Abstract
The “Amyloid Cascade Hypothesis” has dominated the Alzheimer’s disease (AD) field in the last 25 years. It posits that the increase of amyloid-β (Aβ) is the key event in AD that triggers tau pathology followed by neuronal death and eventually, the disease. However, therapeutic approaches aimed at decreasing Aβ levels have so far failed, and tau-based clinical trials have not yet produced positive findings. This begs the question of whether the hypothesis is correct. Here we have examined literature on the role of Aβ and tau in synaptic dysfunction, memory loss, and seeding and spreading of AD, highlighting important parallelisms between the two proteins in all of these phenomena. We discuss novel findings showing binding of both Aβ and tau oligomers to amyloid-β protein precursor (AβPP), and the requirement for the presence of this protein for both Aβ and tau to enter neurons and induce abnormal synaptic function and memory. Most importantly, we propose a novel view of AD pathogenesis in which extracellular oligomers of Aβ and tau act in parallel and upstream of AβPP. Such a view will call for a reconsideration of therapeutic approaches directed against Aβ and tau, paving the way to an increased interest toward AβPP, both for understanding the pathogenesis of the disease and elaborating new therapeutic strategies.
Keywords: Amyloid-β peptide, amyloid-β protein precursor, oligomers, synaptic dysfunction, tau
Alzheimer’s disease (AD) is a neurodegenerative disorder clinically characterized by dementia, defined as a deficit of memory function and at least one other cognitive domain (language, praxis, gnosis, executive function, judgment, and abstract thought) as well as functional impairment, without alteration of the state of consciousness. In the last decades, AD has gained rising attention for its growing prevalence in aging populations, with 46.8 million people affected by the pathology worldwide, a number expected to increase up to 74.7 million in 2030 and 131.5 million in 2050. Besides representing a serious health and social problem, the disease causes exorbitant costs for the healthcare system estimated as 604 billion dollars in 2010 that represented a 35.4% increase in only 5 years [1, 2]. Despite the numerous efforts to counteract the disease, no therapies have so far proven to prevent AD onset or progression.
To date, data from thousands of basic, pre-clinical, and clinical studies have identified amyloid-β peptide (Aβ and tau protein as the key actors in the patho-physiology of AD, mainly because of their deposition in the characteristic histopathological brain lesions, the senile plaques for Aβ and the neurofibrillary tangles (NFTs) for tau, and the increase of their soluble forms in the brain of AD patients. However, therapeutic approaches aimed to decrease Aβ levels that have been attempted so far, have failed. Similarly, tau-based clinical trials have not yet produced positive findings. The overall goal of this review is to provide a critical assessment of the literature on mechanisms underlying disease occurrence and progression. Specifically, we will revisit studies on Aβ and tau, as well as on their interaction, challenging the amyloid hypothesis that has dominated the AD field in the last 25 years. This hypothesis establishes Aβ as the primum movens in a cascade of pathological events that places tau downstream of Aβ. According to this hypothesis, once tau pathology has ensued, therapies against Aβ would no longer work because the disease would progress independently [3]. We propose rearranging the intricate puzzle of AD pathogenesis by placing soluble forms of Aβ and tau in parallel and upstream of amyloid-β protein precursor (AβPP), which would permit the peptides to exert their toxic functions. Such a view will call for a reconsideration of the reasons for the failure of anti-Aβ therapies, no longer attributable to the fact that they were started after triggering of tau pathology, necessarily changing the approach to studies on the etiopathogenesis of AD and paving the way for new therapeutic strategies.
AMYLOID-β PEPTIDE AND ALZHEIMER’S DISEASE: MORE THAN ONE CENTURY OF RESEARCH
Aβ derives from a complex cleavage of AβPP, a type I single-pass transmembrane protein constituted by 639–770 amino acids in humans, and highly expressed in the central nervous system where it exerts a variety of physiological functions [4]. AβPP is initially cleaved by α-secretase or β-secretase, generating soluble and carboxyterminal fragments (CTF). α-secretase activity leads to the formation of sAβPPα and CTF83, whereas β-secretase generates sAβPPβ and CTF99. Then, γ-secretase intervenes, further cleaving CTF83 and CTF99, generating the intracellular peptide AICD/AID (amyloid intracellular domain) and a small p3 peptide from CTF83, and AICD/AID and Aβ from CTF99. Based on this biochemical processing, the cascade initiated by α-secretase has been considered neuroprotective when compared with the β-secretase cleavage, leading to the amyloidogenic cascade and the formation of Aβ [5]. Based on the γ-secretase site of cutting, different isoforms of Aβ can be generated, composed of 38—43 amino acids. Aβ40 is the predominant species, whereas Aβ42 is present at lower concentrations but has received more attention in the AD field due to its high propensity to form aggregates. However, in the brain of AD patients, Aβ38 and truncated forms at N-terminal region, i.e., Aβ15, Aβ16, and Aβ17, have been also detected [6]. Aβ is undoubtedly the most studied protein in AD and its putative role in the pathogenesis of the disease has oriented drug development and clinical trials for several decades. But how and why did the AD amyloidogenic theory emerge?
From a historical perspective, it was at the beginning of the last century when Alois Alzheimer and other European neuropsychiatrists, e.g., Gaetano Perusini, attributed a nosographic identity to a form of “mental” disorder characterized by memory loss, hallucinations, and disorientation. At that time, the most influent personalities in psychiatry, Sigmund Freud and Emilin Kraeplin, fervently disputed on the origin of psychiatric illness, respectively emphasizing the role of the psyche or of organic and genetic factors. The mind/brain diatribe led several scientists to seek for the “material” causes of mental diseases. In this context, Alzheimer and Perusini, strongly supported by Kraeplin, observed that the psychiatric symptoms of dementia could be correlated to peculiar histological lesions in postmortem brains. In the autopsy of the first described AD patient, Auguste Deter, cortical atrophy, neurons filled with neurofibrils, and extracellular miliary foci of an unknown substance were observed. After Alzheimer’s death, research studies on the disease were few until the 1980 s, when epidemiological studies revealed an increase of patients affected by primary dementia. It was during these years that key discoveries were made, fated to influence research in the field until today. Based on Alzheimer’s histological descriptions, Aβ and tau were recognized as the main components of extracellular foci (senile plaques) and intracellular neurofibrils (NFTs), respectively [7–9]. In the same period, the first genetic mutation linked to dementia was identified on chromosome 21 coding for the AβPP [10]. This autosomal dominant disease was responsible for early onset AD (EOAD) characterized by high levels of Aβ. Other genetic mutations were identified in Familiar Alzheimer’s disease (FAD), involving genes responsible for Aβ production such as presenilin 1 (PS1) on chromosome 14, which mutation is the most common cause of EOAD, and presenilin 2 (PS2) on chromosome 1. Consistent with these findings, the presence of AD-like pathology in patients affected by Down’s syndrome, due to a trisomy of chromosome 21, reinforced the idea that the increase of Aβ played a major role in AD pathogenesis. Based on these data, in 1995 the first mouse model of AD carrying an AβPP mutation was engineered [11] and, over time, different models for pre-clinical studies have been generated based on the most common mutations observed in FAD [12].
These findings contributed to the excitement around the “Amyloid Cascade Hypothesis” [13–15], recognized as the pathogenic mechanism underlying AD. Because insoluble fibrils of Aβ were present in AD plaques, and could be formed in vitro from synthetic Aβ, they have dominated the scene until a fundamental breakthrough confirmed by several in vitro and in vivo studies indicated that soluble forms of Aβ were also present in the brain [16, 17]. Aβ soluble aggregates range from monomers to oligomers (molecular aggregates consisting of a few monomer units) and pre-clinical studies confirmed that dimers, trimers, tetramers, dodecamers, and high molecular weight oligomers were all able to induce neurotoxic effects as well as to induce an immediate impairment of synaptic plasticity, and in particular of hippocampal long-term potentiation (LTP), thought to be the electrophysiological correlate of memory (for a review on the role of Aβ oligomers, see [18]). Moreover, Aβ oligomer presence in human cerebrospinal fluid (CSF) could be already recognized decades before AD onset [19]. These data led to the formulation of another theory, the “Oligomer Hypothesis” [20, 21], according to which Aβ oligomers but not monomers or fibrils were responsible for synaptic dysfunction and memory loss in AD [22, 23]. This further influenced AD drug discovery so that new therapies aimed at specifically targeting Aβ oligomers were developed in addition to those clearing Aβ plaques.
Unfortunately, while the “Oligomer Hypothesis” is still a matter of investigation, and data are being gathered to test the grounds of its premises, the clinical failure of most of the anti-Aβ drugs has strongly destabilized this concept. Clinical trials to date show that, despite successful results obtained in animal models of AD, anti-Aβ drugs have not yet been shown to modify cognition in humans although they might be able to reduce plaque or amyloid burden. So far (based on Medline database search and Clinical-Trials.gov): 1) active immunization (i.e., AN-1792, CAD-106, and vanutide cridificar) have not proven effective and several side effects were reported; 2) passive immunization with monoclonal antibodies bapineuzumab, solanezumab, crenezumab, and gantenerumab have not yet succeeded, and although a recent clinical trial with aducanumab has shown a dose-dependent reduction of Aβ plaques, the study was not sufficiently powered to detect clinical changes and the drug is undergoing further investigation [24]; and 3) a number of clinical trials with drugs aimed at preventing Aβ formation by inhibiting β- or γ- secretases have also failed or were interrupted; among these, the γ-secretase inhibitors semagacestat and avagacestat did not show efficacy, and actually induced mild worsening in cognition and severe side effects, whereas the EPOCH trial with the newest β-secretase inhibitor verubecestat was stopped for the lack of any positive effect. Notwithstanding these discouraging results, several scientists are still developing anti-Aβ therapies, convinced that the failure of Aβ tailored drugs might relate to the particular drugs chosen, inadequate dosage, or the fact that treatment was started in a late phase of the disease when Aβ-induced damage cannot be reversed.
This review is written, in turn, with the belief that a careful evaluation of the knowledge in the AD field is due prior to further investing resources with anti-Aβ therapies. Evidences that have been underestimated for a long time are now gaining ground, questioning the way in which the actual role of Aβ in AD pathogenesis is currently thought. First, late onset AD (LOAD), representing 95% of AD cases, is not linked to genetic anomalies leading to a direct overproduction of Aβ, as in FAD, although the phenotype might be comparable. However, pre-clinical studies on AD mouse models have been almost entirely performed on mice presenting FAD-like mutations leading to an increase of Aβ. Second, we know since the 1990 s that there is no correlation between Aβ deposition and clinical degree of dementia among affected individuals [25–28], and plaques might occur in the brains of individuals with no sign of dementia [27, 29, 30]. Third, recent studies have suggested that plaque formation might be a reactive process [31] with a protective role by decreasing oligomer levels [32]. Fourth, a vast literature claims that Aβ exerts a physiological role in the CNS interfering with neuronal growth, neurotransmitter release, synaptic function, and memory formation [33, 34]. Indeed, our group and others have previously demonstrated that administration of low concentration of oligomeric Aβ positively modulate synaptic function [35–37] and, conversely, blocking endogenous Aβ in the healthy brain resulted in an impairment of synaptic plasticity and memory [36, 38]. Finally, even Aβ concentration per se has become a relative concept, as the persistence of a low picomolar Aβ concentration in extracellular fluids provides for detrimental outcomes in synaptic plasticity [39]. In conclusion, taking into account almost one century of research, it emerges that the Aβ model of AD is insufficient [40, 41] and needs to be reconsidered [34].
A REVALUED PLAYER IN ALZHEIMER’S DISEASE PATHOGENESIS: TAU PROTEIN
As described above, the intricate story of Aβ and tau began with the brain of Auguste Deter, but most of the research efforts have been directed toward Aβ. Recently, the discontent generated by too many anti-Aβ therapy failures has induced several groups to re-focus on tau.
Tau is a microtubule-associated protein originally described as a heat stable protein essential for microtubule assembly and stabilization [42]. It is present in the human brain in six main isoforms, deriving from the alternative splicing of exons 2, 3, and 10 of microtubule-associated protein tau (MAPT) gene. This process appears to be of particular interest for exon 10 splicing which determines the presence of tau isoforms containing 3- (3R) or 4-repeats (4R) of a ~32 amino acid sequence in the microtubule binding domain (MBD) [43]. Moreover, the splicing process of exons 2 and 3 determines the number of 29-residue near-amino-terminal inserts which results in isoforms containing 0,1, or 2 inserts (0N, 1N, 2N) [44]. Both R and N repeats are capable of microtubule-binding and assembly-promoting activity, whereas the flanking regions are more likely involved in binding processes [45,46]. In the last decades, many studies have demonstrated tau physiological involvement at different subcellular localizations: 1) at axonal level, by regulating axonal elongation, maturation and transport [47–50]; 2) in dendrites, participating in synaptic plasticity [51, 52]; and 3) in nucleus, maintaining the integrity of genomic DNA, cytoplasmic and nuclear RNA [53,54].
From a functional point of view, tau can be divided in four different regions consisting of a N-terminal domain, a proline-rich domain, a MBD, and a C-terminal domain [3, 55, 56]. The N-terminal domain is rich with negative charges devoted to separation of different microtubules by electrostatic repulsion when tau is bound to a certain microtubule [46, 57, 58], Interestingly, the C-terminal domain, besides its key role in regulation of microtubule polymerization induction and interaction with plasma membranes [59–62], creates an overall asymmetry in the molecule contributing to this microtubule spacing function thanks to its neutral charge. The proline-rich domain and the MBD with their multiple aminoacidic acceptor residues are more involved in interactions with different signaling proteins, which can be scaf-folded by tau or can modify tau conformational status and activity itself [3].
The presence of multiple binding sites confers to tau many interaction possibilities in regards to cell signaling. The flanking region of MBD contains the majority of phosphate acceptor residues, and the phosphorylation of these sites is relevant for regulating microtubule polymerization [63–66], regulation of axonal transport [67] and neurotransmitter receptors [68, 69], interference with vesicles trafficking [70] and apolipoprotein E [71], interaction with Src-family kinases [62, 72–75], and many others [3, 55, 56].
The multiple roles of tau in neuronal physiology have been widely studied and, undoubtedly, a fine regulation is needed to maintain tau structure and function. Accordingly, a wide range of neurodegenerative disorders known as tauopathies have been recognized and classified with respect to the predominant species of tau that accumulates: 1) 3R tauopathies (i.e.. Pick’s disease); 2) 4R tauopathies (i.e., corticobasal degeneration and progressive supranuclear palsy); and 3) 3R + 4R tauopathies (i.e., AD) [43].
Biochemical studies have demonstrated that deposition of insoluble tau aggregates in NFTs depends upon a dysregulated phosphorylation process of the flanking regions of tau. In fact, while two phosphates per molecule of tau are normally present [76], analysis of tau from AD brains has revealed the presence of about eight phosphates per molecule of tau [77]. For this reason, tau phosphorylation abnormalities have been linked to misfolding and deposition of the protein in the diseased brain [78]. Although tau has been defined as a “natively unfolded” protein with a low tendency to aggregation [79], phosphorylation of certain residues or detachment from microtubules [79–81] might change its conformational status and consequently its aggregation propensity. However, the undefined structure of tau in solution has precluded crystallographic analyses leaving a lack of knowledge about the protein structure [82]. Moreover, notwithstanding electron microscopic visualization of tau bound to microtubules demonstrated a linear alignment lengthwise to the protofilament ridges, the protein structure keeps holding a disordered state [83, 84]. Interestingly, when in a solution, tau spontaneously tends to modify its conformation in favor of a paperclip-like structure that might prevent its aggregation [55, 82], unlike Aβ that has a high tendency to aggregate in a solution due to its biochemical properties. Thus, alterations of tau (i.e., hyperphosphorylation, truncated forms) could inhibit the constitution of the paperclip-like structure leading to paired helical filament (PHF) and NFT formation [85]. In this context, tau hyperphosphorylation has been widely studied and the sequence hyperphosphorylation→PHFs→NFTs linked to AD, even if it is unlikely to represent by itself the main pathogenic event for several reasons. First, tau phosphorylation has been demonstrated to be responsible for aggregation only when occurring at certain residues [86], whereas in other sites it has the opposite effect thus preventing aggregation [80]. Moreover, tau hyperphosphorylation is not a prerogative of AD, since it occurs in several other conditions such as hypothermia [87], starvation [88], chronic stress [89], and anesthesia [90, 91].
Interestingly, the amount of PHFs and NFTs is slightly related to the severity of neuronal damage and cognitive impairment in humans. Experiments on regulatable mouse models of tauopathy demonstrated that a variant of human tau with the pro-aggregant mutation ΔK280 developed synaptic and memory impairment as well as tau hyperphosphorylation and pre-tangle formation. However, when the pro-aggregant tau was turned off, synaptic deficit was rescued even if insoluble tau was still present [92]. Other studies on transgenic mice expressing mutant tau (P301L mutation), which could be suppressed with doxycycline, demonstrated that behavioral impairment and neuronal loss were recovered when suppressing transgenic tau, whereas NFTs accumulation continued [93]. Moreover, in the P301S model of tauopathy, synaptic damage and cognitive impairment occurred before the emergence of NFTs [94]. Some authors also reported that, in vitro, abnormally phosphorylated tau can sequester normal tau into tangles of filaments, leading to the hypothesis that tau accumulation into PHFs might initially be neuroprotective until it starts compromising neuronal function as a space-occupying lesion [95].
The observations that synaptic and memory impairment is not mediated by NFTs, and that insoluble deposition of tau might be a compensatory protective mechanism suggested that synaptic failure might be sought in soluble oligomeric species of tau, resembling the “Oligomeric Hypothesis” already formulated for Aβ. Soluble tau was found to be most acutely toxic in animal models of tauopathy [93, 94, 96]. Most importantly, increases in granular tau oligomer levels occur before NFTs form and before individuals manifest clinical symptoms of AD, suggesting that increases in tau oligomer levels may represent a very early sign of brain aging and AD [97]. We have recently demonstrated that an acute exposure to tau oligomers (but not monomers) both in vitro and in vivo is detrimental to LTP and memory [98]. Noteworthy, this toxic effect was exerted by a different preparation of oligomeric tau, i.e., recombinant tau 4R/2N, tau derived from AD patients, tau derived from hTau mice [98]. These results are in agreement with other observations reporting that tau oligomers 1) impair synaptic function and memory in wild type mice [99], 2) correlate with cognitive impairment in rTg4510 mice [100], and 3) accelerate pathology in hTau mice [101].
Pre-clinical findings have been confirmed by studies on humans showing the increase of oligomeric forms of tau in the brain of AD patients compared to controls, occurring before NFT formation and clinical symptoms [97]. Interestingly, tau oligomers have been also found in other tauopathies such as progressive supranuclear palsy, dementia with Lewy bodies, and Huntington’s disease [101–103]. In AD brain, homogenates of tau dimers are also markedly elevated, suggesting that tau aggregation might be a hierarchical process that passes through distinct phases, i.e., monomers, dimers, oligomers, pre-tangles, and tangles [104]. Notably, the time-course leading from monomers to insoluble deposits is comparable to that already described for Aβ, with soluble forms of the peptide increasing in an initial phase of the disease.
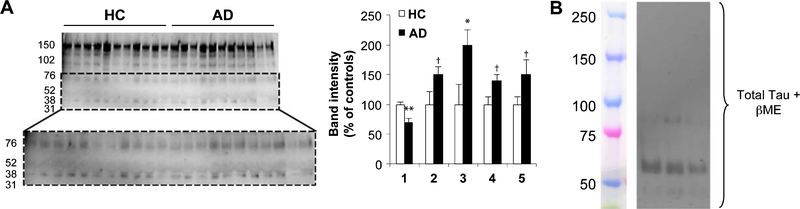
Based on the findings described above and considering the urgent need to find more valuable biomarkers for an early diagnosis, the possibility of detecting tau oligomers in CSF of living patients is appealing. Hence, we have conducted a pilot study to verify that soluble aggregated forms of tau are detectable outside neurons in the CSF of living people and therefore they are not necessarily the byproduct of pathological alterations occurring in postmortem evaluations. We characterized tau immunoreactivity by western blot in CSF samples [105] from a cohort of 11 patients with probable AD and 11 healthy control (HC) individuals at the time of harvesting CSF (Table 1). High molecular immunoreactive species for total tau were observed in all the samples (Fig. 1A, B). However, a significant change in intensity of different bands was found, with an increase in the high molecular weight bands, presumably corresponding to oligomers, coincident with a decrease at 31–38 kDa in AD patient CSF compared to HC (Fig. 1A, B).
Table 1.
Patients characteristics. The diagnosis of probable AD or control for healthy individual was done according to the NINCDS-ADRDA Alzheimer’s Criteria
| Patients # | Diagnosis | Age | Gender | MMSE |
|---|---|---|---|---|
|
| ||||
| 13 | HC | 65 | W | 30 |
| 14 | HC | 64 | W | 27 |
| 15 | HC | 69 | W | 27 |
| 16 | HC | 57 | W | 27 |
| 17 | HC | 66 | M | 29 |
| 18 | HC | 55 | M | 28 |
| 19 | HC | 73 | M | 26 |
| 20 | HC | 58 | W | 30 |
| 21 | HC | 83 | M | 28 |
| 22 | HC | 73 | W | 28 |
| 24 | HC | 79 | W | 29 |
| 2 | AD | 76 | M | 18 |
| 3 | AD | 72 | M | 28 |
| 4 | AD | 58 | M | 23 |
| 5 | AD | 68 | M | 17 |
| 6 | AD | 66 | M | 25 |
| 7 | AD | 54 | M | 25 |
| 8 | AD | 81 | M | 26 |
| 9 | AD | 71 | M | 27 |
| 10 | AD | 69 | M | 24 |
| 11 | AD | 64 | W | 25 |
| 12 | AD | 68 | M | 26 |
Diagnosis was determined after full neurological history and examination including testing of mental status. All diagnoses were made by an experienced neurologist, psychiatrist, or a consensus conference including neurologists and neuropsychologists. Cerebrospinal fluid samples were banked at Columbia University, under protocols approved by the Columbia University and New York State Psychiatric Institute Institutional Review Boards. HC: range 55–83 years, average: 67.45 ± 2.72; probable AD: range: 54–81 years, average: 67.91 ± 2.29 years. MMSE, Mini-Mental State Examination.
Fig. 1.
Oligomeric tau is present in the CSF of AD patients and healthy individuals. A) Western blot showing total tau levels in CSF samples of healthy individuals (HC) and probable AD patients (higher magnification view of the lower molecular weight bands on the lower part of the panel. Different band intensity is quantified on the right graph (31–38 kDa: p = 0.009, 50–54 kDa: p = 0.003, 74–78 kDa: p = 0.04, 100–104 kDa: p = 0.002 and 120–150 kDa: p = 0.003). CSF specimens from subjects listed in Table 1 were thoroughly mixed, de-identified, and underwent one freeze-thaw cycle before standard aliquoting in 1.5 ml portions in polypropylene screw-cap tubes and storage at −80° C. To verify the oligomerization status of tau, we ran samples on western blots. Immunoreactivity toward total tau was measured in each of the CSF aliquots. Equal amounts of protein (8 μg) were fractionated by Tris-Acetate gradient gels (3–8%) and transferred to nitrocellulose membranes (Millipore). Tau immunoreactivity was detected using anti-total tau polyclonal antibody (1:2000; Epitomics). Immunoblot data were quantified by measuring the band intensity using imaging software (NIH ImageJ). Statistical analyses were performed by ANOVA plus post-hoc multiple comparisons test using Prism (GraphPad) software. B) Immunoreactivity for total tau in samples from probable AD patients reduced with β-mercaptoethanol (βME). βME zeroed the high molecular weight signal revealed by tau antibodies while intensifying the signals in the monomeric range.
Interestingly, when we dissociated tau by treating the CSF samples with the reducing agent beta-mercaptoethanol (βME) to disrupt the thiol bonds between tau molecules, the signal intensity of high molecular weight tau immunoreactivity became undetectable, whereas a clear signal was present for monomeric tau, suggesting that the presence of oligomers was linked to disulfide bridges involving tau molecules (Fig. 1C). This study leads to important considerations. First, the possibility to evaluate the presence of extracellular oligomeric tau in clinical lumbar CSF specimens could be useful as a possible early biomarker of the disease, in agreement with other findings [102, 106]. Second, the observations that tau oligomers are also present in HCs and that monomers/oligomers are differently distributed in AD and control CSF suggest that the biological significance of tau species should be further investigated. These aspects should be taken into account when designing new drugs targeting tau to avoid the same issues already experienced with anti-Aβ treatments.
Notwithstanding the increase of tau oligomers in the AD brain and CSF, drugs aimed at inhibiting tau aggregation or dissolving existing aggregates, i.e., methylthioninium chloride and its second-generation derivatives such as TRx0237, have not been proven efficacious in clinical trials. A Phase II study with TRx0237 was terminated after a few months for “administrative” reasons, whereas Phase III studies have reported negative results on cognitive improvement (see clinicaltrials.gov for details). However, it is not clear whether these drugs actually inhibit tau aggregation in humans. Also, this makes us wonder whether the increase of tau oligomers in AD patients should be better considered as a pathogenic marker of the disease rather than a target of therapeutic strategies.
Aβ AND TAU OLIGOMERS: A GAME AT THE SYNAPSE RESULTING IN MEMORY IMPAIRMENT
How do Aβ and tau induce memory loss? According to most of the studies, the answer should be sought at the synapse. Although cortical atrophy and synaptic loss have been reported in AD brains, mainly due to a structural damage imputable to plaques and tangles in a later stage of the disease, a subtle effect exerted by soluble forms of Aβ and tau at the synapse seems to be the earlier event underlying memory loss [98, 99, 107, 108]. Several studies have demonstrated that administration of different preparations of oligomeric Aβ and tau (synthetic, from transgenic mice, from AD brains) impaired synaptic plasticity and memory. The role of soluble oligomers also emerged in studies performed on AD mouse models, since synaptic and memory dysfunction was present before the appearance of plaques or tangles [18,109].
In vitro and in vivo studies have shown that Aβ and tau derange molecular signaling pathways crucial for synaptic plasticity atbothpre- and post-synaptic sites. Both Aβ and tau interfere with the transcription factor cAMP response element-binding protein (CREB), whose phosphorylation at Ser133 is thought to be one of the fundamental events in memory formation [110–112]. In particular, Aβ inhibits the physiological increase of CREB phosphorylation during LTP [113–115], causing a downregulation of both the NO/cGMP/PKG and the cAMP/PKA pathways, two cascades converging on CREB. Tau overexpression and hyperphosphorylation was also found to be accompanied by a reduction of CREB phosphorylation at Ser133, mediated by a decrease of phosphorylation of NR2B (Tyr1472) [116]. Moreover, synaptic plasticity and memory impairment caused by h-tau overexpression was reported to be related to nuclear dephosphorylation/inactivation of CREB [117]. Interestingly, these findings were validated in humans affected by AD showing a decrease in CREB and phospho-CREB levels in hippocampus [118–122].
Aβ and tau also target other molecules upstream of CREB, among which the Ca2+/calmodulin-dependent protein kinase II (CaMKII), another key molecule needed for LTP and memory formation [123]. CaMKII is dysregulated in the hippocampus of AD mouse models and patients (for a review, see [124]) and it has been demonstrated that Aβ oligomers interfere with its phosphorylation leading to AMPA receptor dysfunction [125–127]. On the other hand, evidences for the interaction tau-CaMKII have been reported since the late 1980 s in works analyzing the ability of CaMKII to induce an AD-like tau phosphorylation [128, 129]. CaMKII phosphorylates tau at different sites and this might prevent tau binding to microtubule [130] and modify tau structure leading to PHFs formation [131]. Indeed, CaMKII colocalizes with tau mRNA, PHFs, NFTs in AD brains (for a review, see [124]). Recently, in a drosophila model of tauopathy, suppression of tau phosphorylation at Ser262/356 inhibited tau toxicity through a mechanism involving calcium homeostasis dysregulation driven by CaMKII [132].
The deleterious effects of Aβ and tau also involved BDNF, a critical factor linked to neuronal survival and function that is needed for synaptic plasticity and memory. A decrease of BDNF levels in serum and brains of AD patients correlates with cognitive impairment, and BDNF polymorphisms have been proposed to be involved in AD pathogenesis [133]. Moreover, several in vitro and in vivo studies have confirmed that Aβ-induced LTP and cognitive dysfunction are associated with a reduction of BDNF levels [133]. Recently, a loss of BDNF has been also reported in THY-Tau22 and P301L mouse models of tau pathology [134, 135].
Taken all together, these findings suggest that restoring synaptic-related molecules and second messenger systems regulating memory mechanisms might be a viable therapeutic strategy to counteract AD [115]. Most importantly, these data point at common synapse-related mechanisms affected by both Aβ and tau during memory impairment.
Aβ AND TAU ACTIVITY-DEPENDENT SECRETION, NEURONAL UPTAKE, AND SPREADING OF THE DISEASE
Because Aβ and tau interfere with the synaptic machinery, another relevant subject of investigation has been to determine whether they act via extracellular or intracellular mechanisms. Based on the localization of insoluble deposits, for several years Aβ has been considered an extracellular protein and tau an intracellular one. However, it is now clear that this rigid vision is no more applicable, since both Aβ and tau can be found inside and outside neurons. Notwithstanding most of the studies have been performed on models of disease, the extra- and intracellular presence of Aβ and tau is the result of a physiological dynamic process in which the two proteins are secreted at the synapse and internalized by neurons. A relevant body of data has supported the hypothesis that neurons are able to secrete Aβ in an activity dependent fashion. In vitro studies performed by applying drugs that decrease (i.e., tetrodotoxin or high magnesium) or increase (i.e., picrotoxin) neuronal activity have shown a concomitant decrease or increase of Aβ secretion in organotypic slices overexpressing human AβPP Swedish mutation [136]. An in vivo approach by using microdialysis also revealed an increase of Aβ levels in the brain interstitial fluid concomitant to the increase of synaptic activity [137] or paralleling the neurological status [138]. An increase of Aβ secretion has also been found during learning in healthy wild-type mice [38]. Based on the fact that synaptic activity stimulates Aβ secretion, and that extracellular Aβ is known to reduce synaptic plasticity, it has been proposed a theory according to which an increase of synaptic (and cognitive) activity is linked to AD pathogenesis. However, although an increase of brain activity in AD could be supported by data indicating hyperexcitability in transgenic mice and human AD patients [139, 140], this activity-dependent role of Aβ should be better viewed as a physiological mechanism occurring within the healthy brain, especially because levels of Aβ secreted during activity are in the picomolar range and are not neurotoxic [35, 38, 141]. Thus, the high increase of extracellular Aβ during AD might be due to a derangement of this physiological loop or it could be a consequence of degeneration of neurons that have previously accumulated Aβ at intracellular level (for a review, see [142]). Whether the impairment of synaptic function is directly mediated by these high extracellular Aβ levels or by Aβ accumulated inside neurons, is still a matter of debate. Surely, these two pools are strictly interconnected, since extracellular Aβ induced the accumulation of intracellular Aβ by stimulating AβPP processing [143] or by a direct AβPP-mediated internalization [144]; in turn, intracellular Aβ disrupts synaptic transmission and plasticity [145].
Interestingly, tau also undergoes the same dynamic flux characterized by activity-dependent secretion and neuronal internalization. Indeed, application of KCl or glutamate to cultured neurons resulted in an increase of tau secretion [98, 146] mediated by AMPA receptor activation [146]. In vivo studies reported an increase of tau in brain interstitial fluid when stimulating neurons with high K+ perfusion, or after stimulation of the N-Methyl-d-aspartic acid (NMDA) receptors, or picrotoxin administration [147]. An increase of tau secretion also paralleled the increase of glutamate release induced by an antagonist of metabotropic glutamate receptors 2/3 [147]. The phenomenon was further confirmed in different cultured neural cell lines where extracellular tau levels were modified proportionally to synaptic activity [148]. On the other hand, several pre-clinical studies have demonstrated that exogenously applied tau can be internalized by neurons [98, 149–152] and glial cells [153–155] with different mechanisms involving bulk endocytosis [152], binding to heparan sulfate proteoglycans [156] or to AβPP [144].
Activity dependent secretion and neuronal uptake of Aβ and tau have been related to the spread of the disease throughout the brain, a process known as spreading which refers to the capability of neurotoxic proteins to diffuse from a neuron to another, expanding the disease from a restricted area to the entire brain. This type of dissemination, defined as “trans-synaptic spreading”, is thought to occur among different brain areas functionally connected [157, 158] and is supported by observations on postmortem AD brains as well as by clinical studies exploiting computerized x-ray tomography (CT) and magnetic resonance imaging (MRI) techniques, that allow tracing different neuropathological markers such as atrophy of certain brain areas, brain ventricles enlargement, and deposition of amyloid plaques and NFTs (for a review, see [78]). However, it should be pointed out that imaging biomarkers like fluorodeoxyglucose in PET scans are associated to discrete difficulties in data interpretation, as they are also positive in Suspected Non-Alzheimer Disease Pathophysiology (SNAP) [159].
Evidence for AD spreading and progression throughout the cortex was reported more than 30 years ago, based on tangle distribution in the proximity of the same pyramidal neurons that give connectivity to other brain areas [160]. At the present day, neither the cause that initiates spreading nor its underlying mechanisms have been identified, but useful information has come from pre-clinical studies. Notwithstanding tau has been under the spotlight for many years, one of the first evidence of spreading in AD dates back to the 1990 s and involves Aβ [161, 162]. When trying to unravel the causes of Aβ diffusion, studies have often focused on the first area affected in AD, the medial temporal lobe, and in particular, the entorhinal cortex (EC). EC superficial layer is susceptible to Aβ-dependent neurodegeneration, and this can negatively affect its primary afferent regions resulting in a disruption of the whole circuitry in both mouse models and AD patients [163, 164]. Consistently, an increase of mutant AβPP in layer II/III neurons of EC has been shown to elicit a molecular and functional disruption in the CA1 area of the hippocampus with presence of soluble Aβ in the dentate gyrus, Aβ deposits in the performant pathway, and epileptiform activity in the parietal cortex [165]. Further studies in mutant human AβPP (mhAβPP) mice have reported an age-dependent progressive deterioration of synaptic plasticity and memory spreading from the EC to the hippocampus [166], a phenomenon mediated by microglial RAGE activation and subsequent increase in p38MAPK phosphorylation [166]. Consistently, other studies reported the capability of reactive microglia in secreting Aβ through micro vesicles, which in turn would promote Aβ toxicity to neurons through their axons [167–169]. Accordingly, other supporting evidences indicate that after administration of fluorescent oligomeric Aβ to neurons, a higher percentage of the protein was found surrounding neurons, and this process needed the presence of differentiated neuritis to occur [170]. Cell-to-cell transfer mechanism has been reported for different Aβ species (i.e., oAβ1–42 TMR, oAβ3(pE)—40TMR, oAβ1—40TMR, and oAβ11–42TMR), and this prion-like spreading was attributed to an insufficient activity of cellular clearance degradation systems [171]. Another mechanism proposed for Aβ spreading relies on the presence of tunneling nanotubes (TNTs) consisting of cellular membrane extensions creating a direct connection between cells [172]. TNTs have been demonstrated to mediate high-speed transfer of Aβ among neurons, through a p53/EGFR/Akt/PI3K/mTOR pathway that, in turn, would trigger F-actin polymerization promoting TNTs formation [173]. However, Aβ has been shown to be secreted by neurons through exosomes [174] that could be internalized and stored from the acceptor neuron as lysosomal vesicles through a macroautophagy mediated mechanism [170, 175]. In any case, despite these numerous evidences, there is not a uniform consensus about the causes or mechanisms underlying Aβ spreading.
On the other hand, a growing body of evidence refers to tau spreading as a prion-like propagation, which fascinatingly occurs in different directions among the many forms of tauopathies [176]. Also, tau pathology is likely to begin in EC then move to the hippocampus, and ultimately invading the cortex, following an overlapping path existing among functionally connected areas [55, 157, 158, 177]. These evidences are consistent with data coming from studies on non-human primates in which bilateral lesions of EC induce a functional impairment of declarative memory accompanied by long-lasting hypometabolism in temporal and parietal lobes, demonstrating a functional connection starting from EC [178]. Accordingly, in a transgenic mouse model differentially expressing pathological human tau in EC (EC-tau), the localization of tauopathy was investigated at different time points, demonstrating a progression of the pathology through anatomically and functionally connected brain areas [158]. Interestingly, in vivo chemogenetic stimulation of EC in EC-tau mice induced additional pathology in synaptically connected areas (e.g., dentate gyrus) [148]. Consistent with this finding, tau has been found in exosomes that might lead to its diffusion to adjacent cells [106, 179]. Further work demonstrated that cell-to-cell contact was not necessarily needed for tau spreading in vitro given that the administration of neuronal-derived tau media to neuronal cultures was sufficient for tau transfer and internalization, even though it is not known whether tau in the media was vesicle bound or free [148]. Other studies suggested that pathologic tau requires TNTs to be transferred from a neuron to another one [180]. However, whether the mechanism underlying tau propagation is mediated by TNTs, non-vesicular direct translocation or through secretory lysosomes into extracellular space [106, 162, 181, 182] is still under investigation. Another interesting feature of tau transmission is the possibility that it can move both anterogradely and retrogradely, meaning that it can be internalized both at the somatodendritic compartment and axon terminals, and can be transported in either direction to disseminate tauopathy [152, 162].
While spreading is involved in the progression of the disease among functionally connected brain areas, the transition from oligomers to insoluble deposits has been described as a “nucleation-dependent protein polymerization” and explains the pattern of aggregate formation [183] for proteins with high tendency to organize in β-sheet conformation as for Aβ, tau, or α-synuclein [184]. This process, known as seeding, involves a nucleation phase and a growth phase. In the nucleation phase, the nucleus formation requires the assembly of misfolded monomers, a thermodynamically unfavorable process remarkably dependent on protein concentration [161, 185, 186]. The latter influences the lag time defined as the period before aggregates detection. In fact, supersaturated solutions can drastically shorten the nucleus formation time from years to microseconds [161]. After the nucleus formation, the critical concentration is reached, and a further addition of monomers occurs leading to polymerization, representing the growth phase. Interestingly, if a preformed nucleus, or seed, is added to a solution containing normally folded monomers, an immediate polymerization occurs. This phenomenon is defined as seeding [161, 183] and can be distinguished as homologous or heterologous [183, 187], While homologous seeding involves monomers of the same type, heterologous seeding or cross-seeding takes place when a nucleus formed by a certain misfolded protein promotes polymerization of a different protein [183, 187]. A large body of evidence supports this cross-seeding among tau, α-synuclein and TDP-43 [188]. Some studies in which spreading of tau pathology was significantly accelerated by injecting pre-aggregated Aβ into mouse brain [189, 190] suggested the possibility of Aβ and tau cross-seeding. Consistently, a protein interaction study by surface plasmon resonance demonstrated an affinity constant of tau for Aβ which was almost 1000-fold higher than for tau toward itself [191]. Moreover, confocal immunohistochemical imaging of AD brains showed intracellular aggregates in which Aβ and tau coexisted in the same structure [191]. Also, a recent work showed that tau fibrillization can be induced in a cell-free assay by adding pre-aggregated Aβ, and that Aβ provide an efficient seed to induce tau cross-seeding and a consequent spreading of tau pathology in vivo [192].
In conclusion, seeding and spreading of Aβ and tau and their dynamic flux across the membrane characterized by activity-dependent secretion and neuronal internalization are crucial for the progression of the disease. Most importantly, the commonalities displayed by both Aβ and tau with respect to these phenomena are intriguing and suggest that soluble forms of the two molecules are involved in similar mechanisms of disease etiopathogenesis.
ALZHEIMER’S DISEASE: REARRANGING THE PUZZLE
As described above, Aβ and tau share several features leading to common mechanisms of toxicity, regardless of their different sequence (Table 2). This was predicted by a study showing that all of the soluble oligomers tested including β-synuclein, islet amyloid polypeptide, polyglutamine, lysozyme, human insulin, and prion peptide 106–126, display a common conformation-dependent structure that is unique to soluble oligomers [193], By now, a variety of studies have demonstrated that soluble oligomeric forms of Aβ and tau, more than their aggregates, are increased in the diseased brain, are detectable in the CSF, and are highly correlated with cognitive impairment. The deleterious effect of Aβ and tau occurs at the synapse, where they interfere with molecular pathways needed for synaptic plasticity and memory. The capability of neuronal and glial cells to release and internalize Aβ and tau contributes to spread of the disease from specific areas, such as EC and the hippocampus, to the entire brain. Despite these studies have certainly clarified several aspects of AD onset and progression, the crosstalk between Aβ and tau in the diseased brain is still a matter of debate.
Table 2.
Similarities and differences between Aβ and tau
| Amyloid-β Peptide | Tau Protein | |
|---|---|---|
|
| ||
| Isoforms | • Aβ40, Aβ42, other fragments | • 3R-4R, 0N-1N-2N |
| Secondary structure | • β-sheet | • β-sheet |
| Physiological functions | • Neuronal growth | • Microtubule assembly and stabilization |
| • Neurotransmitter release | • Axon elongation | |
| • Synaptic transmission and plasticity | • Synaptic plasticity | |
| • Memory formation | Nuclear function | |
| • Immune response | ||
| • Anti-oxidant properties | ||
| Aggregation sequence | Monomers → Oligomers → Fibrils → Senile plaques | Tau hyperphosphorylation → PHFs → NFTs |
| Insoluble and soluble forms | • No correlation between senile plaques and cognitive impairment | • Poor correlation between NFTs and cognitive impairment |
| • Oligomers induce synaptic dysfunction and memory loss | • Oligomers induce synaptic dysfunction and memory loss | |
| • Oligomers increase in brains and CSF of AD patients versus controls | • Oligomers increase in brains and CSF of AD patients versus controls | |
| Genetic mutations | AβPP, PS1 and PS2 linked to FAD | MAPT linked to FTDP-17, PSP, CBD |
| Synaptic target | CREB, CamKII, BDNF among others | CREB, CamKII, BDNF among others |
| Extra- and intracellular dynamic | • Activity dependent secretion | • Activity dependent secretion |
| • Neuronal and glia uptake | • Neuronal and glia uptake | |
| • Extracellular toxicity | • Extracellular toxicity | |
| • Intracellular toxicity | • Intracellular toxicity | |
| Spreading | EC → Hippocampus → Cortex | EC → Hippocampus → Cortex |
| AβPP-dependent mechanism | • AβPP binding | • AβPP binding |
| • Neuronal and glial uptake | • Neuronal and glial uptake | |
| • Synaptic plasticity impairment | • Synaptic plasticity impairment | |
| • Memory impairment | • Memory impairment | |
PHFs, paired helical filaments; NFTs, neurofibrillary tangles; CSF, cerebrospinal fluid; AβPP, amyloid-β protein precursor; PS, presenilin; FAD, familiar Alzheimer’s disease; MAPT, microtubule-associated protein tau; FTPD-17, frontotemporal dementia with parkinsonism-17; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; CREB, cAMP response element binding protein; CaMKII, Ca2+/calmodulin-dependent protein kinase II; BDNF, brain-derived neurotrophic factor; EC, entorhinal cortex.
The most common view in the AD field is based on the “Amyloid Cascade Hypothesis” and suggests that the initial increase of Aβ induces amyloid and tau pathology over time (Fig. 2). This temporal sequence derives from studies in patients with FAD, where the genetic-driven increase of Aβ is followed by NFT accumulation [194], whereas the increase of tau, as in tauopathies, is not associated with Aβ deposition. Preclinical studies have confirmed that oligomers of Aβ can trigger tau pathology [195] and, conversely, when knocking down tau, Aβ toxic effects are prevented [196, 197]. Interestingly, recent work has demonstrated that Aβ acutely induces tubulin post-translational modifications and stabilizes dynamic microtubules promoting tau-dependent loss of dendritic spines and tau hyperphosphorylation [52]. Thus, Aβ has been thought to act upstream of tau in the pathogenesis of the disease. However, our recent works have challenged this scenario. We have demonstrated that oligomers of both Aβ and tau produce an immediate reduction of synaptic plasticity and memory when extracellularly applied and that these detrimental effects occur not only with high concentrations of Aβ or tau alone, but also when sub-toxic doses of oligomeric Aβ are combined with sub-toxic doses of oligomeric tau [98]. These observations suggested that: 1) Aβ and tau might act at the same level or on different targets that later converge on a common molecular mechanism; 2) the two proteins are able to impair synaptic plasticity and memory per se, i.e., regardless of the presence of high concentrations of one another; and 3) elevated levels of Aβ are not needed to initiate the tau-mediated toxic events leading to synaptic dysfunction. Inspired by these data, we have recently focused on the possible common mechanism of action for extracellular Aβ and tau oligomers to impair LTP and memory. We found that both oligomers of Aβ and tau require AβPP to exert their deleterious effect at the synapse [144], in agreement with previous observations indicating that A(3PP mediates extracellular Aβ neurotoxicity [143, 198, 199], and a recent study showing that AβPP is required for binding of human brain-derived oligomers to synapses and disruption of synaptic plasticity [200]. Our findings are also consistent with the observation that AβPP is involved in AD hippocampal hyperactivity [140, 201–204]. Previous papers have already shown that oligomeric Aβ is able to bind AβPP [205], whereas AβPP and tau interaction was studied several years ago in the context of NFTs [206–208]. We have now provided evidence that soluble oligomeric tau is able to bind AβPP [144]. This binding might be related to the AβPP-mediated uptake of Aβ and tau, since AβPP influences accumulation of tau fibrils in cultured cells [209] and is needed for the entrance of oligomeric Aβ and tau into neurons [144] and astrocytes [155]. Based on these findings, we hypothesize that AβPP-mediated oligomer uptake plays a role in AD pathogenesis. Indeed, because Aβ and tau do not impair synaptic plasticity and memory in AβPP KO mice, AβPP binding and/or AβPP-mediated internalization of the two proteins should lead to LTP and memory reduction, even if one cannot exclude the possibility that Aβ and tau act on other targets, or that their intraneuronal accumulation does not directly inhibit the synaptic machinery. However, a previous observation indicating that blocking intracellular Aβ rescues the LTP impairment induced by administration of extracellular Aβ [145] supports the hypothesis that Aβ intraneuronal uptake is critical for the impairment of synaptic plasticity. On the other hand, recent studies have evidenced that the AβPP-dependent accumulation of extracellular tau oligomers in astrocytes induces a disruption of calcium signaling which in turn disrupts synaptic function in neighboring neurons [155]. Interestingly, while it has been previously demonstrated that extracellular Aβ requires AβPP cleavage to permit intraneuronal Aβ accumulation [143], our results have excluded that the toxicity of extracellular Aβ and tau oligomers on LTP depends upon amyloidogenic processing of AβPP since BACE KO mice still present the impairment of LTP induced by the two oligomeric proteins [144].
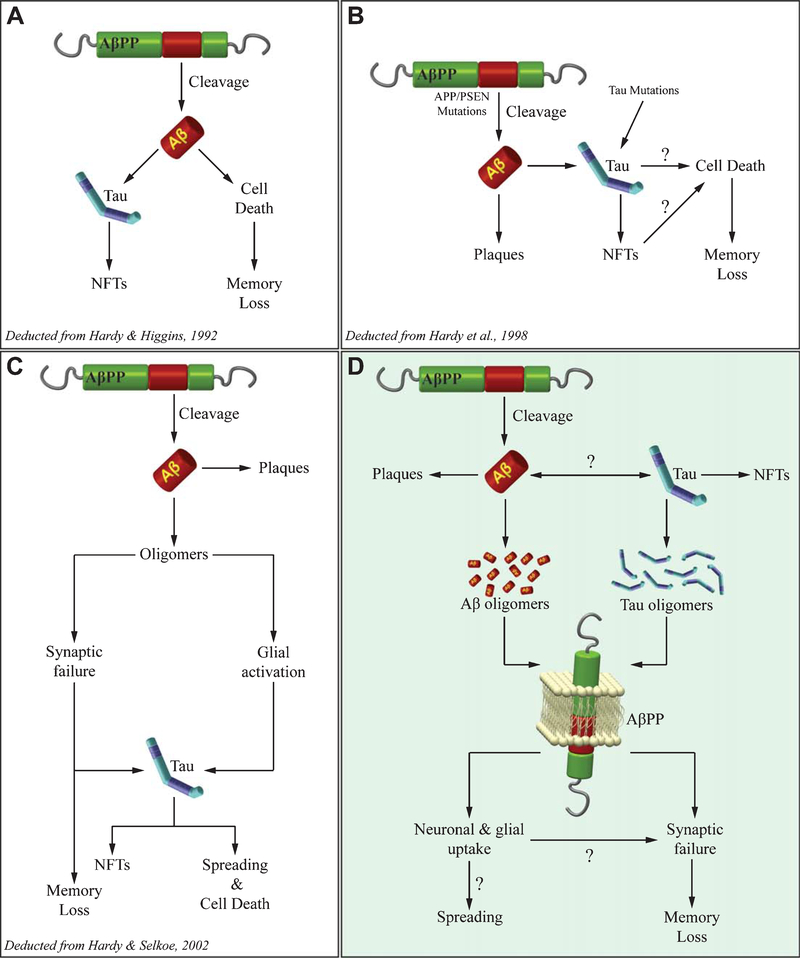
Fig. 2.
Different views of Aβ and tau interaction in AD pathogenesis. The Amyloid Cascade Hypothesis has dominated the AD field for several years. This picture describes how it has been updated over time from the beginning (A), to the discovery of genetic mutations involving both Aβ and tau production (B), to a more complex vision recognizing oligomers as the toxic Aβ species (C). Notably, in A-C Aβ acts upstream tau. D) According to our novel vision, both oligomers of Aβ and tau exert a neurotoxic effect mediated by AβPP leading to synaptic and memory dysfunction. AβPP also mediates oligomers entrance into neurons and glial cells, a mechanism probably contributing to the spreading of the disease throughout the brain.
The requirement for AβPP to lead to intracellular entrance of Aβ and tau oligomers to produce synaptic dysfunction and memory loss begs the question of how this occurs. Whether AβPP acts as a channel permeable to the oligomers [210, 211], or induces the formation of pores across the membrane to let oligomers enter the cell [212], or promotes endocytosis of the oligomers [213], is still under investigation. Another possibility is that when Aβ and tau oligomers bind AβPP, they lead to the activation of its intracellular portion, AID/AICD, triggering either a structural change, for example inducing a different AβPP conformation, or a functional effect, for example activating or inhibiting molecular cascades involved in synaptic plasticity and memory. Interestingly, it is known that AID/AICD might stimulate transcription by forming a multimeric complex with the nuclear adaptor protein Fe65 and the histone acetyltransferase Tip60 [214]. It has been also shown that AβPP-dependent transcription mediated by Fe65 is blocked by the cell death mediator p75, which is able to bind AβPP altering its processing [215]. Another possible mechanism might involve AβPP phosphorylation at specific intracellular sites. For example, it has been demonstrated that AβPP phosphorylation of Thr668, which regulates docking sites for intracellular proteins that interact with AβPP, is increased in AD cases [216] and knock-in mouse bearing a Thr(668)Ala mutation preventing phosphorylation at this site protects against abnormal synaptic plasticity and memory when crossed with a mouse model of dementia [217].
Our model placing extracellular Aβ and tau in parallel and upstream of AβPP does not exclude the possibility that the two proteins involve other molecules to produce detrimental effects in addition to synaptic plasticity and memory impairment, nor the possibility that some deleterious effects need the other protein for the effect itself to be present (i.e., Aβ might require tau for some of the pathologies to occur). Consistent with this scenario, AD is a complex plex condition involving multiple aspects in addition to memory, a phenomenon that is likely dependent upon synaptic activity and that has greatly influenced our critical analysis of the current literature because it represents the clinical hallmark of AD. Furthermore, as shown in Table 2, some of the physiological functions of the two proteins are different with Aβ playing a major role in neuronal growth and synaptic plasticity and tau in axonal elongation and microtubule assembly and stabilization. Then, in light of the different affinities that Aβ has towards its multiple targets, it is likely that as the concentration of the peptide increases with worsening of the pathology new pathways are affected bv the disease.
In any case, demonstrating that AβPP serves as a Trojan horse to mediate synaptic plasticity and memory impairment by extracellular oligomers of both Aβ and tau, challenges the prevailing hypothesis in the AD field stating that Aβ triggers tau pathology. According to our findings, Aβ and tau do not act in series but in parallel, both through AβPP (Fig. 2). Now, it would be desirable to understand whether and how the involvement of AβPP is limited to Aβ and tau entrance into cells or also underlies the derangement of molecular mechanisms involved in synaptic plasticity and memory. In any case, this new player might be taken into consideration when studying the pathogenesis of AD. For example, further studies should be performed to understand the exact mechanisms of AβPP-mediated entrance of oligomers inside neurons and glial cells and whether this might initially represent a compensatory mechanism aimed at clearing toxic oligomers from the synaptic cleft.
The consequences of the model underlying AD pathology proposed in the current review are notable from a drug discovery point of view. The first thought is that therapies targeting tau might not work similarly to the failure of anti-Aβ therapies, as Aβ might still exercise its detrimental effects independent of tau and vice versa. Most important, given the convergence of Aβ and tau onto AβPP, a fascinating possibility is that therapies acting onto AβPP might be more efficacious than those acting solely against Aβ or tau. Furthermore, an approach directed against AβPP would have the advantage of overcoming obstacles offered by the physiological functions of Aβ and tau that might occur independently of their action onto AβPP and might still be present if one decides to simultaneously target Aβ and tau, an approach that is also suggested by our model. A strategy directed against AβPP will likely have its own drawbacks including physiological functions of full length AβPP [4]. Nevertheless, AβPP offers the flexibility of having multiple sites undergoing post-translational modifications that could be exploited as a tool to selectively affect a putative AβPP-dependent toxicity of Aβ and tau oligomers [218]. To this end, the AβPP phosphorylation at Thr668 is very interesting because it has been suggested that averting its noxious role in synaptic plasticity and memory might serve as a therapeutic strategy for human dementias [217]. Consistent with this finding it has been shown that overexpression of the protein phosphatase 2A (PP2A) methyltransferase, leucine carboxyl methyltransferase-1, leads to a decrease in AβPP phosphorylation at the PP2A-sensitive Thr-668 site and protects mice against Aβ-induced damage of synaptic plasticity and memory [219]. Certainly, our hypothesis paves the way to an increased interest toward AβPP, a molecule that has been taken into account mostly for its role as an Aβ generator, being, in our opinion, unfortunately overshadowed bv its own child, Aβ.
In conclusion, after more than one century of research in the AD field, several questions remain to be answered especially on the role of the two main actors, Aβ and tau, in the pathogenesis of the disease. It is certain that their interactions at the synapse need to be further elucidated and new players such as AβPP should enter the stage to get a clearer picture of this intricate disease.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-9935).
REFERENCES
- [1].Prince M, Wimo A, Guerchet M, Ali GC, Wu Y-T, Prina M, Chan KY, Xia Z (2015) World Alzheimer Report 2015. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer Disease International, London. [Google Scholar]
- [2].Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina AM, Winblad B, Jönsson L, Liu Z, Prince M (2017) The world-wide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morris M, Maeda S, Vossel K, Mucke L (2011) The many faces of tau. Neuron 70, 410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Muller UC, Zheng H (2012) Physiological functions of APP family proteins. Cold Spring Harb Perspect Med 2, a006288–a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang Y, Thompson R, Zhang H, Xu H (2011) APP processing in Alzheimer’s disease. Mol Brain 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mawuenyega KG, Kasten T, Sigurdson W, Bateman RJ (2013) Amyloid-beta isoform metabolism quantitation by stable isotope-labeled kinetics. Anal Biochem 440. 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Glenner GG, Wong CW (1984) Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120, 885–890. [DOI] [PubMed] [Google Scholar]
- [8].Glenner GG, Wong CW (1984) Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122, 1131–1135. [DOI] [PubMed] [Google Scholar]
- [9].Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261, 6084–6089. [PubMed] [Google Scholar]
- [10].Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B (1990) Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248, 1124–1126. [DOI] [PubMed] [Google Scholar]
- [11].Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masilah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373, 523–527. [DOI] [PubMed] [Google Scholar]
- [12].Puzzo D, Gulisano W, Palmeri A, Arancio O (2015) Rodent models for Alzheimer’s disease drug discovery. Expert Opin Drug Discov 10, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12, 383–388. [DOI] [PubMed] [Google Scholar]
- [14].Hardy J, Higgins G (1992) Alzheimer’s disease: The amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- [15].Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M (1998) Genetic dissection of Alzheimer’s disease and related dementias: Amyloid and its relationship to tau. Nat Neurosci 1, 355–358. [DOI] [PubMed] [Google Scholar]
- [16].Wisniewski T, Ghiso J, Frangione B (1994) Alzheimer’s disease and soluble A beta. Neurobiol Aging 15, 143–152. [DOI] [PubMed] [Google Scholar]
- [17].Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from A 142 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walsh DM, Selkoe DJ (2007) Aβ oligomers - A decade of discovery. J Neurochem 101, 1172–1184. [DOI] [PubMed] [Google Scholar]
- [19].Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M (2010) High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J 24, 2716–2726. [DOI] [PubMed] [Google Scholar]
- [20].Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–357. [DOI] [PubMed] [Google Scholar]
- [21].Ferreira ST, Klein WL (2011) The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem 96, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ripoli C, Piacentini R, Riccardi E, Leone L, Li Puma DD, Bitan G, Grassi C (2013) Effects of different amyloid βprotein analogues on synaptic function. Neurobiol Aging 34, 1032–1044. [DOI] [PubMed] [Google Scholar]
- [23].Attar A, Ripoli C, Riccardi E, Maiti P, Li Puma DD, Liu T, Hayes J, Jones MR, Lichti-Kaiser K, Yang F, Gale GD, Tseng CH, Tan M, Xie CW, Straudinger JL, Klarner FG, Schrader T, Frautschy SA, Grassi C, Bitan G (2012) Protection of primary neurons and mouse brain from Alzheimer’s pathology by molecular tweezers. Brain 135, 3735–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A (2016) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56. [DOI] [PubMed] [Google Scholar]
- [25].Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30, 572–580. [DOI] [PubMed] [Google Scholar]
- [26].Arriagada P V, Growdon JH, Hedleywhyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639. [DOI] [PubMed] [Google Scholar]
- [27].Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P (1995) Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 16, 285–298. [DOI] [PubMed] [Google Scholar]
- [28].Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR (1997) Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol 94, 471–478. [DOI] [PubMed] [Google Scholar]
- [29].Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A (1988) Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23, 138–144. [DOI] [PubMed] [Google Scholar]
- [30].Delaere P, Duyckaerts C, Masters C, Beyreuther K, Piette F, Hauw JJ (1990) Large amounts of neocortical beta A4 deposits without neuritic plaques nor tangles in a psychometrically assessed, non-demented person. Neurosci Lett 116, 87–93. [DOI] [PubMed] [Google Scholar]
- [31].Reitz C (2012) Alzheimer’s disease and the amyloid cascade hypothesis: A critical review. Int J Alzheimers Dis 2012, 369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puoliväli J, Lesné S, Ashe KH, Muchowski PJ, Mucke L, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, Mucke L (2007) Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem 282, 23818–23828. [DOI] [PubMed] [Google Scholar]
- [33].Puzzo D, Arancio O (2013) Amyloid-β peptide: Dr. Jekyll or Mr. Hyde? J Alzheimers Dis 33(Suppl 1), S111–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Puzzo D, Gulisano W, Arancio O, Palmeri A (2015) The keystone of Alzheimer pathogenesis might be sought in A[3 physiology. Neuroscience 307, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O (2008) Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci 28, 14537–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morley JE, Farr S A, Banks WA, Johnson SN, Yamada KA, Xu L (2010) A physiological role for amyloid-β protein: Enhancement of learning and memory. J Alzheimers Dis 19, 441–449. [DOI] [PubMed] [Google Scholar]
- [37].Lawrence JLM, Tong M, Alfulaij N, Sherrin T, Contarino M, White MM, Bellinger FP, Todorovic C, Nichols RA (2014) Regulation of presynaptic Ca2+, synaptic plasticity and contextual fear conditioning by a N-terminal beta-amyloid fragment. J Neurosci 34, 14210–14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Puzzo D, Privitera L, Fa’ M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, Jung SS, Palmeri A, Arancio O (2011) Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol 69, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Koppensteiner P, Trinchese F, Fá M, Puzzo D, Gulisano W, Yan S, Poussin A, Liu S, Orozco I, Dale E, Teich AF, Palmeri A, Ninan I, Boehm S, Arancio O (2016) Time-dependent reversal of synaptic plasticity induced by physiological concentrations of oligomeric Aβ42: An early index of Alzheimer’s disease. Sci Rep 6, 32553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Glass DJ, Arnold SE (2012) Some evolutionary perspectives on Alzheimer’s disease pathogenesis and pathology. Alzheimers Dement 8, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Herrup K (2015) The case for rejecting the amyloid cascade hypothesis. Nat Neurosci 18, 794–799. [DOI] [PubMed] [Google Scholar]
- [42].Weingarten MD, Lockwood AH, Hwo S Y, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A 72, 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dickson DW, Kouri N, Murray ME, Josephs KA (2011) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45, 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu C, Gotz J (2013) Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PLoS One 8, e84849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Trinczek B, B iernat J, Baumann K, Mandelkow EM, Mandelkow E (1995) Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell 6, 1887–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kar S, Fan J, Smith MJ, Goedert M, Amos LA (2003) Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J 22, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dawson HN (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci 114, 1179–1187. [DOI] [PubMed] [Google Scholar]
- [48].Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP (2007) Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A 104, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yuan A, Kumar A, Peterhoff C, Duff K, Nixon RA (2008) Axonal transport rates in vivo are unaffected by tau deletion or overexpression in mice. J Neurosci 28, 1682–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ittner LM, Ke YD, Gotz J (2009) Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem 284, 20909–20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Frandemiche ML (2014) Activity-dependent tau protein translocation to excitatory synapse is disrupted by exposure to amyloid-[beta] oligomers. J Neurosci 34, 6084–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Qu X, Yuan FN, Corona C, Pasini S, Pero ME, Gundersen GG, Shelanski ML, Bartolini F (2017) Stabilization of dynamic microtubules by mDial drives Tau-dependent Aβ1–42synaptotoxicity. J Cell Biol 216, 3161–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sultan A (2011) Nuclear tau, a key player in neuronal DNA protection. J Biol Chem 286, 4566–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Caillierez R, Talahari S, Nesslany F, Lefebvre B, Bonnefoy E, Buée L, Galas M-C (2014) A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang Y, Mandelkow E (2015) Tau in physiology and pathology. Nat Rev Neurosci 17, 22–35. [DOI] [PubMed] [Google Scholar]
- [56].Arendt T, Stieler JT, Holzer M (2016) Tau and tauopathies. Brain Res Bull 126, 238–292. [DOI] [PubMed] [Google Scholar]
- [57].Mukhopadhyay R, Hoh JH (2001) ARM force measurements on microtubule-associated proteins: The projection domain exerts a long-range repulsive force. FEBS Lett 505, 374–378. [DOI] [PubMed] [Google Scholar]
- [58].Amos LA (2004) Microtubule structure and its stabilisation. Org Biomol Chem 2, 2153. [DOI] [PubMed] [Google Scholar]
- [59].Brandt R, Lee G (1993) Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J Biol Chem 268, 3414–3419. [PubMed] [Google Scholar]
- [60].Maas T, Eidenmiiller J, Brandt R (2000) Interaction of tau with the neural membrane cortex is regulated by phosphorylation at sites that are modified in paired helical filaments. J Biol Chem 275, 15733–15740. [DOI] [PubMed] [Google Scholar]
- [61].Eidenmüller J, Fath T, Maas T, Pool M, Sontag E, Brandt R (2001) Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem J 357, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Reynolds CH, Garwood CJ, Wray S, Price C, Kellie S, Perera T, Zvelebil M, Yang A, Sheppard PW, Varndell IM, Hanger DP, Anderton BH (2008) Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgammal, Grb2, and Src family kinases. J Biol Chem 283, 18177–18186. [DOI] [PubMed] [Google Scholar]
- [63].Lee G, Neve RL, Kosik KS (1989) The microtubule binding domain of tau protein. Neuron 2, 1615–1624. [DOI] [PubMed] [Google Scholar]
- [64].Butner KA, Kirschner MW (1991) Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol 115, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, Feinstein SC (1997) Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell 8, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mukrasch MD, Biernat J, von Bergen M, Griesinger C, Mandelkow E, Zweckstetter M (2005) Sites of tau important for aggregation populate beta-structure and bind to microtubules and polyanions. J Biol Chem 280, 24978–24986. [DOI] [PubMed] [Google Scholar]
- [67].Xia D, Li C, Götz J (2015) Pseudophosphorylation of Tau at distinct epitopes or the presence of the P301L mutation targets the microtubule-associated protein Tau to dendritic spines. Biochim Biophys Acta 1852, 913–924. [DOI] [PubMed] [Google Scholar]
- [68].Cardona-Gomez GP, Arango-Davila C, Gallego-Gomez JC, Barrera-Ocampo A, Pimienta H, Garcia-Segura LM (2006) Estrogen dissociates Tau and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit in postischemic hippocampus. Neuroreport 17, 1337–1341. [DOI] [PubMed] [Google Scholar]
- [69].Miyamoto T, Stein L, Thomas R, Djukic B, Taneja P, Knox J, Vossel K, Mucke L (2017) Phosphorylation of tau at Y18, but not tau-fyn binding, is required for tau to modulate NMDA receptor-dependent excitotoxicity in primary neuronal culture. Mol Neurodegener 12, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E, Mandelkow E (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer’s disease. J Cell Biol 143, 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD (1994) Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proc Natl Acad Sci U S A 91, 11183–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brandt R, Leger J, Lee G (1995) Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol 131, 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bhaskar K, Yen S-H, Lee G (2005) Disease-related modifications in tau affect the interaction between Fyn and Tau. J Biol Chem 280, 35119–35125. [DOI] [PubMed] [Google Scholar]
- [74].Lee G (2005) Tau and src family tyrosine kinases. Biochim Biophys Acta 1739, 323–330. [DOI] [PubMed] [Google Scholar]
- [75].Qi H, Cantrelle F-X, Benhelli-Mokrani H, Smet-Nocca C, Buee L, Lippens G, Bonnefoy E, Galas M-C, Landrieu I (2015) Nuclear magnetic resonance spectroscopy characterization of interaction of tau with DNA and its regulation by phosphorylation. Biochemistry 54, 1525–1533. [DOI] [PubMed] [Google Scholar]
- [76].Kanemaru K, Takio K, Miura R, Titani K, Ihara Y (1992) Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem 58, 1667–1675. [DOI] [PubMed] [Google Scholar]
- [77].Kopke E (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem 268, 24374–24384. [PubMed] [Google Scholar]
- [78].Smith AD (2002) Imaging the progression of Alzheimer pathology through the brain. Proc Natl Acad Sci U S A 99, 4135–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mukrasch MD, Bibow S, Korukottu J, Jeganathan S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M (2009) Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol 7, e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38, 3549–3558. [DOI] [PubMed] [Google Scholar]
- [81].von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow EM, Mandelkow E (2000) Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc Natl Acad Sci U S A 97, 5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E (2006) Global hairpin folding of tau in solution. Biochemistry 45, 2283–2293. [DOI] [PubMed] [Google Scholar]
- [83].Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA (2002) MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol 157, 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Goux WJ, Kopplin L, Nguyen AD, Leak K, Rutkofsky M, Shanmuganandam VD, Sharma D, Inouye H, Kirschner DA (2004) The formation of straight and twisted filaments from short tau peptides. J Biol Chem 279, 26868–26875. [DOI] [PubMed] [Google Scholar]
- [85].Wegmann S, Medalsy ID, Mandelkow E, Muller DJ (2013) The fuzzy coat of pathological human Tau fibrils is a two-layered polyelectrolyte brush. Proc Natl Acad Sci U S A 110, E313–E321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Vega IE (2005) Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates. Brain Res Mol Brain Res 138, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Planel E (2004) Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: Implications for Alzheimer’s disease. J Neurosci 24, 2401–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yanagisawa M, Planel E, Ishiguro K, Fujita SC (1999) Starvation induces tau hyperphosphorylation in mouse brain: Implications for Alzheimer’s disease. FEBS Lett 461, 329–333. [DOI] [PubMed] [Google Scholar]
- [89].Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OFX (2011) Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neuro sci 31, 7840–7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Le Freche H, Brouillette J, Fernandez-Gomez F-J, Patin P, Caillierez R, Zommer N, Sergeant N, Buée-Scherrer V, Lebuffe G, Blum D, Buée L (2012) Tau phosphorylation and sevoflurane anesthesia. Anesthesiology 116, 779–787. [DOI] [PubMed] [Google Scholar]
- [91].Whittington RA, Bretteville A, Dickler MF, Planel E (2013) Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry 47, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Van der Jeugd A (2012) Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol 123, 787–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yoshiyama Y, Higuchi M, Zhang B, Huang S-M, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM-Y (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351. [DOI] [PubMed] [Google Scholar]
- [95].Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 98, 6923–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buée L, Brion J-P (2007) Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol 171, 976–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A (2006) Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer’s disease. Neurosci Res 54, 197–201. [DOI] [PubMed] [Google Scholar]
- [98].Fa M, Puzzo D, Piacentini R, Staniszewski A, Zhang H, Baltrons MA, Li Puma DD, Chatterjee I, Li J, Saeed F, Berman HL, Ripoli C, Gulisano W, Gonzalez J, Tian H, Costa JA, Lopez P, Davidowitz E, Yu WH, Haroutunian V, Brown LM, Palmeri A, Sigurdsson EM, Duff KE, Teich AF, Honig LS, Sierks M, Moe JG, D’Adamio L, Grassi C, Kanaan NM, Fraser PE, Arancio O, D’Adamio L, Grassi C, Kanaan NM, Fraser PE, Arancio O (2016) Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci Rep 6, 19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, Kayed R (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C (2007) Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 27, 3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gerson J, Castillo-Carranza DL, Sengupta U, Bodani R, Prough DS, DeWitt DS, Hawkins BE, Kayed R (2016) Tau oligomers derived from traumatic brain injury cause cognitive impairment and accelerate onset of pathology in hTau mice. J Neurotrauma 33, 2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Sengupta U, Portelius E, Hansson O, Farmer K, Castillo-Carranza D, Woltjer R, Zetterberg H, Galasko D, Blennow K, Kayed R (2017) Tau oligomers in cerebrospinal fluid in Alzheimer’s disease. Ann Clin Transl Neurol 4, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Vuono R, Winder-Rhodes S, de Silva R, Cisbani G, Drouin-Ouellet J, Spillantini MG, Cicchetti F, Barker RA, Barker RA (2015) The role of tau in the pathological process and clinical expression of Huntington’s disease. Brain 138, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Patterson KR, Remmers C, Fu Y, Brooker S, Kanaan NM, Vana L, Ward S, Reyes JF, Philibert K, Glucksman MJ, Binder LI (2011) Characterization of prefibrillar Tau oligomers in vitro and in Alzheimer disease. J Biol Chem 286, 23063–23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Meredith JE, Sankaranarayanan S, Guss V, Lanzetti AJ, Berisha F, Neely RJ, Slemmon JR, Portelius E, Zetterberg H, Blennow K, Soares H, Ahlijanian M, Albright CF (2013) Characterization of novel CSF Tau and ptau biomarkers for Alzheimer’s disease. PLoS One 8, e76523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NCY, Hall GF (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287, 3842–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- [108].Selkoe DJ (2008) Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res 192, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Guerrero-Munoz MJ, Gerson J, Castillo-Carranza DL (2015) Tau oligomers: The toxic player at synapses in Alzheimer’s disease. Front Cell Neurosci 9, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21, 127–148. [DOI] [PubMed] [Google Scholar]
- [111].Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. [DOI] [PubMed] [Google Scholar]
- [112].Carlezon WA, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28, 436–445. [DOI] [PubMed] [Google Scholar]
- [113].Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M (2002) Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A 99, 13217–13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O (2005) Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci 25, 6887–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Teich AF, Nicholls RE, Puzzo D, Fiorito J, Purgatorio R, Fa’ M, Arancio O (2015) synaptic therapy in Alzheimer’s disease: A CREB-centric approach. Neurotherapeutics 12, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Xie M, Li Y, Wang S, Yu Q, Meng X, Liao X (2017) The involvement of NR2B and tau protein in MG132-induced CREB dephosphorylation. J Mol Neurosci 62, 154–162. [DOI] [PubMed] [Google Scholar]
- [117].Yin Y, Gao D, Wang Y, Wang Z-H, Wang X, Ye J, Wu D, Fang L, Pi G, Yang Y, Wang X-C, Lu C, Ye K, Wang J-Z (2016) Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci U S A 113, E3773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pugazhenthi S, Wang M, Pham S, Sze C-I, Eckman CB (2011) Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yamamoto-Sasaki M, Ozawa H, Saito T, Rösler M, Riederer P (1999) Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res 824, 300–303. [DOI] [PubMed] [Google Scholar]
- [120].Yamamoto M, Ozawa H, Saito T, Frölich L, Riederer P, Takahata N (1996) Reduced immunoreactivity of adenylyl cyclase in dementia of the Alzheimer type. Neuroreport 7, 2965–2970. [DOI] [PubMed] [Google Scholar]
- [121].Bartolotti N, Bennett DA, Lazarov O (2016) Reduced pCREB in Alzheimer’s disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol Psychiatry 21, 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yamamoto M, Ozawa H, Saito T, Hatta S, Riederer P, Takahata N (1997) Ca2+/CaM-sensitive adenylyl cyclase activity is decreased in the Alzheimer’s brain: Possible relation to type I adenylyl cyclase. J Neural Transm 104. 721–732. [DOI] [PubMed] [Google Scholar]
- [123].Fink CC, Meyer T (2002) Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol 12, 293–299. [DOI] [PubMed] [Google Scholar]
- [124].Ghosh A, Giese KP (2015) Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol Brain 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhao D, Watson JB, Xie C-W (2004) Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol 92, 2853–2858. [DOI] [PubMed] [Google Scholar]
- [126].Gu Z, Liu W, Yan Z (2009) {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem 284, 10639–10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Whitcomb DJ, Hogg EL, Regan P, Piers T, Narayan P, Whitehead G, Winters BL, Kim D-H, Kim E, St George-Hyslop P, Klenerman D, Collingridge GL, Jo J, Cho K (2015) Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci Rep 5, 10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Baudier J, Cole RD (1987) Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem 262, 17577–17583. [PubMed] [Google Scholar]
- [129].Baudier J, Cole RD (1988) Interactions between the microtubule-associated tau proteins and SlOOb regulate tau phosphorylation by the Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 263, 5876–5883. [PubMed] [Google Scholar]
- [130].Singh TJ, Wang JZ, Novak M, Kontzekova E, Grundke-Iqbal I, Iqbal K (1996) Calcium/calmodulin-dependent protein kinase II phosphorylates tau at Ser-262 but only partially inhibits its binding to microtubules. FEBS Lett 387, 145–148. [DOI] [PubMed] [Google Scholar]
- [131].Steiner B, Mandelkow EM, Biernat J, Gustke N, Meyer HE, Schmidt B, Mieskes G, Soling HD, Drechsel D, Kirschner MW (1990) Phosphorylation of microtubule-associated protein tau: Identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J 9, 3539–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Oka M, Fujisaki N, Maruko-Otake A, Ohtake Y, Shimizu S, Saito T, Hisanaga S-I, Iijima KM, Ando K (2017) Ca2+/calmodulin-dependent protein kinase II promotes neurodegeneration caused by tau phosphorylated at Ser262/356 in a transgenic Drosophila model of tauopathy. J Biochem 162, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Song J-H, Yu J-T, Tan L (2014) Brain-derived neurotrophic factor in Alzheimer’s disease: Risk, mechanisms, and therapy. Mol Neurobiol 52, 1477–1493. [DOI] [PubMed] [Google Scholar]
- [134].Burnouf S, Martire A, Derisbourg M, Laurent C, Belarbi K, Leboucher A, Fernandez-Gomez FJ, Troquier L, Eddarkaoui S, Grosjean M-E, Demeyer D, Muhr-Tailleux A, Buisson A, Sergeant N, Hamdane M, Humez S, Popoli P, Buée L, Blum D (2013) NMDA receptor dysfunction contributes to impaired brain-derived neurotrophic factor-induced facilitation of hippocampal synaptic transmission in a Tau transgenic model. Aging Cell 12, 11–23. [DOI] [PubMed] [Google Scholar]
- [135].Jiao S-S, Shen L-L, Zhu C, Bu X-L, Liu Y-H, Liu C-H, Yao X-Q, Zhang L-L, Zhou H-D, Walker DG, Tan J, Gotz J, Zhou X-F, Wang Y-J (2016) Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl Psychiatry 6, e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) APP processing and synaptic function. Neuron 37, 925–937. [DOI] [PubMed] [Google Scholar]
- [137].Cirri to JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM (2005) Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48, 913–922. [DOI] [PubMed] [Google Scholar]
- [138].Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM (2008) Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321, 1221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Scala F, Fusco S, Ripoli C, Piacentini R, Li Puma DD, Spinelli M, Laezza F, Grassi C, D’Ascenzo M (2015) Intraneuronal Aβ accumulation induces hippocampal neuron hyperexcitability through A-type K+ current inhibition mediated by activation of caspases and GSK-3. Neurobiol Aging 36, 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L (2013) Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70, 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Palmeri A, Ricciarelli R, Gulisano W, Rivera D, Rebosio C, Calcagno E, Tropea MR, Conti S, Das U, Roy S, Pronzato MA, Arancio O, Fedele E, Puzzo D (2017) Amyloid-β peptide is needed for cGMP-induced long-term potentiation and memory. J Neurosci 37, 6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tampellini D (2015) Synaptic activity and Alzheimer’s disease: A critical update. Front Neurosci 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, Lin MT, Gouras GK (2009) Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci 29, 9704–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Puzzo D, Piacentini R, Fa M, Gulisano W, Li Puma DD, Staniszewski A, Zhang H, Tropea MR, Cocco S, Palmeri A, Fraser P, D’Adamio L, Grassi C, Arancio O (2017) LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent. Elife 6, e26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ripoli C, Cocco S, Li Puma DD, Piacentini R, Mastrodonato A, Scala F, Puzzo D, D’Ascenzo M, Grassi C (2014) Intracellular accumulation of amyloid-β (Aβ) protein plays a maj or role in Aβ-induced alterations of glutamatergic synaptic transmission and plasticity. J Neurosci 34, 12893–12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP (2013) Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep 14, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgrafe K, Mandelkow E-M, Holtzman DM (2014) Neuronal activity regulates extracellular tau in vivo. J Exp Med 211, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RACM, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284, 12845–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Guo JL, Lee VM (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286, 15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Guo JL, Lee VMY (2013) Neurofibrillary tangle-like tau pathology induced by synthetic tau fibrils in primary neurons over-expressing mutant tau. FEBS Lett 587, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, Steinberg JI, Margittai M, Kayed R, Zurzolo C, Di Paolo G, Duff KE (2013) Small misfolded tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 288, 1856–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Luo W, Liu W, Hu X, Hanna M, Caravaca A, Paul SM (2015) Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci Rep 5, 11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bolós M, Perea JR, Avila J (2017) Alzheimer’s disease as an inflammatory disease. Biomol Concepts 8, 37–43. [DOI] [PubMed] [Google Scholar]
- [155].Piacentini R, Li Puma DD, Mainardi M, Lazzarino G, Tavazzi B, Arancio O, Grassi C (2017) Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia 65, 1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI (2013) Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A 110, E3138–E3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Braak H, Del Tredici K (2011) Alzheimer’s pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathol 121, 589–595. [DOI] [PubMed] [Google Scholar]
- [158].Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012) Trans-synaptic spread of tau pathology in vivo. PLoS One 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Jack CR, Knopman DS, Chételat G, Dickson D, Fagan AM, Frisoni GB, Jagust W, Mormino EC, Petersen RC, Sperling RA, van der Flier WM, Villemagne VL, Visser PJ, Vos SJB (2016) Suspected non-Alzheimer disease patho-physiology — concept and controversy. Nat Rev Neurol 12, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A 82, 4531–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Jarrett JT, Lansbury PT (1993) Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73, 1055–1058. [DOI] [PubMed] [Google Scholar]
- [162].Lv Z-Y, Tan C-C, Yu J-T, Tan L (2017) Spreading of pathology in Alzheimer’s disease. Neurotox Res 32, 707–722. [DOI] [PubMed] [Google Scholar]
- [163].Palop JJ, Jones B, Kekonius L, Chin J, Yu G-Q, Raber J, Masliah E, Mucke L (2003) Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci U S A 100, 9572–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Scarmeas N, Honig LS, Choi H, Cantero J, Brandt J, Blacker D, Albert M, Amatniek JC, Marder K, Bell K, Hauser WA, Stern Y (2009) Seizures in Alzheimer disease. Arch Neurol 66, 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P, Lo I, Yu G-Q, Palop JJ, Masliah E, Mucke L (2010) Transsynaptic progression of amyloid-$β$-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68, 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, Yan SS, Origlia N (2017) Entorhinal Cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Sci Rep 7, 42370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, Giussani P, Magnani G, Comi G, Legname G, Ghidoni R, Furlan R, Matteoli M, Verderio C (2014) Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Prada I, Amin L, Furlan R, Legname G, Verderio C, Cojoc D (2016) A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. Biotechniques 60, 35–41. [DOI] [PubMed] [Google Scholar]
- [169].Söllvander S, Nikitidou E, Brolin R, Söderberg L, Sehlin D, Lannfelt L, Erlandsson A (2016) Accumulation of amyloid-β by astrocytes result in enlarged endosomes and micro vesicle-induced apoptosis of neurons. Mol Neurodegener 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M (2012) Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci 32, 8767–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Domert J, Rao SB, Agholme L, Brorsson A-C, Marcusson J, Hallbeck M, Nath S (2014) Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis 65, 82–92. [DOI] [PubMed] [Google Scholar]
- [172].Rustom A, Saffrich R, Markovic I, Walther P, Gerdes H-H (2004) Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. [DOI] [PubMed] [Google Scholar]
- [173].Wang Y, Cui J, Sun X, Zhang Y (2011) Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ 18, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103, 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Zheng L, Terman A, Hallbeck M, Dehvari N, Cowburn RF, Benedikz E, Kågedal K, Cedazo-Minguez A, Marcusson J (2011) Macroautophagy-generated increase of lysosomal amyloid (3-protein mediates oxidant-induced apoptosis of cultured neuroblastoma cells. Autophagy 7, 1528–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC, Miller TM, Grinberg LT, Seeley WW, Diamond MI (2014) Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [178].Meunier M, Bachevalier J, Mishkin M, Murray EA, Chavoix C (1993) Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci 13, 5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kiigler S, Ikezu T (2015) Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin J-C, Mannel D, Zurzolo C (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11, 328–336. [DOI] [PubMed] [Google Scholar]
- [181].Simón D, García-García E, Gómez-Ramos A, Falcón-Pérez JM, Díaz-Hernández M, Hernández F, Avila J (2012) Tau overexpression results in its secretion via membrane vesicles. Neurodegener Dis 10, 73–75. [DOI] [PubMed] [Google Scholar]
- [182].Dujardin S, Beégard S, Caillierez R, Lachaud C, Delattre L, Carrier S, Loyens A, Galas M-C, Bousset L, Melki R, Auregan G, Hantraye P, Brouillet E, Buée L, Colin M (2014) Ectosomes: A new mechanism for non-exosomal secretion of tau protein. PLoS One 9, e100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Morales R, Moreno-Gonzalez I, Soto C (2013) Cross-seeding of misfolded proteins: Implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog 9, e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4, 49–60. [DOI] [PubMed] [Google Scholar]
- [185].Hofrichter J, Ross PD, Eaton WA (1974) Kinetics and mechanism of deoxyhemoglobin S gelation: A new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A 71, 4864–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Chothia C, Janin J (1975) Principles of protein-protein recognition. Nature 256, 705–708. [DOI] [PubMed] [Google Scholar]
- [187].Morales R, Green KM, Soto C (2009) Cross currents in protein misfolding disorders: Interactions and therapy. CNS Neurol Disord Drug Targets 8, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Spires-Jones TL, Attems J, Thai DR (2017) Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 134, 187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Götz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P3011 tau transgenic mice induced by Abeta 42 fibrils. Science 293, 1491–1495. [DOI] [PubMed] [Google Scholar]
- [190].Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M (2007) Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP × Tau transgenic mice. Am J Pathol 171, 2012–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Guo J-P, Arai T, Miklossy J, McGeer PL (2006) Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer’s disease. Proc Natl Acad Sci U S A 103, 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Vasconcelos B, Stancu I-C, Buist A, Bird M, Wang P, Vanoosthuyse A, Van Kolen K, Verheyen A, Kienlen-Campard P, Octave J-N, Baatsen P, Moechars D, Dewachter I (2016) Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol 131, 549–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kayed R, Head E, Thompson JL, Mclntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489. [DOI] [PubMed] [Google Scholar]
- [194].Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett EM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (2008) Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid -protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci U S A 108, 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu G-Q, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754. [DOI] [PubMed] [Google Scholar]
- [198].Lorenzo A, Yuan M, Zhang Z, Paganetti PA, Sturchler-Pierrat C, Staufenbiel M, Mautino J, Sol Vigo F, Sommer B, Yankner BA (2000) Amyloid beta interacts with the amyloid precursor protein: A potential toxic mechanism in Alzheimer’s disease. Nat Neurosci 3, 460–464. [DOI] [PubMed] [Google Scholar]
- [199].Shaked GM, Kummer MP, Lu DC, Galvan V, Bredesen DE, Koo EH (2006) Abeta induces cell death by direct interaction with its cognate extracellular domain on APP (APP 597–624). FASEB J 20, 1254–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wang Z, Jackson RJ, Hong W, Taylor WM, Corbett GT, Moreno A, Liu W, Li S, Frosch MP, Slutsky I, Young-Pearse TL, Spires-Jones TL, Walsh DM (2017) Human brain-derived Aβ oligomers bind to synapses and disrupt synaptic activity in a manner that requires APP. J Neurosci 37, 11947–11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, YassaM A, Bassett SS, Shelton AL, Gallagher M (2012) Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A (2012) Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 109 8740–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in alzheimer model. Cell 149, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Fogel H, Frere S, Segev O, Bharill S, Shapira I, Gazit N, O’Malley T, Slomowitz E, Berdichevsky Y, Walsh DM, Isacoff EY, Hirsch JA, Slutsky I (2014) APP homodimers transduce an amyloid-βmediated increase in release probability at excitatory synapses. Cell Rep 7, 1560–1576. [DOI] [PubMed] [Google Scholar]
- [206].Smith MA, Siedlak SL, Richey PL, Mulvihill P, Ghiso J, Frangione B, Tagliavini F, Giaccone G, Bugiani O, Praprotnik D (1995) Tau protein directly interacts with the amyloid beta-protein precursor: Implications for Alzheimer’s disease. Nat Med 1, 365–369. [DOI] [PubMed] [Google Scholar]
- [207].Giaccone G, Pedrotti B, Migheli A, Verga L, Perez J, Racagni G, Smith MA, Perry G, De Gioia L, Selvaggini C, Salmona M, Ghiso J, Frangione B, Islam K, Bugiani O, Tagliavini F (1996) beta PP and Tau interaction. A possible link between amyloid and neurofibrillary tangles in Alzheimer’s disease. Am J Pathol 148, 79–87. [PMC free article] [PubMed] [Google Scholar]
- [208].Islam K, Levy E (1997) Carboxyl-terminal fragments of beta-amyloid precursor protein bind to microtubules and the associated protein tau. Am J Pathol 151, 265–271. [PMC free article] [PubMed] [Google Scholar]
- [209].Takahashi M, Miyata H, Kametani F, Nonaka T, Akiyama H, Hisanaga S ichi, Hasegawa M (2015) Extracellular association of APP and tau fibrils induces intracellular aggregate formation of tau. Acta Neuropathol 129, 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Fraser SP, Suh YH, Chong YH, Djamgoz MBA (1996) Membrane currents induced in xenopus oocytes by the C-terminal fragment of the beta-amyloid precursor protein. J Neurochem 66, 2034–2040. [DOI] [PubMed] [Google Scholar]
- [211].Fraser SP, Suh YH, Djamgoz MB (1997) Ionic effects of the Alzheimer’s disease beta-amyloid precursor protein and its metabolic fragments. Trends Neurosci 20, 67–72. [DOI] [PubMed] [Google Scholar]
- [212].Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG (2004) Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem 279, 46363–46366. [DOI] [PubMed] [Google Scholar]
- [213].Xu W, Weissmiller AM, White JA, Fang F, Wang X, Wu Y, Pearn ML, Zhao X, Sawa M, Chen S, Gunawardena S, Ding J, Mobley WC, Wu C (2016) Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration. J Clin Invest 126, 1815–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Cao X (2001) A transcriptively active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293, 115–120. [DOI] [PubMed] [Google Scholar]
- [215].Fombonne J, Rabizadeh S, Ban wait S, Mehlen P, Bredesen DE (2009) Selective vulnerability in Alzheimer’s disease: Amyloid precursor protein and p75(NTR) interaction. Ann Neurol 65, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Shin R-W, Ogino K, Shimabuku A, Taki T, Nakashima H, Ishihara T, Kitamoto T (2007) Amyloid precursor protein cytoplasmic domain with phospho-Thr668 accumulates in Alzheimer’s disease and its transgenic models: A role to mediate interaction of Abeta and tau. Acta Neuropathol 113, 627–636. [DOI] [PubMed] [Google Scholar]
- [217].Lombino F, Biundo F, Tamayev R, Arancio O, D’Adamio L (2013) An intracellular threonine of amyloid-β precursor protein mediates synaptic plasticity deficits and memory loss. PLoS One 8, e57120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Saito Y, Matsushima T, Suzuki T (2013) Mechanism of Alzheimer amyloid β-protein precursor localization to membrane lipid rafts. In Understanding Alzheimer’s Disease, Zerr I, ed. InTech. [Google Scholar]
- [219].Nicholls RE, Sontag J-M, Zhang H, Staniszewski A, Yan S, Kim CY, Yim M, Woodruff CM, Arning E, Wasek B, Yin D, Bottiglieri T, Sontag E, Kandel ER, Arancio O (2016) PP2A methylation controls sensitivity and resistance to β-amyloid-induced cognitive and electrophysiological impairments. Proc Natl Acad Sci U S A 113, 3347–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]