Highlights
-
•
NTRK fusion-positive fibrosarcoma like tumour of the cervix in a paediatric patient.
-
•
Fertility sparing surgery achieved with the use of a neo-adjuvant TKi.
-
•
Identifying NTRK fusions may afford conservative treatment options in these tumours.
Keywords: NTRK1 fusion, TRK inhibitor, Cervical sarcoma, Fibrosarcoma, Entrectinib, Neo-adjuvant
Abstract
This case report reviews the case of a 13-year-old patient who presented with a 9 cm NTRK1-rearranged cervical sarcoma with fibrosarcoma like morphology. At presentation the lesion filled her vagina and pelvis and any attempt at surgical removal would have been morbid and led to loss of fertility. These neoplasms are extremely rare with 18 cases of the uterine cervix reported in the literature, none of which have occurred in a paediatric patient, and none of whom have received neo-adjuvant therapy prior to excision. Based upon evidence that has shown good tolerability and responses of paediatric NTRK fusion-positive solid tumours to TRK inhibitors, both in the neo-adjuvant and upfront setting, this patient was managed with neo-adjuvant entrectinib. Following a dramatic reduction in tumour size confirmed by imaging, she underwent conservative fertility sparing surgery with final histopathology showing no residual disease.
1. Introduction
Fibrosarcoma like tumours of the uterine cervix affecting premenopausal women with neurotrophic tyrosine kinase receptor (NTRK) gene rearrangements have recently been described in the literature (Rabban et al., 2020). They are extremely rare and to our knowledge there are only 18 cervical cases reported, none of which has occurred in the paediatric population. Solid NTRK fusion-positive tumours are responsive to highly selective TRK inhibitors (TRKi) with entrectinib and larotrectinib having been studied in early phase clinical trials in children and adults showing high response rates with good durability (Shulman & DuBois, 2020). Studies have also shown that paediatric patients with NTRK fusion-positive solid tumours at other sites who have received neo-adjuvant TRKi due to initially unresectable disease, have had good responses to treatment, resulting in less morbid surgical resections with R0 resections being achieved in roughly half of these cohorts (DuBois et al., 2018, Laetsch et al., 2018). We present the case of a 13-year-old patient who presented with a 9 cm NTRK1-rearranged cervical sarcoma with fibrosarcoma like morphology. Informed consent was obtained for the writing of this manuscript.
2. Case report
A 13-year-old patient presented with a short history of vaginal discharge and bleeding, fevers and night sweats. Medical background was otherwise unremarkable and family history included a paternal grandmother with breast cancer, paternal grandfather with prostate cancer and a maternal grandmother with renal cancer.
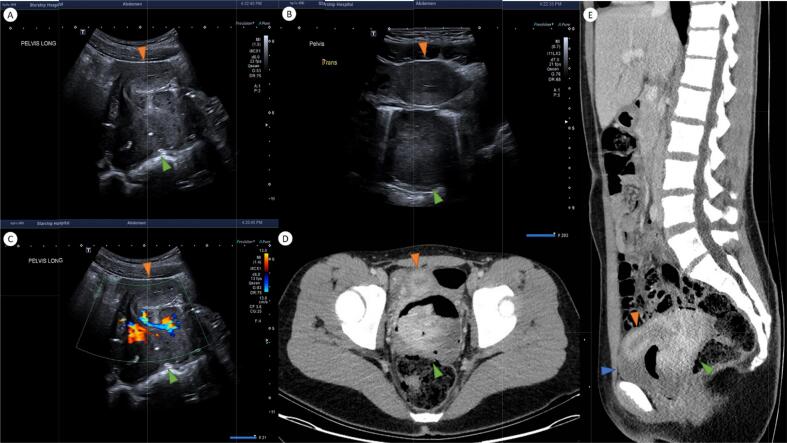
Pelvic ultrasound (Fig. 1 A-C) demonstrated a heterogenous solid vascular mass (green arrowhead) arising from the uterine cervix and filling the vaginal cavity. It exerted mass effect in the pelvis, displacing the uterine body anteriorly (orange arrowhead). CT (Fig. 1 D-E) confirmed a lobulated enhancing mass (green arrowhead) distending the vagina and arising from a vascular pedicle in the right low posterior cervix measuring 9.2 x 8.2 cm. No parametrial linvolvement or nodal disease was evident (Fig. 1).
Fig. 1.
Ultrasound and CT at diagnosis.
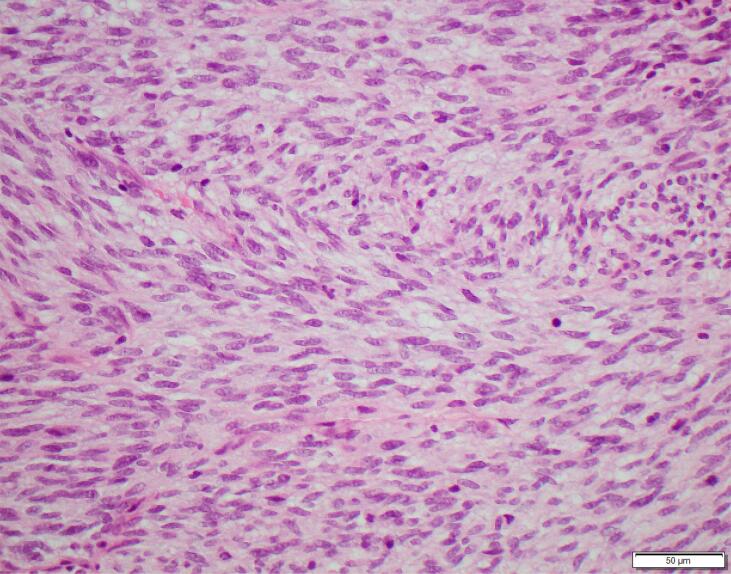
The patient underwent an examination under anaesthesia (EUA) and vaginal biopsy. The histopathology showed monomorphic, mitotically-active spindle cells with a fascicular and herring-bone architecture (Fig. 2). Immunohistochemistry (IHC) showed diffuse S100 and patchy CD34 expression. The tumour was sent overseas for further testing.
Fig. 2.
Bland spindle cells with a herringbone pattern, Haematoxylin and Eosin.
The case was discussed through both the paediatric and gynaecologic oncology multidisciplinary-meetings (MDM). After extensive counselling regarding the potential for iatrogenic infertility as a result of multimodal treatment, the patient was enrolled into a clinical trial, as is offered standardly to all patients with a high risk of infertility at our institution. The patient underwent a laparoscopic right oophorectomy for ovarian cryopreservation and fertility preservation, and was administered a gonadotrophin releasing hormone agonist for ovarian suppression and chemoprotection. Due to the challenging histological diagnosis and ongoing symptoms, the patient was treated with empiric chemotherapy as per the high risk rhabdomyosarcoma protocol with doxorubicin and ifosfamide. The patient received one cycle of doxorubicin and ifosfamide chemotherapy, however, due to toxicity declined ongoing chemotherapy, in addition to this the tumour had enlarged on imaging although did not fulfil RECIST criteria for tumour progression.
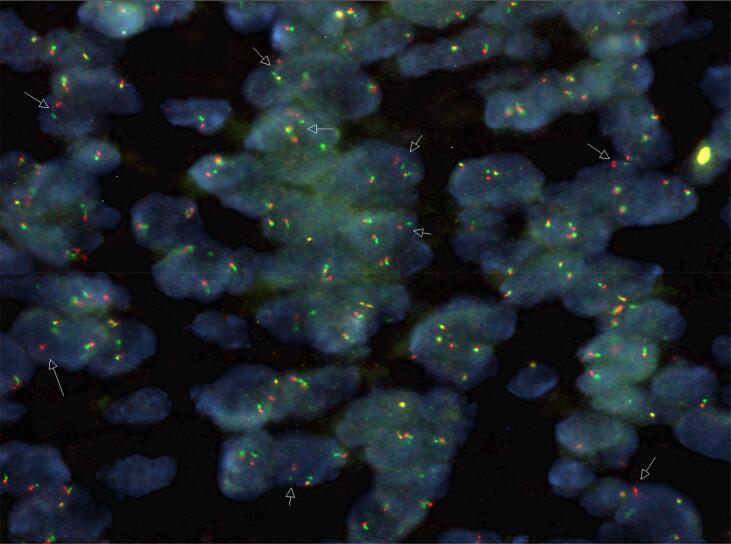
Following this further histopathological investigations showed PanTRK IHC to be diffusely positive and fluorescence in situ hybridization (FISH) with a Zytovision NTRK1 break-apart probe was positive for an NTRK1 rearrangement (Fig. 3). Next generation sequencing showed a TPM3-NTRK1 fusion.
Fig. 3.
Positive NTRK1 break-apart FISH, Zytovision probe. [Credit to Foundation Medicine and Ms Joanne Peverall, Senior Scientist, PathWest Labaroatory Medicine, Perth, Western Australia].
Imaging and histopathology results were reviewed via MDM and it was felt that surgical resection would require a hysterectomy, which would signficantly impact upon the patient's future fertility causing marked morbidity. Based upon the FISH studies the decision was made for a trial of neo-adjuvant entrectinib. The patient was commenced on entrectinib, a TRKi with activities against NTRK fusion-positive solid tumours, and received a total of 27 weeks treatment, during which time the patients symptoms improved, enabling a good quality of life, returning to horse riding four weeks after commencement of therapy.
Regarding duration of treatment, entrectinib has a median progression free survival (PFS) of 11 months (Doebele et al., 2020). Resistance is well described and is inevitable, with one case in the colorectal literature describing resistance as early as 16 weeks (Russo et al., 2016). In order to avoid potential drug resistance and disease progression surveillance with imaging at 8 weekly intervals was initiated.
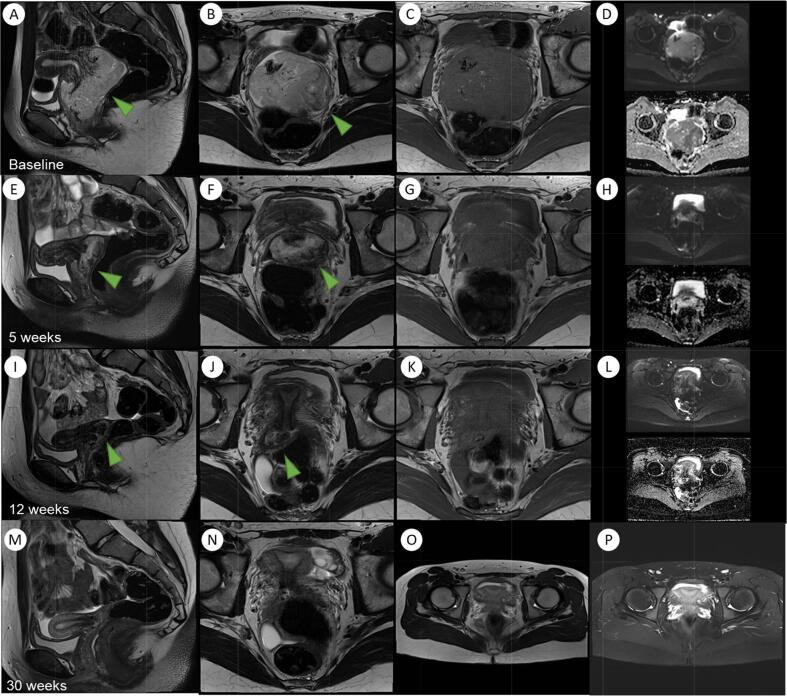
Surveillance of the tumour with MRI confirmed a good response to treatment and at 12 weeks post initiation there was a reduction in size of the tumour to a maximal dimension of 3.6 cm, with no resiual disease seen at 30 weeks post commencement of treatment (Fig. 4). A repeat EUA and biopsy was performed showing a normal vaginal vault and a small exophytic tumour arising from the external cervical os with no evidence of parametrial invasion. Biopsies showed no viable tumour.
Fig. 4.
Serial MRI imaging from diagnosis to post-treatment follow-up.
A cone biopsy of 19x20mm width and 16 mm depth was subsequently performed to excise the tumour bed, which measured 6x6x3mm. There was no residual viable tumour and the tumour bed was excised with clear margins, the closest being 6 mm. At the time of writing this manuscript, the patient remains well with no evidence of disease recurrence four months after completion of treatment.
3. Discussion
NTRK1 was first identified as an oncogene in 1982 (Pulciani et al., 1982), following which three NTRK genes (NTRK1-3) have been identified. The NTRK genes encode the tropomyosin receptor kinases (TRK) TRKA, TRKB and TRKC, which are normally involved in cellular signalling cascades in nervous system development (Rabban et al., 2020). Gene fusions result from chromosomal rearrangements and recurrent gene fusions are oncogenic drivers of tumour growth and survival across a variety of malignancies (Stransky, Cerami, Schalm, Kim, & Lengauer, 2014). Structurally many of these fusions have an intact tyrosine kinase domain fused to an upstream gene partner that promotes ligand-independent dimerization with the resulting chimeric oncoprotein leading to increased cellular signalling and tumour growth and proliferation (Shaw, Hsu, Awad, & Engelman, 2013).
Fusions of the NTRK genes have been identified in a wide spectrum of malignancies in patients of all ages and are commonly associated with infantile fibrosarcoma, cellular and mixed type congenital mesoblastic nephroma, mammary analogue secretory carcinoma and secretory breast carcinoma (Shulman & DuBois, 2020). More recently, mesenchymal tumours with NTRK fusions have been described at multiple sites and across a broad age range (Davis et al., 2019). They comprise a heterogeneous group clinically, morphologically and at a molecular level, and have little in common with infantile fibrosarcoma. They have been described in superficial and deep soft tissues, bone and in the viscera.
Previously these diseases have required morbid therapies and have heralded a poor prognosis, however the introduction of TRKi has shown durable and high responses with modest toxicity profiles and harbours an exciting opportunity for targeted agents in NTRK fusion-positive tumours (Drilon et al., 2018, Shulman and DuBois, 2020, DuBois et al., 2018, Laetsch et al., 2018).
Mesenchymal neoplasms of the uterine cervix are a heterogenous group of tumours and with the advancement of molecular technology newly defined genetic abnormalities are being recognised in this group (Croce et al., 2019). Recently fibrosarcoma like tumours of the uterine cervix affecting premenopausal women with NTRK gene rearrangements have been described (Rabban et al., 2020). To our knowledge, 18 such cases have been reported in the literature, none of which have been in the paediatric population or have received neo-adjuvant TRKi therapy followed by surgical resection (Rabban et al., 2020, Croce et al., 2019, Chiang et al., 2018, Boyle et al., 2020).
Fibrosarcoma like tumours of the uterine cervix tend to present as a cervical polyp or mass in patients in their early twenties to late forties with bleeding and discharge commonly described (Rabban et al., 2020). Histologically they are spindle cell tumours resembling fibrosarcoma, with high mitotic activity and sometimes focal necrosis (Croce et al., 2019). Most NTRK fusion-positive tumours show TRK expression detectable by pan-TRK IHC with one study showing a sensitivity of greater than 95% (Rudzinski et al., 2018). Molecular confirmation is required and this can be done rapidly with FISH or reverse transcription-polymerase chain reaction (RT-PCR), or with Next Generation Sequencing (Shulman & DuBois, 2020). Given the recent emergence of fibrosarcoma like tumours of the uterine cervix in premenopausal patients, the use of IHC and molecular technology to identify NTRK gene rearrangements in these tumours is recommended given the potential for targeted treatments (Croce et al., 2019).
There is a growing body of evidence regarding the efficacy of TRKi for the treatment of NTRK fusion-positive tumours with both larotrectinib and entrectinib. Regarding larotrectinib, in an early combined analysis of three phase I to II studies (n = 55) the overall objective response rate (ORR) across all tumour types was 76%, including a 22% complete response rate (Drilon et al., 2018). In regards to entrectinib, a pooled analysis (n = 54) of patients with advanced or metastatic NTRK fusion-positive solid tumours the ORR was 57% across all tumour types with 7% achieving a complete response, with a median duration of response of 10.4 months and median progression free survival of 11 months (Doebele et al., 2020).
The efficacy and safety of TRKi with solid NTRK fusion-positive tumours in the paediatric population with larotrectinib and entrectinib has also been established in phase I and II clinical trials showing high response rates and good durability of responses (Shulman & DuBois, 2020). In addition to this trials in the paediatric population have shown that neo-adjuvant TRKi can lead to remarkable disease regressions allowing for less morbid surgical resections with good complete resection rates (DuBois et al., 2018, Laetsch et al., 2018). As such, both agents are now approved in the United States for the treatment of paediatric solid NTRK fusion-positive tumours in the setting of relapsed disease, or where no viable alternative systemic treatment is available, and for those patients without an option of complete surgical resection.
There has been one case described in the literature of the use of TRKi in an NTRK fusion-positive cervical sarcoma in the setting of recurrent disease, in which larotrectinib was given following the development of pulmonary metastasis, the patient had a good response and was alive and disease free at the time of publication 15 months later (Rabban et al., 2020). The use of neo-adjuvant TRKi in the paediatric population in this setting has, to our knowledge, not yet been described.
4. Conclusion
We describe the first case of a paediatric patient with a NTRK fusion-positive fibrosarcoma like tumour of the uterine cervix who was successfully managed with neo-adjuvant entrectinib and subsequently went on to have conservative, fertility sparing surgery.
As the molecular profiling of tumours continues to evolve the number of fusion events that are detected is rising, as is the proportion of recurrent gene rearrangements that are clinically relevant, inclusive of recurrent NTRK gene fusions which are amenable to targeted therapies such as TRKi.
This case is significant for several reasons. Firstly, it is the first described case of a NTRK fusion-positive cervical fibrosarcoma like tumour in the paediatric population, as all published cases have been in premenopausal women from their early twenties to late forties (Rabban et al., 2020). Secondly it highlights the importance of the investigation of NTRK fusions in fibrosarcoma like tumours of the uterine cervix, as this may open up treatment options for patients and avoids potentially morbid extensive surgery, which may impair fertility.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Boyle W., Williams A., Sundar S., Yap J., Taniere P., Rehal P. TMP3-NTRK1 rearranged uterine sarcoma: A case report. Case Rep. Womens Health. 2020;28 doi: 10.1016/j.crwh.2020.e00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S., Cotzia P., Hyman D.M., Drilon A., Tap W.D., Zhang L. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018;42(6):791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce S., Hostein I., Longacre T.A., Mills A.M., Perot G., Devouassoux-Shisheboran M. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol. 2019;32(7):1008–1022. doi: 10.1038/s41379-018-0184-6. [DOI] [PubMed] [Google Scholar]
- Davis J.L., Lockwwod C.M., Stohr B., Boecking C., Al-Ibraheemi A., DuBois S.G., Vargas S.O., Black J.O., Cox M.C., Luquette M., Turpin B., Szaba S., Laetsch T.W., Albert C.M., Parham D.M., Hawkins D.S., Ruzinski E.R. Expanding the Spectrum of Pediatric NTRK-rearranged Mesenchymal Tumors. Am. J. Surg. Pathol. 2019;43:435–445. doi: 10.1097/PAS.0000000000001203. [DOI] [PubMed] [Google Scholar]
- Doebele R.C., Drilon A., Paz-Ares L., Siena S., Shaw A.T., Farago A.F. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilon A., Laetsch T.W., Kummar S., DuBois S.G., Lassen U.N., Demetri G.D. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois S.G., Laetsch T.W., Federman N., Turpin B.K., Albert C.M., Nagasubramanian R. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer. 2018;124(21):4241–4247. doi: 10.1002/cncr.31701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch T.W., DuBois S.G., Mascarenhas L., Turpin B., Federman N., Albert C.M. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A.V., Long L.K., Aaronson S.A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982;300(5892):539–542. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- Rabban J.T., Devine W.P., Sangoi A.R., Poder L., Alvarez E., Davis J.L. NTRK fusion cervical sarcoma: a report of three cases, emphasising morphological and immunohistochemical distinction from other uterine safrcomas, including adenosarcoma. Histopathology. 2020;77(1):100–111. doi: 10.1111/his.14069. [DOI] [PubMed] [Google Scholar]
- Rudzinski E.R., Lockwood C.M., Stohr B.A., Vargas S.O., Sheridan R., Black J.O. Pan-Trk Immunohistochemistry Identifies NTRK Rearrangements in Pediatric Mesenchymal Tumors. Am. J. Surg. Pathol. 2018;42(7):927–935. doi: 10.1097/PAS.0000000000001062. [DOI] [PubMed] [Google Scholar]
- Russo M., Misale S., Wei G., Siravegna G., Crisafulli G., Lazzari L. Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer. Cancer Discov. 2016;6(1):36–44. doi: 10.1158/2159-8290.CD-15-0940. [DOI] [PubMed] [Google Scholar]
- Shaw A.T., Hsu P.P., Awad M.M., Engelman J.A. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat. Rev. Cancer. 2013;13(11):772–787. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman D.S., DuBois S.G. The Evolving Diagnostic and Treatment Landscape of NTRK-Fusion-Driven Pediatric Cancers. Paediatr. Drugs. 2020;22(2):189–197. doi: 10.1007/s40272-020-00380-9. [DOI] [PubMed] [Google Scholar]
- Stransky N., Cerami E., Schalm S., Kim J.L., Lengauer C. The landscape of kinase fusions in cancer. Nat. Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]