Abstract
Bone defects often require operative intervention with the use of bone graft. Two sources of autologous bone graft include reamer-irrigator-aspirate (RIA) and bone marrow aspirate concentrate (BMC). Osteoprogenitor cells and osteoconductive proteins have been identified in both sources. This study collected samples of these cells and proteins from a canine model and cultured them on human cancellous allograft bone blocks. Findings suggest that BMC may be preferred for indications that allow for delivery via injection, saturation of the patient's tissues, or an implanted scaffold, whereas RIA may be preferred when the biologic augment is delivered as a scaffold or graft.
Keywords: Bone healing, Autologous bone graft, Reamer-irrigator-aspirate, Bone marrow aspirate concentrate, Pre-clinical animal model
1. Introduction
While most fractures that occur in the United States heal, a significant proportion are at risk for delayed union or nonunion.1 The current gold standard for enhancing bone healing involves use of iliac crest autograft for its osteoconductive, osteoinductive, and osteogenic properties.2 However, iliac crest autograft is limited by the amount of graft that is able to be obtained and is associated with potential morbidity and complications.3 As such, numerous autograft, allograft, and synthetic alternatives for enhancing bone healing have been investigated.
Autograft alternatives that have been reported to safely and effectively enhance bone healing with potential advantages over iliac crest autograft include long bone harvesting using the Reamer-Irrigator-Aspirator (RIA) (DePuy Synthes, West Chester, PA, USA) and bone marrow aspirate concentrate (BMC).4,5 RIA is obtained using the commercially available system to collect intramedullary bone particles and irrigation filtrate from a patient's femur or tibia. RIA bone autograft and filtrate in liquid and lyophilized-powder forms contain osteoprogenitor cells and/or osteoinductive proteins that can enhance bone healing.4,6,7 However, associated complications of RIA include anemia requiring transfusion, iatrogenic fracture, hematoma, infection, and fat embolism.4 BMC is obtained by aspirating bone marrow from any of a number of anatomic sites and concentrating it in one of the commercially available systems to produce a liquid containing nucleated cells and proteins. The concentration process increases the nucleated cell concentration of the BMC by 2–40 times more than the bone marrow aspirate, providing a high concentration of osteoprogenitor cells and osteoinductive factors to deliver to the site of interest.5,8,9 The number of cells obtained via BMC varies by patient, site, aspiration technique, and concentrating system used. Complications of this technique include anemia not requiring transfusion, neuralgia, hematoma, superficial wound infection, and ossification at the aspiration site.10
While RIA and BMC have been compared to iliac crest autograft and evaluated in vitro and in animal models for their bone healing potential in various ways, they have not been directly compared to one another with respect to their osteoprogenitor cell retention and osteoinductive protein release on a clinically relevant substrate.6, 7, 8, 9, 10, 11, 12 Therefore, this study was designed to test the hypothesis that RIA would be associated with significantly higher osteoprogenitor cell concentration and osteoinductive protein elution compared to BMC when cultured on cancellous allograft bone using a preclinical canine model.
2. Methods
2.1. Research animals and bone marrow collection
With animal care and use committee approval from our institution, skeletally mature purpose-bred research hounds (n = 3 females, mean weight = 27 kg) underwent aseptic surgery of one randomly assigned pelvic limb immediately following humane euthanasia (n = 2) in conjunction with another unrelated study or under general anesthesia (n = 1). Bone marrow (42–48 ml) was aspirated (BMA) from the iliac crest using jamshidi needles and 30 ml syringes containing 5 ml Anticoagulant Citrate Dextrose Solution A (ACDA) via a dorsal percutaneous approach. BMA was immediately concentrated in the operating room using a commercially available system (Angel System, Arthrex, Naples, FL, USA; 7% setting) to obtain BMC (3–4 ml). RIA was collected from the ipsilateral femur (RIA2, 10 mm head, DePuy Synthes, Westchester, PA, USA). Using the manufacturer's recommended technique and instrumentation, 3 full proximal-distal-proximal reaming passes were performed in antegrade fashion from the intertrochanteric notch using a total of 1 L of isotonic saline irrigation. Three ml of the RIA bone graft was placed in a sterile syringe. Both samples from each dog were immediately transported to the laboratory for culture.
2.2. Cell culture
Demineralized cancellous bone cubes (Conform Cube, MTF Biologics, Edison, NJ, USA; 3 mm3) were placed in 6-well tissue culture plates and saturated with 250 μl of RIA (n = 18; 6 per dog) or BMC (n = 18; 6 per dog) and cultured for 7 or 14 days in 8 ml of DMEM media containing 10% FBS, PEN/STREP/AMPH, L-glut, ascorbic acid, NA pyruvate, and NE amino acids (ThermoFisher, Waltham, MA, USA) at 37 °C, 5% CO2, and 95% humidity. Media were changed on days 3, 7, 11, and 14 of culture, and collected for biomarker analysis on days 3, 7, and 14. Bone blocks and culture well bottom were assessed for viable osteoprogenitor cell colonization on days 7 and 14 of culture.
2.3. Media analysis
Media samples from days 3, 7, and 14 were analyzed using canine-specific assays to measure adrenocorticotropic hormone (ACTH), dickkopf-related protein 1 (DKK1), leptin, osteoprotegerin (OPG), osteocalcin (OC), osteopontin (OPN), sclerostin (SOST), parathyroid hormone (PTH), epidermal growth factor (EGF), fibroblast growth factor (FGF)-23, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-AA, and PDGF-BB (MyBiosource, San Diego, CA, USA).
2.4. Viable osteoprogenitor cell colonization
On days 7 and 14, each bone block was analyzed for viable cell attachment and infiltration using the fluorescent live stain, calcein acetoxymethyl (AM). Blocks were incubated in phosphate buffered saline (PBS) containing calcein AM (10 μM) for 30 min at 37 °C. After staining, blocks were washed in PBS for 5 min at room temperature to remove excess stain. The outer surface of the block was subjectively assessed using an Olympus BX-51 fluorescent microscope. Images were taken at 4x magnification using an F-view CCD cooled fluorescent camera and the program MicroSuite (Olympus Corp, Shinjuku, Tokyo, Japan). After the surface was evaluated, the block was transected to subjectively assess infiltration of viable cells into blocks during culture. Phase contrast microscopy was used to visualize osteoprogenitor cell adherence to the culture well surface. Images were taken at 20X magnification and subjectively assessed for cell colonization and expansion. Extent of cell adherence to the bone block and culture well plate were determined subjectively by one observer who was blinded to treatment and time point.
2.5. Statistical analysis
A paired t-Test was performed to determine significant differences between BMC and RIA groups for media biomarker concentrations with significance set at P < 0.05.
3. Results
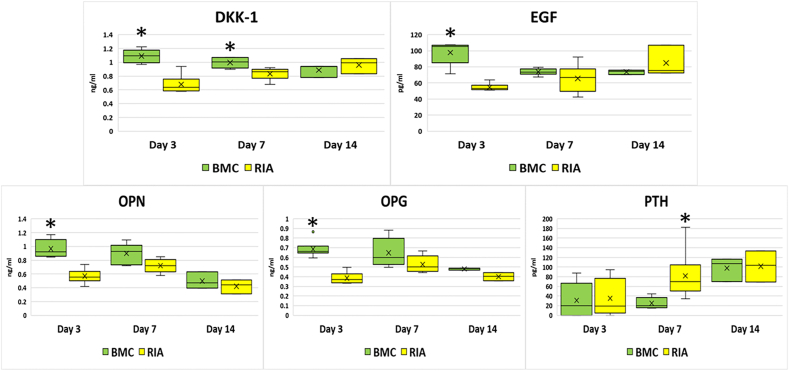
The BMC group was associated with subjectively higher viable cell density on cancellous bone and on the bottom of culture wells compared to the RIA group at both time points (Fig. 1). Media concentrations for most biomarkers analyzed revealed no significant differences between RIA and BMC groups. Statistically significant differences in media concentrations were noted for DKK-1 (higher in the BMC group at days 3 and 7); EGF, osteopontin, and osteoprotegerin (higher in the BMC group at day 3); and PTH (higher the RIA group at day 7) (Fig. 2).
Fig. 1.
Representative 4X fluorescent viability staining (green) and phase contrast images of cells adherent to the bone block (cell viability) or well surface (phase contrast) after treatment with bone marrow concentrate (BMC) or reamer-irrigator-aspirate (RIA) bone material and cultured for 7 or 14 days. Images from the surface and interior of the bone block.
Fig. 2.
Media concentrations of dickkopf-related protein 1 (DKK1), epidermal growth factor (EGF), osteoprotegerin (OPG), osteopontin (OPN), and parathyroid hormone (PTH) from bone blocks treated with bone marrow concentrate (BMC) or reamer-irrigator-aspirate (RIA) on days 3, 7, and 14 of culture. (*) Significantly higher than other group on that day of culture.
4. Discussion
These study results require rejection of the hypothesis that RIA would be associated with significantly higher osteoprogenitor cell concentration and osteoinductive protein elution compared to BMC when cultured on cancellous allograft bone using a preclinical canine model. While both RIA and BMC produced osteoprogenitor cells that infiltrated and remained viable on and within cancellous bone blocks, BMC was consistently associated with higher viable cell adherence to the bone block and the culture well surface. Further, detectable levels of key osteoinductive proteins were identified in the media of RIA and BMC samples, indicating that both methods could be associated with the sustained release of the proteins. However, the data from this study indicate that BMC is associated with higher initial release of some key osteoinductive proteins—including DKK-1, EGF, osteopontin, and osteoprotegerin—when compared to RIA. The higher cell density noted for BMC suggests that the cell concentration and delivery process was more effective in producing viable osteoprogenitor cells with osteoinductive protein synthetic capabilities when compared to cell migration from RIA graft onto demineralized cancellous bone. These findings have important clinical implications for bone healing augmentation indications that allow for delivery via injection or saturation, with potential use in nonunion and avascular necrosis cases.13, 14, 15
Bone healing is a complex biochemical and biomechanical process that requires osteoprogenitor cells and osteoconductive proteins for elaboration of functional extracellular matrix.16 Osteoprogenitor cells are needed to form organic bone matrix, coordinate mineralization, and produce additional signaling molecules.17,18 DKK-1 is an inhibitor of the Wnt pathway and is expressed in osteoblasts and osteocytes.19 It inhibits osteoblast-mediated bone mineralization, and deletion of DKK1 has been found to increase bone mass.20,21 EGF is catabolic to bone production and loss of EGF activity decreases cortical bone thickness.22 Although in vitro EGF promotes osteoclastogenesis, upregulation of EGFR activity decreases bone resorption in mouse models.22 In a rat model of surgically induced femoral head avascular necrosis, Basal et al.23 found that addition of EGF to core decompression promoted bone formation compared to core decompression alone. Osteopontin is primarily secreted by osteoblasts and regulates bone mass.24 It promotes mesenchymal stem cell migration and increases osteoblast and osteoclast adhesion.24,25 Osteoprotegerin is secreted by osteoblasts and inhibits bone resorption by binding receptors on osteoclasts.26 Mice deficient in osteoprotegerin develop osteoporosis.27 Parathyroid hormone is produced by the chief cells in the parathyroid gland and is anabolic to bone healing when administered in an intermittent but not continuous fashion.28 It prevents apoptosis of osteoblasts via a direct pathway and also contributes to increased bone mineral density via activation of IGF-1.29
Previous studies have confirmed presence of many osteoconductive proteins in both RIA and BMC. Schmidmaier et al.30 studied FGFb, PDGFbb, VEGF, IGF, and BMP-2 in reaming aspirate versus iliac crest. Many of these factors were higher in the RIA group; our study may have found the opposite because the aspirate was concentrated. Crist et al.7 compared RIA graft with iliac crest plus RIA waste water. Osteoprotegerin and osteopontin were found at all time points in their study, but interestingly PTH was not found at any time point in either group. In our study, PTH was found in both samples and was found to be higher in the RIA group at day 7. Since PTH is only synthesized and stored in the parathyroid gland, this finding may be due to enhanced retention in RIA as it was harvested in more solid graft form compared to the BMC. In the Crist et al.7 study, the iliac crest group contained higher osteoprotegerin, osteopontin, and EGF at various time points. This is consistent with our findings of higher concentrations of the same proteins in the BMC group. Since their study harvested iliac crest bone graft without concentration of any elements, this similarity may be reflective of the richer environment of the iliac crest donor site compared to the femur.
This study was associated with limitations that must be considered when interpreting and applying the results. The experimental design included a relatively small sample size and used RIA and BMC from an animal model for in vitro assessments on human demineralized cancellous bone allografts. While these design features limit direct clinical application, they allowed for standardized comparisons that do have clinical relevance. Both products were obtained using standard clinical technique and instrumentation, and the use of demineralized bone and canine-specific assays mitigate any confounding multi-species effects. In addition, cell phenotype was not verified and assessments for viable cell density were subjective in nature, such that the conclusions regarding osteoprogenitor cell retention are only qualitative. However, the quantitative assessment of osteoinductive proteins that showed statistically significant differences between groups supports the qualitative conclusions regarding osteoprogenitor cell retention differences that were reported.
Taken together, the data from this preclinical study suggest that both RIA and BMC have potential to serve as effective biological augments for bone healing through delivery of osteoprogenitor cells and osteoinductive proteins. The differences in viable osteoprogenitor cell retention and osteoinductive protein production between these two biologic agents, in conjunction with their material properties and methods of delivery, have important clinical implications for bone healing augmentation indications. BMC may be preferred for indications that allow for delivery via injection or saturation of the patient's tissues or an implanted scaffold, whereas RIA may be preferentially indicated when the biologic augment needs to be delivered as a scaffold or graft. A human trial is underway at our institution comparing RIA and BMC to further elucidate the results of this study.
5. Conclusion
RIA and BMC deliver osteoprogenitor cells and osteoinductive proteins. However, in this preclinical study, BMC had a higher viable cell adherence to the bone block and the culture well surface, as well as higher concentrations of some key osteoinductive proteins including DKK-1, EGF, osteopontin, and osteoprotegerin.
Author contributions statement
All authors were instrumental in study design, acquisition and analysis of study data, manuscript drafting, and final approval of all manuscript-related items.
Declaration of competing interest
This study received grant funding from the AO Trauma North America Fellows Research program. Brett D. Crist, MD, and James P. Stannard, MD, are both paid consultants for DePuy Synthes; the Reamer-Irrigator-Aspirator (RIA) system used in this study is a DePuy Synthes product. The authors also received in-kind support from DePuy Synthes for the RIA products used in this study.
References
- 1.Rupp M., Biehl C., Budak M., Thormann U., Heiss C., Alt V. Diaphyseal long bone nonunions - types, aetiology, economics, and treatment recommendations. Int Orthop. 2018;42(2):247–258. doi: 10.1007/s00264-017-3734-5. [DOI] [PubMed] [Google Scholar]
- 2.Nauth A., Lee M., Gardner M.J. Principles of nonunion management: state of the art. J Orthop Trauma. 2018;32(Suppl 1):S52–S57. doi: 10.1097/BOT.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 3.Loeffler B.J., Kellam J.F., Sims S.H., Bosse M.J. Prospective observational study of donor-site morbidity following anterior iliac crest bone-grafting in orthopaedic trauma reconstruction patients. J Bone Joint Surg Am. 2012;94(18):1649–1654. doi: 10.2106/JBJS.K.00961. [DOI] [PubMed] [Google Scholar]
- 4.Madison R.D., Nowotarski P.J. The reamer-irrigator-aspirator in nonunion surgery. Orthop Clin N Am. 2019;50(3):297–304. doi: 10.1016/j.ocl.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Schottel P.C., Warner S.J. Role of bone marrow aspirate in orthopedic trauma. Orthop Clin N Am. 2017;48(3):311–321. doi: 10.1016/j.ocl.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Wessel A.R., Crist B.D., Stannard J.P. Assessment of Reamer Irrigator Aspirator System (RIA) filtrate for its osteoinductive potential in a validated animal model. Injury. 2018;49(6):1046–1051. doi: 10.1016/j.injury.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Crist B.D., Stoker A.M., Stannard J.P., Cook J.L. Analysis of relevant proteins from bone graft harvested using the reamer irrigator and aspirator system (RIA) versus iliac crest (IC) bone graft and RIA waste water. Injury. 2016;47(8):1661–1668. doi: 10.1016/j.injury.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Gianakos A., Ni A., Zambrana L., Kennedy J.G., Lane J.M. Bone marrow aspirate concentrate in animal long bone healing: an analysis of basic science evidence. J Orthop Trauma. 2016;30(1):1–9. doi: 10.1097/BOT.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler C.G., Van Sloun R., Gonzalez S. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: a prospective analysis. Am J Sports Med. 2019;47(9):2174–2187. doi: 10.1177/0363546519832003. [DOI] [PubMed] [Google Scholar]
- 10.Imam M.A., Holton J., Ernstbrunner L. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017;41(11):2213–2220. doi: 10.1007/s00264-017-3597-9. [DOI] [PubMed] [Google Scholar]
- 11.Uppal H.S., Peterson B.E., Misfeldt M.L. The viability of cells obtained using the Reamer-Irrigator-Aspirator system and in bone graft from the iliac crest. Bone Jt J. 2013;95-B(9):1269–1274. doi: 10.1302/0301-620X.95B9.31756. [DOI] [PubMed] [Google Scholar]
- 12.Bakker A.D., Kroeze R.J., Korstjens C., de Kleine R.H., Frölke J.P.M., Klein-Nulend J. Reaming debris as a novel source of autologous bone to enhance healing of bone defects. J Biomed Mater Res. 2011;97(4):457–465. doi: 10.1002/jbm.a.33080. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P., Poignard A., Manicom O., Mathieu G., Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P., Mathieu G., Poignard A., Manicom O., Beaujean F., Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):322–327. doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- 15.Harford J.S., Dekker T.J., Adams S.B. Bone marrow aspirate concentrate for bone healing in foot and ankle surgery. Foot Ankle Clin. 2016;21(4):839–845. doi: 10.1016/j.fcl.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahian A., Davis D.D. StatPearls. StatPearls Publishing; 2021. Histology, osteoprogenitor cells.http://www.ncbi.nlm.nih.gov/books/NBK559160/ Accessed. [PubMed] [Google Scholar]
- 18.ClinicalKey Cartilage and bone. Elsevier.com. 2021. https://www-clinicalkey-com.eresources.mssm.edu/#!/content/book/3-s2.0-B9780323672726000072?scrollTo=%23hl0000690 Accessed April 26.
- 19.Huang Y., Liu L., Liu A. Dickkopf‐1: current knowledge and related diseases. Life Sci. Published online. 2018 doi: 10.1016/j.lfs.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Sarosi I., Cattley R.C. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39(4):754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Morvan F., Boulukos K., Clément-Lacroix P. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res Off J Am Soc Bone Miner Res. 2006;21(6):934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., Tamasi J., Lu X. Epidermal growth factor receptor plays an anabolic role in bone metabolism in vivo. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(5):1022–1034. doi: 10.1002/jbmr.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basal O., Atay T., Ciris İ.M., Baykal Y.B. Epidermal growth factor (EGF) promotes bone healing in surgically induced osteonecrosis of the femoral head (ONFH) Bosn J Basic Med Sci. 2018;18(4):352–360. doi: 10.17305/bjbms.2018.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Si J., Wang C., Zhang D., Wang B., Hou W., Zhou Y. Osteopontin in bone metabolism and bone diseases. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e919159-1–e919159-9. doi: 10.12659/MSM.919159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Icer M.A., Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Boyce B.F., Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucay N., Sarosi I., Dunstan C.R. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam C.S., Heersche J.N., Murray T.M., Parsons J.A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110(2):506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 29.Miyakoshi N. Effects of parathyroid hormone on cancellous bone mass and structure in osteoporosis. Curr Pharmaceut Des. 2004;10(21):2615–2627. doi: 10.2174/1381612043383737. [DOI] [PubMed] [Google Scholar]
- 30.Schmidmaier G., Lucke M., Wildemann B., Haas N.P., Raschke M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury. 2006;37(Suppl 2):S105–S112. doi: 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]