostic cut-off for moderate to severe OSA
AHI/RDI ≥ 30—diagnostic cut-off for severe OSA
Search methods for identification of studies
Comprehensive literature searches in CINAHL PLUS, Scopus, PubMed, Web of Science, and the Latin American and Caribbean Health Sciences Literature (LILACS) database were conducted from inception to 18 December 2020. Detailed individual search strategies (Online Resource 1 & 2), with appropriate truncation and word combinations, were developed for each database. Additional records were identified from grey literature sources comprising ETHos, OpenGrey, Google Scholar, ProQuest, and New York Grey Literature Report. The reference lists from the final articles for analysis and related review articles were manually searched for references that could have been omitted during the electronic database searches.
Data collection and analysis
Study selection
Two reviewers (LB, EB) screened the titles and abstracts of the electronic search results independently to identify studies eligible for inclusion in the review. Records classified as ‘excluded’ by both reviewers were excluded. The full text of any study about which there was disagreement or uncertainty was assessed independently against the selection criteria and resolved through discussion and consultation with a third reviewer (IS or NR). Duplicates were identified and excluded before recording the selection process in sufficient detail to complete the PRISMA flow diagram and tables describing the characteristics of the excluded studies (Online Resource 3) [20].
Data extraction and management
Two reviewers (LB, EB) independently conducted data extraction on all studies included and extracted the data required to reconstruct the 2 × 2 contingency tables, including true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values. Where these values were not documented, we extrapolated the values from equations when data allowed. A data collection form tailored to the research question and fulfilling the data entry requirements of MetaDTA (Diagnostic Test Accuracy Meta-Analysis v1.43) was utilised [21].
HP and JR extracted the study characteristics and demographic data for all included studies, and LB and EB entered the data into Review Manager 5.3 [22].
No studies with inconclusive results were identified.
Assessment of methodological quality
The quality of studies included was appraised independently by the reviewers (LB, EB) utilising the Quality Assessment for Diagnostic Accuracy Studies tool (QUADAS-2) with disagreements resolved through consultation with a third reviewer (IS or NR) [23].
Statistical analysis and data synthesis
Statistical analysis was performed according to “Chapter 10” of the Cochrane Handbook for Systematic Review of Diagnostic Test Accuracy [24].
Questionnaire screening was considered positive for OSA if the questionnaire score was above the defined threshold specified in the primary study and negative if the questionnaire score was below the defined threshold. The TP, FP, TN, and FN results were produced by cross-classifying the questionnaire results with those of the PSG results. These were based on the ability of screening questionnaires to classify and detect OSA correctly.
The sensitivity and specificity of individual studies were calculated using 2 × 2 contingency tables and presented as forest plots. The meta-analysis was conducted using MetaDTA version 1.43, which models sensitivity and specificity by fitting the random effects bivariate binomial model of Chu and Cole [25, 26]. The summary receiver operating characteristic (SROC) plot was drawn using the hierarchical SROC parameters, which are estimated from the bivariate model parameters using the equivalence equations of Harbord [27]. Following guidance from the Cochrane Handbook for Systematic Review of Diagnostic Test Accuracy, we did not pool the positive and negative predictive values due to the prevalence of OSA varying across studies [24].
As per the Cochrane DTA handbook, we investigated heterogeneity by plotting the observed study results and SROC curve in the ROC space alongside the 95% confidence region [24].
We conducted a meta-regression to investigate differences in sensitivity and specificity between questionnaires, including the type of questionnaire as a covariate. Meta-regression was conducted in R version 4.0.1 using the lme4 package [28].
To assess the robustness of the meta-analysis, sensitivity analyses were conducted by excluding studies based on their QUADAS-2 assessment score [23]. Those identified as high risk in any QUADAS-2 domain or as unclear in four domains were excluded. Different AASM (American Academy of Sleep Medicine) scoring criteria and desaturation (and arousal) thresholds were applied to the included studies. We conducted additional sensitivity analyses by analysing studies that applied the ≥ 3% desaturation scoring criteria together and those that applied the ≥ 4% desaturation scoring criteria (summarised in Table 1).
Table 1.
Study characteristics
| Study | Country | Questionnaire | N | Validation tool | OSA definition | Apnoea definition | Hypopnoea definition | % desaturation | Scoring criteria |
|---|---|---|---|---|---|---|---|---|---|
| Sleep clinic population | |||||||||
| Abdullah 2018 [29] | Malaysia | STOP-Bang | 134 | Level 1—Lab PSG | AHI ≥ 5 | Complete cessation of airflow for ≥ 10 s | A reduction of airflow of ≥ 50% for ≥ 10 s associated with 3% desaturation | 3% | AASM 2012 |
| Alhouqani 2015 [30] | UAE | STOP-Bang | 193 | Level 1—Lab PSG | AHI ≥ 5 | A cessation of airflow for > 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | 3% | AASM 2012 |
| Amra 2013 [31] | Iran | Berlin | 157 | Level 1—Lab PSG | AHI > 5 | Complete cessation of airflow for ≥ 10 s | A reduction of airflow of ≥ 50% for ≥ 10 s associated with ≥ 3% desaturation | ≥ 3% | AASM 1999 |
| Amra 2018 [32] | China | Berlin, STOP-Bang | 400 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline for ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | 3% | AASM 2012 |
| Oktay Arslan 2020 [33] | Turkey | Berlin | 1003 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline for ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | 3% | AASM 2012 |
| Avincsal 2017 [34] | Turkey | STOP-Bang | 162 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| BaHammam 2015 [35] | Saudi Arabia | STOP-Bang | 100 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | ≥ 3% | AASM 2012 |
| Boynton 2013 [36] | USA | STOP-Bang | 219 | Level 1—Lab PSG | AHI > 5 | Complete absence of airflow for ≥ 10 s | ≥ 50% decrease in airflow followed by an arousal, awakening, or ≥ 3% desaturation | ≥ 3% | AASM 2007 |
| Deflandre 2018 [37] | Belgium | STOP-Bang, OSA50 | 159 | Level 1—Lab PSG | AHI > 30 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and associated with ≥ 4% desaturation | ≥ 4% | AASM 2012 |
| Delgado-Vargas 2020 [38] | Spain | STOP-Bang | 193 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and associated with ≥ 4% desaturation | ≥ 4% | AASM 2012 |
| Duarte 2017 [39] | Brazil | STOP-Bang | 456 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | ≥ 3% | AASM 2012 |
| Duarte 2020 [40] | Brazil | STOP-Bang | 3606 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | ≥ 3% | AASM 2012 |
| El Sayed 2012 [41] | Egypt | Berlin, STOP, STOP-Bang | 234 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Ha 2014 [42] | China | Berlin, STOP, STOP-Bang, ASA checklist | 141 | Level 1—Lab PSG | RDI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Hu 2019 [43] | China | STOP-Bang | 196 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | 3% | AASM 2012 |
| Kashaninasab 2017 [44] | Iran | Berlin, STOP, STOP-Bang | 250 | Level 1—Lab PSG | AHI ≥ 5 | A decrease in airflow that lasted ≥ 10 s | A reduction in airflow by ≥ 50% or a 3% desaturation | ≥ 3% | AASM 2005 |
| Khaledi-Paveh 2016 [45] | Iran | Berlin | 100 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Kim 2015 [46] | Korea | Berlin, STOP-Bang, SA-SDQ | 592 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Ong 2010 [47] | Singapore | STOP-Bang | 314 | Level 1—Lab PSG | AHI > 5 | Cessation of airflow for > 10 s | ≥ 30% decrease in airflow with 4% oxygen desaturation or with 3% oxygen desaturation and arousal | Unclear | Rechtschaffen & Kales 1968 |
| Pataka 2016 [48] | Greece | STOP, STOP-Bang, Berlin | 204 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Pecotic 2012 [49] | Croatia | STOP | 425 | Level 1—Lab PSG | AHI > 5 | Complete cessation of airflow for ≥ 10 s | A decrease in airflow > 50% from baseline for ≥ 10 and ≥ 3% desaturation | ≥ 3% | Not referenced |
| Pereira 2013 [50] | Canada | Berlin, STOP-Bang | 128 | Level 1—Lab PSG | AHI ≥ 5 | A cessation of airflow ≥ 50% for ≥ 10 s | A decrease in airflow of > 50% for ≥ 10 s followed by ≥ 3% oxygen desaturation | ≥ 3% | AASM 2007 |
| Perumalsamy 2017 [51] | Chennai | STOP-Bang, Berlin | 62 | Level 1—Lab PSG | AHI ≥ 5 | Not described | Not described | Not described | AASM Manual—not referenced |
| Sadeghniiat-Haghighi 2015 [52] | Iran | STOP, STOP-Bang | 603 | Level 1—Lab PSG | AHI > 5 | Total cessation of airflow for ≥ 10 s | Reduction of airflow for > 50% for ≥ 10 s with 3% desaturation or with arousal | 3% | AASM 2007 |
| Saleh 2011 [53] | Egypt | Berlin | 100 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Sangkum 2017 [54] | USA | STOP, STOP-Bang | 208 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow with 3% oxygen desaturation and/or arousal | ≥ 3% | AASM 2012 |
| Suksakorn 2014 [55] | Thailand | Berlin | 132 | Level 1—Lab PSG | AHI > 5 | Not reported or referenced | Not reported or referenced | Not reported or referenced | Not reported or referenced |
| Vana 2013 [56] | USA | STOP-Bang | 47 | Level 1—Lab PSG | AHI > 5 |
Complete cessation of airflow > 10 s In paper |
≥ 30% reduction in airflow that lasted ≥ 10 s and associated with 4% desaturation or arousal | 4% | AASM 2007 |
| Yüceege 2015 [57] | Turkey | Berlin | 433 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | A ≥ 50% drop in flow for ≥ 10 associated with ≥ 3% oxygen desaturation | ≥ 3% | AASM 2007 |
| Surgical population | |||||||||
| Chung 2008a [58] | Canada | Berlin, STOP, ASA checklist | 177 | Level 1—Lab PSG | AHI > 5 | Complete cessation of breathing | A decrease of > 50% of airflow for ≥ 10 s and > 3% desaturation or an arousal | > 3% | AASM1999 |
| Chung 2008b [59] | Canada | STOP, STOP-Bang | 177 | Level 1—Lab PSG | AHI ≥ 5 | Complete cessation of breathing | A decrease of > 50% of airflow for ≥ 10 s and > 3% desaturation or an arousal | ≥ 3% | AASM 1999 |
| Chung 2013 [60] | Canada | STOP-Bang | 384 | Level 2—portable PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasts ≥ 10 s and ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Chung 2014 [61] | Canada | STOP-Bang | 516 | Level 2—portable PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 50% reduction in airflow that lasted ≥ 10 s followed by ≥ 3% oxygen desaturation | ≥ 3% | AASM 2007 |
| Deflandre 2017 [62] | Belgium | STOP-Bang, OSA50 | 150 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% decrease in airflow for ≥ 10 s with ≥ 3% oxygen desaturation and/or arousal | ≥ 3% | AASM 2012 |
| Nunes 2015 [63] | Brazil | STOP-Bang, Berlin | 81 | Level 1—Lab PSG | AHI ≥ 15 | Apnoea was defined as complete cessation of airflow for ≥ 10 s | ≥ 50% reduction in airflow for 10 s associated with desaturation of > 3% or an arousal | > 3% | AASM 2007 |
| Xia 2018 [64] | China | STOP-Bang | 790 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in air flow and ≥ 4% desaturation | ≥ 4% | AASM 2012 |
| Resistant hypertension | |||||||||
| Giampá 2018 [65] | Brazil | Berlin | 119 | Level 1—Lab PSG | AHI > 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | At least 30% decrease in airflow with 3% desaturation and/or arousal | 3% | AASM 2007 |
| Margello 2014 [66] | Brazil | Berlin | 422 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | At least 30% decrease of airflow for ≥ 10 s accompanied by desaturation ≥ 4% | ≥ 4% | AASM 2007 |
| Asthma population | |||||||||
| Lu 2017 [67] | China | Berlin, STOP-Bang | 123 | Level 1—Lab PSG | AHI ≥ 5 | Apnoea was defined as the absence of oronasal airflow for ≥ 10 s | ≥ 50% reduction in airflow accompanied by oxygen desaturation ≥ 4% | ≥ 4% | AASM 2007 |
| Community clinic | |||||||||
| Gantner 2010 [68] | China | Berlin | 143 | Level 1—Lab PSG | AHI ≥ 15 | Not considered necessary to distinguish apnoeas from hypopnoeas | > 50% reduction in airflow for ≥ 10 s associated with > 3% desaturation or arousal | > 3% | AASM 1999 |
| Highway bus drivers | |||||||||
| Firat 2012 [69] | Turkey | Berlin, STOP, STOP-Bang, OSA50 | 85 | Level 1—Lab PSG (daytime) | AHI > 15 | ≥ 90% decrease in airflow persisting for ≥ 10 s | ≥ 50% decrease in the airflow with 3% desaturation or arousal, persisting for at least 10 s | ≥ 3% | AASM 2007 |
| Neurology population | |||||||||
| ElKholy 2017 [70] | Egypt | Berlin | 30 | Level 1—Lab PSG | AHI ≥ 5 | Not reported | Not reported | Not reported | Rechstchaffen and Kales (1968) |
| Primary care | |||||||||
| Bouloukaki 2013 [71] | Crete | Berlin | 129 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
| Respiratory population | |||||||||
| Yunus 2013 [72] | Malaysia | Berlin | 144 | Level 1—Lab PSG | AHI ≥ 5 | Not reported | Not reported | Not reported | Not reported |
| Snoring clinic | |||||||||
| Banhiran 2014 [73] | Thailand | STOP, STOP-Bang | 303 | Level 1—Lab PSG | AHI ≥ 5 | ≥ 90% drop in airflow from baseline, which lasted ≥ 10 s | ≥ 30% reduction in airflow that lasted ≥ 10 s and was associated with ≥ 4% desaturation | ≥ 4% | AASM 2007 |
We neither explored reporting bias, nor assessed publication bias due to the uncertainty about the determinants of publication bias for diagnostic accuracy studies, and the inadequacy of tests for detecting funnel plot asymmetry [74].
Results
Search results and study characteristics
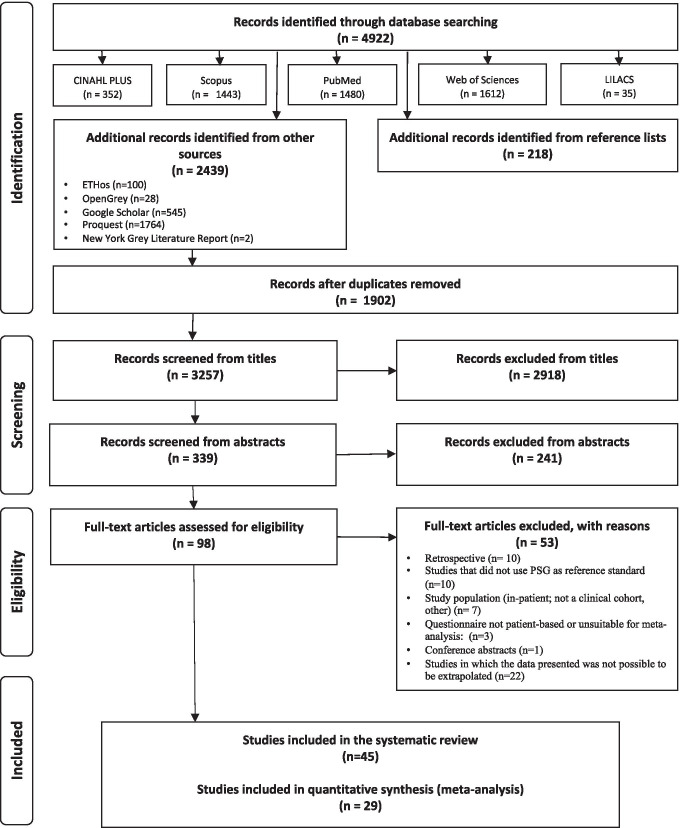
Search results are summarised in Fig. 1.
Fig. 1.
Flowchart of search results
Of 45 studies, 29 were included for meta-analysis in the sleep clinic population (n = 10,951), 7 were included for meta-analysis in the surgical population (n = 2275), and 2 were included in the resistant hypertension population (n = 541). The remaining 7 studies were excluded from the meta-analysis due to heterogeneity of included populations. Study characteristics and demographic data of the included studies are summarised in Tables 1 and 2 [29–73]. Overall, 10 clinical settings were identified, of which the sleep clinic, surgical, and resistant hypertension cohorts had sufficient studies for inclusion in the meta-analysis.
Table 2.
Demographic data
| Study | Age (mean ± SD) | Gender (%), male | BMI (kg/m2) | NC (cm) (mean ± SD) | WC (cm) (mean ± SD) | AHI (mean ± SD) |
|---|---|---|---|---|---|---|
| Sleep clinic population | ||||||
| Abdullah 2018 [29] | 41.22 ± 12.66 | 63.4 | n/a | n/a | n/a | n/a |
| Alhouqani 2015 [30] | 42.87 ± 11.838 | 77.7 | 34.9 ± 8.602 | 39.5 ± 3.463 | n/a | 34.87 ± 31.273 |
| Amra 2013 [31] | 52.3 ± 13.6 | 55.4 | 31.5 ± 6 | n/a | n/a | 37.8 ± 30.8 |
| Amra 2018 [32] | 49.29 ± 9.75 | 58.5 | 32.4 ± 7.43 | 40.8 ± 3.13 | n/a | n/a |
| Oktay Arslan 2020 [33] | 50.65 ± 11.38 | 70 | 32.5 ± 5.9 | 41.6 ± 3.3 | n/a | n/a |
| Avincsal 2017 [34] | 50 ± 0.79 | 69.8 | 34.2 ± 0.42 | 41.3 ± 0.32 | n/a | n/a |
| BaHammam 2015 [35] | 46.6 ± 14 | 61.0 | 34.4 ± 7.8 | 38.0 ± 3.81 | n/a | 50 ± 37 |
| Boynton 2013 [36] | 46.3 ± 13.9 | 44.8 | 33.4 ± 8.76 | 39.9 ± 5.4 | n/a | n/a |
| Deflandre 2018 [37] | 55.8 ± 14 | 68.0 | 31.8 ± 12.07 | n/a | n/a | n/a |
| Delgado-Vargas 2020 [38] | 50.42 ± 12.05 | 73 | 29.83 ± 6.12 | n/a | n/a | n/a |
| Duarte 2017 [39] | 43.7 ± 12.5 | 63.0 | 32.1 ± 7.8 | 40.8 ± 4.3 | n/a | 24.6 ± 25.2 |
| Duarte 2020 [40] | 45.7 ± 14.6 | 54 | 32.9 ± 7.7 | 40.5 ± 4.8 | n/a | n/a |
| El Sayed 2012 [41] | 50.38 ± 11.29 | 85.5 | 37.8 ± 9.54 | 42.4 ± 4.26 | n/a | 45.57 ± 32.74 |
| Ha 2014 [42] | 45 ± 11 | 81.6 | 26.0 ± 4 | n/a | n/a | 25 ± 24 |
| Hu 2019 [43] | 18–70 (range) | 84 | 27.23 ± 4.03 | 40.41 ± 3.7 | n/a | n/a |
| Kashaninasab 2017 [44] | 48.1 ± 12 | 76.0 | n/a | 40.2 ± 3.8 | n/a | 44.1 ± 31.2 |
| Khaledi-Paveh 2016 [45] | 47.8 ± 14.1 | 60.0 | 29.5 ± 6.1 | n/a | n/a | 24 ± 21.5 |
| Kim 2015 [46] | 47.6 ± 12.7 | 83.3 | 24.7 ± 3.5 | 39.2 ± 3.5 | n/a | 25.9 ± 21.8 |
| Ong 2010 [47] | 46.8 ± 15 | 70.5 | 27.9 ± 6 | 39.8 ± 4.1 | n/a | 26.2 ± 26.9 |
| Pataka 2016 [48] | 51.8 ± 13.8 | 77.5 | 32.8 ± 6.2 | 41.6 ± 3.9 | 1 ± 0.4 | 29.7 ± 24.7 |
| Pecotic 2012 [49] | 55 | 70.0 | 30.1 ± 4.7 | n/a | n/a | 31.4 ± 22.6 |
| Pereira 2013 [50] | 50 ± 12.3 | 65.6 | 31.0 ± 6.6 | 41.0 ± 4.4 | n/a | 33.1 ± 28 |
| Perumalsamy 2017 [51] | 53 | 59.7 | n/a | n/a | n/a | n/a |
| Sadeghniiat-Haghighi 2015 [52] | 45.8 ± 12.7 | 74.8 | 29.2 ± 5.9 | 39.7 ± 3.6 | n/a | n/a |
| Saleh 2011 [53] | 45 | 51.0 | 33.1 | n/a | n/a | n/a |
| Sangkum 2017 [54] | 52 ± 0.9 | 36.0 | 36.9 ± 0.7 | 40.6 ± 0.4 | 0.95 ± 0.01 | n/a |
| Suksakorn 2014 [55] | 48.15 ± 8.8 | 68.2 | 29.2 ± 6.8 | n/a | n/a | 28 ± 29.7 |
| Vana 2013 [56] | 46.4 ± 13.2 | 34.0 | 36.3 ± 9.2 | n/a | n/a | n/a |
| Yüceege 2015 [57] | 47.5 ± 10.5 | 65.8 | 31.1 ± 5.6 | 39.4 ± 3.9 | 102.9 ± 12.9 | 28.27 ± 26.5 |
| Surgical population | ||||||
| Chung 2008a [58] | 55 ± 13 | 49.7 | 30.0 ± 6 | 39.0 ± 6 | n/a | 20 ± 6 |
| Chung 2008b [59] | 55 ± 13 | 49.7 | 30.0 ± 6 | 39.0 ± 6 | n/a | 20 ± 6 |
| Chung 2013 [60] | 60 ± 11 | 46.0 | 31.2 ± 7 | 39.1 ± 4 | n/a | n/a |
| Chung 2014 [61] | 59.5 ± 12 | 54.0 | 30.6 ± 7 | 39.0 ± 4 | n/a | n/a |
| Deflandre 2017 [61] | 59.66 ± 12.41 | 70.0 | 32.4 ± 2.26 | 42.0 ± 4.64 | n/a | n/a |
| Nunes 2015 [63] | 56 ± 07 | 70.0 | 29.5 ± 5 | n/a | n/a | n/a |
| Xia 2018 [64] | 41.4 ± 10 | 58.5 | 28.5 ± 4.7 | 40.0 ± 4 | n/a | 13.1 ± 4.4 |
| Resistant hypertension | ||||||
| Giampá 2018 [65] | 52 ± 9 | 43 | 52 ± 9 | 40.0 ± 4 | 104 ± 14 | 27 ± 24 |
| Margello 2014 [66] | 62.4 ± 9.9 | 31.3 | 37.8 ± 3.7 | 37.8 ± 3.7 | 101 ± 12.1 | n/a |
| Asthma population | ||||||
| Lu 2017 [67] | 47.56 ± 12.12 | 57.7 | 26.4 ± 2.99 | 36.3 ± 2.97 | n/a | 15.07 ± 12.87 |
| Community clinic | ||||||
| Gantner 2010 [68] | 62.2 ± 7.6 | 40.5 | 26.6 ± 3.7 | 37.5 ± 4 | n/a | n/a |
| Highway bus drivers | ||||||
| Firat 2012 [69] | 48 ± 5.7 | 100 | 29.1 ± 3.8 | 41.1 ± 2.8 | 99.6 ± 9.8 | 21.1 ± 17.4 |
| Neurology population | ||||||
| ElKholy 2017 [70] | 50.67 ± 14.94 | 60 | n/a | n/a | n/a | 29.11 ± 33.16 |
| Primary care | ||||||
| Bouloukaki 2013 [71] | 47 ± 13 | 61.9 | 35.0 ± 25.1 | n/a | n/a | 41 ± 32 |
| Respiratory population | ||||||
| Yunus 2013 [72] | 44.7 ± 11.5 | 64 | 36.3 ± 11 | 39.3 ± 4.9 | 94.1 ± 16.9 | 38.8 ± 31.9 |
| Snoring clinic | ||||||
| Banhiran 2014 [73] | 49.6 | 61.4 | 27.5 | 37.05 | n/a | n/a |
OSA obstructive sleep apnoea, AHI apnoea-hypopnoea index, RDI respiratory disturbance index, Lab laboratory, PSG polysomnography, AASM American Academy of Sleep Medicine.
SD standard deviation, kg kilogramme, m metre, cm centimetre, NC neck circumference, WC waist circumference, AHI apnoea-hypopnoea index, n/a not applicable.
Methodological quality of included studies
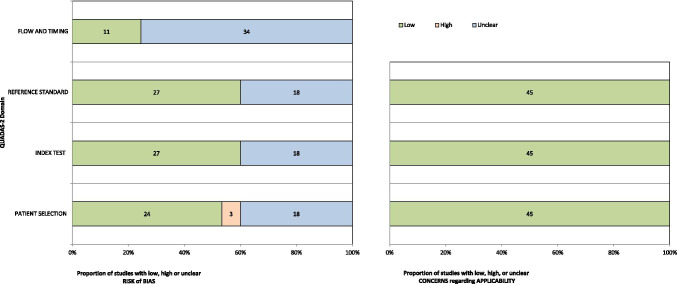
Results of the QUADAS-2 assessment are summarised in Fig. 2 and Online Resource 4 [29–73].
Fig. 2.
Risk of bias summary using the QUADAS-2 tool
In the patient selection domain, 3 studies were rated as high risk of bias due to the case–control study design. For both the index test and reference standard domains, 18 studies were rated as unclear risk of bias due to inadequate information related to blinding; it was unclear if the index test and reference standard findings were interpreted without the knowledge of the other. Thirty-four studies were rated as unclear risk of bias in the flow and timing domain due to lack of reporting on the time interval between the index test and the reference standard. Applicability was rated as low risk in all 45 studies.
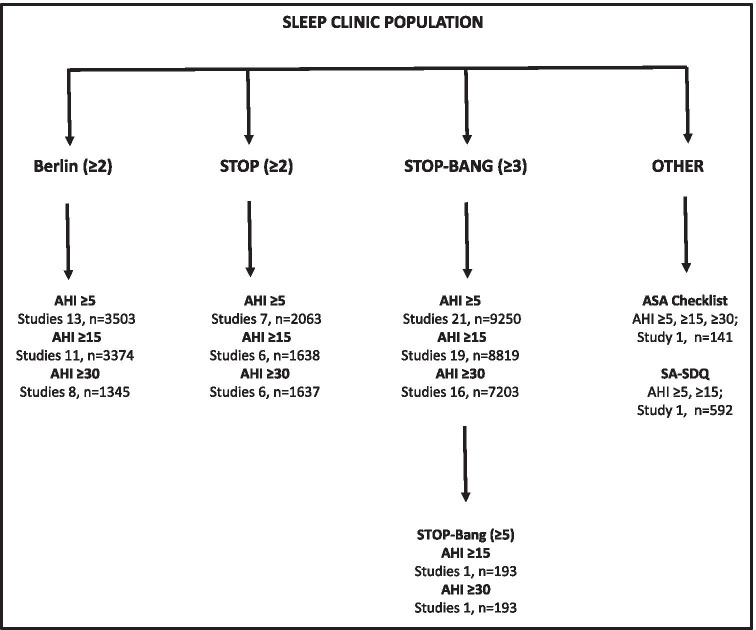
Sleep clinic population
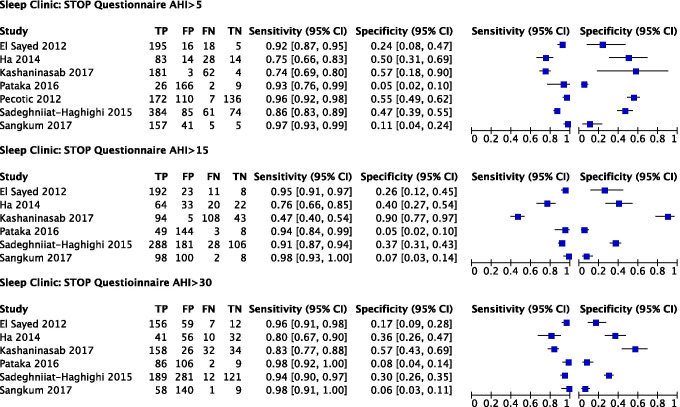
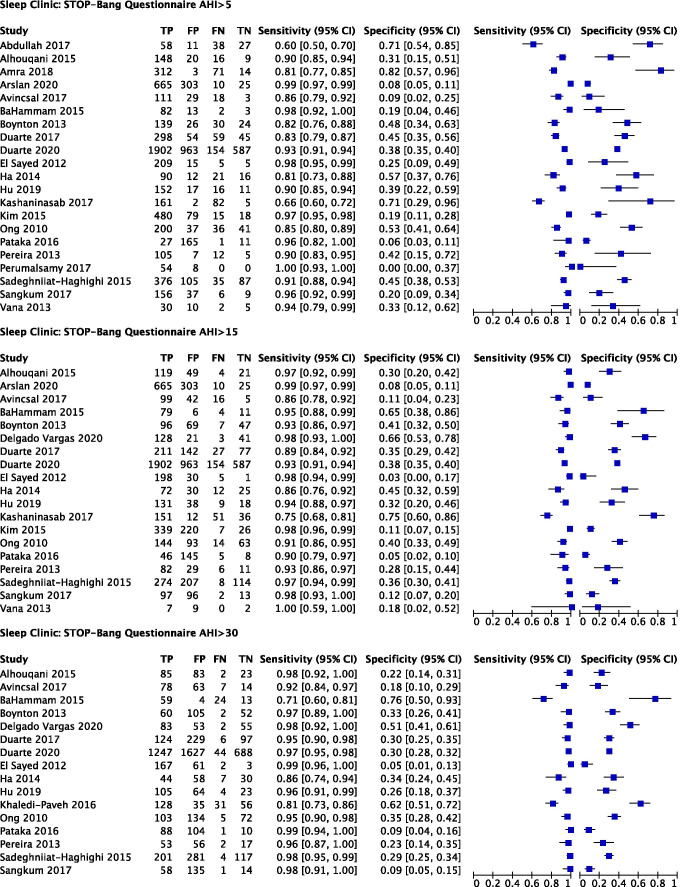
In the sleep clinic population (N = 10,951) (Fig. 3), the Berlin (score cut-off ≥ 2) (Online Resource 5), STOP (score cut-off ≥ 2), and STOP-Bang (score cut-off ≥ 3) (Online Resource 6) questionnaires were included in the meta-analysis [58, 75]. The ASA checklist, SA-SDQ, and STOP-Bang (cut-off ≥ 5) questionnaires were excluded due to insufficient studies.
Fig. 3.
Questionnaire studies in sleep clinic population
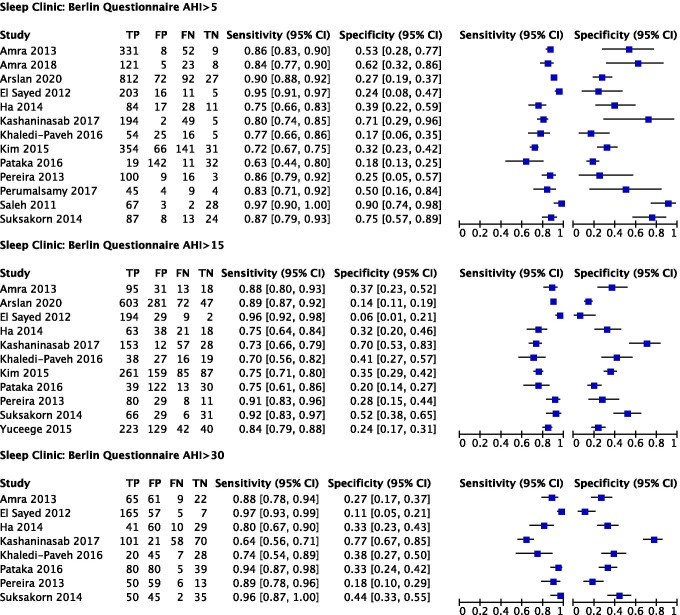
Predictive parameters of the Berlin questionnaire (score cut-off ≥ 2)
The prevalence of AHI ≥ 5 (all OSA), AHI ≥ 15 (moderate to severe), and AHI ≥ 30 (severe) OSA was 84%, 64%, and 50% respectively. The pooled sensitivity of the Berlin questionnaire to predict all OSA, moderate–severe, and severe OSA was 85% (95% confidence interval (CI): 79%, 89%), 84% (95% CI: 79%, 89%), and 89% (95% CI: 80%, 94%) respectively. Pooled sensitivity remained consistent across OSA severity. Pooled specificity was 43% (95% CI: 30%, 58%), 30% (95% CI: 20%, 41%), and 33% (95% CI: 21%, 46%) respectively. The corresponding diagnostic odds ratio (DOR) were 4.3 (95% CI: 0.7, 7.8), 2.3 (95% CI: 1.3, 3.3), and 3.9 (95% CI: 2.1, 5.7) (Fig. 4, Table 3).
Fig. 4.
Forest plots for Berlin questionnaire in sleep clinic population (generated using the software Review Manager 5.3, The Cochrane Collaboration)
Table 3.
Summary statistics for Berlin, STOP, and STOP-Bang questionnaires in the sleep clinic population
| Predictive parameters | Berlin AHI ≥ 5 | STOP AHI ≥ 5 | STOPB AHI ≥ 5 | Berlin AHI ≥ 15 | STOP AHI ≥ 15 | STOPB AHI ≥ 15 | Berlin AHI ≥ 30 | STOP AHI ≥ 30 | STOPB AHI ≥ 30 |
|---|---|---|---|---|---|---|---|---|---|
| 13 studies; n = 3503 | 7 studies; n = 2063 | 21 studies; n = 9250 | 11 studies; n = 3374 | 6 studies; n = 1638 | 19 studies; n = 8819 | 8 studies; n = 1345 | 6 studies; n = 1637 | 16 studies; n = 7203 | |
| Prevalence (% and range) | 83.8 (14.71–97.2) | 66.94 (13.79–97.20) | 79.98 (13.73–97.20) | 64 (25.49–86.72) | 58.42 (25.49–86.75) | 58.78 (25.00–86.75) | 50.11 (27.0–72.65) | 45.94 (28.37–76.00) | 39.25 (28.31–83.0) |
| Sensitivity (95% CI) | 0.848 (0.79, 0.891) | 0.904 (0.824, 0.95) | 0.919 (0.874, 0.949) | 0.843 (0.785, 0.887) | 0.903 (0.754, 0.966) | 0.945 (0.920, 0.963) | 0.886 (0.804, 0.936) | 0.945 (0.883, 0.975) | 0.959 (0.930, 0.976) |
| Specificity (95% CI) | 0.433 (0.296, 0.582) | 0.306 (0.148, 0.528) | 0.345 (0.248, 0.457) | 0.298 (0.204, 0.413) | 0.29 (0.098, 0.606) | 0.271 (0.181, 0.384) | 0.334 (0.211, 0.458) | 0.214 (0.104, 0.391) | 0.282 (0.199, 0.384) |
| False positive rate | 0.567 (0.418, 0.704) | 0.694 (0.472, 0.852) | 0.655 (0.543, 0.752) | 0.702 (0.587, 0.796) | 0.71 (0.394, 0.902) | 0.729 (0.616, 0.819) | 0.666 (0.515, 0.789) | 0.786 (0.609, 0.896) | 0.718 (0.616, 0.801) |
| Log LR + ve (95% CI) | 1.497 (1.066, 1.927) | 1.304 (0.970, 1.637) | 1. 403 (1.232, 1.598) | 1.201 (1.049, 1.353) | 1.273 (0.904, 1.642) | 1.296 (1.125, 1.466) | 1.330 (0.110, 1.550) | 1.203 (1.027, 1.279) | 1.336 (1.184, 1.488) |
| Log LR − ve (95% CI) | 0.350 (0.155–0.546) | 0.312 (0.118–0.506) | 0.235 (0.183, 0.301) | 0.527 (0.361, 0.693) | 0.333 (0.194, 0.472) | 0.203 (0.123, 0.466) | 0.343 (0.210, 0.475) | 0.256 (0.154, 0.357) | 0.146 (0.095, 0.196) |
| DOR (95% CI) | 4.270 (0.718, 7.822) | 4.174 (0.767, 7.581) | 5.969 (4.410, 7.529) | 2.279 (1.309, 3.249) | 3.825 (1.7, 5.949) | 6.383 (3.255, 9.511) | 3.882 (2.06, 5.704) | 4.704 (2.615, 6.794) | 9.168 (5.932, 12.405) |
AHI apnoea-hypopnoea index, STOPB STOP-Bang, CI confidence interval, LR likelihood ratio, DOR diagnostic odds ratio, LR likelihood ratio, + positive, − negative.
Predictive parameters of the STOP questionnaire (score cut-off ≥ 2)
The prevalence of AHI ≥ 5 (all OSA), AHI ≥ 15 (moderate to severe), and AHI ≥ 30 (severe) OSA was 67%, 58%, and 46% respectively. The pooled sensitivity of the STOP questionnaire to predict all OSA, moderate–severe, and severe OSA was 90% (95% CI: 82%, 95%), 90% (95% CI: 75%, 97%), and 95% (95% CI: 88%, 98%) respectively. The pooled specificity was 31% (95% CI: 15%, 53%), 29% (95% CI: 10%, 61%), and 21% (95% CI: 10%, 39%) respectively. The corresponding DOR were 4.2 (95% CI: 0.8, 7.6), 3.8 (95% CI: 1.7, 5.9), and 4.7 (95% CI: 2.6, 6.8) respectively (Fig. 5, Table 3). Greater uncertainty and variability in specificity were noted in the CI width and scatter of individual study estimates.
Fig. 5.
Forest plots for STOP questionnaire in sleep clinic population (generated using the software Review Manager 5.3, The Cochrane Collaboration)
Predictive parameters of the STOP-Bang questionnaire (score cut-off ≥ 3)
The prevalence of AHI ≥ 5 (all OSA), AHI ≥ 15 (moderate to severe), and AHI ≥ 30 (severe) OSA was 80%, 59%, and 39%, respectively. The pooled sensitivity of the STOP-Bang questionnaire to predict all OSA, moderate–severe, and severe OSA was 92% (95% CI: 87%, 95%), 95% (95% CI: 92%, 96%), and 96% (95% CI: 93%, 98%) respectively. The pooled specificity was 35% (95% CI: 25%, 46%), 27% (95% CI: 18%, 34%), and 28% (95% CI: 20%, 38%) respectively. The corresponding DOR were 6.0 (95% CI: 4.4, 7.6), 6.4 (95% CI: 3.3, 9.5), and 9.2 (95% CI: 5.9, 12.4) respectively (Fig. 6, Table 3). Greater uncertainty and variability in specificity were noted in the CI width and scatter of individual trial estimates, particularly for AHI ≥ 5.
Fig. 6.
Forest plots for STOP-Bang questionnaire in sleep clinic population (generated using the software Review Manager 5.3, The Cochrane Collaboration)
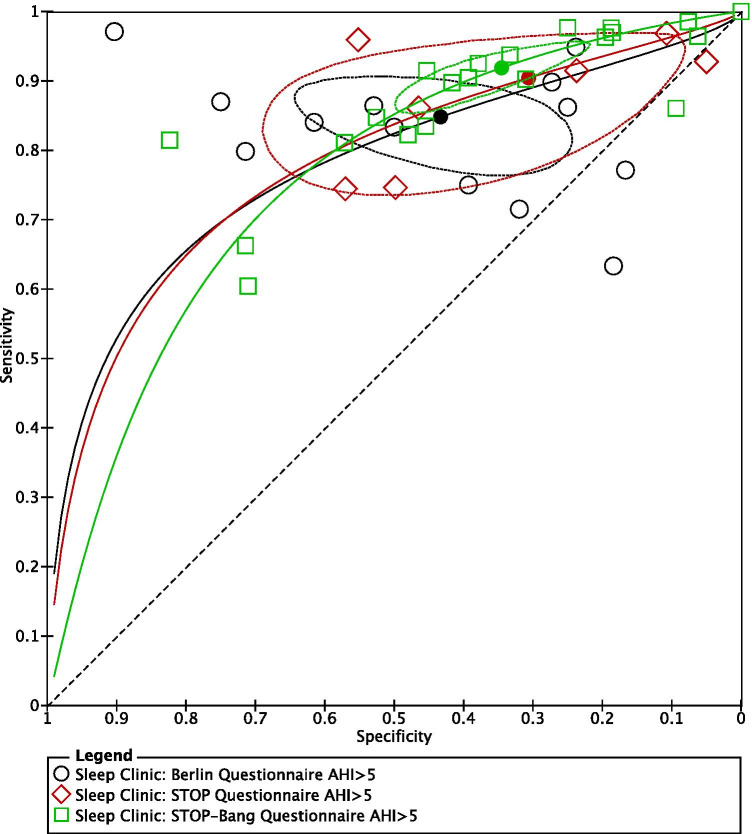
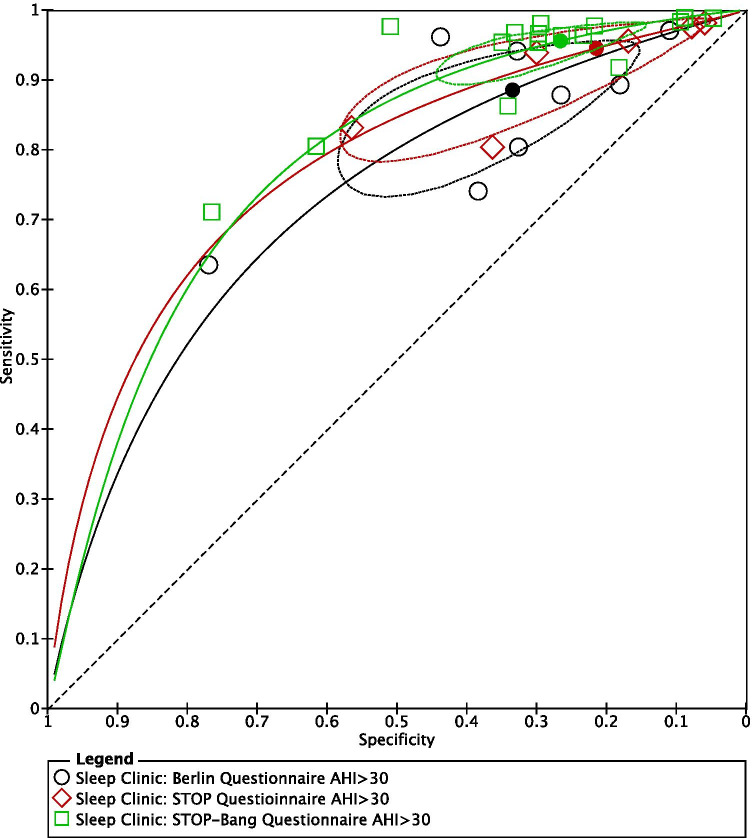
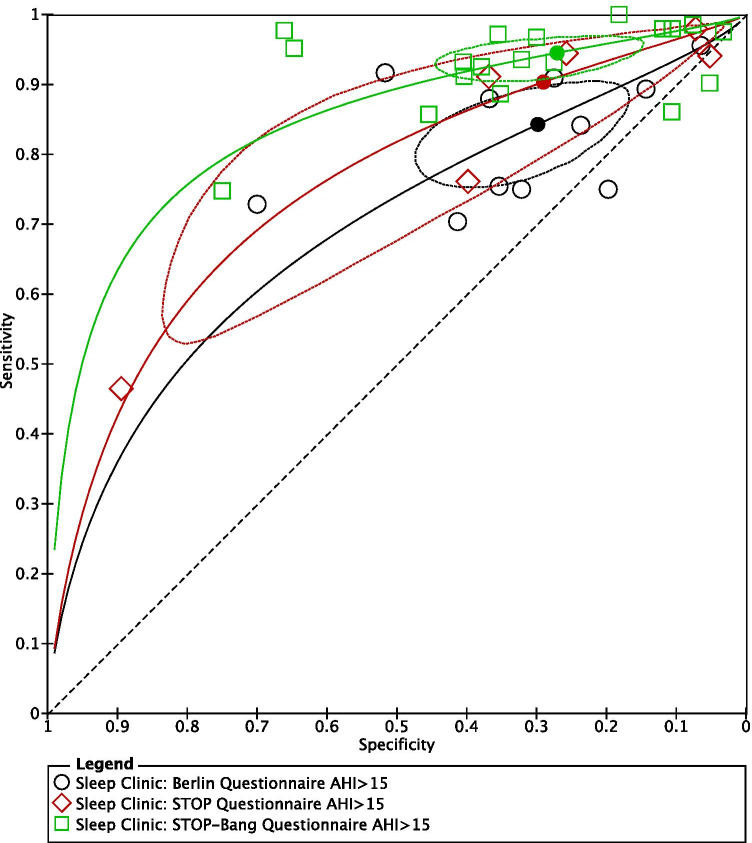
SROC plots were used to display the results of individual questionnaires in the ROC space, plotting each questionnaire as a single sensitivity–specificity point [24]. When we plotted the SROC for all three questionnaires on the same axes, the confidence regions of the Berlin, STOP, and STOP-Bang questionnaires, for all OSA (AHI ≥ 5) (Fig. 7) and severe OSA (AHI ≥ 30) (Fig. 9), overlapped, suggesting that there was no statistically significant difference in sensitivity among the 3 questionnaires.
Fig. 7.
Summary ROC for Berlin, STOP, and STOP-Bang questionnaires AHI ≥ 5 (generated using the software Review Manager 5.3, The Cochrane Collaboration)
Fig. 9.
Summary ROC for Berlin, STOP, and STOP-Bang questionnaires AHI ≥ 30 (generated using the software Review Manager 5.3, The Cochrane Collaboration)
Figure 8 shows no overlap of the confidence regions for the Berlin and STOP-Bang questionnaires, suggesting a possible difference in sensitivity between the two questionnaires. A meta-regression model assuming equal variances for logit sensitivity and logit specificity suggested that the expected sensitivity or specificity differed between the two tests (chi-square = 14.1, 2df, p = 0.0008) (Fig. 9).
Fig. 8.
Summary ROC for Berlin, STOP, and STOP-Bang questionnaires AHI ≥ 15 (generated using the software Review Manager 5.3, The Cochrane Collaboration)
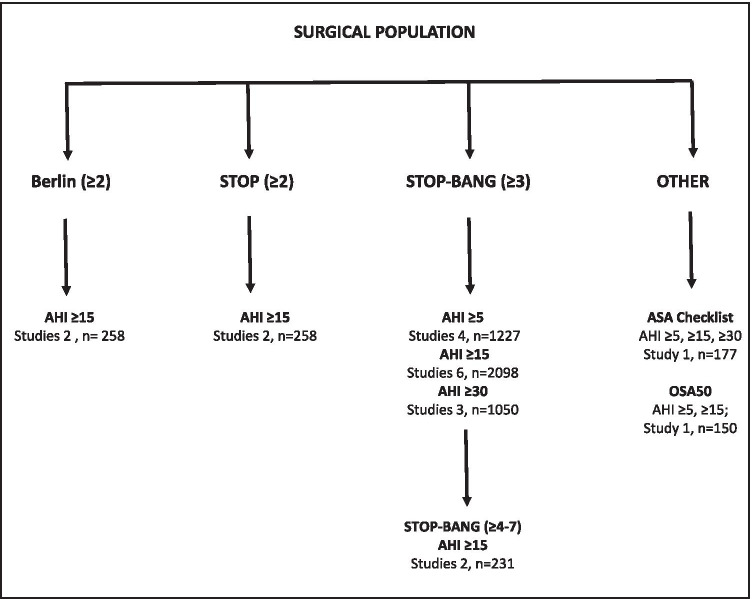
Surgical population
In the surgical population (n = 2710) (Fig. 10), we identified the Berlin, STOP, and STOP-Bang questionnaires for inclusion in the meta-analysis. The ASA checklist and OSA50 questionnaires were excluded from meta-analysis due to an insufficient number of studies. Nunes included two surgical cohorts, abdominal and coronary artery bypass grafting, which were entered as separate cohorts [63].
Fig. 10.
Questionnaire studies in surgical population
Predictive parameters of the Berlin questionnaire (score cut-off ≥ 2)
Two studies were included in the meta-analysis of the Berlin Questionnaire for moderate to severe OSA (AHI ≥ 15) (Fig. 11). Due to insufficient data, we were unable to conduct a meta-analysis for all (AHI > 5) and severe OSA (AHI > 30).
Fig. 11.
Forest plot for Berlin questionnaire in surgical population for AHI ≥ 15 (generated using the software Review Manager 5.3, The Cochrane Collaboration)
The prevalence of moderate to severe OSA or AHI of ≥ 15 was 42%. The pooled sensitivity of the Berlin questionnaire to predict moderate to severe OSA (AHI ≥ 15) was 76% (95% CI: 66%, 84%), and the pooled specificity was 47% (95% CI: 32%, 62%). The DOR was 2.9 (95% CI: 0.2, 5.5) (Table 4).
Table 4.
Pooled predictive parameters of Berlin and STOP-Bang questionnaires in surgical population for AHI ≥ 15
| Parameter (95% CI) | Berlin, AHI ≥ 15 | STOP-Bang, AHI > 5 | STOP-Bang, AHI ≥ 15 | STOP-Bang, AHI ≥ 30 |
|---|---|---|---|---|
| 2 studies, n = 258 | 4 studies, n = 1227 | 6 studies, n = 2098 | 3 studies, n = 1050 | |
| Prevalence (% and range) | 41.86 (39.55–52.50) | 71.56 (67.05–89.33) | 32.79 (17.22–62.00) | 21.24 (16.67–42.00) |
| Sensitivity (95% CI) | 0.764 (0.661, 0.843) | 0.846 (0.811, 0.876) | 0.903 (0.871, 0.927) | 0.96 (0.924, 0.979) |
| Specificity (95% CI) | 0.468 (0.320, 0.623) | 0.394 (0.298, 0.498) | 0.269 (0.189, 0.367) | 0.261 (0.232, 0.292) |
| False positive rate (95% CI) | 0.532 (0.377, 0.680) | 0.606 (0.502, 0.702) | 0.731 (0.633, 0.811) | 0.739 (0.708, 0.768) |
| Log LR + ve (95% CI) | 1.437 (0.929, 1.945) | 1.395 (1.187, 1.603) | 1.235 (1.093, 1.377) | 1.299 (1.236, 1.362) |
| Log LR − ve (95% CI) | 0.504 (0.206, 0.802) | 0.391 (0.304, 0.479) | 0.361 (0.235, 0.487) | 3.169 (2.502, 3.836) |
| DOR (95% CI) | 2.849 (0.194, 5.505) | 3.564 (2.311, 4.817) | 3.421 (1.892, 4.949) | 8.406 (2.65, 14.162) |
CI confidence interval, LR likelihood ratio, DOR diagnostic odds ratio, + positive, − negative.
Predictive parameters of the STOP questionnaire (score cut-off ≥ 2)
Two studies were eligible for inclusion in the STOP questionnaire meta-analysis for moderate to severe OSA (AHI ≥ 15). However, due to insufficient studies and large heterogeneity around the specificity, the STOP questionnaire was excluded from the meta-analysis (Fig. 12).
Fig. 12.
Forest plot for STOP questionnaire in surgical population for AHI ≥ 15 (generated using the software Review Manager 5.3, The Cochrane Collaboration)
Predictive parameters of the STOP-Bang questionnaire (score cut-off ≥ 3)
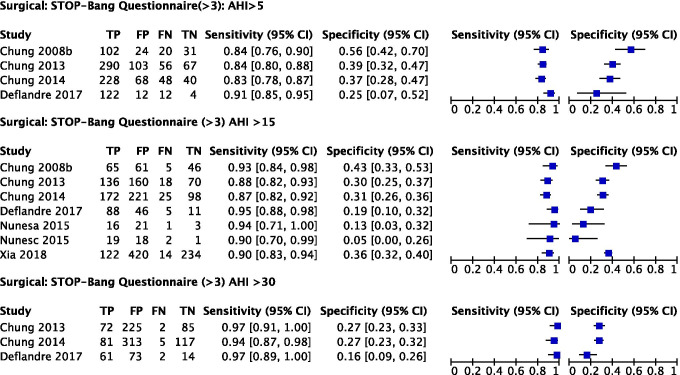
We included 6 studies in the meta-analysis of the STOP-Bang questionnaire for moderate to severe OSA (AHI ≥ 15) (Fig. 13).
Fig. 13.
Forest plots for STOP-Bang questionnaire in surgical population (generated using the software Review Manager 5.3, The Cochrane Collaboration)
The prevalence of AHI ≥ 5 (all OSA), AHI ≥ 15 (moderate to severe), and AHI ≥ 30 (severe) OSA was 72%, 33%, and 21%, respectively. The pooled sensitivity of the STOP-Bang questionnaire to predict all OSA, moderate–severe, and severe OSA was 85% (95% CI: 81%, 88%), 90% (95% CI: 87%, 93%), and 96% (95% CI: 92%, 98%) respectively. The pooled specificity was 40% (95% CI: 30%, 50%), 27% (95% CI: 19%, 37%), and 26% (95% CI: 21%, 46%). The corresponding DOR were 3.6 (95% CI: 2.3, 4.8), 3.4 (95% CI: 1.9, 4.9), and 8.4 (95% CI: 2.7, 14.2), respectively (Table 4). Compared to the Berlin and STOP questionnaires, individual trial estimates of sensitivity appeared to be more homogeneous for the STOP-Bang questionnaire (Figs. 11, 12, and 13).
Predictive performance of STOP-Bang questionnaires at various questionnaire scores
In the surgical population, two of six studies reported data at multiple cut-off points for the STOP-Bang questionnaire for moderate-to-severe OSA (AHI ≥ 15) [62, 63]. Increasing the threshold from 4 to 7 increased specificity from 31% (95% CI: 0.2, 0.4) to 96% (95% CI: 0.89, 0.99) and was greatest at cut-off values ≥ 6 and ≥ 7 (Table 5). However, increase in specificity was at the expense of a reduction in sensitivity.
Table 5.
STOP-Bang questionnaire at different questionnaire cut-offs for moderate–severe OSA (AHI ≥ 15) in the surgical population
| Predictive parameters | SBQ ≥ 4, AHI ≥ 15 | SBQ ≥ 5, AHI ≥ 15 | SBQ ≥ 6, AHI ≥ 15 | SBQ ≥ 7, AHI ≥ 15 |
|---|---|---|---|---|
| 2 studies; n = 231 | ||||
| Prevalence (% and range) | 56.71 (41.46–62) | |||
| Sensitivity (95% CI) | 0.893 (0.828, 0.936) | 0.640 (0.480, 0.774) | 0.372 (0.211, 0.567) | 0.120 (0.033, 0.353) |
| Specificity (95% CI) | 0.310 (0.227, 0.407) | 0.575 (0.458, 0.684) | 0.807 (0.676, 0.894) | 0.958 (0.889, 0.985) |
| False positive rate (95% CI) | 0.690 (0.593, 0.773) | 0.425 (0.316, 0.542) | 0.193 (0.106, 0.324) | 0.042 (0.015, 0.111) |
| Log LR + ve (95% CI) | 1.294 (1.108, 1.481) | 1.505 (1.052, 1.958) | 1.930 (0.859, 3.000) | 2.875 (− 2.580, 7.647) |
| Log LR − ve (95% confidence interval) | 0.345 (0.147, 0.543) | 0.627 (0.372, 0.881) | 2.481 (0.547, 4.414) | 0.918 (0.756, 1.080) |
| DOR (95% CI) | 3.755 (1.135, 6.374) | 2.402 (0.763, 4.041) | 8.406 (2.650, 14.162) | 3.131 (− 2.580, 8.841) |
CI confidence interval, LR likelihood ratio, DOR diagnostic odds ratio, + positive, − negative, OSA obstructive sleep apnoea, AHI apnoea-hypopnoea index.
Resistant hypertension population
We included 2 studies (n = 517) in the meta-analysis of the Berlin questionnaire (cut-off ≥ 2) for all OSA (AHI of ≥ 5) [65, 66]. Due to insufficient study data, we were unable to conduct a meta-analysis for moderate–severe (AHI > 15) and severe OSA (AHI > 30).
The prevalence of all OSA or an AHI of ≥ 5 was 80%. The Berlin questionnaire’s pooled sensitivity to predict all OSA or AHI of ≥ 5 was 80% (95% CI: 60%, 92%), and the pooled specificity was 36% (95% CI: 21%, 55%). The DOR was 2.2 (95% CI: 0.7, 3.8).
Other cohorts
Asthma, community clinic, highway bus drivers, neurology clinic, primary care, respiratory and snoring clinic cohorts were identified but were excluded from the meta-analysis due to having only one study per cohort (Online Resource 7) [67–73].
Sensitivity analyses
Risk of bias
No studies were evaluated as high risk in the surgical and resistant hypertension populations; therefore, no sensitivity analyses were conducted.
In the sleep clinic population, sensitivity analyses were conducted for the Berlin (Online Resource 8), STOP-Bang (Online Resource 9), and the STOP questionnaires for AHI > 5, AHI ≥ 15, and AHI ≥ 30 (Online Resource 10) excluding studies identified as high risk in any QUADAS-2 domain, unclear in four domains or outliers.
We excluded one study for the STOP questionnaire for AHI > 5 [49], AHI ≥ 15 [44], and AHI ≥ 30 [44]. For the STOP-Bang questionnaire, we excluded five studies for AHI > 5 [29–31, 34, 46] and four studies for AHI ≥ 15 [30, 35, 38, 44] and AHI ≥ 30 [30, 35, 38, 44]. For the Berlin questionnaire AHI > 5 [45, 46, 53, 55] and AHI ≥ 15 [45, 46, 53, 55], we excluded four studies, and for an AHI ≥ 30 [44, 45, 55], we excluded three studies.
Across all three questionnaires, exclusion of studies was associated with stable or slightly increased sensitivity. In contrast, sensitivity analysis was associated with reduced specificity (Online Resources 8–10). The STOP-Bang questionnaire remained the most effective questionnaire with the highest sensitivity compared to the Berlin and STOP questionnaires. Specificity among all three questionnaires remained low.
Desaturation and arousal criteria
Due to an insufficient number of studies, no sensitivity analysis was conducted in the resistant hypertension population.
In the surgical population, the Berlin and STOP questionnaire studies utilised the ≥ 3% desaturation scoring criteria; therefore, no sensitivity analyses were conducted. For the STOP-Bang questionnaire, studies applied either ≥ 3% or ≥ 4% desaturation criteria. When we applied the ≥ 3% desaturation criteria to the STOP-Bang questionnaire, we excluded one study for AHI > 5 [60], two studies for AHI ≥ 15 [60, 64], and one study for AHI ≥ 30 [60]. In turn, when we applied the ≥ 4% desaturation criteria, we excluded four studies for AHI ≥ 15 [59, 61–63]. Across the three AHI thresholds, sensitivity remained stable, compared to a stable or slightly decreased sensitivity with application of the ≥ 3% desaturation criteria. For AHI ≥ 15, application of the ≥ 4% desaturation criterion was associated with a slight reduction in sensitivity and an increase in specificity (Online Resource 11).
We conducted a sensitivity analysis in the sleep clinic population for the Berlin, STOP, and STOP-Bang questionnaires, applying both the ≥ 3% and ≥ 4% desaturation criteria respectively. Studies were excluded on the basis of high risk of bias, scoring criteria not specified, and desaturation criteria (≥ 3% or ≥ 4%) (Online Resource 12).
Across all three questionnaires in the sleep clinic population, exclusion of studies was associated with stable sensitivity and reduced specificity, particularly when applying the ≥ 4% desaturation criterion (Online Resources 13, 14, 15). Overall, the STOP-Bang questionnaire remained the most effective questionnaire with the highest sensitivity compared to the Berlin and STOP questionnaires. Specificity among all three questionnaires remained low.
Discussion
This systematic review and meta-analysis investigated questionnaires’ accuracy and clinical utility as screening tools for OSA in adults in different clinical cohorts.
Consistent with previous studies, our findings showed that the STOP-Bang questionnaire (score cut-off ≥ 3) suggested the highest sensitivity to detect OSA and the highest diagnostic odds ratio in both the sleep clinic and surgical populations [12, 18, 76]. However, the STOP-Bang questionnaire was limited by consistently low specificity across all AHI thresholds, resulting in high false positive rates. The Berlin questionnaire (score cut-off ≥ 2) appeared to be the least useful, demonstrating overall low sensitivity and low specificity across all three cohorts [12, 18, 77]. Although there was no comparison with other questionnaires in the resistant hypertension cohort, findings were comparable with the sleep clinic and surgical cohorts.
OSA screening questionnaires are intended to provide the information required to identify patients most likely to benefit from downstream management decisions, such as onward referral for objective sleep testing and possible treatment following a positive full diagnostic test. The potential utility of OSA screening questionnaires in risk stratification of patients has been demonstrated in several cohorts. Not only has OSA been associated with risk of peri-operative complications and consequent longer length of hospital stay, but it has also been linked to poor clinical outcomes including higher rates of post CABG atrial fibrillation [78–80]. In the context of the ongoing coronavirus disease 2019 (Covid-19) pandemic, a recent study reported worse clinical outcomes in patients with Covid-19 classified by the Berlin questionnaire as high risk, compared to those at low risk, of OSA [81]. The study also highlighted the challenges with objective assessment of OSA with PSG during the Covid-19 pandemic, emphasising the need for alternative approaches beyond PSG, such as validated screening questionnaires. In this context, we would encourage the assessment and validation of OSA screening questionnaires, in particular STOP-Bang, as screening tools for risk stratification appropriate clinical settings, with the aim of improving outcomes for patients.
Although sensitivity and specificity provide us with the necessary information to discern between the available screening questionnaires, the clinical value and application of the screening questionnaires are demonstrated by means of the positive and negative predictive values which are dependent on the prevalence of the disease in the given clinical population. Although we were unable to pool the predictive values of individual questionnaires due to variation in prevalence across studies, the point estimates of PPV and negative predictive value (NPV) for the STOP-Bang questionnaire in both the sleep clinic and surgical population (Online Resource 16) demonstrated an increase in NPV as OSA severity increases. The combination of high sensitivity and NPV of the STOP-Bang questionnaire is therefore useful to help clinicians exclude patients with low risk of clinically significant OSA.
At the same time, the low specificity of the STOP-Bang questionnaire (and therefore its relative inability to correctly identify patients without OSA) leads to a high rate of false positive findings; this may have emotional and cognitive implications for individual patients with added consequences for clinical services, not least cost [80, 82].
This systematic review’s main strength lies in our comprehensive literature search with stringent eligibility criteria to identify all relevant studies reporting on the accuracy and clinical utility of existing OSA screening questionnaires that were validated against the gold standard PSG. Our inclusion of the LILACS database expanded our search to include Latin America and the Caribbean studies. Of previous reports, the review by Ramachandran [17] was limited to a search of two databases, English publications only, and omitted any grey literature sources in their search strategy. Additionally, it was unclear if Ross [16] and Abrishami [12] included any grey literature sources in their searches.
Two independent reviewers completed data extraction, and we used the QUADAS-2 tool to assess rigorously all included studies for risk of bias. To evaluate the robustness of the meta-analysis, we conducted sensitivity analyses to investigate the potential influence on our findings from studies at high, or unclear, risk of bias. Although our study did not explore source differences from an ethnicity or geographical perspective, we conducted a further sensitivity analysis to evaluate the impact of varying scoring criteria on our study findings. The utilisation of different AASM scoring criteria and desaturation (and arousal) thresholds across studies created a source of variability [83–85]. Although the definition for apnoeas remained stable, there has been much controversy about the definition of hypopnoeas, specific to flow reduction, oxygen desaturation, and the presence or absence of arousal [86]. Varying definitions of hypopnoea not only impacts on prevalence estimates but is likely to underestimate OSA in patients who may benefit from treatment [86]. A study by Guilleminault et al. (2009) showed that by using the 30% flow reduction and 4% desaturation without arousal criteria would have missed 40% of patients who were identified using the criteria with arousal and who were responsive to CPAP therapy with reduction in AHI and symptomatic improvement [87].
On this background, our review is based on a larger number of studies than prior analyses [12, 16, 17]. Although the review by Chiu [18] encompassed a larger dataset, that report carried a greater risk of bias due to the inclusion of retrospective studies and studies that used PSG and portable monitoring as the reference standard.
This review considered all existing OSA screening questionnaires for inclusion. In contrast, Chui [18] pre-selected four questionnaires, including the ESS, which was not developed as a screening questionnaire, but as a measure of daytime sleepiness.
Similar to Abrishami [12] and Chui [18], our review focused on questionnaires only, in contrast to Ross [16] and Ramachandran [17], who also included portable monitoring and clinical prediction tools, respectively.
There are a number of limitations to this work. Our findings are influenced by the limitations of the included studies. In several, the true risk of bias was unclear in several of the QUADAS-2 domains due to underreporting in the index test, reference standard, and flow and timing domains. Similarly, it was often unclear if the results of the index test and the reference standard were interpreted independently. Very few studies provided adequate information to determine if the time interval between the index test and the reference standard was appropriate.
Our decision to exclude seven additional clinical cohorts may be considered a limitation; however, in the context of unclear, and possibly substantial, differences among these studies in the patient spectrum and disease prevalence, we felt it appropriate not to include these in the meta-analysis. Because the accuracy of screening tools varies according to the spectrum of disease, this further reiterates the need for validation studies in similar clinical cohorts.
There was a high degree of heterogeneity among included studies with the possibility of selection bias, especially in the sleep clinic population. Consequently, reported sensitivity estimates will be higher than lower-risk populations, making it difficult to extrapolate the true utility of the questionnaire in clinical practice.
In conclusion, our review investigated the accuracy and clinical utility of existing OSA screening questionnaires in different clinical cohorts. While the STOP-Bang questionnaire had a high sensitivity to detect OSA in both the sleep clinic and surgical cohorts, it lacked adequate specificity. This review highlights the issue of low specificity across OSA screening questionnaires. Research is required to explore reasons for low specificity and strategies for improvement, ideally without reducing sensitivity. The validation of screening questionnaires in sleep clinic populations is limited by possible selection and spectrum bias, reiterating the need for diagnostic validation studies in clinically similar cohorts. Additionally, further research is needed in resistant hypertension and other at-risk populations that we could not include in the meta-analysis. Improvement in the conduct and reporting of diagnostic validation studies must ensure quality and low risk of bias.
Finally, to enable the extrapolation of the true accuracy and clinical utility of screening questionnaires, validation studies of high methodological quality in comparable, clinically relevant cohorts are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AHI
Apnoea-hypopnoea index
- CI
Confidence interval
- DOR
Diagnostic odds ratio
- FN
False negative
- FP
False positive
- NPV
Negative predictive values
- OSA
Obstructive sleep apnoea
- PPV
Positive predictive values
- PRISMA
Reporting Items for Systematic Reviews and Meta-analysis
- PSG
Polysomnography
- QUADAS
Quality Assessment for Diagnostic Accuracy Studies
- RDI
Respiratory disturbance index
- ROC
Receiver operating characteristic
- SROC
Summary receiver operating characteristic
- TN
True negative
- TP
True positive
Author contribution
Protocol development: LB, EMB, NR, IBS, GDC.
Selection of studies and data extraction: LB, EMB, HP, JCR, NR, IBS.
Quality assessment: LB, EMB, HP, JCR, NR, IBS.
Statistical analysis and interpretation of data: LB, EMB, SCF, NR, IBS.
Drafting of the manuscript: LB, EMB, SCF, NR, IBS.
Critical revision: LB, EMB, SCF, HP, JCR, CFM, GDC, NR, IBS.
Guarantor(s) of the paper: LB, IS.
All authors have seen and approved the manuscript.
Funding
This study was co-funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM), now recommissioned as NIHR Applied Research Collaboration East Midlands (ARC EM) and Leicestershire Partnership NHS Trust—Raising Health Charitable Funds Award and supported by the NIHR Leicester Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Data availability
Available on request.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Notation of prior abstract publication/presentation
Part of this article was presented as a poster at the World Sleep Congress, 22 September 2019, Vancouver, Canada, and MEMTAB2020 Virtual Symposium on 10 December 2020.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunex CM, Patel SR, Penzel T, Pepin JL, Peppard P, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(10):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Pepperell JCT, Davies RJO, Stradling JR. Systemic hypertension and obstructive sleep apnoea. Sleep Med Rev. 2002;6(3):157–173. doi: 10.1053/smrv.2001.0189. [DOI] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 5.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57(2):119–127. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 6.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnoea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–76. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayas, N.T., Taylor, C.M. & Laher, I. (2016) Cardiovascular consequences of OSA. Curr Opinion Cardiol. (6), 599–605. Available from: 10.1097/HCO.0000000000000329 [DOI] [PubMed]
- 8.Moore, A. (2012) Primary care supplement: the big sleep problem. Health Service J. Available from: https://www.hsj.co.uk/supplement-archive/primary-care-supplement-the-big-sleep-problem/5065470.article. Accessed 17 Mar 2017
- 9.NHS North of England Specialist Commissioning Group (NW office) 2012: Sleep-related breathing disorders
- 10.Morsy, N.E., Farrag, N.S., Zaki, N.F.W., Badasy, A.Y., Abdelhafez, S.A., El-Gilany, a.H., El Shafey, M.M., Pandi-Perumal, S.R., Spence, D.W. & BaHammam, A.S. (2019) Obstructive sleep apnea: personal, societal, public health, and legal implications. Rev Environ Health. 34(2):153–169. 10.1515/reveh-2018-0068 [DOI] [PubMed]
- 11.Rejón-Parrilla, J.C., Garau, M. and Sussex, J. (2014) Obstructive sleep apnoea health economics. Consulting Report for the British Lung Foundation. Available from: https://www.ohe.org/publications/obstructive-sleep-apnoea-health-economics-report. Accessed 30 Jun 2017
- 12.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea/Une revue méthodique des questionnaires de dépistage de l’apnée obstructive du sommeil. Can J Anesth. 2010;57:423–438. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 13.Costa LE, Uchôa CHG, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101(16):1288–1292. doi: 10.1136/heartjnl-2014-307276. [DOI] [PubMed] [Google Scholar]
- 14.Malaweera A.S.N, Riley J.P., Simonds, A.D.& Cowie, M.C. (2015) Barriers to diagnosis and treatment of sleep disordered breathing in patients with heart failure: perception and experiences of healthcare professionals. Eur J Heart Failure. 17, 171. Available from: ISSN: 1388–9842
- 15.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23(7):929–938. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 16.Ross SD, Sheinhait IA, Harrison KJ, Kvasz M, Connelly JE, Shea SA, Allen IE. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23(4):1–14. doi: 10.1093/sleep/23.4.1f. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110:928–939. doi: 10.1097/ALN.0b013e31819c47b6. [DOI] [PubMed] [Google Scholar]
- 18.Chiu H-Y, Chen P-Y, Chuang L-P, Chen N-H, Tu Y-K, Hsieh Y-J, Wang Y-C, Guilleminault C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann Intern Med. 2002;137:598–602. doi: 10.7326/0003-4819-137-7-200210010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T., Mulrow C.D., Shamseer L. & Moher D. (2020) Updating guidelines for reporting systematic reviews and meta-analyses: development of the PRISMA 2020 statement. Advances in Evidence Synthesis: special issue Cochrane Database of Systematic Reviews. 9 (supplement 1) 10.1002/14651858.CD202001
- 21.Freeman, S.C., Kerby, C.R., Patel, A., Cooper, N.J., Quinn T. & Sutton, A.J. (2019) Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med Res Methodol.19 (81). Available from: 10.1186/s12874-019-0724-x [DOI] [PMC free article] [PubMed]
- 22.RevMan. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014
- 23.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MMG, Sterne JAC, Bossuyt PMM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Macaskill, P., Gatsonis, C., Deeks, J.J., Harbord, R.M. & Takwoingi, Y. Chapter 10: analysing and presenting results. In: Deeks J.J., Bossuyt P.M., Gatsonis C. (2010) Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0. The Cochrane Collaboration. Available from: https://methods.cochrane.org/sdt/handbook-dta-reviews. Accessed 16 Mar 2018
- 25.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalised linear mixed model approach. J Clin Epidemiol. 2006;12:1331–1332. doi: 10.1016/j.jclinepi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8:239–251. doi: 10.1093/biostatistics/kxl004. [DOI] [PubMed] [Google Scholar]
- 28.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 29.Abdullah B, Idris AI, Mohammad ZW, Mohamad H. Validation of Bahasa Malaysia STOP-BANG questionnaire for identification of obstructive sleep apnea. Sleep and Breath. 2018;22:1235–1239. doi: 10.1007/s11325-018-1663-1. [DOI] [PubMed] [Google Scholar]
- 30.Alhouqani, S., Al Manhali, M., Al Essa, A. & Al-Houqani, M. (2015) Evaluation of the Arabic version of STOP-Bang questionnaire as a screening tool for obstructive sleep apnea. Sleep Breathing. 9(4), 1235–1240. Available from: http://link.springer.com/10.1007/s11325-015-1150-x [DOI] [PubMed]
- 31.Amra B, Nouranian E, Golshan M, Fietze I, Penzel T. Validation of the Persian version of Berlin Sleep Questionnaire for diagnosing obstructive sleep apnea. Int J Prev Med. 2013;4(3):334–339. [PMC free article] [PubMed] [Google Scholar]
- 32.Amra B, Javani M, Soltaninejad F, Penzel T, Fietze I, Schoebel C, Farajzadegan Z. Comparison of Berlin questionnaire, STOP-Bang, and Epworth sleepiness scale for diagnosing obstructive sleep apnea in Persian patients. Int J Prev Med. 2018;9:1–5. doi: 10.4103/ijpvm.ijpvm_131_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oktay Arslan B, UçarHoşgör ZZ, Orman MN. Which screening questionnaire is best for predicting obstructive sleep apnea in the sleep clinic population considering age, gender, and comorbidities? Turkish Thoracic J. 2020;21(6):383–389. doi: 10.5152/TurkThoracJ.2019.19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avincsal, M.O., Dinc, M.E., Ulusoy, S., Dalgic, A., Ozdemir, C. & Develioglu, O.N. (2017) Modified Mallampati score improves specificity of STOP-BANG questionnaire for obstructive sleep Apnea. J Craniofacial Surg. 28(4), 904–908. 10.1097/SCS.0000000000003513 [DOI] [PubMed]
- 35.BaHammam, A.S., Al-Aqueel, A.M., Alhedyani, A.A., Al-Obaid, G.I., Al-owais, M.M. & Olaish, A.H. (2015) The validity and reliability of an Arabic version of the STOP-Bang Questionnaire for identifying obstructive sleep apnea. The Open Respiratory Medicine Journal. 9(1), 22–9. Available from: http://benthamopen.com/ABSTRACT/TORMJ-9-22 [DOI] [PMC free article] [PubMed]
- 36.Boynton, G., Vahabzadeh, Hammoud, S., Ruzicka, D.L. & Chervin, R.D. (2013) Validation of the STOPBANG Questionnaire among patients referred for suspected obstructive sleep apnea. Journal of Sleep Disorders: Treatment & Care. 2(4). Available at: 10.4172/2325-9639.1000121 [DOI] [PMC free article] [PubMed]
- 37.Deflandre E, Piette N, Bonhomme V, Degey S, Cambron L, Poirrier R, Richant J-F, Joris J. Comparison of clinical scores in their ability to detect hypoxemic severe OSA patients. PLoS ONE. 2018;13(5):1–11. doi: 10.1371/journal.pone.0196270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado-Vargas B, Acle-Cervera L, Narciso López G. Validation of the Spanish version of the STOP-Bang Questionnaire: usefulness as a screening tool for obstructive sleep apnea in adults. Ear Nose Throat J. 2020;18:1–7. doi: 10.1177/0145561320932334. [DOI] [PubMed] [Google Scholar]
- 39.Duarte, R.L.M. de M., Fonseca, L.B. de M., Magalhães-da-Silveira, F.J., Da Silveira, E. A. & Rabahi, M.F. (2017) Validation of the STOP-Bang questionnaire as a means of screening for obstructive sleep apnea in adults in Brazil [Validação do questionário STOP-Bang para a identificação de apneiaobstrutiva do sono em adultos no Brasil]. J Brasileiro Pneumol. 43(6), 456–463. Available from: 10.1590/s1806-37562017000000139 [DOI] [PMC free article] [PubMed]
- 40.Duarte RL, Magalhães-da-Silveira FJ, Oliveira-E-Sá TS, Silva JA, Mello FC, Gozal D. Obstructive sleep apnea screening with a 4-item instrument, named GOAL questionnaire: development, validation and comparative study with No-Apnea, STOP-Bang, and NoSAS. Nat Sci Sleep. 2020;23(12):57–67. doi: 10.2147/NSS.S238255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Sayed IH. Comparison of four sleep questionnaires for screening obstructive sleep apnea. Egypt J Chest Dis Tuberc. 2012;61(4):433–441. doi: 10.1016/j.ejcdt.2012.07.003. [DOI] [Google Scholar]
- 42.Ha SCN, Lee DLY, Abdullah VJ, Van Hasselt CH. Evaluation and validation of four translated Chinese questionnaires for obstructive sleep apnea patients in Hong Kong. Sleep Breathing. 2014;18(4):715–721. doi: 10.1007/s11325-013-0889-1. [DOI] [PubMed] [Google Scholar]
- 43.Hu YY, Yu Y, Wang ZB, Liu C, Cui YH, Xiao WM. Reliability and validity of simplified Chinese STOP-BANG questionnaire in diagnosing and screening obstructive sleep apnea hypopnea syndrome. Curr Med Sci. 2019;39(1):127–133. doi: 10.1007/s11596-019-2010-x. [DOI] [PubMed] [Google Scholar]
- 44.Kashaninasab F, Alavi K, Farhadi M, Salehi M, Bandi MFGA. Comparative study of four Persian versions of sleep questionnaires for screening obstructive sleep apnea syndrome (OSAS) Med J Islamic Repub Iran. 2017;31(1):810–816. doi: 10.1007/s11596-019-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khaledi-Paveh B, Khazzaie H, Nasouri M, Ghadami MR, Tahmasian M. Evaluation of Berlin questionnaire validity for sleep apnea risk in sleep clinic populations. Basic Clin Neurosci. 2016;7(1):43–48. [PMC free article] [PubMed] [Google Scholar]
- 46.Kim B, Lee EM, Chung Y-S, Kim W-S, Lee S-A. The utility of three screening questionnaires for obstructive sleep apnea in a sleep clinic setting. Yonsei Med J. 2015;56(3):684–690. doi: 10.3349/ymj.2015.56.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong TH, Raudha S, Fook-Chong S, Lew N, Hsu AAL. Simplifying STOP-BANG: use of a simple questionnaire to screen for OSA in an Asian population. Sleep Breathing. 2010;14(4):371–376. doi: 10.1007/s11325-010-0350-7. [DOI] [PubMed] [Google Scholar]
- 48.Pataka A, Zarogoulidis P, Hohenforst-Schmidt W, Tsiouda T, Tsavlis D, Kioumis DI, Papakala E, Karapantzos I, Karapantzou C, Rapati A, Tsakiridis K, Zarogoulidis K, Argyropoulou P. During economic crisis can sleep questionnaires improve the value of oximetry for assessing sleep apnea? Ann Transl Med. 2016;4(22):443–443. doi: 10.21037/atm.2016.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pecotic R, Dodig IP, Valic M, Ivkovic N, Dogas Z. The evaluation of the Croatian version of the Epworth sleepiness scale and STOP questionnaire as screening tools for obstructive sleep apnea syndrome. Sleep Breathing. 2012;16(3):793–802. doi: 10.1007/s11325-011-0578-x. [DOI] [PubMed] [Google Scholar]
- 50.Pereira EJ, Driver HS, Stewart SC, Fitzpatrick MF. Comparing a combination of validated questionnaires and level III portable monitor with polysomnography to diagnose and exclude sleep apnea. J Clin Sleep Med. 2013;9(12):1259–1266. doi: 10.5664/jcsm.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perumalsamy, P., Charles, A.K., Reddy, G., Chokkalingam, C. & Balakrishnan, R. (2017) Comparison of three sleep questionnaires in screening obstructive sleep apnoea. J Evol Med Dental Sci. 6(88), 6132–6136. Available from: https://www.jemds.com/data_pdf/preethi-meena-giri-Nov-6-.pdf
- 52.Sadeghniiat-Haghighi K, Montazeri A, Khajeh-Mehrizi A, Ghajarzadeh M, Alemohammad ZB, Aminian O, Sedaghat M. (2015) The STOP-BANG questionnaire: reliability and validity of the Persian version in sleep clinic population. Qual Life Res. 2015;24(8):2025–2030. doi: 10.1007/s11136-015-0923-9. [DOI] [PubMed] [Google Scholar]
- 53.Saleh ABM, Ahmad MA, Awadalla NJ. Development of Arabic version of Berlin questionnaire to identify obstructive sleep apnea at risk patients. Ann Thorac Med. 2011;6(4):212. doi: 10.4103/1817-1737.84775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sangkum, L., Klair, I., Limsuwat, C., Bent, S., Myers, L. & Thammasitboon, S. (2017) Incorporating body-type (apple vs. pear) in STOP-BANG questionnaire improves its validity to detect OSA. J Clin Anaesthesia. 41, 126–131. Available from: 10.1016/j.jclinane.2016.12.019https://linkinghub.elsevier.com/retrieve/pii/S0952818016311369 [DOI] [PMC free article] [PubMed]
- 55.Suksakorn S, Rattanaumpawan P, Banhiran W, Cherakul N, Chotinaiwattarakul W. Reliability and validity of a Thai version of the Berlin questionnaire in patients with sleep disordered breathing. J Med Assoc Thai. 2014;97(3):S46–56. [PubMed] [Google Scholar]
- 56.Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84–94. doi: 10.1002/nur/21512. [DOI] [PubMed] [Google Scholar]
- 57.Yüceege M, Firat H, Sever Ö, Demir A, Ardiç S. The effect of adding gender item to Berlin Questionnaire in determining obstructive sleep apnea in sleep clinics. Ann Thorac Med. 2015;10(1):25–28. doi: 10.4103/1817-1737.146856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung F, Yeneswaran B, Liao P, Chung SA, Vairavananthan S, Islam S, Khajehdehi A, Shapiro CM. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 59.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP Questionnaire: a tool to screen patient for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 60.Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesis B. Serum bicarbonate level improves specificity of STOP-Bang screening for obstructive sleep apnea. Chest. 2013;143(5):1284–1293. doi: 10.1378/chest.12-1132. [DOI] [PubMed] [Google Scholar]
- 61.Chung F, Yang Y, Brown R, Liao P. Alternative scoring models of STOP-Bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):951–958. doi: 10.5664/jcsm.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deflandre E, Degey S, Brichant J-F, Conneau A-F, Frognier R, Poirrier R, Bonhomme V. Pre-operative ability of clinical scores to predict obstructive sleep apnea (OSA) severity in susceptible surgical patients. Obesity Surgery. 2017;27(3):716–729. doi: 10.1007/s11695-016-2352-4. [DOI] [PubMed] [Google Scholar]
- 63.Nunes FS, Danzi-Soares NJ, Genta PR, Drager LF, Cesar LAM, Lorenzi-Filho G. Critical evaluation of screening questionnaires for obstructive sleep apnea in patients undergoing coronary artery bypass grafting and abdominal surgery. Sleep Breathing. 2014;19(1):115–122. doi: 10.1007/s11325-s11325-014-0971-3. [DOI] [PubMed] [Google Scholar]
- 64.Xia, M., Liu, S., Ji, N., Xu, J., Zhou, Z., Tong, J. & Zhang Y. (2018) BMI 35 kg/m2 does not fit everyone: a modified STOP-Bang questionnaire for sleep apnea screening in the Chinese population. Sleep and Breathing. 1–8. Available from: 10.1007/s11325-017-1610-6 [DOI] [PubMed]
- 65.Giampá SQC, Pedrosa RP, Gonzaga CC, Bertolami A, Amodeo C, Furlan SF, Bortolotto LA, Lorenzi-Filho G, Drager LF. Performance of NoSAS score versus Berlin questionnaire for screening obstructive sleep apnoea in patients with resistant hypertension. J Hum Hypertens. 2018;32(7):518–523. doi: 10.1038/s41371-018-0072-z. [DOI] [PubMed] [Google Scholar]
- 66.Margallo VS, Muxfeldt ES, Guimarães GM, Salles GF. Diagnostic accuracy of the Berlin questionnaire in detecting obstructive sleep apnea in patients with resistant hypertension. J Hypertens. 2014;32(10):2030–2037. doi: 10.1097/HJH.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 67.Lu H, Fu C, Li W, Jiang H, Wu Y, Li S. Screening for obstructive sleep apnea syndrome in asthma patients: a prospective study based on Berlin and STOP-Bang questionnaires. J Thoracic Dis. 2017;9(7):1945–1958. doi: 10.21037/jtd.2017.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gantner D, Ge J-Y, Antic N, Windler S, Wong K, Heeley E, Huang S-G, Ciu P, Anderson C, Wang J-G, McEvoy D. Diagnostic accuracy of a questionnaire and simple home monitoring device in detecting obstructive sleep apnoea in a Chinese population at high cardiovascular risk. Respirology. 2010;15(6):952–960. doi: 10.1111/j.1440-1843.2010.01797.x. [DOI] [PubMed] [Google Scholar]
- 69.Firat H, Yuceege M, Demir A, Ardic S. Comparison of four established questionnaires to identify highway bus drivers at risk for obstructive sleep apnea in Turkey. Sleep and Biological Rhythms. 2012;10(3):231–236. doi: 10.1111/j.1479-8425.2012.00566.x. [DOI] [Google Scholar]
- 70.Elkholy SH, Amer HA, Nada MM, Nada MAF, Labib A. Sleep-related breathing disorders in cerebrovascular stroke and transient ischemic attacks: a comparative study. J Clin Neurophysiol. 2012;29(2):194–198. doi: 10.1097/WNP.0b013e31824d96b0. [DOI] [PubMed] [Google Scholar]
- 71.Bouloukaki I, Komninos ID, Mermigkis C, Micheli K, Komninou M, Moniaki V, Mauroudi E, Siafakas NM, Schiza S. Translation and validation of Berlin questionnaire in primary health care in Greece. BMC Pulmonary Medicine. 2013;13(1):6. doi: 10.1186/1471-2466-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yunus, A., Seet, W., Mohamad Adam, B. & Haniff, J. (2014) Validation of the Malay version of Berlin questionnaire to identify Malaysian patients for obstructive sleep apnea. Malaysian Family Physic. 8(1), 5–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25606261 [PMC free article] [PubMed]
- 73.Banhiran, W., Durongphan, A., Saleesing, C.K. & Chongkolwatana, C. (2014) Diagnostic properties of the STOP-bang and its modified version in screening for obstructive sleep apnea in Thai patients. J Med Assoc Thailand. 97(6), 644–654. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25137882 [PubMed]
- 74.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 75.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 76.Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, Mokhlesi B, Chung F. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS ONE. 2015;10(12):e0143697. doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seneratna CV, Perret JL, Matheson MC, Lodge CJ, Lowe AJ, Cassim R, Russell MA, Burgess JA, Hamilton GS, Dharmage SC. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–124. doi: 10.1016/j.smrv.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Kaw R, Michota F, Jaffer A, Ghamande S, Auckley D, Golish J. Unrecognized sleep apnea in the surgical patient: implications for the peri-operative setting. Chest. 2006;129:198–e205. doi: 10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]
- 79.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Canadian Journal of Anaesthesia. 2009;56:819–e828. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 80.Van Oosten EM, Hamilton A, Pesikas D, Payne D, Redfearn DP, Zhang S, Hopman WM, Baranchuk A. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2014;113(6):919–923. doi: 10.1016/j.majcard.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 81.Peker, Y., Celik, Y., Arbatli, S., Isik, S.R., Balcan, B., Karataş, F., Uzel, F.I., Tabak, L., Çetin, B., Baygül, A., Öztürk, A.B., Altuğ, E., İliaz, S., Atasoy, C., Kapmaz, M., Yazici, D., Bayram, H., Çetin, B.D. & Çağlayan, B. The OSACOVID-19 Study Collaborators (2021) Effect of high-risk obstructive sleep apnea on clinical outcomes in adults with coronavirus disease 2019: a multicenter, prospective, observational cohort study. Ann Am Thorac Soc. Available from10.1513/AnnalsATS.202011-1409OC [DOI] [PMC free article] [PubMed]
- 82.Bossuyt PMM, Reitsma JB, Linnet K, Moons KGM. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58(12):1636–1643. doi: 10.1373/clinchem.2012.182576. [DOI] [PubMed] [Google Scholar]
- 83.American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. doi: 10.1093/sleep/22.5.667. [DOI] [PubMed] [Google Scholar]
- 84.Iber, C., Ancoli-Israel, S., Chesson, A.L. & Quan, S.F. for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. First Edition. Westchester, IL: Am Acad Sleep Med. Available from10.1186/s41606-018-0023-1
- 85.Berry RB, Budhiraja R, Gottlieb DB, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strolh KP, Davidson SL, Tangredi MM. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shamim-Uzzaman A, Singh S, Chowdhuri S. Hypopnea definitions, determinants and dilemmas: a focused review. Sleep Sci Practice. 2018;2(7):1–12. [Google Scholar]
- 87.Guilleminault C, Hagen CC, Huynh NT. Comparison of hypopnea definitions in lean patients with known obstructive sleep apnea hypopnea syndrome (OSAHS) Sleep Breath. 2009;13:341–7. doi: 10.1007/s11325-009-0253-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available on request.
Not applicable.