ABSTRACT
Transient modification of the environment involves the expression of specific genes and degradation of mRNAs and proteins. How these events are linked is poorly understood. CCR4-NOT is an evolutionary conserved complex involved in transcription initiation and mRNA degradation. In this paper, we report that the yeast Not4 localizes in cytoplasmic foci after cellular stress. We focused our attention on the functional characterization of the C-terminus of the Not4 protein. Molecular dissection of this region indicates that the removal of the last 120 amino acids, does not affect protein localization and function, in that the protein is still able to suppress the thermosensitivity observed in the not4Δ mutant. In addition, such shortened form of Not4, as well its absence, increases the transcription of stress-responsive genes conferring to the cell high resistance to the oxidative stress. On the contrary, the last C-terminal 211 amino acids are required for proper Not4 localization at cytoplasmic foci after stress. This truncated version of Not4 fails to increase the transcription of the stress genes, is more stable and seems to be toxic to cells undergoing oxidative stress.
Keywords: yeast, stress response, E3 ubiquitin ligase, gene expression, protein aggregation
The C-terminal region of Not4 ubiquitin ligase mediates the formation of cytoplasmic foci after stress. In addition, this region contains positive and negative elements that influence cell resistance to hydrogen peroxide.
INTRODUCTION
NOT proteins are part of CCR4-NOT multifunctional complex that controls gene expression at the level of both transcription initiation and mRNA degradation. Several genetic and biochemical interactions have been observed between yeast Not proteins and transcriptional complexes in the initiation and elongation of mRNAs (Denis and Chen 2003; Collart and Timmer 2004). The multiple functions of the CCR4-NOT complex have been reviewed (Collart, Panasenko and Nikolaev 2013; Collart 2016).
The complex is highly conserved through the evolution and orthologs of most of the genes of the mammalian CCR4-NOT complex are also present in yeast.
In this unicellular eukaryotic organism, the CCR4-NOT complex dysfunction affects histone methylation, suggesting that transcriptional regulation by the complex in the nuclei involves chromatin modification (Laribee et al. 2007; Mulder et al. 2007). Besides its role in nuclear transcription regulation, the CCR4-NOT complex participates to mRNA decay. In fact, human CNOT6 (hCcr4) as its yeast orthologue Ccr4 possess a 3′- 5′ exonuclease activity which shortens the poly(A) tails of mRNAs (Denis and Chen 2003; Collart 2003; Collart and Timmer 2004; Yamashita et al. 2005). It has been reported that a substantial fraction of human and yeast CCR4-NOT proteins is localized in the cytoplasm (Collart and Timmer 2004; Yamashita et al. 2005) and that cell-cycle progression regulates the distribution of human CNOT7 (hCaf1) subunit between the nucleus and the cytoplasm (Morel et al. 2003).
Together, these findings indicate that the CCR4-NOT complex exerts its functions on transcription regulation both in the cytoplasm and in the nucleus. However, how Not proteins participate in these events during stress response remains unclear.
Yeast Not4 is a protein of 588 amino acids and its amino-terminal region, containing the RING finger domain, has been deeply studied. Through these studies several targets, substrates of Not4-E3 ubiquitin ligase, have been identified: Egd2p, involved in protein translation (Panasenko et al. 2006), Jhd2p, the demethylase responsible for chromatin modification (Mersman et al. 2009), Cdc17p, involved in DNA replication (Haworth et al. 2010), Yap1 (Gulshan, Thommandru and Moye-Rowley 2012) and cyclin C (Cooper et al. 2012). It has also been reported that Not4 plays a role in protein quality control and for proper assembly of the proteasome (Panasenko and Collart 2011; Halter, Collart and Panasenko 2014; Panasenko 2014).
It has also been shown that the absence of Not4 affected global translational repression upon nutrient withdrawal, enhanced the expression of arrested nascent polypeptides and caused constitutive protein folding stress and aggregation (Preissler et al. 2015).
In contrast, little information is available about the carboxy-terminus functions of the Not4 protein. It has been reported that the region between amino acids 430 and 480 is responsible for the interaction with some components of the CCR4-NOT complex (Panasenko and Collart 2011). In the same study, it has been described a role for the C-terminal region in regulating the amount of Ecm29, a protein known to enhance proteasome stability (Panasenko and Collart 2011).
In this paper, we studied the effects of progressive deletions of the Not4 carboxy-terminus in the cellular response to stress. We show that Not4, when yeast cells are exposed to H2O2 and ethanol stress, and after glucose deprivation, localize in cytoplasmic foci. Furthermore, we show that Not4 association in cytoplasmic foci under stress conditions, depends on amino acids localized between positions 378 and 419 of its C-terminus. Progressive deletions in the C-terminal region of the Not4 protein abolished its capacity to aggregate and increased protein stability and toxicity, suggesting a regulatory role of the C-terminus for Not4 protein functions.
MATERIALS AND METHODS
Yeast strains, plasmids and growth conditions
The yeast strains used in this study are BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0 and ura3Δ0; Brachmann et al. 1998) and its derivative deletion strain not4::kanMX4.
The pNot4-GFP plasmid was obtained as previously described (Serafini 2004), briefly: inserting the EcoRI/SalI fragment (about 1750 bp) carrying the entire NOT4 ORF, amplified by PCR using primers NOT4forGFP (cccccgaattcatgatgaatccacacgttcaagaaaat) and NOT4revGFP (cccccgtcgacattaccggcgataattttccttc), into the EcoRI/SalI sites of pUG35 vector (U. Guldener and J. H. Hegemann, Institut fur Mikrobiologie, Heinrich Heine University Dusseldorf, Germany; unpublished results; plasmid information available online at http://mips.gsf.de/proj/yeast/info/tools/hegemann/gfp.html).
The functionality of the Not4-GFP fusion protein, was tested by verifying its ability to suppress thermosensitivity, one of the phenotypes observed in the not4 null mutant.
The strain carrying the construction Not4-GFP integrated into the genome was from Thermofisher GFP collection https://clones.thermofisher.com/cloneinfo.php?clone=yeastgfp. Yeast transformation was performed by lithium acetate (Gietz et al. 1992).
Cells were grown in liquid or solid SD (Synthetic Dextrose: 0.67% yeast nitrogen base without amino acids, 2% glucose and auxotrophic requirements as needed) or YPD (Yeast extract Peptone Dextrose) medium at 28°C. For glucose deprivation experiments, exponential cells grown in SD, were collected, washed twice, resuspended in the same medium deprived of glucose and incubated for 15′ before fluorescence microscopy observation.
CELL VIABILITY
The determination of chronological lifespan was done by the method of microcolonies as already described (Palermo, Falcone and Mazzoni 2007). Briefly, cell suspensions (5 μL) containing approximately 6×106 cells/mL were poured on a thin layer of YPD agar on a microscope slide. A cover slip was placed over the samples and, after 24 h at 28°C, viable and unviable cells were scored on the basis of their ability to form microcolonies.
To determine the sensitivity to hydrogen peroxide, exponentially growing cells were exposed to 0.8, 1.2 and 3 mM H2O2 at 28°C for 4 h. Cell viability was determined by counting the number of microcolonies formed.
Western analysis
Cells grown at 28°C were harvested at OD600 = 0.4. Samples of total proteins, extracted by the TCA method as previously described (Knop et al. 1999), were separated on 10% acrylamide gel containing SDS. Western blot and immunodetection by enhanced chemiluminescence (ECL; SuperSignal‚ system, Pierce, Waltham, Massachusetts, USA) was performed following standard methods. αGFP antibody was from Boehringer-Mannheim (Germany), αTubulin antibody was from Santa-Cruz Biotechnolgy (Sc-530300).
Microscopy fluorescence
To better appreciate fluorence, cells were observed without fixation. For image acquisition we used a Zeiss Axio Imager Z1 Fluorescence Microscope with AxioVision 4.8 Digital Image Processing System, and the objective lens used was 63 oil. The fluorescence was observed using filter sets for GFP (470/40-nm excitation and 525/50-nm emission) and RFP (550/25-nm excitation and 605/670-nm emission). For all experiments, at least three independent cultures were examined and about 400 cells were counted at each point.
Real-time quantitative PCR analysis
Total RNA was prepared as already described (Schmitt, Brown and Trumpower 1990) from exponential cells grown at 28°C in minimal media SD (yeast nitrogen base without amino acids and auxotrophic requirement as needed) and after 40′ exposure to 0.4 mM H2O2. Nucleic acid was quantified by measuring the absorbance at 260 nm using nanodrop technology (Thermo-Fisher Scientific, Waltham, Massachusetts, USA) and 2 μg of RNAs were transcribed to cDNA using M-MuLV Reverse Transcriptase (BioLabs, Ipswich, MA, USA). Sample preparation and amplification reaction were carried out as previously described (De Luca et al. 2006). Briefly, samples were prepared by adding to 50 ng of cDNA to12.5 μL of the reaction mixture, containing 1 × iQ SYBR Green Supermix (BioRad) and 0.1 μM of the oligonucleotides indicated in Table 1, in a final volume of 25 μL Amplification was carried out in the iCycler apparatus from Biorad, as follow: cycle 1: 95°C for 10 min (1X), cycles 2: 95°C for 30″, 65°C for 30″ (35X) and 72°C for 45″.
Table 1.
List of primers used in this study.
| Name | Molecular fuction | Primer sequence | Amplicon size |
|---|---|---|---|
| TDH3 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), isozyme 3 | F:CGGTAGATACGCTGGTGAAGTTTCR:TGGAAGATGGAGCAGTGATAACAAC | 153–373 (220 bp) |
| SOD1 | Cytosolic copper-zinc superoxide dismutase | F:ATGGTGTGGCCAAGGGCTCCR:AGTCATCTTGGCCGGCGTGG | 278–379 (101 bp) |
| SOD2 | Mitochondrial manganese superoxide dismutase | F:CTCCGGTCAAATCAACGAATR:CCTTGGCCAGAAGATCTGAG | 132–242 (110 bp) |
| YAP1 | Basic leucine zipper (bZIP) transcription factor | F:CAATCGGTACCAGGAAATGAAAGCR:CCTTCCTTAGATGGAACGACATCA | 1654–1779 (125 bp) |
| MSN2 | Stress-responsive transcriptional activator | F:AGTGTCAACTCAACTGGCAATGGTR:CTTCTGGACGGTGTCATTGATTTTC | 1807–1893 (86 bp) |
| MSN4 | Stress-responsive transcriptional activator | F:AATTGGCGACTTCTGGTGTTGATAR:GTTGATGATGTTGAGCTGCATGG | 1406–1540 (134 bp) |
The reporter signals were analysed using the iCycler iQ software (BioRad). These values can be translated into a quantitative result by constructing a standard curve, with the standard sample values. A melting curve was obtained after completion of the cycles to verify the presence of a single amplicon. Quantification was performed using a comparative CT method (CT = threshold cycle value). Briefly, the differences between the mean CT value of each sample and the CT value of the housekeeping gene (TDH3) were calculated: ΔCTsample = CTsample—CTTDH3. Final result was determined as 2^− ΔΔCT where ΔΔCT = ΔCTsample (H2O2 treated)—ΔCTcontrol (not treated). A melting curve was obtained after completion of the cycles to verify the presence of a single amplicon. The presented RT-PCR results are mean values of at least three independent experiments.
The primers and their respective amplicons, are shown in Table 1.
RESULTS AND DISCUSSION
Functionality and stability of truncated Not4 proteins
The sequence alignment of the yeast Not4 protein with HsNot4-Np, the most studied form of the human CCR4-NOT complex (Hanzawa et al. 2001; Mazzoni, Serafini and Falcone 2005) revealed the conservation of the most important domains. Among them the RING-finger, the coiled-coil domains known to mediate subunit oligomerization of a large number of proteins (Burkhard, Stetefeld and Strelkov 2001), the Pham:rrm RNA binding domain and PEST sequences, which are a signal for proteolytic degradation (Rechsteiner and Rogers 1996). In addition, Not4 proteins present several serine and threonine phosphorylation sites (Lau et al. 2010).
To identify new functional domains, we studied the properties of Not4 proteins harboring progressive deletions at the C terminus and fused to the GFP under the control of the MET25 promoter (Not4Δ1- Δ2 and Δ3 in Fig. 1A).
Figure 1.
Not4 C-terminus analysis. (A) Schematic representation of truncated Not4 proteins used in this study. (B) Complementation analysis by dilution spot assay of not4 strains expressing the entire NOT4-GFP gene fusion (Not4) and its truncated forms (Not4Δ1, Δ2 and Δ3). Wild type strain (BY4741) and Pug35 (vector) were used as a control. (C) Protein extracts from not4 mutant cells expressing GFP (lane 1) and the Not4–GFP fusion proteins (lanes 2–5) were separated on SDS-acrylamyde gel and probed with an anti-GFP antibody. Additional bands of lower intensity and faster migration might represent degradation products of the fusion proteins. (D) Protein stability. Not4Δ cells transformed with the entire and the truncated forms of Not4 fused to GFP were grown exponentially. After blocking translation with cycloheximide (CHX), proteins were extracted at the indicated time, separated on SDS–PAGE and probed with anti-GFP and anti-tubulin antibodies. Ponceau red staining of membranes is also shown for quantization of transferred proteins. In the bottom part is reported the relative quantity of the Not4-GFP proteins compared to tubulin.
In the Not4Δ1, we eliminated the last 120 amino acids to obtain a fused protein of about 52.4 kDa. This truncated protein maintains the regions required for the interaction with Not1 (Bhaskar et al. 2013) while lacks the putative phosphorylation site T543 and a Q/N rich-region indicated as possible prion site (Reijns et al. 2008). Not4Δ2 lacks the last 169 amino acids and is very similar in length to KlNot4Δ2 that we studied previously in the related yeast Kluyveromyces lactis (Mazzoni, Serafini and Falcone 2005). In Not4Δ3, we eliminated the last 211 amino acids, including the PEST sequence, obtaining a fused protein of about 42.2 KDa.
We expressed the three truncated forms of the protein into a not4 null strain and verified their ability to complement the thermosensitivity of this mutant. We found that all of them were able to restore cell growth at 37°C, indicating that the first 377 amino acids of Not4 are required for function in yeast viability (Fig. 1B). In Fig. 1C, it is shown that all the fused proteins were expressed in yeast showing the expected size.
We also checked the stability of these proteins. As shown in Fig. 1D, following the arrest of protein synthesis by cycloheximide, Not4 and Not4Δ1 were barely detectable after the first 2 h while Not4Δ 2 and Not4Δ 3 were still abundant after 3-4 h.
In the case of Not4Δ3, we observed an additional band, possibly due to phosphorylation (Lau et al. 2010) or autoubiquitination (Panasenko and Collart 2011) of the protein. These results, in agreement with previous findings indicating that Not4 truncated proteins lacking C-terminal regions have higher stability (Panasenko and Collart 2011), show that the latter two forms are more stable than the entire protein.
Cellular localization of Not4 proteins under normal and stress conditions
We then determined if the Not4 C-terminus had any role in the localization of Not4 in normal conditions and after H2O2 and ethanol treatments, and glucose deprivation.
Not4-GFP, as previously reported for the native protein (Tucker et al. 2001; Huh et al. 2003), has a dispersed distribution in the cytosol with very rare foci (Fig. 2A, NT). After exposure of cells to 3 mM H2O2, 7% ethanol or glucose deprivation, the protein clearly localized in multiple cytoplasmic foci per cell (Fig. 2A).
Figure 2.

Protein localization (A) Not4 localization in cells not treated (NT) and after 15′ treatment with 3 mM hydrogen peroxide (H2O2), 7% ethanol (EtOH) and after 10′ of glucose deprivation. (B) Percentage of not4Δ cells transformed with the NOT4-GFP constructs showing foci under the indicated conditions. (C) Not4 localization in cells expressing the Not4-GFP fusion integrated into the chromosome under the native promoter after 10′ of glucose deprivation (-Glu), 15′ treatment with 7% ethanol (EtOH) or 3 mM hydrogen peroxide (H2O2). NT: not treated.
To be sure that the formation of foci after stress was not a consequence of possible protein over-expression from the MET25 promoter, we also used a strain expressing the Not4–GFP fusion under the control of its own promoter integrated into the genome. As shown in Fig. 2C, also in this case Not4–GFP foci were clearly visible after stress treatments.
We observed that cells expressing Not4Δ1 formed foci at a level almost similar to that of the full-length protein in all three conditions tested. On the contrary, cells expressing Not4Δ2 showed a reduced capability to form foci that were very rare, if not absent, as also in the case of Not4Δ3 (Fig. 2A and B).
Studies of the protein sequences of all CCR4/NOT components, as well as of P-bodies components, revealed Q and/or N-rich stretches of varying length in many of them, suggesting an intrinsic feature of these proteins to form aggregates (Parker and Sheth 2007; Mazzoni, D'Addario and Falcone 2007; Reijns et al. 2008; Falcone and Mazzoni 2018).
Although the nature of these foci that we observed is still unclear, our preliminary evidences suggest that after cellular stresses Not4 might also come in contact with P-bodies. As cellular complexes containing Not4 and the P-bodies component Dcp2 can be found either together or separately in the cell, there is the possibility of a dynamic interaction between Not4 and P-bodies, depending on cellular conditions (Figure S1, Supporting Information).
Stress resistance and transcription of stress responsive genes
We next analyzed the effects of the different truncations in the cellular stress responses following H2O2 treatment and acute heat shock.
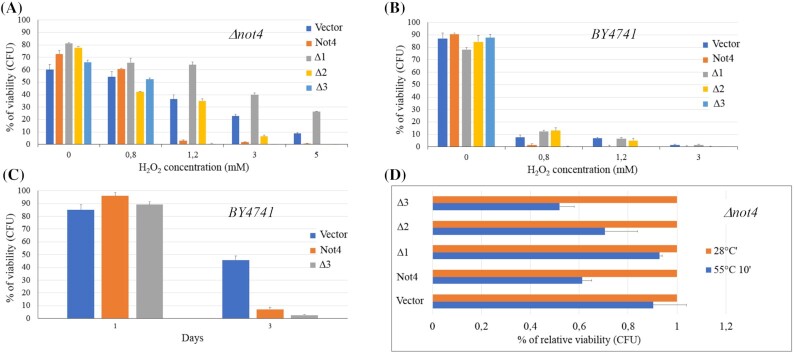
As shown in Fig. 3A, the absence of the Not4 protein rendered the cells more resistant to hydrogen peroxide. In fact, increasing the concentration of H2O2 up to 1.2 mM, cells expressing the wild type Not4 protein showed 1% viability after 4h of incubation while, in the same conditions, the not4 null mutant showed 35% viability. The absence of the last 120 amino acids (Not4Δ1) conferred high resistance to oxidative stress and, after treatments with 5 mM H2O2, more than 25% of cell population was still alive suggesting that in such a construct, important elements for oxidative stress sensitivity had been removed. Cells expressing Not4Δ2 showed higher resistance compared to the strain expressing Not4 but lower to that of the not4 null mutant. Cells expressing the truncated Not4 protein lacking the PEST sequence (Not4Δ3) showed high sensitivity to H2O2 in that, at the concentration of 1.2 mM, viability was three times lower compared to cells expressing the entire protein and almost undetectable at 3 mM.
Figure 3.
Effect of the expression of Not4 truncated forms on viability and stress resistance. Viability of the not4Δ strain (A) and BY4741 (B) transformed with NOT4-GFP fusions and its truncated forms was measured after exposure of cells to H2O2 at the indicated concentration for 4 h. The BY4741/not4Δ strain expressing the pUG35 vector was used as a control. Average and standard deviations, obtained from three independent experiments, are indicated. (C) Viability of transformants during aging. Viability of BY4741 transformed with Not4 and Not4Δ3 GFP fusions was measured during exponential (day 1) and stationary phase (day 3). BY4741 strain expressing the pUG35 (Vector) was used as a control. Average and standard deviations, obtained from three independent experiments, are indicated. (D) The same strains as in A were tested for acute heat shock stress. Viability is expressed as the fraction of viable cells compared to the corresponding untreated cells (28°C) set to 1.
These results suggested us that the persistence of Not4Δ3, stabilized by the absence of the PEST element and not associated in foci, could be toxic to cells undergoing oxidative stress.
To verify this hypothesis, we performed the same experiment in the wild type strain (BY4741), which harbors the endogenous NOT4 gene.
Unexpectedly, the ectopic expression of Not4 and Not4Δ3 in the presence of the wild type NOT4 gene, resulted in a dominant negative effect on oxidative and stationary phase stresses in that cells expressing these constructs were more sensitive to H2O2 and showed reduced viability during ageing (Fig. 3B and C).
In this direction, it has been reported that C-terminal truncations of Not4 have a dominant negative phenotype (Collart and Struhl 1994; Panasenko and Collart 2012). Intriguingly, the loss of the capability to associate in foci of some truncated forms of the Not4 protein, together with the increased sensitivity of cells to H2O2, suggests that the presence of free Not4 fragments in the cytoplasm could be toxic during stress. Actually, the formation of aggregates is a known cellular strategy observed in some neurodegenerative diseases for sequestering soluble toxic protein fragments (Taylor et al. 2003; Takalo et al. 2013; Vanderweyde et al. 2013). Recently, it has been reported that mutations that increase the aggregation of the TDP-43 protein, which occurs in nearly all cases of amyotrophic lateral sclerosis (ALS), strongly decrease their toxicity in yeast cells, suggesting a mechanism which titrates the protein away from a toxic liquid-like phase (Bolognesi et al. 2019).
It has been reported that not4 null strains, or strains mutated in the RING finger domain, show a high resistance to the acute heat shock (Collart 2016). We found that the Not4Δ1 truncated protein conferred resistance to this kind of stress, just as in the absence of the protein, suggesting that the removed region could contain important sequence that mediate the acute heat shock response (Fig. 3D).
In cells expressing Not4Δ2 and Not4Δ3, the effect of the acute heat shock was more severe compared to that observed with Not4Δ1, suggesting that regions important for the maintenance of viability during heat shock were deleted in such constructs.
It is known that Not4, together with other proteins of the CCR4–NOT complex, is involved in the expression of stress responsive genes (Collart and Struhl 1994; Collart 2016). In particular, not4Δ mutants show high transcription levels of genes under the control of Msn2-4-dependent STRE elements (Lenssen et al. 2005). Moreover, the protein level of another important regulator of the stress response, namely Yap1, is degraded by Not4 during H2O2 cells treatment (Gulshan, Thommandru and Moye-Rowley 2012).
To investigate if the different H2O2 sensitivity showed by the strains expressing the Not4 forms was related to an altered transcription of the stress responsive genes, we analyzed the expression of SOD1, SOD2, MSN2, MSN4 and YAP1 in such strains.
Figure 4 shows the fold of induction of these genes after 0.4 mM H2O2 treatment for 40′, compared to the untreated samples. As can be seen, cells harboring the control plasmid pUG35 or the Not4Δ1 and Not4Δ2 constructs showed higher transcription levels essentially for all the tested genes compared to cells expressing the entire NOT4 gene.
Figure 4.
Gene expression in yeast cells expressing the entire Not4 protein and its truncated forms Not4Δ1, Not4Δ2 and Not4Δ3 after oxidative stress. Total RNA was isolated before and after 40′ incubation in 0.4 mM H2O2 from not4Δ cells expressing the indicated forms of Not4 and reverse-transcribed to cDNA. Real time PCR was used to analyze the expression levels of SOD1, SOD2, MSN2, MSN4 and YAP1 using the specific primers indicated in Table 1. The expression of the TDH3 gene was used as an internal control for normalization of the real time PCR data and mRNA relative quantity are expressed each vs the own untreated strain cells (values are expressed as Log2). Vector: not4Δ strain expressing pUG35 plasmid. Data represent the mean of three independent experiments.
The huge increase of these genes transripts after H2O2 treatment, may account for the higher resistance showed by the not4 null mutant to oxidative stress (Fig. 3A).
The transcription levels of essentially all these genes, compared to cells expressing the entire Not4, were higher also in the case of Not4Δ1 and Not4Δ2, at least at H2O2 concentrations up to 1.2 mM for the latter. Again, the higher resistance to H2O2 treatments shown by cells expressing these truncated proteins could be due to the increased capability to induce the stress genes. In particular, one can notice that the high level of the YAP1 transcript after stress enhances the transcription of SOD1, a well-known specific target of YAP1. Differently, cells expressing Not4Δ3 were unable to induce the stress genes after oxidative stress, undergoing cell death very quickly (Figs 3A and 4).
It will be interesting to study the effects of the truncated forms of Not4 on another target of this gene, namely Cyclin C, which is a negative regulator of stress response genes (Cooper et al. 1997). This protein localizes to cytoplasm and is degraded only upon H2O2 induction through an ubiquitine-dependent mechanism mediated by NOT4 (Cooper et al. 2012).
In conclusion, our results suggest that the C-terminus of Not4 plays an important role for the aggregation of the protein and for the cellular response to different stress.
Glucose deprivation, ageing, H2O2 and ethanol treatments, trigger the appearance in the cell of Not4 aggregates the formation of which requires the amino acid residues from 378 to 419 and not the last 120 amino acids of the protein harboring Q/N-reach motifs.
It will be interesting to ascertain the existence of dynamic interactions between Not4 and other cell granules, raising the possibility to use yeast cells as a model for the study of the formation of cellular protein aggregates under different stresses. In respect to the latter point, it has been reported that the Saccharomyces cerevisiae lysine acetyltransferase complex NuA4 is required for stress granule formation upon glucose deprivation but not heat stress (Rollins et al. 2017).
Recent advances clearly demonstrate the existence of various subtypes of stress granules, heterogeneous in their RNA and protein content, suggesting that the biology of different SG subtypes may be directly implicated in neurodegeneration (Advani and Ivanov 2020).
Our deletion approach in the region from position 378 to 588, suggested that Not4 has both a recessive positive (Not4Δ1 and Not4Δ2) and a dominant negative (Not4Δ3) effect on cell viability after H2O2 treatments. These traits seem to be linked to the transcriptional activation of stress genes in response to oxidative stress.
In the case of the Not4Δ3 construct, carrying a larger deletion encompassing the PEST sequences, the protein shows high stability, does not associate in foci, fails to activate the transcription of STRE genes thus conferring higher sensitivity to H2O2 and resulting toxic in cells undergoing oxidative stress.
At present, it is not clear if the failure to activate the cell stress response depends on the transcriptional arrest of the stress genes or on the degradation of their mRNAs.
Further experiments are required to answer these questions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Professor Roy Parker for kindly providing the pRP1155/CEN/LEU plasmid expressing a tagged version of Dcp2p with RFP (Dcp2p-RFP) and Dr Agnese Serafini for providing the pNot4-GFP plasmid expressing a tagged version of Not4 with GFP (Not4-GFP).
Contributor Information
Vanessa Palermo, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Mariarita Stirpe, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Michele Maria Bianchi, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Teresa Rinaldi, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Angela Cirigliano, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Antonella Ragnini-Wilson, Department of Biology, University of Tor Vergata Rome, Viale Della Ricerca Scientifica, 00133 Rome, Italy.
Claudio Falcone, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
Cristina Mazzoni, Department of Biology and Biotechnology “C. Darwin”, Pasteur Institute-Cenci Bolognetti Foundation, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy.
FUNDING
This work was supported by funds from the Sapienza University of Rome ‘La Sapienza’ and from the Italian Ministry of Research.
Conflicts of interest
None declared.
REFERENCES
- Advani VM, Ivanov P. Stress granule subtypes: an emerging link to neurodegeneration. Cell Mol Life Sci. 2020;77:4827–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V, Roudko V, Basquin Jet al. Structure and RNA-binding properties of the Not1-Not2-Not5 module of the yeast Ccr4-Not complex. Nat Struct Mol Biol. 2013;20:1281–8. [DOI] [PubMed] [Google Scholar]
- Bolognesi B, Faure AJ, Seuma Met al. The mutational landscape of a prion-like domain. Nat Commun. 2019;10:4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJet al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–32. [DOI] [PubMed] [Google Scholar]
- Burkhard P, Stetefeld J, Strelkov SV. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–8. [DOI] [PubMed] [Google Scholar]
- Collart MA, Panasenko OO, Nikolaev SI. The Not3/5 subunit of the Ccr4-Not complex: a central regulator of gene expression that integrates signals between the cytoplasm and the nucleus in eukaryotic cells. Cell Signal. 2013;25:743–51. [DOI] [PubMed] [Google Scholar]
- Collart MA, Struhl K. NOT1(CDC39), NOT2(CDC36), NOT3, and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 1994;8:525–37. [DOI] [PubMed] [Google Scholar]
- Collart MA, Timmers HT. The eukaryotic Ccr4-not complex: a regulatory platform integrating mRNA metabolism with cellular signaling pathways?. Prog Nucleic Acid Res Mol Biol. 2004;77:289–322. [DOI] [PubMed] [Google Scholar]
- Collart MA. Global control of gene expression in yeast by the Ccr4-Not complex. Gene. 2003;313:1–16. [DOI] [PubMed] [Google Scholar]
- Collart MA. The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip Rev RNA. 2016;7:438–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Mallory MJ, Smith JBet al. Stress and developmental regulation of the yeast C-type cyclin Ume3p (Srb11p/Ssn8p). EMBO J. 1997;16:4665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Scarnati MS, Krasley Eet al. Oxidative-stress-induced nuclear to cytoplasmic relocalization is required for Not4-dependent cyclin C destruction. J Cell Sci. 2012;125:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C, Besagni C, Frontali Let al. Mutations in yeast mt tRNAs: specific and general suppression by nuclear encoded tRNA interactors. Gene. 2006;377:169–76. [DOI] [PubMed] [Google Scholar]
- Denis CL, Chen J. The CCR4-NOT complex plays diverse roles in mRNA metabolism. Prog Nucleic Acid Res Mol Biol. 2003;73:221–50. [DOI] [PubMed] [Google Scholar]
- Falcone C, Mazzoni C. RNA stability and metabolism in regulated cell death, aging and diseases. FEMS Yeast Res. 2018;18. DOI: 10.1093/femsyr/foy050. [DOI] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RAet al. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulshan K, Thommandru B, Moye-Rowley WS. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J Biol Chem. 2012;287:26796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter D, Collart MA, Panasenko OO. The Not4 E3 ligase and CCR4 deadenylase play distinct roles in protein quality control. PLoS ONE. 2014;9:e86218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa H, de Ruwe MJ, Albert TKet al. The structure of the C4C4 ring finger of human NOT4 reveals features distinct from those of C3HC4 RING fingers. J Biol Chem. 2001;276:10185–90. [DOI] [PubMed] [Google Scholar]
- Haworth J, Alver RC, Anderson Met al. Ubc4 and Not4 regulate steady-state levels of DNA polymerase-alpha to promote efficient and accurate DNA replication. Mol Biol Cell. 2010;21:3205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WK, Falvo JV, Gerke LCet al. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. [DOI] [PubMed] [Google Scholar]
- Knop M, Siegers K, Pereira Get al. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast. 1999;15:963–72. [DOI] [PubMed] [Google Scholar]
- Laribee RN, Shibata Y, Mersman DPet al. CCR4/NOT complex associates with the proteasome and regulates histone methylation. Proc Natl Acad Sci. 2007;104:5836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Mulder KW, Brenkman ABet al. Phosphorylation of Not4p functions parallel to BUR2 to regulate resistance to cellular stresses in Saccharomyces cerevisiae. PLoS ONE. 2010;5:e9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenssen E, James N, Pedruzzi Iet al. The Ccr4-Not complex independently controls both Msn2-dependent transcriptional activation–via a newly identified Glc7/Bud14 type I protein phosphatase module–and TFIID promoter distribution. Mol Cell Biol. 2005;25:488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C, D'Addario I, Falcone C. The C-terminus of the yeast Lsm4p is required for the association to P-bodies. FEBS Lett. 2007;581:4836–40. [DOI] [PubMed] [Google Scholar]
- Mazzoni C, Serafini A, Falcone C. The inactivation of KlNOT4, a Kluyveromyces lactis gene encoding a component of the CCR4-NOT complex, reveals new regulatory functions. Genetics. 2005;170:1023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersman DP, Du HN, Fingerman IMet al. Polyubiquitination of the demethylase Jhd2 controls histone methylation and gene expression. Genes Dev. 2009;23:951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel AP, Sentis S, Bianchin Cet al. BTG2 antiproliferative protein interacts with the human CCR4 complex existing in vivo in three cell-cycle-regulated forms. J Cell Sci. 2003;116:2929–36. [DOI] [PubMed] [Google Scholar]
- Mulder KW, Inagaki A, Cameroni Eet al. Modulation of Ubc4p/Ubc5p- mediated stress responses by the RING-finger-dependent ubiquitin-protein ligase Not4p in Saccharomyces cerevisiae. Genetics. 2007;176:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo V, Falcone C, Mazzoni C. Apoptosis and aging in mitochondrial morphology mutants of S. cerevisiae. Folia Microbiol (Praha). 2007;52:479–83. [DOI] [PubMed] [Google Scholar]
- Panasenko O, Landrieux E, Feuermann Met al. The yeast Ccr4-Not complex controls ubiquitination of the nascent- associated polypeptide (NAC-EGD) complex. J Biol Chem. 2006;281:31389–98. [DOI] [PubMed] [Google Scholar]
- Panasenko OO, Collart MA. Not4 E3 ligase contributes to proteasome assembly and functional integrity in part through Ecm29. Mol Cell Biol. 2011;31:1610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko OO, Collart MA. Presence of Not5 and ubiquitinated Rps7A in polysome fractions depends upon the Not4 E3 ligase. Mol Microbiol. 2012;83:640–53. [DOI] [PubMed] [Google Scholar]
- Panasenko OO. The role of the E3 ligase Not4 in cotranslational quality control. Front Genet. 2014:5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–46. [DOI] [PubMed] [Google Scholar]
- Preissler S, Reuther J, Koch Met al. Not4-dependent translational repression is important for cellular protein homeostasis in yeast. EMBO J. 2015;34:1905–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–71. [PubMed] [Google Scholar]
- Reijns MA, Alexander RD, Spiller MPet al. A role for Q/N-rich aggregation-prone regions in P-body localization. J Cell Sci. 2008;121:2463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins M, Huard S, Morettin Aet al. Lysine acetyltransferase NuA4 and acetyl-CoA regulate glucose-deprived stress granule formation in Saccharomyces cerevisiae. PLos Genet. 2017;13:e1006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt ME, Brown TA, Trumpower BL. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini A. Lo studio di NOT4, un componente del complesso CCR4-NOT, rivela nuove funzioni nei lieviti Saccharomyces cerevisiae e Kluyveromyces lactis. Ph.D. Thesis. Università di Roma La Sapienza, Italy. 2004. [Google Scholar]
- Takalo M, Salminen A, Soininen Het al. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Tanaka F, Robitschek Jet al. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet. 2003;12:749–57. [DOI] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RRet al. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell. 2001;104:377–86. [DOI] [PubMed] [Google Scholar]
- Vanderweyde T, Youmans K, Liu-Yesucevitz Let al. Role of stress granules and RNA-binding proteins in neurodegeneration: a mini-review. Gerontology. 2013;59:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Chang TC, Yamashita Yet al. Concerted action of poly(A) nucleases and decapping enzyme in mammalian mRNA turnover. Nat Struct Mol Biol. 2005;12:1054–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.