Abstract
Objective
Senile patients often experience neurocognitive disturbance after non-cardiac surgery. Several clinical trials have investigated if the perioperative intravenous use of dexmedetomidine has a positive effect on the prevention of neurocognitive dysfunction, but the results have been inconsistent. We performed a meta-analysis to investigate the effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients.
Methods
The PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure databases were comprehensively searched for all randomized controlled trials published before 1 February 2020 that investigated the efficacy of dexmedetomidine in the prevention of postoperative delirium (POD) or postoperative cognitive dysfunction (POCD).
Results
Sixteen studies involving 4376 patients were included in this meta-analysis. Compared with the control (i.e., saline), the perioperative intravenous use of dexmedetomidine significantly reduced the incidence of POD and POCD. However, patients in the dexmedetomidine group were more likely to develop bradycardia and hypotension during the administration of dexmedetomidine than patients in the control group. There were no differences between the two groups in the incidence of nausea and vomiting or mortality rate.
Conclusion
Dexmedetomidine has a positive effect on the prevention of neurocognitive disturbance in senile patients after elective non-cardiac surgery.
Keywords: Anesthesiology, postoperative cognitive disturbance, non-cardiac surgery, dexmedetomidine, postoperative delirium, senile
Introduction
Postoperative neurocognitive disturbance includes postoperative delirium (POD) and postoperative cognitive dysfunction (POCD). In general, POD occurs within 3 days after surgery,1 whereas POCD is characterized by long-term cognitive impairment, such as the loss of memory and impaired comprehension.2–5 It was reported that 13% to 50% of senile patients develop neurocognitive disturbance after surgery,6–8 which is an important risk factor for many undesirable outcomes, such as a longer length of hospital stay, disability, even higher postoperative mortality rates and others.9–12 Therefore, many clinicians have attempted to identify better approaches to prevent POD/POCD in senile patients after surgery.6,13
Dexmedetomidine, a selective α2-adrenaline receptor agonist, has a dose-dependent sedative effect on respiration with minimal depressive effects. It also shows a modest analgesic effect through the inhibition of pain signals.14–16 The efficacy of the perioperative use of dexmedetomidine in the prevention of POD/POCD after cardiac surgery has been clearly demonstrated.17,18
Scientists have found that dexmedetomidine improves the cognitive function of older rats after splenectomy.19 Several clinical trials have investigated the efficacy and safety of dexmedetomidine in the prevention of POD and POCD; however, there are inconsistencies among the results of these studies. For instance, both Deiner et al20 and Ma et al21 concluded that the perioperative intravenous use of dexmedetomidine did not significantly reduce POD among elderly patients after non-cardiac surgery. In contrast, other clinicians have reported opposite findings.22,23 Furthermore, two meta-analyses showed the efficacy of the perioperative intravenous use of dexmedetomidine in the prevention of POD in elderly patients after non-cardiac surgery.24,25 However, they both included a relatively small number of samples, and neither mentioned POCD. Therefore, we performed a meta-analysis with a larger sample size to investigate the effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients and draw a more convincing conclusion, which may provide guidelines for future clinical work.
Methods and materials
Study design
We conducted this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.26 PRISMA is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses that can be used as a basis for reporting systematic reviews of different types of research. Because our study did not involve any interventions in patients or animals, ethical approval was not applicable.
Search strategies
The PubMed, Cochrane Library, EMBASE and China National Knowledge Infrastructure databases were comprehensively searched for randomized controlled clinical trials (RCTs) published before February 2020 that investigated the effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients. In addition, the reference lists of all included studies were checked for any potential additional publications. We used the keywords of dexmedetomidine, postoperative delirium, postoperative cognitive dysfunction, POD and POCD. The detailed search strategies for each database were presented in the Supplemental materials (Supplementary Table 1).
Inclusion and exclusion criteria
For a published article to be included in our study, it had to meet the following criteria: (1) RCT design, (2) investigated the effects of dexmedetomidine on POD/POCD after elective non-cardiac surgery in senile patients, (3) compared dexmedetomidine with normal saline and (4) full text available.
Studies were excluded if they were duplicate publications, reviews, editorials, abstracts, commentaries, case reports, meetings, involved animals or included patients younger than 60 years old.
Data extraction
Two reviewers (Bi and Wei) independently screened the titles and abstracts of papers and selected the relevant studies. The same two reviewers (Bi and Wei) independently extracted the data from the studies according to a prespecified protocol with any disagreement settled by a third reviewer (Zhang). If data were missing, we e-mailed the corresponding author of the original article and asked if they could provide the required information.
The following items were extracted: name of the first author; publication year; type of surgery; sample size (classified by the participants’ sex); participants’ age; anesthesia technique; measurement method for POD/POCD; method of dexmedetomidine administration; and incidence of POD/POCD, bradycardia and hypotension during the intervention, postoperative nausea and/or vomiting (PONV) and postoperative mortality.
The primary outcome of the meta-analysis was the incidence of POD/POCD. The descriptions of instruments used in the enrolled trials were summarized in Supplementary Table 3. The secondary outcome was the incidence of bradycardia and hypotension during the intervention, PONV and postoperative mortality. Postoperative mortality was defined as all-cause mortality within 30 days after surgery. The raw data were presented in the supplementary materials.
Data synthesis and statistical analysis
This meta-analysis was performed using Review Manager (RevMan) Version 5.3 (Copenhagen, The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). Cochran’s Q test and the I2 statistical test were used to assess the statistical heterogeneity of the pooled results. If 0% ≤ I2 < 25%, the results showed no heterogeneity; if 25% ≤ I2 < 50%, the results showed a low level of heterogeneity; if 50% ≤ I2 < 75%, the results showed a medium level of heterogeneity; and if 75% ≤ I2 ≤ 100%, the results showed a high level of heterogeneity. Data from all eligible RCTs were pooled, and the Mantel–Haenszel method was used to calculate the risk ratio (RR) with 95% confidence intervals (CI) for these dichotomous outcomes. A pooled estimate of RR was computed using the DerSimonian and Laird random-effects model. This model provides an appropriate estimate of the average treatment effect when studies are statistically heterogeneous, and it typically yields relatively wide CIs resulting in a more conservative statistical claim.
The risk of bias assessment was performed using the Cochrane Collaboration tool (Cochrane, London, UK). Subgroup analyses were conducted by classifying these included studies according to their medication timing and dose and their primary outcome (POD or POCD). In addition, a sensitivity analysis was used to assess the robustness of the results by excluding specific studies, and a funnel plot was used to assess potential publication bias. Two reviewers (Bi and Wei) independently synthesized the data with any disagreement settled by a third reviewer (Zhang). A p value of <0.05 was considered statistically significant.
Results
Literature search
The literature search identified 1144 articles, of which 16 articles20–23,27–38 met the inclusion criteria (Supplementary Figure 1). The characteristics of the 16 studies involving 4376 participants were summarized in Supplementary Table 2. All raw data extracted from the original articles were available online.
Primary outcome
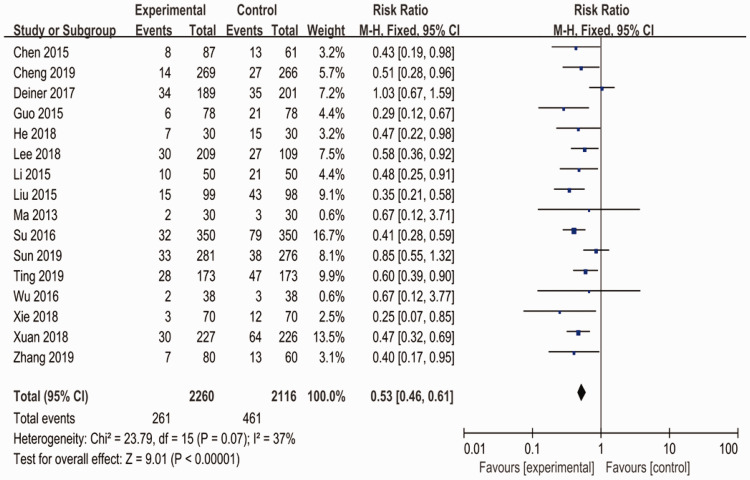
After synthesizing the data, the results showed that the perioperative intravenous use of dexmedetomidine significantly reduced the incidence of POD and POCD in senile patients after non-cardiac surgery compared with the control group (RR: 0.53; 95% CI: 0.46–0.61; p < 0.001; I2 = 37%) (Figure 1).
Figure 1.
Meta-analysis of the perioperative intravenous use of dexmedetomidine in the prevention of POD and POCD in senile patients after non-cardiac surgery. Each diamond represents the summary of all presented data.
POD, postoperative delirium; POCD, postoperative cognitive dysfunction; CI, confidence interval.
Subgroup analyses
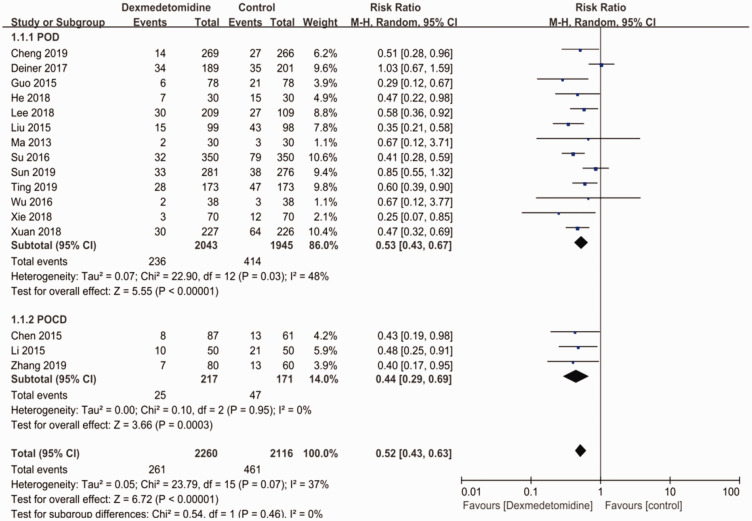
Of the 16 studies, 3 studies28,32,33 investigated the relationship between the perioperative use of dexmedetomidine and POCD, and the other 13 studies20–23,27–31,34–38 assessed whether the perioperative use of dexmedetomidine could prevent POD. As shown in Figure 2, the perioperative intravenous use of dexmedetomidine significantly reduced the incidence of POD (RR: 0.53; 95% CI: 0.43–0.67; p < 0.001; I2 = 48%) and POCD (RR: 0.44; 95% CI: 0.29–0.69; p < 0.001; I2 = 0%).
Figure 2.
Subgroup analysis based on the type of postoperative neurocognitive disturbance.
CI, confidence interval.
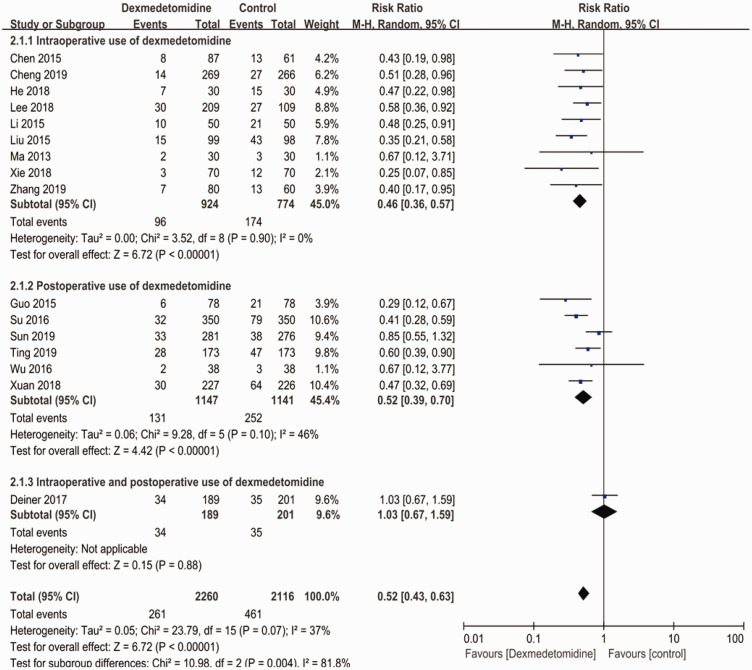
Regarding the timing of dexmedetomidine use, 9 studies21,23,28,29,31–34,37 reported the intraoperative use of dexmedetomidine, the patients in 6 studies22,27,30,35,36,38 were given dexmedetomidine postoperatively, and Deiner et al21 administered a continuous infusion of dexmedetomidine from the start of surgery to 24 hours after surgery. As shown in Figure 3, both intraoperative (RR: 0.46; 95% CI: 0.36–0.57; p < 0.001; I2 = 0%) and postoperative (RR: 0.52; 95% CI: 0.39–0.70; p < 0.001; I2 = 46%) use of dexmedetomidine significantly reduced the incidence of postoperative neurocognitive cognitive disturbance compared with the control group. However, the only study that investigated the continuous use of dexmedetomidine showed that there was no significant difference between the experimental group and the control group (RR: 1.03; 95% CI: 0.67–1.59; I2 = not applicable).
Figure 3.
Subgroup analysis based on the timing of dexmedetomidine use.
CI, confidence interval.
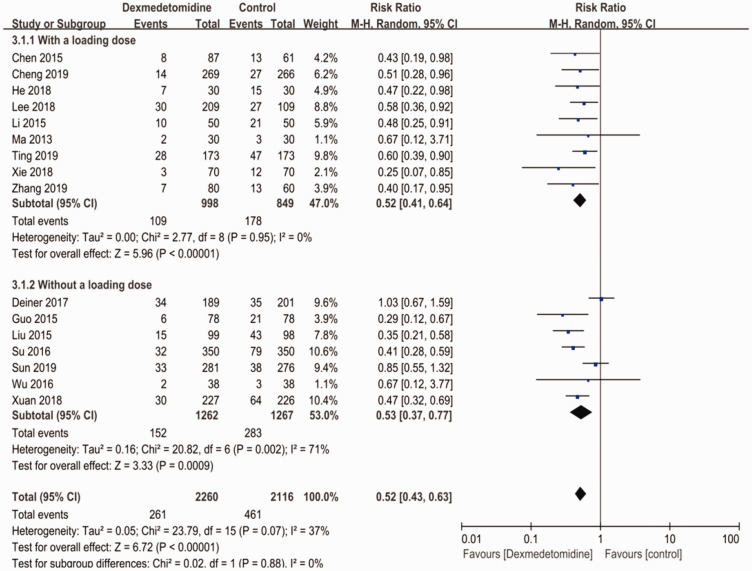
After classifying all studies by whether there was a loading dose or not, we found that patients in the dexmedetomidine group experienced a lower rate of postoperative neurocognitive cognitive disturbance (RR: 0.52; 95% CI: 0.43–0.63; p < 0.001; I2=37%) regardless of whether a loading dose was applied (Figure 4).
Figure 4.
Subgroup analysis based on the use of a loading dose.
CI, confidence interval.
Secondary outcomes
Adverse events related to the use of dexmedetomidine were analyzed as our secondary outcomes. Of note, we e-mailed the corresponding author of Ting et al30 to ask for the data regarding adverse events because they only summarized the adverse events and did not present them separately.
Patients in the dexmedetomidine group were more likely to experience hypotension during dexmedetomidine use (RR: 1.29; 95% CI: 1.12–1.49; p=0.0006; I2=3%) (Supplementary Figure 2). As shown in Supplementary Figure 3, patients in the dexmedetomidine group had a higher incidence of bradycardia than those in the control group (RR: 1.39; 95% CI: 1.15–1.67; p=0.0008; I2=0%). The synthesized data showed that there was no significant difference between the dexmedetomidine group and the control group in the incidence of PONV (RR: 0.83; 95% CI: 0.58–1.17; I2=11%) (Supplementary Figure 4). Last, patients in both groups exhibited the same mortality rate after surgery (RR: 0.72; 95% CI: 0.28–1.84; I2=0%) (Supplementary Figure 5).
Sensitivity analysis
Three studies20,23,32 included patients with preoperative mild cognitive impairment, which we thought might be the source of the heterogeneity. Therefore, we performed a sensitivity analysis by excluding these 3 studies, and the heterogeneity decreased to zero (RR: 0.50; 95% CI: 0.42–0.59; I2=0%) (Supplementary Figure 6).
Bias assessment
As shown in the risk of bias summary (Supplementary Figure 7) and risk of bias graph (Supplementary Figure 8), two studies31,33 had a high risk for performance bias, one study38 had a high risk for reporting bias, and one study28 was rated as high risk for selection bias. Regarding publication bias, there was no significant asymmetry in the funnel plot (Supplementary Figure 9), indicating that no significant publication bias existed.
Discussion
We concluded that the perioperative intravenous use of dexmedetomidine has a positive effect on the prevention of postoperative cognitive disturbance based on the results of our meta-analysis. Only one study20 reported both the intraoperative and postoperative use of dexmedetomidine (i.e., continuous infusion of dexmedetomidine from the start of surgery to 24 hours after surgery) and showed that there was no significant difference between the experimental group and the control group.
Postoperative neurocognitive disturbance is closely related to the age of patients.6,35 Additionally, studies have shown that postoperative cognitive disturbance has a certain relationship with anesthesia, especially the use of general anesthetic drugs.39
Dexmedetomidine is a derivative of medetomidine, which inhibits the sympathetic nervous system and reduces the release of norepinephrine.40 Promisingly, its brain-protective effect in senile patients is currently being widely investigated by clinicians.41
Nine studies reported the use of a loading dose. The most frequently used loading dose was 0.5 µg/kg for 10 to 15 minutes before or after induction, whereas the other studies reported a loading dose of 1 µg/kg for 10 to 15 minutes before or after induction. The maintenance rate of dexmedetomidine ranged from 0.2 to 0.7 µg/kg/minute throughout the entire surgery or for 30 minutes before the end of surgery. Six studies reported the postoperative use of dexmedetomidine at a rate of 0.1 to 0.2 µg/kg/minute for several hours or even as long as 3 days. The dose was relatively smaller when patients were awake after anesthesia. Generally, the perioperative intravenous use of dexmedetomidine prevented postoperative cognitive disturbance regardless of whether a loading dose was applied.
Hypotension and bradycardia are the most common side effects of dexmedetomidine and are caused by its inhibition of the sympathetic nervous system.42 It is not surprising that patients in the experimental group experienced a higher incidence of hypotension and bradycardia. Importantly, these are transient side effects and have no significant impact on the prognosis of patients. Previous studies have demonstrated that the perioperative use of dexmedetomidine improved patient prognosis and shortened their length of hospital stay.43 Nevertheless, the use of dexmedetomidine had no effect on reducing PONV.
The effects of dexmedetomidine on delirium have been evaluated in different patient populations, including non-cardiac surgical elderly patients and cardiac surgical patients. An article published in 2020 in The Lancet showed no benefit from perioperative dexmedetomidine use in cardiac surgery regarding the postoperative complications of delirium and atrial fibrillation.44 The results varied among different participants.
A medium level of heterogeneity was observed among all studies included in this meta-analysis (I2=37%). Three studies20,23,31 included patients with preoperative mild cognitive impairment, which we thought might be the source of the heterogeneity. After the exclusion of these three studies, the I2 was decreased to zero, demonstrating that the heterogeneity was derived from these three studies.
This study has several limitations. First, we only found one study21 that reported the intraoperative and postoperative use of dexmedetomidine, and it showed that dexmedetomidine did not prevent postoperative cognitive disturbance. Therefore, further studies should focus on the timing of the combined intraoperative and postoperative use of dexmedetomidine. Second, only four studies reported the occurrence of PONV, and the results showed that the perioperative intravenous use of dexmedetomidine did not reduce the incidence of PONV. This result contradicted our hypothesis that dexmedetomidine reduces the incidence of PONV due to its inhibition of the sympathetic nervous system and sedative effect. Thus, further studies should be performed to investigate the efficacy of the perioperative use of dexmedetomidine in the prevention of PONV.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211014294 for Effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients: a systematic review and meta-analysis by Xiaobo Bi, Jingxia Wei and Xia Zhang in Journal of International Medical Research
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The study was supported by the Sichuan Science and Technology Department Foundation (2019YJ0692).
Author contributions: Xiaobo Bi participated in designing the study, screening the articles, data extraction and data synthesis, and preparing the first draft of the manuscript. Xia Zhang participated in designing the study, solving the discrepancies between different reviewers, critically revising the manuscript, and providing funding support. Jingxia Wei participated in screening the articles, data extraction, and data synthesis.
ORCID iD: Xia Zhang https://orcid.org/0000-0002-1939-4885
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Erdem AF, Kayabasoglu G, Tas Tuna A, et al. Effect of controlled hypotension on regional cerebral oxygen saturation during rhinoplasty: a prospective study. J Clin Monit Comput 2016; 30: 655–660. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph JL, Marcantonio ER, Culley DJ, et al. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia 2008; 63: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017; 34: 192–214. [DOI] [PubMed] [Google Scholar]
- 4.Evered L, Silbert B, Knopman DS, et al. , Nomenclature Consensus Working G. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology 2018; 129: 872–879. [DOI] [PubMed] [Google Scholar]
- 5.Hughes CG, Boncyk CS, Culley DJ, et al. Perioperative Quality Initiative W. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg 2020; 130: 1572–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, Westendorp RGJ, Saczynski JS.Delirium in elderly people. Lancet 2014; 383: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto Y, Yoshimura M, Utada K, et al. Prediction of postoperative delirium after abdominal surgery in the elderly. J Anesth 2009; 23: 51–56. [DOI] [PubMed] [Google Scholar]
- 8.Fu H, Fan L, Wang T.Perioperative neurocognition in elderly patients. Curr Opin Anaesthesiol 2018; 31: 24–29. [DOI] [PubMed] [Google Scholar]
- 9.Joost W, Eurelings LSM, Jonghe JFM, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010; 304: 443–451. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249: 173–178. [DOI] [PubMed] [Google Scholar]
- 11.Krogseth M, Watne LO, Juliebø V, et al. Delirium is a risk factor for further cognitive decline in cognitively impaired hip fracture patients. Arch Gerontol Geriatr 2016; 64: 38–44. [DOI] [PubMed] [Google Scholar]
- 12.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–861. [DOI] [PubMed] [Google Scholar]
- 13.Hshieh T, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 2015; 175: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannitti JA, Thoms SM, Crawford JJ.Alpha–2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog 2015; 62: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Xiong M, Nadavaluru PR, et al. Dexmedetomidine attenuates neurotoxicity induced by prenatal propofol exposure. J Neurosurg Anesthesiol 2016; 28: 51–64. [DOI] [PubMed] [Google Scholar]
- 16.Blaudszun G, Lysakowski C, Elia N, et al. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 2012; 116: 1312–1322. [DOI] [PubMed] [Google Scholar]
- 17.Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology 2016; 124: 362–368. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Xie G, Zhang K, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care 2017; 38: 190–196. [DOI] [PubMed] [Google Scholar]
- 19.Xiong B, Shi Q, Fang H.Dexmedetomidine alleviates postoperative cognitive dysfunction by inhibiting neuron excitation in aged rats. Am J Transl Res 2016; 8: 70–80. [PMC free article] [PubMed] [Google Scholar]
- 20.Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg 2017; 152: e171505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma P, Piao M, Wang Y, et al. Influence of dexmedetomidine and sub-anesthetic dose of ketamine on postoperative delirium in elderly orthopedic patients under total intravenous anesthesia. J Jilin Univ (Med Ed) 2013; 39: 128–132. [Google Scholar]
- 22.Guo Y, Sun L, Chen Z, et al. Preventive effect of dexmedetomidine on postoperative delirium in elderly patients with oral cancer (Chinese). Shanghai Kou Qiang Yi Yao 2015; 24: 236–239. [PubMed] [Google Scholar]
- 23.Liu Y, Ma L, Gao M, et al. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin Exp Res 2016; 28: 729–736. [DOI] [PubMed] [Google Scholar]
- 24.Zeng H, Li Z, He J, et al. Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: A meta-analysis of randomized controlled trials. PLoS One 2019; 14: e0218088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan H, Liu C, Ma X, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can J Anesth 2019; 66: 1489–1500. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Jiang M, Ji Y, et al. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther 2019; 13: 2911–2922. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Zhang J, Liu G, Zhang F, et al. Analysis of postoperative cognitive dysfunction and influencing factors of dexmedetomidine anesthesia in elderly patients with colorectal cancer. Oncol Lett 2019; 18: 3058–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie S, Xie M.Effect of dexmedetomidine on postoperative delirium in elderly patients undergoing hip fracture surgery. Pak J Pharm Sci 2018; 31: 2277–2281. [PubMed] [Google Scholar]
- 30.Ting H, Hu X, Peng H, et al. Perioperative dexmedetomidine reduces delirium in elderly patients after lung cancer surgery. Psychiatria Danubina 2019; 31: 95–101. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Lee CH, Lee G, et al. The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. J Clin Anesth 2018; 47: 27–32. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, He R, Chen S, et al. Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri-operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp Ther Med 2015; 10: 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W, Liu B, Zhang F, et al. The effects of dexmedetomidine on post-operative cognitive dysfunction and inflammatory factors in senile patients. Int J Clin Exp Med 2015; 8: 4601–4605. [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng X, Mei B, Zuo Y, et al. A multicentre randomised controlled trial of the effect of intra-operative dexmedetomidine on cognitive decline after surgery. Anaesthesia 2019; 74: 741–750. [DOI] [PubMed] [Google Scholar]
- 35.Su X, Meng Z, Wu X, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016; 388: 1893–1902. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Cui F, Zhang C, et al. Low-dose Dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit. A pilot randomized controlled trial. Anesthesiology 2016; 125: 979–991. [DOI] [PubMed] [Google Scholar]
- 37.He F, Shen L, Zhong J.A study of dexmedetomidine in the prevention of postoperative delirium in elderly patients after vertebral osteotomy. Int J Clin Exp Med 2018; 11: 4984–4990. [Google Scholar]
- 38.Xuan Y, Fan R, Chen J, et al. Effects of dexmedetomidine for postoperative delirium after joint replacement in elderly patients: a randomized, double-blind, and placebo-controlled trial. Int J Clin Exp Med 2018; 11: 13147–13157. [Google Scholar]
- 39.De Tournay-Jetté E, Dupuis G, Bherer L, et al. The relationship between cerebral oxygen saturation changes and postoperative cognitive dysfunction in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011; 25: 95–104. [DOI] [PubMed] [Google Scholar]
- 40.Pavlov VA, Tracey KJ.The cholinergic anti-inflammatory pathway. Brain Behav Immun 2005; 19: 493–499. [DOI] [PubMed] [Google Scholar]
- 41.Maldonado JR, Wysong A, Van Der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009; 50: 206–217. [DOI] [PubMed] [Google Scholar]
- 42.Kamibayashi T, Maze M.Clinical uses of alpha2-adrenergic agonists. Anesthesiology 2000; 93: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, Liang Y, Dai Z, et al. Perioperative dexmedetomidine reduces delirium after cardiac surgery: a meta-analysis of randomized controlled trials. J Clin Anesth 2018; 50: 33–42. [DOI] [PubMed] [Google Scholar]
- 44.Turan A, Duncan A, Leung S, et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): a randomized placebo-controlled trial. Lancet 2020; 396: 177–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211014294 for Effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients: a systematic review and meta-analysis by Xiaobo Bi, Jingxia Wei and Xia Zhang in Journal of International Medical Research