Graphical abstract
Keywords: Atrial myxoma, ASD device occlusion, Echocardiography, Minimally invasive cardiac surgery, Three-dimensional echocardiography
Highlights
-
•
Device occlusion is the method of choice to treat atrial septal defects.
-
•
Atrial myxoma on an atrial septal defect occlusion device is rare but does occur.
-
•
Echocardiography plays an essential role in confirming an atrial myxoma.
-
•
Echocardiography helps to exclude a left atrial thrombus.
-
•
Echocardiography contributes to surgical planning.
Introduction
Atrial myxoma is the most common cardiac tumor, constituting 83% of all primary tumors of the human heart.1 Cardiac myxoma used to be a clinical diagnostic challenge.2 In the era of multimodality imaging, especially the advent of high-resolution transthoracic (TTE) and transesophageal echocardiography (TEE), it is relatively straightforward to detect and diagnose an atrial myxoma.3 Ostium secundum atrial septal defect (ASDII) is the most common congenital heart disease diagnosed in adults after the fourth decade of life.4 Catheter-based device occlusion of ASDII has been established as a safe and effective intervention for most patients.5
To the best of our knowledge, neither an association between ASDII and the development of an atrial myxoma nor an increased risk of atrial myxoma occurrence in patients undergoing atrial septal defect (ASD) device closure has been reported. In this paper, we reported a 62-year-old patient who developed a left atrial myxoma 2 years after successful ASDII device occlusion. With this case report, we emphasize the essential role of echocardiography in diagnosing atrial myxoma and planning an appropriate surgical approach to remove the tumor.
Case Presentation
A 62-year-old man was diagnosed with an ASDII with good rims and underwent a successful transcatheter ASD device closure (Amplatzer Septal Occluder 24 mm; Abbott. Abbott Park, IL) at our center in 2018. At the time of intervention, TTE and TEE did not show any abnormal mass on the interatrial septum. The patient was discharged healthy and has been regularly checked at the outpatient clinic. The latest TTE performed 6 months before this appointment (2 years after the device closure) confirmed an ASD device in good position, no residual shunt, and no abnormal intra-atrial mass.
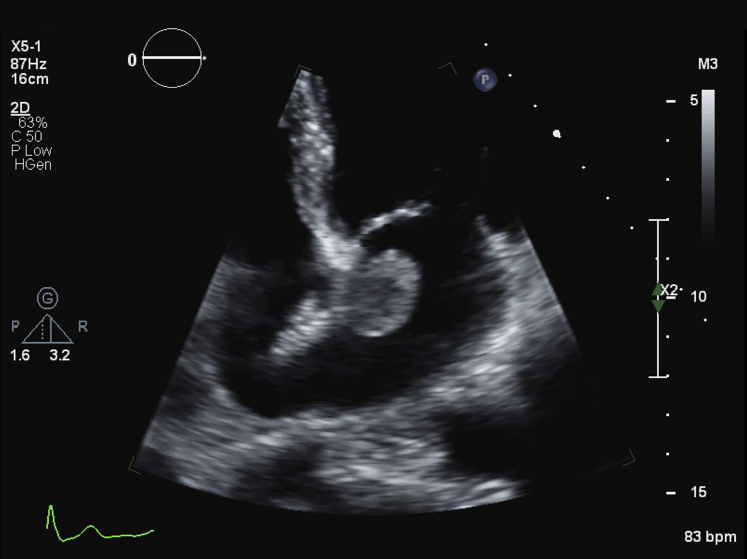
The patient was asymptomatic on daily activity and quite cheerful. The clinical examination did not pick up any abnormality. Unexpectedly, we found a mobile mass in the left atrium, next to the edge of the ASD device on TTE (Figure 1, Videos 1 and 2).
Figure 1.
Transthoracic apical four-chamber view echocardiography showing a well-delineated and homogeneous mass in the left atrium.
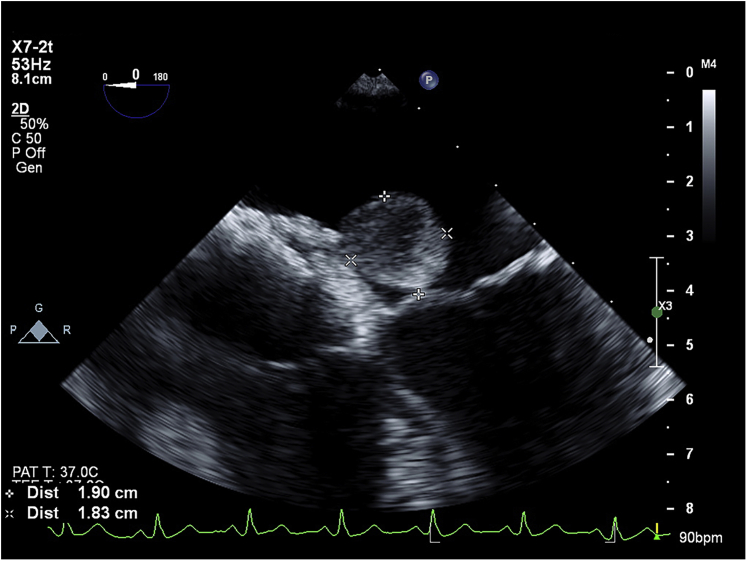
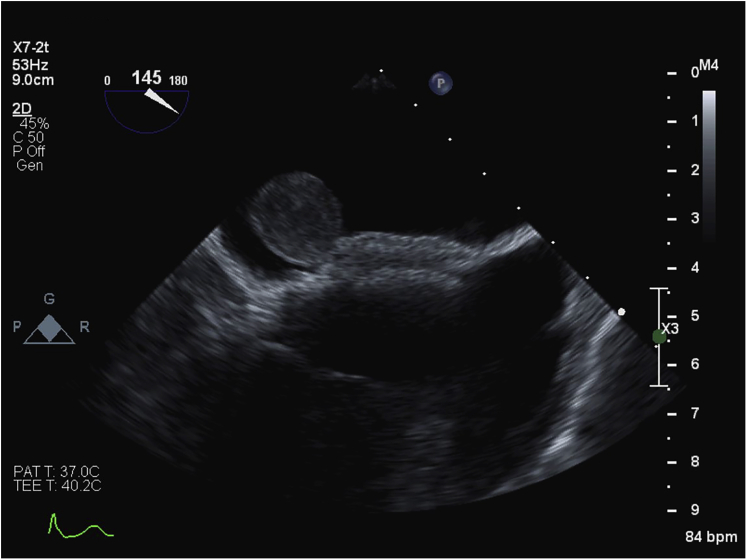
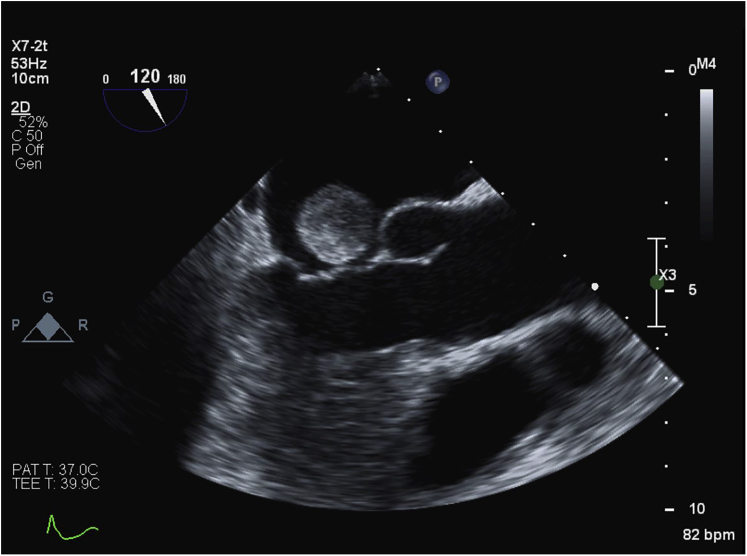
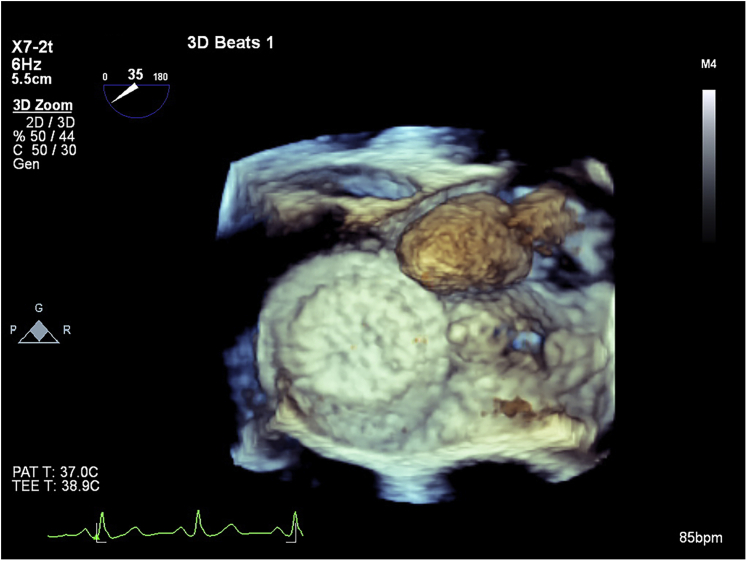
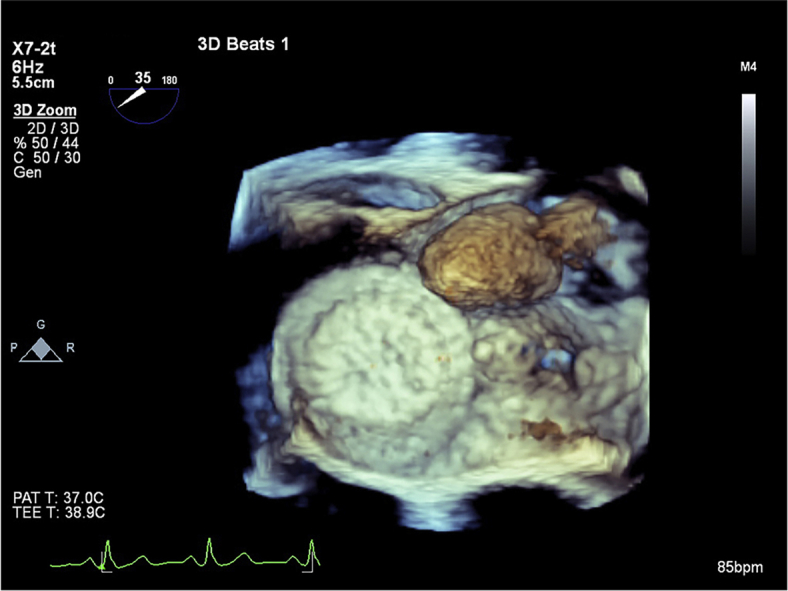
A TEE was performed, showing a well-delineated mass 19.0 × 18.3 mm in diameter and a tumor stalk sandwiched between the two discs of the ASD device (Figures 2 and 3, Videos 3 and 4). This left atrial mass was mobile but not obstructing the mitral valve (Figures 4 and 5, Videos 5 and 6).
Figure 2.
Midesophageal transesophageal four-chamber view echocardiography showing a round mass of 19 × 18.3 mm in diameter.
Figure 3.
Transesophageal echocardiography located the myxoma stalk sandwiched between the discs of the ASD device.
Figure 4.
The atrial myxoma was mobile but did not obstruct the mitral valve.
Figure 5.
The ASD device and the atrial myxoma seen from the left side of the interatrial septum.
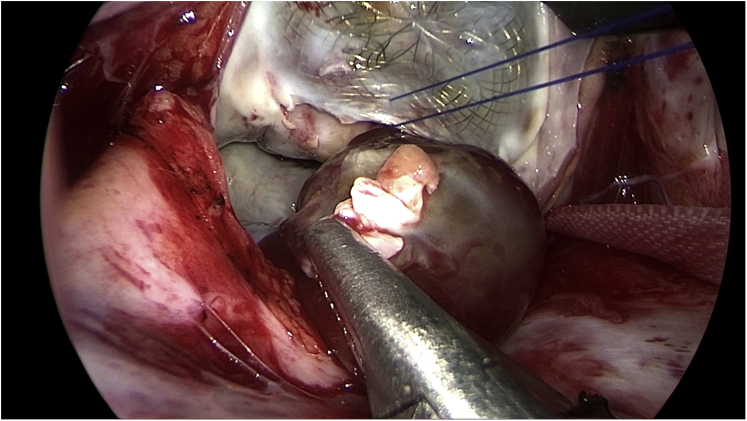
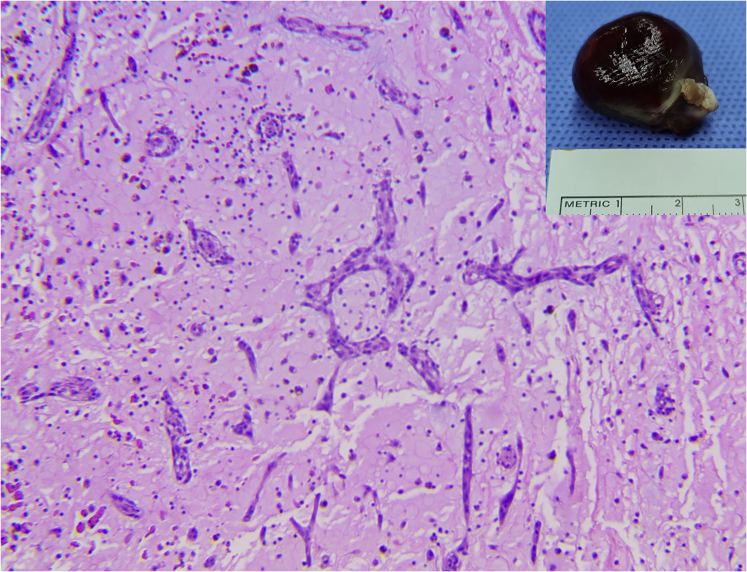
The patient underwent an elective minimally invasive cardiac surgery for removal of the left atrial myxoma. With the preoperative echocardiography, especially the three-dimensional TEE that pinpointed the stalk of the myxoma, our cardiac surgeons were able to easily locate and expose the stalk (Figure 6). The myxoma, its stalk, and limited surrounding endocardial tissue were removed successfully without dislocating the ASD device. The edge of the ASD device was stabilized by a reinforced suture. Intraoperative TEE showed no residual shunt. Pathological examination confirmed an atrial myxoma (Figure 7). The postoperative care proceeded uneventfully, and the patient was discharged on postoperative day 7. The patient was healthy and cheerful at his regular checkups 7 days and 1 month after discharge from the hospital.
Figure 6.
Intraoperative picture. The edge of the ASD device was lifted to better expose and excise the myxoma stalk.
Figure 7.
Pathological examination of the removed tumor confirmed the diagnosis of cardiac myxoma.
Discussion
Ostium ASDII is still the most common congenital heart disease in adults, with a predominance in female patients of approximately 2:1.4 Transcatheter device closure of ASDII is currently the method of choice.5 By investigating 1,000 consecutive patients undergoing ASD device occlusion with the use of TEE 4 weeks and 6 months after the procedure, Krumsdorf et al.6 have concluded that the incidence of thrombus formation on closure devices was low and that the thromboses usually resolved themselves under anticoagulation therapy. However, Ruge et al.7 reported a case of left atrial thrombus on the occlusion device of a patent foramen ovale as the source of cerebral emboli 3 years after closure. When the left atrial mass was detected in our patient, the question was raised, Was it a myxoma or a thrombus? In view of the technical challenge and the risk of biopsy of cardiac masses, a diagnosis is typically made using an imaging modality.8 Even though the characteristics of the mass were suggestive of an atrial myxoma on TTE, we could not rule out with certainty the possibility of a thrombus on the device. The TEE, with its higher resolution and three-dimensional reconstruction, played an essential role in the diagnosis of an atrial myxoma. In this case, we could visualize the myxoma stalk, which really facilitated the surgical plan.
In the current case, the atrial myxoma was not bulky and did not obstruct the blood flow, which explained the asymptomatic clinical presentation. The latest TTE done 6 months before this admission was normal, indicating that the tumor had grown quickly in size. The growth rate of the atrial myxoma was calculated in one patient to be 1.36 × 0.3 cm/month.9 Depending on the tumor size, intracardiac obstruction, constitutional symptoms, infected myxomas, and systemic embolism have all been described.10,11 With all these risks, our decision to remove the myxoma was reasonable.
There is no clear risk factor for myxoma recurrence. The recurrence rate reported in the literature ranges from 5% to 14%.12 Tumor recurrence was more likely to occur in the first 10 postoperative years, in younger patients, in patients with a smaller tumor, and in patients with ventricular myxoma.13 Our surgeons, as a matter of routine, asked for the precise insertion site of the myxoma stalk so that they could carry out complete removal of the tumor tissue. The echocardiographic quality was good enough to pinpoint the stalk sandwiched between the device discs.
The development of an atrial myxoma on an ASD occlusion device has not been described, while thrombus formation in these patients has been long recognized.6 A left atrial thrombus is considered an apparent risk for systemic embolism5 and must be treated by medical thrombolysis or anticoagulation, which are not devoid of serious complications. The TTE for the detection and the TEE for the confirmation of a rare but real atrial myxoma on the ASD occlusion device played a crucial role in the management of our patient.
Conclusion
We report for the first time the development of an atrial myxoma on an ASD occlusion device. This combination was very rare but did in fact occur. Transthoracic echocardiography helped to detect the presence of a tumor in an otherwise healthy person. Transesophageal echocardiography with high-resolution and three-dimensional reconstruction was crucial for confirming an atrial myxoma and excluding a left atrial thrombus. In addition, echocardiography played an important role in planning an appropriate surgical approach to remove the tumor safely.
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2021.05.004.
Supplementary Data
Mobile left atrial myxoma on TTE.
Mobile left atrial myxoma on biplane TTE.
Midesophageal four-chamber view on TEE with an atrial myxoma. The tumor stalk can be visualized sandwiched between the device discs.
The perpendicular incidence of the ultrasound beam helps to delineate the ASD device and the myxoma.
Midesophageal long-axis view showing a mobile myxoma at the mitral valve annular plane.
Three-dimensional TEE with the ASD device and the mobile atrial myxoma.
References
- 1.Blondeau P. Primary cardiac tumors—French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38(Suppl 2):192–195. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 2.O’Neil M.B., Jr., Grehl T.M., Hurley E.J. Cardial myxomas: a clinical diagnostic challenge. Am J Surg. 1979;138:68–76. doi: 10.1016/0002-9610(79)90244-7. [DOI] [PubMed] [Google Scholar]
- 3.Tripodi V.F., Rao C.M., Covino A., Fratto P., Benedetto F.A. The importance of ultrasound in a case of stroke caused by left atrial myxoma. J Cardiovasc Echogr. 2020;30:113–115. doi: 10.4103/jcecho.jcecho_51_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuijpers J.M., Mulder B.J., Bouma B.J. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205–211. doi: 10.1007/s12471-015-0663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akagi T. Current concept of transcatheter closure of atrial septal defect in adults. J Cardiol. 2015;65:17–25. doi: 10.1016/j.jjcc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Krumsdorf U., Ostermayer S., Billinger K., Trepels T., Zadan E., Horvath K. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Ruge H., Wildhirt S.M., Libera P., Vogt M., Holper K., Lange R. Images in cardiovascular medicine. Left atrial thrombus on atrial septal defect closure device as a source of cerebral emboli 3 years after implantation. Circulation. 2005;112:e130–e131. doi: 10.1161/CIRCULATIONAHA.104.492017. [DOI] [PubMed] [Google Scholar]
- 8.Poterucha T.J., Kochav J., O’Connor D.S., Rosner G.F. Cardiac tumors: clinical presentation, diagnosis, and management. Curr Treat Options Oncol. 2019;20:66. doi: 10.1007/s11864-019-0662-1. [DOI] [PubMed] [Google Scholar]
- 9.Karlof E., Salzberg S.P., Anyanwu A.C., Steinbock B., Filsoufi F. How fast does an atrial myxoma grow? Ann Thorac Surg. 2006;82:1510–1512. doi: 10.1016/j.athoracsur.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Bernatchez J., Gaudreault V., Vincent G., Rheaume P. Left atrial myxoma presenting as an embolic shower: a case report and review of literature. Ann Vasc Surg. 2018;53:266.e13–266.e20. doi: 10.1016/j.avsg.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Dell’Aquila M., Carbone A., Pennacchia I., Stigliano E., Oliva A., Arena V. Sudden death by massive systemic embolism from cardiac myxoma. Role of the clinical autopsy and review of literature. Cardiovasc Pathol. 2020;49:107244. doi: 10.1016/j.carpath.2020.107244. [DOI] [PubMed] [Google Scholar]
- 12.Pinede L., Duhaut P., Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Shah I.K., Dearani J.A., Daly R.C., Suri R.M., Park S.J., Joyce L.D. Cardiac myxomas: a 50-year experience with resection and analysis of risk factors for recurrence. Ann Thorac Surg. 2015;100:495–500. doi: 10.1016/j.athoracsur.2015.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mobile left atrial myxoma on TTE.
Mobile left atrial myxoma on biplane TTE.
Midesophageal four-chamber view on TEE with an atrial myxoma. The tumor stalk can be visualized sandwiched between the device discs.
The perpendicular incidence of the ultrasound beam helps to delineate the ASD device and the myxoma.
Midesophageal long-axis view showing a mobile myxoma at the mitral valve annular plane.
Three-dimensional TEE with the ASD device and the mobile atrial myxoma.