Abstract
It was recently reported that hepatocellular carcinoma (HCC) patients with non-alcoholic steatohepatitis (NASH) are not responsive to immune-checkpoint inhibitor (ICI) treatment. The present study aimed to evaluate the therapeutic efficacy of lenvatinib in patients with non-alcoholic fatty liver disease (NAFLD)/NASH-related unresectable-HCC (u-HCC). Five hundred thirty u-HCC patients with Child–Pugh A were enrolled, and divided into the NAFLD/NASH (n = 103) and Viral/Alcohol (n = 427) groups. Clinical features were compared in a retrospective manner. Progression-free survival (PFS) was better in the NAFLD/NASH than the Viral/Alcohol group (median 9.3 vs. 7.5 months, P = 0.012), while there was no significant difference in overall survival (OS) (20.5 vs. 16.9 months, P = 0.057). In Cox-hazard analysis of prognostic factors for PFS, elevated ALT (≥ 30 U/L) (HR 1.247, P = 0.029), modified ALBI grade 2b (HR 1.236, P = 0.047), elevated AFP (≥ 400 ng/mL) (HR 1.294, P = 0.014), and NAFLD/NASH etiology (HR 0.763, P = 0.036) were significant prognostic factors. NAFLD/NASH etiology was not a significant prognostic factor in Cox-hazard analysis for OS (HR0.758, P = 0.092), whereas AFP (≥ 400 ng/mL) (HR 1.402, P = 0.009), BCLC C stage (HR 1.297, P = 0.035), later line use (HR 0.737, P = 0.014), and modified ALBI grade 2b (HR 1.875, P < 0.001) were significant. Lenvatinib can improve the prognosis of patients affected by u-HCC irrespective of HCC etiology or its line of treatment.
Subject terms: Cancer, Gastroenterology, Oncology
Introduction
Molecular targeted agents (MTAs) have recently been introduced for unresectable hepatocellular carcinoma (u-HCC), with sorafenib developed first in 2009 as a first-line MTA based on results presented in the SHARP1 and Asia–Pacific2 trials. Following development of that drug, lenvatinib received approval as another first-line treatment in 20183. Moreover, atezolizumab plus bevacizumab treatment (Atezo + Bev), an immune-checkpoint inhibitor (ICI) and anti-vascular endothelial growth factor (anti-VEGF) combination, was recently introduced in September 2020 as a first-line treatment option for u-HCC4.
Despite the good therapeutic response noted for Atezo + Bev in the IMbrave 150 trial, a recent report noted that therapeutic responses to ICI treatments differed according to the etiology of the background liver disease5. A meta-analysis of findings in that study indicated that patients with HCC with a viral etiology showed therapeutic benefits from ICI use [HR 0.64], whereas those with a nonviral etiology did not [HR 0.92] (P = 0.03). Most importantly, results obtained in that investigation of two validation cohorts treated with ICI clearly showed that overall survival (OS) for non-alcoholic fatty liver disease or non-alcoholic steatohepatitis (NAFLD/NASH)-related HCC patients was significantly worse than that for the non-NAFLD/NASH-related HCC group (11.0 vs. 5.4 months, P = 0.023 and 17.7 vs. 8.8 months, P = 0.034, respectively). Those striking epoch-making results showed that ICI treatment response differs depending on background liver disease etiology, and especially that NAFLD/NASH-related HCC patients lack immune response as well as immune surveillance related to tumor-associated antigens.
Lenvatinib, which was approved after showing non-inferior therapeutic efficacy as compared to sorafenib, has recently come to play a large role as a first-line MTA drug in clinical practice throughout the world for u-HCC cases. However, therapeutic response in non-viral u-HCC patients given lenvatinib, especially those with NAFLD/NASH-related HCC, has not been adequately elucidated. This study aimed to evaluate differences among background hepatic disease etiology factors for therapeutic response in patients treated with lenvatinib.
Materials and methods
Patients
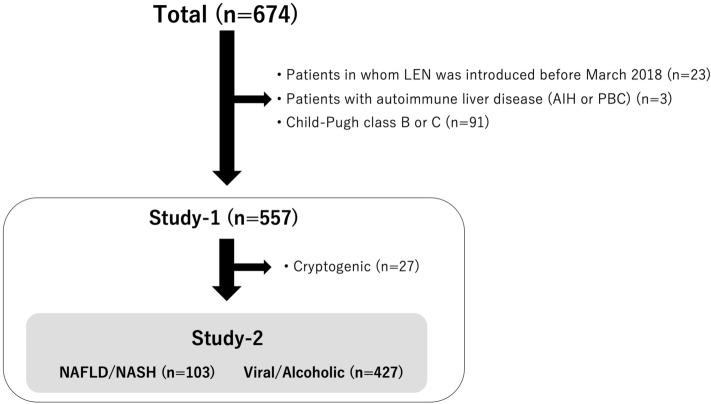
The records of 674 patients with u-HCC and treated with lenvatinib at various institutions in Japan between March 2018 and February 2021 (Ehime Prefectural Central Hospital, Kindai University Hospital, Himeji Red Cross Hospital, Kagawa University Hospital, Okayama City Hospital, Osaka Medical School, Nippon Medical School, Ehime University Graduate Hospital, Teine Keijinkai Hospital, Saiseikai Niigata Hospital, Kagawa Prefectural Central Hospital, Asahi General Hospital, Toyama University Hospital, Otakanomori Hospital, Tokushima Prefectural Central Hospital, Matsuyama Red Cross Hospital, Hamamatsu University School of Medicine Hospital, Ogaki Municipal Hospital) were obtained. Those in whom lenvatinib was introduced before March 2018 as part of a clinical trial (n = 23), classified as Child–Pugh class B or C (n = 91), or with autoimmune liver disease [autoimmune hepatitis (AIH) or primary biliary cirrhosis (PBC)] (n = 3) were excluded, thus 557 cases were subjected to evaluations performed in a retrospective manner (Fig. 1).
Figure 1.
Flow of patient enrollment. LEN lenvatinib, AIH autoimmune hepatitis, PBC primary biliary cirrhosis.
Patients positive for hepatitis B virus surface antigen (HBsAg) were judged to have HCC due to the presence of hepatitis B virus (HBV), while those positive for anti-hepatitis C virus (HCV) were judged to have HCC due to HCV. Two patients positive for both HCV and HBV were included in the HCV group for this study because HBV DNA levels were below the detection level. For patients with a history of alcohol abuse of 60 g/day or more6,7, background liver disease was judged as alcoholic. NAFLD/NASH diagnosis was determined by a medical interview [history of obesity, hyperlipidemia, hypertension, etc., and/or no/low alcohol intake (< 30 g/day in males, < 20 g/day in females)] of fatty liver patients and/or based on pathological findings8. Burned-out NASH liver cirrhosis was diagnosed clinically based on the clinical course (e.g., no history of alcohol abuse, history of obesity and/or fatty liver, or past pathological diagnosis) by each institution. Patients without autoimmune liver disease (AIH or PBC) other than the above, or those in whom hepatic fibrosis was not observed pathologically were classified as cryptogenic liver disease. Positive for severe fibrosis was defined based on elevated FIB-4 index (≥ 3.25)9.
The therapeutic effects of lenvatinib in all 557 patients with Child–Pugh class A were examined as Study-1. Furthermore, therapeutic responses were compared between patients with NAFLD/NASH (n = 103), and those with chronic hepatic viral infection or alcohol abuse (Viral/Alcohol group) (n = 427), after exclusion of cryptogenic patients (n = 27), as Study-2 (Fig. 1).
HCC diagnosis
HCC was diagnosed based on an increasing trend of alpha-fetoprotein (AFP), as well as typical findings obtained in dynamic CT10, MRI11,12, and contrast enhanced ultrasonography (CEUS) with perflubutane (Sonazoid®, Daiichi Sankyo Co., Ltd., Tokyo, Japan) examinations13,14, and/or pathological findings. To evaluate tumor progression, Barcelona Clinic Liver Cancer (BCLC) stage15 and tumor node metastasis (TNM) stage were used, and determined as previously reported in a study for TNM staging of HCC conducted by the Liver Cancer Study Group of Japan (LCSGJ) 6th edition (TNM-LCSGJ)16.
Assessment methods for hepatic reserve function and therapeutic response
Child–Pugh classification17 and albumin-bilirubin (ALBI) grade were used for assessment of hepatic reserve function18–20. To perform more detailed evaluations of patients with the middle ALBI grade of 2, a revised grading system was used that consisted of four levels, with sub-grading for the middle grade of 2 (2a and 2b) based on an ALBI score of − 2.27 as the cut-off (modified ALBI, mALBI grade), which was previously reported to result in a predictive value for indocyanine green retention after 15 min (ICG-R15) of 30%21,22. Progression-free survival (PFS) was analyzed according to the modified Response Evaluation Criteria In Solid Tumors (mRECIST) criteria23,24, based on results of dynamic CT examinations performed at intervals of 8–12 weeks.
Lenvatinib treatment and assessment of adverse events
After obtaining written informed consent from each patient, lenvatinib treatment was started. The drug was orally administered at 8 mg/day in patients weighing < 60 kg or 12 mg/day in those ≥ 60 kg, and discontinued when any unacceptable or serious adverse event (AE) occurred (any grade 3 or more severe AE, or any unacceptable grade 2 drug-related AE), or radiological tumor progression was observed, according to the guidelines for administration of lenvatinib. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.025. When a drug-related AE was noted, dose reduction or temporary interruption was maintained until the symptom was resolved to grade 1 or 2, according to the guidelines provided by the manufacturer. AEs of grade 3 or more were defined as severe, and the worst grade for each AE during the present observation period was recorded.
Ethical approval
Written informed consent for lenvatinib treatment was obtained from each patient. This was a retrospective analysis of records stored in a database and official approval was received based on the Guidelines for Clinical Research issued by the Ministry of Health and Welfare of Japan. All procedures complied with the declaration of Helsinki. The study protocol was granted approval by the Institutional Ethics Committee of Ehime Prefectural Central Hospital (IRB No. 30-66) (UMIN000043219).
Statistical analysis
Continuous variables are expressed as median values (first-third quartile). Statistical analyses were performed using Welch’s t-test, Student’s t-test, Fischer’s exact test, or Mann–Whitney’s U test, as appropriate. Cox hazard analysis (stepwise regression method), the Kaplan–Meyer method, and a log-rank test were used to analyze prognosis factors.
A P value less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Easy R (EZR) version 1.53 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)26, a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study 1
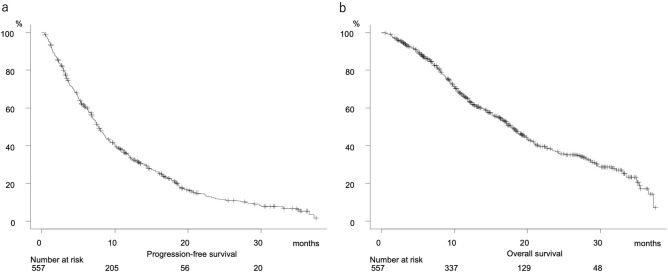
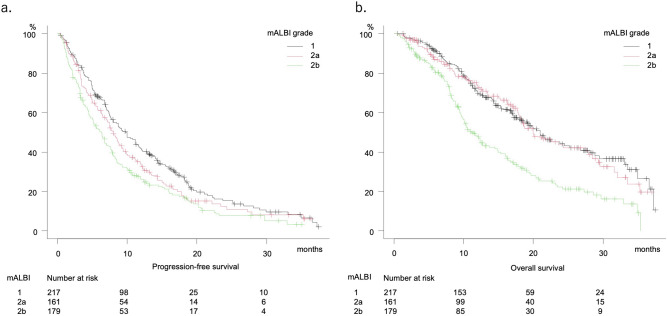
Clinical features of all 557 patients are shown in Table 1. PFS and OS were 7.8 months (95%CI 7.0–8.6 months) and 17.8 months (95%CI 16.3–19.5 months), respectively (Fig. 2a,b), and were well stratified according to mALBI grade (median PFS and OS: grade 1:2a:2b = 9.8:8.0:6.3 months, P = 0.002, and 21.0:20.0:11.2 months, P < 0.001, respectively) (Fig. 3a,b), while there were no significant differences for those according to treatment line (first, second, third or greater) using lenvatinib (median PFS and OS: 7.6:8.2:7.7 months, P = 0.080, and 16.7:18.3:23.2 months, P = 0.091, respectively) (Supplemental Fig. S1a,b)]. A comparison between initial and later line (second or greater) showed no significant difference regarding PFS (7.6 vs. 8.1 months, P = 0.752), while a significant difference was noted for OS (16.7 vs. 19.6 months, P = 0.029) (Supplemental Fig. S1c,d).
Table 1.
Clinical features of all u-HCC patients.
| n = 557 | |
|---|---|
| Age, years* | 73.0 (67.0 to 79.0) |
| Gender, male:female | 430:127 |
| Etiology, HCV:HBV:alcohol:NAFLD/NASH:cryptogenic | 236:88:103:103:27 |
| ECOG PS, 0:1:2:3 | 474:73:9:1 |
| Body mass index (kg/m2) | 22.98 (20.74 to 25.55) |
| ALBI score* | − 2.49 (− 2.18 to − 2.74) |
| (mALBI grade 1:2a:2b) | (217:161:179) |
| Child–Pugh score, 5:6 | 359:198 |
| AFP, ≥ 400 ng/mL (%) | 167 (30.0%) |
| TNM-LCSGJ, I:II:III:IVa:IVb | 6:74:207:81:189 |
| BCLC stage, 0:A:B:C:D | 4:10:221:321:1 |
| Lenvatinib treatment line, first:second:third:fourth:fifth | 355:132:63:6:1 |
| Deaths (%) | 301 (54.0%) |
| Observation period, months | 12.2 (6.9 to 19.2) |
HCV hepatitis C virus, HBV hepatitis B virus, NAFLD: non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, ECOG PS Eastern Cooperative Oncology Group performance status, ALBI score albumin-bilirubin score, mALBI grade modified ALBI grade, AFP alpha-fetoprotein, TNM LCSGJ 6th tumor node metastasis stage by Liver Cancer Study Group of Japan 6th edition, BCLC stage Barcelona Clinic Liver Cancer stage.
*Median (interquartile range).
Figure 2.
Progression-free and overall survival for all unresectable hepatocellular carcinoma patients with Child–Pugh class A (n = 557). (a) Progression-free survival (median 7.8 months, 95% CI 7.0–8.6). b. Overall survival (median 17.8 months, 95% CI 16.3–19.5).
Figure 3.
Progression-free and overall survival according to modified ALBI grade. (a) Progression-free survival divided by mALBI grade. mALBI 1 (median 9.8 months, 95% CI 7.8–11.6), mALBI 2a (median 8.0 months, 95% CI 6.6–9.3), mALBI 2b (6.3 months, 95% CI 4.8–7.4) (P = 0.002). (b) Overall survival divided by mALBI grade. mALBI 1 (median 21.0 months, 95% CI 17.2–26.8), mALBI 2a (median 20.0 months, 95% CI 17.8–27.9), mALBI 2b (median 11.2 months, 95% CI 9.7–14.5) (P < 0.001).
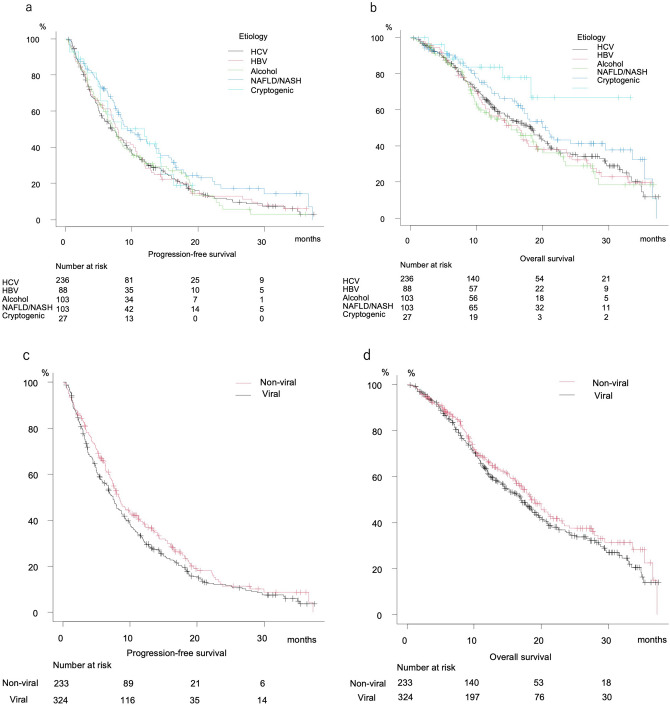
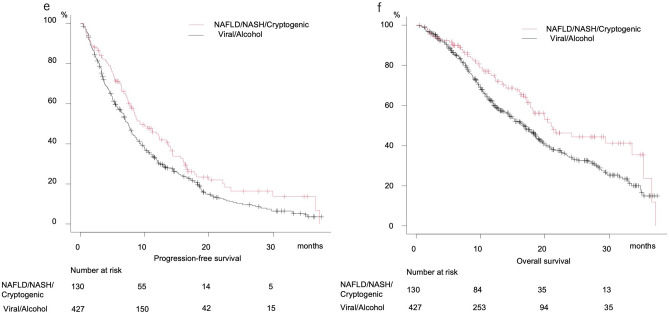
Median PFS after dividing patients into HCV, HBV, alcohol, NAFLD/NASH, and cryptogenic groups was 7.0, 7.9, 7.4, 9.3, and 11.9 months, respectively, (P = 0.154) (Fig. 4a), while median OS was 18.3, 16.3, 15.3, 20.5 months, and not reached, respectively (P = 0.052) (Fig. 4b). There were no significant differences in regard to PFS (7.5 vs. 8.3 months, P = 0.092) or OS (17.2 vs. 18.5 months, P = 0.226) between the viral HCC and nonviral (NAFLD/NASH, cryptogenic, alcohol) groups (Fig. 4c,d). Since median PFS and OS for the alcohol group were similar to those for the viral group, patients associated with alcohol abuse were included in the viral group, and comparisons between the Viral/Alcohol group and others (NAFLD/NASH, cryptogenic) were performed. Those results showed that both PFS and OS for the Viral/Alcohol group were significantly worse (median PFS: 7.5 vs. 9.3 months, P = 0.012; median OS: 16.9 vs. 21.0 months, P = 0.009) (Fig. 4e,f).
Figure 4.
Progression-free and overall survival according to basal liver disease etiology. (a) Progression-free survival divided by basal liver disease etiology. HCV (median 7.0 months, 95% CI 5.5–8.7), HBV (median 7.9 months, 95% CI 6.8–10.5), alcohol (median 7.4 months, 95% CI 5.8–8.6), NAFLD/NASH (median 9.3 months, 95% CI 7.8–13.5), cryptogenic (median 11.9 months, 95% CI 5.2–14.4) (P = 0.154). (b) Overall survival divided by basal liver disease etiology. HCV (median 18.3 months, 95% CI 14.3–20.4), HBV (median 16.3 months, 95% CI 11.4–19.3), alcohol (median 15.3 months, 95% CI 10.5–19.2), NAFLD/NASH (median 20.5 months, 95% CI 16.8–29.5), cryptogenic (median not reached, 95% CI 18.3-not reached) (P = 0.052). (c) Progression-free survival of Viral and Non-viral (NAFLD/NASH, Cryptogenic, Alcohol) groups. Viral (median 7.5 months, 95% CI 6.4–8.5), Non-viral (median 8.3 months, 95% CI 7.4–10.0) (P = 0.092). (d) Overall survival of Viral and Non-viral (NAFLD/NASH, Cryptogenic, Alcohol) groups. Viral (median 17.2 months, 95% CI 14.4–19.3), Non-viral (median 18.5 months, 95% CI 16.3–21.7) (P = 0.226). (e) Progression-free survival of Viral/Alcohol and NAFLD/NASH/Cryptogenic groups. Viral/Alcohol (median 7.5 months, 95% CI 6.8–8.0), NAFLD/NASH/Cryptogenic (median 9.3, 95% CI 7.8–13.4) (P = 0.012). (f) Overall survival of Viral/Alcohol and NAFLD/NASH/Cryptogenic groups. Viral/Alcohol (median 16.9 months, 95% CI 14.5–18.6), NAFLD/NASH/Cryptogenic (median 21.0 months, 95% CI 17.8–33.5) (P = 0.009).
Study-2
In comparisons between the NAFLD/NASH and Viral/Alcohol groups, platelet count and FIB-4 index were better in the former (15.3 × 104/µL vs. 13.1 × 104/µL and 3.81 vs. 4.39, P = 0.005 and P = 0.038, respectively). On the other hand, a larger percentage of patients in the NAFLD/NASH group started lenvatinib at a reduced dose (36.9% vs. 26.0%, P = 0.037), while there was no significant difference observed in regard to hepatic function (Child–Pugh score, ALBI score, mALBI grade), tumor burden (TNM-LCSGJ, BCLC stage), or malignancy grade of HCC (elevated AFP: ≥ 400 ng/mL) between them (Table 2).
Table 2.
Comparison of clinical features between NAFLD/NASH and Viral/Alcohol groups after exclusion of cryptogenic HCC.
| NAFLD/NASH (n = 103) | Viral/alcohol (n = 427) | P value | |
|---|---|---|---|
| Age, years * | 75 (69 to 80) | 73 (66 to 79) | 0.078 |
| Gender, male:female | 77:26 | 334:93 | 0.434 |
| Etiology, HCV:HBV:alcohol:NAFLD/NASH | 0:0:0:103 | 236:88:103:0 | < 0.001 |
| ECOG PS, 0:1:2 | 89:11:3 | 364:58:5 | 0.270 |
| Body mass index, kg/m2 | 24.93 (22.79 to 27.41) | 22.65 (20.32 to 25.04) | < 0.001 |
| Platelets, 104/µL* | 15.3 (11.2 to 20.0) | 13.1 (9.5 to 17.6) | 0.005 |
| AST, U/L* | 40 (28 to 57) | 41 (29 to 62) | 0.273 |
| ALT, U/L* | 28 (21 to 41) | 30 (19 to 47) | 0.403 |
| T-bilirubin, mg/dL* | 0.68 (0.50 to 0.90) | 0.70 (0.54 to 1.00) | 0.094 |
| Albumin, g/dL* | 3.70 (3.50 to 4.00) | 3.80 (3.45 to 4.10) | 0.998 |
| Prothrombin time, %* | 89.0 (84.0 to 101.0) | 88.0 (80.0 to 97.0) | 0.142 |
| eGFR, nL/min/1.73 m2* | 66.0 (50.2 to 77.3) | 66.4 (56.0 to 79.4) | 0.367 |
| ALBI score* | − 2.48 (− 2.21 to − 2.48) | − 2.48 (− 2.17 to − 2.76) | 0.603 |
| mALBI grade 1:2a:2b:3 | 41:31:31:0 | 164:123:140:0 | 0.869 |
| Child–Pugh score, 5:6 | 72:31 | 269:158 | 0.208 |
| AFP, ≥ 400 ng/mL | 25 (24.3%) | 136 (31.9%) | 0.152 |
| MVI (portal vein), none:Vp1:Vp2:Vp3:Vp4 | 89:1:4:7:2 | 350:12:29:24:12 | 0.663 |
| MVI (hepatic vein), none:Vv1:Vv2:Vv3 | 90:8:4:1 | 391:22:9:5 | 0.417 |
| Positive for EHM | 40 (38.8%) | 140 (32.8%) | 0.249 |
| TNM-LCSGJ, I:II:III:IVa:IVb | 2:12:37:12:40 | 4:60:157:66:140 | 0.537 |
| BCLC stage, 0:A:B:C | 1:2:39:61 | 3:8:171:245 | 0.933 |
| Initial dose of lenvatinib, 4:8:12 mg* | 5:55:43 | 36:240:151 | 0.174 |
| Reduced starting dose | 38 (36.9%) | 111 (26.0%) | 0.037 |
| Lenvatinib treatment line: first:second:third:fourth:fifth | 73:18:11:0:1 | 262:108:51:6:0 | 0.093 |
| FIB-4 index | 3.81 (2.31 to 5.55) | 4.39 (2.80 to 6.34) | 0.038 |
| Pathological diagnosis of NAFLD/NASH during clinical course (%) | 27 (26.2%) | NA | NA |
| Deaths (%) | 51 (49.5%) | 244 (57.1%) | 0.185 |
| Observation period, months | 13.5 (7.5 to 21.3) | 11.9 (6.8 to 18.9) | 0.124 |
HCV hepatitis C virus, HBV hepatitis B virus, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, ECOG PS Eastern Cooperative Oncology Group performance status, AST aspartate transaminase, ALT alanine aminotransferase, ALBI score albumin-bilirubin score, mALBI grade modified ALBI grade, AFP alpha-fetoprotein, MVI macrovascular invasion, EHM extra-hepatic metastasis, TNM LCSGJ 6th tumor node metastasis stage by Liver Cancer Study Group of Japan 6th edition, BCLC stage Barcelona Clinic Liver Cancer stage, NA not applicable.
*Median (interquartile range).
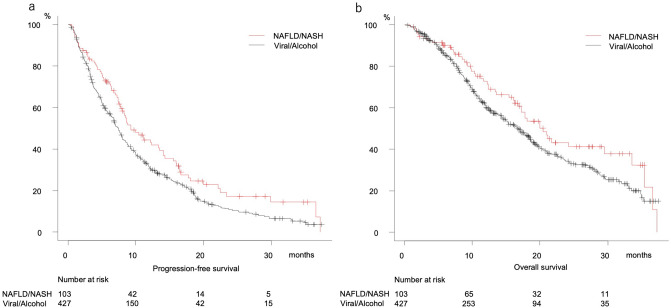
Following exclusion of cryptogenic patients, PFS was better in the NAFLD/NASH than the Viral/Alcohol group (median 9.3 vs. 7.5 months, P = 0.012) (Fig. 5a), while there was no significant difference in regard to OS (median 20.5 vs. 16.9 months, P = 0.057) (Fig. 5b). Cox-hazard analysis for prognostic factors of PFS showed elevated ALT (≥ 30 U/L) (HR 1.247, P = 0.029), mALBI grade 2b (HR 1.236, P = 0.047), elevated AFP (≥ 400 ng/mL) (HR 1.294, P = 0.014), and NASH/NAFLD (HR 0.763, P = 0.036) to be significant prognostic factors (Table 3a). Although NAFLD/NASH was not a significant prognostic factor in that analysis for OS (HR 0.758, P = 0.092), the factors AFP (≥ 400 ng/mL) (HR 1.402, P = 0.009), BCLC C stage (HR 1.297, P = 0.035), later line introduction of lenvatinib (HR 0.737, P = 0.014), and mALBI grade 2b (HR 1.875, P < 0.001) were significant (Table 3b). Additionally, FIB-4 index was lower in the NAFLD/NASH group, though elevated FIB-4 index (≥ 3.25) was not a significant prognostic factor in regard to either PFS or OS (Table 3a,b).
Figure 5.
Progression-free and overall survival of NAFLD/NASH and Viral/Alcohol groups. (a) Progression-free survival. Viral/Alcohol (median 7.5 months, 95% CI 6.8–8.0), NAFLD/NASH (median 9.3 months, 95% CI 7.8–13.5) (P = 0.012). (b) Overall survival. Viral/Alcohol (median 16.9 months, 95% CI 14.5–18.6), NAFLD/NASH (median 20.5 months, 95% CI 16.8–29.5). (P = 0.057).
Table 3.
Prognostic factors for progression-free survival and overall survival in Study-2.
| a. Cox hazard analysis for PFS | HR | 95%CI | P value |
|---|---|---|---|
| Age ≥ 75 years | 1.059 | 0.863–1.300 | 0.583 |
| Female gender | 1.013 | 0.802–1.280 | 0.912 |
| ECOG PS 2 | 0.946 | 0.377–2.374 | 0.906 |
| NAFLD/NASH | 0.730 | 0.562–0.978 | 0.019 |
| ALT ≥ 30 U/L | 1.240 | 1.022–1.522 | 0.030 |
| Platelet count ≥ 10 (104/µL) | 0.935 | 0.732–1.194 | 0.588 |
| Elevated FIB-4 index (≥ 3.25) | 1.122 | 0.892–1.412 | 0.326 |
| mALBI grade 2b | 1.172 | 0.945–1.453 | 0.148 |
| AFP ≥ 400 ng/mL | 1.272 | 1.031–1.569 | 0.025 |
| TNM-LCSGJ stage IV | 1.194 | 0.818–1.742 | 0.358 |
| BCLC stage C | 1.043 | 0.713–1.526 | 0.827 |
| Reduced starting dose | 1.215 | 0.980–1.505 | 0.076 |
| Treatment as later line | 0.880 | 0.718–1.079 | 0.219 |
| Results of stepwise regression method | |||
| NAFLD/NASH | 0.763 | 0.594–0.982 | 0.036 |
| ALT ≥ 30 U/L | 1.247 | 1.023–1.520 | 0.029 |
| mALBI grade 2b | 1.236 | 1.003–1.523 | 0.047 |
| AFP ≥ 400 ng/mL | 1.294 | 1.055–1.588 | 0.014 |
| b. Cox hazard analysis for OS | HR | 95%CI | P value |
|---|---|---|---|
| Age ≥ 75 years | 1.167 | 0.904–1.505 | 0.236 |
| Female gender | 1.141 | 0.862–1.510 | 0.358 |
| ECOG PS 2 | 1.106 | 0.394–3.105 | 0.848 |
| NAFLD/NASH | 0.758 | 0.550–1.046 | 0.092 |
| ALT ≥ 30 U/L | 1.188 | 0.934–1.512 | 0.160 |
| Platelet count ≥ 10 (104/µL) | 1.038 | 0.772–1.396 | 0.805 |
| Elevated FIB-4 index (≥ 3.25) | 1.102 | 0.808–1.468 | 0.506 |
| mALBI grade 2b | 1.764 | 1.376–2.262 | < 0.001 |
| AFP ≥ 400 ng/mL | 1.367 | 1.059–1.765 | 0.016 |
| TNM-LCSGJ stage IV | 1.183 | 0.762–1.837 | 0.454 |
| BCLC stage C | 1.174 | 0.756–1.824 | 0.475 |
| Reduced starting dose | 1.055 | 0.807–1.380 | 0.694 |
| Treatment as later line | 0.737 | 0.574–0.946 | 0.017 |
| Results of stepwise regression method | |||
| mALBI grade 2b | 1.875 | 1.481–2.375 | < 0.001 |
| AFP ≥ 400 ng/mL | 1.402 | 1.089–1.805 | 0.009 |
| BCLC stage C | 1.297 | 1.019–1.652 | 0.035 |
| Treatment as later line | 0.737 | 0.578–0.939 | 0.014 |
ALT alanine aminotransferase, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, ECOG PS Eastern Cooperative Oncology Group performance status, ALBI score: albumin-bilirubin score, mALBI grade modified ALBI grade, AFP alpha-fetoprotein, TNM LCSGJ 6th tumor node metastasis stage by Liver Cancer Study Group of Japan 6th edition, BCLC stage Barcelona Clinic Liver Cancer stage.
Finally, examination of AEs (over 20%) showed that hypothyroid and urine protein conditions were more common in the NAFLD/NASH as compared to the Viral/Alcohol group (P = 0.013 and P = 0.032, respectively) (Supplemental Table S1).
Discussion
The present analyses of u-HCC patients who received lenvatinib showed that PFS and OS in those in the NAFLD/NASH group were favorable as compared with those in the Viral/Alcohol group (median PFS: 9.3 vs. 7.5 months, P = 0.012) (median OS: 20.5 vs. 16.9 months, P = 0.057). Also, when cryptogenic HCC was included in the NAFLD/NASH group, both PFS and OS were better in those patients (median PFS: 9.3 vs. 7.5 months, P = 0.012) (median OS: 21.0 vs. 16.9 months, P < 0.001). An interesting meta-analysis article recently reported by Pfister showed that patients with a viral etiology demonstrated therapeutic benefits with ICI treatment [HR 0.64], whereas those with nonviral etiology HCC did not [HR 0.92] (P = 0.03)5. That report also presented results of two different validation studies of ICI treatment for HCC, in which NAFLD-HCC cases showed significantly worse OS than cases of HCC with another etiology (HR 2.6, 95% CI 1.2–5.6, P = 0.017; median 8.8 vs. 17.7 months, P = 0.034). In patients undergoing ICI treatment, background liver disease etiology might be a biomarker of efficacy. As for a reason for that phenomenon, it has been reported that CD8 + T cells in HCC patients with NASH are increased and activated by IL-15-induced Fas-ligand dependent apoptosis through tumor necrotic factor (TNF) and acetate in the tumor, unlike MHC class-I dependent CD8 + T cell activation27, thus immune response to tumor antigens is impaired28,29. In contrast, the effectiveness of MTA is not related with mode of CD8 + T cell activation, but rather inhibition of multi-tyrosine kinase activity, thus MTA should be effective irrespective of HCC etiology including in NAFLD/NASH-related HCC cases.
Patients in the IMbrave150 study who underwent treatment with Atezo + Bev, a newly developed ICI and anti-VEGF-antibody combination, showed an overwhelmingly superior therapeutic efficacy as compared with those who received sorafenib (median OS: 19.2 vs. 13.4 months, HR 0.66, 95%CI 0.52–0.85) (ORR/CR by mRECIST: 35%/12% vs. 14%/3%)30. Although pooled analysis of the SHARP and Asia–Pacific trials found that positive for HCV was a predictive factor for therapeutic response to sorafenib [HR 0.47, 95% CI 0.32–0.69, P = 0.035]31, the IMbrave150 study showed superiority for the therapeutic effect (both OS and PFS) of Atezo + Bev as compared with sorafenib in HCV-HCC cases (HR 0.43, 95% CI 0.25–0.73 and HR 0.68, 95% CI 0.42–1.10, respectively)30. On the other hand, that study did not demonstrate superior findings for Atezo + Bev in regard to OS in HCC with nonviral etiology (HR 1.05, 95% CI 0.68–1.63 and HR 0.80, 95% CI 0.55–1.17) as compared to viral HCC cases. However, these results do not indicate that Atezo + Bev is not effective for non-viral HCC, as the OS in patients who received that treatment was 17.0 months, similar to that in patients with HBV HCC (19.0 months). Rather, the worse OS HR can be attributed to better efficacy of sorafenib even in non-viral HCC (18.1 months) as compared with HBV-HCC (12.4 months) and HCV-HCC (12.6 months) cases, though the reasons are unknown. The present results suggested a similar phenomenon. Although liver fibrosis in the background of HCC patients with NAFLD/NASH may be milder as compared to that in those with Viral/Alcohol, elevated FIB-4 index was not significant prognostic factor both in PFS and OS. An explanation for these findings is not clear.
Despite the retrospective nature of this analysis, it is possible to speculate that cryptogenic HCC is a subgroup of NAFLD/NASH HCC. In the present Study-1, patients with u-HCC due to alcohol abuse had PFS and OS similar to those with viral HCC, thus viral and alcoholic HCC were treated as a single group in comparisons of PFS and OS with those of NAFLD/NASH/cryptogenic HCC patients. In Study-2, after excluding cryptogenic HCC, the NAFLD/NASH and other etiology (Viral/Alcohol) groups were compared to confirm response to lenvatinib in clinically diagnosed NAFLD/NASH patients. The NAFLD/NASH group showed better PFS (P = 0.012). Although there was no significant difference in OS (P = 0.057), OS in the NAFLD/NASH-HCC patients treated with lenvatinib was very favorable (20.5 months) and tended to be better than that in the Viral/Alcohol HCC cases (16.9 months). It was recently proposed by Hessheier et al. that metabolic factors may be risk factors for development of liver diseases and cirrhosis32, while Eguchi et al. found “lean-NASH” (non-obese NASH, body mass index: BMI < 25 kg/m2) existing in 20% to > 35% in patients in Japan33. Of the present cryptogenic HCC patients (n = 26), diabetes was observed in 44.4% (n = 12), hypertension in 51.9% (n = 14), and overweight (BMI ≥ 25 kg/m2) in 25.9% (n = 7), while 70.4% (n = 19) had at least one of those co-factors (Supplemental Table S2). Thus, cryptogenic HCC might be categorized as NAFLD/NASH HCC. When cryptogenic HCC cases are included with NAFLD/NASH, in other words, without hepatitis viral infection or alcohol abuse history, such patients may receive benefit from lenvatinib treatment (Fig. 4e,f).
Since 2004, the number of adults with NASH awaiting liver transplantation in the United States has nearly tripled and NASH has become the second leading etiology of liver disease among such cases34. In meta-analysis results, the NAFLD incidence rate was reported to be 25.24% (all regions, 95% CI 22.1–28.65) and pooled overall NASH prevalence among biopsied NAFLD patients was estimated to be 59.10% (95%CI 47.55‐69.73), while the annual rate of liver carcinogenesis from NAFLD was estimated to be approximately 0.04% (95% CI 0.29–0.66)35. Similarly in Japan, a rapidly increasing rate of HCC patients without hepatitis viruses has been reported36, with most cases of non-B, non-C HCC shown to be related to lifestyle/metabolic factors, such as obesity or diabetes, including cryptogenic HCC37. Recently, liver-related diseases, such as cirrhosis and HCC, have been reported to be the third leading cause of death in patients in Japan with type 2 diabetes mellitus, which is associated with NAFLD38. Furthermore, a recent review article of cases of HCC related to NAFLD mentioned that the impact of metabolic syndrome and its relevance in those patients is not clear39. Nevertheless, establishment of an effective treatment strategy for u-HCC related with NAFLD/NASH is considered to be a critical clinical issue. It is anticipated that the number and percentage of NAFLD-HCC cases will continue to increase, though liver cirrhosis is not present in all of those. However, HCC is often detected in an advanced stage because no surveillance program for NAFLD-HCC patients has been established. As a result, it is important to confirm which systemic treatment (e.g. MTAs or ICI combination) is a more effective therapeutic option for patients with NAFLD HCC as well as those with viral hepatitis-related HCC. Moreover, some favorable results regarding OS in u-HCC patients receiving lenvatinib as post-progression treatment following ICI have been reported. Aoki noted that the median OS of lenvatinib was 15.8 months (95% CI 8.49–23.17) after ICI failure40, while Yoo reported that patients who received lenvatinib as post-progression treatment after Atez/Bev failure showed good OS (median 16.6 months)41. In the present analysis, lenvatinib showed a good therapeutic effect with both first and later line administration. Thus, not only for NAFLD/NASH u-HCC cases but also those with ICI treatment failure, lenvatinib can be selected for administration as an effective subsequent therapeutic option at any time, especially in patients with good hepatic function.
The present study has some limitations, including its design as a retrospective multicenter study. Furthermore, the pathological diagnosis of disease etiology for the present patients without viral hepatitis was not adequately assessed. A future study in which prospective comparisons between lenvatinib and ICI treatment in NASH/NAFLD HCC patients is needed.
In conclusion, lenvatinib was found to be effective for improving the prognosis of u-HCC patients irrespective of HCC etiology or line of treatment.
Supplementary Information
Author contributions
A.H., T.K., and M.K. conceived the study, and participated in its design and coordination. A.H., T.K., T.o.T., J.T., K.k.a., S.F., M.A., M.H., K.T.s., T.I., K.Tak., E.I., K.Taj., N.S., H.S., H.Oc., K.K., S.Y., H.T., T.Ao., Ta.T., H.Oh., K.N., A.T., T.N., N.I., K.H., T.Ar., M.I., Y.K., S.N., K.M., K.J., and Y.H. performed data curation. A.H. performed statistical analyses and interpretation. A.H., T.K., and M.K. drafted the text. All authors have read and approved the final version of the manuscript.
Data availability
The datasets generated and/or analyzed for the current study are not publicly available because of privacy reasons.
Competing interests
Atsushi Hiraoka, MD, PhD—Lecture fees: Bayer, Eisai, Eli Lilly, Otsuka. Takashi Kumada, MD, PhD—Lecture fees: Eisai. Masatoshi Kudo, MD, PhD—Advisory role: Eiasi, Ono, MSD, Bristol-Myers Squibb, Roche; Lecture fees: Eisai, Bayer, MSD, Bristol-Myers Squibb, Eli Lilly, EA Pharma; Research funding: Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, Abbvie, Eisai. None of the other authors have potential conflicts of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96089-x.
References
- 1.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/s1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/s0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Pfister D, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021 doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL clinical practical guidelines Management of alcoholic liver disease. J. Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J. Clin. Gastroenterol. 2013;47(Suppl):S2–6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalasani N, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J. Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.Di Martino M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 12.Sano K, et al. Imaging study of early hepatocellular carcinoma: Usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834–844. doi: 10.1148/radiol.11101840. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka A, et al. Clinical translation in the treatment of hepatocellular carcinoma following the introduction of contrast-enhanced ultrasonography with Sonazoid. Oncol. Lett. 2010;1:57–61. doi: 10.3892/ol_00000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraoka A, Hiasa Y, Onji M, Michitaka K. New contrast enhanced ultrasonography agent: Impact of Sonazoid on radiofrequency ablation. J. Gastroenterol. Hepatol. 2011;26:616–618. doi: 10.1111/j.1440-1746.2011.06678.x. [DOI] [PubMed] [Google Scholar]
- 15.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/s0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 16.The Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 6. Kanehara; 2015. [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Johnson PJ, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015;33:550–558. doi: 10.1200/jco.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraoka A, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016;31:1031–1036. doi: 10.1111/jgh.13250. [DOI] [PubMed] [Google Scholar]
- 20.Hiraoka A, et al. Albumin-bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan Society of Hepatology: A comparison with the liver damage and child-pugh classifications. Liver Cancer. 2017;6:204–215. doi: 10.1159/000452846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraoka A, et al. Validation and potential of Albumin-Bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6:325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraoka A, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer. 2019;8:121–129. doi: 10.1159/000488778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National, Cancer & Institute. https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm. Accessed 23 Jan 2021.
- 26.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudek M, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–449. doi: 10.1038/s41586-021-03233-8. [DOI] [PubMed] [Google Scholar]
- 28.Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 30.Finn, R. et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label Phase III study of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). EASL Liver Cancer Summit 2021, #O-05 (2021).
- 31.Bruix J, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Hessheimer AJ, Forner A, Varela M, Bruix J. Metabolic risk factors are a major comorbidity in patients with cirrhosis independent of the presence of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2010;22:1239–1244. doi: 10.1097/MEG.0b013e32833aa19b. [DOI] [PubMed] [Google Scholar]
- 33.Eguchi Y, et al. Epidemiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in Japan: A focused literature review. JGH Open. 2020;4:808–817. doi: 10.1002/jgh3.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong RJ, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Younossi ZM, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 36.Tateishi R, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019;54:367–376. doi: 10.1007/s00535-018-1532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tateishi R, et al. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J. Gastroenterol. 2015;50:350–360. doi: 10.1007/s00535-014-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura J, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Investig. 2017;8:397–410. doi: 10.1111/jdi.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geh D, Manas DM, Reeves HL. Hepatocellular carcinoma in non-alcoholic fatty liver disease: A review of an emerging challenge facing clinicians. Hepatobiliary Surg. Nutr. 2021;10:59–75. doi: 10.21037/hbsn.2019.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki T, et al. Exploratory analysis of lenvatinib therapy in patients with unresectable hepatocellular carcinoma who have failed prior PD-1/PD-L1 checkpoint blockade. Cancers. 2020 doi: 10.3390/cancers12103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo C, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: A multinational multicenter retrospective study. Liver Cancer. 2021;10:107–114. doi: 10.1159/000512781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed for the current study are not publicly available because of privacy reasons.