Abstract
All vertebrates share a canonical retina with light-sensitive photoreceptors in the outer retina. These photoreceptors are of two kinds: rods and cones, adapted to low and bright light conditions, respectively. They both show a peculiar morphology, with long outer segments, comprised of ordered stacks of disc-shaped membranes. These discs host numerous proteins, many of which contribute to the visual transduction cascade. This pathway converts the light stimulus into a biological signal, ultimately modulating synaptic transmission. Recently, the zebrafish (Danio rerio) has gained popularity for studying the function of vertebrate photoreceptors. In this review, we introduce this model system and its contribution to our understanding of photoreception with a focus on the cone visual transduction cascade.
Keywords: Zebrafish, Visual transduction, Photoreceptors, Cones
Introduction
All vertebrates share a canonical retina with light-sensitive photoreceptors in the outer retina. These photoreceptors are of two kinds: rods and cones. Rod photoreceptors are characterized by higher light sensitivity and slower kinetics, mainly mediating monochromatic low-light vision [191, 50, 57, 105]. Cone photoreceptors on the other hand function under bright light, conveying luminance and color information. In vertebrates, they come in up to four different subtypes, depending on their peak absorption. Both photoreceptor types share a peculiar morphology with a large outer segment comprised of an ordered stack of discs, which contain the proteins of the visual transduction cascade. This biochemical pathway transforms the physical stimulus of light into a biological signal. Outer segments are modified primary cilia that are connected via an axoneme to the mitochondrium-rich inner segment [84]. Synapses of the photoreceptors are among the most complex synapses in the vertebrate brain, featuring ribbons that are thought to enable tonic glutamate release into the synapse [162, 175].
Photoreceptors have been intensively studied in different model organisms. Biochemists favor large bovine eyes for their large yield of proteins. Electrophysiologists favor the amphibians for their comparatively large photoreceptors and geneticists have mainly focused on rodent eyes due to the genetic amenities available in these systems.
More recently, the small tropical teleost zebrafish (Danio rerio) joined the ranks of model system for retinal research. Besides their favorable biological properties, such as small body size, easy maintenance, and large number of offspring, there are several properties of their visual system that have endeared this model system to visual scientists [157]. Unlike the rod-dominant amphibian or rodent retina, the majority of photoreceptors in zebrafish are cones, with about 92% in zebrafish larvae and about 60% in the adult [49, 2, 222]. The larval retina also serves as a model for the primate fovea, featuring a cone-rich acute zone responsible for prey detection [212]. Moreover, more than 70% of human genes have direct orthologues in the zebrafish genome [69], making zebrafish an ideal model to study eye or more specifically cone diseases in humans [61, 11, 113]. The genetic toolbox to manipulate zebrafish has massively expanded during the past decade, including DNA insertion, precisely controlled transgene expression, and CRISPR/Cas genome editing [131]. Because the zebrafish retina starts to transmit visual information at very early stages (3 days post fertilization (dpf)), the function of the visual system can be assessed at early larval stages. Finally, zebrafish larvae are transparent, making them well suited for live imaging (e.g., [135, 222]).
Zebrafish retina signaling with related ocular and retinal diseases have been reviewed recently [8, 123, 113, 126, 18, 11]. In this review, we will provide an overview of biochemical and physiological processes in zebrafish photoreceptors with a focus on the visual transduction cascade, the very first step of image-forming vision.
Zebrafish outer retina
The zebrafish retina possesses one rod type and four morphologically and spectrally distinct cone subtypes, namely short single cones (ultraviolet (UV)-sensitive), long single cones (blue-sensitive), double cone accessory members (green-sensitive), and double-cone principle members (red-sensitive). Double cones exist in most vertebrates, but are absent in most placental mammals, elasmobranches, and catfish [44]. Zebrafish photoreceptors are coupled by gap junction, mainly mediated by Connexin 35 (the zebrafish homologue of mammalian Cx36) [111]. Fish photoreceptor coupling is regulated by the circadian clock, with cone-cone and rod-cone coupling being increased during nighttime [151, 111].
In the absence of pupillary reflexes, many lower vertebrates developed retinomotor movements to adapt to changes in light conditions. In darkness, a mobile part of photoreceptor inner segment, called the myoid, drives cones to elongate and rods to contract [124, 67]. Meanwhile, pigment granules (melanosomes) of the retinal pigment epithelium (RPE) concentrate at the basal part of the RPE. In this way, cone outer segments are buried deeply inside basal RPE while rod outer segments are optimally exposed to incoming light, by being situated far from pigment granules. During light adaptation, cones contract while rods elongate concomitant to pigment granule translocation towards the apical part of the RPE. Therefore, cone outer segments are exposed to light and the rod outer segments are protected by the RPE, akin to sunglasses [1]. The zebrafish retina shows adult-like retinomotor movement from 28 dpf on. Pigment granules take about an hour to migrate to fully light adapted position, while double cone outer segment contraction finishes in about 20 minutes [124, 67]
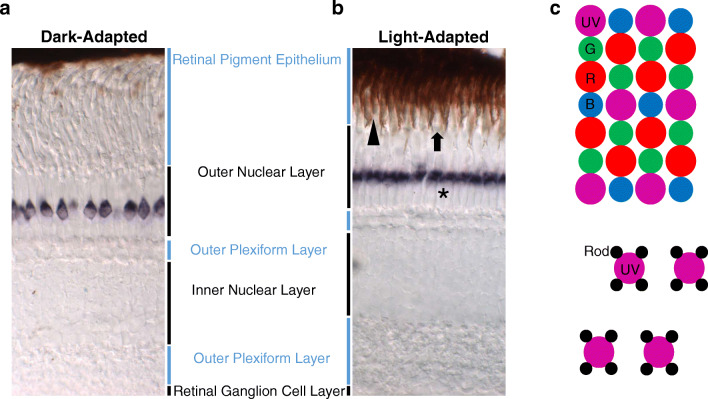
Longitudinal sections of adult retina demonstrate that different photoreceptors are organized into different layers in zebrafish [153, 20] (Fig. 1a, b). The nuclei of rods are located distal to all cone nuclei. The nuclei of UV cones, blue cones, and double cones are located in the distal, medial, and proximal zones of the outer retina, respectively. Cone photoreceptors in the adult zebrafish retina are orderly arranged into a row mosaic pattern (Fig. 1c), in which a red cone neighbors a blue cone while a green cone neighbors a UV cone [2, 107]. Rods project into a square shape around each UV cone to form an integral photoreceptor mosaic [49].
Fig. 1.
Adult zebrafish retina and photoreceptor mosaic. Dark-adapted adult zebrafish retina section (a) and light-adapted section (b) are organized into different cellular layers. The nuclei of rod and cone photoreceptors are located in the outer nuclear layer. During light adaptation, photoreceptor myoid drives cones to contract and rods to elongate to protect rods from over-bleaching, known as retinomotor movement. UV opsin (sws1) is labeled by in situ hybridization. Arrowhead denotes double cone. Arrow denotes blue cone. Star denotes cell body of rod. Schematic of the zebrafish photoreceptor planar mosaic arrangement (c) [153, 2, 49]. UV, UV cone; R, red cone; G, green cone; B, blue cone
The situation is different in the larval retina, where photoreceptors are anisotropically distributed [222]. All cone types are concentrated at the horizon and lower visual field, which may mediate color vision. UV cone density shows a peak at around 30° above the horizon, which is essential for visual prey hunting [212]. The upper visual field is dominated by rods, supporting the effectively achromatic vision towards the sky. This anisotropic distribution is adapted to the spectral content in the natural visual environment serving behavioral demands.
Visual transduction cascade
The main function of photoreceptors is the capture of photons of visual light and the subsequent transformation of this physical stimulus into a biological signal, ultimately modifying the release of the neurotransmitter glutamate by the photoreceptor synapse [50, 105, 57, 22]. The visual transduction cascade and its regulation are among the best-understood trimeric G protein signaling pathways. All reactions take place in the outer segments of photoreceptors, most of them associated with the membrane (Fig. 2). Zebrafish genes involved in visual transduction cascade are summarized in Table 1.
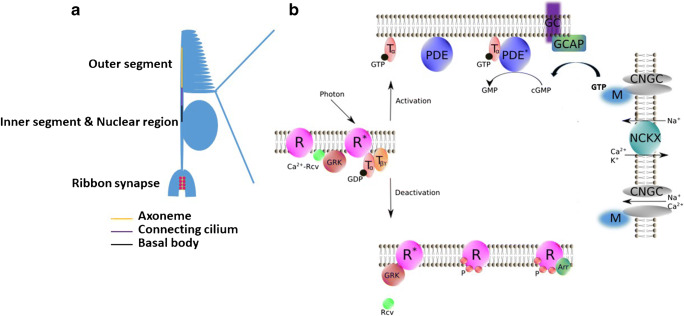
Fig. 2.
Cone photoreceptor morphology (a). Schematic representation of vertebrate visual transduction cascade and Ca2+-regulated deactivation processes (b). Photon absorption activates R. R* then triggers the exchange of GTP for GDP on the Tα. Tα-GTP binds to cyclic nucleotide PDE. Stimulated PDE hydrolyzes free cyclic guanosine monophosphate (cGMP). In darkness, CNGC allows an influx of Na+ and Ca2+, while during illumination CNGC is shut off by cGMP decrease. NCKX is not affected by light, which results in a light-induced intracellular Ca2+ concentration decline. Rcv modulates phosphorylation of R* via GRK in a Ca2+-dependent manner. Phosphorylated R then is fully deactivated by the binding of Arr. R, visual pigment (inactive); R*, light-activated visual pigment; Tα, transducin α subunit; Tβγ, transducin β and γ subunits; PDE, phosphodiesterase (inactive); PDE*, PDE-transducin α complex: NCKX, Na+/Ca2+, K+ exchanger; Arr, arrestin; GRK, G protein–coupled receptor kinase; Rcv, Recoverin; CNGC, cyclic nucleotide–gated ion channel; P, phosphorylation; M, CNG-modulin; GC, guanylate cyclase; GCAP, guanylate cyclase activating protein. Figure was drawn using Inkscape. Inkscape http://www.inkscape.org/. Reproduced with permission from Zang and Neuhauss [217]
Table 1.
Summary of zebrafish genes involved in visual transduction cascade
| Gene name | Protein encoded | Expression pattern in photoreceptor layer | Phenotype in zebrafish with abnormal gene expression | Mouse homologs | Associated human eye diseases |
|---|---|---|---|---|---|
| opn1sw1 | UV opsin | UV cones | Opn1sw | Tritan color blindness [200, 201] | |
| opn1sw2 | Blue opsin | Blue cones | |||
| opn1mw1 | Green opsin | Green cones | Achromatopsia [130] | ||
| opn1mw2 | Green opsin | Green cones | |||
| opn1mw3 | Green opsin | Green cones | |||
| opn1mw4 | Green opsin | Green cones | |||
| opn1lw1 | Red opsin | Red cones | Opn1mw | Achromatopsia [130] | |
| opn1lw2 | Red opsin | Red cones | Opn1mw | ||
| rho | Rod opsin | Rods | Rod photoreceptor degeneration [218] | Rho | Night blindness [168], retinitis pigmentosa [43] |
| gnat1 | Transducin α subunits | Rods and UV cones | Gnat1 | Night blindness [128] | |
| gnb1a | Transducin β subunits | Rods and UV cones | Gnb1 | ||
| gnb1b | Transducin β subunits | Rods and UV cones | Gnb1 | ||
| gngt1 | Transducin γ subunits | Rods and UV cones | Gngt1 | ||
| gnat2 | Transducin α subunits | Cones | Largely reduced photoresponse [21] | Gnat2 | Achromatopsia [91] |
| gnb3a | Transducin β subunits | Cones | Gnb3 | ||
| gnb3b | Transducin β subunits | Cones | Gnb3 | ||
| gngt2a | Transducin γ subunits | Cones | Gngt2 | ||
| gngt2b | Transducin γ subunits | Cones | Gngt2 | ||
| pde6a | PDE catalytic α subunit | Rods and UV cones | Pde6a | Autosomal recessive retinitis pigmentosa [72] | |
| pde6b | PDE catalytic β subunit | Rods and UV cones | Pde6b | Autosomal recessive retinitis pigmentosa [122] | |
| pde6ga | PDE inhibitory γ subunit | Rods and UV cones | Pde6g | ||
| pde6gb | PDE inhibitory γ subunit | Rods and UV cones | Pde6g | ||
| pde6c | PDE catalytic α′ subunit | Cones | Diminished cone ERG and OKR, and cone degeneration [134, 172] | Pde6c | Cone dysfunction and achromatopsia [27, 59, 186] |
| pde6ha | PDE inhibitory γ′ subunit | Cones | Pde6h | ||
| pde6hb | PDE inhibitory γ′ subunit | Cones | Pde6h | ||
| pde6i | PDE inhibitory γ′ subunit | ||||
| cnga1a | CNG channel α1 subunit | Rods | Cnga1 | Autosomal recessive retinitis pigmentosa [42] | |
| cnga1b | CNG channel α1 subunit | Rods | Cnga1 | ||
| cngb1a | CNG channel β1 subunit | Rods | Cngb1 | Autosomal recessive retinitis pigmentosa [5, 14, 93] | |
| cngb1b | CNG channel β1 subunit | Rods | Cngb1 | ||
| cnga3a | CNG channel α3 subunit | Cones | Cnga3 | ||
| cnga3b | CNG channel α3 subunit | Cones | Cnga3 | ||
| cngb3.1 | CNG channel β3 subunit | Cones | Cngb3 | ||
| cngb3.2 | CNG channel β3 subunit | Cones | Cngb3 | ||
| grk1a | G protein–coupled receptor kinase 1a | Rods | Overexpression of grk1a in rods shows minor effect [194] | Grk1 | Oguchi disease [209] |
| grk1b | G protein–coupled receptor kinase 1b | Cones | Delayed ERG response recovery and reduced temporal contrast sensitivity[31] | Grk1 | |
| grk7a | G protein–coupled receptor kinase 7a | Cones | grk7a knockdown [152], grk7a knockout [31], ectopic expression of grk7a in rods [194] | ||
| grk7b | G protein–coupled receptor kinase 7b | UV cones | |||
| arrSa | ArrestinSa | Rods and UV cones | Arr1 | Oguchi disease [58] | |
| arrSb | ArrestinSb | Rods and UV cones | Arr1 | ||
| arr3a | Arrestin3a | Double cones | Delayed ERG response recovery and decreased temporal contrast sensitivity [148] | Arr3 | |
| arr3b | Arrestin3b | Blue and UV cones | Arr3 | ||
| rgs9a | Regulators of G protein signaling 9a | Cones | Rgs9 | Bradyoposia [133] | |
| rgs9b | Regulators of G protein signaling 9b | Rods | Rgs9 | ||
| gucy2e | Guanylate cyclase E | Rods and UV cones | Outer segment loss and shortening, OMR defects [176] | Gucy2e | Leber congenital amaurosis 1 [142] |
| gucy2f | Guanylate cyclase F | Rods and UV cones | Gucy2f | ||
| gucy2d | Guanylate cyclase D | Cones | OKR and OMR impairments [127], PDE6c downregulation [79] | Gucy2d | |
| slc24a1 | Na+/Ca2+, K+ exchanger 1 | Slc24a1 | Congenital stationary night blindness [150] | ||
| slc24a2 | Na+/Ca2+, K+ exchanger 2 | Slc24a2 | |||
| rcv1a | Recoverin 1a | Rods and UV cones | Accelerates photoresponse recovery [215] | Rcv1 | |
| rcv1b | Recoverin 1b | Cones | |||
| rcv2a | Recoverin 2a | Cones | Accelerates photoresponse recovery [215] | ||
| rcv2b | Recoverin 2b | Cones | Accelerates photoresponse recovery [215] | ||
| gcap1 | Guanylate cyclase activation protein 1 | Rods and UV cones | Guca1a | Autosomal dominant cone dystrophy [140] | |
| gcap2 | Guanylate cyclase activation protein 2 | Rods and UV cones | Guca2b | Autosomal dominant retinal dystrophies [160] | |
| gcap3 | Guanylate cyclase activation protein 3 | Cones | Prolonged photoresponse recovery [9] | ||
| gcap4 | Guanylate cyclase activation protein 4 | Cones | |||
| gcap5 | Guanylate cyclase activation protein 5 | Cones | |||
| gcap7 | Guanylate cyclase activation protein 7 | Cones | |||
| eml1 | CNG-modulin | Cones | Reduced light sensitivity [94] | Eml1 |
The photoreceptor outer segment is a cylindrical structure comprised of an ordered stack of disc-shaped membranes, allowing a high concentration of transmembrane visual pigments and increasing the probability of photon catch [92].
Rod and cone photoreceptors share a generally similar visual transduction cascade, but adopt rod- or cone-specific protein isoforms for many of the cascade’s components. The evolution of these photoreceptor-specific paralogues is a well-studied paradigm for the fate of duplicated genes in evolution [103]. This is particularly true for teleost genomes that underwent a lineage-specific whole-genome duplication, following two rounds of whole-genome duplications in the early vertebrate lineage [7, 190, 184].
The generation, deletion, and fate of these duplicated genes add a fascinating complexity to the teleost visual transduction cascade that is beyond the scope of this review. However, the multitude of gene variants to be discussed in the following is the direct consequence of whole-genome duplications in the past [63, 103, 104, 106, 99, 100, 60].
The visual transduction cascade is initiated by the absorption of photons by opsins. These G protein–coupled 7-transmembrane receptors are covalently bound to a light-sensitive chromophore via a Schiff base forming the photopigment complex [73]. Upon the absorption of a photon, chromophore (most commonly vitamin A1 11-cis-retinal) in the photopigment complex isomerizes to all-trans-retinal, which activates the opsin (now referred to R*) by inducing a transformational change [51, 52]. Zebrafish cones express a total of 8 cone opsins, namely opn1sw1 (also known as sws1), opn1sw2 (also known as sws2), opn1mw1 (also known as rh2-1), opn1mw2 (also known as rh2-2), opn1mw3 (also known as rh2-3), opn1mw4 (also known as rh2-4), opn1lw1 (also known as lws1), and opn1lw2 (also known as lws2) [2, 145, 182]. Hence, there are four green (short wave length) and two red (long wavelength) opsin variants. These variants have slightly different peak absorption properties potentially allowing a bewildering range of fine-tuning of color perception [30, 23]. The expressions of these multiple rh2 and lws genes follow a spatiotemporal order during development [182]. Rod photoreceptors express only a single-rod opsin gene rho (also known as rh1) [218]. Mutations in human rod opsin may produce night blindness or retinal degeneration, while cone opsin defects may lead to achromatopsia [168, 43, 130, 200, 201]. For years, vitamin A1-based photopigment has been recognized as the sole photopigment existing in zebrafish photoreceptors under standard laboratory conditions [23, 30]. The peak absorption spectra (λmax) of A1-based photopigments differ markedly and cover a wide spectrum from 355 nm (UV) to 558 nm (red) in vivo. However, thyroid hormone (TH) treatment or colder water temperature may result in a transition from A1 to vitamin A2 (11-cis 3,4-didehydroretinal)-based photopigments. This demonstrates a functional A1-A2 photopigment interchange system in zebrafish [159, 3, 48]. The λmax of A2-based photopigments shifts towards longer wavelength relative to A1-based photopigment [115, 66]. This interchange system is frequently observed in freshwater fishes and amphibians, and may be adapted to the red-shifted light environment in fresh water compared with marine and terrestrial environments [149, 197, 222]. Another mechanism to tune photopigments is to change opsin expression levels. TH treatment has been reported to reduce lws2 (548 nm) and rh2-1 (467 nm), while increasing lws1 (558 nm) and rh2-2 (488 nm) in larvae, favoring the opsins with longer λmax [119]. Both the mechanisms red-shift the zebrafish photoreceptor spectral sensitivity. Moreover, in TH receptor-defective fish, retinal progenitors designed to become red cones are transfated into UV cones, providing another mechanism for TH to regulate long-wavelength vision [180, 195, 37].
Besides the visual opsins, the zebrafish genome harbors 32 nonvisual opsin genes, which encode opsins forming functional photopigments with different chromophores [35, 34, 56]. Many, but not all of them, are expressed in the photoreceptor layer. Their functions in photoreceptors are largely unknown, but a role in circadian light entrainment is discussed [56, 174, 26].
Activated opsin (R*) interacts with the trimeric G protein transducin [22, 50, 105]. Binding of R* to transducin results in the replacement of GDP by GTP at the active site of the transducin α subunit. This nucleotide exchange dissociates the activated α subunit (Gα*) and the heterodimer of β and γ subunits (Gβγ). Gα* then binds to the cGMP Phosphodiesterase 6 (PDE6) [91, 128].
Zebrafish rod and cone photoreceptors express different variants of three subunits [99, 28]. In rods, gnat1 encodes transducin α subunits, gnb1a and gnb1b encode β subunits, and gngt1 encodes γ subunits (all these variants possibly also in UV cones), while in cones, gnat2 encodes α subunits, gnb3a and gnb3b encode β subunits, and gngt2a and gngt2b encode γ subunits.
Surprisingly, a zebrafish mutant defective in the cone-specific gnat2 gene (no optokinetic response f (nof)) shows a residual photoresponse that needs to be mediated by an unknown transducin-independent mechanism [21].
Interestingly, both Gα and Gβ show massive light-induced translocation from rod outer segment to inner segment in mice, which may contribute to light adaptation in rods [170]. However, Gα translocation has not been observed in zebrafish cones (or mouse cone), indicating light adaptation mechanisms may vary between rods and cones [85, 46, 114].
When Gα* binds to PDE6, two PDE6 inhibitory subunits dissociate from the active sites and allow the activation of PDE6 to hydrolyze cGMP [32]. The rod PDE6 variant is expressed as a heterotetramer consisting of two catalytic α and β subunits encoded by pde6a and pde6b, and two identical inhibitory γ subunits encoded by pde6g. Cone PDE6 comprises two homodimers of two catalytic α′ subunits encoded by pde6c and two inhibitory γ′ subunits encoded by pde6h [106, 62, 32, 72, 122].
Zebrafish retain the same set of catalytic subunit genes as in humans (pde6a, pde6b, and pde6c), while inhibitory subunits are encoded by duplicated paralogues: pde6ga and pde6gb in rods and possibly UV cones and pde6ha and pde6hb in all cones [100, 134]. An additional inhibitory subunit gene pde6i has also been found in zebrafish, and some other lower vertebrates including fish (teleost and non-teleost) and amphibians [100].
Mutations in the cone-specific pde6c gene are associated with cone dysfunction in human patients with achromatopsia [27, 59, 186]. Mutations in cone-catalytic subunit pde6c result in almost diminished cone electroretinogram (ERG) and optokinetic response (OKR), and cone photoreceptor degeneration in zebrafish [134, 172]. The mechanism underlying cone degeneration is unknown and is not linked to increased cytosolic Ca2+ levels [118].
Ultimately, the visual transduction cascade regulates the opening of cyclic nucleotide-gated (CNG) ion channels. These non-selective cation channels are opened by cGMP binding [210]. Falling cGMP concentration due to cGMP hydrolysis by PDE6 leads to the closure of these CNG channels, suppressing the circulating dark current and resulting in photoreceptor hyperpolarization. CNG channels are heteromeric proteins consisting of α and β subunits [81, 125]. Rod channels are assembled from 3 CNGA1 subunits and 1 CNGB1, while cone channels are assembled from 2 CNGA3 subunits and 2 CNGB3 subunits [202, 220, 221, 102].
Mutations in CNGA1 and CNGB1 have been identified in human patients with autosomal-recessive retinitis pigmentosa [14, 5, 93, 42]. In zebrafish, all visual CNG channel genes have retained two paralogues, but no additional information is available.
Regulation of visual transduction
At the biochemical level, visual transduction is mainly regulated by its deactivation kinetics. To deactivate the visual transduction cascade, deactivation of both R* and Gα-PDE* complex and the restoration of cGMP concentrations are required [22, 50].
The lifetime of R* is tightly regulated by arrestin proteins that efficiently inactivate photopigmet by binding to its phosphorylated form. Therefore, the first step of R* inactivation is phosphorylation. R* is phosphorylated by G protein–coupled receptor kinases (GRKs). Mice and rats express only GRK1 in both rods and cones, while humans express GRK1 in rods and GRK1 and GRK7 in cones [219, 117, 165, 199]. In zebrafish, both visual grk genes are present as two paralogues. grk1a is expressed exclusively in rods, grk1b and grk7a in all cones, and grk7b only in UV cones [152, 196] (unpublished data). GRK deficiency in humans leads to Oguchi disease, which is characterized by a delay of rod recovery [209]. A grk7a knockdown model produces largely delayed ERG response recovery and reduced temporal contrast sensitivity in the OKR [152]. Another study demonstrates similar but more modest effects in either grk1b or grk7a mutants [31].
Overexpression of grk1a in zebrafish rods shows minor effect on rod photoresponse, suggesting that endogenous GRK1a protein is already at saturation levels. Ectopic expression of cone grk7a in rods resulted in cone-like rod responses [194].
The binding of arrestin completely deactivates the phosphorylated photopigment [98, 203]. In the mouse retina, both rod (ARR1) and cone (ARR3) arrestins are co-expressed in cone photoreceptors [132, 203]. Mutations in ARR1 are a cause of Oguchi disease in human [58]. In zebrafish, arrsa and arrsb (orthologues of Arr1) are expressed in rods while arr3a exists in double cones and arr3b exists in blue and UV cones, indicating subfunctionalization of the two paralogues. arr3a knockdown resulted in a severe delay in ERG response recovery and decreased temporal contrast sensitivity [148].
Regulators of G protein signaling 9 (RGS9) act as GTPase activating protein to deactivate Gα*-PDE complex [17]. Mammals have a single Rgs9 gene, while zebrafish have two rgs9 genes, with rgs9a being expressed in cones and rgs9b in rods [33, 104] (unpublished data). Inactivating mutations in humans lead to bradyopsia, a rare condition characterized by slower photoreceptor deactivation [133]. A landmark study using Rgs9 overexpression in mice demonstrated its crucial role to rate-limit rod visual transduction recovery [96].
To restore the dark current, cGMP needs to be resynthesized by membrane-bound guanylate cyclases (GCs) [88, 167]. Photoreceptor-specific GCs are regulated by the small Ca2+-binding guanylate cyclase activation proteins (CGAPs) [90].
Mammals have two photoreceptor-specific GCs, GC-E (known as GC1) and GC-F (known as GC2), both of which are co-expressed in rods and cones [103, 88, 60]. GC-E is more concentrated in cones, while the expression of GC-F is more prominent in rods. Mutations in GC-E have been shown to cause Leber congenital amaurosis 1 (LCA1), a severe form of pediatric blindness in humans [142]. The zebrafish possess 3 GCs. GC-E (known as GC1), GC-F (known as GC2), and GC-D (known as GC3) are encoded by gucy2e (previous name gucy2f), gucy2f (previous name gc2), and gucy2d (previous name gc3), respectively. Both gucy2e and gucy2f are expressed in rods and UV cones, while gucy2d encodes the only cone-specific GC in all cone subtypes [55, 144].
A zebrafish gucy2d mutant has been identified in behavioral screen by displaying OKR and optomotor response (OMR) impairments [127]. PDE6c protein levels are downregulated in gucy2d knockdown larvae, indicating the interdependence between these two regulators of cGMP metabolism [79]. A knockdown of the gucy2d gene results in the loss and shortening of outer segments and defects in the OMR [176].
In darkness, the open non-selective CNG channels mediate a Ca2+ influx into the photoreceptor outer segment. Ca2+ efflux via Na+/Ca2+, K+ exchanger (NCKX) balances this influx, producing a moderately high intracellular Ca2+ concentration as shown in rods of different species [101, 207]. Under light illumination, CNG channels are closed due to the decrease in cGMP concentration, while Ca2+ efflux continues, resulting in a decrease of intracellular Ca2+ concentration in the outer segment [211]. This light-induced Ca2+ decline can be simultaneously measured with light response in zebrafish UV cones, demonstrating similar kinetics of Ca2+ extrusion via NCKX to that of CNG channel current [109].
NCKX proteins are encoded by SLC24 gene family members. They show a cell-type-specific expression with NCKX1 being expressed in rods and NCKX2 in cones [193, 147, 143, 150]. NCKX2-deficient mice show no or only mild functional defect, suggesting that compensating transporters may mediate ion exchange as well [112, 156]. A recent study proposed that NCKX2 and NCKX4 cooperated to facilitate the rapid and efficient extrusion of Ca2+ from mouse cones. NCKX4 has its well-established function in olfactory sensory neurons and is similarly expressed in all cones in the zebrafish retina [192]. The expression pattern of the other NCKX coding genes is unknown in zebrafish, but studies in the striped bass show expression of nckx1 in rods and four splice variants of nckx2 in cones [137].
The reduction of cytoplasmic Ca2+ negatively feedbacks to the phototransduction cascade, triggering the rapid photoresponse recovery and facilitating photoreceptor adaptation to background light [120, 129]. During light adaptation, photoreceptor light sensitivity is reduced and response kinetics is accelerated, to avoid saturation and to operate across a wide range of environmental light intensity [50]. This has been achieved by mechanisms that primarily involve the regulation of GRKs by Recoverin, GCs by GCAPs, and CNG channels by CNG-modulin (or Calmodulin) [138, 191].
Recoverin (RCV) is a small neuronal calcium sensor (NCS), which is primarily located in vertebrate photoreceptors. Upon Ca2+ binding, RCV undergoes a pronounced conformational change, the so-called Ca2+-myristoyl switch, which translocates the proteins from a cytosolic form to a membrane tethered conformation, allowing targeting and inhibiting GRK proteins [82, 166, 183, 6, 40, 83, 217]. Light stimulation reduces intracellular Ca2+ concentration, allowing the Ca2+-free RCV releasing GRK. GRK disinhibition accelerates R* phosphorylation, enabling arrestin binding.
While there is only one RCV isoform in mammals (RCV1), four rcv genes are encoded in the zebrafish genome (rcv1a, rcv1b, rcv2a, and rcv2b) [215]. rcv1b, rcv2a, and rcv2b are cone RCV, while rcv1a is expressed in rods and UV cones. Mouse RCV1 experiences a remarkable light-induced translocation from outer and inner segment towards synaptic terminals in rods, which has not been observed in zebrafish photoreceptors by studying all zebrafish RCVs [177] (unpublished observation). Downregulation of cone RCV accelerates photoresponse recovery, but this effect is abolished when cone GRK7a is simultaneously knocked-down. This result not only indicates that RCV regulates opsin deactivation via GRK, but also demonstrates that the cone opsin deactivation kinetics dominates the overall photoresponse shut off kinetics in vivo [215]. Interestingly, different RCVs contribute at distinct light intensities. This implies different Ca2+ sensitivities for these RCVs, since intracellular Ca2+ concentration correlates with light levels [158]. Indeed, a recent biochemical work demonstrated distinct Ca2+ affinities, Ca2+-dependent membrane binding, and Ca2+-induced conformational changes among zebrafish isoforms [45]. Furthermore, salamander cone photoresponse, but not rod response, is also dominated by a Ca2+-sensitive mechanism [121, 216]. If the Ca2+-sensitive dominance is a general feature in cone photoresponse, it may contribute to the more powerful light adaptation of cones compared to rods.
To restore the dark current, cGMP needs to be resynthesized by GC, which is under the regulation of small Ca2+-binding proteins called GCAPs [90, 39]. GCAPs belong to the superfamily of EF-hand Ca2+-binding proteins, harboring four EF-hand Ca2+-binding motifs, three of which are functional [89]. Unlike RCVs, GCAPs do not undergo a classical Ca2+-myristoyl switch, but the myristoyl group does play an important role to regulate GCAP properties, including Ca2+ sensitivity, GC affinity, and the catalytic efficiency of the enzyme. Ca2+-binding GCAPs together with GCs form GC/GCAP complex in darkness. Ca2+ reduction during light exposure triggers a conformational change in GCAPs, which results in a transformational change within the GC/GCAP complex, increases GC catalytic activity and reopens the CNG channels. During light adaptation, the Ca2+-sensitive GCAP activity will also prevent the closure of all CNG channels and keep photoreceptors responsive.
GCAP1 and 2 are expressed in mammalian rods and cones. The human (but not the mouse) genome also processes a cone-specific CGAP3 [75, 140, 160]. Zebrafish photoreceptors express six GCAPs, of which gcap3, 4, 5, and 7 are restricted to cones and gcap1 and 2 are exclusively expressed in rods and UV cones [76, 144, 54]. These isoforms show distinct Ca2+ sensitivities of GC activation, Ca2+/Mg2+-dependent conformational changes, and Ca2+-binding affinities [164, 179]. Light exposure allows intracellular Ca2+ fluctuating to different levels, in which distinct CGAPs may reach their optimal working range.
GCAP3 is first expressed in a non-myristoylated form in larvae and then becomes myristoylated in the adult retina [54]. Although GCAP3 has been shown to produce the highest Ca2+-dependent activation of GCs in native zebrafish retina, gcap3 knockdown does not induce any visual behavioral abnormalities [55]. In another study, GCAP3 in green cone was inactivated by antibody injections. Whole-cell patch clamp recordings demonstrated that the photoresponse recovery is strongly prolonged, confirming GCAP3 function to activate GC to restore CNG channel current in cones [9].
cGMP affinity of CNG channels is regulated in a Ca2+-dependent manner in all sensory neurons [19]. Ca2+ cannot directly bind to the channels but work via modulator proteins, which have been identified as calmodulin in mammalian rods and CNG-modulin in fish cones [70, 146]. However, the contribution of CNG channel modulation by Ca2+ in regulating light adaptation is very limited in rods [29, 95]. On the other hand, CNG-modulin has been shown to regulate the cGMP dependence of CNG channels in a Ca2+-sensitive manner, and to modulate the light response kinetics in striped bass cone [146]. CNG-modulin is encoded by the eml1 gene in zebrafish. eml1 knockdown reduces the light sensitivity of dark-adapted and light-adapted cones; the sensitivity cannot be restored to wild-type levels [94]. These experiments demonstrate a stronger Ca2+ feedback to CNG channels in cones compared to rods.
Outer segment: a specialized primary cilium
Photoreceptor outer segments are strongly modified specialized primary cilia, sharing many general structural and biochemical features of cilia [77]. Outer segment stacked discs are arranged on the side of a microtubule-based axoneme, anchoring inside the inner segment through a connecting cilium and its basal body. Therefore, the connecting cilium, known as the transition zone in other cell types, connects outer and inner segment, mediating bi-directional protein trafficking [181]. Dysfunctions of primary cilia result in human disorders referred to as ciliopathies, which were reviewed elsewhere [11].
Outer segments are constantly bombarded by photons and their integrity is endangered by radical oxygen species. Since photoreceptors, like most neurons of the central nervous system, cannot be replaced, photoreceptors constantly rejuvenate themselves by renewing their outer segments. New discs are synthesized by ciliary membrane evagination at the base of the outer segment as the ciliary ectosomes, which then is elongated, flattened, and enclosed inside the outer segment [87, 173, 38, 171]. The tips of the outer segments, containing the oldest and potentially damaged membranes, are phagocytosed and digested by RPE cells. Although outer segment renewal/shedding is essential for photoreceptor homeostasis and survival, molecular mechanisms underlying its regulation are still poorly understood.
Recent works on zebrafish have contributed significantly to our understanding of the molecular mechanisms behind photoreceptor outer segment shedding and renewal. The zebrafish lends itself ideally to transgenically label cellular structures or cells, as Willoughby and colleagues have used elegantly for the outer segment [205]. They devised a stable line with heat shock–inducible fluorescent membrane protein that allowed them to follow the renewal and shedding of the rod outer segments as an updated experimental approach to the classic radioactive labeling method [214]. This line was then used in a high-content small-molecule screens that among others identified an involvement of cyclooxygenase in outer segment growth, gamma secretase in outer segment shedding, and mTOR in RPE phagocytosis [25].
Some earlier studies demonstrated that disc shedding in frog and cat was initiated by light [15, 53]. A recent zebrafish study using PDE6 inhibitors to block the visual transduction cascade mimicking constant dark conditions indeed inhibited rod outer segment shedding [24]. Interestingly, mammalian rod outer segment shedding remains in constant darkness, instead showing circadian clock controlling disc shedding mechanism [108, 185, 64, 74].
Given the nature of the outer segment, it comes as no surprise that many genes associated with intracellular and ciliary trafficking are involved in outer segment generation and maintenance.
The most abundant protein that needs to be shipped out to the outer segment is rhodopsin. Every second, around 70 rhodopsin molecules are trafficked from the inner to the outer segment [213, 204, 141]. Detailed studies of rhodopsin transport in frogs showed that RAB8, a small GTPase, coats rhodopsin-carrier vesicles and directs them to a selective barrier at the base of connecting cilium [139, 36]. In live imaging experiments in zebrafish, RAB8-directed rhodopsin trafficking in rods has been directly visualized in vivo [135]. The correct localization of RAB8 at the base of the outer segment is regulated by components of the connecting cilium itself, such as CC2D2A and further interaction partners, such as Ninl and MICAL3 [10, 12].
About 10% of outer segment is renewed every day in mammalian photoreceptors [108]. Therefore, intraflagellar transport (IFT), which contributes primarily to traffic visual transduction proteins into the outer segment, is important for outer segment development and structure [77]. IFT-B complex and kinesin motors mediate anterograde movement towards the distal outer segment, while IFT-A and dynein motors mediate retrograde movement towards the cell body [154].
A series of zebrafish studies contributed greatly to our understanding of the mechanism underlying IFT. Mutations affecting the IFT-B complex (IFT52, IFT57, IFT88, IFT172) lead to defects in outer segment formation and/or maintenance, finally resulting in both rod and cone degeneration [188, 41, 65]. Biochemical assays indicated that IFT20, a IFT-A member, requires IFT57 to associate with the IFT particle [97]. In another study, TNF receptor-associated factor 3 interacting protein 1 (TRAF3IP1) was shown to bind to IFT20. It can also interact with RAB8 via Rabaptin5, an endocytosis regulator. This demonstrates a connection between the IFT particle and the GTPase pathway, known to facilitate protein complex assembling [136].
Moreover, microtubular motors play an essential role in transporting IFT complexes. KIF17, kinesin-2 family member, is involved in ciliogenesis [206]. It is located all over zebrafish cones but concentrates at the basal body and the distal tip of the axoneme [13]. Knockdown of kif17 disrupts outer segment structure and mislocates visual transduction proteins [78]. Disc shedding is also promoted by KIF17 and eliminated in its absence [110].
Ribbon synapses
Non-spiking photoreceptors respond and adapt to a wide range of light intensities. The light-induced CNG channel closure generates the graded changes in membrane potential, which in turn regulates tonic neurotransmitter glutamate release at the presynaptic terminals [175, 163, 187]. This graded signaling is facilitated by specialized ribbon synapses, which hold a dense array of synaptic vesicles near active zones along their surface and were firstly identified by electron microscopy as electron dense structures in guinea pig rod synapses [169].
Work on zebrafish has helped to identify the key components of ribbon synapses and their function in signal transmission.
Ribeye is the most abundant protein in the synaptic ribbon [163]. In the zebrafish retina, both ribeyea and ribeyeb are present in the photoreceptors while ribeyea also shows expression in bipolar cells. Downregulation of ribeyea diminishes OKR and reduces ribbon length and number [198, 116].
Synaptojanin (Synj1) is a polyphosphoinositide phosphatase regulating clathrin-mediated endocytosis in conventional synapses [155]. A zebrafish synj1 null mutation (nrc) shows unanchored “floating” ribbons and reduced synaptic vesicles in cone but not rod synapses[189, 68], associated with defect in vision [4].
Photoreceptor L-type voltage-dependent calcium channels (Cav1.4) are located in the vicinity of synaptic ribbons and mediate exocytosis [187]. In darkness, they are opened by the depolarized photoreceptor membrane potential, resulting in calcium-dependent glutamate release. Cav1.4 are heteromultimeric protein complexes comprising of a pore-forming α1F subunit, encoded by CACNA1F, and accessory β and α2δ subunits, encoded by CACNB2 and CACNA2D4, respectively. Mutations in CACNA1F gene result in X-linked congenital stationary night blindness type 2 and cone-rod dystrophy in human [16, 178]. Two paralogues, cacna1fa and cacna1fb are identified in zebrafish with cacna1fa being expressed in photoreceptors while cacna1fb only existing in the inner retina [80]. CACNA1Fa protein exclusively accumulates at the outer plexiform layer and its null mutants (wud) present thinner outer plexiform layer, defective ERG, completely absent of synaptic ribbons, and mislocalized Ribeyeb.
Mutations in human CACNA2D4 are related to autosomal recessive cone dystrophy, while rods in different CACNA2D4 knockout mouse lines are even more severely affected, showing missing or largely defective scotopic and photopic ERG response [208, 86, 71]. More recently, another study focused on zebrafish cacna2d4 encoding Cav1.4 α2δ subunit [116]. cacna2d4 is duplicated in zebrafish as cacna2d4a and cacna2d4b. Double KO shows reduced pore-forming CACNA1Fa expression and minor defects in both visual function and ribbon structure. The zebrafish KO model is associated with similar moderate phenotype in human patients, providing a comprehensive tool to study the related human eye disorders.
Zebrafish show a peculiar phenomenon of disassembled ribbon synapses at least in the larval retina during the night. At light onset, the presynaptic structure is rapidly reassembled for function [47]. This unusual mechanism may have evolved to save energy in rapidly growing larvae.
Conclusion
The zebrafish retina serves as an important model of cone photoreceptor and has already contributed significantly to our understanding of photoreceptor maintenance and function. With its ever-increasing toolbox of imaging and genetic techniques, it will continue to crucially help us further in investigating the outer retina and its diseases.
Acknowledgements
Open Access funding provided by Universität Zürich.
Funding
This work was supported by the Swiss National Science foundation (31003A_173083).
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
This article is part of the special issue on Function and Dysfunction in Vertebrate Photoreceptor Cells in Pflügers Archiv—European Journal of Physiology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ali MA. Retinomotor response: characteristics and mechanisms. Vision Res. 1971;11:1225–1288. doi: 10.1016/0042-6989(71)90010-1. [DOI] [PubMed] [Google Scholar]
- 2.Allison WT, Barthel LK, Skebo KM, Takechi M, Kawamura S, Raymond PA. Ontogeny of cone photoreceptor mosaics in zebrafish. J Comp Neurol. 2010;518:4182–4195. doi: 10.1002/cne.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison WT, Haimberger TJ, Hawryshyn CW, Temple SE. Visual pigment composition in zebrafish: evidence for a rhodopsin-porphyropsin interchange system. Vis Neurosci. 2004;21:945–952. doi: 10.1017/S0952523804216145. [DOI] [PubMed] [Google Scholar]
- 4.Allwardt BA, Lall AB, Brockerhoff SE, Dowling JE. Synapse formation is arrested in retinal photoreceptors of the zebrafish nrc mutant. J Neurosci. 2001;21:2330–2342. doi: 10.1523/JNEUROSCI.21-07-02330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alshamrani AA, Raddadi O, Schatz P, Lenzner S, Neuhaus C, Azzam E, Abdelkader E. Severe retinitis pigmentosa phenotype associated with novel CNGB1 variants. Am J Ophthalmol Case Rep. 2020;19:100780. doi: 10.1016/j.ajoc.2020.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 7.Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH. Zebrafish hox clusters and vertebrate genome evolution. Science. 1998;282:1711–1714. doi: 10.1126/science.282.5394.1711. [DOI] [PubMed] [Google Scholar]
- 8.Angueyra JM, Kindt KS. Leveraging zebrafish to study retinal degenerations. Front Cell Dev Biol. 2018;6:110. doi: 10.3389/fcell.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aquila M, Dell’Orco D, Fries R, Koch KW, Rispoli G. Incorporating phototransduction proteins in zebrafish green cone with pressure-polished patch pipettes. Biophys Chem. 2019;253:106230. doi: 10.1016/j.bpc.2019.106230. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann-Gagescu R, Dona M, Hetterschijt L, Tonnaer E, Peters T, de Vrieze E, Mans DA, van Beersum SE, Phelps IG, Arts HH, Keunen JE, Ueffing M, Roepman R, Boldt K, Doherty D, Moens CB, Neuhauss SC, Kremer H, van Wijk E. The Ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 2015;11:e1005575. doi: 10.1371/journal.pgen.1005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann-Gagescu R, Neuhauss SC. The photoreceptor cilium and its diseases. Curr Opin Genet Dev. 2019;56:22–33. doi: 10.1016/j.gde.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011;20:4041–4055. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bader JR, Kusik BW, Besharse JC. Analysis of KIF17 distal tip trafficking in zebrafish cone photoreceptors. Vision Res. 2012;75:37–43. doi: 10.1016/j.visres.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M. Segregation of a mutation in CNGB1 encoding the beta-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet. 2001;108:328–334. doi: 10.1007/s004390100496. [DOI] [PubMed] [Google Scholar]
- 15.Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194:1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- 16.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 17.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 18.Bibliowicz J, Tittle RK, Gross JM. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog Mol Biol Transl Sci. 2011;100:287–330. doi: 10.1016/B978-0-12-384878-9.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15:343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Branchek T, Bremiller R. The development of photoreceptors in the zebrafish. Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- 21.Brockerhoff SE, Rieke F, Matthews HR, Taylor MR, Kennedy B, Ankoudinova I, Niemi GA, Tucker CL, Xiao M, Cilluffo MC, Fain GL, Hurley JB. Light stimulates a transducin-independent increase of cytoplasmic Ca2+ and suppression of current in cones from the zebrafish mutant nof. J Neurosci. 2003;23:470–480. doi: 10.1523/JNEUROSCI.23-02-00470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 23.Cameron DA. Mapping absorbance spectra, cone fractions, and neuronal mechanisms to photopic spectral sensitivity in the zebrafish. Vis Neurosci. 2002;19:365–372. doi: 10.1017/s0952523802192121. [DOI] [PubMed] [Google Scholar]
- 24.Campbell LJ, Jensen AM. Phosphodiesterase inhibitors sildenafil and vardenafil reduce zebrafish rod photoreceptor outer segment shedding. Invest Ophthalmol Vis Sci. 2017;58:5604–5615. doi: 10.1167/iovs.17-21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell LJ, West MC, Jensen AM. A high content, small molecule screen identifies candidate molecular pathways that regulate rod photoreceptor outer segment renewal. Sci Rep. 2018;8:14017. doi: 10.1038/s41598-018-32336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavallari N, Frigato E, Vallone D, Fröhlich N, Lopez-Olmeda JF, Foà A, Berti R, Sánchez-Vázquez FJ, Bertolucci C, Foulkes NS. A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol. 2011;9:e1001142. doi: 10.1371/journal.pbio.1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang B, Grau T, Dangel S, Hurd R, Jurklies B, Sener EC, Andreasson S, Dollfus H, Baumann B, Bolz S, Artemyev N, Kohl S, Heckenlively J, Wissinger B. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci U S A. 2009;106:19581–19586. doi: 10.1073/pnas.0907720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Leung T, Giger KE, Stauffer AM, Humbert JE, Sinha S, Horstick EJ, Hansen CA, Robishaw JD. Expression of the G protein gammaT1 subunit during zebrafish development. Gene Expr Patterns. 2007;7:574–583. doi: 10.1016/j.modgep.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Woodruff ML, Wang T, Concepcion FA, Tranchina D, Fain GL. Channel modulation and the mechanism of light adaptation in mouse rods. J Neurosci. 2010;30:16232–16240. doi: 10.1523/JNEUROSCI.2868-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinen A, Hamaoka T, Yamada Y, Kawamura S. Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics. 2003;163:663–675. doi: 10.1093/genetics/163.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrispell JD, Dong E, Osawa S, Liu J, Cameron DJ, Weiss ER. Grk1b and Grk7a both contribute to the recovery of the isolated cone photoresponse in larval zebrafish. Invest Ophthalmol Vis Sci. 2018;59:5116–5124. doi: 10.1167/iovs.18-24455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cote RH. Characteristics of photoreceptor PDE (PDE6): similarities and differences to PDE5. Int J Impot Res. 2004;16(Suppl 1):S28–S33. doi: 10.1038/sj.ijir.3901212. [DOI] [PubMed] [Google Scholar]
- 33.Cowan CW, Fariss RN, Sokal I, Palczewski K, Wensel TG. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc Natl Acad Sci U S A. 1998;95:5351–5356. doi: 10.1073/pnas.95.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies WI, Tamai TK, Zheng L, Fu JK, Rihel J, Foster RG, Whitmore D, Hankins MW. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25:1666–1679. doi: 10.1101/gr.189886.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies WI, Zheng L, Hughes S, Tamai TK, Turton M, Halford S, Foster RG, Whitmore D, Hankins MW. Functional diversity of melanopsins and their global expression in the teleost retina. Cell Mol Life Sci. 2011;68:4115–4132. doi: 10.1007/s00018-011-0785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108(Pt 1):215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- 37.Deveau C, Jiao X, Suzuki SC, Krishnakumar A, Yoshimatsu T, Hejtmancik JF, Nelson RF. Thyroid hormone receptor beta mutations alter photoreceptor development and function in Danio rerio (zebrafish) PLoS Genet. 2020;16:e1008869. doi: 10.1371/journal.pgen.1008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding JD, Salinas RY, Arshavsky VY. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J Cell Biol. 2015;211:495–502. doi: 10.1083/jcb.201508093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 40.Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- 41.Doerre G, Malicki J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech Dev. 2002;110:125–138. doi: 10.1016/s0925-4773(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 42.Dryja TP, Finn JT, Peng YW, McGee TL, Berson EL, Yau KW. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92:10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 44.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 45.Elbers D, Scholten A, Koch KW. Zebrafish recoverin isoforms display differences in calcium switch mechanisms. Front Mol Neurosci. 2018;11:355. doi: 10.3389/fnmol.2018.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- 47.Emran F, Rihel J, Adolph AR, Dowling JE. Zebrafish larvae lose vision at night. Proc Natl Acad Sci U S A. 2010;107:6034–6039. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enright JM, Toomey MB, Sato SY, Temple SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y, How MJ, Johnson SL, Roberts NW, Kefalov VJ, Guengerich FP, Corbo JC. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr Biol. 2015;25:3048–3057. doi: 10.1016/j.cub.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258:277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 50.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 51.Farahbakhsh ZT, Hideg K, Hubbell WL. Photoactivated conformational changes in rhodopsin: a time-resolved spin label study. Science. 1993;262:1416–1419. doi: 10.1126/science.8248781. [DOI] [PubMed] [Google Scholar]
- 52.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 53.Fisher SK, Pfeffer BA, Anderson DH. Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci. 1983;24:844–856. [PubMed] [Google Scholar]
- 54.Fries R, Scholten A, Säftel W, Koch KW. Operation profile of zebrafish guanylate cyclase-activating protein 3. J Neurochem. 2012;121:54–65. doi: 10.1111/j.1471-4159.2011.07643.x. [DOI] [PubMed] [Google Scholar]
- 55.Fries R, Scholten A, Säftel W, Koch KW. Zebrafish guanylate cyclase type 3 signaling in cone photoreceptors. PLoS One. 2013;8:e69656. doi: 10.1371/journal.pone.0069656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frøland Steindal IA, Whitmore D (2019) Circadian clocks in fish-what have we learned so far? Biology (Basel) 8. 10.3390/biology8010017 [DOI] [PMC free article] [PubMed]
- 57.Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchs S, Nakazawa M, Maw M, Tamai M, Oguchi Y, Gal A. A homozygous 1-base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat Genet. 1995;10:360–362. doi: 10.1038/ng0795-360. [DOI] [PubMed] [Google Scholar]
- 59.Georgiou M, Robson AG, Singh N, Pontikos N, Kane T, Hirji N, Ripamonti C, Rotsos T, Dubra A, Kalitzeos A, Webster AR, Carroll J, Michaelides M. Deep phenotyping of PDE6C-associated achromatopsia. Invest Ophthalmol Vis Sci. 2019;60:5112–5123. doi: 10.1167/iovs.19-27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gesemann M, Neuhauss SCF. Selective gene loss of visual and olfactory guanylyl cyclase genes following the two rounds of vertebrate-specific whole-genome duplications. Genome Biol Evol. 2020;12:2153–2167. doi: 10.1093/gbe/evaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gestri G, Link BA, Neuhauss SC. The visual system of zebrafish and its use to model human ocular diseases. Dev Neurobiol. 2012;72:302–327. doi: 10.1002/dneu.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gillespie PG, Beavo JA. Characterization of a bovine cone photoreceptor phosphodiesterase purified by cyclic GMP-sepharose chromatography. J Biol Chem. 1988;263:8133–8141. doi: 10.1016/S0021-9258(18)68452-2. [DOI] [PubMed] [Google Scholar]
- 63.Glasauer SM, Neuhauss SC. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Genet Genomics. 2014;289:1045–1060. doi: 10.1007/s00438-014-0889-2. [DOI] [PubMed] [Google Scholar]
- 64.Grace MS, Wang LM, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735:93–100. doi: 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- 65.Gross JM, Perkins BD, Amsterdam A, Egaña A, Darland T, Matsui JI, Sciascia S, Hopkins N, Dowling JE. Identification of zebrafish insertional mutants with defects in visual system development and function. Genetics. 2005;170:245–261. doi: 10.1534/genetics.104.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hárosi FI. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 1994;34:1359–1367. doi: 10.1016/0042-6989(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 67.Hodel C, Neuhauss SC, Biehlmaier O. Time course and development of light adaptation processes in the outer zebrafish retina. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:653–662. doi: 10.1002/ar.a.20329. [DOI] [PubMed] [Google Scholar]
- 68.Holzhausen LC, Lewis AA, Cheong KK, Brockerhoff SE. Differential role for synaptojanin 1 in rod and cone photoreceptors. J Comp Neurol. 2009;517:633–644. doi: 10.1002/cne.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Eliott D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- 71.Huang L, Zhang Q, Li S, Guan L, Xiao X, Zhang J, Jia X, Sun W, Zhu Z, Gao Y, Yin Y, Wang P, Guo X, Wang J. Exome sequencing of 47 chinese families with cone-rod dystrophy: mutations in 25 known causative genes. PLoS One. 2013;8:e65546. doi: 10.1371/journal.pone.0065546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang SH, Pittler SJ, Huang X, Oliveira L, Berson EL, Dryja TP. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet. 1995;11:468–471. doi: 10.1038/ng1295-468. [DOI] [PubMed] [Google Scholar]
- 73.Hubbard R, Kropf A. The action of light on rhodopsin. Proc Natl Acad Sci U S A. 1958;44:130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikarashi R, Akechi H, Kanda Y, Ahmad A, Takeuchi K, Morioka E, Sugiyama T, Ebisawa T, Ikeda M. Regulation of molecular clock oscillations and phagocytic activity via muscarinic Ca. Sci Rep. 2017;7:44175. doi: 10.1038/srep44175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imanishi Y, Li N, Sokal I, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci. 2002;15:63–78. doi: 10.1046/j.0953-816x.2001.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imanishi Y, Yang L, Sokal I, Filipek S, Palczewski K, Baehr W. Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP1-8) in pufferfish (Fugu rubripes) J Mol Evol. 2004;59:204–217. doi: 10.1007/s00239-004-2614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iribarne M, Nishiwaki Y, Nakamura S, Araragi M, Oguri E, Masai I. Aipl1 is required for cone photoreceptor function and survival through the stability of Pde6c and Gc3 in zebrafish. Sci Rep. 2017;7:45962. doi: 10.1038/srep45962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jia S, Muto A, Orisme W, Henson HE, Parupalli C, Ju B, Baier H, Taylor MR. Zebrafish Cacna1fa is required for cone photoreceptor function and synaptic ribbon formation. Hum Mol Genet. 2014;23:2981–2994. doi: 10.1093/hmg/ddu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 82.Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- 83.Kawamura S, Kuwata O, Yamada M, Matsuda S, Hisatomi O, Tokunaga F. Photoreceptor protein s26, a cone homologue of S-modulin in frog retina. J Biol Chem. 1996;271:21359–21364. doi: 10.1074/jbc.271.35.21359. [DOI] [PubMed] [Google Scholar]
- 84.Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–2138. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41:915–928. doi: 10.1016/S0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- 86.Kerov V, Laird JG, Joiner ML, Knecht S, Soh D, Hagen J, Gardner SH, Gutierrez W, Yoshimatsu T, Bhattarai S, Puthussery T, Artemyev NO, Drack AV, Wong RO, Baker SA, Lee A. α2δ-4 is required for the molecular and structural organization of rod and cone photoreceptor synapses. J Neurosci. 2018;38:6145–6160. doi: 10.1523/JNEUROSCI.3818-16.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinney MS, Fisher SK. The photoreceptors and pigment epithelium of the larval xenopus retina: morphogenesis and outer segment renewal. Proc R Soc Lond B Biol Sci. 1978;201:149–167. doi: 10.1098/rspb.1978.0037. [DOI] [PubMed] [Google Scholar]
- 88.Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. doi: 10.1023/A:1014209711793. [DOI] [PubMed] [Google Scholar]
- 89.Koch KW, Duda T, Sharma RK. Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol Cell Biochem. 2010;334:105–115. doi: 10.1007/s11010-009-0330-z. [DOI] [PubMed] [Google Scholar]
- 90.Koch KW, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- 91.Kohl S, Baumann B, Rosenberg T, Kellner U, Lorenz B, Vadalà M, Jacobson SG, Wissinger B. Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia. Am J Hum Genet. 2002;71:422–425. doi: 10.1086/341835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolb H, Fernandez E, Nelson R (1995) Webvision: the organization of the retina and visual system. In. doi:NBK482309 [PubMed]
- 93.Kondo H, Qin M, Mizota A, Kondo M, Hayashi H, Hayashi K, Oshima K, Tahira T. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest Ophthalmol Vis Sci. 2004;45:4433–4439. doi: 10.1167/iovs.04-0544. [DOI] [PubMed] [Google Scholar]
- 94.Korenbrot JI, Mehta M, Tserentsoodol N, Postlethwait JH, Rebrik TI. EML1 (CNG-modulin) controls light sensitivity in darkness and under continuous illumination in zebrafish retinal cone photoreceptors. J Neurosci. 2013;33:17763–17776. doi: 10.1523/JNEUROSCI.2659-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koutalos Y, Nakatani K, Yau KW. The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J Gen Physiol. 1995;106:891–921. doi: 10.1085/jgp.106.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 97.Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kühn H, Hall SW, Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984;176:473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- 99.Lagman D, Callado-Pérez A, Franzén IE, Larhammar D, Abalo XM. Transducin duplicates in the zebrafish retina and pineal complex: differential specialisation after the teleost tetraploidisation. PLoS One. 2015;10:e0121330. doi: 10.1371/journal.pone.0121330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lagman D, Franzén IE, Eggert J, Larhammar D, Abalo XM. Evolution and expression of the phosphodiesterase 6 genes unveils vertebrate novelty to control photosensitivity. BMC Evol Biol. 2016;16:124. doi: 10.1186/s12862-016-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog Retin Eye Res. 2013;36:52–119. doi: 10.1016/j.preteyeres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Lamb TD, Hunt DM (2018) Evolution of the calcium feedback steps of vertebrate phototransduction. Open Biol 8. 10.1098/rsob.180119 [DOI] [PMC free article] [PubMed]
- 104.Lamb TD, Patel HR, Chuah A, Hunt DM (2018) Evolution of the shut-off steps of vertebrate phototransduction. Open Biol 8. 10.1098/rsob.170232 [DOI] [PMC free article] [PubMed]
- 105.Lamb TD, Pugh EN. Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5137–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 106.Larhammar D, Nordström K, Larsson TA. Evolution of vertebrate rod and cone phototransduction genes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2867–2880. doi: 10.1098/rstb.2009.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larison KD, Bremiller R. Early onset of phenotype and cell patterning in the embryonic zebrafish retina. Development. 1990;109:567–576. doi: 10.1242/dev.109.3.567. [DOI] [PubMed] [Google Scholar]
- 108.LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 109.Leung YT, Fain GL, Matthews HR. Simultaneous measurement of current and calcium in the ultraviolet-sensitive cones of zebrafish. J Physiol. 2007;579:15–27. doi: 10.1113/jphysiol.2006.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lewis TR, Kundinger SR, Link BA, Insinna C, Besharse JC. Kif17 phosphorylation regulates photoreceptor outer segment turnover. BMC Cell Biol. 2018;19:25. doi: 10.1186/s12860-018-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H, Chuang AZ, O’Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li XF, Kiedrowski L, Tremblay F, Fernandez FR, Perizzolo M, Winkfein RJ, Turner RW, Bains JS, Rancourt DE, Lytton J. Importance of K+-dependent Na+/Ca2+-exchanger 2, NCKX2, in motor learning and memory. J Biol Chem. 2006;281:6273–6282. doi: 10.1074/jbc.M512137200. [DOI] [PubMed] [Google Scholar]
- 113.Link BA, Collery RF. Zebrafish models of retinal disease. Annu Rev Vis Sci. 2015;1:125–153. doi: 10.1146/annurev-vision-082114-035717. [DOI] [PubMed] [Google Scholar]
- 114.Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW, Arshavsky VY. Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci. 2010;30:6815–6824. doi: 10.1523/JNEUROSCI.0613-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loew ER, Dartnall HJ. Vitamin A1/A2-based visual pigment mixtures in cones of the rudd. Vision Res. 1976;16:891–896. doi: 10.1016/0042-6989(76)90217-0. [DOI] [PubMed] [Google Scholar]
- 116.Lv C, Gould TJ, Bewersdorf J, Zenisek D. High-resolution optical imaging of zebrafish larval ribbon synapse protein RIBEYE, RIM2, and CaV 1.4 by stimulation emission depletion microscopy. Microsc Microanal. 2012;18:745–752. doi: 10.1017/S1431927612000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lyubarsky AL, Chen C, Simon MI, Pugh EN. Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J Neurosci. 2000;20:2209–2217. doi: 10.1523/JNEUROSCI.20-06-02209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma EY, Lewis A, Barabas P, Stearns G, Suzuki S, Krizaj D, Brockerhoff SE. Loss of Pde6 reduces cell body Ca(2+) transients within photoreceptors. Cell Death Dis. 2013;4:e797. doi: 10.1038/cddis.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mackin RD, Frey RA, Gutierrez C, Farre AA, Kawamura S, Mitchell DM, Stenkamp DL. Endocrine regulation of multichromatic color vision. Proc Natl Acad Sci U S A. 2019;116:16882–16891. doi: 10.1073/pnas.1904783116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matthews HR, Murphy RL, Fain GL, Lamb TD. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988;334:67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- 121.Matthews HR, Sampath AP. Photopigment quenching is Ca2+ dependent and controls response duration in salamander L-cone photoreceptors. J Gen Physiol. 2010;135:355–366. doi: 10.1085/jgp.200910394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4:130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 123.Meier A, Nelson R, Connaughton VP. Color processing in zebrafish retina. Front Cell Neurosci. 2018;12:327. doi: 10.3389/fncel.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menger GJ, Koke JR, Cahill GM. Diurnal and circadian retinomotor movements in zebrafish. Vis Neurosci. 2005;22:203–209. doi: 10.1017/S0952523805222083. [DOI] [PubMed] [Google Scholar]
- 125.Michalakis S, Becirovic E, Biel M (2018) Retinal cyclic nucleotide-gated channels: from pathophysiology to therapy. Int J Mol Sci:19. 10.3390/ijms19030749 [DOI] [PMC free article] [PubMed]
- 126.Molday RS, Moritz OL. Photoreceptors at a glance. J Cell Sci. 2015;128:4039–4045. doi: 10.1242/jcs.175687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, Gahtan E, Xiao T, Nevin LM, Gosse NJ, Staub W, Finger-Baier K, Baier H. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Naeem MA, Chavali VR, Ali S, Iqbal M, Riazuddin S, Khan SN, Husnain T, Sieving PA, Ayyagari R, Hejtmancik JF, Riazuddin SA. GNAT1 associated with autosomal recessive congenital stationary night blindness. Invest Ophthalmol Vis Sci. 2012;53:1353–1361. doi: 10.1167/iovs.11-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakatani K, Yau KW. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- 130.Nathans J, Piantanida TP, Eddy RL, Shows TB, Hogness DS. Molecular genetics of inherited variation in human color vision. Science. 1986;232:203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- 131.Niklaus S, Neuhauss SCF. Genetic approaches to retinal research in zebrafish. J Neurogenet. 2017;31:70–87. doi: 10.1080/01677063.2017.1343316. [DOI] [PubMed] [Google Scholar]
- 132.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nishiguchi KM, Sandberg MA, Kooijman AC, Martemyanov KA, Pott JW, Hagstrom SA, Arshavsky VY, Berson EL, Dryja TP. Defects in RGS9 or its anchor protein R9AP in patients with slow photoreceptor deactivation. Nature. 2004;427:75–78. doi: 10.1038/nature02170. [DOI] [PubMed] [Google Scholar]
- 134.Nishiwaki Y, Komori A, Sagara H, Suzuki E, Manabe T, Hosoya T, Nojima Y, Wada H, Tanaka H, Okamoto H, Masai I. Mutation of cGMP phosphodiesterase 6alpha'-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech Dev. 2008;125:932–946. doi: 10.1016/j.mod.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 135.Ojeda Naharros I, Gesemann M, Mateos JM, Barmettler G, Forbes A, Ziegler U, Neuhauss SCF, Bachmann-Gagescu R. Loss-of-function of the ciliopathy protein Cc2d2a disorganizes the vesicle fusion machinery at the periciliary membrane and indirectly affects Rab8-trafficking in zebrafish photoreceptors. PLoS Genet. 2017;13:e1007150. doi: 10.1371/journal.pgen.1007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 137.Paillart C, Winkfein RJ, Schnetkamp PP, Korenbrot JI. Functional characterization and molecular cloning of the K+-dependent Na+/Ca2+ exchanger in intact retinal cone photoreceptors. J Gen Physiol. 2007;129:1–16. doi: 10.1085/jgp.200609652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Palczewski K, Polans AS, Baehr W, Ames JB. Ca(2+)-binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 139.Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- 140.Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Warren MJ, Bird AC, Bhattacharya SS. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genet. 1998;7:273–277. doi: 10.1093/hmg/7.2.273. [DOI] [PubMed] [Google Scholar]
- 141.Pearring JN, Salinas RY, Baker SA, Arshavsky VY. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res. 2013;36:24–51. doi: 10.1016/j.preteyeres.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perrault I, Rozet JM, Gerber S, Ghazi I, Ducroq D, Souied E, Leowski C, Bonnemaison M, Dufier JL, Munnich A, Kaplan J. Spectrum of retGC1 mutations in Leber’s congenital amaurosis. Eur J Hum Genet. 2000;8:578–582. doi: 10.1038/sj.ejhg.5200503. [DOI] [PubMed] [Google Scholar]
- 143.Prinsen CF, Szerencsei RT, Schnetkamp PP. Molecular cloning and functional expression of the potassium-dependent sodium-calcium exchanger from human and chicken retinal cone photoreceptors. J Neurosci. 2000;20:1424–1434. doi: 10.1523/JNEUROSCI.20-04-01424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rätscho N, Scholten A, Koch KW. Expression profiles of three novel sensory guanylate cyclases and guanylate cyclase-activating proteins in the zebrafish retina. Biochim Biophys Acta. 2009;1793:1110–1114. doi: 10.1016/j.bbamcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 145.Raymond PA, Barthel LK. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int J Dev Biol. 2004;48:935–945. doi: 10.1387/ijdb.041873pr. [DOI] [PubMed] [Google Scholar]
- 146.Rebrik TI, Botchkina I, Arshavsky VY, Craft CM, Korenbrot JI. CNG-modulin: a novel Ca-dependent modulator of ligand sensitivity in cone photoreceptor cGMP-gated ion channels. J Neurosci. 2012;32:3142–3153. doi: 10.1523/JNEUROSCI.5518-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reiländer H, Achilles A, Friedel U, Maul G, Lottspeich F, Cook NJ. Primary structure and functional expression of the Na/Ca,K-exchanger from bovine rod photoreceptors. EMBO J. 1992;11:1689–1695. doi: 10.1002/j.1460-2075.1992.tb05219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Renninger SL, Gesemann M, Neuhauss SC. Cone arrestin confers cone vision of high temporal resolution in zebrafish larvae. Eur J Neurosci. 2011;33:658–667. doi: 10.1111/j.1460-9568.2010.07574.x. [DOI] [PubMed] [Google Scholar]
- 149.Reuter TE, White RH, Wald G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J Gen Physiol. 1971;58:351–371. doi: 10.1085/jgp.58.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Riazuddin SA, Shahzadi A, Zeitz C, Ahmed ZM, Ayyagari R, Chavali VR, Ponferrada VG, Audo I, Michiels C, Lancelot ME, Nasir IA, Zafar AU, Khan SN, Husnain T, Jiao X, MacDonald IM, Riazuddin S, Sieving PA, Katsanis N, Hejtmancik JF. A mutation in SLC24A1 implicated in autosomal-recessive congenital stationary night blindness. Am J Hum Genet. 2010;87:523–531. doi: 10.1016/j.ajhg.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rinner O, Makhankov YV, Biehlmaier O, Neuhauss SC. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron. 2005;47:231–242. doi: 10.1016/j.neuron.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 153.Robinson J, Schmitt EA, Hárosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A. 1993;90:6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999;144:385–388. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sakisaka T, Itoh T, Miura K, Takenawa T. Phosphatidylinositol 4,5-bisphosphate phosphatase regulates the rearrangement of actin filaments. Mol Cell Biol. 1997;17:3841–3849. doi: 10.1128/mcb.17.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sakurai K, Vinberg F, Wang T, Chen J, Kefalov VJ. The Na(+)/Ca(2+), K(+) exchanger 2 modulates mammalian cone phototransduction. Sci Rep. 2016;6:32521. doi: 10.1038/srep32521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Samardzija M., Neuhauss S.C.F., Joly S. K-LM, C. G (2009) Animal models for retinal degeneration.
- 158.Sampath AP, Matthews HR, Cornwall MC, Fain GL. Bleached pigment produces a maintained decrease in outer segment Ca2+ in salamander rods. J Gen Physiol. 1998;111:53–64. doi: 10.1085/jgp.111.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saszik S, Bilotta J. The effects of temperature on the dark-adapted spectral sensitivity function of the adult zebrafish. Vision Res. 1999;39:1051–1058. doi: 10.1016/s0042-6989(98)00237-5. [DOI] [PubMed] [Google Scholar]
- 160.Sato M, Nakazawa M, Usui T, Tanimoto N, Abe H, Ohguro H. Mutations in the gene coding for guanylate cyclase-activating protein 2 (GUCA1B gene) in patients with autosomal dominant retinal dystrophies. Graefes Arch Clin Exp Ophthalmol. 2005;243:235–242. doi: 10.1007/s00417-004-1015-7. [DOI] [PubMed] [Google Scholar]
- 161.Schlegel DK, Glasauer SMK, Mateos JM, Barmettler G, Ziegler U, Neuhauss SCF. A new zebrafish model for CACNA2D4-dysfunction. Invest Ophthalmol Vis Sci. 2019;60:5124–5135. doi: 10.1167/iovs.19-26759. [DOI] [PubMed] [Google Scholar]
- 162.Schmitz F. Presynaptic [Ca(2+)] and GCAPs: aspects on the structure and function of photoreceptor ribbon synapses. Front Mol Neurosci. 2014;7:3. doi: 10.3389/fnmol.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- 164.Scholten A, Koch KW. Differential calcium signaling by cone specific guanylate cyclase-activating proteins from the zebrafish retina. PLoS One. 2011;6:e23117. doi: 10.1371/journal.pone.0023117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sears S, Erickson A, Hendrickson A. The spatial and temporal expression of outer segment proteins during development of Macaca monkey cones. Invest Ophthalmol Vis Sci. 2000;41:971–979. [PubMed] [Google Scholar]
- 166.Senin II, Fischer T, Komolov KE, Zinchenko DV, Philippov PP, Koch KW. Ca2+-myristoyl switch in the neuronal calcium sensor recoverin requires different functions of Ca2+-binding sites. J Biol Chem. 2002;277:50365–50372. doi: 10.1074/jbc.M204338200. [DOI] [PubMed] [Google Scholar]
- 167.Sharma RK. Evolution of the membrane guanylate cyclase transduction system. Mol Cell Biochem. 2002;230:3–30. doi: 10.1023/A:1014280410459. [DOI] [PubMed] [Google Scholar]
- 168.Sieving PA, Richards JE, Naarendorp F, Bingham EL, Scott K, Alpern M. Dark-light: model for nightblindness from the human rhodopsin Gly-90-->Asp mutation. Proc Natl Acad Sci U S A. 1995;92:880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sjostrand FS. Ultrastructure of retinal rod synapses of the guinea pig eye as revealed by three-dimensional reconstructions from serial sections. J Ultrastruct Res. 1958;2:122–170. doi: 10.1016/s0022-5320(58)90050-9. [DOI] [PubMed] [Google Scholar]
- 170.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 171.Spencer WJ, Lewis TR, Pearring JN, Arshavsky VY. Photoreceptor discs: built like ectosomes. Trends Cell Biol. 2020;30:904–915. doi: 10.1016/j.tcb.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stearns G, Evangelista M, Fadool JM, Brockerhoff SE. A mutation in the cone-specific pde6 gene causes rapid cone photoreceptor degeneration in zebrafish. J Neurosci. 2007;27:13866–13874. doi: 10.1523/JNEUROSCI.3136-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- 174.Steindal IAF, Whitmore D. Zebrafish circadian clock entrainment and the importance of broad spectral light sensitivity. Front Physiol. 2020;11:1002. doi: 10.3389/fphys.2020.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 176.Stiebel-Kalish H, Reich E, Rainy N, Vatine G, Nisgav Y, Tovar A, Gothilf Y, Bach M. Gucy2f zebrafish knockdown--a model for Gucy2d-related leber congenital amaurosis. Eur J Hum Genet. 2012;20:884–889. doi: 10.1038/ejhg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005;280:29250–29255. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- 178.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 179.Sulmann S, Vocke F, Scholten A, Koch KW. Retina specific GCAPs in zebrafish acquire functional selectivity in Ca2+-sensing by myristoylation and Mg2+-binding. Sci Rep. 2015;5:11228. doi: 10.1038/srep11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Suzuki SC, Bleckert A, Williams PR, Takechi M, Kawamura S, Wong RO. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc Natl Acad Sci U S A. 2013;110:15109–15114. doi: 10.1073/pnas.1303551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Szymanska K, Johnson CA. The transition zone: an essential functional compartment of cilia. Cilia. 2012;1:10. doi: 10.1186/2046-2530-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Takechi M, Kawamura S. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. J Exp Biol. 2005;208:1337–1345. doi: 10.1242/jeb.01532. [DOI] [PubMed] [Google Scholar]
- 183.Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- 184.Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–1679. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Terman JS, Remé CE, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605:256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- 186.Thiadens AA, den Hollander AI, Roosing S, Nabuurs SB, Zekveld-Vroon RC, Collin RW, De Baere E, Koenekoop RK, van Schooneveld MJ, Strom TM, van Lith-Verhoeven JJ, Lotery AJ, van Moll-Ramirez N, Leroy BP, van den Born LI, Hoyng CB, Cremers FP, Klaver CC. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet. 2009;85:240–247. doi: 10.1016/j.ajhg.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.tom Dieck S, Brandstätter JH (2006) Ribbon synapses of the retina. Cell Tissue Res 326:339-346. doi:10.1007/s00441-006-0234-0 [DOI] [PubMed]
- 188.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 189.Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vandepoele K, De Vos W, Taylor JS, Meyer A, Van de Peer Y. Major events in the genome evolution of vertebrates: paranome age and size differ considerably between ray-finned fishes and land vertebrates. Proc Natl Acad Sci U S A. 2004;101:1638–1643. doi: 10.1073/pnas.0307968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vinberg F, Chen J, Kefalov VJ. Regulation of calcium homeostasis in the outer segments of rod and cone photoreceptors. Prog Retin Eye Res. 2018;67:87–101. doi: 10.1016/j.preteyeres.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vinberg F, Wang T, De Maria A, Zhao H, Bassnett S, Chen J, Kefalov VJ (2017) The Na+/Ca2+, K+ exchanger NCKX4 is required for efficient cone-mediated vision. Elife 6. 10.7554/eLife.24550 [DOI] [PMC free article] [PubMed]
- 193.Vinberg F, Wang T, Molday RS, Chen J, Kefalov VJ. A new mouse model for stationary night blindness with mutant Slc24a1 explains the pathophysiology of the associated human disease. Hum Mol Genet. 2015;24:5915–5929. doi: 10.1093/hmg/ddv319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vogalis F, Shiraki T, Kojima D, Wada Y, Nishiwaki Y, Jarvinen JL, Sugiyama J, Kawakami K, Masai I, Kawamura S, Fukada Y, Lamb TD. Ectopic expression of cone-specific G-protein-coupled receptor kinase GRK7 in zebrafish rods leads to lower photosensitivity and altered responses. J Physiol. 2011;589:2321–2348. doi: 10.1113/jphysiol.2010.204156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Volkov LI, Kim-Han JS, Saunders LM, Poria D, Hughes AEO, Kefalov VJ, Parichy DM, Corbo JC. Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proc Natl Acad Sci U S A. 2020;117:15262–15269. doi: 10.1073/pnas.1920086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wada Y, Sugiyama J, Okano T, Fukada Y. GRK1 and GRK7: unique cellular distribution and widely different activities of opsin phosphorylation in the zebrafish rods and cones. J Neurochem. 2006;98:824–837. doi: 10.1111/j.1471-4159.2006.03920.x. [DOI] [PubMed] [Google Scholar]
- 197.Wald G. The porphyropsin visual system. J Gen Physiol. 1939;22:775–794. doi: 10.1085/jgp.22.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wan L, Almers W, Chen W. Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J Neurosci. 2005;25:941–949. doi: 10.1523/JNEUROSCI.4657-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J Neurosci. 2001;21:9175–9184. doi: 10.1523/JNEUROSCI.21-23-09175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weitz CJ, Miyake Y, Shinzato K, Montag E, Zrenner E, Went LN, Nathans J. Human tritanopia associated with two amino acid substitutions in the blue-sensitive opsin. Am J Hum Genet. 1992;50:498–507. [PMC free article] [PubMed] [Google Scholar]
- 201.Weitz CJ, Went LN, Nathans J. Human tritanopia associated with a third amino acid substitution in the blue-sensitive visual pigment. Am J Hum Genet. 1992;51:444–446. [PMC free article] [PubMed] [Google Scholar]
- 202.Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- 203.Wilden U, Hall SW, Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res. 2002;42:455–462. doi: 10.1016/s0042-6989(01)00228-0. [DOI] [PubMed] [Google Scholar]
- 205.Willoughby JJ, Jensen AM. Generation of a genetically encoded marker of rod photoreceptor outer segment growth and renewal. Biol Open. 2012;1:30–36. doi: 10.1242/bio.2011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wong-Riley MT, Besharse JC. The kinesin superfamily protein KIF17: one protein with many functions. Biomol Concepts. 2012;3:267–282. doi: 10.1515/bmc-2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci. 2006;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- 209.Yamamoto S, Sippel KC, Berson EL, Dryja TP. Defects in the rhodopsin kinase gene in the Oguchi form of stationary night blindness. Nat Genet. 1997;15:175–178. doi: 10.1038/ng0297-175. [DOI] [PubMed] [Google Scholar]
- 210.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 211.Yau KW, Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985;317:252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- 212.Yoshimatsu T, Schröder C, Nevala NE, Berens P, Baden T. Fovea-like photoreceptor specializations underlie single UV cone driven prey-capture behavior in zebrafish. Neuron. 2020;107:320–337.e326. doi: 10.1016/j.neuron.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zang J, Keim J, Kastenhuber E, Gesemann M, Neuhauss SC (2015) Recoverin depletion accelerates cone photoresponse recovery. Open Biol 5. 10.1098/rsob.150086 [DOI] [PMC free article] [PubMed]
- 216.Zang J, Matthews HR. Origin and control of the dominant time constant of salamander cone photoreceptors. J Gen Physiol. 2012;140:219–233. doi: 10.1085/jgp.201110762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zang J, Neuhauss SCF. The binding properties and physiological functions of recoverin. Front Mol Neurosci. 2018;11:473. doi: 10.3389/fnmol.2018.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zelinka CP, Sotolongo-Lopez M, Fadool JM. Targeted disruption of the endogenous zebrafish rhodopsin locus as models of rapid rod photoreceptor degeneration. Mol Vis. 2018;24:587–602. [PMC free article] [PubMed] [Google Scholar]
- 219.Zhao X, Huang J, Khani SC, Palczewski K. Molecular forms of human rhodopsin kinase (GRK1) J Biol Chem. 1998;273:5124–5131. doi: 10.1074/jbc.273.9.5124. [DOI] [PubMed] [Google Scholar]
- 220.Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–896. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- 221.Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zimmermann MJY, Nevala NE, Yoshimatsu T, Osorio D, Nilsson DE, Berens P, Baden T. Zebrafish differentially process color across visual space to match natural scenes. Curr Biol. 2018;28:2018–2032.e2015. doi: 10.1016/j.cub.2018.04.075. [DOI] [PubMed] [Google Scholar]