Abstract
This Practice Guidelines document has been co-published in Phlebology [DOI: 10.1177/0268355521999559] and Journal of Vascular Surgery: Venous and Lymphatic Disorders [DOI: 10.1016/j.jvsv.2020.12.084]. The publications are identical except for minor stylistic and spelling differences in keeping with each journal’s style. The contribution has been published under a Attribution-Non Commercial 4.0 International (CC BY-NC 4.0), (https://creativecommons.org/licenses/by-nc/4.0/)
With the support of the American College of Obstetricians and Gynecologists, the American Vein & Lymphatic Society, the American Venous Forum, the Canadian Society of Phlebology, the Cardiovascular and Interventional Radiology Society of Europe, the European Venous Forum, the International Pelvic Pain Society, the International Union of Phlebology, the Korean Society of Interventional Radiology, the Society of Interventional Radiology, and the Society for Vascular Surgery
As the importance of pelvic venous disorders (PeVD) has been increasingly recognized, progress in the field has been limited by the lack of a valid and reliable classification instrument. Misleading historical nomenclature, such as the May-Thurner, pelvic congestion, and nutcracker syndromes, often fails to recognize the interrelationship of many pelvic symptoms and their underlying pathophysiology. Based on a perceived need, the American Vein and Lymphatic Society convened an international, multidisciplinary panel charged with the development of a discriminative classification instrument for PeVD. This instrument, the Symptoms-Varices-Pathophysiology (“SVP”) classification for PeVD, includes three domains—Symptoms (S), Varices (V), and Pathophysiology (P), with the pathophysiology domain encompassing the Anatomic (A), Hemodynamic (H), and Etiologic (E) features of the patient’s disease. An individual patient’s classification is designated as SVPA,H,E. For patients with pelvic origin lower extremity signs or symptoms, the SVP instrument is complementary to and should be used in conjunction with the Clinical-Etiologic-Anatomic-Physiologic (CEAP) classification. The SVP instrument accurately defines the diverse patient populations with PeVD, an important step in improving clinical decision making, developing disease-specific outcome measures and identifying homogenous patient populations for clinical trials.
Keywords: Venous insufficiency, Varicose veins, Pelvic pain, May Thurner syndrome, Renal nutcracker syndrome
Article Highlights
Type of Research: Multispecialty, intersocietal development of a discriminative classification instrument.
Key Findings: The clinical presentation of patients with pelvic venous disorders can be accurately and fully characterized by a discriminative instrument that includes presenting symptoms (S), the involved variceal reservoirs (V), and the underlying pathophysiology (P), which includes the anatomic (A), hemodynamic (H), and etiologic (E) features of the disease. A patient’s presentation is summarized as SVPA,H,E.
Take Home Message: The use of historical nomenclature for pelvic venous disorders fails to recognize the complex and interrelated pelvic venous circulation, contributes to misdiagnosis and poor treatment outcomes, and hinders clinical research. In defining homogenous patient populations, the Symptoms-Varices-Pathophysiology instrument will facilitate clinical communication, allow treatment to be more precisely directed, and facilitate the development of patient-reported outcome measures and clinical trials.
The importance of venous disorders of the abdomen and pelvis has become increasingly recognized over the past decade. Unfortunately, progress has been hindered by the use of historical syndromic nomenclature—for example the May-Thurner, pelvic congestion, and nutcracker syndromes—which has often confused the underlying pathophysiology and led to diagnostic errors and suboptimal treatment outcomes. Furthermore, the lack of a robust classification system defining homogenous patient populations limits clinical communications, makes interpretation of the literature difficult, and hinders the development of appropriate clinical trials. The existence of pelvic venous disorders (PeVD) and their appropriate treatment has also been questioned owing to the lack of validated definitions and imaging criteria as well as rigorous randomized clinical trials.1 There is a critical need for a classification system for PeVD that recognizes the variable, but often overlapping, clinical presentations, as well as the underlying pathophysiology. A multidisciplinary panel has ranked the development of validated diagnostic criteria and a discriminative classification instrument as the most important research priorities for PeVDs.1
For venous disorders of the lower extremities, the Clinical-Etiologic-Anatomic-Physiologic (CEAP) classification, originally published in 19962 and revised in 20043 and 2020,4 has become the international standard for classification of these disorders. By defining patient groups with similar clinical presentations and pathophysiologic features, the instrument has facilitated clinical communication regarding individual patients and is recognized as a reporting standard for clinical research. Despite its usefulness and general acceptance, the CEAP classification system is limited to lower extremity venous disorders. Since its original description, rapid advancements in diagnostic imaging and catheter-based interventions have improved our understanding of disorders arising from veins other than those in the legs, particularly those of pelvic and abdominal origin.
Venous disorders of the pelvis are associated with a spectrum of symptoms arising from both reflux, most commonly involving the gonadal and internal iliac veins, and obstruction, usually of the left renal and iliac veins. These hemodynamic patterns are associated with at least four broad clinical presentations, including (a) left flank or abdominal pain and hematuria (left renal vein compression), (b) chronic pelvic pain (pelvic varicosities associated with primary reflux in the ovarian/internal iliac veins or obstruction of the left renal or common iliac veins), (c) venous claudication (iliac venous obstruction), and (d) symptomatic lower extremity varicosities in either atypical (vulva/testicles, medial and posterior thigh, sciatic nerve) or typical saphenous distributions, the latter frequently recurring after initial treatment.
The relationship between pelvic symptoms and venous pathology is far more complex than in the lower extremity. Multiple symptoms may be present concurrently and several potential pathophysiologic mechanisms, such as left renal and iliac venous compression, may be simultaneously present. Additionally, similar symptoms may arise from disparate underlying causes (eg, chronic pelvic pain can arise from primary ovarian vein reflux, left common iliac vein compression, or left renal vein compression), and similar anatomic derangements may lead to different symptoms (eg, left renal vein compression may be associated with either left flank pain and hematuria or chronic pelvic pain). This can lead to diagnostic errors and may be responsible for the suboptimal results of many interventions.5,6 From a research perspective, appropriate patient classification is also important in ensuring homogenous patient populations for the development of disease-specific outcome instruments and clinical trials. There is thus a critical need for precise classification of PeVDs that has implications for both individual patient management and future clinical research.
Methods
Based on the need for a classification instrument for PeVD, the American Vein and Lymphatic Society convened an International Working Group on Pelvic Venous Disorders in Chicago, Illinois, on July 27, 2018. International societies representing the broad spectrum of specialties involved in the care of patients with PeVD, including gynecologists, interventional radiologists, vascular surgeons, and phlebologists, were invited to participate either in-person or remotely. Invited societies and their representatives are listed in Table 1.
Table 1.
International Working Group on Pelvic Venous Disorders (PeVDs) Participants
| Contributor | Affiliation |
|---|---|
| Diana Atashroo, MD | International Pelvic Pain Society (IPPS) |
| Antonio Basile, MD | Cardiovascular and Interventional Radiological Society of Europe (CIRSE) |
| Antonio Gasparis, MD | American Venous Forum (AVF) |
| Kathleen Gibson, MD | American Vein and Lymphatic Society (AVLS) |
| Milka Greiner, MD, PhD | European Venous Forum (EVF) |
| Nicos Labropoulos, PhD | International Union of Phlebology (UIP) |
| Zaza Lazarashvilli, MD | International Union of Phlebology (UIP) |
| Lee Learman, MD, PhD | American College of Obstetricians and Gynecologists (ACOG) |
| Joanne Lohr, MD | American Venous Forum (AVF) |
| Neil Khilnani, MD | Society of Interventional Radiology (SIR) |
| Man-Deuk Kim, MD, PhD | Korean Society of Interventional Radiology |
| Fedor Lurie, MD, PhD | Society for Vascular Surgery |
| Mark Meissner, MD | American Vein and Lymphatic Society (AVLS) |
| Philippe Nicolini, MD | European Venous Forum (EVF) |
| Waleska Pabon-Ramos, MD, MPH | Society of Interventional Radiology (SIR) |
| Marc Passman, MD | Society for Vascular Surgery |
| Mel Rosenblatt, MD | American Vein and Lymphatic Society (AVLS) |
The specific goal of the group was to develop a discriminative classification instrument for PeVDs. Discriminative instruments are designed to measure cross-sectional differences between individuals at a single point in time, as opposed to evaluative instruments that measure longitudinal changes within people over time.7,8 Discriminative instruments include key components of the disease that are stable, at least over short periods of time, have a limited number of options and clear definitions that enable uniform interpretation, and have large and stable between-subject variation.8 From a simplistic standpoint, discriminative instruments place patients into homogenous groups with similar clinical features, natural histories, and responses to treatment.
At the initial meeting, the clinical, anatomic, and pathophysiologic aspects of PeVD were presented and discussed among panel members, incorporating the views of the various subspecialties included on the panel. The methodology underlying instrument development was then reviewed and alternative approaches discussed. Based on this discussion, it was agreed that the instrument should be based on the following principles.
The instrument should be patient-centric, that is, focused on the primary concerns of the patient rather than simply the underlying pathophysiology.
In addition to patient-important clinical features, complete characterization of a patient’s presentation requires a precise description of the underlying anatomy and pathophysiology.
Asymptomatic patients with pelvic venous disease should be included in the classification, although among symptomatic patients, only those with a recognized venous etiology should be included. Similar clinical presentations that are not of venous origin (eg, chronic pelvic pain owing to other causes) are not included in this classification.
Several nuances of PeVD, particularly the observation that PeVD are primarily symptom rather than sign based, preclude a purely CEAP-based approach. However, because venous disorders of the pelvis and lower extremity are a continuum, the instrument should, as much as feasible, follow the conventions of and be complementary to CEAP. Accordingly, the pelvic instrument should avoid duplication of lower extremity signs that are included in CEAP. For example, although localized pelvic origin extrapelvic symptoms, such as tenderness associated with pelvic origin varicosities, should be included in the pelvic instrument, more generalized lower extremity signs, such as swelling continue to be best classified with CEAP.
Guided by these principles, the domains to be included were discussed and precise definitions developed, emphasizing the importance of optimizing the validity and reproducibility of the instrument. Small groups were then formed to craft an initial strategy for each domain, which was then discussed among the entire group. Based on the discussion, a draft instrument (the SVP classification) was developed and three rounds of simulated patient classification performed by the writing group (M.H.M., N.K., N.L., A.G., K.G., and M.G.) to identify potential problems with the definitions and ensure reproducibility of the instrument. Definitions were further refined based on the simulated classification exercises and review of the literature, striving to make them as evidence based as possible. The final draft was then circulated to all participants for revision.
Results: the classification of PeVD
Definitions
Minimizing interobserver variability through precise definitions is critical to the reproducibility of a discriminative instrument. The following definitions were developed and should be utilized for the purpose of pelvic venous classification. When possible, efforts were made to make these definitions congruent with lower extremity CEAP.
Symptoms
PeVD—The spectrum of symptoms and signs arising from the veins of the pelvis (the gonadal veins, the internal iliac veins and their tributaries, and the venous plexuses of the pelvis) and their primary drainage pathways (the left renal vein, the iliac veins, and the pelvic escape points).
This includes symptoms historically ascribed to the May-Thurner, nutcracker, and pelvic congestion syndromes. Given their imprecise and overlapping nature, these historical terms should no longer be used.1
Venous origin renal symptoms—Symptoms arising from renal venous hypertension secondary to left renal vein obstruction.
These include microhematuria or macrohematuria and left flank or abdominal pain that is worsened by activities such as standing, sitting, or walking.9
Chronic pelvic pain—Pain symptoms perceived to originate from pelvic organs/structures typically lasting more than 6 months. It is often associated with negative cognitive, behavioral, sexual, and emotional consequences as well as with symptoms suggestive of lower urinary tract, sexual, bowel, pelvic floor, myofascial, or gynecologic dysfunction.10
Although there has historically been a lack of consensus11 regarding the definition of chronic pelvic pain, we have adopted that proposed by the American College of Obstetricians and Gynecologists. Causes of chronic pelvic pain include a wide range of disorders of the reproductive, urinary, gastrointestinal, neurologic, and musculoskeletal systems,12 often with overlapping symptoms in an individual patient.13 PeVD are included in the range of somatic, visceral and neurologic pain generators that are often associated with chronic pelvic pain.
Data regarding the demographics and symptomatology of women with venous origin pelvic pain are largely derived from small case series of those presenting for treatment and there is a clear need for larger studies comparing women with chronic pelvic pain of venous and nonvenous origin. Such limited case series suggest that venous origin pelvic pain most commonly occurs in multiparous women of reproductive age.12,14–16 Despite this general observation, a somewhat older population with iliac venous obstruction has recently been described in which pelvic pain often occurs in conjunction with leg symptoms,17,18 implying that patient demographics and associated symptoms may depend on the underlying etiology.
Because chronic pelvic pain includes a spectrum of symptoms, there is substantial overlap between women with pain secondary to venous and nonvenous causes. Descriptions of the typical characteristics of venous origin pelvic pain come largely from a single dated but well-done study comparing women with pelvic pain and varices on transuterine venography to women with either pelvic pain owing to other pathology or without pelvic pain undergoing elective sterilization.15 Most of the signs and symptoms associated with venous-origin pelvic pain have been found to be relatively sensitive, but nonspecific.19 Pelvic pain of venous origin is often characterized as dull unilateral or bilateral pain with occasional sharp flares. Bimanual examination, demonstrating focal adnexal tenderness, often reproduces the pain. Symptoms are often worse with activities such as walking and prolonged standing, and improve with lying down. Although deep dyspareunia is common among women with pelvic pain from a variety of causes, venous origin pain is more likely to be associated with prolonged postcoital ache.12,15,19 The combination of postcoital ache and tenderness over the ovarian point (the junction of the upper and middle thirds of a line drawn from the umbilicus to the anterior superior iliac spine) has been reported to be 94% sensitive and 77% specific for distinguishing a venous origin from other causes of pelvic pain.15
Although chronic pelvic pain also occurs in males,20,21 there is currently little evidence to suggest that pelvic venous disease is an important contributing factor. This is likely due to both differences in venous anatomy as well as the role of pregnancy in PeVDs in women. The gonadal veins follow an extrapelvic course in males and the arrangement of the visceral pelvic venous plexuses are substantially different.
Pelvic origin extrapelvic symptoms—Symptoms localized to the external genitalia or lower extremities that arise from either reflux through recognized escape points in the pelvic floor22 or from iliocaval venous obstruction.
In females, reflux-related symptoms may include pain, discomfort, tenderness, itching, bleeding, and superficial venous thrombosis associated with nonsaphenous varicosities. These may be localized to the vulva or the posteromedial thigh in the distribution of the perineal and inferior gluteal escape points. In males, these include testicular discomfort and infertility related to a varicocele. Extrapelvic reflux arising from the inferior gluteal vein may also rarely be associated with sciatic or tibial nerve symptoms. Symptoms associated with sciatic nerve varices include pain radiating from the buttock to the lateral aspect of the leg, often worsened with sitting.23,24 Anecdotal reports suggest tibial nerve symptoms are milder, often including only paresthesias on compression of the nerve. Obstruction-related extrapelvic symptoms include venous claudication.
Venous claudication—Exertional pain in the lower extremities frequently described as a tight, “bursting” pain, in the thigh, buttock, or leg not associated with a specific walking distance or confined to specific muscle groups, but relieved by rest and elevation of the legs.25–28 Symptoms of venous claudication are most commonly associated with iliocaval venous obstruction.
HASTI (Provensis, Uxbridge, UK) symptoms—Nonspecific symptoms typically associated with lower extremity venous disease including heaviness (H), aching (A), swelling (S), throbbing (T), and itching (I).27,29
Such symptoms are usually generalized to the lower extremity rather than localized to any pelvic origin extrapelvic lower extremity varices. Although the responsible pathology may arise in the pelvis, generalized signs of lower extremity venous disease are not included in the SVP classification and should be accounted for by the concurrent use of CEAP.
Signs
Left renal vein obstruction—Compression of the left renal vein at the crossing of the abdominal aorta associated with symptoms related either to (a) renal venous hypertension (hematuria and/or abdominal/flank pain) or (b) if decompressed by collaterals, pelvic varices and chronic pelvic pain or a left-sided varicocele.
Symptomatic obstruction of the left renal vein is usually attributed to compression of the renal vein between the abdominal aorta and superior mesenteric artery (anterior nutcracker syndrome), although compression may also arise from a retroaortic course of the left renal vein (posterior nutcracker syndrome) or stretching of the renal vein over the abdominal aorta.9 Symptoms of flank pain and hematuria are presumed secondary to renal venous hypertension, often defined as a transrenal pressure gradient of 3 or more mm Hg at the time of venography.30–33 Hematuria in such cases is often attributed to renal varices, which are often asymptomatic, effect predominantly the left kidney, and have been identified in 10% of left renal venograms performed for a variety of indications.34 However, such a gradient may be absent it there is significant decompression via refluxing collaterals including the left gonadal, ascending lumbar, adrenal, periureteral, capsular, or intrarenal veins.9,31 In such cases, pelvic varices or a varicocele may be associated with secondary gonadal vein reflux.
A variety of imaging modalities including ultrasound, venography (with or without intravascular ultrasound [IVUS] and measurement of pressure gradients), computed tomography (CT), and magnetic resonance imaging (MRI) have been used in the evaluation of left renal vein compression. Although mean renal vein diameter reduction by CT is significantly higher in patients with symptoms related to renal venous hypertension (74.5 ± 1.9%) than in controls (25.4 ± 2.4%)35 and a transrenal pressure gradient of 3 or more mm Hg has been associated with hematuria,30–32 definitive diagnostic criteria and cut-points are lacking and may vary between patients. Furthermore, asymptomatic 50% or greater compression of the left renal vein (nutcracker phenomenon) is seen in 51% to 72% of CT angiograms.32 Given the lack of definitive anatomic and hemodynamic criteria across a variety of clinical settings, we have not included them in the definition, which instead relies on correlating the patient’s symptoms and imaging studies.
Pelvic varicose veins—Tortuous, dilated veins 5 mm or more in diameter around the ovary and uterus.36
Pelvic varices may involve both the ovarian (pampiniform) and uterovaginal venous plexuses, which communicate through the broad ligament.12,22,37–39 There may also be extensive communication with the vesicular and external rectal plexus.22 Although venography has historically been the reference standard for the diagnosis of pelvic varices,14,37,39 it remains an invasive study associated with the risks of ionizing radiation and is now often limited to definitive imaging at the time of planned intervention. Several noninvasive imaging studies,37,40 more suitable for initial evaluation, have been suggested including transabdominal ultrasonography, transvaginal ultrasonography, CT, and MR imaging. Among these, pelvic ultrasound, either transabdominal or transvaginal, is the most widely available, has been the most extensively investigated, and allows an evaluation of both venous diameter and reflux. We have accordingly defined pelvic varices based on commonly cited ultrasound criteria.36 Other diagnostic criteria have been proposed, including greater than 4 tortuous, dilated veins greater than 4 mm in diameter surrounding the ovaries and uterus,41 the appearance of dilated transuterine veins (arcuate and/or myometrial veins) connecting the left and right uterine veins,37 and reversed flow direction or disappearance of flow with Valsalva.37,40,42 However, Park et al36 found transuterine crossing veins in only 25% of patients with symptomatic pelvic varicosities in comparison with 8.6% of controls. Similarly, reversal of Doppler flow direction during a Valsalva maneuver was identified in only 26.9% of symptomatic patients, in comparison with 8.8% of controls.36
Position does influence the ability to detect pelvic venous pathology. Investigators have reported ultrasound evaluation in the supine,36 30° to 45° reverse Trendelenburg position,42,43 semi-erect,44 and upright positions.43 CT and MR imaging are obligatorily performed in the supine position. Because there is no consensus regarding positioning for noninvasive examinations, it has not been included in the definitions of pelvic varicose veins or reflux. However, clinicians should be aware of the role that position may have in the interpretation of all imaging studies.
Gonadal vein reflux—Retrograde flow in either gonadal vein, spontaneously or in response to a Valsalva’s maneuver, as documented by ultrasound, venography, or time resolved magnetic resonance angiography.
Retrograde flow is the primary criteria for the definition of venous reflux and in the left ovarian vein, has been identified in 100% of patients with symptomatic pelvic varices in comparison with 25% of controls.41 Although some investigators45 have defined pelvic reflux as retrograde flow greater than 1 second in duration and persisting until the end of the maneuver, other investigators41,46 have noted no validated cut-point for pathologic duration of reflux in the ovarian veins. Still other investigators have noted variable reflux patterns, including spontaneous, intermittent retrograde flow; retrograde flow only during a Valsalva maneuver; and continuous retrograde flow.47 Given the conflicting evidence, we have chosen not to include reflux duration in the definition.
Gonadal vein diameter, in the presence of pelvic varices is often used as a surrogate for retrograde flow. Although some investigators44,45,48 have reported ovarian vein diameter to be an insensitive maker of reflux, other investigators36 have reported positive predictive values of 71.2%, 83.3%, 81.8%, and 75.8% for diameters of 5, 6, 7, and 8 mm, respectively. Other investigators41 have similarly found pelvic varices to be present in all patients with a left ovarian vein diameter of more than 6 mm by ultrasound assessment. Diameter criteria have also been reported for CT and MR.40 However, in view of the conflicting evidence, we have not included diameter as a criteria for gonadal vein reflux.
Iliac venous obstruction—Greater than 50% cross-sectional area reduction by IVUS or a 50% or greater diameter reduction by multiplanar venography of the common or external iliac veins in association with appropriate lower extremity or pelvic symptoms.
This definition was derived from those commonly used in the literature, although it must be acknowledged that there is currently no validated method of defining a clinically or hemodynamically significant venous stenosis49–51 and that this value may differ between patients.52 In evaluating the predictors of clinical improvement after iliac venous stenting, a cross sectional area reduction of more than 54% by IVUS examination had the highest sensitivity (83% sensitivity, 47% specificity), whereas a greater than 52% diameter decrease by multiplanar venography had the highest specificity (50% sensitivity, 71% specificity).49 Notably, the thresholds for clinical improvement after stenting were somewhat higher for nonthrombotic lesions. However, because a 50% or greater iliac stenosis may be present in one-quarter to one-third of the general population,52,53 it is critical that anatomic stenosis alone not be considered a criterion for intervention and that any measurement of stenosis be interpreted in the context of the patient’s clinical presentation. Both cross-sectional imaging and transabdominal ultrasound examination have been used in the initial evaluation of iliac obstruction and a number of ultrasound criteria for detection of a 50% or greater iliac venous obstruction have been developed.51,53
Internal iliac venous reflux—Retrograde flow in the internal iliac vein or its tributaries, either spontaneously or in response to a provocative Valsalva’s maneuver.
Reflux can be demonstrated by antegrade or selective descending venography, transabdominal/transperineal ultrasound,43,47 or transvaginal ultrasound.42,44 Pathologic flow patterns observed with ultrasound include retrograde flow isolated to the main internal iliac trunk, cephalad flow in the main trunk and reflux in the tributaries, or retrograde flow in both the main trunk and tributaries.
Pelvic origin extrapelvic varices—Retrograde flow in extrapelvic veins arising from reflux exiting the pelvis through recognized escape points.22
Pelvic origin extrapelvic varices include refluxing veins in either atypical locations (vulva in females and pampiniform plexus in males, perineum, gluteal cleft, and posterior thighs), or, through communication with saphenous tributaries, in a typical saphenous distribution. Extrapelvic varices also include intra/perineural (sciatic and tibial) varices arising from the inferior gluteal tributary of the internal iliac vein.22,54
As elsewhere, this is an ultrasound-derived definition that includes both visible varicosities as well as refluxing pelvic-origin tributaries that are seen only with ultrasound. Protocols for visualization of these refluxing tributaries are well-defined elsewhere.43
Pelvic origin extrapelvic varices may arise from either pelvic reflux or obstruction. However, by definition, collateral veins from the lower extremity to the pelvis that demonstrate antegrade flow at rest and function to bypass an iliocaval venous obstruction are not pelvic origin extrapelvic varices.
Lower extremity varices—As defined in CEAP,3 subcutaneous, dilated veins ≥ 3 mm in diameter which demonstrate reflux in the upright position and involve the named saphenous and accessory saphenous trunks, their tributaries and nonsaphenous superficial leg veins.
Classification of PeVDs—The SVP Instrument
Discriminative instruments for venous disorders consist of descriptive domains or categories, such as the clinical (C), etiologic (E), anatomic (A), and pathophysiologic (P) domains of CEAP, with precisely defined responses within each domain. The proposed classification for PeVDs has been designated the SVP classification and includes three domains: symptoms (S), varices (V), the primary sign of PeVD, and a composite anatomic-pathophysiologic domain (P). The composite P domain is composed of three subdomains, including the anatomy of the involved abdominal and pelvic veins (A), the associated hemodynamic abnormalities (H), and the underlying etiology (E), which are listed as subscripts after the P domain (PA,H,E). An individual patient’s pelvic classification is thus designated as SVPA,H,E.
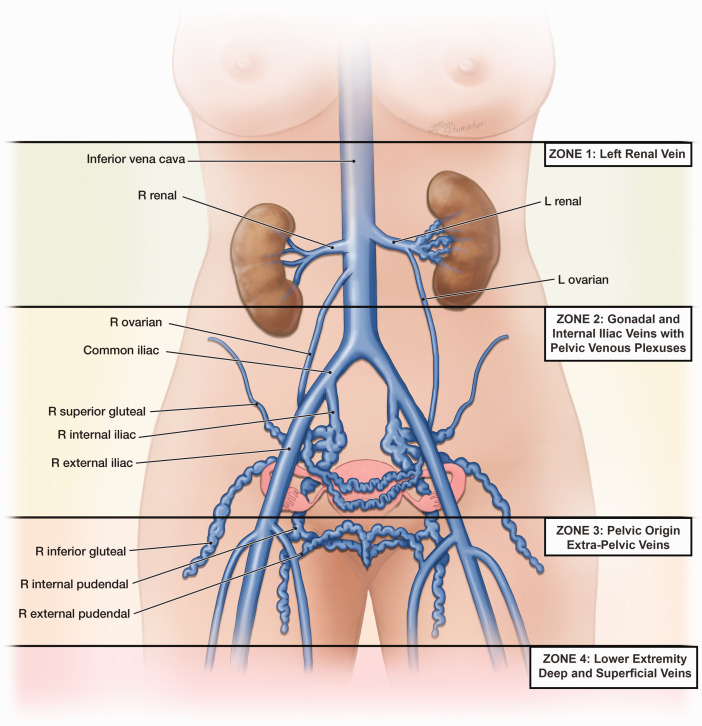
Symptoms (S) and varices (V) associated with PeVD are considered to occur in 4 anatomic zones extending in a descending fashion from the renal veins to the lower extremities (Figure 1). Three of these zones—(1) the left renal vein, (2) the gonadal and internal iliac veins and associated pelvic venous plexuses, and (3) the pelvic origin extrapelvic transitional veins arising from reflux exiting the pelvis through recognized escape points—are included in the SVP classification. Although often communicating with zone 3, the fourth zone, the superficial and deep veins of the lower extremity and their tributaries, is optimally classified with CEAP and is not included in the SVP instrument.
Figure 1.
The symptoms, signs (varices), and pathophysiologic manifestations of pelvis venous disorders (PeVD) occur in four anatomic zones of the abdomen and pelvis. These are arranged in descending order from the renal veins to the lower extremities and include symptoms and varices associated with (1) the left renal vein, (2) the gonadal, internal iliac, and pelvic veins, (3) the pelvic origin extrapelvic veins arising in the pelvis and refluxing through the pelvic escape points to the genitalia and lower extremity veins, and (4) the lower extremity veins. The first three zones are included in the Symptoms-Varices-Pathophysiology (SVP) classification while the fourth zone, associated with the superficial and deep veins of the lower extremity and their tributaries, is optimally classified with CEAP and is not included. L, left; R, right.
Each of the three primary domains—symptoms (S), varices (V), and pathophysiology (P) with its 3 subdomains—is discussed in this section.
Symptoms (S)
Pelvic venous classification begins with the patient’s clinical symptoms (S) designated by subscripts from 0 through 3 (Table 2). As discussed elsewhere in this article, responses are arranged in descending anatomic zones from the renal veins to the lower extremities. Although some complaints may occur in either sex, others such as pelvic pain and varicocele occur predominantly or exclusively in one sex. Venous origin extrapelvic symptoms (S3) are further subdivided into those involving the external genitalia, those related to pelvic origin nonsaphenous varicosities of the leg (posteromedial thigh and sciatic/tibial nerve), and those of venous claudication.
Table 2.
Symptoms (“S”)
| S0 | No symptoms of a PeVD (no renal, pelvic, or extrapelvic symptoms) |
| S1 | Renal symptoms of venous origin |
| S2 | Chronic pelvic pain of venous origin |
| S3 | Extrapelvic symptoms of venous origin |
| a | Localized symptoms (pain, discomfort, tenderness, itching, bleeding and superficial venous thrombosis) associated with veins of the external genitalia (vulva and scrotum) |
| b | Localized symptoms associated with pelvic origin nonsaphenous veins of the leg. These include those related to pelvic origin varices of the posteromedial thigh (pain, discomfort, tenderness, itching, superficial venous thrombosis) as well as those related to sciatic/tibial nerve varices (pain, paresthesias). More generalized lower extremity symptoms and signs, such as heaviness and swelling, are classified with CEAP not SVP.* |
| c | Venous claudication.* |
PeVD, Pelvic venous disorder; SVP, symptoms-Varices-Pathophysiology.
*Must include CEAP classification for full characterization of lower extremity symptoms.
The pelvic origin extrapelvic veins of the thigh may communicate with the superficial and deep veins of the lower extremity and be associated with any of the manifestations of C2 through C6 disease. Although localized symptoms such as discomfort, pruritis, bleeding, and superficial thrombosis are included in S3a and S3b, to avoid redundancy and potentially compromised reproducibility, generalized lower extremity signs (eg, swelling) and symptoms (eg, HASTI symptoms associated with C2S) are not specifically included in SVP and must be further classified using CEAP. Patients presenting with more than one clinical symptom should have all presenting features included as subscripts, separated by commas, following the S designation.
Varices (V)
The venous system of the pelvis can be considered to consist of 3 reservoirs where varices may develop—(1) the renal hilum, (2) the venous plexuses of the pelvis, and (3) the pelvic origin extrapelvic veins. The lower extremity veins comprise a fourth reservoir, which may communicate with pelvic origin extrapelvic varices. However, as with symptoms, the lower extremity reservoir is optimally defined with CEAP and is not included in SVP.
Increased venous pressures, arising from proximal reflux or obstruction, are transmitted to these reservoirs, where symptoms related to either varices or increased venous pressure may develop. Most therapeutic interventions are directed toward decreasing venous pressure in these reservoirs. The variceal reservoirs of the pelvis are designated V and are again denoted in a descending fashion by the subscripts 0 to 3 (Figureure 1, Table 3).
Table 3.
Varices (“V”)
| V0 | No abdominal, pelvic, or pelvic origin extrapelvic varices on clinical or imaging examination |
| V1 | Renal hilar varices |
| V2 | Pelvic varices |
| V3 | Pelvic origin extrapelvic varices. |
| a | Genital varices (vulvar varices and varicocele) |
| b | Pelvic origin lower extremity varicose veins arising from the pelvic escape points and extending into the thigh. Includes visible varicosities, typically over the posteromedial thigh, as well as sciatic varices and other refluxing veins transitioning the pelvic floor which are visualized only with ultrasound examination.* |
*Must include CEAP classification for full characterization of lower extremity varices.
Although some varices (eg, pelvic origin varices of the vulva or posteromedial thigh) may be apparent on physical examination, others (renal hilar, pelvic, and some pelvic origin extrapelvic varices) are identified only through imaging studies. The V classification should, therefore, include the full extent of varices defined by both physical examination and imaging studies. As with symptoms, patients presenting with varices in more than one reservoir should have all of their presenting features included as multiple subscripts, separated by commas, to V. Finally, because the pelvic and lower extremity venous systems are in continuity, patients with lower extremity signs and symptoms arising in the pelvis should be described using both SVP and CEAP as complementary instruments.
Pathophysiology (P)
The pathophysiology domain (P) is a composite of the anatomic (A), hemodynamic (H), and etiologic (E) subdomains. Involved anatomic segments in the abdomen and pelvis are designated by anatomic abbreviations that include laterality (Table 4).
Table 4.
Anatomy
| Abbreviation | Expansions |
|---|---|
| IVC | Inferior vena cava |
| LRV | Left renal vein |
| GV | Gonadal (testicular, ovarian) veins |
| LGV | Left gonadal vein |
| RGV | Right gonadal vein |
| BGV | Bilateral gonadal veins |
| CIV | Common iliac veins |
| LCIV | Left common iliac vein |
| RCIV | Right common iliac vein |
| BCIV | Bilateral common iliac veins |
| EIV | External iliac veins |
| LEIV | Left external iliac vein |
| REIV | Right external iliac vein |
| BEIV | Bilateral external iliac veins |
| IIV | Internal iliac veins |
| LIIV | Left internal iliac vein and tributaries |
| RIIV | Right internal iliac vein and tributaries |
| BIIV | Bilateral internal iliac veins and tributaries |
| PELV | Pelvic escape veins22 (“escape points”); inguinal, obturator, pudendal, and/or gluteal |
As in CEAP, the underlying hemodynamic (H) derangements—reflux (R), obstruction O), or both (R,O)—are designated by a subscript to the P category (Table 5). Obstruction, which may be thrombotic or nonthrombotic in origin, primarily involves the left renal, common iliac, and external iliac veins. Reflux occurs most commonly in the gonadal veins, internal iliac veins, and pelvic escape points with their associated pelvic origin extrapelvic veins. By convention, the hemodynamic subscript should immediately follow the designation of each involved anatomic segment. In contrast with the lower extremities, concurrent reflux and obstruction in a single pelvic venous segment is unusual but, if present, should be designated by both the R and O subscripts. Also, some congenital malformations, may not be associated with either reflux or obstruction, in which case the H subscript should be omitted.
Table 5.
Hemodynamics
| Obstruction (O) | Thrombotic or nonthrombotic (venous compression) venous obstruction |
| Reflux (R) | Thrombotic or nonthrombotic reflux |
The etiology (E) of pelvic venous pathology is defined as being thrombotic (T), nonthrombotic (NT), or congenital (C) (Table 6). Venous obstruction can arise from either a previous episode of deep venous thrombosis (thrombotic) or extrinsic compression by adjacent arterial structures or mass lesions (nonthrombotic). Thrombotic reflux can similarly develop after an episode of deep venous thrombosis, whereas nonthrombotic reflux is presumed to represent a degenerative process of the vein wall leading to venous dilation and valvular incompetence. Congenital etiologies include vascular malformations, either venous or mixed. The designated etiology (E) should be denoted by a subscript to the P category, immediately after the designation of the involved anatomic segments and the hemodynamic derangements.
Table 6.
Etiology (E)
| Thrombotic (T) | Venous reflux or obstruction arising from a previous episode of DVT |
| Nonthrombotic (NT) | Reflux arising from a degenerative process of the vein wall or proximal obstruction; Obstruction arising from extrinsic compression |
| Congenital (C) | Congenital venous or mixed vascular malformations |
DVT, Deep vein thrombosis.
Using the SVP Classification
For the purposes of documenting reproducibility of the instrument and for recording data in clinical studies, all five domains and subdomains of SVP—S, V, A, H, and E—should be documented independently. However, such a system is overly complicated for routine clinical use and communication. For such purposes, the A, H, and E subdomains are collapsed into a single anatomic-pathophysiological domain P. By convention, this single term should include the anatomic segment(s) involved, the underlying hemodynamics, and the etiology in this order. That is, notation for the P domain should be Panatomic segment, hemodynamics, etiology. If multiple anatomic segments are involved, each venous segment after the P should be specified in this fashion, separating the full anatomic-pathophysiologic description of each segment with a semicolon. In such cases, the anatomic segments and associated pathology should be listed beginning at the inferior vena cava and proceeding caudally. For example, nonthrombotic obstruction of the left common iliac vein associated with internal iliac reflux should be designated as PLCIV,O,NT; LIIV,R,NT. The historic syndromes of the abdomen and pelvis would be now be designated as follows in the SVP classification,
Pelvic congestion syndrome with chronic pelvic pain due to bilateral ovarian reflux: S2V2PBGV,R,NT
Nutcracker syndrome with flank pain and hematuria: S1V1PLRV,O,NT
May-Thurner syndrome with left lower extremity edema: S0V0PLCIV,O,NT; Left C3sEseAdPo(CIV)
Clinical examples of the SVP classification are shown in Figures 2 to 9. The use of a scoring sheet as shown in Table 7 may aid in early application of the instrument. Smart phone applications to assist in classification are available on the AVLS website (https://myavls.org/svp).
Figure 2.
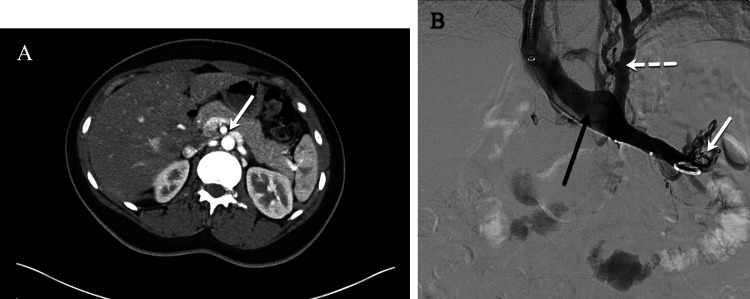
Left renal vein compression associated with symptoms of left flank pain and hematuria. (A) Computed tomography (CT) demonstrates compression of the left renal vein (white arrow) over the abdominal aorta. (B) Venography demonstrates contrast attenuation over the abdominal aorta (black arrow), renal hilar varices (white arrow), and ascending collaterals (dashed white arrow) consistent with renal vein compression. The Symptoms-Varices-Pathophysiology (SVP) classification is S1V1PLRV,O,NT.
Figure 3.
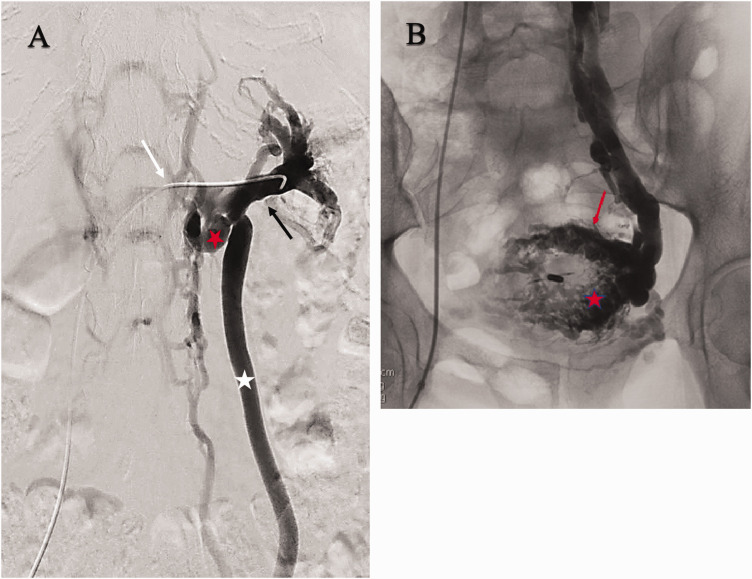
Chronic pelvic pain due to compression of the left renal vein with secondary reflux in the left ovarian vein. (A) Selective renal venography demonstrates compressive obstruction (white arrow) of the central left renal vein (black arrow) associated with renal hilar varices. The left renal vein is drained through the renal-azygous trunk (red star) and a refluxing left ovarian vein (white star). (B) Selective left ovarian venography demonstrates associated pelvic varices, myometrial veins (red star) and small arcuate veins (red arrow). The Symptoms-Varices-Pathophysiology (SVP) classification is S2V1,2PLRV,O,NT; LGV,R,NT.
Figure 4.
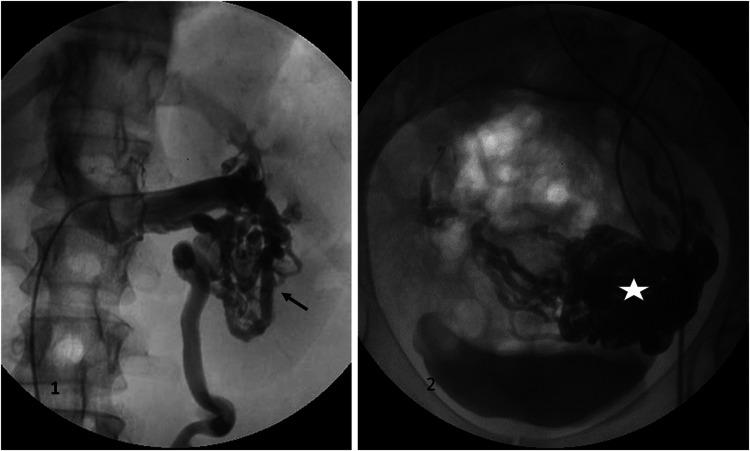
Left flank pain associated with chronic microscopic hematuria and pelvic pain. Selective renal venography (1) demonstrates a left inferior pole renal venous malformation (black arrow) drained by a left ovarian vein with no visible connection to the renal vein. Pelvic venography (2) shows associated pelvic varicosities (white star). The Symptoms-Varices-Pathophysiology (SVP) classification is S1,2V1,2PLRV,C; LGV,R,NT.
Figure 5.
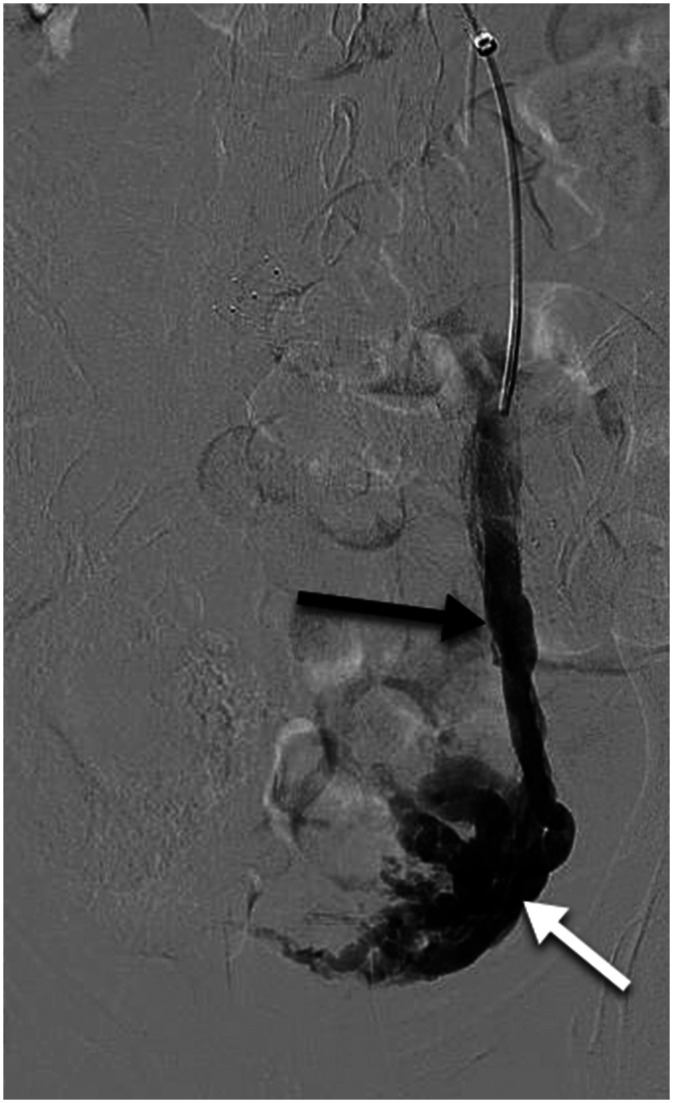
Chronic pelvic pain due to bilateral primary ovarian vein reflux. A dilated, refluxing left ovarian vein (black arrow) is associated with multiple pelvic varicosities (white arrow). Right ovarian vein reflux is also present, but not demonstrated in this image. No obstruction of the left renal or common iliac veins or internal iliac reflux is present by ultrasound examination. The Symptoms-Varices-Pathophysiology (SVP) classification is S2V2PBGV,R,NT.
Figure 6.
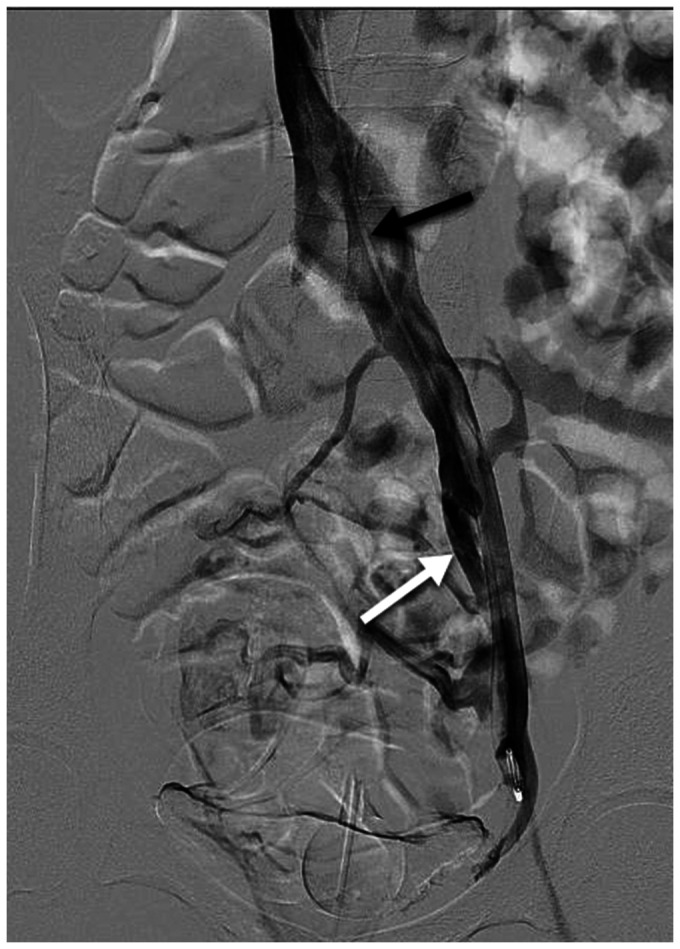
Chronic pelvic pain due to left common iliac compression. The patient has no lower extremity symptoms. Transabdominal ultrasound examination (not shown) demonstrates >50% compression of the left common iliac vein, retrograde flow in the left internal iliac vein, and periuterine varices. Intravascular ultrasound (IVUS) (not shown) demonstrates 70% cross-sectional area reduction of the left common iliac vein at the crossing of the right common iliac artery. Antegrade venography demonstrates flattening of the left common iliac vein with contrast attenuation at the arterial crossing (black arrow) and left internal iliac reflux (white arrow). Associated pelvic varices are better seen on delayed imaging (not shown). The Symptoms-Varices-Pathophysiology (SVP) classification is S2V2PLCIV,O,NT; LIIV,R,NT.
Figure 7.
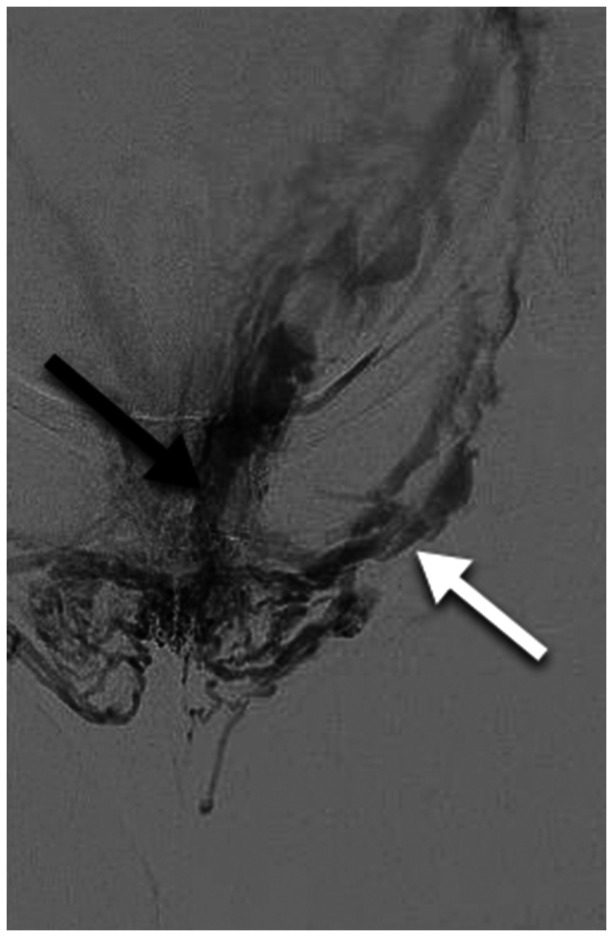
Symptomatic vulvar varicosities with associated pelvic pain due to bilateral ovarian and internal iliac venous reflux. There are no associated lower extremity varices. Transabdominal ultrasound (not shown) shows periuterine varices with bilateral ovarian and internal iliac reflux and no evidence of left renal or common iliac venous obstruction. Balloon occlusion venography performed from a left internal iliac injection demonstrating vulvar varicosities associated with the internal (black arrow) and external (white arrow) pudendal veins. Similar reflux through the pudendal veins is present on the right. Ovarian and right internal iliac vein injections not shown. The Symptoms-Varices-Pathophysiology (SVP) classification is S2,3aV2,3aPBGV,R,NT; BIIV,R,NT; BPELV,R,NT.
Figure 8.
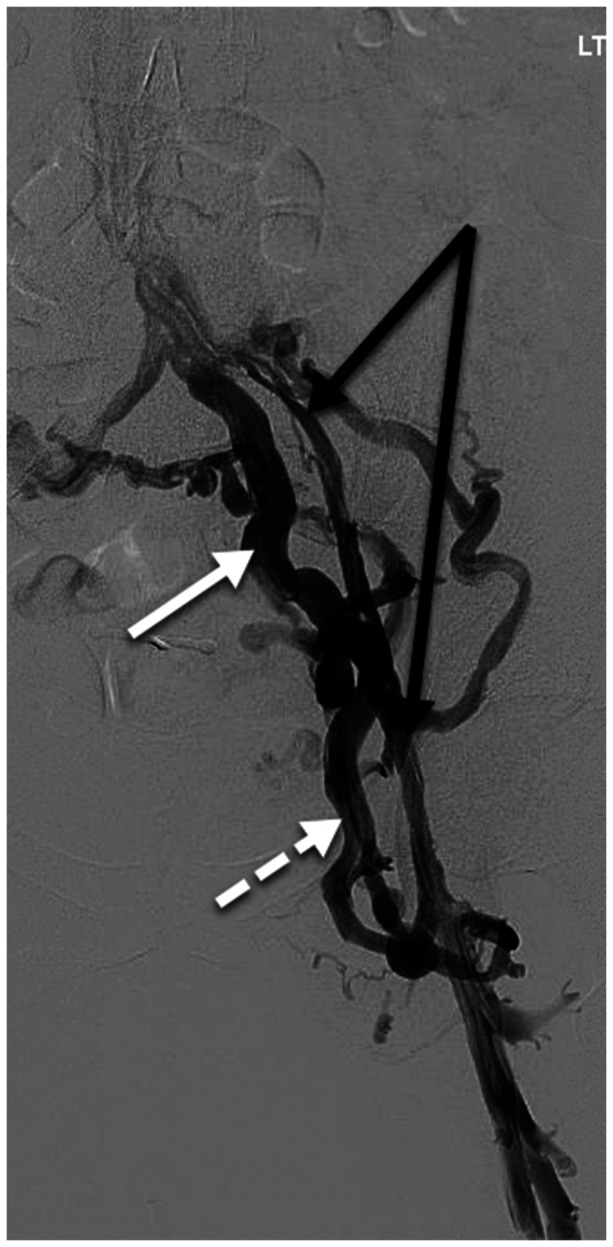
Post-thrombotic venous claudication and left lower extremity swelling without visible lower extremity varices. Ultrasound (not shown) demonstrates post-thrombotic reflux with partial obstruction in the left common femoral, femoral, and popliteal veins, and no superficial venous reflux. The Figure shows post-thrombotic changes in the left common and external iliac veins (black arrows) with large obturator collaterals (dashed white arrow) draining into the left internal iliac vein (solid white arrow). Collateral veins with antegrade flow bypassing an obstruction are not considered varices by the Symptoms-Varices-Pathophysiology (SVP) instrument. Because the presentation involves lower extremity symptoms and signs, the SVP classification should be used in conjunction with the CEAP classification. The SVP classification is S3cV0PLCIV,O,T; LEIV,O,T; Left C3sEsiAdP(o)CIV, EIV; (r,o)CFV,FV,POPV
Figure 9.
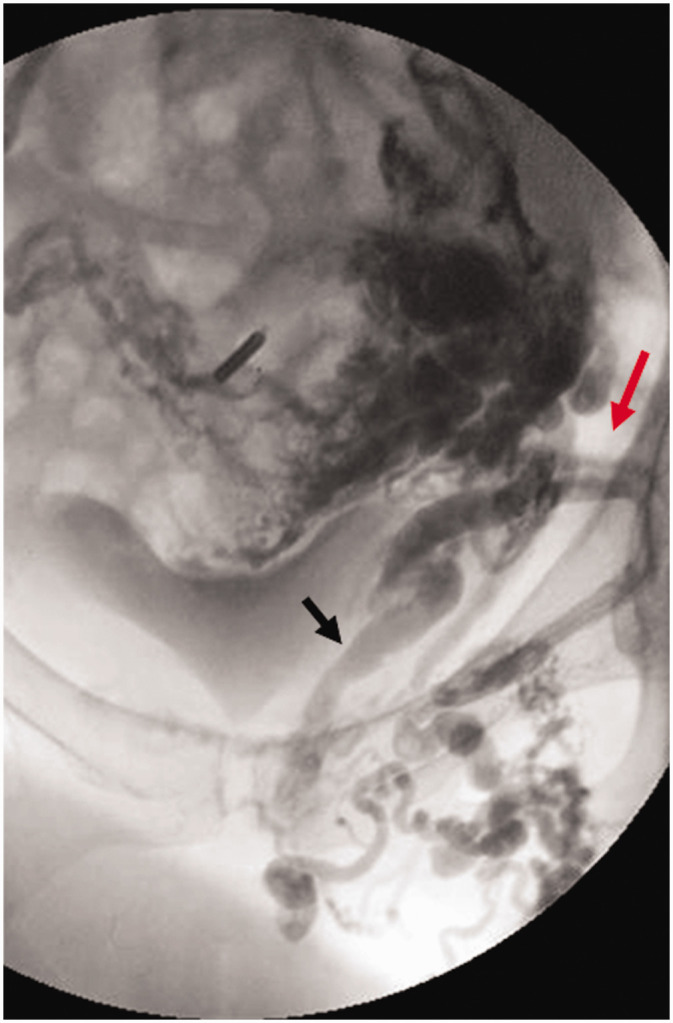
Locally painful, recurrent, left medial thigh varicosities in 56-year-old G3P3 female 21 years after great saphenous stripping. She has no pelvic symptoms. Ultrasound examination (not shown) demonstrates reflux in the bilateral ovarian and left internal iliac veins associated with pelvic varices communicating with the extrapelvic varices over the left medial thigh. No right internal iliac or superficial or deep lower extremity reflux is seen on ultrasound. Venography demonstrates pelvic origin varices over the medial thigh communicating with pudendal (black arrow) and inguinal (red arrow) tributaries of the left internal iliac vein. The Symptoms-Varices-Pathophysiology (SVP) classification is S3bV2,3bPBGV,R,NT; LIIV,R,NT; LPELV,R,NT ; Left C2s,rEpAs,dP(r) IIV,Pelvic,NSV.
Table 7.
Symptoms-Varices-Pathophysiology (SVP) classification scoring sheet
| Symptoms (S) | Varices (V) | Anatomy/pathophysiology (P) | |||||
|---|---|---|---|---|---|---|---|
| A | H | E | |||||
| No pelvic symptoms | 0 | No pelvic varices | 0 | IVC | O | T | |
| Renal | 1 | Renal | 1 | NT | |||
| Pelvic | 2 | Pelvic | 2 | C | |||
| Extrapelvic | 3 | Extrapelvic | 3 | L | RV | O | T |
| Genital | 3a | Genital | 3a | NT | |||
| Leg symptoms | 3b | Leg varices | 3b | C | |||
| Venous claudication | 3c | R | GV | O R | T | ||
| L | NT | ||||||
| B | C | ||||||
| R | CIV | O R | T | ||||
| L | NT | ||||||
| B | C | ||||||
| R | IIV | O R | T | ||||
| L | NT | ||||||
| B | C | ||||||
| R | EIV | O R | T | ||||
| L | NT | ||||||
| B | C | ||||||
| R | PELV | O R | T | ||||
| L | NT | ||||||
| B | C | ||||||
| S | V | Psegment1,H,E;segment 2,H,E | |||||
A, Anatomic; H, hemodynamic; C, congenital; CIV, common iliac veins; E, etiologic; EIV, external iliac veins; GV, gonadal (testicular, ovarian) veins; IIV, internal iliac veins; IVC, inferior vena cava; L, left; NT, nonthrombotic; O, obstruction; PELV, pelvic escape veins22 (“escape points”); inguinal, obturator, pudendal, and/or gluteal; R, reflux; RV, renal vein; S, symptoms; T, thrombotic; V, varices.
All components of the instrument, that is S, V, and PA,H,E are to be used in designating a patient’s final SVP classification. This presumes imaging (abdominal/transperineal ultrasound, transvaginal ultrasonography, cross-sectional imaging, venography/IVUS, laparoscopy) has been done as part of the classification, recognizing that some components of the classification may change as the evaluation progresses from noninvasive to more definitive imaging such as venography. It is acceptable to use an interim designation (x) as a subscript for those domains where evaluation is not yet complete (eg, S0-3VxPx).
Discussion
Despite technical advances, progress in the diagnosis and management of PeVDs has been hampered by the use of historic nomenclature—the May-Thurner, pelvic congestion, and nutcracker syndromes—to describe underlying anatomic lesions that often have variable clinical presentations. The use of these terms ignores the complex and interrelated abdominal and pelvic venous circulation, as well as the observation that similar clinical presentations may have different underlying pathophysiologies while identical pathology may have different clinical presentations. Inaccuracy in precisely characterizing a patient’s clinical presentation has often led to misdiagnosis and suboptimal treatment outcomes and has hindered progress in the field. The use of the historical syndromic terms should be abandoned in favor of a more precise characterization of the patient’s clinical presentation, including symptoms, signs (varices), and the underlying venous anatomy and pathophysiology.1 Although incomplete, our understanding has progressed to the point that a discriminative instrument is needed to characterize patients with PeVD.
Discriminative instruments characterize a patient’s clinical presentation at a particular point in time. From a pragmatic standpoint, such instruments place patients into categories with similar clinical features, natural histories, and responses to treatment. By virtue of their fundamental features (large between subject variability), these instruments are not designed to quantitatively measure either severity or change over time or in response to treatment, which is the role evaluative instruments. Although both types of instrument depend on a high ratio of signal to noise (low measurement error), for discriminative instruments the signal is differences between subjects, whereas for evaluative instruments it is longitudinal changes within subjects.7 Responsiveness to change is not a primary concern for discriminative instruments. This dichotomy is well-illustrated for lower extremity venous disorders. CEAP2-4 was designed as a purely discriminative instrument, whereas the Venous Clinical Severity Score55,56 is its evaluative complement. The development of disease-specific evaluative instruments for PeVD is in its infancy but depends on defining homogenous patient populations with instruments such as the SVP classification. For example, patient-reported outcomes for symptomatic left common iliac venous obstruction associated with lower extremity symptoms would be very different than if associated with chronic pelvic pain.
Because the pelvic venous system is in continuity with that of the lower extremities and can be the origin of lower extremity signs, compatibility with the CEAP classification was considered to be important. This factor was thoroughly considered by the panel, which ultimately concluded that, although the basic clinical, etiologic, anatomic, and pathophysiologic domains of CEAP are equally relevant to PeVD, many unique considerations prevent a precise alignment between discriminative instruments for PeVD and chronic lower extremity venous disease. Most importantly, whereas the CEAP clinical classification (C) focuses on the signs of venous disease, patient-important features of pelvic venous disease necessarily include both symptoms and signs (varices). Furthermore, although lower extremity varices largely develop in the distribution of the saphenous trunks and their tributaries, symptomatic varices in the abdomen and pelvis may occur in multiple beds or reservoirs, including the renal hilum, the pelvic venous plexus, the transition (escape) points between the pelvis and lower extremities, and the lower extremities.
The situation is further complicated by the observation that symptoms of pelvic reflux or obstruction may be related to the development of increased venous pressure in the immediately upstream (considering normally directed venous flow from peripheral to central) venous reservoir or, if decompressed from one reservoir to another via refluxing collaterals, to more caudal venous reservoirs. Although occurring between all variceal reservoirs,57 this phenomenon has been most thoroughly described for symptomatic compression of the left renal vein, which may be associated with either an elevated (noncompensated) or normal to borderline abnormal (compensated) transrenal pressure gradient in the presence of collaterals.30,31 Left renal vein obstruction may accordingly be associated with symptoms of flank pain and hematuria (noncompensated obstruction) or with chronic pelvic pain (compensated obstruction) if decompressed by left ovarian vein collaterals. In a similar fashion, increased venous pressure owing to reflux or obstruction in any of the three anatomic zones included in the SVP instrument, may be transmitted to a more caudal zone by collateral reflux flow (compensated reflux or obstruction).57 The clinical implication is that similar symptoms, such as venous origin chronic pelvic pain, may arise from diverse anatomic-pathophysiologic patterns, whereas, depending on the degree of collateralization, similar anatomic-pathophysiologic lesions may be associated with variable symptoms.
Despite these differences, the manifestations of pelvic and lower extremity venous disease are a continuum that frequently coexist and there is a clear need to use CEAP as a complement to any proposed pelvic venous classification. The SVP classification has the granularity needed to account for the complex and interrelated nature of pelvic symptoms and pathophysiology, whereas CEAP accurately characterizes the signs of lower extremity venous disease, even if the pathophysiologic derangements arise in the pelvis. Reasonable attempts have been made to make the instruments congruent by incorporating the anatomic and physiologic conventions that are familiar to users of CEAP. The overlap between the two instruments are (a) refluxing veins traversing the pelvic escape points and (b) the transmission of increased venous pressure from iliocaval venous obstruction to the lower extremities. These veins, as well as their pathophysiologic origins are precisely described in SVP (eg, V3bPPELV,R,NT) and more generally in the recent revision of CEAP (eg, P(r)Pelv).4 In contrast, CEAP more precisely defines the subsequent communications and clinical manifestations of these veins in the legs. The instruments are, therefore, to be used together in limbs with pelvic origin lower extremity symptoms (S3b and S3c) and signs (V3b).
The SVP instrument characterizes a patient’s presenting features in terms of signs, symptoms, and the underlying pathophysiology. However, there are some caveats to be considered in using the instrument. The instrument is a purely discriminative instrument and carries no implication of disease severity. As with CEAP, the responses within each domain are categorical variables that should be described by absolute numbers and percentages rather than by a mean score. Furthermore, the SVP presumes an underlying venous etiology to the patient’s clinical presentation and does not include similar clinical presentations that are nonvenous in origin. Finally, although interim designations are allowed, complete classification will usually only be possible once initial diagnostic studies are completed. Abbreviated forms of SVP were considered, similar to basic CEAP,3 but truncating the full anatomic-pathophysiologic description of a patient’s presentation resulted in potentially misleading overlaps in classification. For example, if the classification was abbreviated to SVPH, chronic pelvic pain due to either left renal vein or iliac vein compression would be identically classified as S2V2PR,O.
The SVP instrument attempts to comprehensively describe a patient’s clinical presentation. The inclusion of additional descriptive subdivisions beneath the elements of some domains was considered, but ultimately deferred due to concerns of making the instrument overly complicated and limiting initial adoption. Additional subdivisions that were considered included the following.
Subcategorization of S1 (venous origin renal symptoms) to include separate designations for flank pain and hematuria.
Subcategorization of S2 (chronic pelvic pain) to include sexual, menstrual, urinary, and defacatory symptoms.
Subcategorization of S3 to include hemorrhoids. Some investigators have reported a relationship between PeVDs and hemorrhoids. For example, hemorrhoids on transvaginal ultrasound have been noted in 36.3% of women presenting with pelvic origin lower extremity reflux.58 Although the internal rectal (hemorrhoidal) plexus drains primarily through the inferior mesenteric vein via the superior rectal vein, there is some contribution from the middle rectal tributary of the internal iliac vein. The external rectal plexus drains through the middle and inferior rectal tributaries of the internal iliac vein. However, there are communications between all three rectal veins, allowing drainage into both the portal and systemic circulation.22,59 There are also anecdotal reports of improvement in hemorrhoidal symptoms after pelvic venous embolization,60 although the effectiveness of phlebotonic agents, such as micronized purified flavonoid fraction, has been inconsistent.61,62 Despite these observations, the pathophysiology of hemorrhoids is more complex than simple venous dilation59,61,63 and their relationship to other PeVDs is not clear. Although at present there is insufficient evidence to support a strong relationship between hemorrhoids and PeVDs, this area warrants further investigation.
More precisely characterizing lower extremity venous symptoms and signs, beyond those of pelvic origin extrapelvic varices (S3b, V3b), by adding additional subdivisions of each. That is, more precisely defining signs and symptoms arising from each of the pelvic escape points.
The strengths of the SVP instrument include its collaborative multidisciplinary development, ensuring that the spectrum of clinical presentations encountered by multiple specialties is well-represented. In addition to accurately describing and classifying the spectrum of clinical presentations, the other goals of instrument development were to ensure that it included patient important domains and that it had high reproducibility. The instrument’s domains and responses are, therefore, precisely defined with minimal overlap between groups and have clinical relevance to the patient. Efforts were made to ensure the definitions were evidence based and as precise as possible, recognizing that there are deficiencies in the current literature. The underlying pathophysiology and involved anatomic segments are similarly precisely described.
The SVP instrument does have some limitations. Although members of the multidisciplinary panel were all experts in their respective fields, patient representatives were not included and may have identified other factors of importance to patients. Additionally, the knowledge base with respect to PeVD is rapidly advancing and it is fully recognized that future revisions with be required. For example, there are no consistent and widely accepted diagnostic criteria for most PeVD.46 Because many definitions are based on noninvasive imaging studies with variable diagnostic criteria, definitions were occasionally problematic and it is anticipated that these will be refined as the field advances. Although every effort was made to ensure that definitions were precise and that reproducibility was acceptable in simulated classification exercises, the instrument awaits clinical validation.
It is also anticipated that there will be resistance to abandoning the historic nomenclature for PeVD and that the SVP classification will be criticized as being overly complex for clinical use. Despite bringing much-needed clarity to lower extremity venous disorders, the CEAP classification has been similarly criticized. However, with increasing familiarity, CEAP has been successfully adopted by most clinicians and investigators and has become the international standard for the classification of lower extremity venous disorders. Despite efforts to make the classification of PeVDs as simple as possible, it must be appreciated that PeVD are quite complex with variable, but interrelated hemodynamic and clinical features that cannot be adequately described by the current nomenclature. As with CEAP, the nuances of the SVP classification cannot be appreciated from simply reading this manuscript. Comfort and familiarity with the classification, as well as identification of additional limitations, can only come with routine use. It is hopeful that use of Table 7, as well as an electronic version that is available through https://myavls.org/svp, will aid in initial adoption of the SVP classification.
The SVP instrument is a starting point in bringing greater scientific rigor to PeVDs. It is presumed that, much like lower extremity CEAP, the instrument will be carefully studied and any deficiencies addressed in future revisions. However, it is only through the precise definition of homogenous patient populations that clinical care can be optimized, appropriate outcome instruments developed, and rigorous clinical trials conducted.
Acknowledgment
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: N.K. receives consulting fees from Medtronic, Inc. N.L. receives consulting fees from Medtronic, Inc, Cook Medical, Phillips, Bard, and Tactile. K.G. receives consulting fees from Medtronic, Boston Scientific, Gore, Vesper Medical and Phillips, receives research support from Medtronic, Bayer, Bard, Vesper Medical, and the National Institutes of Health; and is a speaker for Medtronic, Bristol Meyers Squib, Jansen Pharmaceuticals, and Boston Scientific. W.P.B. receives royalties from Medtronic and NXT and nonfinancial research support from Guerbet.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by the American Vein and Lymphatic Society (AVLS) and the Peter Gloviczki Chair of Venous and Lymphatic Disorders.
References
- 1.Khilnani NM, Meissner MH, Learman LA, Gibson KD, Daniels JP, Winokur RS, et al. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol 2019; 30:781–9. [DOI] [PubMed] [Google Scholar]
- 2.Beebe HG, Bergan JJ, Bergqvist D, Eklof B, Eriksson I, Goldman MP, et al. Classification and grading of chronic venous disease in the lower limbs. A consensus statement. Eur J Vasc Endovasc Surg 1996; 12:487–91. [DOI] [PubMed] [Google Scholar]
- 3.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004; 40:1248–52. [DOI] [PubMed] [Google Scholar]
- 4.Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020; 8:342–52. [DOI] [PubMed] [Google Scholar]
- 5.Meissner MH, Gibson K.Clinical outcome after treatment of pelvic congestion syndrome: sense and nonsense. Phlebology 2015; 30:73–80. [DOI] [PubMed] [Google Scholar]
- 6.Greiner M, Dadon M, Lemasle P, Cluzel P.How does the patho-physiology influence the treatment of pelvic congestion syndrome and is the result long-lasting? Phlebology 2012; 27(Suppl 1):58–64. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Kirshner B, Jaeschke R.A methodologic framework for health status measures: clarity or oversimplification? J Clin Epidemiol 1992; 45:1353–5. [DOI] [PubMed] [Google Scholar]
- 8.Kirshner B, Guyat G.A methodological framework for assessing health indices. J Chron Dis 1985; 38:27–36. [DOI] [PubMed] [Google Scholar]
- 9.Kurklinsky AK, Rooke TW.Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010; 85:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists. ReVITALize. Gynecology data definitions (version 1.0) Washington, D.C.: American College of Obstetricians and Gynecologists; 2018. Available at: www.acog.org/-/media/project/acog/acogorg/files/pdfs/publications/revitalize-gyn.pdf. (Accessed March 10, 2021). [Google Scholar]
- 11.Williams RE, Hartmann KE, Steege JF.Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol 2004; 103:686–91. [DOI] [PubMed] [Google Scholar]
- 12.Phillips D, Deipolyi AR, Hesketh RL, Midia M, Oklu R.Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management. J Vasc Interv Radiol 2014; 25:725–33. [DOI] [PubMed] [Google Scholar]
- 13.Zondervan KT, Yudkin PL, Vessey MP, Jenkinson CP, Dawes MG, Barlow DH, et al. Chronic pelvic pain in the community–symptoms, investigations, and diagnoses. Am J Obstet Gynecol 2001; 184:1149–55. [DOI] [PubMed] [Google Scholar]
- 14.Beard RW, Highman JH, Pearce S, Reginald PW.Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet 1984; 2:946–9. [DOI] [PubMed] [Google Scholar]
- 15.Beard RW, Reginald PW, Wadsworth J.Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol 1988; 95:153–61. [DOI] [PubMed] [Google Scholar]
- 16.Scultetus AH, Villavicencio JL, Gillespie DL, Kao TC, Rich NM.The pelvic venous syndromes: analysis of our experience with 57 patients. J Vasc Surg 2002; 36:881–8. [DOI] [PubMed] [Google Scholar]
- 17.Santoshi RKN, Lakhanpal S, Satwah V, Lakhanpal G, Malone M, Pappas PJ.Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord 2018; 6:202–11. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty SF, Gillespie DL.Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord 2015; 3:283–9. [DOI] [PubMed] [Google Scholar]
- 19.Herrera-Betancourt AL, Villegas-Echeverri JD, Lopez-Jaramillo JD, Lopez-Isanoa JD, Estrada-Alvarez JM.Sensitivity and specificity of clinical findings for the diagnosis of pelvic congestion syndrome in women with chronic pelvic pain. Phlebology 2018; 33:303–8. [DOI] [PubMed] [Google Scholar]
- 20.Rana N, Drake MJ, Rinko R, Dawson M, Whitmore KE.The fundamentals of chronic pelvic pain assessment, based on international continence society recommendations. Neurourol Urodyn 2018; 37:S32–8. [DOI] [PubMed] [Google Scholar]
- 21.Potts JM.Chronic pelvic pain syndrome: a non-prostatocentric perspective. World J Urol 2003; 21:54–6. [DOI] [PubMed] [Google Scholar]
- 22.Kachlik D, Pechacek V, Musil V, Baca V.The venous system of the pelvis: new nomenclature. Phlebology 2010; 25:162–73. [DOI] [PubMed] [Google Scholar]
- 23.Labropoulos N, Tassiopoulos AK, Gasparis AP, Phillips B, Pappas PJ.Veins along the course of the sciatic nerve. J Vasc Surg 2009; 49:690–6. [DOI] [PubMed] [Google Scholar]
- 24.Ricci S, Georgiev M, Jawien A, Zamboni P.Sciatic nerve varices. Eur J Vasc Endovasc Surg 2005; 29:83–7. [DOI] [PubMed] [Google Scholar]
- 25.Meissner MH, Eklof B, Smith PC, Dalsing MC, DePalma RG, Gloviczki P, et al. Secondary chronic venous disorders. J Vasc Surg 2007; 46Suppl S:68S–83S. [DOI] [PubMed] [Google Scholar]
- 26.Delis KT, Bountouroglou D, Mansfield AO.Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg 2004; 239:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrin M, Eklof B, van Rij A, Labropoulos N, Vasquez M, Nicolaides A, et al. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. Int Angiol 2016; 35:374–98. [PubMed] [Google Scholar]
- 28.Gloviczki P, Cho J-S.Surgical treatment of chronic occlusions of the iliac veins and inferior vena cava. In: Rutherford RB, editor. Vascular Surgery. 6th ed. Philadelphia (PA): Elsevier, Inc; 2005, p. 2303–2320. [Google Scholar]
- 29.Paty J, Elash CA, Turner-Bowker DM.Content validity for the VVSymQ(®) Instrument: a new patient-reported outcome measure for the assessment of varicose veins symptoms. Patient 2017; 10:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KW, Cho JY, Kim SH, Yoon JH, Kim DS, Chung JW, et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol 2011; 80:648–54. [DOI] [PubMed] [Google Scholar]
- 31.Takebayashi S, Ueki T, Ikeda N, Fujikawa A.Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol 1999; 172:39–43. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH.Doppler US and CT diagnosis of Nutcracker Syndrome. Korean J Radiol 2019; 20:1627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beinart C, Sniderman KW, Tamura S, Vaughan ED, Sos TA.Left renal vein to inferior vena cava pressure relationship in humans. J Urol 1982; 127:1070–1. [DOI] [PubMed] [Google Scholar]
- 34.Beckmann CF, Abrams HL.Idiopathic renal vein varices: incidence and significance. Radiology 1982; 143:649–52. [DOI] [PubMed] [Google Scholar]
- 35.Hangge PT, Gupta N, Khurana A, Quencer KB, Albadawi H, Alzubaidi SJ, et al. Degree of left renal vein compression [predicts Nutcracker Syndrome. J Clin Med. 2018; 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SJ, Lim JW, Ko YT, Lee DH, Yoon Y, Oh JH, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol 2004; 182:683–8. [DOI] [PubMed] [Google Scholar]
- 37.Steenbeek MP, van der Vleuten CJM, Schultze Kool LJ, Nieboer TE.Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand 2018; 97:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray HR.Gray’s Anatomy. 15th ed. Pick TP, Howden R, editors. New York (NY): Barnes & Noble; 2010. [Google Scholar]
- 39.Kauppila A.Uterine phlebography with venous compression. A clinical and roentgenological study. Acta Obstet Gynecol Scand 1970; 3(Suppl 3):1–66. [DOI] [PubMed] [Google Scholar]
- 40.Arnoldussen CW, de Wolf MA, Wittens CH.Diagnostic imaging of pelvic congestive syndrome. Phlebology 2015; 30:67–72. [DOI] [PubMed] [Google Scholar]
- 41.Malgor RD, Adrahtas D, Spentzouris G, Gasparis AP, Tassiopoulos AK, Labropoulos N.The role of duplex ultrasound in the workup of pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord 2014; 2:34–8. [DOI] [PubMed] [Google Scholar]
- 42.Whiteley MS, Dos Santos SJ, Harrison CC, Holdstock JM, Lopez AJ.Transvaginal duplex ultrasonography appears to be the gold standard investigation for the haemodynamic evaluation of pelvic venous reflux in the ovarian and internal iliac veins in women. Phlebology 2015; 30:706–13. [DOI] [PubMed] [Google Scholar]
- 43.Labropoulos N, Jasinski PT, Adrahtas D, Gasparis AP, Meissner MH.A standardized ultrasound approach to pelvic congestion syndrome. Phlebology 2017; 32:608–19. [DOI] [PubMed] [Google Scholar]
- 44.Hansrani V, Dhorat Z, McCollum CN.Diagnosing of pelvic vein incompetence using minimally invasive ultrasound techniques. Vascular 2017; 25:253–9. [DOI] [PubMed] [Google Scholar]
- 45.Dos Santos SJ, Holdstock JM, Harrison CC, Lopez AJ, Whiteley MS.Ovarian vein diameter cannot be used as an indicator of ovarian venous reflux. Eur J Vasc Endovasc Surg 2015; 49:90–4. [DOI] [PubMed] [Google Scholar]
- 46.Champaneria R, Shah L, Moss J, Gupta JK, Birch J, Middleton LJ, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess 2016; 20:1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemasle P, Greiner M.Duplex ultrasound investigation in pelvic congestion syndrome: technique and results. Phlebolymphology 2017; 24:79–87. [Google Scholar]
- 48.Black CM, Thorpe K, Venrbux A, Kim HS, Millward SF, Clark TW, et al. Research reporting standards for endovascular treatment of pelvic venous insufficiency. J Vasc Interv Radiol 2010; 21:796–803. [DOI] [PubMed] [Google Scholar]
- 49.Gagne PJ, Gasparis A, Black S, Thorpe P, Passman M, Vedantham S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018; 6:48–56e41. [DOI] [PubMed] [Google Scholar]
- 50.Neglen P, Raju S.Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002; 35:694–700. [DOI] [PubMed] [Google Scholar]
- 51.Labropoulos N, Borge M, Pierce K, Pappas PJ.Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg 2007; 46:101–7. [DOI] [PubMed] [Google Scholar]
- 52.Raju S, Kirk O, Davis M, Olivier J.Hemodynamics of "critical" venous stenosis. J Vasc Surg Venous Lymphat Disord 2014; 2:52–9. [DOI] [PubMed] [Google Scholar]
- 53.Metzger PB, Rossi FH, Kambara AM, Izukawa NM, Saleh MH, Pinto IM, et al. Criteria for detecting significant chronic iliac venous obstructions with duplex ultrasound. J Vasc Surg Venous Lymphat Disord 2016; 4:18–27. [DOI] [PubMed] [Google Scholar]
- 54.Choudur HN, Joshi R, Munk PL.Inferior gluteal vein varicosities: a rare cause of sciatica. J Clin Rheumatol 2009; 15:387–8. [DOI] [PubMed] [Google Scholar]
- 55.Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL.Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg 2000; 31:1307–12. [DOI] [PubMed] [Google Scholar]
- 56.Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg 2010; 52:1387–96. [DOI] [PubMed] [Google Scholar]
- 57.Meissner MH, Gloviczki P.Pelvic venous disorders. In: Almeida JI, ed. Atlas of endovascular venous surgery. 2nd ed. Philadelphi (PA): Elsevier; 2019. p. 567–99. [Google Scholar]
- 58.Holdstock JM, Dos Santos SJ, Harrison CC, Price BA, Whiteley MS.Haemorrhoids are associated with internal iliac vein reflux in up to one-third of women presenting with varicose veins associated with pelvic vein reflux. Phlebology 2015; 30:133–9. [DOI] [PubMed] [Google Scholar]
- 59.Margetis N.Pathophysiology of internal hemorrhoids. Ann Gastroenterol 2019; 32:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Vleuten CJ, van Kempen JA, Schultze-Kool LJ.Embolization to treat pelvic congestion syndrome and vulval varicose veins. Int J Gynaecol Obstet 2012; 118:227–30. [DOI] [PubMed] [Google Scholar]
- 61.Zagriadskii EA, Bogomazov AM, Golovko EB.Conservative treatment of hemorrhoids: results of an observational multicenter study. Adv Ther 2018; 35:1979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aziz Z, Huin WK, Badrul Hisham MD, Tang WL, Yaacob S.Efficacy and tolerability of micronized purified flavonoid fractions (MPFF) for haemorrhoids: a systematic review and meta-analysis. Complement Ther Med 2018; 39:49–55. [DOI] [PubMed] [Google Scholar]
- 63.Pata F, Sgro A, Ferrara F, Vigorita V, Gallo G, Pellino G. Anatomy, physiology and pathophysiology of haemorrhoids. Rev Recent Clin Trials 2020. April 6 [Epub ahead of print]. [DOI] [PubMed]