Abstract
Background:
Exposure to environmental metals can cause nephrotoxicity. There is an international epidemic of chronic kidney disease of unknown cause (CKDu). Whether metal exposures contribute to kidney dysfunction in populations at-risk for CKDu remains unresolved.
Objective:
Urinary metals (arsenic, cadmium, nickel, and uranium) were examined in 222 sugarcane cutters in Guatemala at three time points over one year.
Methods:
We explored the relationships between metal concentrations and markers of kidney function using multivariable linear mixed-effect models.
Results:
Arsenic, cadmium, and nickel were detected in the majority of the 340 urine samples and were generally within limits previously considered to be non-nephrotoxic. Nevertheless, higher urine cadmium was inversely associated with estimated glomerular filtration rate (eGFR) (β: −4.23, 95% confidence interval [CI]: −6.92, −1.54) and positively associated with neutrophil gelatinase-associated lipocalin (NGAL) (β: 2.92, 95% CI: 1.20, 4.64). Higher urine arsenic was also inversely associated with eGFR (β: −4.36, 95% CI: −7.07, −1.64).
Significance:
Our findings suggest that exposures to metals, including cadmium and arsenic, might contribute to kidney toxicity seen in workers at risk for CKDu. These findings are consistent with the potential for metal nephrotoxicity at lower than expected levels in the setting of manual work in a very hot environment.
Keywords: metal exposure, kidney function, agricultural workers
Introduction:
A severe epidemic of kidney disease, called chronic kidney disease of unknown etiology (CKDu) emerged in Central America decades ago (1). The classic presentation of CKDu (also coined Mesoamerican Nephropathy in Central America) has been found mainly among young, otherwise healthy male agricultural workers. The etiology for CKDu remains unclear as it is not explained by traditional risk factors such as age, diabetes, and hypertension (1). Environmental exposures (including metals), possibly in concert with recurrent heat stress and dehydration, are hypothesized to play a role in the development of CKDu (1-3). Metal-induced renal toxicity can be characterized by proximal tubular dysfunction which may result in tubulointerstitial disease (4, 5), a key characteristic with CKDu (1). Several nephrotoxic metals (e.g., arsenic, cadmium, uranium, lead, mercury, and nickel) are commonly found in affected agricultural communities in the region due to anthropogenic activity or naturally from soil or bedrock (6). As of yet, evidence for metal exposures as a contributing factor has been weak and has not explained CKDu epidemics in Central America (1, 7).
Metals have been considered as a possible cause of CKDu in Sri Lanka (8-10) and there have been several studies examining the possible relationship between metal exposures and CKDu in that region (10-13). However, in Central America, there is very limited quantitative biomarker data on metals. The few studies that have examined the relationship between exposure to nephrotoxic metals and acute or chronic renal effects have yielded varying results (14-18). To our knowledge there have been a total of four studies conducted in at-risk CKDu populations in Latin America that have measured biomarkers of metal concentrations and examined their relationship with kidney dysfunction, with three studies conducted in Nicaragua (15-17) and one study conducted in Mexico (14). In one Nicaraguan study, among 99 sugarcane workers, the researchers observed some evidence that workers with the highest urinary arsenic exposure had significantly worse kidney function and found no evidence that urinary lead, cadmium, or uranium were associated with biomarkers of kidney function (15). However in a case-control study in a Nicaraguan community cohort, researchers observed no differences in 12 urinary metal concentrations between a stable kidney function group and a declining kidney function group using Student’s t-test at two separate times (before the harvest [n=263] and at the end of the harvest [n=213] (16). In contrast, Fischer et al. (2020) examined toenail clippings in a recent case-control study from 18 Nicaraguan renal disease patients with an acute form of kidney injury, researchers detected elevated nickel concentrations relative to 36 healthy controls and nickel concentrations were correlated with serum creatinine (17). The study conducted in Mexico measured arsenic, cadmium, mercury and lead concentrations in 100 hair samples (50 CKDu cases and 50 controls) and observed a significant difference between cases and controls in arsenic concentrations (14). Due to conflicting findings and variations in exposure assessment from these studies, further measurement of exposure is needed in order to explore relationships between exposures and kidney parameters in agriculture populations at-risk for CKDu.
Our goals were to measure exposure to nephrotoxic metals, specifically arsenic, cadmium, uranium, and nickel, and to evaluate the potential associations between metals and markers of nephrotoxicity in a cohort of Guatemalan sugarcane workers who have been shown to be at-risk for CKDu. We conducted a 9-month study, spanning the harvest and post-harvest season. We concurrently measured three kidney parameters - estimated glomerular filtration rate (eGFR), urine albumin and neutrophil gelatinase-associated lipocalin (NGAL) - and urinary concentrations of the metals of interest at three time points.
Methods:
a. Study Design
This was a repeated cross-sectional analysis examining the association between metal exposure and markers of kidney function. Data were collected at three time points during the period from November 2017 to July 2018. The study population included 222 sugarcane cutters from a sugarcane plantation in southwest Guatemala. At the start of the 6-month harvest and study (November), we recruited and collected data from four work groups using stratified random sampling based on home of residence. Two groups comprised of local workers and two groups comprised of workers who live in the highland regions during the off-season, see Figure 1. All participants were male sugarcane cutters who: (1) were 18 years of age or older; (2) were employed at the sugarcane agribusiness during the 2017-2018 harvest; (3) were free of known kidney dysfunction at the start of the study based on an eGFR > 90 ml/min/1.73 m2; and (4) provided urine and capillary blood samples at least once out of the three sampling occasions.
Figure 1:
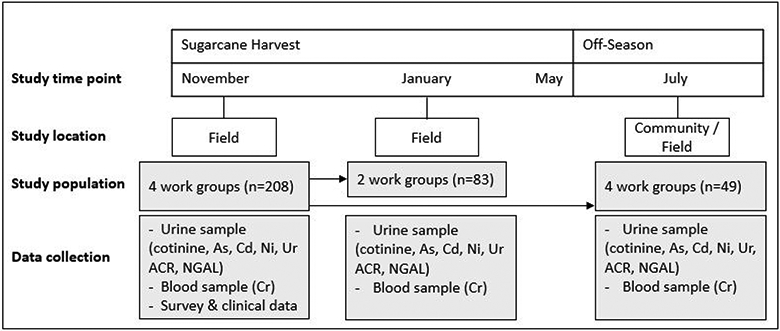
Study timeline and design. Abbreviations: As: arsenic; Cd: Cadmium; Ni: Nickel; NGAL: urine neutrophil gelatinase-associated lipocalin; ACR: urine albumin-to-creatinine ratio; Cr: capillary blood creatinine.
For each study participant, urine and capillary blood samples were collected concurrently up to three time points depending on their work group. In November, data were collected from all four work groups in the sugarcane fields (samples were collected in the morning with two groups and in the afternoon with two groups). In January, data were collected from only two of the work groups (one local group and one highland group) in the morning in the fields. In July, the off-season, data were collected from a convenience sample of 49 of the study participants from all four groups either in their communities or in fields throughout the day (half in the morning between 9:00 and 12:00 and half in the afternoon between 12:00 and 17:00). Work setting, worker population, and work practice details have been previously described (19, 20). Participants provided written informed consent at the time of recruitment, and IRB approval for this study was obtained from the Institutional Review Board of the University of Colorado and Comité de Ética Independiente ZUGUEME in Guatemala.
b. Metal Exposure Assessment
We analyzed urine samples from the three study time points for total arsenic, cadmium, uranium, and nickel. Spot urine samples were collected and placed on ice and transported to an on-site clinic where the urine was aliquoted into Fisherbrand sterile polypropylene tubes without preservatives and frozen at −20°C. The urine aliquots for metal analyses were shipped to Columbia University. Urine samples were analyzed for arsenic, cadmium, uranium, and nickel using a PerkinElmer NexION 350S ICP-MS equipped with an Autosampler SC-4 DX, manufactured by Elemental Scientific, highly automated, and set up as a FAST System for NexION measurements. ICP-MS-DRC method for metals in urine was developed according to published procedures (21, 22), CDC method (23), and with modifications suggested by PerkinElmer application laboratory. The detection limits were 0.07, 0.01, 0.01, and 0.04 μg/L for arsenic, cadmium, uranium, and nickel, respectively. Samples were analyzed in batches of 20 with a set of calibration blanks, sample preparation blanks, and commercially available reference urine sample quality controls from Institut de Sante Publique du Quebec, and from National Institute of Technology (NIST) with a broad range of metal concentrations.
c. Markers of Kidney Function
We measured three markers of kidney function including capillary blood creatinine in order to calculate eGFR as a marker of overall kidney function, urine albumin to serve as a marker of glomerular injury (24), and urinary NGAL as a marker of distal tubular injury (25). NGAL has been shown to be more sensitive than serum creatinine in detecting certain forms of kidney damage (26) and has been of particular interest in CKDu because of the tubulointerstitial pathology associated with this illness (1).
At all three time points, eGFR was calculated from capillary blood creatinine using the CKD-EPI equation (27). To measure creatinine, blood was collected by finger prick at the same time as urine collection and read instantly in the field with the point-of-care device Nova® Statscan (Stat Sensor Creatinine Meter, Nova Biomedical Corporation, Waltham, MA, USA), adjusting for time of sampling as previously published (10).
At two time points (November and January), urinary NGAL and urinary albumin were measured. NGAL was measured using Quantikine ELISA kits, human lipocalin-2/NGAL (R&D Systems, Minneapolis, MN, USA) with two-fold diluted urine. Urinary albumin was measured with florescence immunoassay (Boditech, I-Chroma). Urinary creatinine was measured at all three time points via kinetic alkaline picrate and urine sodium concentrations using an automatic biochemical analyzer (Roche Cobas Integra 400 Plus) with the ion-selective method.
d. Covariates
We analyzed urine samples for cotinine, a metabolite of nicotine, in November and January. Besides occupational exposures and diet, tobacco smoke is a potential source of metal exposure, specifically for cadmium and arsenic (28). Urine cotinine concentrations were determined using the Calbiotech Cotinine ELISA CO096D (Calbiotech, El Cajon, California). All samples were run in duplicate, per manufacturer’s recommendation. In November, we collected 1) interviewer-administered survey data about age, lifestyle factors, health history, and occupational history, 2) clinical data, which included body mass index (BMI) and blood pressure (at least 3 minutes of seated rest before the measurement), and 3) venous blood samples from each participant by a trained phlebotomist and subsequently analyzed for blood hemoglobin A1c (HbA1c).
A variable “time of collection” was created since samples were collected at varying times of day in November and July. Urine samples collected before 12:00 were categorized as morning samples and samples collected between 12:00 and 17:00 were categorized as afternoon samples. We compared urinary creatinine concentrations between time of collection and found that urine creatinine concentrations were significantly higher (more concentrated) in the morning samples in November but not July (p <0.01 and p=0.93, respectively) so we included time of collection in the multivariable models.
e. Statistical Analyses
Levels below the limit of detection (LOD) were entered as the LOD divided by the square root of two for each biomarker (29). We calculated medians and interquartile ranges (IQR, 25th percentile and 75th percentile) for urine total arsenic, cadmium, uranium, and nickel concentrations in micrograms analyte per liter urine (μg/L). Metal concentrations were skewed to the right, so they were transformed by the natural log to reduce the influence of outliers.
We calculated Spearman correlation coefficients to compare urine metal concentrations and markers of kidney function by combining all time points. We performed repeated measure analyses to analyze cross-sectional associations between metal concentrations and kidney function using linear mixed-effect (LME) models, estimating β and 95% confidence intervals (CI). We used a random intercept for each study subject to account for the repeated measurements on individual. We applied LME regression analyses for natural log-transformed arsenic, cadmium, and nickel concentrations individually as continuous exposures, as well as categorized as quartiles, with each outcome (eGFR, NGAL, and urine albumin). To account for urinary concentration, the models included urinary creatinine as an independent covariate as recommended by Barr et al. (2005) (30). Urine albumin was used as an outcome in lieu of albumin-to-creatinine ratio (ACR) since urine creatinine was included as a covariate in the model. The repeated measures include time points 1-3 for eGFR and time points 1-2 for NGAL and urine albumin. All models were adjusted for potential kidney disease risk factors including continuous variables: age, HbA1c, systolic blood pressure, BMI, “time of collection” (morning or afternoon), and time point (November, January, or July). Due to the high number of samples that were below the LOD for cotinine and the lack of correlations between cotinine and any metal concentrations or kidney parameters, we did not include cotinine as a covariate in the LME models. Based on the findings with the LME models, we examined an interaction term between urinary cadmium and arsenic with eGFR. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, North Carolina).
For a sensitivity analysis, the analyses were repeated after removing urine creatinine concentrations that were less than 30 mg/dL (n=101 of 332 total urine creatinine samples, 30%) as recommended by the World Health Organization (WHO) for valid urine samples in occupational monitoring (31). The WHO recommends that if a sample is too dilute (creatinine concentration < 30 mg/dL) or too concentrated (creatinine concentration > 300 mg/dL), another urine void should be collected (30, 31). None of the urine samples had a urine creatinine > 300 mg/dL. The rationale behind these guidelines is that urine samples with extremely low creatinine concentrations may be too dilute, resulting in falsely low measured levels of toxicants.
Results:
a. Study Population Characteristics
Urine samples and capillary blood creatinine were collected from 208 cane cutters in November, 83 cutters in January, and 49 cutters in July, totaling 340 urine and blood samples. November demographic and clinical data are shown in Table 1. Among the 222 participants, 51% had samples collected at one time point, 44% at samples collected at two time points, and 5% had samples at three time points. The mean age was 29 years (range 18-57). The average BMI was 23 mg/kg2, one person had diabetes (≥ 6.5% HbA1c), and 5 participants (2%) were hypertensive defined as having a systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥90 mmHg in November. Due to the study design, we had subsets of the study population in January and July. We examined possible differences in demographic and clinical variables related to participants at each time point including baseline age, BMI, HbA1c, systolic and diastolic blood pressure, home of residence, self-reported current smokers, and urine cotinine concentrations (Supplemental Table 1). We observed no significant differences in these variables with the study population across the time points except for diastolic blood pressure which was slightly lower in January compared to November and July (68 mmHg [SD: 8]; 71 mmHg [SD: 9]; and 73 mmHg [SD:8], respectively).
Table 1:
November demographics and clinical data for study sugarcane cutters, N=208.
| Variables | Mean (SD) |
|---|---|
| Age, years | 29 (8) |
| BMI, mg/kg2 | 23 (2) |
| HbA1c, % | 5.4 (0.3) |
| Systolic blood pressure, mmHg | 106 (10) |
| Diastolic blood pressure, mmHg | 71 (9) |
| N (%) | |
| Local home of residence (vs. highland) | 99 (48%) |
| Diabetic (≥ 6.5% HbA1c) | 1 (1%) |
| Hypertensive A | 5 (2%) |
| Self-reported current smoker (vs. former/never) | 25 (12%) |
| Current smoker (≥ 50 ng/mL cotinine concentration) | 69 (34%) |
BMI, body mass index. Values are mean (standard deviation) and frequency (percentage).
Defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥90 mmHg.
b. Metal Exposure Concentrations and Correlations
Urine metal concentrations are presented as μg/L (Table 2). Over the three time points, median arsenic concentrations were 6.88, 7.13 and 8.05 μg/L and were detected in 100% of the total 340 urine samples. Median cadmium concentrations were 0.14, 0.10, and 0.09 across the time points and were detected in 89% of the samples. Median nickel concentrations ranged from 1.4, 1.8, and 2.2 μg/L and were detected in 99% of the samples. Uranium was detected in only 10% of the urine samples. As such, we excluded uranium data from the tables and analyses.
Table 2:
| Biomarker | November (n=208) | January (n=83) | July (n=49) | |||
|---|---|---|---|---|---|---|
| Percent detected |
Median (IQR) | Percent detected |
Median (IQR) | Percent detected |
Median (IQR) | |
| Arsenic, μg/L | 208 (100%) | 6.88 (2.43, 12.07), GM: 5.32 (SE: 0.4) | 83 (100%) | 7.13 (4.18, 12.41), GM: 7.10 (SE: 0.7) | 49 (100%) | 8.05 (3.51, 17.63), GM: 8.10 (SE: 1.4) |
| Cadmium, μg/L | 177 (85%) | 0.14 (0.02, 0.26), GM: 0.09 (SE: 0.01) | 77 (93%) | 0.10 (0.05, 0.15), GM 0.08 (SE: 0.01) | 47 (96%) | 0.09 (0.05, 0.16), GM: 0.09 (SE: 0.02) |
| Nickel, μg/L | 204 (99%) | 1.42 (0.51, 2.44), GM: 1.13 (SE:0.09) | 83 (100%) | 1.84 (1.12, 2.95), GM: 1.62 (SE: 0.15) | 48 (98%) | 2.20 (0.88, 3.54), GM: 1.68 (SE: 0.31) |
| Urine creatinine, mg/dL | 208 (100%) | 75.40 (22.61, 129.20) | 83 (100%) | 61.30 (41, 103.70) | 49 (100%) | 22.0 (12.0, 68.0) |
| Urine cotinine, ng/mL | 94 (46%) | <LOD (<LOD, 109.31) | 35 (42%) | <LOD (<LOD, 90.31) | N/A | N/A |
| eGFR, mL/min/1.73 m2 | 208 (100%) | 119.84 (105.81, 129.16) | 83 (100%) | 125.40 (117.49, 133.88) | 49 (100%) | 100.38 (81.28, 125.71) |
| NGAL, ng/mL | 188 (93%) | 4.29 (1.76, 9.23) | 80 (96%) | 5.49 (3.23, 10.51) | N/A | N/A |
| Urine albumin, mg/L | 160 (80%) | 4.63 (2.17, 8.67) | 31 (38%) | <LOD (<LOD, 4.57) | N/A | N/A |
| ACR, μg/mg | N/A | 7.18 (3.59, 18.25) | N/A | 3.76 (2.38, 7.41) | N/A | N/A |
IQR: Interquartile range, 25th, 75th percentiles; GM: geometric mean; SE: standard error; eGFR: estimated glomerular filtration rate; NGAL: urine neutrophil gelatinase-associated lipocalin; ACR: urine albumin-to-creatinine ratio.
Normal urine metal values: arsenic: < 50 μg/L; cadmium: < 1 μg/L, and nickel: < 4 μg/L) (43).
Percentage of frequency of detection (%) for each compound measured above the limit of detection (LOD). LOD: 0.07, 0.01, 0.04, 0.2, 5.0, 0.04, 2.0 for arsenic, cadmium, nickel, creatinine, cotinine, NGAL, and albumin, respectively.
Levels <LOD imputed with LOD/sqrt (2).
We observed moderate to strong significant correlations between the metal concentrations when we combined all time points together (Supplemental Table 2). Metal correlation coefficients varied from 0.58 for arsenic and nickel to 0.76 for arsenic and cadmium. None of the metal concentrations were correlated with urine cotinine concentrations. In addition, self-reported smoking was not associated with the metal concentrations (cadmium: p=0.78; arsenic: p=0.09, and nickel: p=0.45, based on the Wilcoxon-Mann-Whitney test).
c. Kidney Function Outcomes
Median eGFR levels ranged from 100 to 125 mL/min/1.73 m2 across the three time points. We observed a 1% prevalence (n = 3) of moderately reduced kidney function (eGFR: 30–59 mL/min/1.73 m2) in November, 0% prevalence in January, and 13% prevalence (n=6) in July. The median urine albumin concentration was 4.6 mg/L in November, with a prevalence of moderately increased albuminuria (ACR: 30-300 mg/g creatinine) of 16% (n = 33) and with one person displaying severely increased albuminuria (ACR: >300 mg/g creatinine). The median urine NGAL concentrations were 4.29 ng/mL in November and 5.46 ng/mL in January.
d. Repeated Cross-Sectional Associations Between Urine Metal Concentrations and Kidney Function Outcomes
There were positive associations between cadmium and NGAL (2.92, 95% CI: 1.20, 4.64) and negative associations between cadmium and eGFR (−4.23, 95% CI: −6.92, −1.54 ) after controlling for age, systolic blood pressure, HbA1c, BMI, time of collection, time point, and urine creatinine (Table 3). We observed a negative association between arsenic and eGFR (−4.36, 95% CI: −7.07, −1.64). The results showed no significant interaction between urinary arsenic and cadmium (−0.27, 95% CI: −1.72, 1.17). We observed a similar pattern of effect with nickel on NGAL, however, the magnitude of effect was not as strong (1.90, 95% CI: −0.15, 3.95). As a post-hoc analysis, all models were run with urine creatinine-corrected metal concentrations (μg/g creatinine) with urine creatinine excluded as a covariate. We observed the same relationships with arsenic and cadmium (data not shown).
Table 3:
Adjusted β-coefficient (95% CI) of eGFR, NGAL, and urine albumin by natural log transformed urinary arsenic, cadmium, and nickel concentrations.
| eGFR (n=311) | NGAL (n=269) | Albumin (n=269) | |
|---|---|---|---|
| Ln urine arsenic | −4.36 (−7.07, −1.64)* | 1.82 (−0.96, 4.60) | −2.82 (−6.55, 0.90) |
| Ln urine cadmium | −4.23 (−6.92, −1.54)* | 2.92 (1.20, 4.64) * | −0.13 (−3.09, 2.82) |
| Ln urine nickel | 0.55 (−2.24, 3.34) | 1.90 (−0.15, 3.95) | 2.20 (−1.70, 6.11) |
eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase-associated lipocalin.
Model includes In urine metal concentration (μg/L) with adjustment for baseline age, HbA1c, systolic blood pressure, BMI, time of collection, time point and urine creatinine.
Significance at a p-value <0.05.
Table 4 presents the mean difference in eGFR, NGAL, and albumin between quartile levels of arsenic, cadmium, and nickel after controlling for age, systolic blood pressure, HbA1c, BMI, time of collection, time point, and urine creatinine. The three highest cadmium quartiles showed inverse associations with eGFR, with an increase in the strength of association from lowest quartile to the highest quartile, indicating a dose-response trend. The cadmium quartiles were positively associated with NGAL, with a slight dose-response trend between the lowest and highest quartiles. The highest arsenic quartile showed a negative association with eGFR compared to the lowest quartile. We observed an increase in the strength of association moving from the low arsenic quartiles to the highest quartile.
Table 4:
Difference in kidney parameters associated with urinary arsenic, cadmium, and nickel concentrations in quartiles.
| Quartiles, min-max (μg/L) | eGFR (n=311) | NGAL (n=269) | Albumin (n=269) |
|---|---|---|---|
| Arsenic | |||
| Q1: 0.50-2.73 | referent | referent | referent |
| Q2: 2.73-7.12 | −5.55 (−12.23, 1.12) | 4.43 (−1.5, 10.35) | 0.91 (−8.13, 9.94) |
| Q3: 7.12-13.13 | −5.60 (−12.63, 1.43) | 1.08 (−4.02, 6.18) | −4.69 (−10.63, 1.25) |
| Q4: 13.18-129.19 | −11.18 (−19.68, −2.69) * | 5.22 (−1.17, 11.6) | −5.31 (−15.2, 4.59) |
| Cadmium | |||
| Q1: 0.01-0.03 | referent | referent | referent |
| Q2: 0.03-0.12 | −4.11 (−10.55, 2.33) | 4.69 (0.81, 8.58) * | −1.2 (−7.64, 5.25) |
| Q3: 0.12-0.23 | −13.55 (−21.1, −5.99) * | 10.44 (1.81, 19.08) * | 1.53 (−12.97, 16.02) |
| Q4: 0.23-2.29 | −14.32 (−22.72, −5.93) * | 9.39 (3.88, 14.9) * | −1.12 (−12.36, 10.11) |
| Nickel | |||
| Q1: 0.02-0.72 | referent | Referent | referent |
| Q2: 0.72-1.61 | 4.29 (−1.56, 10.15) | 5.15 (1.63, 8.67) * | −0.09 (−5.04, 4.86) |
| Q3: 1.61-2.64 | 3.27 (−4.4, 10.94) | 6.42 (−3.27, 16.11) | 3.74 (−4.42, 11.91) |
| Q4: 2.70-29.93 | 5.62 (−2.82, 14.06) | 3.58 (−3.21, 10.36) | 3.85 (−7.85, 15.54) |
eGFR, estimated glomerular function; NGAL, neutrophil gelatinase-associated lipocalin.
Q1, quartile 1: the lowest 25% of samples; Q2: between 26% and 50% ; Q3: between 51% and 75%; Q4: the highest 25% of the samples.
Data provided as the difference in outcome measure (95% confidence interval), with metal concentration quartiles (lowest quartile is reference category).
Models include LN Urine metal concentration (μg/L) with adjustment for baseline age, HbA1c, systolic blood pressure, BMI, time of collection, time point, and urine creatinine.
Significance at a p-value <0.05.
For the sensitivity analysis, we removed urine samples with a urine creatinine less than 30 mg/dL (n=101). Arsenic, cadmium, and nickel concentrations (μg/L) were significantly higher for urine samples with a creatinine ≥ 30 mg/dL compared to < 30 mg/dL. We ran the same models with the continuous natural log transformed metal concentrations (Table 5). Cadmium was negatively associated with eGFR (−7.29, 95% CI: −10.94, −3.64) and positively associated with NGAL (3.28, 95% CI: 0.23, 6.34) with slight increases in effect size. Removing the dilute samples increased the median concentrations of the metals, which may have increased the effect sizes.
Table 5:
Sensitivity analysis model (urine creatinine values <30 ng/mL were removed).
| eGFR (n=216) | NGAL (n=198) | Albumin (n=198) | |
|---|---|---|---|
| Ln urine arsenic | −2.98 (−7.01, 1.05) | 0.90 (−4.78, 6.57) | −5.17 (−11.45, 1.1) |
| Ln urine cadmium | −7.29 (−10.94, −3.64) * | 3.28 (0.23, 6.34) * | −0.27 (−3.89, 3.36) |
| Ln urine nickel | −2.16 (−4.89, 0.58) | 1.97 (−0.49, 4.43) | 2.50 (−2.44, 7.45) |
eGFR: estimated glomerular filtration rate; NGAL, neutrophil gelatinase-associated lipocalin.
Adjusted β-coefficient (95% CI) of eGFR, NGAL, and urine albumin by log-natural transformed urinary arsenic, urinary cadmium, and urinary nickel concentrations.
Model includes In urine metal concentration (μg/L) with adjustment for baseline age, HbA1c, systolic blood pressure, BMI, time of collection, time point, and urine creatinine.
Significance at a p-value <0.05.
Discussion:
We investigated the relationships between urine biomarkers of metal exposure and markers of kidney function using serial measurements within a longitudinal study conducted among healthy male sugarcane workers in Guatemala. Here we report two interesting findings: 1) urine arsenic and cadmium concentrations were significantly associated with lower eGFR, and 2) urine cadmium concentrations were significantly associated with higher urine NGAL excretion. While we observed levels below those customarily thought to cause renal injury, based on our data, as well as others (32-34), the importance of these low levels of urinary metals should not be discounted. High exposure to metals in occupational settings have been shown to increase risk for acute kidney injury (35-37), however, there is increasing recognition that chronic exposures below standard levels of nephrotoxicants found in the environment may also contribute to kidney injury and increased risk for kidney disease (32, 38, 39). Though not within populations at-risk for CKDu, there is previous evidence that toxic effects may occur at low cadmium exposure levels, levels less than 2 μg/g creatinine (33, 34). The importance of these lower levels of exposure should be examined further, ideally in relation to concomitant exertion in hot and humid environmental conditions. In fact, our data lend support to the hypothesis that CKDu may result from the nephrotoxicant exposures combined with the recurrent dehydration and renal hypoperfusion experienced by workers exerting themselves in hot climates (1, 40). It raises the need for future research examining the heat x nephrotoxin axis in CKDu pathogenesis.
To our knowledge, this is the largest study to explore associations of urinary metals with kidney parameters among sugarcane workers and one of the first to examine these relationships at multiple time points across the harvest season and off-season. Importantly, we assessed multiple biomarkers of kidney dysfunction, including NGAL, a biomarker reflective of tubular kidney injury, which is the predominant site of injury in CKDu (1). Also, we examined urine metal concentrations both as continuous variables and as quartiles in order to explore a dose-response effect. With this study, we are the first to report significant associations between NGAL and an environmental toxicant in populations at-risk for CKDu.
Arsenic, cadmium, and nickel are widespread and naturally occurring in the environment in Central America. These metals are established causes of kidney damage characterized by tubular injury. To our knowledge, there have been three previous studies examining the relationships between urinary metal exposures and kidney function in populations at-risk for CKDu in Central America (15-17). Similar to our findings, McClean et al (2012) observed an association between urine arsenic concentrations and eGFR among 99 workers in Nicaragua (15). They found, on average, the eGFR among workers with high arsenic was 9.0 mL/min/1.73 m2 lower than workers with low arsenic. However, they found no association between urine cadmium and markers of kidney function. Differences in worker populations and time of data collection may explain why our results differ. With the Nicaragua study, biomarkers were collected from workers with several job types (cane cutters, irrigators, seeders, factory workers, and miners) prior to the start of the harvest and associations were analyzed cross-sectionally. This current study collected biomarkers among only cane cutters, a worker population who is at a high risk of dehydration and heat stress due to labor conditions. We analyzed cross-sectional relationships at multiple time points. The second study was a case-control community study conducted in Nicaragua (16) and observed no associations between metal exposure and two subgroups of community members (stable vs. declining function). Again, study design differences may explain differences in results compared to this current study. Researchers collected urinary metal exposure at two time points among community members, before and after the harvest, and these time points are analyzed separately. Their two renal function trajectory subgroups were derived from eGFR measures across 2 years. Furthermore, cadmium was also associated with NGAL in our study, which may suggest several mechanisms of injury for cadmium. In contrast, McClean et al. (2012) did not observe an association between urine lead, cadmium, arsenic or uranium with NGAL. NGAL has shown to be a useful early clinical biomarker of acute kidney injury and CKD progression in adults and children (41). Another study conducted among welding workers exposed to metal fumes observed significantly elevated NGAL after exposure to PM2.5(42). Fischer et al. (2020) observed higher nickel concentrations in toenail clipping among 18 renal disease patients in Nicaragua with an acute form of kidney injury compared to 36 healthy controls (17). While this toenail study was also conducted among agricultural workers, the 18 patients with acute nephritis appear to have a different phenotype of clinical illness compared to this current study’s cohort and nickel toenail concentrations reflect a different period of exposure than our urinary nickel concentrations.
The median urinary levels of metals in our study are higher than those reported in a previous study among 23 adults from four communities in western Guatemala (arsenic: 0.06 μg/L; cadmium: 0.11 μg/L; nickel 0.07 μg/L), however our study observed metal concentrations are within standard reference ranges (arsenic: < 50 μg/L; cadmium: < 1 μg/L, and nickel: < 4 μg/L) (43). The median urinary levels of arsenic (6.88-8.05 μg/L) and cadmium (0.09-0.14 μg/L) in these Guatemalan workers were higher than those reported for a Mexican-American population reported by the U.S. National Health and Nutrition Examination Survey (NHANES) in 2015-2016 (5.65 μg/L for As; 0.098 μg/L for Cd) (44). We cannot compare nickel levels as it has not been measured in the NHANES population.
We hypothesized that tobacco smoke might contribute to the elevated levels observed in our study subjects. Tobacco is a well-established source of arsenic and cadmium exposure, along with water, diet, and the natural environment (28). We found no correlations between these metals and urine cotinine concentrations in these workers, suggesting that future research should further investigate smoking as well as other sources.
Our study focused on the potential contribution of metals and was not able to examine the potential combined effects of metals and heat extremes. Recent research in Central America has demonstrated the importance of heat stress and dehydration as contributors to CKDu (45). One hypothesis it that dehydration and volume depletion may increase the risk of kidney injury from nephrotoxicants such as agrochemicals or metals (46). We have observed previously that recurrent dehydration is associated with renal hypoperfusion among Guatemalan sugarcane cutters (similar to the current study population) (40). In the face of dehydration, chronic low-level metal exposures could contribute to kidney injury. Nephrotoxicants may lead to reabsorption of metals and agrochemicals in the renal tubules (13) and induce injury at levels below referenced toxic levels. The relevance of low-level metal exposures in people with urinary metal levels at or below standard reference ranges should be reconsidered in the context of heat-related dehydration and kidney hypoperfusion.
There are several limitations to our study. First, causal inference between metals exposure and renal outcomes cannot be made because of the cross-sectional design. For example, it remains possible that the reduction in kidney function might have influenced the urinary heavy metal concentrations (i.e., a reverse causation mechanism). The association observed between urinary cadmium and NGAL (a urinary protein) may be consistent with the hypothesis of reverse causality put forward by Chaumont et al. (2012)(47). Chaumont et al. state that the physiological variation in tubular reabsorption may affect excretion of urinary cadmium and low molecular weight urinary proteins (i.e., NGAL) in the same direction and caution is needed when interpreting the renal effects of urinary cadmium at low-levels of exposure (47). While we acknowledge that reverse causality is a concern, several lines of evidence strengthen our findings. First, it should be noted that participants evaluated in this study did not have abnormal levels of biomarkers that could indicate kidney damage, although we cannot exclude the possibility of subclinical kidney conditions. Decreased glomerular filtration may indicate an occurrence whereby reduced renal function may decrease the urinary excretion of environmental chemicals, including metals. Secondly, the observation that urinary cadmium is associated with both urinary NGAL and serum eGFR may very well reflect true changes in kidney function. Thirdly, our results demonstrate that there was a dose-response relationship between urinary cadmium and eGFR as well as urinary cadmium and NGAL. A second limitation is that there may be intra-individual variability with eGFR due to extrinsic factors such as protein intake, fluid intake, and exercise (48). However, we used serial measurements of eGFR at three time points to help address this issue. Third, we relied on both morning and afternoon single spot urine samples. While using spot samples is common practice for epidemiological studies, correction for urine dilution is necessary. By including urine creatinine as an independent variable, the associations of the kidney outcomes with urine metal concentrations are not influenced by a relationship with urinary creatinine (30). Of note, we ran the models with urine creatinine-adjusted metals and observed the same results. Another limitation is that we measured total arsenic without specifically measuring the inorganic toxic form. Seafood in the diet may increase urinary total arsenic measurements based on organic arsenic compounds (the nontoxic form) (49). While speciation data would provide more accurate risk assessment, we believe the total arsenic measurement is not a reflection of arsenic ingestion from consumption of seafood based on previous nutrition surveys in these communities (50) and the worksite cafeteria menu for the highland workers. Fish is on the menu one or two times per month during the harvest season. However, given the interesting findings for arsenic, future research should be done to speciate. Finally, we are studying the relationship of urinary metal concentrations to kidney parameters but not directly testing the hypothesis that metal concentrations are part of the pathway for CKDu, since we have not defined cases of CKDu in this paper. Another limitation is that we had very few subjects with kidney dysfunction (eGFR of less than 60 ml/min/1.73 m2). Nevertheless, sugarcane workers, as a whole, are at a high risk for both acute and chronic kidney disease (1, 20). A final limitation is that were not able to identify the source of the cadmium or arsenic in this study.
In conclusion, we have observed a relationship between urine cadmium and arsenic with lower kidney function in a cohort of Guatemalan sugarcane workers. Importantly, the concentrations were not at a level typically associated with renal injury. However, it is possible that these levels are clinically significant and that they may represent injurious effects in the setting of high temperatures and intense labor, possibly due to enhanced tubular absorption. Consistent with this finding, higher concentrations of urine cadmium were positively associated with urinary NGAL, a marker for the type of tubular injury seen in CKDu. Notably, the urinary levels of these metals were not elevated compared to standard normal ranges. The results from the present study suggest, but do not prove, that renal tubular damage might develop at lower systemic concentrations of metals than previously thought. Additionally, our data suggest that use of NGAL, a more sensitive biomarker than creatinine, may allow for early diagnosis and facilitate early intervention strategies. We conclude that metal exposures should be explored in further research as well as the sources of these exposures, specifically in susceptible populations such as sugarcane workers. If tubular dysfunction from cadmium and arsenic exposure is occurring, this dysfunction might increase susceptibility to the future development of CKDu.
Supplementary Material
Acknowledgements:
We wish to thank all our collaborators including Hillary Yoder, MS, Stephen Brindley, MS, Nicholas Smith, and all the workers who have made this work possible. We would also like to acknowledge Trace Metals Core Facility at the Mailman School of Public Health, Columbia University for the analysis of the urine samples.
Funding Sources: This study was supported by Centers for Disease Control and Prevention (CDC) (U19 OH011227) and National Institutes of Health (NIH) (R21 ES028826), and in part by Pantaleon and the Chancellor, University of Colorado, CU Anschutz Campus. Metal laboratory analysis was supported by NIH grants P30 ES009089 and P42 ES010349. Funders had no role in data analysis, interpretation of data, writing the manuscript, or the decision to submit the findings for publication.
Footnotes
Conflict of Interests: The University of Colorado has a memorandum of agreement with Pantaleon, a Guatemala-based agribusiness. Pantaleon provides partial financial support for research through a contract with the university and has provided access to the employees who volunteered to participate in this research project. The University of Colorado employed appropriate research methods in keeping with academic freedom, based conclusions on critical analysis of the evidence and reported findings fully and objectively. The terms of this arrangement have been reviewed and approved by the University of Colorado in accordance with its conflict of interest policies.
References
- 1.Johnson RJ, Wesseling C, Newman LS. Chronic Kidney Disease of Unknown Cause in Agricultural Communities. New England Journal of Medicine. 2019;380(19):1843–52. [DOI] [PubMed] [Google Scholar]
- 2.Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environmental Health. 2015;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesseling C, Crowe J, Hogstedt C, Jakobsson K, Lucas R, Wegman DH, et al. Resolving the Enigma of the Mesoamerican Nephropathy: A Research Workshop Summary. American Journal of Kidney Diseases. 2014;63(3):396–404. [DOI] [PubMed] [Google Scholar]
- 4.Vervaet BA, D'Haese PC, Verhulst A. Environmental toxin-induced acute kidney injury. Clinical Kidney Journal. 2017;10(6):747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler BA. Mechanisms of kidney cell injury from metals. Environ Health Perspect. 1993;100:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Experientia supplementum (2012). 2012;101:133–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce N, Caplin B. Let's take the heat out of the CKDu debate: more evidence is needed. Occupational and Environmental Medicine. 2019;76(6):357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rango T, Jeuland M, Manthrithilake H, McCornick P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Science of the Total Environment. 2015;518:574–85. [DOI] [PubMed] [Google Scholar]
- 9.Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? International Journal of Environmental Research and Public Health. 2014;11(2):2125–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulathunga M, Wijayawardena MAA, Naidu R, Wijeratne AW. Chronic kidney disease of unknown aetiology in Sri Lanka and the exposure to environmental chemicals: a review of literature. Environmental Geochemistry and Health. 2019;41(5):2329–38. [DOI] [PubMed] [Google Scholar]
- 11.Bandara JMRS, Senevirathna DMAN, Dasanayake DMRSB, Herath V, Bandara JMRP, Abeysekara T, et al. Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environmental Geochemistry and Health. 2008;30(5):465–78. [DOI] [PubMed] [Google Scholar]
- 12.Herath HMAS, Kawakami T, Nagasawa S, Serikawa Y, Motoyama A, Chaminda GGT, et al. Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. Journal of Water and Health. 2018;16(2):212–22. [DOI] [PubMed] [Google Scholar]
- 13.Jayasumana C, Gunatilake S, Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. Bmc Nephrology. 2015;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bustamante-Montes LP, Flores-Polanco JA, Isaac-Olive K, Hernandez-Tellez M, Campuzano-Gonzalez ME, Ramirez-Duran N. Exploratory study on the association of heavy metals and the nephropathy of unknown etiology in the western state of mexico. Revista Internacional De Contaminacion Ambiental. 2018;34(4):555–64. [Google Scholar]
- 15.McClean M, Laws R, Kaufman JS, Weiner DE, Rodriguez JMS, Ramirez-Rubio O, et al. Biological Sampling Report: Investigating Biomarkers of Kidney Injury and Chronic Kidney Disease Among Workers in Western Nicaragua, 2012 [report], Boston University; 2012. [Google Scholar]
- 16.Smpokou ET, Gonzalez-Quiroz M, Martins C, Alvito P, Le Blond J, Glaser J, et al. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occupational and Environmental Medicine. 2019;76(12):920–6. [DOI] [PubMed] [Google Scholar]
- 17.Fischer RSB, Unrine J, Vangala C, Sanderson WT, Mandayam S, Murray KO. Evidence of nickel and other trace elements and their relationship to clinical findings in acute Mesoamerican Nephropathy: A case-control analysis.: PLoS One; 2020. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wijkstrom J, Gonzalez-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, et al. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. American Journal of Kidney Diseases. 2017;69(5):626–36. [DOI] [PubMed] [Google Scholar]
- 19.Butler-Dawson J, Krisher L, Asensio C, Cruz A, Tenney L, Weitzenkamp D, et al. Risk Factors for Declines in Kidney Function in Sugarcane Workers in Guatemala. Journal of Occupational and Environmental Medicine. 2018;60(6):548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler-Dawson J, Krisher L, Yoder H, Dally M, Sorensen C, Johnson RJ, et al. Evaluation of heat stress and cumulative incidence of acute kidney injury in sugarcane workers in Guatemala. International Archives of Occupational and Environmental Health. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruszkowski E, Neubauer K, Thomas R. An overview of clinical applications by inductively coupled plasma mass spectrometry. Atomic Spectroscopy. 1998;19(4):111–5. [Google Scholar]
- 22.Nixon D ES, Butz J, Burrit M, Neubauer K,, Schneider C. Determination of Arsenic, Lead, Cadmium, and Mercury in Whole Blood and Urine Using Dynamic Reaction Cell ICP-MS. Application Notes, Perkin-Elmer SCIEX Instruments [Google Scholar]
- 23.Jarrett JM, Cadwell KL, Jones RL. Urine Multi-Element ICP-DRC-MS:Antimony, Arsenic, Barium, Beryllium, Cadmium, Cesium, Cobalt, Lead, Manganese, Molybdenum, Platinum, Strontium, Thallium, Tin, Tungsten, and Uranium. CDC-Division of Laboratory Sciences, Laboratory Protocol. Adopted October 01 1994, Updated September 15 2014. [Google Scholar]
- 24.Fiseha T, Tamir Z. Urinary Markers of Tubular Injury in Early Diabetic Nephropathy. International journal of nephrology. 2016;2016:4647685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devarajan P Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomarkers in medicine. 2010;4(2):265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YP, Castro AF, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Annals of Internal Medicine. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caruso RV, O'Connor RJ, Stephens WE, Cummings KM, Fong GT. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: results from the International Tobacco Control (ITC) United States survey cohort. International journal of environmental research and public health. 2013;11(1):202–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg Jan 1990. p. 46–51. [Google Scholar]
- 30.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Office of Occupational H. Biological monitoring of chemical exposure in the workplace: guidelines. Geneva: World Health Organization; 1996. [Google Scholar]
- 32.Weaver VM, Kim N-S, Jaar BG, Schwartz BS, Parsons PJ, Steuerwald AJ, et al. Associations of low-level urine cadmium with kidney function in lead workers. Occupational and environmental medicine. 2011;68(4):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environmental health perspectives. 2005;113(11):1627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, et al. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38(5):1001–8. [DOI] [PubMed] [Google Scholar]
- 35.Adams RG, Harrison JF, Scott P. The development of cadmium-induced proteinuria, impaired renal function, and osteomalacia in alkaline battery workers. Q J Med. 1969;38(152):425–43. [PubMed] [Google Scholar]
- 36.Navarro-Moreno LG, Quintanar-Escorza MF, Gonzalez S, Mondragon R, Cerbon-Solorzano J, Valdes J, et al. Effects of lead intoxication on intercellular junctions and biochemical alterations of the renal proximal tubule cells. Toxicol In Vitro. 2009. Oct;23(7):1298–304. [DOI] [PubMed] [Google Scholar]
- 37.Gerhardt RE, Hudson JB, Raghunatha RN, Sobel RE. Chronic renal insufficiency from cortical necrosis induced by arsenic poisoning. Arch Intern Med. 1978;138(8):1267–1269. [PubMed] [Google Scholar]
- 38.Hambach R, Lison D, D'Haese P, Weyler J, Francois G, De Schryver A, et al. Adverse effects of low occupational cadmium exposure on renal and oxidative stress biomarkers in solderers. Occup Environ Med. 2013;70(2):108–13. [DOI] [PubMed] [Google Scholar]
- 39.Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, et al. Urine arsenic and prevalent albuminuria: evidence from a population-based study. Am J Kidney Dis. 2013. Mar;61(3):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, et al. Risk Factors and Mechanisms Underlying Cross-Shift Decline in Kidney Function in Guatemalan Sugarcane Workers. J Occup Environ Med. 2019;61(3):239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang KJ, Pan CH, Su CL, Lai CH, Lin WY, Ma CM, et al. Urinary neutrophil gelatinase-associated lipocalin is associated with heavy metal exposure in welding workers. Scientific Reports. 2015;5(1):18048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordberg GF, Fowler BA, Nordberg M. Handbook on the Toxicology of Metals 3rd Edition. Academic Press; 2007. [Google Scholar]
- 44.CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 45.Roncal-Jimenez C, Lanaspa MA, Jensen T, Sanchez-Lozada LG, Johnson RJ. Mechanisms by Which Dehydration May Lead to Chronic Kidney Disease. Annals of Nutrition and Metabolism. 2015;66:10–3. [DOI] [PubMed] [Google Scholar]
- 46.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of Unknown Origin in Central America: The Case for a Mesoamerican Nephropathy. American Journal of Kidney Diseases. 2014;63(3):506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaumont A, Nickmilder M, Dumont X, Lundh T, Skerfving S, Bernard A. Associations between proteins and heavy metals in urine at low environmental exposures: Evidence of reverse causality. Toxicology Letters. 2012;210(3):345–52. [DOI] [PubMed] [Google Scholar]
- 48.Stevens LA, Coresh J, Greene T, Levey AS. Assessing Kidney Function — Measured and Estimated Glomerular Filtration Rate. New England Journal of Medicine. 2006;354(23):2473–83. [DOI] [PubMed] [Google Scholar]
- 49.National Research Council. Arsenic in Drinking Water: 2001 Update. Washington, DC: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 50.Krisher L, Butler-Dawson J, Asensio C, Yoder H, Cruz A, Pilloni D, et al. A Total Worker Health approach to assessing kidney health in sugarcane workers in Guatemala: an opportunity for nutrition intervention. Poster presented at the Third International Workshop on Chronic Kidney Diseases of Uncertain/Non-Traditional Etiology in Mesoamerica and Other Regions, Costa Rica. March 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


