Abstract
Co-infection with malaria and human immunodeficiency virus (HIV) increases the severity and mortality rates of both diseases. A better understanding of the effects of co-infections could help in the diagnosis, prompt treatment, prevention, and control of malarial parasites among HIV-infected patients. In this systematic review and meta-analysis, we estimated the prevalence and characteristics of severe malaria (SM) caused by co-infection with HIV. We included relevant studies that were conducted between the years 1991 and 2018 and reporting on SM. We pooled the prevalence of SM in patients with co-infection, pooled odds ratios of SM in patients with co-infection and Plasmodium mono-infection, and differences in laboratory parameters such as parasite density and leucocyte counts, between co-infected and Plasmodium mono-infected patients. The meta-analysis included 29 studies (1126 SM cases). The pooled prevalence of SM in co-infected patients using the data of 23 studies (SM = 795 cases, all co-infection cases = 2534 cases) was 43.0% (95% confidence interval [CI] 31.0–56.0%; I2, 98.0%). Overall, the odds of SM from 18 studies were pooled. The odds of SM were significantly higher in co-infected patients than in Plasmodium mono-infected patients (OR 2.41; 95% CI 1.43–4.08; I2 = 85%; P = 0.001) and also significantly higher in children (OR 9.69; 95% CI 5.14–18.3; I2, 0%; P < 0.0001; two studies) than in adults (OR 2.68; 95% CI 1.52–4.73; I2, 79.0%; P = 0.0007; 12 studies). Co-infected patients with SM had a higher parasite density than those with Plasmodium mono-infection when the data of seven studies were analysed (SMD, 1.25; 95% CI 0.14–2.36; I2, 98.0%; P = 0.03) and higher leukocyte counts when the data of four studies were analysed (MD, 1570 cells/µL; 95% CI 850–2300 cells/µL; I2, 21.0%; P < 0.0001). Thus, the prevalence of SM among patients co-infected with Plasmodium spp. and HIV is high. Because co-infections could lead to SM, patients with Plasmodium spp. and HIV co-infection should be identified and treated to reduce the prevalence of SM and the number of deaths.
Subject terms: Malaria, Parasitic infection
Introduction
Malaria remains one of the most dangerous diseases affecting the world’s population with about 228 million cases and 405,000 deaths from malaria globally1; most of the malaria cases (93%) and deaths (94%) were found in the African Region1. In areas with stable malaria, human immunodeficiency virus infection (HIV) and acquired immune deficiency syndrome (AIDS) increase the risk of malaria infection, especially in adults with advanced immunosuppression2,3. HIV infection remains a major health problem with approximately 37.9 million people living with HIV and 770,000 deaths observed at the end of 20184.
Severe malaria (SM) is defined by the World Health Organization 2014 by the presence of malaria parasites in the blood of patients with potentially fatal manifestations, including impaired consciousness, acidosis, hypoglycaemia, severe malarial anaemia (SMA), renal impairment, jaundice, pulmonary oedema, significant bleeding, shock, and hyperparasitaemia5. In Africa, many children develop three overlapping syndromes—cerebral malaria, severe malarial anaemia, and respiratory distress—and the prognoses and ages at presentation differ6. The sequestration of infected red blood cells (RBCs) in the microvascular system of patients with Plasmodium falciparum infections is the main factor of severe malaria7. SM can be caused not only by P. falciparum but also by Plasmodium knowlesi8, Plasmodium vivax9,10, Plasmodium malariae11, and Plasmodium ovale12, although in fewer people. However, the mechanism remains poorly understood.
Co-infection with Plasmodium spp. and HIV is likely to occur because of the high prevalence of both infections in the same areas, particularly in Sub-Saharan African regions. Data suggest that Plasmodium spp. and HIV co-infection result in adverse outcomes particularly in pregnant women and their infants13. Previous studies demonstrated that adults infected with HIV were at increased risk of developing severe malaria14–16. Moreover, almost all patients with Plasmodium spp. and HIV co-infection develop anaemia17. A previous meta-analysis of 23 studies demonstrated that the development of anaemia increased by 49% in co-infected pregnant women compared with those who had HIV in mono-infection18. In addition, mono-infection with either malaria or HIV was associated with haematological alterations, such as anaemia, leukopenia, leucocytosis, thrombocytopenia, monocytosis, and eosinophilia19,20. However, there is limited information on the impact of Plasmodium spp. and HIV co-infection on SM and a better understanding of the impact of co-infections could help in the diagnosis, prompt treatment, prevention, and control of malaria parasites among HIV-infected patients. Thus, the primary aim of our study was to generate a pooled prevalence estimate of SM among patients co-infected with Plasmodium spp. and HIV. Our secondary aim was to compare the odds of SM caused by Plasmodium spp. and HIV co-infections with those of SM caused by Plasmodium mono-infection. The third aim was to identify the differences in laboratory parameters between patients with Plasmodium spp. and HIV co-infection and those with Plasmodium mono-infection.
Results
Study selection
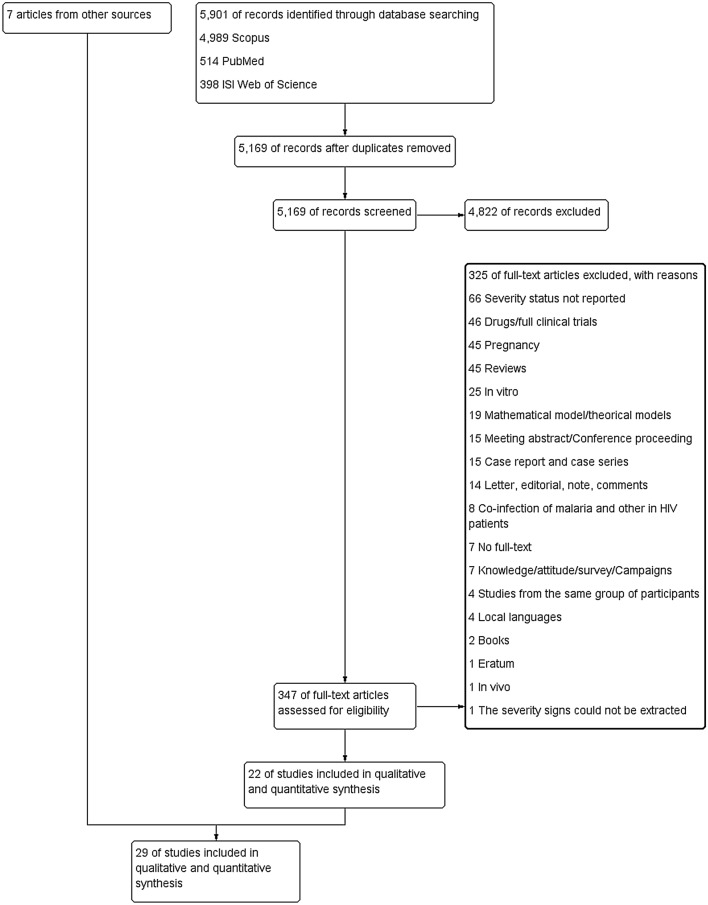
A total of 5901 articles were identified by the initial search. After removing duplicates, 5169 articles remained for further consideration. Article titles and abstracts were screened, leading to the exclusion of additional 4822 articles. Further assessments of 347 full-text articles were performed, and 22 of these met the inclusion criteria2,21–41, whereas 325 were excluded (Fig. 1). After reviewing the reference list of eligible articles and additional searches, seven additional articles15,17,42–46 were included in the present study. Eventually, a total of 29 studies were included in the systematic review and meta-analysis.
Figure 1.
PRISMA diagram. Flowchart for study selection.
Study characteristics
Data from 2534 patients with Plasmodium spp. and HIV co-infections across the 23 included studies2,15,17,21–26,28–30,32,33,36–43,46 (range 9–1071) and six studies27,31,34,35,44,45 reporting SM in patients with Plasmodium spp. and HIV co-infection were analysed in the present study (Table 1). Of the 29 included studies, 28 (96.6%) were conducted in African countries: 6 (20.7%) in Malawi27,31,34,35,37,44; 5 (17.2%) in Mozambique2,23,24,30,40; 3 (10.30%) in Kenya25,29,46; 2 (6.90%) each in Ghana17,41, Cameroon38,39, South Africa15,42 and Ethiopia21,26 and 1 (3.40%) each in Nigeria22, Zambia28, Gabon32, Uganda43, Congo33 and Burundi45. The other study involved patients in France36. Most of the included studies were cross-sectional studies (20/29, 69%)2,17,21–27,29,30,33,35,37–42,45, whereas two were prospective cohort studies15,46, four were case–control studies28,31,34,43, and one was retrospective study36. Most of the studies had included adults aged > 15 years2,15,21,23,24,28,32,37,38,40–42,45, children aged < 15 years (10/29, 34.5%)22,25,27,29,31,34,35,43,44,46 and any age group17,26,30,33,36,39, respectively. Most participants at enrolment reflected patients with malaria (16/29, 55.2%)15,21,24,25,27–31,33–37,43,45, patients with HIV/AIDS17,22,26,32,39,41, with undefined23,38,40,46 febrile2,42 and other conditions/diseases44. The most common diagnostic method for the detection of Plasmodium spp. among the included studies was microscopy (25/29, 86.2%), whereas the most common method for the identification of HIV was polymerase chain reaction (12/29, 41.4%). P. falciparum was the only Plasmodium spp. reported among HIV-positive patients2,15,21–25,27–32,34–38,40–46; three reports did not specify the Plasmodium spp.17,33,39 and one study26 focused on mixed infection with P. falciparum and P. vivax among HIV-positive patients but did not specify the exact Plasmodium spp. among patients with SM. Table 2 lists the laboratory data on parasite density, leukocyte counts and differential counts of Plasmodium spp. and HIV co-infected patients with SM versus Plasmodium spp. mono-infected patients with SM.
Table 1.
Characteristics of the included studies.
| No. | Author, year | Study area (years of the survey) | Study design | Age range | Gender | Participants | HIV status | Plasmodium mono–infections | Detection method for Plasmodium sp. | Severe malaria (mono-infections) | HIV mono-infections | Detection method for HIV | Co-infections |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Addissie et al. (2007)21 | Ethiopia (2003–2004) | Cross-sectional study | 15–34 | Male (203), Female (104) |
337 P. falciparum Severe 62 Prostration 18 Hyperparasitemia 17 Cerebral manifestations 16 Other 18 |
Recently diagnosed | 323 (P. falciparum) | Microscopy |
Severe malaria 52 Cerebral malaria 16, prostration 18, hyperparasitemia, and other complications (estimated 18) |
NA | ELISA |
14 (P. falciparum) Severe malaria 3 (P. falciparum) |
| 2. | Amodu-Sanni et al. (2020)22 | Nigeria (2016) | Cross-sectional study | 3 months to 15 years | Male (143), Female (137) | 140 HIV positive, 140 HIV negative | Undertreated | 132 (P. falciparum) | Microscopy | Severe malaria 84 | 40 | ELISA, PCR |
100 (P. falciparum) Severe malaria 46 (P. falciparum) |
| 3. | Berg et al. (2014)2 | Mozambique (2011–2012) | Cross-sectional study | 18–84 | Male (142), Female (126) | 212 adults with fever and/or suspected malaria, 56 healthy controls | Recently diagnosed | 61 (P. falciparum) | Microscopy, RDT, PCR |
Severe malaria 24/52 Hypotension (1/52), Respiratory distress (3/52), Hyperpyrexia (6/50), GCS < 11 and/or convulsions (5/61), Bleeding disturbances and/or hemolysis (1/61), Jaundice (3/61) Severe anemia (3/55), Hypoglycaemia (0/47), Renal failure (3/46), Hyperparasitaemia (16/53) |
NA | RDT, PCR |
70 (P. falciparum) Severe malaria 55/66 (P. falciparum) Hypotension (0/63), Respiratory distress (15/61), Hyperpyrexia (3/54), GCS < 11 and/or convulsions (6/70), Bleeding disturbances and/or hemolysis (9/70), Jaundice and/or se-bilirubin (13/70), Severe anemia (10/67), Hypoglecemia (5/62), Renal failure (15/63), Hyperparasitemia (33/64) |
| 4. | Berg et al. (2008)23 | Mozambique (2006) | Cross-sectional study | 16–92 | Male (167), Female (166) | 333 adult patients | Immunosuppressed | 8 (P. falciparum) | Microscopy |
Severe malaria 4 Jaundice 1, Renal failure 2, fatal 1 |
NA | RDT |
12 (P. falciparum) Severe malaria 9 (P. falciparum) Jaundice 2, Renal failure 6, Fatal 1 |
| 5. | Berg et al. (2020)24 | Mozambique (2011–2012) | Cross-sectional study | ≥ 18 years | Male (71), Female (60) | 131 P. falciparum | Undertreated | 61 (P. falciparum) | Microscopy, RDT |
Severe malaria 28 Bleeding disturbances and/or haemolysis 1, Fatal 1 |
NA | PCR |
70 (P. falciparum) Severe malaria 57 (P. falciparum) Bleeding disturbances and/or hemolysis 9, Fetal 9 |
| 6. | Berkley et al. (2009)25 | Kenya (1998–2002) | Cross-sectional study | Children aged ≥ 60 days | NA | 3,068 severe P. falciparum, 592 healthy control | Recently diagnosed | P. falciparum | Microscopy | Severe malaria 938/1071 |
119/684 10/592 |
ELISA | Severe malaria 133/1071 (P. falciparum) |
| 7. | Beyene et al. (2017)26 | Ethiopia (2012–2013) | Cross-sectional study | < 27 years (139), 27.00–31.99 years (141), 32.0–39.74 years (113), ≥ 39.75 (131) | Male (250), Female (284) | 528 people living with HIV/AIDS | Recently diagnosed | NA | Microscopy, RDT | NA |
436 Severe anemia 12 |
NA |
92 (52 P. falciparum, 37 P. vivax, and 3 mixed infections) Severe anemia 25 (Plasmodium spp.) |
| 8. | Bronzan et al. (2007)27 | Malawi (1996–2005) | Cross-sectional study | ≥ 6 months old | Male (250), Female (541) | 1388 severe malaria | Recently diagnosed | 941 severe malaria (P. falciparum) | NA |
941 severe malaria Cerebral malaria 541/627 Cerebral malaria and severe anemia 291/355, severe anemia 109/137 |
RDT |
Severe malaria 178 (P. falciparum) Cerebral malaria 86/627 Cerebral malaria and severe anemia 64/355, severe anemia 28/137 |
|
| 9. | Chalwe et al. (2009)28 | Zambia (2004–2005) | Case–control study |
15–49 years 15–19, 20–29, 30–39, 40–49 |
Male (42), Female (45) | 29 severe malaria, 29 uncomplicated malaria, 29 asymptomatic community controls | Immunosuppressed | 32 (P. falciparum) | Microscopy, RDT |
Severe 2 Jaundice 1 |
NA | RDT, ELISA, Western blot |
55 (P. falciparum) Severe malaria 27 (P. falciparum) Impaired consciousness 15, Severe anemia 5, Convulsions 6, Jaundice 3, Hypoglycemia 11, Hyperparasitemia 6 |
| 10. | Cohen et al. (2005)15 | South Africa (2001–2002) | A prospective cohort study |
15–49 years: Malaria-mono-infection: 29 (17–49) Co-infection: 30 (17–49) |
Malaria-mono-infection: Male (178), Female (48) Co-infection: Male (85), Female (25) |
502 P. falciparum patients | Immunosuppressed | 226 (P. falciparum) | Microscopy, RDT |
Severe malaria 14: Cerebral malaria 4, Severe anemia 0 Renal impairment 9, Shock 2, Acidosis 3, Hypoglycemia 0, Hepatic dysfunction 8 |
NA | RDT, ELISA |
110 (P. falciparum) Severe malaria 18 (P. falciparum): Cerebral malaria 3 Severe anemia 3, Renal impairment 14, Shock 2, Acidosis 11, Hypoglycemia 1, Hepatic dysfunction 6 |
| 11. | Davenport et al. (2010)29 | Kenya (2004–2006) | Cross-sectional study | 3–36 months |
Malaria-mono-infection: Male (219), Female (187) Co-infection: Male (14), Female (10) |
542 with P. falciparum | Undertreated | 406 (P. falciparum) | Microscopy | Severe anemia 44 | NA | RDT, PCR |
24 (P. falciparum) Severe anemia 14 (P. falciparum) |
| 12. | Grimwade et al. (2004)42 | South Africa (2000) | Cross-sectional study |
Adults (> 14 y): Malaria-mono-infection: 28 (18–43) Co-infection: 30 (22–42) |
Malaria-mono-infection: Male (211), Female (222) Co-infection: Male (70), Female (110) |
1,109 febrile adults | Recently diagnosed | 433 (P. falciparum) | Microscopy, RDT |
Severe malaria 62: Severe malaria 18: Impaired renal function 32, Coma 16, Severe anemia 22, Pulmonary edema 2, Bleeding 0, Acidosis 13, Confusion 18, Jaundice 3 |
152 | ELISA |
180 (P. falciparum) Severe malaria 18 (P. falciparum): Renal failure 28, Coma 16, Severe anemia 14, Pulmonary edema 4, Bleeding 2, Acidosis 15, Confusion 7, Jaundice 9 |
| 13. | Hendriksen et al. (2012)30 | Mozambique (2005–2010) | Cross-sectional study |
Malaria-mono-infection: 2.5–23 Co-infection: 3–38 Age group < 15 and > 15 years |
Malaria-mono-infection: Male (6314), Female (286) Co-infection: Male (68), Female (55) |
896 with suspected severe malaria | Recently diagnosed | 600 (P. falciparum) | Microscopy, RDT |
Severe malaria 549 Coma 454, Convulsions 516, Prostration 137, Shock 21, Severe respiratory distress 38, Severe acidosis 109, Hypoglycemia 33, Severe anemia with respiratory distress 67, Black water fever 28, Severe jaundice 17, Hyperparasitemia 109 |
NA | RDT, PCR |
123 (P. falciparum) Severe malaria 107 (P. falciparum) Coma 89, Convulsions 74, Prostration 31, Shock 6, Severe respiratory distress 20, Severe acidosis 38, Hypoglycemia 10, Severe anemia with respiratory distress 18, Black water fever 15, Severe jaundice 9, Hyperparasitemia 33 |
| 14. | Hochman et al. (2015)31 | Malawi (1996–2010) | Case–control study | 6 months to 12 years |
Malaria-mono-infection: Male (39), Female (18) Co-infection: Male (7), Female (8) |
103 autopsy tissues | Recently diagnosed | 57 (P. falciparum) | ELISA | Cerebral malaria 57 | NA | IHC | Cerebral malaria 15 (P. falciparum) |
| 15. | Huson et al. (2015)32 | Gabon (2012–2013) | Prospective observational study | age ≥ 18 years |
Malaria-mono-infection: Male (44), Female (69) Co-infection: Male (0), Female (14) |
103 patients with sepsis 127 patients with malaria, 60 HIV infected control | Undertreated | 133 (P. falciparum) | Microscopy |
0 Fetal 0 |
RDT, PCR |
14 (P. falciparum) Fetal 1 (P. falciparum) |
|
| 16. | Imani et al. (2011)43 | Uganda (2006–2007) | Case–control study | < 12 years | Cerebral malaria: Male (70), Female (30), uncomplicated malaria: Male (92), Female (40), non-malaria: Male (84), Female (36) |
352 children: 100 cerebral malaria, 132 uncomplicated malaria, 120 non-malaria |
Recently diagnosed | 220 (P. falciparum) | Microscopy |
220 Cerebral malaria 91/220 |
NA | RDT |
12 (P. falciparum) Cerebral malaria 9/12 (P. falciparum) |
| 17. | Jacques et al. (2019)33 | Congo (2017–2018) | Cross-sectional study |
12–60 years Co-infection 12–60 years |
Male (111), Female (114) | 225 undernourished children (200 malaria) | Recently diagnosed | NA | Microscopy | NA | NA | RDT, ELISA, PCR |
168 (Plasmodium spp.) Severe anemia 86 (Plasmodium spp.) |
| 18. | Joice et al. (2016)34 | Malawi (1996–2011) | Case–control study |
Malaria-mono-infection: 3.1–8.8 Co-infection: 1.7–3.6 |
Malaria-mono-infection: Male (38), Female Co-infection: Male (9), Female (11) |
103 autopsy cases | NA | 75 (P. falciparum) | IHC | Cerebral malaria 75 | NA | PCR | Cerebral malaria 20/95 (P. falciparum) |
| 19. | Kyeyune et al. (2014)44 | Malawi (2002–2004) | Prospective observational study | aged < 2 years | NA | 391 children with severe anemia | Recently diagnosed | 183/312 (P. falciparum) | Microscopy | severe anemia 183 | 19 | RDT, PCR |
26/45 (P. falciparum) Severe anemia 26 (P. falciparum) |
| 20. | Mandala et al. (2018)35 | Malawi (2005–2006) | Cross-sectional study | 1.2–4.6 years | Male (5), Female (33) | 38 Cerebral malaria, 35 severe malarial anemia, control 42, HIV positive 4 | Recently diagnosed | Severe malaria 59 (P. falciparum) | Microscopy |
59 Cerebral malaria 29, Severe anemia 30 |
4 | RDT, PCR |
14 (P. falciparum) Cerebral malaria 9, Severe anemia 5 |
| 21. | Mouala et al. (2008)36 | France (1996–2003) | Retrospective study | < 30, 30–39, 40–49, > 50 years | Male (99), Female (91) |
190 imported malaria P. falciparum 178, other species 12 |
Undertreated | 150 (P. falciparum) | Microscopy | Severe malaria 54 | NA | PCR |
28 (P. falciparum) Severe malaria 11 (P. falciparum) |
| 22. | Munyenyembe et al. (2018)37 | Malawi (2016–2017) | Cross-sectional study |
Malaria-mono-infection: 20–67 Co-infection: 18–66 |
Male (50), Female (57) | 107 participants with malaria | Undertreated | 76 (P. falciparum) | Microscopy, RDT | Severe malaria 18 | NA | RDT |
30 (P. falciparum) Severe malaria 12 (P. falciparum) |
| 23. | Niyongabo et al. (1994)45 | Burundi (1991–1992) | Cross-sectional study | Adults | Male (22), Female (9) | 31 cerebral malaria (P. falciparum) | Recently diagnosed | 29 (P. falciparum) | Microscopy | Severe malaria 17 | NA | ELISA, Western blot | Severe malaria 12 (P. falciparum) |
| 24. | Nkuo-Akenji et al. (2008)38 | Cameroon (2006) | Cross-sectional study | 15–49 years: 15–25, 26–35 and 36–49 years | Male (183), Female (501) | 684 outpatients | Undertreated | 324 (P. falciparum) | Microscopy | Hyperparasitemia 84 | 57 | RDT |
201 (P. falciparum) Hyperparasitemia 102 (P. falciparum) |
| 25. | Otieno et al. (2006)46 | Kenya | A prospective cohort study |
Children < 2 years: Malaria-mono-infection: 11.66 (0.45) Co-infection: 12.04 (1.34) |
Malaria-mono-infection: Male (109), Female (85) Co-infection: Male (12), Female (12) |
317 Children | Recently diagnosed | 194 (P. falciparum) | Microscopy | Severe anemia 37 | NA | RDT, PCR |
23 (P. falciparum) Severe anemia 15 (P. falciparum) |
| 26. | Sandie et al. (2019)39 | Cameroon (2014) | Cross-sectional study | 1–72 years | Male (112), Female (299) | HIV positive patients | Recently diagnosed and undertreated | 34 (Plasmodium spp.) | Microscopy | Severe anemia 1/3 | 285 | RDT |
24 (Plasmodium spp.) Severe anemia 1/9 (Plasmodium spp.) |
| 27. | Saracino et al. (2012)40 | Mozambique (2010) | Cross-sectional study | > 15 years | Male (262), Female (168) | 330 adult patients | Undertreated | 39 (P. falciparum) | Microscopy, RDT | Severe malaria 17 | NA | RDT |
51 (P. falciparum) Severe malaria 29 (P. falciparum) |
| 28. | Tagoe DNA and Boachie (2012)41 | Ghana | Cross-sectional study | 18–65 years | Male (59), Female (161) | 220 adults with HIV | Undertreated | NA | Microscopy, RDT | NA | 186 | NA |
34 (P. falciparum) Severe anemia 6 (P. falciparum) |
| 29. | Tay et al. (2015)17 | Ghana (2011–2012) | Cross-sectional study | 1–73 years: 1–4 (12), 5–9 (2), 10–14 (9), 15–24 (37), 25–34 (147), 35–44 (138), 45–60 (69), > 60 (5) | Male (108), Female (292) | 400 HIV sero-positive participants | Undertreated and immunosuppressed | NA | Microscopy | NA | 326 | RDT |
47 (Plasmodium spp.) Severe anemia 11 (Plasmodium spp.) |
ELISA enzyme-linked immunosorbent assay, GCS Glasgow Coma Scale, IHC immunohistochemistry, NA not applicable, P. Plasmodium, PCR polymerase chain reaction, RDT rapid diagnostic testing.
Table 2.
Parasitemia level and leukocyte differential counts in co-infections and Plasmodium mono-infections.
| No. | Author, year | Parasitemia level (cells/µL) | Leukocyte counts (103 cells/µL) | Neutrophil counts (103 cells/µL) | Lymphocyte counts (103 cells/µL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Co-infection | Plasmodium mono-infection | Co-infection | Plasmodium mono-infection | Co-infection | Plasmodium mono-infection | Co-infection | Plasmodium mono-infection | ||
| 1. | Addissie et al. (2007)21 | NA | NA | NA | NA | NA | NA | NA | NA |
| 2. | Amodu-Sanni et al. (2020)22 | NA | NA | NA | NA | NA | NA | NA | NA |
| 3. | Berg et al. (2014)2 | NA | NA | NA | NA | NA | NA | NA | NA |
| 4. | Berg et al. (2008)23 | NA | NA | NA | NA | NA | NA | NA | NA |
| 5. | Berg et al. (2020)24 | NA | NA | NA | NA | NA | NA | NA | NA |
| 6. | Berkley et al. (2009)25 | 37,500 (2680–172,150) (n = 133) | 22,352 (2213–142,590) (n = 938) | NA | NA | NA | NA | NA | NA |
| 7. | Beyene et al. (2017)26 | NA | NA | NA | NA | NA | NA | NA | NA |
| 8. | Bronzan et al. (2007)27 | 52,048 (n = 178) | 40,356 (n = 941) | 10.5 | 11.4 | NA | NA | NA | NA |
| 9. | Chalwe et al. (2009)28 | 43,314 (25,467–81,145) (n = 27) | 11,745 and 38,942 (n = 2) | 6.9 (3.9) | 4.2 and 8.7 | NA | NA | 1.73 (0.43) | 1.16 (0.58) |
| 10. | Cohen et al. (2005)15 | NA | NA | NA | NA | NA | NA | NA | NA |
| 11. | Davenport et al. (2010)29 | 16,220 (43,127) (n = 24) | 22,281 (51,064) (n = 406) | 13.3 (8.4) | 11.2 (6.6) | 35.6 (21.1) | 41.0 (22.8) | 49.4 (16.7) | 50.0 (19.2) |
| 12. | Grimwade et al. (2004)42 | NA | NA | NA | NA | NA | NA | NA | NA |
| 13. | Hendriksen et al. (2012)30 |
Aged < 15: 47,141 (38,005–58,474) (n = 74) Aged ≥ 15: 133,653 (59,082–302,343) (n = 581) |
Aged < 15: 68,320 (37,680–123,874) (n = 49) Aged ≥ 15: 61,525 (24,628–153,704) (n = 19) |
NA | NA | NA | NA | NA | NA |
| 14. | Hochman et al. (2015)31 |
CM1: 98,300 (48,200–324,800) (n = 7) CM2: 56,400 (28,800–308,700) (n = 7) |
CM1: 49,200 (5,100–717,600) (n = 5) CM2: 13,200 (7,500–433,300) (n = 33) |
CM1: 14.5 (12.1–17.1) CM2: 13.2 (10.9–19.3) |
CM1: 11.2 (7.3–15.7) CM2: 12.4 (9.1–21.5) |
NA | NA |
CM1: 2.4 (1.5, 5.1) CM2: 2.7 (1.5–5.3) |
CM1: 2.3 (2–5.5) CM2: 5.3 (2.2–7.8) |
| 15. | Huson et al. (2015)32 | 54,000 (17,340–134,700) (n = 14) |
4,740 (1,300– 18,300) (n = 113) |
5.1 (3.3–6.6) | 5.3 (3.9–7.2) | NA | NA | NA | NA |
| 16. | Imani et al. (2011)43 |
Cerebral malaria 240,000 (3080–583,520) (n = 9) |
Cerebral malaria 21,440 (1600–133,080) (n = 91) |
NA | NA | NA | NA | NA | NA |
| 17. | Jacques et al. (2019)33 | NA | NA | NA | NA | NA | NA | NA | NA |
| 18. | Joice et al. (2016)34 | 20,200 (2900–32,500) (n = 20) | 7400 (800–42,400) (n = 75) | 13.6 (11.9–14.7) | 11.2 (8.8–18.4) | NA | NA | NA | NA |
| 19. | Kyeyune et al. (2014)44 | NA | NA | NA | NA | NA | NA | NA | NA |
| 20. | Mandala et al. (2018)35 | NA | NA | 10.20 (7.85–13.75) (n = 9) | 11.20 (7.90–15.30) (n = 29) | 6.30 (5.23–12.35) | 7.40 (4.6–12.50) | 1.10 (0.85–1.73) | 2.01 (1.50–3.2) |
| 21. | Mouala et al. (2008)36 | NA | NA | NA | NA | NA | NA | NA | NA |
| 22. | Munyenyembe et al. (2018)37 | NA | NA | Severe malaria 6.15 (2.9–14.2) (n = 12), uncomplicated malaria 4.3 (2.4–9) (n = 18) | Severe malaria 5 (2.4–7.25) (18), uncomplicated malaria 5.6 (3.9–10.5) (n = 58) | Severe malaria 3.46 (0.9–12.4), uncomplicated malaria 2.25 (2.1–4.1) | Severe malaria 2.72 (1.1–3.74), uncomplicated malaria 3.03 (1.7–7.7) | Severe malaria 2.25 (1.1–2.8), uncomplicated malaria 1.4 (0.62–3.15) | Severe malaria 1.26 (0.6–3.3), uncomplicated malaria 1.58 (0.74–3.04) |
| 23. | Niyongabo et al. (1994)45 | NA | NA | NA | NA | NA | NA | NA | NA |
| 24. | Nkuo-Akenji et al. (2008)38 | NA | NA | NA | NA | NA | NA | NA | NA |
| 25. | Otieno et al. (2006)46 | NA | NA | NA | NA | NA | NA | NA | NA |
| 26. | Sandie et al. (2019)39 | NA | NA | NA | NA | NA | NA | NA | NA |
| 27. | Saracino et al. (2012)40 | 1.8 ± 1.1 (n = 51) | 2.3 ± 1.2 (n = 39) | 7.7 ± 2.5 | 7.7 ± 3.7 | NA | NA | NA | NA |
| 28. | Tagoe DNA and Boachie (2012)41 | NA | NA | NA | NA | NA | NA | NA | NA |
| 29. | Tay et al. (2015)17 | NA | NA | NA | NA | NA | NA | NA | NA |
NA not applicable.
Severe complications in patients with Plasmodium spp. and HIV co-infection
The total number of 1,171 severe complications were derived from 19 studies 2,15,17,23,24,26,27,29,30,32,34,35,38–42,44,46. The following severe complications were frequently reported in patients with Plasmodium spp. and HIV co-infection: severe anaemia (25.7%, 301/1171), hyperparasitaemia (15.1%, 177/1171), cerebral malaria (14.4%, 168/1171), coma (7.60%, 89/1171), convulsion (6.83%, 80/1171), and acute renal failure (6.15%, 72/1171). Among co-infected patients, ten patients who were undertreated died as reported by Berg et al.’s study (9 cases)24 and Huson et al. (1 case)32, while one patient who was immunosuppressed died as reported by Berg et al.’s study23. Other severe complications reported in patients co-infected with Plasmodium spp. and HIV are listed in Table 3.
Table 3.
Severe complications of co-infected patients.
| No. | Authors, year | Hypotension/shock | Hyperparasitemia | Severe anemia | Acute renal failure | Metabolic acidosis | Respiratory distress | Severe anemia and respiratory distress | Hypoglycemia | Cerebral malaria | Impaired consciousness/convulsion | Impaired consciousness | Coma | Convulsion | Prostration | Bleeding | Black water fever | Jaundice | Fatal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Addissie et al. (2007)21 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 2. | Amodu-Sanni et al. (2020)22 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 3. | Berg et al. (2014)2 | NS | 33 | 15 | 24 | NS | 25 | NS | 8 | NS | 9 | NS | NS | NS | NS | 13 | NS | 17 | NS |
| 4. | Berg et al. (2008)23 | NS | NS | NS | 6 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 2 | 1 |
| 5. | Berg et al. (2020)24 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 9 | NS | NS | 9 |
| 6. | Berkley et al. (2009)25 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 7. | Beyene et al. (2017)26 | NS | NS | 25 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 8. | Bronzan et al. (2007)27 | NS | NS | 28 | NS | NS | NS | NS | NS | 86 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 9. | Chalwe et al. (2009)28 | NS | 6 | 5 | NS | NS | NS | NS | 11 | NS | NS | 15 | NS | 6 | NS | NS | NS | 3 | NS |
| 10. | Cohen et al. (2005)15 | 2 | 3 | 3 | 14 | 11 | NS | NS | 1 | 3 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 11. | Davenport et al. (2010)29 | NS | NS | 14 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 12. | Grimwade et al. (2004)42 | NS | NS | 14 | 28 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
NS NS |
9 | NS |
| 13. | Hendriksen et al. (2012)30 | 6 | 33 | 18 | NS | 38 | 20 | 18 | 10 | NS | NS | NS | 89 | 74 | 31 | NS | 15 | 9 | NS |
| 14. | Huson et al. (2015)32 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 1 |
| 15. | Hochman et al. (2015)31 | NS | NS | NS | NS | NS | NS | NS | NS | 15 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 16. | Imani et al. (2011)43 | NS | NS | NS | NS | NS | NS | NS | NS | 9 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 17. | Jacques et al. (2019)33 | NS | NS | 86 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 18. | Joice et al. (2016)34 | NS | NS | NS | NS | NS | NS | NS | NS | 20 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 19. | Kyeyune et al. (2014)44 | NS | NS | 26 | NS | NS | NS | NS | NS | 26 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 20. | Mandala et al. (2018)35 | NS | NS | 5 | NS | NS | NS | NS | NS | 9 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 21. | Mouala et al. (2008)36 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 22. | Munyenyembe et al. (2018)37 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 23. | Niyongabo et al. (1994)45 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 24. | Nkuo-Akenji et al. (2008)38 | NS | 102 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 25. | Otieno et al. (2006)46 | NS | NS | 15 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 26. | Sandie et al. (2019)39 | NS | NS | 1 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 27. | Saracino et al. (2012)40 | NS | NS | 29 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 28. | Tagoe DNA and Boachie (2012)41 | NS | NS | 6 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 29. | Tay et al. (2015)17 | NS | NS | 11 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Total complications (1171) | 8 | 177 | 301 | 72 | 49 | 45 | 18 | 30 | 168 | 9 | 15 | 89 | 80 | 31 | 22 | 15 | 31 | 11 | |
| Percentage | 0.68 | 15.1 | 25.7 | 6.15 | 4.18 | 3.84 | 1.54 | 2.56 | 14.35 | 0.77 | 1.28 | 7.60 | 6.83 | 2.65 | 1.88 | 1.28 | 2.65 | 0.94 |
NS Not specified.
Risk of bias in individual studies
Of the 29 studies included, all included studies were judged to be of high quality (≥ 7 stars). Twenty-three studies were rated with nine stars, whereas six studies27,31,34,35,44,45 were rated with eight stars because they did not report the information on several non-SM patients with co-infection, which was the primary outcome of the present study. Table 4 provides the data on the risk of bias of the included studies.
Table 4.
Quality of the included studies.
| No. | Reference | Selection | Compatibility | Exposure | Total score (9) | Rating (high, moderate, low quality) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non–response rate | |||||
| 1. | Addissie et al. (2007)21 | * | * | * | * | ** | * | * | * | 9 | High |
| 2. | Amodu-Sanni et al. (2020)22 | * | * | * | * | ** | * | * | * | 9 | High |
| 3. | Berg et al. (2014)2 | * | * | * | * | ** | * | * | * | 9 | High |
| 4. | Berg et al. (2008)23 | * | * | * | * | ** | * | * | * | 9 | High |
| 5. | Berg et al. (2020)24 | * | * | * | * | ** | * | * | * | 9 | High |
| 6. | Berkley et al. (2009)25 | * | * | * | * | ** | * | * | * | 9 | High |
| 7. | Beyene et al. (2017)26 | * | * | * | * | ** | * | * | * | 9 | High |
| 8. | Bronzan et al. (2007)27 | * | * | * | * | * | * | * | * | 8 | High |
| 9. | Chalwe et al. (2009)28 | * | * | * | * | ** | * | * | * | 9 | High |
| 10. | Cohen et al. (2005)15 | * | * | * | * | ** | * | * | * | 9 | High |
| 11. | Davenport et al. (2010)29 | * | * | * | * | ** | * | * | * | 9 | High |
| 12. | Grimwade et al. (2004)42 | * | * | * | * | ** | * | * | * | 9 | High |
| 13. | Hendriksen et al. (2012)30 | * | * | * | * | ** | * | * | * | 9 | High |
| 14. | Hochman et al. (2015)31 | * | * | * | * | * | * | * | * | 8 | High |
| 15. | Huson et al. (2015)32 | * | * | * | * | ** | * | * | * | 9 | High |
| 16. | Imani et al. (2011)43 | * | * | * | * | ** | * | * | * | 9 | High |
| 17. | Jacques et al. (2019)33 | * | * | * | * | ** | * | * | * | 9 | High |
| 18. | Joice et al. (2016)34 | * | * | * | * | * | * | * | * | 8 | High |
| 19. | Kyeyune et al. (2014)44 | * | * | * | * | * | * | * | * | 8 | High |
| 20. | Mandala et al. (2018)35 | * | * | * | * | * | * | * | * | 8 | High |
| 21. | Mouala et al. (2008)36 | * | * | * | * | ** | * | * | * | 9 | High |
| 22. | Munyenyembe et al. (2018)37 | * | * | * | * | ** | * | * | * | 9 | High |
| 23. | Niyongabo et al. (1994)45 | * | * | * | * | * | * | * | * | 8 | High |
| 24. | Nkuo-Akenji et al. (2008)38 | * | * | * | * | ** | * | * | * | 9 | High |
| 25. | Otieno et al. (2006)46 | * | * | * | * | ** | * | * | * | 9 | High |
| 26. | Sandie et al. (2019)39 | * | * | * | * | ** | * | * | * | 9 | High |
| 27. | Saracino et al. (2012)40 | * | * | * | * | ** | * | * | * | 9 | High |
| 28. | Tagoe DNA and Boachie (2012)41 | * | * | * | * | ** | * | * | * | 9 | High |
| 29. | Tay et al. (2015)17 | * | * | * | * | ** | * | * | * | 9 | High |
Pooled prevalence of SM in patients with Plasmodium spp. and HIV co-infection
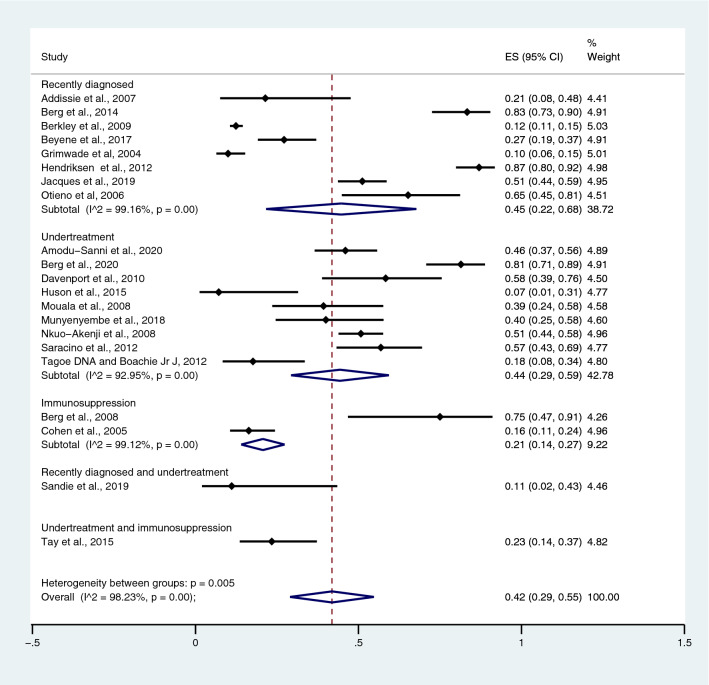
The number of Plasmodium spp. and HIV co-infected patients with SM and the total number of co-infected patients were analysed to estimate the pooled prevalence of SM in patients with co-infection. Overall, the pooled prevalence of SM in patients with Plasmodium spp. and HIV co-infection was 42.0%, according to 21 studies (95% CI 29.0–55.0%; I2, 98.2%) (Fig. 2). The highest prevalence estimate (87%) was found in the study by Hendriksen et al.30, whereas the lowest prevalence estimate (7%) was observed in the study by Huson et al.32. Prevalence estimates were stratified by the time of detection of HIV infection; the prevalence of SM among co-infected patients in whom HIV had been recently diagnosed was 45.0%, according to eight studies (95% CI 22.0–68.0%; I2, 99.2%); among those who received undertreatment, 44.0% according to nine studies (95% CI, 29.0%–59.0%; I2, 93.0%) and among those who were immunosuppressed, 21.0% according to two studies (95% CI 14.0–27.0%; I2, 99.1%).
Figure 2.
The pooled prevalence estimate of severe malaria in Plasmodium spp. and HIV co-infected patients.
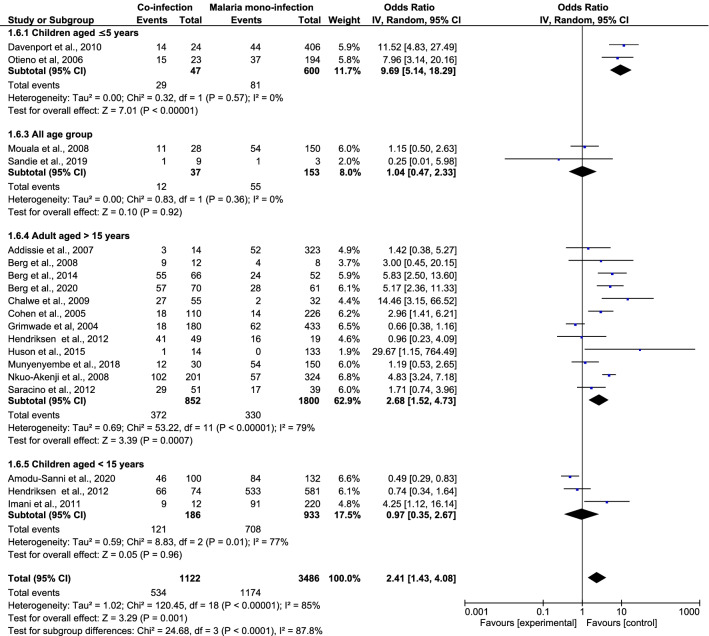
The odds of SM in Plasmodium spp. and HIV co-infected patients
When the number of Plasmodium spp. and HIV co-infected patients with SM were compared with the number of malaria mono-infected patients with SM, a significantly increased odds of SM were found in the former group, according to 19 studies (OR 2.41; 95% CI 1.43–4.08; I2 = 85.0%; P = 0.001; 19 studies) (Fig. 3). As heterogeneity was high (I2 statistic = 87.0%), the Random Effects model was used in the present analysis. The source of heterogeneity was identified by a subgroup analysis of the patients’ age. The subgroup analysis revealed that the odds of developing SM were significant in children aged < 5 years according to two studies (OR 9.69; 95% CI 5.14–18.3; I2, 0%; P < 0.0001) and in adults aged > 15 years who were co-infected with two pathogens according to 12 studies (OR 2.68; 95% CI 1.52–4.73; I2, 79.0%; P = 0.0007). The odds of malaria did not differ between co-infected patients and those with Plasmodium mono-infection in three studies that included children < 15 years of age (OR, 0.97; 95% CI, 0.35–2.67; I2, 77%; P = 0.96) or among all age groups in three studies (OR, 1.04; 95% CI, 0.47–2.33; I2, 0%; P = 0.92).
Figure 3.
The risk of severe malaria in Plasmodium spp. and HIV co-infected patients.
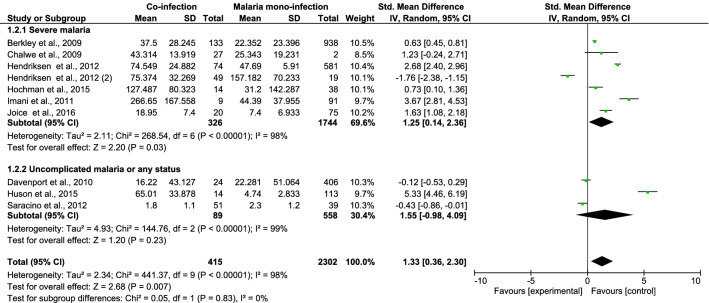
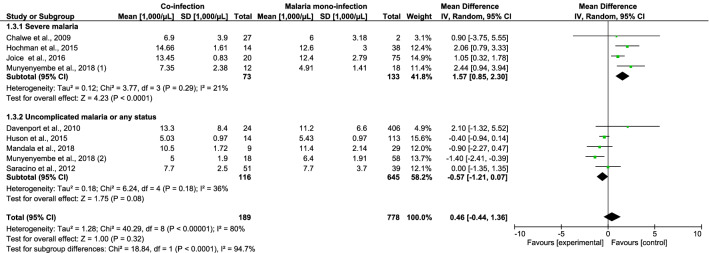
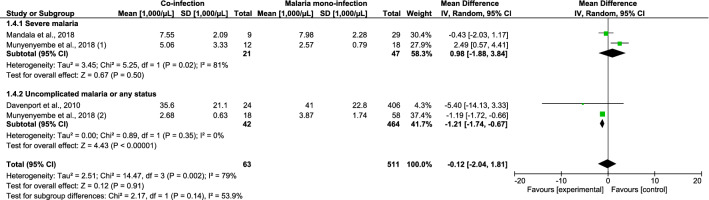
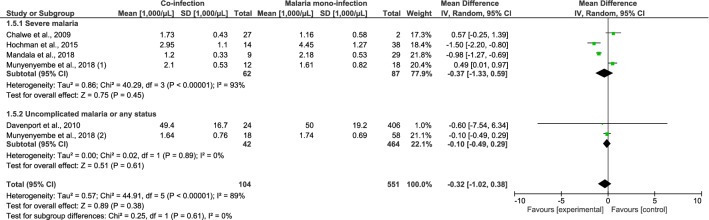
Parasite density, leukocyte count, and differential counts
The differences in parasite density, leukocyte counts, and differential counts of Plasmodium spp. and HIV co-infected and malaria mono-infected patients with SM were estimated. Patients co-infected with Plasmodium spp. and HIV who had SM had a higher mean parasite density than patients with Plasmodium mono-infection, according to six studies (standardised mean difference [SMD], 1.25; 95% CI 0.14–2.36; I2, 97%; P = 0.03) (Fig. 4). Co-infected patients with SM had higher leukocyte counts than patients with Plasmodium mono-infection, according to four studies (mean difference [MD], 1570 cells/µL; 95% CI 850–2300 cells/µL; I2, 21%; P < 0.0001) (Fig. 5). The mean neutrophil counts of patients with Plasmodium and HIV co-infection and SM as well as patients with Plasmodium mono-infection did not significantly differ according to two studies (MD, 980 cells/µL; 95% CI − 1880 to 3840 cells/µL; I2, 81.0%; P = 0.5; Fig. 6). The lymphocyte counts in Plasmodium spp. and HIV co-infected individuals with SM and those in Plasmodium mono-infected individuals were also similar according to four studies (MD, 370 cells/µL; 95% CI − 1330 to 590 cells/µL; I2, 93.0%; P = 0.45; Fig. 7).
Figure 4.
The pooled MD of parasite density between Plasmodium spp. and HIV co-infected and Plasmodium spp. patients.
Figure 5.
The pooled MD of total leukocyte between Plasmodium spp. and HIV co-infected and Plasmodium spp. patients.
Figure 6.
The pooled MD of neutrophil counts between Plasmodium spp. and HIV co-infected and Plasmodium spp. patients.
Figure 7.
The pooled MD of lymphocyte counts between Plasmodium spp. and HIV co-infected and Plasmodium spp. patients.
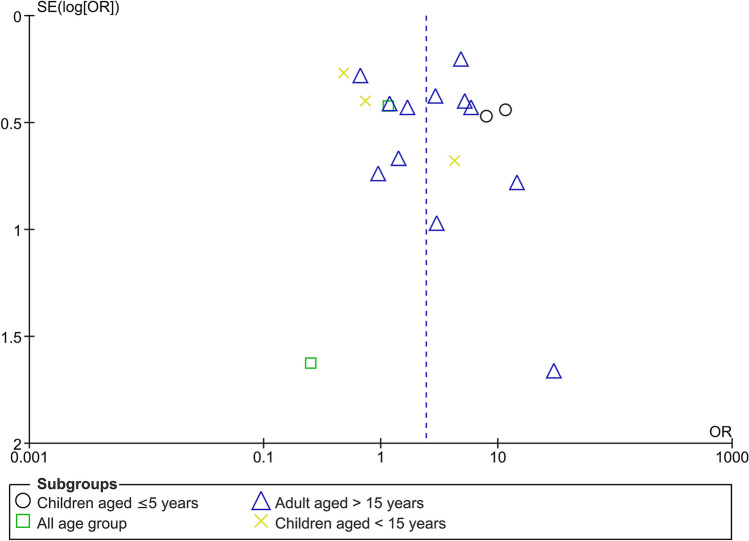
Publication bias
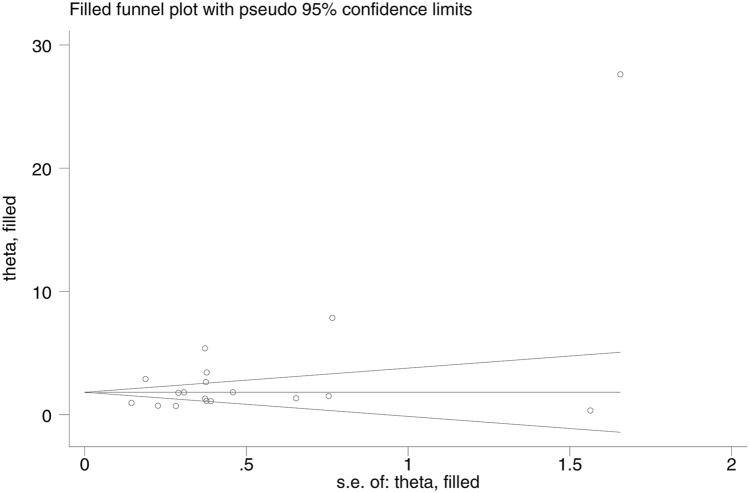
There was an indication of publication bias across the included studies, as demonstrated by the asymmetrical distribution of the funnel plot (Fig. 8).
Figure 8.
The funnel plot demonstrating publication bias across the included studies.
Sensitivity analysis
Because of the publication bias indicated in Fig. 8, we used the trim-and-fill method to evaluate the odds of SM caused by malaria and HIV co-infection in 18 studies. We found that the OR for the Fixed Effects model was 1.82 (P < 0.001; 95% CI 1.67–1.96), whereas the OR for the Random Effects model was 3.01 (P < 0.001; 95% CI 2.14–3.88; Fig. 9). We also used the trim-and-fill method to conduct the sensitivity analysis for the pooled prevalence of SM among co-infected patients. The pooled prevalence estimated by the Fixed Effects model was 13.5% (95% CI 12.2–14.8%), and that estimated by the Random Effects model was 16% (95% CI 2–29.9%).
Figure 9.
The funnel plot after trim-and-fill method was performed.
Discussion
Most studies reporting on co-infection with Plasmodium spp. and HIV were performed in Sub-Saharan Africa. The geographical overlap of these two types of infections has raised several research gaps as to how one infection influences the severity of the other. Our meta-analysis demonstrated a high prevalence of SM (43.0%) among patients with Plasmodium spp. and HIV co-infection, according to the data from 23 of 29 included studies. When the pooled prevalence of SM among co-infected patients was stratified by the time of detection of HIV infection, the prevalence of SM among co-infected patients recently diagnosed with HIV infection (48.0%), that among patients who were undertreated (44.0%), and that among immunosuppressed patients (45.0%) did not significantly differ, and the degree of SM severity in each subgroup was highly heterogeneous. Our sensitivity analysis of prevalence of SM among co-infected patients showed the prevalence of SM among co-infected patients were 13.5% by the Random Effects model, and 16% by the Fixed Effects model. This results suggested that the meta-analysis had the robustness of the conclusions that patients with Plasmodium spp. and HIV co-infection developed SM. The severity of malaria in patients with Plasmodium spp. and HIV co-infection may be caused by a low immune response, particularly a lower of CD4+ T cells in patients with HIV, leading to the uncontrolled number of malaria parasites, which may lead to SM23,36,47–49. A previous study suggested that the incidence of clinical malaria episodes was reported to be higher in patients with a CD4 cell count of < 200 cells/μL compared with those with a CD4 cell count of > 500 cells/µL50. Previous studies suggested that co-infection can facilitate the rate of malaria transmission by the process of CD4 cell activation, up-regulation of pro-inflammatory and cytokine production, and T-cell activation resulting in a reduction in the immune response49,51.
The study conducted in Mozambique demonstrated the highest prevalence of SM among children co-infected with Plasmodium spp. and HIV, who were characterised by undernourishment, severe acidosis, severe anaemia, respiratory distress, and elevated blood urea nitrogen concentrations30. The high prevalence of SM in that study might be attributable to the fact that 896 patients suspected of having SM were enrolled. Contrarily, the study with the lowest prevalence (7.0%), that of Huson et al.32, was a prospective observational study of 103 patients with sepsis and 127 with malaria and 60 HIV-infected individuals as a control group.
Our meta-analysis showed a significantly increased odds of SM in patients with Plasmodium spp. and HIV co-infection compared with those with Plasmodium spp. mono-infection. Our meta-analysis the odds of developing SM in patients co-infected with Plasmodium spp. and HIV depend on age. Although the higher odds of developing SM in adults than in children had been reported25,27,29,47,52, our meta-analysis demonstrated that the odds of developing SM were higher in children younger than five years and in children younger than 15 years. In addition, the odds of SM among co-infected children younger than five years (OR 9.69) were higher than those among co-infected adults older than 15 years (OR 2.68). Our sensitivity analysis of odds of SM in patients with Plasmodium spp. and HIV co-infection compared with those with Plasmodium spp. mono-infection showed the odds of SM among co-infected patients were higher than those among mono-infected patients (OR, 1.82 by the Fixed Effect model; OR, 3.0 by the Random Effects model). These results suggested that the meta-analysis had the robustness of the conclusions that patients with Plasmodium spp. and HIV co-infection increased odds of SM compared with those with Plasmodium spp. mono-infection. The development of SM among adults could be reflected by a failure to acquire immunity, which resulted in a higher parasite density among patients co-infected with Plasmodium spp. and HIV47. Conversely, Plasmodium spp. and HIV co-infection in children was associated with the rapid onset of cerebral malaria mediated by defects in macrophage phagocytosis34. This was supported by a previous study demonstrating lower absolute counts of CD4+ T cells, B cells, and NK cells in co-infected children who developed cerebral malaria35. That previous study demonstrated that HIV-positive patients are prone to additional opportunistic infections and febrile illnesses, which may be difficult to clinically distinguish from malaria23. Co-infection with Plasmodium spp. and HIV has been associated with a reduction in anticoagulant protein S and markers of endothelial activation, resulting in increased morbidity among co-infected patients32.
Our meta-analysis found that Plasmodium spp. and HIV co-infected patients with SM had a higher parasite density than Plasmodium spp. mono-infected patients with SM. We found that children younger than 5 years25,34 and children younger than 15 years31,43 who were co-infected with Plasmodium spp. and HIV and had SM had higher parasite densities than children with Plasmodium mono-infection. However, the study of adults aged 15–49 years that was conducted in Zambia28 demonstrated no difference in the mean parasite densities, whereas the study of both co-infected children younger than 15 years and adults older than 15 years that was conducted in Mozambique demonstrated that the SMD of parasite density was higher in children and lower in adults30. In patients with Plasmodium spp. and HIV co-infection, it was reported that malaria caused an increase in transitory HIV viral load53 and that HIV infection caused an increased susceptibility to malaria infection53 as well as induced more severe parasitaemia and higher rates of treatment failure13. These likely effects of HIV infection lead to impairment of the immune system, resulting in reduced control of parasite multiplication50.
Only a few studies have reported on the effects of co-infection on haematological parameters such as leukocytes, platelet counts, and haemoglobin levels. Our meta-analysis showed that Plasmodium spp. and HIV co-infected patients with SM had higher leukocyte counts than patients with Plasmodium spp. mono-infection. The leukocyte counts, particularly the neutrophil count, were significantly higher in patients with high parasitaemia compared with those with low and moderate parasitaemia, whereas lymphocyte counts were significantly lower in patients with high parasitaemia54. Our meta-analysis revealed higher leucocyte counts among studies conducted in Malawi during the periods of 1996–201134, 1996–201031, and 2016–201737, whereas the study conducted in Zambia during 2004–200528 demonstrated no differences in leucocyte counts. This difference might be explained by the fact that the study conducted in Zambia included HIV-infected patients who were immunosuppressed28. Although our meta-analysis demonstrated the differences in leucocyte counts, no difference in neutrophil counts or lymphocyte counts was observed. Among individual studies, the neutrophil counts were higher in the study conducted in Malawi in 2016–201737 but did not differ in the study conducted in Malawi during 2005–200635. Only these two studies, however, contained information on neutrophil counts. Therefore, the difference in the leucocyte counts should be investigated further.
Our meta-analysis of lymphocyte counts showed lower lymphocyte counts in two studies conducted in Malawi during the periods of 2005–200635 and 1996–201031 but higher lymphocyte counts in the study conducted in Malawi during 2016–201737. The heterogeneity of lymphocyte counts among the three studies might be explained by the fact that two of these studies included patients who have recently been diagnosed with HIV31,35, and the other study included HIV-infected patients who were undertreated37. These findings were in agreement with that of a previous study that demonstrated that a lower lymphocyte count in HIV-infected patients was associated with a more clinically advanced disease55. For other haematological changes in patients with Plasmodium spp. and HIV co-infection, such as red blood cell parameters, another previous study demonstrated that severe anaemia was caused by a reduction in erythropoiesis29.
Previous studies have shown that the mortality risk among individuals with Plasmodium spp. and HIV co-infection was twice as high as those with HIV mono-infection38,42,56. The mortality caused by the Plasmodium spp. and HIV co-infection was reported to be 282% higher in children and 64% higher in adults with SM compared to HIV-negative patients30. A previous study suggested that the severity and mortality of immunosuppression by HIV might be associated with hypoglycaemia and hypotension23.
Our study had several limitations. First, we excluded full clinical drug trials because our objective was to investigate the odds of SM in co-infected patients who did not receive any malaria treatment. A further meta-analytic study of the risk of SM in full clinical trials should be conducted. Second, patients with HIV status who rejected malaria testing or were not tested for malaria may have resulted in the underreporting of HIV and malaria co-infection, because HIV patients may present with atypical signs and symptoms of malaria57. Third, the difference in the CD4 cell count between patients with co-infection and those with Plasmodium mono-infection could not be meta-analysed as the CD4 data reported by some included studies were insufficient. Clinicians in the regions where both Plasmodium spp. and HIV are endemic should carefully consider co-infection as a differential diagnosis to prevent SM. Moreover, an early evaluation of HIV patients with suspected malaria may help reduce disease severity and mortality. Further longitudinal studies should focus on the impact of HIV on malaria infection to inform the management of co-infected individuals living with HIV/AIDS. In conclusion, our systematic review and meta-analysis demonstrated that Plasmodium spp. and HIV co-infection could lead to SM. As patients with Plasmodium spp. and HIV co-infection had a greater risk of developing SM than those with Plasmodium spp. mono-infection, it is necessary to diagnose and treat patients with Plasmodium spp. and HIV co-infection to reduce the number of cases of SM and death from co-morbidities.
Methods
Data sources and search strategy
The present systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)58. The searches were performed systematically in three databases, including PubMed, Scopus, and the Web of Science. The search terms: ‘(malaria or Plasmodium) AND HIV AND (coinfection OR co-infection)’ were used for the searches, applying search strategies relevant to each of the individual databases. Table S1 describes the details of the search strategy for all research databases. The end date for the search was 5 May 2020. All relevant articles (no limitation in the year of publication but limited to the English language) reporting on SM in patients with Plasmodium spp. and HIV co-infection were screened for eligibility. The reference lists of included studies and review articles were examined for additional studies. Searches in other sources, including Google Scholar, were also performed to maximise the number of included studies.
Study selection
The eligibility criteria for study inclusion were as follows: (1) cross-sectional studies, case–control or prospective studies reporting SM caused by Plasmodium spp. and HIV co-infection; (2) studies published in the English language, and (3) studies involving human samples. Any reports of a small number of cases (fewer than five), such as case reports, case series, commentaries, letters to editors, short reports, and research notes, were excluded from this study. As we aim to investigate the pooled prevalence of severe malaria in patients with malaria and HIV co-infection patients who did not receive any malaria treatment, rather than the incidence of severe malaria caused by co-infection, therefore, clinical drug trials were excluded from the present study. Two independent authors (MK and AM) screened abstract titles and evaluated the full-text articles according to the inclusion and exclusion criteria. Disagreements were resolved by requesting the third author (KUK) to reach a consensus.
Data extraction
Two authors (AM and MK) extracted the data from the included studies. The following data were extracted: author’s name, publication year, study location, study period, study design, age range, sex, type and number of participants enrolled, the detection method for Plasmodium spp. and HIV, number of Plasmodium spp. and HIV co-infection, number of Plasmodium spp. mono-infections, number of cases of SM caused by Plasmodium spp. and HIV co-infection and Plasmodium spp. mono-infection. The extracted data were entered in a standardised form of an Excel spreadsheet (Microsoft Corporation, USA).
Risk of bias in individual studies
The risk of bias of individual studies included in the present analysis was assessed independently by two authors (MK and FRM) using the Newcastle–Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses59. All included studies were judged based on three broad parameters, namely the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest59. A star system was developed for rating the quality of each included study with a ranging system from 1 to 9. The risk of bias was high if the study was rated < 7 stars, and the risk of bias was low if the study was rated ≥ 7 stars.
Statistical analysis
The primary outcome of the present study was to estimate the pooled prevalence of SM among patients with Plasmodium spp. and HIV co-infection. The pooled prevalence of SM among patients with Plasmodium spp. and HIV co-infection was estimated using the Random Effects model (method of DerSimonian and Laird)60. The results were demonstrated as the pooled prevalence estimate and 95% confidence intervals (CIs) using a forest plot. The meta-analysis of pooled prevalence was performed using Stata version 12.1 (StataCorp LP, College Station, TX, USA). As mentioned above, the secondary aim of the present study was to determine whether Plasmodium spp. and HIV co-infection is associated with higher odds of SM when compared with Plasmodium spp. mono-infection. The pooled odds ratio (OR) and 95% CI was estimated using (1) the number of patients with SM in the presence of Plasmodium spp. and HIV co-infection and those with Plasmodium spp. mono-infection; (2) the total number of patients with Plasmodium spp. and HIV co-infection and those with Plasmodium spp. mono-infections. The pooled mean differences (MDs) and 95% CI between laboratory parameters, including parasite density, and leukocyte and differential counts were estimated based on the means and standard deviations (SDs) between the two groups. Medians and ranges/interquartile ranges reported by included studies were transformed to means and SDs as described elsewhere61. Meta-analyses of the pooled ORs and MDs were performed using Review Manager (RevMan) 5.3 software (Version 5.3, London, UK). The heterogeneity among included studies was tested and quantified by the Cochrane chi-square, and I2 statistics were presented in the forest plots. If the I2 statistic was higher than 50%, indicating substantial heterogeneity62, the Random Effects model was used in the meta-analysis. A subgroup analysis of age groups and locations of participants was also performed to identify any difference in the odds of SM among subgroups.
Publication bias
Publication bias was evaluated by visual inspection of funnel plot asymmetry. Generally, if symmetry is observed, this indicates no publication bias, whereas asymmetry suggests publication bias across the included studies. If the results indicated a publication bias, we revised the estimate of the prevalence and the odds ratio after correcting for such publication bias in the sensitivity analysis using the trim-and-fill method26 utilizing Stata ver. 14 (Stata Corporation, College Station, TX, USA).
Supplementary Information
Author contributions
M.K., A.M., K.U.K., G.D.M., and F.R.M. participated in the study design, data analysis, and writing of the paper. All authors have read and approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95591-6.
References
- 1.WHO. World Malaria Report 2019 (2019). https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 25 July 2020.
- 2.Berg A, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: A prospective cross-sectional study. PLoS ONE. 2014;9:e88257. doi: 10.1371/journal.pone.0088257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Malaria in HIV/AIDS Patients (2017). http://www10.who.int/malaria/areas/high_risk_groups/hiv_aids_patients/en/. Accessed 25 July 2020.
- 4.WHO. (2019). HIV/AIDS. Available from https://www.who.int/news-room/fact-sheets/detail/hiv-aids Accessed 31 June 2020.
- 5.WHO Severe malaria. Trop. Med. Int. Health. 2014;19:7–131. doi: 10.1111/tmi.12313_2. [DOI] [PubMed] [Google Scholar]
- 6.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 7.Brejt JA, Golightly LM. Severe malaria: Update on pathophysiology and treatment. Curr. Opin. Infect. Dis. 2019;32:413–418. doi: 10.1097/QCO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 8.Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Prevalence of severe Plasmodium knowlesi infection and risk factors related to severe complications compared with non-severe P. knowlesi and severe P. falciparum malaria: A systematic review and meta-analysis. Infect. Dis. Poverty. 2020;9:106. doi: 10.1186/s40249-020-00727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naing C, Whittaker MA, Nyunt Wai V, Mak JW. Is Plasmodium vivax malaria a severe malaria?: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014;8:e3071. doi: 10.1371/journal.pntd.0003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotepui M, Kotepui KU, Milanez GJ, Masangkay FR. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: A systematic review, meta-analysis, and analysis of case reports. BMC Infect. Dis. 2020;20:363. doi: 10.1186/s12879-020-05046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Global prevalence and mortality of severe Plasmodium malariae infection: A systematic review and meta-analysis. Malar. J. 2020;19:274. doi: 10.1186/s12936-020-03344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Severity and mortality of severe Plasmodium ovale infection: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0235014. doi: 10.1371/journal.pone.0235014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 14.Cuadros DF, Branscum AJ, Crowley PH. HIV-malaria co-infection: Effects of malaria on the prevalence of HIV in East sub-Saharan Africa. Int. J. Epidemiol. 2011;40:931–939. doi: 10.1093/ije/dyq256. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin. Infect. Dis. 2005;41:1631–1637. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- 16.Renia L, Potter SM. Co-infection of malaria with HIV: An immunological perspective. Parasite Immunol. 2006;28:589–595. doi: 10.1111/j.1365-3024.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 17.Tay SC, Badu K, Mensah AA, Gbedema SY. The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels. Ann. Clin. Microbiol. Antimicrob. 2015;14:10. doi: 10.1186/s12941-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naing C, Sandhu NK, Wai VN. The effect of malaria and HIV co-infection on anemia: A meta-analysis. Medicine (Baltimore) 2016;95:e3205. doi: 10.1097/MD.0000000000003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: A systematic review of the literature. Am. J. Med. 2004;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Kotepui M, Kotepui KU, Milanez GD, Masangkay FR. Reduction in total leukocytes in malaria patients compared to febrile controls: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0233913. doi: 10.1371/journal.pone.0233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addissie A, Sellassie FE, Deressa W. Malaria and HIV co-infection in Hadya Zone, southern Ethiopia. Ethiop. Med. J. 2007;45:9–17. [PubMed] [Google Scholar]
- 22.Amodu-Sanni M, et al. Prevalence and clinical forms of malaria among febrile HIV-infected children seen at Usmanu Danfodiyo University teaching hospital, Sokoto, Nigeria. Afr. J. Infect. Dis. 2020;14:24–32. doi: 10.21010/ajid.v14i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg A, Patel S, Langeland N, Blomberg B. Falciparum malaria and HIV-1 in hospitalized adults in Maputo, Mozambique: Does HIV-infection obscure the malaria diagnosis? Malar. J. 2008 doi: 10.1186/1475-2875-7-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg A, et al. Plasma parasitemia as assessed by quantitative PCR in relation to clinical disease severity in African adults with falciparum malaria with and without HIV co-infection. Infection. 2020 doi: 10.1007/s15010-020-01399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkley JA, et al. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin. Infect. Dis. 2009;49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyene HB, Tadesse M, Disassa H, Beyene MB. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in Northern Ethiopia. Acta Trop. 2017;169:8–13. doi: 10.1016/j.actatropica.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Bronzan RN, et al. Bacteremia in Malawian children with severe malaria: Prevalence, etiology, HIV coinfection, and outcome. J. Infect. Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 28.Chalwe V, et al. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerg. Infect. Dis. 2009;15:749–755. doi: 10.3201/eid1505.081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport GC, et al. Hematological predictors of increased severe anemia in Kenyan children coinfected with Plasmodium falciparum and HIV-1. Am. J. Hematol. 2010;85:227–233. doi: 10.1002/ajh.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendriksen IC, et al. Diagnosis, clinical presentation, and in-hospital mortality of severe malaria in HIV-coinfected children and adults in Mozambique. Clin. Infect. Dis. 2012;55:1144–1153. doi: 10.1093/cid/cis590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hochman SE, et al. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio. 2015;6:e01390. doi: 10.1128/mBio.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huson MAM, et al. Impact of HIV infection on the haemostatic response during sepsis and malaria. Br. J. Haematol. 2016;173:918–926. doi: 10.1111/bjh.14006. [DOI] [PubMed] [Google Scholar]
- 33.Jacques M, Salissou MM, Kaswiyi L, Guan F, Lei JH. Co-infection of malaria and HIV infection in severely undernourished children in the Democratic Republic of the Congo: A cross-sectional study. Parasitology. 2020;147:248–253. doi: 10.1017/s0031182019001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joice R, et al. Evidence for spleen dysfunction in malaria-HIV co-infection in a subset of pediatric patients. Mod. Pathol. 2016;29:381–390. doi: 10.1038/modpathol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandala WL, et al. HIV infection compounds the lymphopenia associated with cerebral malaria in Malawian children. J. Blood Med. 2019;10:9–18. doi: 10.2147/JBM.S187081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouala C, et al. Imported malaria in HIV-infected patients enrolled in the ANRS CO4 FHDH study. J. Acquired Immune Defic. Syndr. 2008;49:55–60. doi: 10.1097/QAI.0b013e31817e635b. [DOI] [PubMed] [Google Scholar]
- 37.Munyenyembe AU, et al. HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria. J. Blood Med. 2018;9:153–162. doi: 10.2147/jbm.S172869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nkuo-Akenji T, Tevoufouet EE, Nzang F, Ngufor N, Fon E. High prevalence of HIV and malaria co-infection in urban Douala, Cameroon. Afr. J. AIDS Res. 2008;7:229–235. doi: 10.2989/ajar.2008.7.2.8.525. [DOI] [PubMed] [Google Scholar]
- 39.Sandie SM, Sumbele IUN, Tasah MM, Kimbi HK. Malaria parasite prevalence and Haematological parameters in HIV seropositive patients attending the regional hospital Limbe, Cameroon: A hospital-based cross-sectional study. BMC Infect. Dis. 2019;19:988. doi: 10.1186/s12879-019-4629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saracino A, et al. Prevalence and clinical features of HIV and malaria co-infection in hospitalized adults in Beira, Mozambique. Malar. J. 2012;11:8. doi: 10.1186/1475-2875-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tagoe DNA, Boachie J., Jr Assessment of the impact of malaria on cd4+ T Cells and haemoglobin levels of HIV-malaria co-infected patients. J. Infect. Dev. Ctries. 2012;6:660–663. doi: 10.3855/jidc.2124. [DOI] [PubMed] [Google Scholar]
- 42.Grimwade K, et al. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS (London, England) 2004;18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 43.Imani PD, Musoke P, Byarugaba J, Tumwine JK. Human immunodeficiency virus infection and cerebral malaria in children in Uganda: A case-control study. BMC Pediatr. 2011;11:5. doi: 10.1186/1471-2431-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyeyune FX, et al. The interaction between malaria and human immunodeficiency virus infection in severely anaemic Malawian children: A prospective longitudinal study. Trop. Med. Int. Health. 2014;19:698–705. doi: 10.1111/tmi.12295. [DOI] [PubMed] [Google Scholar]
- 45.Niyongabo T, et al. Prognostic indicators in adult cerebral malaria: A study in Burundi, an area of high prevalence of HIV infection. Acta Trop. 1994;56:299–305. doi: 10.1016/0001-706x(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 46.Otieno RO, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS (London, England) 2006;20:275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 47.Whitworth J, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: A cohort study. Lancet. 2000;356:1051–1056. doi: 10.1016/s0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 48.French N, et al. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS (London, England) 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 49.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laufer MK, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J. Infect. Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 51.Chavale H, Santos-Oliveira JR, Da-Cruz AM, Enosse S. Enhanced T cell activation in Plasmodium falciparum malaria-infected human immunodeficiency virus-1 patients from Mozambique. Mem. Inst. Oswaldo Cruz. 2012;107:985–992. doi: 10.1590/s0074-02762012000800004. [DOI] [PubMed] [Google Scholar]
- 52.Korenromp EL, et al. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerg. Infect. Dis. 2005;11:1410–1419. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kublin JG, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: A prospective cohort study. Lancet. 2005;365:233–240. doi: 10.1016/S0140-6736(05)17743-5. [DOI] [PubMed] [Google Scholar]
- 54.Kotepui M, et al. Effects of malaria parasite density on blood cell parameters. PLoS ONE. 2015;10:e0121057. doi: 10.1371/journal.pone.0121057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavu EK, Kutson N, Connie C, Tau G, Sims P. Total lymphocyte counts in adult HIV/AIDS patients in Port Moresby General Hospital. P. N. G Med. J. 2004;47:31–38. [PubMed] [Google Scholar]
- 56.Quigley MA, et al. The effect of malaria on mortality in a cohort of HIV-infected Ugandan adults. Trop. Med. Int. Health. 2005;10:894–900. doi: 10.1111/j.1365-3156.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- 57.Brentlinger PE, Behrens CB, Kublin JG. Challenges in the prevention, diagnosis, and treatment of malaria in human immunodeficiency virus infected adults in sub-Saharan Africa. Arch. Intern. Med. 2007;167:1827–1836. doi: 10.1001/archinte.167.17.1827. [DOI] [PubMed] [Google Scholar]
- 58.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wells, G., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses (2013). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 25 July 2020.
- 60.Nyaga VN, Arbyn M, Aerts M. Metaprop: A stata command to perform meta-analysis of binomial data. Arch. Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019) (2019). www.training.cochrane.org/handbook. Accessed 25 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.