Abstract
The role of the mammalian auditory olivocochlear efferent system in hearing has long been the subject of debate. Its ability to protect against damaging noise exposure is clear, but whether or not this is the primary function of a system that evolved in the absence of industrial noise remains controversial. Here we review the behavioral consequences of olivocochlear activation and diminished olivocochlear function. Attempts to demonstrate a role for hearing in noise have yielded conflicting results in both animal and human studies. A role in selective attention to sounds in the presence of distractors, or attention to visual stimuli in the presence of competing auditory stimuli, has been established in animal models, but again behavioral studies in humans remain equivocal. Auditory processing deficits occur in models of congenital olivocochlear dysfunction, but these deficits likely reflect abnormal central auditory development rather than direct effects of olivocochlear feedback. Additional proposed roles in age-related hearing loss, tinnitus, hyperacusis, and binaural or spatial hearing, are intriguing, but require additional study. These behavioral studies almost exclusively focus on medial olivocochlear effects, and many relied on lesioning techniques that can have unspecific effects. The consequences of lateral olivocochlear and of corticofugal pathway activation for perception remain unknown. As new tools for targeted manipulation of olivocochlear neurons emerge, there is potential for a transformation of our understanding of the role of the olivocochlear system in behavior across species.
Keywords: olivocochlear, auditory efferent, psychoacoustics, selective attention, hearing in noise, spatial hearing
1. Introduction
1.1. OC system overview
Our ability to effectively perceive and interact with the environment integrates the activity of efferent pathways that can modulate the signals transmitted by the afferent sensory systems. In the auditory system, the efferent pathways form a neural network comprised of several feedback loops with numerous subcortical nuclei, including the thalamus, inferior colliculus, superior olivary complex, and cochlear nucleus (Malmierca and Ryugo, 2011). The auditory efferent pathways extend from auditory cortex to the peripheral sensory organ via the olivocochlear (OC) system (Figure 1). The OC system, originally described by Rasmussen (1946), is formed by two neuronal groups: (i) the medial olivocochlear neurons (MOC) and (ii) the lateral olivocochlear neurons (LOC) (Warr and Guinan, 1979).
Figure 1.
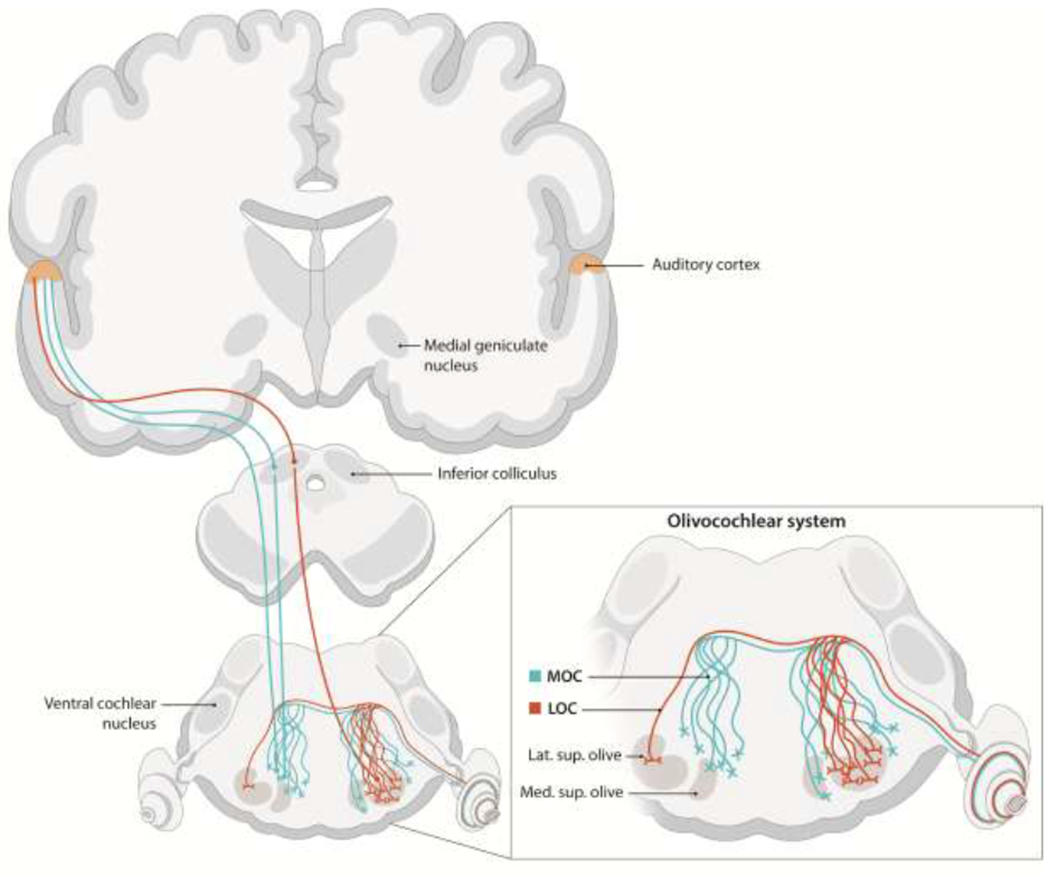
Schematic diagram of the descending auditory pathways from cortex to cochlea in mammals. Inset shows olivocochlear pathways.
Even though the precise anatomical location of these two groups varies depending on the species, in general MOC neurons can be found in the medial periolivary regions, while the LOCs originate in or around the lateral superior olive (Brown, 2011). The MOC synapses are organized along a tonotopic gradient in the periphery, with greater density in the middle regions of the cochlea (Guinan, 1996; Maison et al., 2003). In addition, most of the MOC neurons send collaterals that reach the CN of the same side as the target cochlea (Benson & Brown, 1990). MOC neurons primarily release acetylcholine, leading to the hyperpolarization of the OHCs and, consequently, a reduction of the gain of the cochlear amplifier (Blanchet et al. 1996; Dallos et al. 1997; Evans et al. 2000).
LOC neurons are on average smaller and more numerous than MOCs and are characterized by having fine, non-myelinated fibers (Guinan, 1996). As with the MOCs, they also project via the vestibular nerve, but synapse with the dendrites of type I cochlear afferents just below the inner hair cells (Guinan, 1996). These projections are tonotopically organized and almost all of them (95-100%) are ipsilateral (Schofield, 2010). LOC neurons express a greater diversity of neurotransmitters than MOCs. While the majority of LOC neurons are cholinergic, they have been observed to express other neurotransmitters within the same synaptic terminal including dopamine (DA), calcitonin gene-related peptide (CGRP), GABA and opioid peptides such as enkephalin (Ciuman, 2010; Eybalin, 1993; Reijntjes and Pyott, 2016; Wu et al. 2020). Furthermore, in mice there is some evidence of subgroups of dopaminergic LOC neurons that are not cholinergic (Darrow et al., 2006b).
The physiological effects of activating the OC system have been reviewed in detail in recent reviews (e.g., Terreros and Delano 2015; Guinan 2018; Lopez-Poveda 2018; Fuchs and Lauer 2018). In spite of the fact that the OC system is composed of both MOC and LOC neurons, most of the knowledge about OC physiology has been obtained by electrical stimulation of MOC fibers (e.g., Galambos 1956; Fex 1959; Gifford and Guinan 1988, Cooper and Guinan, 2006; Elgueda et al., 2011). The electrical activation of MOC fibers at the floor of the fourth ventricle reduced the amplitude of auditory nerve responses (Galambos, 1956) and increases the magnitude of cochlear microphonics (CM) potentials (Fex, 1959; Elgueda et al., 2011). MOC neurons can be reflexively activated by ipsilateral and contralateral sounds (Buño, 1978; Liberman, 1989) through a brainstem circuit that includes auditory nerve, cochlear nucleus and MOC neurons (Thompson and Thompson 1991; DeVenecia et al., 2005). In contrast to the middle ear muscle reflexes (stapedius and tensor tympani), the MOC reflex can be elicited by lower level sounds (< 60 dB), producing a suppression of cochlear responses that can be measured non-invasively with otoacoustic emissions or with electrocochleography (Liberman and Guinan, 1998; Aedo et al., 2015). One important caveat is that this reflex is highly variable among different individuals, ranging from large suppressions (up to 10 dB of effective attenuation) to no effect or even enhancements, although most studies show a limited range of otoacoustic suppression effects within only 1-2 dB in humans (Puria, 1996; Maison and Liberman, 2000). This may be due to the relatively weak innervation of outer hair cells by MOC neurons in humans compared to common laboratory species (Liberman and Liberman 2019). The inter-individual variability has been correlated with levels of resistance to acoustic injury and to different capacities to suppress auditory distractors during selective attention (Maison and Liberman 2000; Bowen et al., 2020). Otoacoustic suppression effects may underestimate the true size of the effect. Some studies measuring MOC-induced CAP suppression in humans show much larger effects (Smith et al. 2017), whereas other studies have only shown small suppressive effects only after many hours of testing (Lichtenhan et al. 2016). The size of the observed effects likely depends on the specific testing parameters used (Verschooten et al. 2017).
Much of what we know about how the OC system affects behavior comes from studies of its dysfunction. Conflicting results have sometimes been reported in behavioral studies performed in humans and animals. Here we focus on the behavioral effects of OC efferent activation, de-efferentation, and genetic manipulation. We include evoked potential studies in cases where little or no behavioral evidence is available, as these data are useful in making predictions about behavioral function.
2. Medial Olivocochlear effects
2.1. Detection and discrimination of sounds in quiet and noisy backgrounds
Physiological effects of OC bundle stimulation in animals suggest that the system should enhance detection and discrimination of sounds in noise and enhance frequency discrimination (Geisler 1974; Dolan and Nutall 1988; Winslow and Sachs 1987, 1988; Kawase and Liberman 1993; Kawase et al. 1993; Seluakumaran et al. 2008; Smalt et al. 2014). An early study in guinea pigs showed that the electrical stimulation of MOC fibers at the floor of the fourth ventricle increases auditory nerve responses to clicks in background noise, a result that was later confirmed in cats (Nieder and Nieder 1970; Winslow and Sachs 1987; Kawase and Liberman, 1993). Tuning curves determined with forward and simultaneous masking of tone-evoked CAPs in the presence of a tonal masker show an elevation of the ‘tip’ of the curve, with greater effects observed for the forward masking stimuli, after crossed OC bundle sectioning in guinea pigs (Bonfils et al., 1986).
Direct evidence that the olivocochlear system contributes to perception of sounds in noise, at least under certain conditions, comes primarily from animal studies in which the OC bundle is surgically lesioned. The lesions are typically made at the floor of the fourth ventricle along the midline, which leaves the ipsilateral OC projections intact. Lesions of the crossed OC bundle result in small and nonsignificant increases in tone in noise detection thresholds in cats under some testing conditions (Trahiotis and Elliott, 1970; Igarashi et al. 1972). These results are surprising in light of the hypothesized role of the olivocochlear system in enhancing detection of transient signals in noise, as described above, and evidence in humans that stimulation of the MOC system with contralateral noise shifts both tone-in-noise detection thresholds (Micheyl and Collet 1995). Several animal studies have reported diminished frequency discrimination for pure tones or vowel formats presented in quiet or noisy backgrounds in subjects with OC bundle lesions (Capps and Ades 1968; Dewson 1968; Hienz et al. 1998), whereas other studies have not (Igarashi 1979a). Intensity discrimination does not appear to be diminished with OC bundle lesions (Igarashi 1979b), despite evidence in humans that the OC system affects intensity discrimination (Micheyl et al. 1997; Roverud and Strickland 2015). Together, these results in different animal models and humans suggest that the OC system is relevant for detection and discrimination of sounds in noisy environments. The major caveats to all these studies are, unsurprisingly, the potential for non-specific effects of lesions, overly large lesions affecting other pathways, or incomplete lesions. Small sample sizes and variability in performance across subjects are also characteristic of these studies. Nevertheless, with the emergence of more refined neurostimulation and silencing tools that can, in theory, specifically target OC neurons, the discrepancies in the role of the OC system in sound discrimination may be resolved.
Psychoacoustic measurements of sound detection and discrimination performed in mouse strains with genetic alterations affecting the olivocochlear system have primarily focused on mouse models of MOC-induced outer hair cell inhibition via alpha-9 nicotinic acetylcholine receptor subunit deletion (alpha9-knockout) or enhancement (alpha9 knockin) (Elgoyhen and Katz 2012). Alpha9-knockouts show normal detection of tones in quiet and noise and normal intensity discrimination in quiet and noise when tested with an operant conditioning task (Prosen et al. 2000; May et al. 2002). Alpha9-knockout mice also show abnormal temporal processing and responses to tones in quiet when tested using acoustic startle-based prepulse inhibition measures, whereas alpha9-knockin mice with a point mutation in the alpha-9 receptor that enhances outer hair cell inhibition show enhanced prepulse inhibition by tones in quiet backgrounds (Lauer and May 2011; Luebke and Allen 2017). Interestingly, neither mutant model shows abnormal prepulse inhibition to tones in noise background, although alpha-CGRP knockout mice do show deficits in this behavior, possibly due to LOC dysfunction (Luebke and Allen 2017).
It must be recognized that the central auditory pathways in the alpha-9 mutant strains, and probably the CGRP mutants, are abnormally developed. Thus, it is impossible to parse out the effects of abnormal organization of central circuits from the specific effects of OC activity in these models. In addition, LOC synapses show some evidence of disorganization in alpha9 knockout mice (Vetter et al. 1999). Models with conditionally expressed gene mutations or optogenetic activation or silencing of OC neurons offer great potential for teasing out the specific contributions of OC activity to sound in noise perception and behaviors that are affected by sound, but such studies have not yet been reported in the literature.
It has proven difficult to establish a clear and consistent correlation between OC activity and speech perception or amplitude modulation detection in human listeners (Giraud et al. 1997; Wagner et al. 2008; deBoer et al. 2012, Guinan 2014; Mishra and Lutman, 2014; Marrufo-Perez et al. 2018; Wojtczak et al. 2019). This may be due to the small (~1-2 dB) observable effects of contralateral noise suppression of OAEs, which remain the primary means of inferring MOC activation strength in humans. Other contributing factors may be related to task characteristics, other regions of the auditory system compensating for abnormal OC activity, that the OC system itself demonstrates experience-dependent plasticity that obscures the emergence of clear correlations between behavioral performance and indirect physiological measurements, or that redundant mechanisms in the central nervous system exist for maintaining acute performance in traditional psychoacoustic tasks (i.e., deBoer and Thornton 2008; Mishra and Lutman, 2014; Wojtczak 2014; Verschooten et al. 2017; Mertes et al. 2019). Importantly, these tasks may be affected by mechanisms that include descending projections from the auditory cortex, which in turn can modulate the OC reflex magnitude (Dragicevic et al., 2015; Aedo et al., 2016).
Some additional reasons that may explain the diversity of results in speech perception in noise and auditory efferent function in human listeners are: (i) there is still no consensus about a paradigm for specifically stimulating olivocochlear neurons with contralateral or ipsilateral sounds in humans (Boothalingam et al., 2018); (ii) it is well known that the olivocochlear reflex is highly variable among individuals, suggesting that for a group of listeners it may be important for improving speech recognition in noise, while for others it may add no further aid; (iii) studying OC-mediated perception only in adults misses developmental effects such as development of listening in noise during childhood (Mishra 2020); (iv) OC function is associated with the slope of the psychometric function for speech recognition in noise, and most studies only glimpse behavior at one or two signal-to-noise ratios (Mertes et al., 2018). In summary, the role of OC activation in noise in humans is still debated. Standardized protocols to study the OC reflex in humans among different research groups are needed.
2.2. Selective attention
The biological filtering of sensory distractors is one of the proposed mechanisms for selective attention. The neural circuits comprising top-down networks are essential to accomplish this function. In line with this, the circuitry of the auditory efferent system allows the brain to filter the most peripheral auditory responses through OC neurons. A seminal work by Hernandez-Peon proposed that corticofugal pathways of the auditory system can suppress neural responses to irrelevant auditory stimuli when animals attend to visual or olfactory stimuli (Hernandez-Peon 1956). Later, in the 1970’s, Oatman implanted cats at the round window of the cochlea, showing that during attention to visual stimuli there is a reduction in amplitude and increase in latency of the N1 component of auditory nerve compound action potentials (CAP) to unattended clicks and tones (Oatman 1971; Oatman 1976). A similar experiment performed in cats showed evidence of increased susceptibility to auditory distractors during a visual task in subjects with OC bundle lesions (Igarashi et al., 1974).
Experiments also showed suppression of the auditory frequency following response during a visual task, particularly in the middle frequency range, as well as enhancement of the theta component of cortically-evoked EEGs (Oatman and Anderson 1980; Oatman 1982). These early works inspired new experiments with a similar research question, but with faster computing and better temporal resolution performed in chinchillas (Delano et al., 2007). In this study, chinchillas were trained in a visual selective attention task with clicks and tones as auditory distractors, while simultaneously CAP and CM responses were recorded. Similar to the Oatman studies CAP amplitudes were reduced during visual selective attention, while CM amplitude was increased. These effects in CAP and CM are analogous to the physiological experiments using electrical stimulation of MOC fibers performed by Galambos (1956) and Fex (1959) in anesthetized animals (section 1.1). A recent study provides further evidence for a role of the MOC neurons in selective attention in the presence of natural sounds. Individual variability in the magnitude of the MOC reflex at a range of frequencies between 1 and 8 kHz is an important factor for predicting behavioral performance during visual attention with chinchilla distress vocalizations as distractors (Bowen et al., 2020). An important caveat is that the MOC system aids in ignoring irrelevant auditory distractors but is not required to perform selective attention behaviors in the alpha-9 KO mice, showing that other neural circuits can compensate for the lack of MOC function (Terreros et al., 2016). Together, these works show that the auditory efferent system aids in the biological filtering of ecological auditory distractors, and that individual variability in MOC reflex magnitude can predict the effect of auditory distractors in a visual selective attention task.
The effects of OC activation during selective attention have been more difficult to demonstrate in experiments performed in human subjects. The auditory nerve component of the auditory brainstem response is reduced when auditory distractors are presented during performance of a visual attention task (Lukas 1980). Selective attention to visual stimuli can likewise reduce otoacoustic emission amplitudes (Puel et al. 1988). However, these suppressive effects are not observed consistently across individuals or studies, and they may be specific to visual attention in the presence of auditory distractors (Froehlich et al. 1990 a, 1990b, 1993; Michie et al. 1996; Srinivasan et al. 2012). Experiments using otoacoustic emissions as physiological readouts of OC activity are subject to a number of methodological and interpretation issues; therefore, studies that carefully control for middle ear muscle reflex activation, ipsilateral and contralateral OC stimulation, tonic versus transient OC activation, effects of task difficulty, and other factors are needed to resolve these discrepancies (Guinan 2014). In addition, most of the human studies of OC effects on selective attention analyzed the amplitude of otoacoustic emissions to search for an auditory efferent effect. This strategy is limited, and new types of analysis are needed. For instance, Dragicevic et al., (2019) found that switching between auditory and visual attention modulates the amplitude and latency of low-frequency oscillations obtained from the envelope of the DPOAE amplitude. Other studies have demonstrated OC effects on otoacoustic emission fine structure and phase, and a similar analysis could be applied in the context of selective attention (e.g., Abdala et al. 2009; Deeter et al. 2009; Wagner et al. 2007; Zhao et al. 2015. In order to find corticofugal effects, new types of analyses, perhaps incorporating artificial intelligence, should be encouraged in future studies.
2.3. Spatial hearing
In animal models, MOC system dysfunction is accompanied by deficits in the spatial localization of sounds. For example, acute lesions of the OC bundle in cats, primarily affecting the MOC neurons, are accompanied by an increase in MAAs for sound localization in the vertical plane in background noise (May et al., 2004). Intriguingly, MAAs returned to normal after about a week of consecutive test sessions, indicating that compensatory pathways can make up for deficits associated with OC bundle lesions. In addition, alpha9-knockout mice trained to identify the location of a sound azimuth showed impaired MAAs compared to WT mice (Clause et al., 2017). In ferrets, MOC lesions, but not LOC lesions, prevented learning of altered binaural cues in the horizontal plane in ear plugged ferrets (Irving et al., 2011). The reasons underlying the discrepancy between learning effects in the vertical and azimuthal sound localization experiments remain unclear.
Supplementing this loss of function evidence, studies in healthy humans have described a correlation between MOC feedback strength and the ability to discriminate sound location in noise. Listeners with stronger MOC reflexes showed lesser effects of noise on the capacity to accurately locating a sound in the vertical plane (Andeol et al., 2011). Application of an olivocochlear-inspired processing strategy can enhance inter-aural level differences and improve lateralization of virtual sound sources in cochlear implant users (Lopez-Poveda et al. 2019). On the other hand, other data indicate that the MOC function does not influence the location of sounds in the horizontal plane (Boothalingam et al., 2016). The reasons for these discrepancies between the vertical and horizontal planes are still unknown. Further studies are needed to examine these effects in detail and to determine how specific they are regarding the task or the animal species used.
3. LOC effects
The perceptual/behavioral effects of LOC efferent activation are completely unknown. At present, there is no assay that provides a specific measurement of LOC activity in an awake, behaving organism. We do not even have much of an understanding of how these neurons function at the cellular level because of the technical difficulty of performing recordings from unmyelinated axons. Because LOC neurons are unmyelinated, any effects on the perception of sounds presumably occur on a slow scale. Given the diverse array of neurotransmitters and neuroactive substances in LOC neurons (Eybalin, 1993; Reijntjes and Pyott, 2016; Vetter et al., 1991), perceptual effects could manifest as increased or decreased hearing sensitivity. We know that LOC neurons show plasticity in response to acoustic experience and hearing loss (Liberman et al. 2015; Wu et al. 2020; Kobrina et al. 2020). Thus, perceptual effects may prove difficult to detect if the system is constantly adapting to the acoustic environment. In fact, this may be one of the primary functions of the system—to modulate the set point of auditory nerve fibers to adapt to changing acoustic environments and hearing loss. An additional hypothesis is that the LOC system is responsible for balancing activity from the two ears; however, studies of these effects are limited and contradictory (Darrow et al. 2006a; Larsen et al. 2010). Much remains to be discovered in this area.
4. Mixed MOC/LOC effects and indeterminate effects
4.1. Vestibular neurectomy/Meniere’s patients
Animal studies have shown that the OC bundle is routed from brainstem to the cochlea through the inferior vestibular nerve, crossing to the auditory nerve in the Oort anastomosis located in the internal ear canal (Liberman and Brown, 1986; Warren and Liberman, 1989). The Oort anastomosis has also been found in human temporal bones (Arnesen 1984), however it is important to highlight that a histological demonstration of the brainstem origin of olivocochlear neurons crossing through the human Oort anastomosis is still lacking (Labrousse et al., 2004; Tian et al., 2008).
In neurotology clinics, surgical sectioning of the vestibular nerve (vestibular neurectomy) is used in a group of selected cases with refractory vertigo, usually in patients with Meniere’s disease (Kitahara, 2018). Several researchers have taken advantage of this surgical technique which can be used as a clinical model of human auditory de-efferentation (Baguley et al., 2002). The study of patients with vestibular neurectomy provide functional evidence suggesting that the OC fibers run inside the Oort anastomosis, as the magnitude of contralateral noise suppression of otoacoustic emissions is reduced in the ear with vestibular neurectomy (Williams et al., 1994; Giraud et al., 1995, 1997).
Scharf et al. (1994, 1997) measured performance on an array of psychoacoustical tasks in Meniere’s patients before and after vestibular neurectomy. Performance on the majority of the psychoacoustic tests did not change after surgery. The only significant difference that they found was that after surgery individuals were unable to filter unattended tones in an auditory attention task. Regarding the other psychoacoustic tasks, there were no differences in the detection and discrimination of tonal signals, frequency selectivity, and loudness adaptation. A later study reported that vestibular neurectomy patients show minimal perceptual deficits in quiet, but variably showed abnormal loudness perception, masking, and hearing in noise after surgery (Zeng et al. 2000). Some patients show improved hearing and speech discrimination after surgery (Rosenberg et al. 1996). Caveats to these experiments include nonspecific or incomplete effects of surgery, nonspecific effects of the disease necessitating the surgery, and difficulty parsing out the effects of hearing loss from the effects of sectioning the OC bundle.
Another research group studied 14 patients (12 Meniere’s) with vestibular neurectomy and found that tinnitus loudness was reduced in nearly 80% after surgery (Kubo et al., 1995). The amplitude ratio of the summating potential to the action potential of the electrocochleogram also increased somewhat, which they speculated could be an effect of OC section. Importantly, it is still not known whether Meniere’s disease itself affects olivocochlear function, and it would be important to study a greater number of cases with vestibular neurectomy.
4.2. Suppression of self-vocalizations
The generation of self-vocalizations presents significant challenges to the auditory system. Among these are: (i) being able to differentiate the self-generated signal from exogenous stimuli, (ii) to protect the sensory system from potential acoustic trauma, and (iii) to maintain sensitivity to the external auditory signals. The ability to selectively attenuate the cochlear response to self-generated vocalizations contributes to addressing these issues. In this context, the OC system, along with the middle ear muscles, may be involved in both suppressing the cochlear response to self-vocalizations and in preventing desensitization to the other stimuli (Goldberg and Henson 1998). Chronic electrode recordings in bats have observed a decrease in cochlear gain during their vocalizations (Goldberg and Henson 1998). Such an effect could be explained by the action of the MOC system. Furthermore, single unit recordings in awake, behaving, and vocalizing squirrel monkeys identified neuronal populations of the OC system capable of integrating audio-vocal signals (Hage et al. 2006). Within these populations, distinct patterns of activity between MOC and LOC neurons were identified. Audio-vocal MOC neurons appear to be involved in protecting the cochlea from overstimulation by own sounds, while audio-vocal LOC neurons are likely to be modulating the activity of cochlear nerve fibers (Hage et al. 2007). Thus, the LOC system may be participating in preserving sensitivity to exogenous acoustic signals.
5. Neurological, psychological, developmental, and sensory disorders
The knowledge about the involvement of the auditory efferent system in neuropsychiatric conditions is largely limited to the use of the available non-invasive tool for assessing MOC reflex function in humans: contralateral sounds with otoacoustic emissions. Again, most of what it is known is about MOC reflex function, while the involvement of LOC function and of the corticofugal projections in neuropsychiatric disorders is largely unknown.
5.1. Neurodevelopmental disorders, migraine, and traumatic brain injury
Olivocochlear effects on perception have not been investigated systematically in neurodivergent versus neurotypical listeners; however, a number of studies indicate that these effects might differ from average for people with a number of neurological and psychiatric conditions. The associations between efferent function and several neuropsychiatric and neurological disorders may be related to the diversity of neurotransmitters affecting the MOC and LOC systems (acetylcholine, dopamine, GABA, serotonin, neuropeptides) or to the influence of top-down networks on the cochlear function. A number of studies have shown weaker or abnormal asymmetry of MOC reflexes estimated as suppression of otoacoustic emissions by contralateral noise in children and adults with autism spectrum diagnoses and selective mutism (Collet et al. 1993; Khalfa et al. 2001; Bar-Haim et al. 2004; Danesh and Kaf 2012; Muchnik et al. 2013; Wilson et al. 2017). A variety of auditory processing differences in listeners on the autism spectrum have been reported in the literature, including increased reactivity or intolerance to loud sounds, which is regarded as a form of hyperacusis (Danesh et al 2015; Stefanelli et al. 2020). In one study, medial olivocochlear efferent suppression strength was positively correlated with hyperacusis scores in children with autism spectrum disorders, and those with the most severe autism spectrum disorders had the strongest MOC reflexes (Wilson et al. 2017). People with Williams Syndrome, a developmental disorder associated with hyperacusis, show hyperactive MOC reflexes, abnormal auditory evoked potentials, reduced loudness discomfort levels, abnormal acoustic reflexes, and evidence of high frequency hearing loss. In general, these studies suggest systemic auditory system dysregulation in which olivocochlear hyperfunction is one symptom (Gothelf et al. 2006; Attias et al. 2008).
Intriguingly, people with migraine headaches and vestibular migraines, which are often associated with decreased tolerance of loud sounds, sometimes show weakened contralateral suppression of otoacoustic emissions (Murdin et al. 2010; Joffley et al. 2016). Some studies have also shown reduced otoacoustic emission amplitudes in migraineurs without contralateral stimulation (Bolay et al. 2008; Hamed et al. 2012). This could indicate an underlying cochlear deficit or tonically activated MOC suppression, which would limit the ability to detect an effect of contralateral noise suppression on already reduced responses.
People with traumatic brain injury often present with a constellation of auditory symptoms, including hyperacusis, tinnitus, and difficulty listening in noise—despite having normal or near-normal audiograms. A higher prevalence of spontaneous otoacoustic emissions, larger sound-evoked otoacoustic emissions, and reduced contralateral suppression of otoacoustic emissions have been reported in these listeners (Ceranic et al. 1998; Attias et al. 2005). Nevertheless, the relationship between auditory symptoms and OC dysfunction in this population remains unclear. It is possible that the hearing in noise difficulties reported by many of these patients is due to reduced MOC strength.
Other disorders affecting the nervous system that have been associated with abnormal auditory function and probable OC dysfunction include multiple sclerosis, Parkinson’s disease—reduced contralateral suppression, increased or decreased otoacoustic emission amplitude without dopaminergic medication (Coelho et al 2007; Pisani et al. 2015; De Keyser et al. 2019), schizophrenia—lack of suppression asymmetry in left versus right ears (Veuillet et al. 2001), and type 2 diabetes (Jacobs et al. 2012), as well as in animal models of diabetes (Wu et al. 2010).; De Keyser et al. 2019). In all of these cases, it is unclear if the effects are specific to auditory brainstem-mediated reflexes or reflect systemic differences in peripheral auditory or brain function. That such a diverse assortment of diseases and disorders may affect OC system activity indicates that OC dysfunction may be more widespread in the population than previously realized. Associations between additional disorders linked to both auditory perceptual deficits and abnormal OC reflex strength will likely emerge over time and may provide a useful screen for brainstem lesions or dysfunction that is not revealed with brain imaging techniques or traditional audiometric tests. A more complete characterization of OC dysfunction in these patient groups is important because chronically reduced MOC suppression of outer hair cell activity could lead to accelerated hearing loss as well as hearing in noise deficits.
5.2. Auditory processing disorders in children
Children with normal hearing thresholds, but with listening difficulties and learning impairments can be classified as having auditory processing disorders (Moore et al. 2018). Dysfunction of the auditory efferent system has been proposed as a possible contributor to auditory processing disorders in children (Mishra et al 2014.) Some studies have shown that children with auditory processing disorders and specific language impairment had a reduced suppressive effect of contralateral noise on otoacoustic emissions compared to normal hearing controls (Muchnik et al. 2004; Sanches and Carvallo 2006). However, other authors have failed to replicate these findings. For instance, Clarke et al., (2006) found no differences in the magnitude of contralateral noise suppression on otoacoustic emissions between a group of children with and without specific language impairments. Smart et al. (2019) compared olivocochlear and middle ear reflex function in a group diagnosed with auditory processing disorders and controls. They found no differences on MOC suppression, while they reported a significant elevation of middle ear reflex thresholds at 2 kHz in children with auditory processing disorders. There is still an ample field to study whether efferent dysfunction is an important factor in the acquisition of auditory skills in children with auditory processing disorders. As with other otoacoustic emission suppression studies in humans, further studies controlling for middle ear muscle reflexes and using more robust OC reflex-eliciting stimuli are needed to clarify whether or not OC dysfunction is prevalent in this heterogeneous population (Mishra 2014; Boothalingam 2019). Additionally, possible differences in diagnostic criteria and disordered performance on specific auditory processing tasks should be taken into account in future studies.
5.3. Tinnitus, hyperacusis, and sensorineural hearing loss
The role between sensorineural hearing loss, central auditory disorders such as tinnitus and hyperacusis, and OC function is complex to sort out because of the interplay between these conditions. Cochlear damage can lead to changes in the central auditory system, such as the loss of inhibition and central gain compensation purported to underlie tinnitus, hyperacusis, and loudness recruitment (Auerbach et al. 2019; Lauer et al. 2019; Sheppard et al. 2020). This in turn could lead to abnormal OC activation. Reduced OC suppression of the auditory periphery could then render the ear more susceptible to noise exposure, which could in turn increase susceptibility to or severity of sensorineural hearing loss, tinnitus, and hyperacusis.
Further complicating effort to tease apart these relationships are heterogeneity in patients reporting tinnitus and hyperacusis, the possibility of subclinical cochlear impairment in these patients, variable methodologies, and the relatively small MOC reflex effects observed (Geven et al. 2014; Riga et al. 2015). It stands to reason that the MOC reflex cannot function normally if outer hair cells and auditory nerve fibers are damaged and afferent drive to the olivocochlear pathway is reduced with peripheral damage. An additional complication is that LOC neurons may contribute to tinnitus and hyperacusis in yet to be understood ways. Nevertheless, several papers have reported either weakened (Chery-Croze et al. 1993,1994), hyperactive (Knudson et al. 2014), or no change (Cheng et al. 2020) in contralateral suppression of otoacoustic emissions in listeners with tinnitus. Increased MOC strength may also be related to reduced loudness tolerance in tinnitus sufferers, though not to self-reported hyperacusis, and the MOC enhancement may simply be a byproduct of decreased central inhibition (Knudson et al. 2014). Much remains to be discovered regarding the role of OC function/dysfunction in tinnitus, hyperacusis, and hearing loss.
An additional consideration in the context of these studies is that the OC system shows age-, noise-, and hearing loss-related synaptic reorganization (Kraus and Illing 2004; Lauer et al. 2012; Liberman et al. 2015; Radtke-Schuller Zachary and Fuchs 2015; Liberman and Liberman 2019; Suthakar and Ryugo 2017; Jeng et al. 2020; Kobrina et al. 2020; Wu et al. 2020). These studies show that peripheral OC synapses, OC neuron cell bodies, and their central inputs change with age and atypical acoustic experience, but how this reorganization affects perception is not known for certain. Most likely, these changes contribute to diminished hearing in noise capability that is commonly experienced by listeners with hearing loss, but further studies are needed to confirm this hypothesis. Additionally, strengthened OC feedback appears to protect against peripheral hearing damage, and, consequently, may protect against hyperacusis and tinnitus (Boero et al. 2018, 2020). However, strengthened OC feedback may have unintended consequences for perception that have yet to be revealed.
6. Conclusions and areas for future investigation
Despite sometimes conflicting evidence, the OC system seems to play a role in optimizing hearing under a number of challenging conditions and in selective attention to sensory stimuli. In some cases, these effects may not be apparent because compensatory or redundant processes are likely in play. A more complete understanding of OC-mediated effects on behavior is important in light of emerging hearing regenerative and reparative therapies, since normal OC connectivity may be required for normal perception of sounds with restored hair cells and auditory nerve fibers.
As modern circuit manipulation tools are brought online for specific and conditional manipulation of the OC neurons, the precise role of this system in behavior will become less murky. There is also much to learn about species diversity in the role of the OC system in hearing. Such studies not only provide important information about biological auditory specialization, but also may yield insights into potential strategies for augmenting hearing through OC-inspired processing strategies. There are many exciting areas for future investigation into the effects of plasticity of the system on behavior, how developmental and natural aging affect OC-mediated perception, and how acquired damage from noise or ototoxic substances affect these processes.
Highlights.
Efferent auditory pathways reach the cochlea through the olivocochlear (OC) system.
The OC system plays a role in hearing in noise and selective attention.
Methodological limitations and compensatory processes limit observed effects.
Additional studies of the effects of the OC system on behavior are necessary.
Acknowledgments
Funding: NIH DC017620, NIH DC006476, ANID BASAL FB008, Iniciativa Cientifica Milenio ICN09_015, Vicerrectoría de Investigación y Desarrollo de la Universidad de Chile ENL 19/20, Fundación Guillermo Puelma, David M. Rubenstein Fund for Hearing Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdala C, Mishra SK, Williams TL 2009. Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex. J. Acoust. Soc. Am 125 (3) 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aedo C, Terreros G, Leon A, Delano PH 2016. The Corticofugal Effects of Auditory Cortex Microstimulation on Auditory Nerve and Superior Olivary Complex Responses Are Mediated via Alpha-9 Nicotinic Receptor Subunit. PLoS One 11 (5) e0155991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aedo C, Tapia E, Pavez E, Elgueda D, Delano PH, Robles L 2015. Stronger efferent suppression of cochlear neural potentials by contralateral acoustic stimulation in awake than in anesthetized chinchilla. Front. Sys. Neurosci 9 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PD, Luebke AE 2017. Reflex Modification Audiometry Reveals Dual Roles for Olivocochlear Neurotransmission. Front. Cell. Neurosci 11 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andeol G, Guillaume A, Micheyl C, Savel S, Pellieux L, Moulin A 2011. Auditory efferents facilitate sound localization in noise in humans. J. Neurosci 31 (18) 6759–6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesen AR, Osen KK 1984. Fibre population of the vestibulocochlear anastomosis in the cat. Acta Otolaryngol. 98 (3-4) 255–269. [DOI] [PubMed] [Google Scholar]
- Attias J, Raveh E, Ben-Naftali NF, Zarchi O, Gothelf D 2008. Hyperactive auditory efferent system and lack of acoustic reflexes in Williams syndrome. J. Basic Clin. Physiol. Pharmacol 19 (3-4) 193–207. [DOI] [PubMed] [Google Scholar]
- Attias J, Zwecker-Lazar I, Nageris B, Keren O, Groswasser Z 2005. Dysfunction of the auditory efferent system in patients with traumatic brain injuries with tinnitus and hyperacusis. J. Basic Clin. Physiol. Pharmacol 16 (2-3) 117–126. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Radziwon K, Salvi R 2019. Testing the Central Gain Model: Loudness Growth Correlates with Central Auditory Gain Enhancement in a Rodent Model of Hyperacusis. Neurosci. 407 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM, Axon P, Winter IM, Moffat DA 2002. The effect of vestibular nerve section upon tinnitus. Clin. Otolaryngol. Allied Sci 27 (4) 219–226. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Henkin Y, Ari-Even-Roth D, Tetin-Schneider S, Hildesheimer M, Muchnik C 2004. Reduced auditory efferent activity in childhood selective mutism. Biol. Psychiatry 55 (11) 1061–1068. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC 1990. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J. Comp. Neurol 295 (1) 52–70. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D 1996. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J. Neurosci 16 (8) 2574–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boero LE, Castagna VC, Di Guilmi MN, Goutman JD, Elgoyhen AB, Gomez-Casati ME 2018. Enhancement of the Medial Olivocochlear System Prevents Hidden Hearing Loss. J. Neurosci 38 (34) 7440–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boero LE, Castagna VC, Terreros G, Moglie MJ, Silva S, Maass JC, Fuchs PA, Delano PH, Elgoyhen AB, Gomez-Casati ME 2020. Preventing presbycusis in mice with enhanced medial olivocochlear feedback. Proc. Natl. Acad. Sci. U. S. A 117 (21) 11811–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolay H, Bayazit YA, Gunduz B, Ugur AK, Akcali D, Altunyay S, Ilica S, Babacan A 2008. Subclinical dysfunction of cochlea and cochlear efferents in migraine: an otoacoustic emission study. Cephalalgia 28 (4) 309–317. [DOI] [PubMed] [Google Scholar]
- Bonfils P, Remond MC, & Pujol R 1986. Efferent tracts and cochlear frequency selectivity. Hear. Res 24(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Boothalingam S, Allan C, Allen P, Purcell DW 2019. The medial olivocochlear reflex Is unlikely to play a role in listening difficulties in children. Trends Hear. 23 2331216519870942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothalingam S, Kurke J, Dhar S 2018. Click-evoked auditory efferent activity: Rate and level effects. J. Assoc. Res. Otolaryngol 19 (4) 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M, Terreros G, Moreno-Gómez FN, Ipinza M, Vicencio S, Robles L, Delano PH 2020. The olivocochlear reflex strength in awake chinchillas is relevant for behavioural performance during visual selective attention with auditory distractors. Scientific Rep. 10 (1) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC 2011. Anatomy of olivocochlear neurons. In: Ryugo DK, Fay R, Popper AN (Eds) Auditory and Vestibular Efferents. Springer, pp. 17–37. [Google Scholar]
- Capps MJ, Ades HW 1968. Auditory frequency discrimination after transection of the olivocochlear bundle in squirrel monkeys. Exp. Neurol 21 (2) 147–158. [DOI] [PubMed] [Google Scholar]
- Ceranic BJ, Prasher DK, Raglan E, Luxon LM 1998. Tinnitus after head injury: evidence from otoacoustic emissions. J. Neurol. Neurosurg. Psychiatry 65 (4) 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LH, Wang CH, Lu RH, & Chen YF 2020. Evaluating the function of the medial olivocochlear bundle in patients with bilateral tinnitus. J. Speech, Lang., Hear. Res, 1–10. [DOI] [PubMed] [Google Scholar]
- Chery-Croze S, Collet L, Morgon A 1993. Medial olivo-cochlear system and tinnitus. Acta Otolaryngol. 113 (3) 285–290. [DOI] [PubMed] [Google Scholar]
- Chery-Croze S, Moulin A, Collet L, Morgon A 1994. Is the test of medial efferent system function a relevant investigation in tinnitus? Br. J. Audiol 28 (1) 13–25. [DOI] [PubMed] [Google Scholar]
- Ciuman RR 2010. The efferent system or olivocochlear function bundle-fine regulator and protector of hearing perception. International journal of biomedical science: IJBS 6 (4) 276. [PMC free article] [PubMed] [Google Scholar]
- Clarke EM, Ahmmed A, Parker D, Adams C 2006. Contralateral suppression of otoacoustic emissions in children with specific language impairment. Ear Hear. 27 (2) 153–160. [DOI] [PubMed] [Google Scholar]
- Clause A, Lauer AM, Kandler K 2017. Mice lacking the alpha9 subunit of the nicotinic acetylcholine receptor exhibit deficits in frequency difference limens and sound localization. Front. Cell. Neurosci 11 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A, Ceranic B, Prasher D, Miller DH, Luxon LM 2007. Auditory efferent function is affected in multiple sclerosis. Ear Hear. 28 (5) 593–604. [DOI] [PubMed] [Google Scholar]
- Collet L, Roge B, Descouens D, Moron P, Duverdy F, Urgell H 1993. Objective auditory dysfunction in infantile autism. Lancet 342 (8876) 923–924. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ Jr 2006. Efferent-mediated control of basilar membrane motion. J. Physiol 576 (1)49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN 1997. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J. Neurosci 17 (6) 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh AA, Kaf WA 2012. DPOAEs and contralateral acoustic stimulation and their link to sound hypersensitivity in children with autism. Int. J. Audiol 51 (4) 345–352. [DOI] [PubMed] [Google Scholar]
- Danesh AA, Lang D, Kaf W, Andreassen WD, Scott J, Eshraghi AA 2015. Tinnitus and hyperacusis in autism spectrum disorders with emphasis on high functioning individuals diagnosed with Asperger’s Syndrome. Int. J. Pediatr. Otorhinolaryngol 79 (10) 1683–1688. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC 2006a. Cochlear efferent feedback balances interaural sensitivity. Nat. Neurosci 9 (12) 1474–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, & Liberman MC 2006b. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J. Comp. Neurol, 498(3), 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser K, De Letter M, De Groote E, Santens P, Talsma D, Botteldooren D, Bockstael A 2019. Systematic audiological assessment of auditory functioning in patients with Parkinson’s Disease. J. Speech Lang. Hear. Res 62 (12) 4564–4577. [DOI] [PubMed] [Google Scholar]
- Deeter R, Abel R, Calandruccio L, Dhar S 2009. Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J. Acoust. Soc. Am 126 (5) 2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L 2007. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J. Neurosci 27 (15) 4146–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson JH, Bund EO 1968. sone relationships to stimulus discrimination in noise. J. Neurophysiol 31 (1) 122–130. [DOI] [PubMed] [Google Scholar]
- Dolan DF, Nuttall AL 1988. Masked cochlear whole-nerve response intensity functions altered by electrical stimulation of the crossed olivocochlear bundle. J. Acoust. Soc. Am 83 (3) 1081–1086. [DOI] [PubMed] [Google Scholar]
- Dragicevic CD, Marcenaro B, Navarrete M, Robles L, Delano PH 2019. Oscillatory infrasonic modulation of the cochlear amplifier by selective attention. PLoS One 14 (1) e0208939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic CD, Aedo C, León A, Bowen M, Jara N, Terreros G, Robles L, Delano PH 2015. The olivocochlear reflex strength and cochlear sensitivity are independently modulated by auditory cortex microstimulation. J. Assoc. Res. .Otolaryngol 16 (2) 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Katz E 2012. The efferent medial olivocochlear-hair cell synapse. J. Physiol 106 (1-2) 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgueda D, Delano PH, Robles L 2011. Effects of electrical stimulation of olivocochlear fibers in cochlear potentials in the chinchilla. J.Assoc. Res. Otolaryngol 12 (3) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P, Mammano F 2000. Cholinergic control of membrane conductance and intracellular free Ca2 in outer hair cells of the guinea pig cochlea. Cell Calcium 28 (3) 195–203. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Charachon G, Renard N 1993. Dopaminergic lateral efferent innervation of the guinea-pig cochlea: immunoelectron microscopy of catecholamine-synthesizing enzymes and effect of 6-hydroxydopamine. Neurosci. 54 (1) 133–142. [DOI] [PubMed] [Google Scholar]
- Fex J 1959. Augmentation of cochlear microphonic by stimulation of efferent fibres to the cochlea; preliminary report. Acta Otolaryngol. 50 540. [DOI] [PubMed] [Google Scholar]
- Froehlich P, Collet L, Chanal JM, Morgon A 1990a. Variability of the influence of a visual task on the active micromechanical properties of the cochlea. Brain Res. 508 (2) 286–288. [DOI] [PubMed] [Google Scholar]
- Froehlich P, Collet L, Morgon A 1990b. Effect of attention on audition. Contribution of the study of the olivocochlear efferent system. Ann. Otolaryngol. Chir. Cervicofac 107 (8) 519–520. [PubMed] [Google Scholar]
- Froehlich P, Collet L, Morgon A 1993. Transiently evoked otoacoustic emission amplitudes change with changes of directed attention. Physiol. Behav 53 (4) 679–682. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Lauer AM 2018. Efferent inhibition of the cochlea. Cold Spring Harbor perspectives in medicine a033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R 1956. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J. Neurophysiol 19 (5) 424–437. [DOI] [PubMed] [Google Scholar]
- Geisler CD 1974. Hypothesis on the function of the crossed olivocochlear bundle. J. Acoust. Soc. Am 56 (6) 1908–1909. [DOI] [PubMed] [Google Scholar]
- Geven LI, Koppl C, de Kleine E, van Dijk P 2014. Plasticity in tinnitus patients: a role for the efferent auditory system? Otol. Neurotol 35 (5) 796–802. [DOI] [PubMed] [Google Scholar]
- Guinan JJ Jr, Gifford ML. 1988. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hear Res.33(2):97–113. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Collet L, Chery-Croze S, Magnan J, Chays A 1995. Evidence of a medial olivocochlear involvement in contralateral suppression of otoacoustic emissions in humans. Brain Res. 705 (1-2) 15–23. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Garnier S, Micheyl C, Lina G, Chays A, Chéry-Croze S 1997. Auditory efferents involved in speech-in-noise intelligibility. Neuroreport 8 (7) 1779–1783. [DOI] [PubMed] [Google Scholar]
- Goldberg RL, Henson OW 1998. Changes in cochlear mechanics during vocalization: evidence for a phasic medial efferent effect. Hear. Res 122 (1-2) 71–81. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Farber N, Raveh E, Apter A, Attias J 2006. Hyperacusis in Williams syndrome: characteristics and associated neuroaudiologic abnormalities. Neurol 66 (3) 390–395. [DOI] [PubMed] [Google Scholar]
- Guinan JJ Jr 2018. Olivocochlear efferents: Their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear. Res 362 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ Jr, Gifford ML 1988. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and thresholds at CF. Hear. Res 37 (1) 29–45. [DOI] [PubMed] [Google Scholar]
- Guinan JJ 2014. Olivocochlear efferent function: issues regarding methods and the interpretation of results. Front. Syst. Neurosci 8 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ 1996. Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fa RR. The Cochlea. Springer, pp. 435–502. [Google Scholar]
- Hage SR, Jurgens U, Ehret G 2006. Audio-vocal interaction in the pontine brainstem during self-initiated vocalization in the squirrel monkey. Eur. J. Neurosci 23 (12) 3297–3308. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Youssef AH, Elattar AM 2012. Assessment of cochlear and auditory pathways in patients with migraine. Am. J. Otolaryngol 33 (4) 385–394. [DOI] [PubMed] [Google Scholar]
- Hernandez-Peon R, Scherrer H, Jouvet M 1956. Modification of electric activity in cochlear nucleus during attention in unanesthetized cats. Science 1 (956) 123. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Stiles P, May BJ 1998. Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear. Res 116 (1-2) 10–20. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Alford BR, Nakai Y, Gordon W. 1972. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat: I. pure-tone threshold and perceptual signal-to-noise ratio. Acta Otolaryngol. 73 (2-6) 455–466. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Alford BR, Gordon WP, & Nakai Y 1974. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. Acta oto-laryngol. 77(1-6), 311–317. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Cranford JL, Allen EA, Alford BR 1979. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat V. Pure-tone intensity discrimination. Acta Otolaryngol. 87 (3-6) 429–433. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Cranford JL, Nakai Y, Alford BR 1979. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat IV. study on pure-tone frequency discrimination. Acta Otolaryngol. 87 (1-2) 79–83. [DOI] [PubMed] [Google Scholar]
- Irving S, Moore DR, Liberman MC, Sumner CJ 2011. Olivocochlear efferent control in sound localization and experience-dependent learning. J. Neurosci 31 (7) 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs PG, Konrad-Martin D, McMillan GP, McDermott D, Fausti SA, Kagen D, Wan EA 2012. Influence of acute hyperglycemia on otoacoustic emissions and the medial olivocochlear reflex. J. Acoust. Soc. Am 131 (2) 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng JY, Johnson SL, Carlton AJ, De Tomasi L, Goodyear RJ, De Faveri F, Furness DN, Wells S, Brown SDM, Holley MC, Richardson GP, Mustapha M,Bowl MR, Marcotti W 2020. Age-related changes in the biophysical and morphological characteristics of mouse cochlear outer hair cells. J. Physiol 598(18), 3891–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas JH. Human auditory attention: the olivocochlear bundle may function as a peripheral filter. 1980. Psychophysiology. 17(5):444–52. [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC 1993. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J. Neurophysiol 70 (6) 2533–2549. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC 1993. Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones. J. Neurophysiol 70 (6) 2519–2532. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien JL, Barthelemy C, Collet L 2001. Peripheral auditory asymmetry in infantile autism. Eur. J. Neurosci 13 (3) 628–632. [DOI] [PubMed] [Google Scholar]
- Kitahara T 2018. Evidence of surgical treatments for intractable Meniere’s disease. Auris Nasus Larynx 45 (3) 393–398. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Shera CA, Melcher JR 2014. Increased contralateral suppression of otoacoustic emissions indicates a hyperresponsive medial olivocochlear system in humans with tinnitus and hyperacusis. J. Neurophysiol 112 (12) 3197–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrina A, Schrode KM, Screven LA, Javaid H, Weinberg MM, Brown G, Board R, Villavisanis DF, Dent ML, Lauer AM 2020. Linking anatomical and physiological markers of auditory system degeneration with behavioral hearing assessments in a mouse (Mus musculus) model of age-related hearing loss. Neurobiol. Aging 96 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Illing RB 2004. Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J. Comp. Neurol 475 (3) 374–390. [DOI] [PubMed] [Google Scholar]
- Kubo T, Doi K, Koizuka I, Takeda N, Sugiyama N, Yamada K, Kohmura E, Hayakawa T 1995. Assessment of auditory and vestibular functions after vestibular neurectomy for Meniere’s disease. Acta Otolaryngol. 115 (2) 149–153. [DOI] [PubMed] [Google Scholar]
- Labrousse M, Cucherousset J, Avisse C, Delattre JF, Chays A 2004. Anatomohistologic study of von Oorťs vestibulocochlear anastomosis. Ann. Otolaryngol. Chir. Cervicofac 121 (4) 205–212. [DOI] [PubMed] [Google Scholar]
- Larsen E, Liberman MC 2010. Contralateral cochlear effects of ipsilateral damage: no evidence for interaural coupling. Hear. Res 260 (1-2) 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Dent ML, Sun W, Xu-Friedman MA 2019. Effects of Non-traumatic Noise and Conductive Hearing Loss on Auditory System Function. Neurosci. 407 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Fuchs PA, Ryugo DK, Francis HW 2012. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol. Aging 33 (12) 2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, May BJ 2011. The medial olivocochlear system attenuates the developmental impact of early noise exposure. J. Assoc. Res. Otolaryngol 12 (3) 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Liberman MC 2019. Cochlear efferent innervation is sparse in humans and decreases with age. J. Neurosci 39 (48) 9560–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Brown MC 1986. Physiology and anatomy of single olivocochlear neurons in the cat. Hear. Res 24 (1) 17–36. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Guinan JJ 1998. Feedback control of the auditory periphery: anti-masking effects of middle ear muscles vs. olivocochlear efferents. J. Commun. Disord 31 (6) 471–82. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF 2015. Chronic conductive hearing loss leads to cochlear degeneration. PLoS One 10 (11) e0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC 1989a. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear. Res 38 (1–2) 47–56. [DOI] [PubMed] [Google Scholar]
- Liberman MC 1989b. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear. Res 38 (1–2) 47–56. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA 2018. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front. Neurol 9 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martín A, Fumero MJ, Stohl JS, Schatzer R, Nopp P, … & Wilson BS. 2019. Lateralization of virtual sound sources with a binaural cochlear-implant sound coding strategy inspired by the medial olivocochlear reflex. Hear. Res, 379, 103–116. [DOI] [PubMed] [Google Scholar]
- Lichtenhan JT, Wilson US, Hancock KE, & Guinan JJ Jr (2016). Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses. Hear. Res 333 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas JH 1981. The role of efferent inhibition in human auditory attention: an examination of the auditory brainstem potentials. Int. J. Neurosci 12 (2) 137–145. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC 2000. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J. Neurosci 20 (12) 4701–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. 2003. Olivocochlear innervation in the mouse: immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol. 455(3):406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Ryugo DK 2011. Descending connections of auditory cortex to the midbrain and brain stem. In: Weiner JA, Schreiner CE. The Auditory Cortex. Springer, pp. 189–208. [Google Scholar]
- May BJ, Budelis J, Niparko JK 2004. Behavioral studies of the olivocochlear efferent system: learning to listen in noise. Arch. Otolaryngol. Head. Neck. Surg 130 (5) 660–664. [DOI] [PubMed] [Google Scholar]
- May BJ, Prosen CA, Weiss D, Vetter D 2002. Behavioral investigation of some possible effects of the central olivocochlear pathways in transgenic mice. Hear. Res 171 (1-2) 142–157. [DOI] [PubMed] [Google Scholar]
- Mertes IB, Johnson KM, Dinger ZA 2019. Olivocochlear efferent contributions to speech-in-noise recognition across signal-to-noise ratios. J. Acoust. Soc. Am 145 (3) 1529–1540. [DOI] [PubMed] [Google Scholar]
- Mertes IB, Wilbanks EC, Leek MR 2018. Olivocochlear efferent activity is associated with the slope of the psychometric function of speech recognition in noise. Ear Hear. 39 (3) 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Perrot X, Collet L 1997. Relationship between auditory intensity discrimination in noise and olivocochlear efferent system activity in humans. Behav. Neurosci 111 (4) 801. [DOI] [PubMed] [Google Scholar]
- Michie PT, LePage EL, Solowij N, Haller M, Terry L 1996. Evoked otoacoustic emissions and auditory selective attention. Hear. Res 98 (1-2) 54–67. [DOI] [PubMed] [Google Scholar]
- Mishra SK 2014. Medial efferent mechanisms in children with auditory processing disorders. Front. Hum. Neurosci 8 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK 2020. The role of efferents in human auditory development: efferent inhibition predicts frequency discrimination in noise for children. J. Neurophysiol 123 (6) 2437–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Lutman ME 2014. Top-down influences of the medial olivocochlear efferent system in speech perception in noise. PLoS One 9 (1) e85756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Sieswerda SL, Grainger MM, Bowling A, Smith N, Perdew A, Eichert S, Alston S, Hilbert LW, Summers L, Lin L, Hunter LL 2018. Referral and diagnosis of developmental auditory processing disorder in a large, United States hospital-based audiology service. J. Am. Acad. Audiol 29 (5) 364–377. [DOI] [PubMed] [Google Scholar]
- Muchnik C, Ari-Even Roth D, Hildesheimer M, Arie M, Bar-Haim Y, Henkin Y 2013. Abnormalities in auditory efferent activities in children with selective mutism. Audiol. Neurootol 18 (6) 353–361. [DOI] [PubMed] [Google Scholar]
- Muchnik C, Ari-Even Roth D, Othman-Jebara R, Putter-Katz H, Shabtai EL, Hildesheimer M 2004. Reduced medial olivocochlear bundle system function in children with auditory processing disorders. Audiol. Neurootol 9 (2) 107–114. [DOI] [PubMed] [Google Scholar]
- Murdin L, Premachandra P, Davies R 2010. Sensory dysmodulation in vestibular migraine: an otoacoustic emission suppression study. Laryngoscope 120 (8) 1632–1636. [DOI] [PubMed] [Google Scholar]
- Nieder P, Nieder I. 1970. Stimulation of efferent olivocochlear bundle causes release from low level masking. Nature 227(5254):184–5. [DOI] [PubMed] [Google Scholar]
- Oatman LC 1971. Role of visual attention on auditory evoked potentials in unanesthetized cats. Exp. Neurol 32 (3) 341–356. [DOI] [PubMed] [Google Scholar]
- Oatman LC 1976. Effects of visual attention on the intensity of auditory evoked potentials. Exp. Neurol 51 (1) 41–53. [DOI] [PubMed] [Google Scholar]
- Oatman LC, Anderson BW 1977. Effects of visual attention on tone burst evoked auditory potentials. Exp. Neurol 57 (1) 200–211. [DOI] [PubMed] [Google Scholar]
- Oatman LC, & Anderson BW 1980. Suppression of the auditory frequency following response during visual attention. Electroencephalog. Clinical Neurophysiol 49 (3-4), 314–322. [DOI] [PubMed] [Google Scholar]
- Oatman LC 1982. Spectral analysis of cortical EEG activity during visual attention. Physiol. Psychol, 10(3), 336–342. [Google Scholar]
- Pisani V, Sisto R, Moleti A, Di Mauro R, Pisani A, Brusa L, Altavista MC, Stanzione P, Di Girolamo S 2015. An investigation of hearing impairment in de-novo Parkinson’s disease patients: A preliminary study. Parkinsonism Relat. Disord 21 (8) 987–991. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Bath KG, Vetter DE, and May BM (2000). Behavioral assessments of auditory sensitivity in transgenic mice. J. Neurosci. Meth 97 (1) 59–67. [DOI] [PubMed] [Google Scholar]
- Puel J, Bonfils P, Pujol R 1988. Selective attention modifies the active micromechanical properties of the cochlea. Brain Res. 447 (2) 380–383. [DOI] [PubMed] [Google Scholar]
- Puria S, Guinan JJ Jr, Liberman MC 1996. Olivocochlear reflex assays: effects of contralateral sound on compound action potentials versus ear-canal distortion products. J. Acoust. Soc. Am 99 (1) 500–507. [DOI] [PubMed] [Google Scholar]
- Radtke-Schuller S, Seeler S, Grothe B 2015. Restricted loss of olivocochlear but not vestibular efferent neurons in the senescent gerbil (Meriones unguiculatus). Front. Aging Neurosci 7 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen GL 1946. The olivary peduncle and other fiber projections of the superior olivary complex. J. Comp. Neurol 84 (2) 141–219. [DOI] [PubMed] [Google Scholar]
- Reijntjes DO, Pyott SJ 2016. The afferent signaling complex: regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear. Res 336 1–16. [DOI] [PubMed] [Google Scholar]
- Riga M, Katotomichelakis M, Danielides V 2015. The potential role of the medial olivocochlear bundle in the generation of tinnitus: controversies and weaknesses in the existing clinical studies. Otol. Neurotol 36 (2) 201–208. [DOI] [PubMed] [Google Scholar]
- Rosenberg SI, Silverstein H, Hoffer ME, Thaler E 1996. Hearing results after posterior fossa vestibular neurectomy. Otolaryngol. Head Neck Surg 114 (1) 32–37. [DOI] [PubMed] [Google Scholar]
- Roverud E, Strickland EA 2015. The effects of ipsilateral, contralateral, and bilateral broadband noise on the mid-level hump in intensity discrimination. J. Acoust. Soc. Am 138 (5) 3245–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches SG, Carvallo RM 2006. Contralateral suppression of transient evoked otoacoustic emissions in children with auditory processing disorder. Audiol. Neurootol 11 (6) 366–372. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Chays A 1997. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear. Res 103 (1-2) 101–122. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Collet L, Ulmer E, Chays A 1994. On the role of the olivocochlear bundle in hearing: a case study. Hear. Res 75 (1-2) 11–26. [DOI] [PubMed] [Google Scholar]
- Schofield BR 2010. Structural organization of the descending auditory pathway. In: (palmer AR, Rees A (Eds.) The Auditory Brain 43–64. [Google Scholar]
- Seluakumaran K, Mulders WH, Robertson D 2008. Unmasking effects of olivocochlear efferent activation on responses of inferior colliculus neurons. Hear. Res 243 (1-2) 35–46. [DOI] [PubMed] [Google Scholar]
- Sheppard A, Stocking C, Ralli M, Salvi R 2020. A review of auditory gain, low-level noise and sound therapy for tinnitus and hyperacusis. Int. J. Audiol 59 (1) 5–15. [DOI] [PubMed] [Google Scholar]
- Smalt CJ, Heinz MG, Strickland EA 2014. Modeling the time-varying and level-dependent effects of the medial olivocochlear reflex in auditory nerve responses. J. Assoc. Res. Otolaryngol 15 (2) 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart JL, Kuruvilla-Mathew A, Kelly AS, Purdy SC 2019. Assessment of the efferent auditory system in children with suspected auditory processing disorder: the Middle ear muscle reflex and contralateral inhibition of OAEs. Int. J. Audiol 58 (1) 37–44. [DOI] [PubMed] [Google Scholar]
- Smith SB, Lichtenhan JT, & Cone BK (2017). Contralateral inhibition of click-and chirp-evoked human compound action potentials. Front. Neurosci, 11, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Keil A, Stratis K, Woodruff Carr KL, Smith DW. 2012. Effects of cross-modal selective attention on the sensory periphery: cochlear sensitivity is altered by selective attention. Neurosci. 223 325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli A C GF, Zanchetta S, Furtado EF. 2020. Auditory hyper-responsiveness in autism spectrum disorder, terminologies and physiological mechanisms involved: systematic review. Codas 32 (3) e20180287–1782/20192018287. eCollection 2020. [DOI] [PubMed] [Google Scholar]
- Suthakar K, Ryugo DK 2017. Descending projections from the inferior colliculus to medial olivocochlear efferents: Mice with normal hearing, early onset hearing loss, and congenital deafness. Hear. Res 343 34–49. [DOI] [PubMed] [Google Scholar]
- Terreros G, Jorratt P, Aedo C, Elgoyhen AB, Delano PH 2016. Selective Attention to Visual Stimuli Using Auditory Distractors Is Altered in Alpha-9 Nicotinic Receptor Subunit Knock-Out Mice. J. Neurosci 36 (27) 7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terreros G, Delano PH 2015. Corticofugal modulation of peripheral auditory responses. Front. Sys. Neurosci 9 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC 1991. Posteroventral cochlear nucleus projections to olivocochlear neurons. J. Comp. Neurol 303 (2) 267–285. [DOI] [PubMed] [Google Scholar]
- Tian GY, Xu DC, Huang DL, Liao H, Huang MX 2008. The topographical relationships and anastomosis of the nerves in the human internal auditory canal. Surg. Radiol. Anat 30 (3) 243–247. [DOI] [PubMed] [Google Scholar]
- Trahiotis C, Elliott DN 1970. Behavioral investigation of some possible effects of sectioning the crossed olivocochlear bundle. J. Acoust. Soc. Am 47 (2B) 592–596. [DOI] [PubMed] [Google Scholar]
- Verschooten E, Strickland EA, Verhaert N, Joris PX 2017. Assessment of ipsilateral efferent effects in human via ECochG. Front. Neurosci 11 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB 1999. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23 (1) 93–103. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Mugnaini E 1992. Distribution and dendritic features of three groups of rat olivocochlear neurons. A study with two retrograde cholera toxin tracers. Anat. Embryol 185 (1) 1–16. [DOI] [PubMed] [Google Scholar]
- Veuillet E, Georgieff N, Philibert B, Dalery J, Marie-Cardine M, Collet L 2001. Abnormal peripheral auditory asymmetry in schizophrenia. J. Neurol. Neurosurg. Psychiatry 70 (1) 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Frey K, Heppelmann G, Plontke SK, Zenner HP 2008. Speech-in-noise intelligibility does not correlate with efferent olivocochlear reflex in humans with normal hearing. Acta Otolaryngol. 128 (1) 53–60. [DOI] [PubMed] [Google Scholar]
- Wagner W, Heppelmann G, Muller J, Janssen T, Zenner HP 2007. Olivocochlear reflex effect on human distortion product otoacoustic emissions is largest at frequencies with distinct fine structure dips. Hear. Res 223 (1-2) 83–92. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ Jr 1979. Efferent innervation of the organ of Corti: two separate systems. Brain Res. 173 (1) 152–155. [DOI] [PubMed] [Google Scholar]
- Warren EH, Liberman MC 1989. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear. Res 37 (2) 89–104. [DOI] [PubMed] [Google Scholar]
- Williams EA, Brookes GB, Prasher DK 1994. Effects of olivocochlear bundle section on otoacoustic emissions in humans: efferent effects in comparison with control subjects. Acta Otolaryngol. 114 (2) 121–129. [DOI] [PubMed] [Google Scholar]
- Wilson US, Sadler KM, Hancock KE, Guinan JJ, Lichtenhan JT 2017a. Efferent inhibition strength is a physiological correlate of hyperacusis in children with autism spectrum disorder. J. Neurophysiol 118 (2) 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson US, Sadler KM, Hancock KE, Guinan JJ, Lichtenhan JT 2017b. Efferent inhibition strength is a physiological correlate of hyperacusis in children with autism spectrum disorder. J. Neurophysiol 118 (2) 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB 1988. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear. Res 35 (2–3) 165–189. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB 1987. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J. Neurophysiol 57 (4) 1002–1021. [DOI] [PubMed] [Google Scholar]
- Wojtczak M, Klang AM, Torunsky NT 2019. Exploring the Role of Medial Olivocochlear Efferents on the Detection of Amplitude Modulation for Tones Presented in Noise. Journal of the Association for Research in Otolaryngology 20 (4) 395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HP, Guo YL, Cheng TJ, Hsu CJ 2010. Chronological changes in compromised olivocochlear activity and the effect of insulin in diabetic Wistar rats. Hear. Res 270 (1-2) 173–178. [DOI] [PubMed] [Google Scholar]
- Wu JS, Yi E, Manca M, Javaid H, Lauer AM, Glowatzki E 2020. Sound exposure dynamically induces dopamine synthesis in cholinergic LOC efferents for feedback to auditory nerve fibers. Elife 9 10.7554/eLife.52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Yi E, Manca M, Javaid H, Lauer AM, Glowatzki E 2020. Sound exposure dynamically induces dopamine synthesis in cholinergic LOC efferents for feedback to auditory nerve fibers. Elife 9 e52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary SP, Fuchs PA 2015. Re-Emergent Inhibition of Cochlear Inner Hair Cells in a Mouse Model of Hearing Loss. J. Neurosci 35 (26) 9701–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Martino KM, Linthicum FH, Soli SD 2000. Auditory perception in vestibular neurectomy subjects. Hear. Res 142 (1–2) 102–112. [DOI] [PubMed] [Google Scholar]
- Zhao W, Dewey JB, Boothalingam S, Dhar S 2015. Efferent Modulation of Stimulus Frequency Otoacoustic Emission Fine Structure. Front. Syst. Neurosci 9 168. [DOI] [PMC free article] [PubMed] [Google Scholar]


