ABSTRACT
This review provides an overview of the most important novel treatment strategies against Streptococcus pneumoniae infections published over the past 10 years. The pneumococcus causes the majority of community-acquired bacterial pneumonia cases, and it is one of the prime pathogens in bacterial meningitis. Over the last 10 years, extensive research has been conducted to prevent severe pneumococcal infections, with a major focus on (i) boosting the host immune system and (ii) discovering novel antibacterials. Boosting the immune system can be done in two ways, either by actively modulating host immunity, mostly through administration of selective antibodies, or by interfering with pneumococcal virulence factors, thereby supporting the host immune system to effectively overcome an infection. While several of such experimental therapies are promising, few have evolved to clinical trials. The discovery of novel antibacterials is hampered by the high research and development costs versus the relatively low revenues for the pharmaceutical industry. Nevertheless, novel enzymatic assays and target-based drug design, allow the identification of targets and the development of novel molecules to effectively treat this life-threatening pathogen.
Keywords: Streptococcus pneumoniae, virulence, drug development, novel drug targets, immunotherapy, antibiotics
This review discusses recent progress in anti-pneumococcal treatment strategies, with a focus on novel drug targets.
Key Points
Analogues of already marketed antibiotics are in development to overcome increasing pneumococcal resistance. Several of these are currently undergoing clinical trials.
Antibacterial targets, consisting of pneumococcal enzymes essential for viability and survival, are being identified. Target-specific rational drug design, including high-throughput screening (HTS) against a major enzyme followed by elucidation of the structure-activity relationship (SAR) and subsequent lead optimization, shows promise in creating novel antibacterials. Contrarily, discovery of natural antibacterials is being hampered by the difficult growth conditions for microbes producing antibacterials. Furthermore, due to potentially low revenues the pharmaceutical sector is showing less interest in the development of novel antibacterials. Therefore, alternative strategies including modulating the host immune system or inhibiting pneumococcal virulence are gaining scientific attention. However, none of these therapies have currently evolved to clinical trials.
INTRODUCTION
Streptococcus pneumoniae, also called the pneumococcus, is a major human pathogen. It is the leading cause of community-acquired bacterial pneumonia (CABP) and can cause otitis media (OM) and meningitis in children, the elderly and immunocompromised patients (Lundbo and Benfield 2017; Peyrani et al. 2019). In the USA, pneumonia was the eight leading cause of death in 2015 (Jindal et al. 2015). While incidence rates of CABP vary worldwide, overall incidences between 20 and 100 per 10 000 person-years have been observed, with outliers up to 164.3 per 10 000 person-years in patients older than 80 in the USA and up to 294 per 10 000 person-years in Latin-America (Ferreira-Coimbra, Sarda and Rello 2020). For acute OM, 300 million cases are estimated to be caused by S. pneumoniae every year (Monasta et al. 2012; Bergenfelz and Hakansson 2017).
In the USA, current therapy for pneumococcal infections in infants and children typically consists of amoxicillin or, in case of non-IgE-mediated allergy, a cephalosporin. Alternatively, levofloxacin, linezolid, clindamycin or vancomycin can be used (Bradley et al. 2011). Treatment of CABP in adults is generally done with amoxicillin, doxycycline or a macrolide. In case of comorbidities, a fluoroquinolone or combination therapies such as amoxicillin and a cephalosporin or a macrolide and doxycycline are advised by the America Thoracic Society and Infectious Diseases Society of America (Metlay et al. 2019). A similar therapy schedule is proposed for patients with pneumococcal meningitis (van de Beek et al. 2016). In Europe, amoxicillin or a tetracycline is primarily advised in patients with lower respiratory tract infections, while macrolides can only be used in countries with low resistance rates. Alternatively, levofloxacin or moxifloxacin may be considered for general use, while cephalosporins are reserved for hospital use only (Woodhead et al. 2011; Wiersinga et al. 2018). While therapy is in general successful, antibiotic resistance is increasingly observed. According to the United States Centre for Disease Control (CDC), resistance to one or more antibiotics is observed in 30% of pneumococcal infections, and it is estimated that 1.2 million infections per year are caused by resistant pneumococcal strains (Centre for Disease Control (CDC) 2013; Cherazard et al. 2017). Similarly in Europe, 10% of invasive S. pneumoniae isolates reported in 2008 were not susceptible to penicillin, and large regional differences in pneumococcus prevalence have been observed, from less than 5% in Northern Europe to over 40% in some Southern European countries (Woodhead et al. 2011). In 2018, these numbers were confirmed. Resistance occurred in 2.5%–32.3% of all cases in Europe for treatment with macrolides (European Centre for Disease Prevention and Control (ECDC) 2019). Also recently, country-wide resistance rates over 25% have been observed in the USA for macrolides. In Europe, variation is higher, ranging from less than 10% in the northern parts to over 25% in parts of Eastern and Southern Europe (Peyrani et al. 2019). So far, resistance to fluoroquinolones remains low in the USA and European Union (Woodhead et al. 2011; Kim et al. 2016).
Pneumococci asymptomatically colonize the nasopharynx, from where they can migrate to other parts of the airway, thereby generating inflammatory responses and disease. They possess a variety of virulence factors, both bound to the cell wall and excreted, that modulate their virulence. A general overview on the importance of virulence factors is described in detail elsewhere and will not be discussed here (Kadioglu et al. 2008; Brooks and Mias 2018).
The polysaccharide (PS) capsule is considered the most important pneumococcal virulence factor, as it is part of the first recognition by the immune system. This capsule is known to be diverse, giving rise to over 90 different pneumococcal serotypes. Current vaccines consist of PS fragments of a selection of these serotypes to induce an immune response (Kim, Seon and Rhee 2017). The first PS capsule vaccine to be licensed was a 14-valent PS vaccine in 1977. This vaccine was quickly expanded to include 23 serotypes, and it is still in use today (Briles et al. 2019). Unfortunately, immunogenicity of this vaccine is rather poor (Westerink, Schroeder and Nahm 2012). To overcome this issue, the heptavalent pneumococcal conjugate vaccine (PCV7) was the first conjugate vaccine to be licensed in the USA in 2000. Since then, it has been followed by a 13-valent conjugate vaccine (Briles et al. 2019). Currently, a 15-valent and a 20-valent conjugate vaccine are in development (Lee et al. 2019; Hurley et al. 2020). The introduction of conjugate vaccines dramatically reduced the rates of pneumococcal meningitis through direct and indirect (herd) protection (van de Beek et al. 2016; Kwambana-Adams et al. 2020). However, replacement of vaccine serotypes by non-vaccine serotypes is occurring, thereby lowering vaccine effectiveness. While in the USA this effect is limited, in the UK non-PCV13 serotypes were responsible for over 40% of invasive pneumococcal diseases in 2017 (Deng et al. 2013; Kwambana-Adams et al. 2020). Still, global vaccination programs are considered essential in the battle against pneumococcal diseases (World Health Organisation (WHO) 2019; Kwambana-Adams et al. 2020).
A recent review by Koulenti et al. describes in depth all antibacterial agents against Gram-positive bacteria currently in clinical trials (phase I to phase III) (Koulenti et al. 2020). Table 1 lists all evaluated compound libraries based on in-use antibiotics since 2010. Such an approach leads to a better understanding of the structure-activity relationship (SAR) of current antibiotics and can therefore lead to the identification of a novel antibiotic-analogue. As such, Table 2 lists a comprehensive overview of the more extensively studied novel antibiotic-analogues since 2010. Their mechanism of action is similar to that of an already marketed antibiotic, but in most cases resistance mechanisms differ and/or increased activity towards resistant strains is observed. Several of these analogues are currently undergoing clinical trials.
Table 1.
Overview of known antibiotics for which derivatives have been constructed and evaluated against S. pneumoniae since 2010.
| Library derived from | Antibiotic class | Number of tested molecules in library | References |
|---|---|---|---|
| Vancomycin | Glycopeptides | 22 | (Chang et al. 2013) |
| 31 | (Shao et al. 2011) | ||
| Lincomycin | Lincosamides | 14 | (Kumura et al. 2016) |
| 13 | (Umemura et al.2013) | ||
| Azithromycin | Macrolides | 23 | (Fajdetić et al. 2011) |
| 17 | (Pavlović and Mutak 2016 ) | ||
| 30 | (Ma et al. 2011b) | ||
| 28 | (Li et al. 2013) | ||
| 36 | (Wang et al.2017b ) | ||
| 8 | (Wang et al.2017a ) | ||
| 13 | (Čipčić Paljetak et al. 2016) | ||
| Clarithromycin | Macrolides | 10 | (Čipčić Paljetak et al.2016) |
| 18 | (Jia et al. 2018) | ||
| 24 | (Jia et al. 2017) | ||
| 26 | (Qin et al.2018) | ||
| 14 | (Liang et al. 2012) | ||
| 67 | (Kumar et al. 2012) | ||
| 18 | (Cong et al. 2011) | ||
| 33 | (Ma et al. 2011a) | ||
| Erythromycin A | Macrolides | 11 | (Qi et al. 2010) |
| 8 | (Zheng et al. 2016) | ||
| 26 | (Sugimoto et al. 2012) | ||
| 11 | (Bukvić Krajačić et al. 2011) | ||
| Ketolide | Macrolides | 10 | (Pereira and Fernandes 2011) |
| 3 | (Chen et al. 2012) | ||
| 36 | (Ma et al. 2019) |
Table 2.
Overview of novel antibiotic-analogues against S. pneumoniae discovered since 2010. NDA: New drug application; FDA: US Food and Drug Administration.
| Name | Year of discovery of anti-pneumococcal activity | Antibiotic class | Clinical trials | References |
|---|---|---|---|---|
| Avarofloxacin (JNJ-Q2, acorafloxacin) | 2010 | Fluoroquinolones | Phase I | (Morrow et al. 2010; Fernandez et al. 2011; Covington et al. 2013) |
| Solithromycin (CEM-101) | 2010 | Ketolides (Macrolides) | Phase III | (McGhee et al. 2010; Rodgers, Frazier and Champney 2013; Farrell, Mendes and Jones 2015; Zhanel et al. 2016; Kato et al. 2019) |
| MX-2401 | 2011 | Lipopeptides | None | (Dugourd et al. 2011; Rubinchik et al. 2011) |
| Cefilavancin (TD-1792) | 2012 | Glycopeptide-cephalosporin conjugate | Phase III | (Hegde et al. 2012) |
| Lefamulin | 2013 | Pleuromutilins | FDA approved | (Ross et al. 2012; Paukner et al. 2013; Mendes et al. 2016; Paukner and Riedl 2017) |
| Contezolid (MRX-1) | 2014 | Oxazolidinones | Phase III | (Shinabarger 1999; Li et al. 2014) |
| Omadacycline (PTK 0796) | 2014 | Tetracyclines | FDA approved | (Draper et al. 2014; Macone et al. 2014) |
| RBx 14 255 | 2014 | Ketolides (Macrolides) | Preclinical | (Raj et al. 2014; Barman et al. 2019) |
| Eravacycline (TP-434) | 2015 | Tetracyclines | NDA filed | (Grossman et al. 2012, 2015) |
| Lascufloxacin | 2017 | Fluoroquinolones | NDA filed | (Kishii, Yamaguchi and Takei 2017) |
| Nafithromycin (WCK 4873) | 2017 | Ketolides (Macrolides) | Phase II | (Zhanel et al. 2002; Flamm, Rhomberg and Sader 2017) |
| TP-271 | 2017 | Fluorocyclines (Tetracyclines) | Phase I | (Grossman et al. 2017) |
| KBP-7072 | 2019 | Tetracyclines | Phase I | (Lepak et al. 2019) |
While evaluating derivatives of known antibiotics can be useful, evaluation of new antibacterial targets may also help to overcome resistance mechanisms. As such, targeting bacterial virulence instead of bacterial physiology should be considered (Rasko and Sperandio 2010). Identifying these novel targets is often done using computational screening, in which bacterial and human genome sequences are screened to identify essential bacterial proteins, without affecting the human host and/or its microbiome (Wadood et al. 2018; Nayak et al. 2019).
Clearly, with antibiotic resistance still increasing worldwide, the search for novel antibacterials remains of utmost importance with several ingoing discovery and development programs. In this review, we will provide a comprehensive overview of the current pneumococcal drug pipeline, hereby excluding known antibiotic analogues and focusing on the discovery of novel drug targets. Overall, novel therapies can be divided into three main categories: (i) modulating the host immune system to lower pneumococcal disease, (ii) interference with pneumococcal virulence and (iii) development of novel antibiotics.
Modulating the host immune system
Several recently investigated anti-pneumococcal drug targets focus on enhancing host immune responses after infection. Interfering with these responses is challenging, as this might provoke an unwanted immune cascade leading to an increase in inflammatory damage on the one hand or it might overly inhibit the immune system leading to an uncontrolled growth and invasion of pathogens. Figure 1 shows a schematic overview of all discussed therapies. Detailed results regarding the in vivo data of these therapies are listed in Table 3.
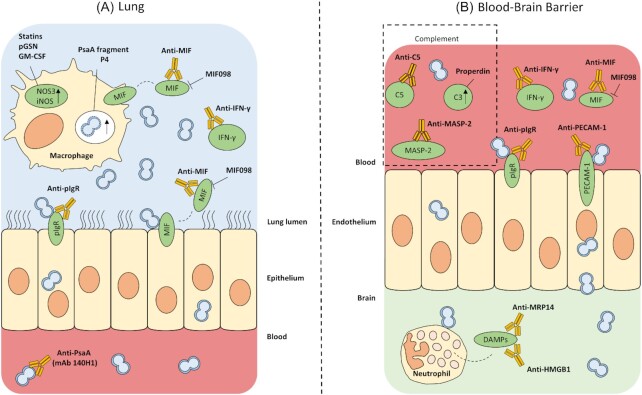
Figure 1.
Overview of drug targets and novel therapies focusing on modulating the host immune system at different locations of the body. (A), Drug targets present at air-blood interface. (B), Drug targets present at blood-brain barrier. Drug targets in and on macrophages, on epithelial and endothelial cells, in the blood and in the brain are labeled in green. pGSN: plasma gelsolin, GM-CSF: granulocyte/macrophage-colony stimulating factor, iNOS: inducible nitric oxide synthase, NOS3: nitric oxide synthase-3, PsaA: pneumococcal surface antigen A, MIF: macrophage inhibitory factor, IFN-γ: interferon-γ, pIgR: polymeric immunoglobulin receptor, mAb: monoclonal antibody, MASP-2: mannose-binding lectin-associated serine protease, PECAM-1: platelet endothelial cell adhesion molecule.
Table 3.
In vivo results of therapies modulating the host immune system. p.i.: post-infection.
| Compound | In vivo model | Treatment schedule | Endpoint | References |
|---|---|---|---|---|
| Pravastatin | Murine post-influenza secondary pneumonia model | 100 mg/kg intraperitoneally, starting one day before pneumococcal challenge, repeated once daily | Increase in murine survival from 0% to 50% 13 days p.i. | (Yang et al. 2014b) |
| pGSN | Murine post-influenza secondary pneumonia model | 400 mg/kg subcutaneously, starting one day before pneumococcal challenge, repeated once daily | Reduction in bacterial burden, 50% reduced acute inflammation 24 h p.i. | (Yang et al. 2015) |
| pGSN | Murine pneumonia model | 5–10 mg/mice intraperitoneally, 2 and 3 days p.i. | Increase in murine survival from 15% to 50% 10 days p.i. | (Yang et al. 2019b) |
| pGSN + penicillin | Murine pneumonia model | 5–10 mg/mice intraperitoneally + 0.1–2 mg penicillin intramuscularly, starting one day p.i., repeated once daily | Increase in murine survival from 30% to 80% 10 days p.i., decrease in weight loss and overall morbidity score | (Yang et al. 2019b) |
| GM-CSF | Murine pneumonia model | 20 µg/mouse orotracheally, 6 h p.i. | 1-log reduction in bacterial burden, 2-fold increase in macrophages present in murine lung exudate 24 h p.i. | (Steinwede et al. 2011) |
| MIF antibodies | Lethal murine sepsis model | 2 mg/mouse intraperitoneally, 2 h prior to infection | Increase in murine survival from 25% to 53.6% 15 days p.i., almost 4-log reduction in bacterial burden 48 h p.i. | (Savva et al. 2016) |
| MIF098 | Murine pneumonia model | 40 mg/kg intraperitoneally, twice daily | Increase in murine survival from 10% to 50% 7 days p.i., 2-log reduction in bacterial burden 48 h p.i., reduced neutrophil and monocyte infiltration 48 h p.i. | (Weiser et al. 2015) |
| anti-pIGR and anti-PECAM-1 antibodies | Murine meningitis model | 4 µg/mouse intravenously, 1 or 5 h p.i. | Prolonged survival of mice, 1-log reduction in bacterial burden after succumbing | (Iovino, Thorsdottir and Henriques-Normark 2018) |
| anti-pIGR and anti-PECAM-1 antibodies + ceftriaxone | Murine meningitis model | 4 µg/mouse antibodies + 100 mg/kg ceftriaxone intravenously, 1 h p.i. | Increase in murine survival from 60% to 100%, reduction in bacterial burden, prevention from passing the BBB in 60% of all cases, reduced neuroinflammation 5 days p.i. | (Iovino, Thorsdottir and Henriques-Normark 2018) |
| C5 antibodies | Murine meningitis model | 1 mg/mouse intraperitoneally, 24 h p.i. | Increase in murine survival from 66% to 100%, visible reduction in cerebral hemorrhages 48 h p.i. | (Woehrl et al. 2011) |
| C5 antibodies | Murine meningitis model | 1 mg/mouse intraperitoneally, 20 h p.i. | Increase in murine survival from 10% to 30% 72 h p.i. | (Kasanmoentalib et al. 2015) |
| MASP-2 antibodies | Murine meningitis model | 1 mg/kg intraperitoneally, 20 h p.i. | Increase in murine survival from 64% to 86%, brain burden unaffected 68 h p.i. | (Kasanmoentalib et al. 2017) |
| Recombinant FH | Murine sepsis model | 600 µg/mouse intraperitoneally + 25 mg/kg ceftriaxone, 17 h p.i. | No effect of treatment on disease score, cytokine production or vascular leakage in the liver 26 h p.i. | (Van Der Maten et al. 2016) |
| Recombinant FH | Murine meningitis model | 1 mg/mouse intraperitoneally, 16 h p.i. | No effect of treatment on murine survival 72 h p.i. or bacterial burden 24 h p.i. | (Kasanmoentalib et al. 2019) |
| Properdin | Murine intranasal infection model | 100 µg/mouse intraperitoneally, at time of infection | Increase in murine survival from 0% to 90% 60 h p.i., 2-log reduction in blood burden 24 h p.i. | (Ali et al. 2014) |
| IFN-γ antibodies | Murine meningitis model | 30 µg/mouse intracranially, at time of infection | Increase in murine survival from 33% to 83% 4 days p.i., brain burden unaffected 48 h p.i. | (Pettini et al. 2015) |
| Anti-DR5 antibodies | Murine pneumonia model | 75 µg/mouse intratracheally, 6 h p.i. | Increase in murine survival from 30% to 70% 8 days p.i., 1-log reduction in bacterial lung burden 72 h p.i. | (Steinwede et al. 2012) |
| Paquinimod | Murine meningitis model | 10 mg/kg intracranially, 24 h p.i. | Reduction of cranial inflammation with over 50% reduction in white blood cell count in the brain and CXCL2 levels 48 h p.i. | (Wache et al. 2015) |
| HMGB1 antibodies | Murine meningitis model | 100 µg/mouse intraperitoneally, 21 h p.i. | Increase in murine survival from 25% to 100%, improved clinical parameters (e.g. temperature), 58% reduction in white blood cell count in the brain 45 h p.i. | (Masouris et al. 2017) |
| Peptide P4 | Murine intranasal infection model | 100 µg/mouse intravenously, 48 h and 72 h p.i. | Increase in murine survival from 45% to 95% in 11-month-old mice, from 20% to 73% in 15-month old mice and from 30% to 80% in 6 to 10 weeks old mice 144 h p.i. | (Rajam et al. 2010) |
| mAB 140H1 (PsaA antibody) | Murine pneumonia model | 200 µg/mouse intraperitoneally, 6 h p.i. | Increase in murine survival from 0% to 54% 15 days p.i., 2-log reduction in lung burden and full clearance in blood 24 h p.i. | (Kristian et al. 2016) |
| mAB 140H1 (PsaA antibody) + ceftriaxone | Lethal murine sepsis model | 100 µg/mouse antibody + 50 mg/kg ceftriaxone intraperitoneally, 24 h p.i. | Increase in murine survival from 50% to 100% 15 days p.i. | (Kristian et al. 2016) |
Induction of nitric oxide synthase (NOS) activity
After macrophage activation by a variety of pro-inflammatory cytokines, constitutively expressed nitric oxide synthase-3 (NOS3) and inducible NOS (iNOS) expression are increased. These synthases show bactericidal activity through the production of nitric oxide (NO) (Hernansanz-Agustín et al. 2013). NO is used as a signaling molecule in low concentrations, while in high concentrations (e.g. during the oxidative burst in neutrophils) it shows direct antimicrobial properties by binding to DNA, proteins and lipids (Schairer et al. 2012). Although an excess production of NO can lead to immunosuppression, NOS inhibitors have previously been reported to reduce tissue injury and mortality in pneumococcal meningitis models. (Fang 2004) It is clear that a strict regulation of the amount of NO is needed to battle an infection. NOS3 expression is mediated by estrogen (Yang et al. 2014b, 2015). This estrogen-dependency leads to a greater risk of developing pneumonia for males than females (Casimir et al. 2013; Yang et al. 2014b). Statins, known to boost NOS3 activity, have been shown to improve bacterial clearance and survival from secondary pneumococcal pneumonia (Yang et al. 2014b). Furthermore, the use of statins has proven to be beneficial to patients suffering from pneumonia and is associated with a lower risk of hospitalization and mortality (Nielsen et al. 2012; Nishimoto, Rosch and Tuomanen 2020). Administration of sub-cutaneous plasma gelsolin (pGSN), a human blood protein, has also been shown to activate NOS3 and improve outcome of secondary bacterial pneumonia in mice, with reduced acute inflammation and improved bacterial clearance after 24 hours (Yang et al. 2015). The positive effects of pGSN treatment were confirmed in a more clinically relevant murine treatment model using an antibiotic-resistant pneumococcal strain (Yang et al. 2019b). Furthermore, in a 2019 study, patients suffering from community-acquired pneumonia (CAP) admitted to the hospital with low pGSN concentrations were more at risk for developing severe, short-term clinical outcomes such as higher risk of death, septic shock and respiratory failure (Self et al. 2019). Recently, a phase 1 clinical trial was finalized showing recombinant pGSN was generally safe and well tolerated in patients with mild CAP symptoms and a clinical trial studying the use of recombinant pGSN for the treatment of Covid-19 patients is currently recruiting (Tannous et al. 2020). Apart from NOS3, increased levels of iNOS are also known to contribute to the antibacterial activity of alveolar macrophages against pneumococci. It has also been shown that granulocyte/macrophage-colony stimulating factor (GM-CSF), used in the treatment of leukemia, is able to induce iNOS induction in response to infection, leading to a reduction in bacterial load and inflammation in the lungs of mice (Steinwede et al. 2011).
Macrophage inhibitory factor (MIF) inhibitors
MIF is a component of the innate immune system and constitutively expressed by immune and epithelial cells. It is released rapidly after exposure to bacteria and pro-inflammatory cytokines, promotes expression of numerous other pro-inflammatory molecules and thereby amplifies the response. Apart from its role in inflammation and immunity, it is also important in cell proliferation and oncogenesis (Roger et al. 2007; Bewersdorf et al. 2018). While MIF is essential for pneumococcal clearance after nasopharyngeal colonization (Das et al. 2014), high MIF levels in cerebrospinal fluid (CSF) are associated with poor patient outcome. Moreover, mice treated with MIF-neutralizing antibodies show lower bacterial burdens in lungs and a higher survival in a lethal pneumococcal sepsis model (Savva et al. 2016). Similarly, in another study using a murine lung infection model, treatment with the small-molecule receptor antagonist MIF098 improved survival, decreased bacterial burdens by 2 logs and reduced inflammation (Weiser et al. 2015).
Blocking of brain endothelial receptors
Adhesion to and invasion of endothelial and epithelial cells, mediated by several cellular receptors, is an important aspect of invasive pneumococcal disease, such as meningitis. First, the platelet-activating factor (PAF) receptor on activated epithelial and endothelial cells enables the bacteria to enter the basal membrane of the host epithelial cell. Secondly, the pneumococcal choline-binding protein PspC can bind to the epithelial polymeric immunoglobulin receptor (pIgR). Analogous to the PAF receptor pathway, bacteria are transported into the cell. Concordantly, when crossing the blood-brain barrier (BBB), the PAF receptor is used again (Koedel, Scheld and Pfister 2002; Mook-Kanamori et al. 2011). Furthermore, also pIgR is expressed by brain endothelial cells and can be used by pneumococci to adhere. Lastly, platelet endothelial cell adhesion molecule (PECAM-1), one of the major endothelial adhesion molecules, can mediate adhesion of pneumococci to the BBB endothelium (Iovino et al. 2017). Using an in vivo meningitis model, treatment of infected mice with anti-pIgR and anti-PECAM-1 antibodies 1 hour post infection increased survival time and lowered bacterial brain burden, yet all mice eventually still succumbed. A co-treatment strategy with ceftriaxone was however more successful. Ceftriaxone was capable of clearing the blood infection while the anti-pIgR and anti-PECAM-1 antibodies prevented most bacteria from passing the BBB, leading to a decrease in bacterial burdens and an increase in survival. Moreover, neuroinflammation was significantly lower in the combination therapy group compared to untreated mice or mice treated with ceftriaxone alone (Bewersdorf et al. 2018; Iovino, Thorsdottir and Henriques-Normark 2018).
Modulation of complement activity
The complement system is important in human immunity and comprises several recognition proteins activated in response to pathogens. Activation is triggered by a proteolytic cleavage amplification cascade, which generates fragments capable of binding to microbial surfaces as opsonins and bacterial cell destructions. Opsonins can also promote pathogen phagocytosis and induce inflammatory responses (Andre et al. 2017). In pneumococcal meningitis, complement however has a dual role. While it is needed to initiate complement-mediated bacterial killing, uncontrolled activation can occur and often leads to a worse disease outcome (Bewersdorf et al. 2018). High levels of complement component 5 (C5) have been shown to worsen patient outcome for bacterial meningitis. Furthermore, adjuvant therapy with C5 antibodies showed beneficial effects on survival rates, brain damage and clinical severity in several murine in vivo studies (Woehrl et al. 2011; Kasanmoentalib et al. 2015). Similar results have been found using mannose-binding lectin-associated serine protease (MASP-2), an important activator in the lectin pathway. While MASP-2 has proven to be important in avoiding nasopharyngeal carriage of pneumococci, it is also associated with worsened meningitis outcomes. As with C5, treatment with MASP-2 antibodies after pneumococcal infection increased in vivo murine survival (Kasanmoentalib et al. 2017). Complement factor H (FH), a regulatory protein inhibiting complement component 3 (C3), is known to be important in moderating pneumococcal disease. However, in several mouse models, combination therapy of recombinant FH with ceftriaxone showed no beneficial effects (Van Der Maten et al. 2016; Kasanmoentalib et al. 2019). Lastly and contradictorily to aforementioned therapies, Ali et al. showed increased in vitro opsonization of pneumococci after properdin treatment, i.e. a known positive regulator of complement activation naturally present in humans. Furthermore, animals infected with pneumococci and treated with properdin show a higher chance of survival and lower bacterial blood burden compared to non-treated animals (Ali et al. 2014). Clearly, functions of complement in pneumococcal pathogenesis are diverse. Interfering with its working mechanism might result in major and unforeseen changes in immune responses, and therefore, this interference should be done in a controlled and temporarily manner. Currently, one anti-C5 antibody, eculizumab, is on the market. However, side effects to this drug include increased risk of severe meningococcal meningitis, demonstrating the delicate role of the complement system in bacterial infections. Two other antibodies are currently undergoing clinical trials (Koelman, Brouwer and Van De Beek 2019).
Interferon-gamma (IFN-γ) inhibitors
Similarly to the aforementioned molecules, IFN-γ also plays a dual role in pneumococcal disease. As a powerful mediator of different innate and adaptive immune pathways during inflammation and infection, it is required for generating an effective response upon bacterial invasion. However, it is also involved in long-term neurological sequelae after pneumococcal meningitis and interfering with IFN-γ responses can lead to a higher survival rate of patients (Too et al. 2014; Yau et al. 2017; Bewersdorf et al. 2018). IFN-γ antibody treatment improved survival after pneumococcal meningitis and overall clinical symptoms were less compared to non-treated animals. Interestingly, bacterial burdens were unaffected by this treatment (Pettini et al. 2015).
Death receptor (DR) agonists
Tumor necrosis factor-related inducing ligand (TRAIL) is a member of the TNF superfamily. There are five human TRAIL receptors known, of which receptors DR4 and DR5 are involved in apoptosis of neutrophils, amongst other effects (Sag et al. 2019). It has been shown that TRAIL deficiency leads to increased inflammation following pneumococcal challenge (Hoffmann et al. 2007). In a murine pneumonia model, treatment with agonistic anti-DR5 antibodies led to a small but significant decrease in lung burden 72 h p.i. and an increase in survival from 30% to 70% 8 days p.i (Steinwede et al. 2012). However, TRAIL is also considered important in protection against viral lung infection, which often precede pneumococcal infections. Therefore, interference with this molecule is considered difficult, making it less favorable as therapeutic target (Braithwaite, Marriott and Lawrie 2018).
Danger-associated molecular pattern (DAMP) inhibitors
Meningitis is triggered after recognition of pathogen-associated molecular patterns (PAMPs). As a result, neutrophils are recruited and release toxins in order to kill the pathogens present. However, these neutrophil-derived toxins can, in turn, cause stress and damage to host cells. These injured cells subsequently release DAMPs, responsible for tissue damage (Wache et al. 2015; Masouris et al. 2017). HMGB1 and MRP14 are DAMPs known to be secreted in large amounts in de CSF of meningitis patients and could thus be a therapeutic target. Recently, the MRP14 inhibitor paquinimod that could be used in autoimmune diseases has been shown to reduce inflammation in a pneumococcal meningitis model, further supporting its potential for meningitis therapy (Wache et al. 2015; Bewersdorf et al. 2018). Similarly, HMGB1 antibodies were also shown to lower inflammation, and improve the outcome of pneumococcal infection (Masouris et al. 2017). As for other immunomodulatory therapies, inhibiting DAMPs can only be done in a very tightly controlled and balanced manner to assure controlled inflammation and subsequent elimination of bacteria (Land 2020).
Pneumococcal surface antigen A (PsaA) and pneumococcal surface protein A (PspA) as targets for therapy
PsaA and PspA are well-known pneumococcal virulence factors and have been studied as potential vaccine candidates. PsaA is an indispensable protein for Mn2+ transport, protecting against oxidative stress and for adherence to endothelial cells. PspA is important in evasion of the host immune system by interfering with the complement C3 cascade (Tai 2006). Mutants lacking PsaA are significantly less virulent compared to their parent strains, which could be attributed to growth impairment, reduced adherence capacity or hypersensitivity to oxidative stress (Rajam et al. 2008a). PsaA however, seems also essential in the induction of a protective host immune response, since peptide P4, a short amino acid fragment of PsaA, was capable of binding to nasopharyngeal epithelial cells and eliciting an inflammatory response (Rajam et al. 2008b). Furthermore, ex vivo human alveolar macrophages and neutrophils demonstrated improved bacterial killing after P4 exposure and treatment of intranasal infected mice with P4 was shown to increase survival (Rajam et al. 2009, 2010; Bangert et al. 2013; Morton et al. 2016). No follow-up studies involving P4 have been reported so far. Monoclonal antibodies (mAbs) against PspA have also been studied for therapeutic use. Several mAbs against PspA were produced in mice, and their in vitro opsonization capacity to pneumococci was evaluated. One selected mAb (mAb 140H1) was evaluated in vivo, and administration after infection with pneumococci reduced bacterial lung and blood burden and improved survival rate. Furthermore, combination therapy with the standard antibiotic ceftriaxone showed a synergistic effect (Kristian et al. 2016). Currently, PspA is studied as a vaccine candidate rather than a therapeutic target (Wagner-Muñiz et al. 2018; Akbari et al. 2019).
Interfering with pneumococcal virulence
Next to modulating host immunity, research focusing on reducing pneumococcal virulence to enable the immune system to overcome the infection shows growing interest. Most proposed targets are based on virulence factors specific to pneumococci and thus potential novel therapies will be pathogen-specific. A schematic overview of the drug targets is shown in Fig. 2. Table 5 lists detailed results regarding the current in vivo data of these therapies.
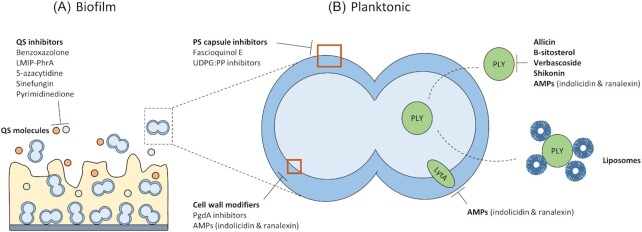
Figure 2.
Novel therapies interfering with pneumococcal virulence. (A), Drug targets involved in biofilm formation are targeting quorum-sensing mechanisms. (B), Drug targets present on/in individual pneumococci. Drugs specific for these targets aim at inhibition of polysaccharide capsule, pneumolysin and LytA and modification of the pneumococcal cell wall. QS: quorum sensing, LMIP: linear molecularly imprinted polymer, PS: polysaccharide, UDPG:PP: uridine diphosphate glucose pyrophosphorylase, PgdA: peptidoglycan N-acetylglycosamine deacetylase A, AMPs: antimicrobial peptides, PLY: pneumolysin.
Table 5.
In vivo results of therapies interfering with pneumococcal virulence. p.i.: post-infection.
| Compound | In vivo model | Treatment schedule | Endpoint | References |
|---|---|---|---|---|
| B-sitosterol | Murine intranasal infection model | 80 mg/kg subcutaneously, 1 h p.i., repeated every 4 h for 48 h | Increase in murine survival from 10% to 70% 120 h p.i., 2-log reduction in bacterial burden 48 h p.i., decrease in pulmonary inflammation 48 h p.i. | (Li et al. 2015) |
| Verbascoside | Murine intranasal infection model | 100 mg/kg subcutaneously, 2 h p.i. | Increase in murine survival from 25% to 75% 120 h p.i., 1-log reduction in lung burden 48 h p.i., visual pulmonary inflammation is reduced 48 h p.i. | (Zhao et al. 2016) |
| Shikonin | Murine intranasal infection model | 50 mg/kg orally, 2 h p.i., repeated once daily | Increase in murine survival from 10% to 60% 5 days p.i., 1-log reduction in lung burden, reduction in inflammatory cell infiltration and cell damage 3 days p.i. | (Zhao et al. 2017) |
| EGCG | Murine intranasal infection model | 50 mg/kg, subcutaneously, directly after infection, repeated in 8 h intervals | Increase in murine survival from 40% to 60% 120 h p.i., 1-log reduction in lung burden 48 h p.i., reduction in overall inflammatory reactions in the lung 48 h p.i. | (Song et al. 2017b) |
| Liposomes | Murine intranasal infection model | 100 mg/kg intranasally, 30 min p.i. | Increase in murine survival from 40% to 80%, 1-log reduction in lung and blood burden, reduction in inflammatory responses in the lungs 24 h p.i. | (Henry et al. 2015) |
| Liposomes | Lethal murine sepsis model | 100 mg/kg intravenously, 6 h p.i. | Increase in murine survival from 0% to 50–60%, 4-log reduction in bacterial blood burden, reduction in inflammatory responses in the lungs, 2-fold reduction in blood TNF-alpha levels 24 h p.i. | (Henry et al. 2015) |
| indolicidin and ranalexin analogues | Murine pneumonia model | 20 mg/kg intraperitoneally, 1 h, 12 h and 24 h p.i. | Increase in murine survival from 0% to 30–50%, clearance of bacteria in blood, reduction in tissue damage in lungs and spleen 7 days p.i. | (Jindal et al. 2017) |
| indolicidin and ranalexin analogues | Lethal murine sepsis model | 10 mg/kg intraperitoneally, 1 h, 12 h and 24 h p.i. | Increase in murine survival from 0% to 60%, reduction in bacterial burden, decrease in tissue damage in lungs and spleen 7 days p.i. | (Jindal et al. 2017) |
| Benzoxazolone | Guinea pig otitis media model | 12 mg/kg intraperitoneally, twice daily for 3 months | Prevention of biofilm formation on cochlear implants after 3 months | (Cevizci et al. 2015) |
| LMIP-PhrA | Murine intranasal infection model | 100 nM/50 µL intranasally, at time of infection | Increase in murine survival from 37 h to 65 h, 2-log reduction in bacterial blood burden 24 h p.i. | (Motib et al. 2017) |
| Sinefungin | Rat otitis media model | 1,75 µg/rat in the middle ear, at time of infection | 0.7 log reduction in burden on bulla 1 week p.i. | (Yadav et al. 2014) |
PS capsule formation inhibitors
As mentioned earlier, the PS capsule is the most important virulence factor. It inhibits macrophage phagocytosis, which forms the first line of defense to pneumococcal invasion (Dockrell et al. 2003; Dockrell, Whyte and Mitchell 2012). Deletion of the capsule increases phagocytosis rates in vitro and decreases virulence in vivo (Preston and Dockrell 2008). However, downregulation of the capsule is needed to initiate nasopharyngeal colonization (Gilley and Orihuela 2014). Also during pneumonia and OM, downregulation of the capsule and subsequent formation of a biofilm is considered part of the pneumococcal immune evasion strategy (Moscoso, Garcia and Lopez 2006; Domenech et al. 2013). In contrast, when the change from a commensal to a pathogenic lifestyle occurs, pneumococci upregulate their PS capsule production (Gilley and Orihuela 2014). The pneumococcal capsule is formed through two pathways, the Wzy-dependent pathway used by most, and the synthase-dependent pathway, only used in serotypes 3 and 37 (Geno et al. 2015). The capsule of serotype 3 consists of glucose (Glc) and glucuronic acid (GlcA) and is the result of 3 genes (cps3D, cps3S and cps3U), of which only the first two are essential genes for capsule production. Serotype 37 only requires one gene, tts, to form its capsule, consisting solely of Glc chains. The Wzy-dependent pathway is more complex. In general, sugar-1-phosphate is transferred to a lipid carrier on the cytoplasmic side of the cell membrane by a glycosyltransfersase. From there, the repeat unit is built and translocated to the extracellular side by flippase Wzx before being polymerized by Wzy. Lastly, the final sugar (usually Glc) is covalently bound to N-acetylglucosamine residue of the cell wall peptidoglycan (Geno et al. 2015; Paton and Trappetti 2019). While these pathways differ, they both use the cps gene locus to build the correct sugar conformation. As the loci slightly differ for each serotype, interfering with them or their products is difficult. Thus far, only CpsB, a tyrosine phosphatase encoded by cpsB, has been suggested to be a potential novel anti-virulence drug target, as cpsB mutants are avirulent in several animal models of infection (Morona et al. 2004; Standish et al. 2012; Monteiro Pedroso et al. 2017). Furthermore, the molecule fascioquinol E—an extract derived from the marine sponge Fasciospongia spp.—inhibited CpsB phosphatase activity and increased macrophage attachment in vitro (Standish et al. 2012).
Lastly, regardless of the pneumococcal serotype or capsule pathway, uridine diphosphate glucose (UDP:Glc) is generally considered a key component in the formation of PS capsule. UDP:Glc is part of the Glc and galactose (Gal) metabolism and is made through the interconversion of glucose-1-phosphate (Glc-1-P) by uridine diphosphate glucose pyrophosphorylase (UDPG:PP) (Mollerach, López and García 1998). It has been described that mutants lacking a functional galU gene form a lower amount of PS capsule, are more prone to macrophage phagocytosis in vitro and are less virulent in a Galleria mellonella in vivo model. (Mollerach, López and García 1998; Cools et al. 2018) As the pneumococcal UDPG:PP crystal structure is unknown, modeling of new molecules is challenging. However, recently, two attempts have been made, either using the purified enzyme in an enzymatic assay or using a computational molecular docking model based on other bacterial UDPG:PP's (Zavala et al. 2017; Cools et al. 2020).
Bacterial cell wall modifiers
The cell wall of Gram-positive bacteria, such as the pneumococcus, consists of a thick peptidogylycan (PG) layer, to which teichoic acids and capsular PS are covalently attached. Due to its importance, PG biosynthesis has always been an interesting drug target. Currently, beta-lactam antibiotics and vancomycin are the two most used PG inhibitors (Vollmer, Blanot and De Pedro 2008; Rajagopal and Walker 2017; Vollmer, Massidda and Tomasz 2019).
Lysozyme, a major bacteriolytic component of the immune system, hydrolyses PG chains, which results in lysis of bacteria. Pathogens such as S. pneumoniae, however, modify their glycan strands through deacetylation by peptidoglycan N-acetylglycosamine deacetylase A (PgdA), thereby increasing resistance to lysozyme. Mutant strains lacking PgdA are significantly more susceptible to lysozyme in vitro and show a reduction in virulence in vivo (Vollmer and Tomasz 2002). Since then, several publications have described in vitro inhibitors of PgdA enzymatic activity, yet no follow-up studies on the activity towards the bacterial cell or in vivo use have been performed (Bui et al. 2011; Ariyakumaran et al. 2015; DiFrancesco, Morrison and Nitz 2018).
Pneumolysin (PLY) inhibitors
PLY is formed intracellularly and released in the environment through bacterial lysis. It is known to be cytolytic to all human cell types through the formation of pores. Furthermore, it promotes pro-inflammatory immune responses through activation of the classical and lectin pathways of complement activation (Anderson and Feldman 2017). As PLY is known as an essential virulence factor for bacterial survival in the respiratory tract, inhibiting this enzyme might prove beneficial (Kadioglu et al. 2008; Kim, Seon and Rhee 2017). A variety of natural compounds has been successfully tested for their anti-PLY activity (Table 4). Also statins were proven to have a direct effect on PLY cytotoxicity in vitro and in vivo and could be valuable as adjuvant therapy to existing antibiotics. However, further studies are needed to study the mechanism of action and potential place in pneumococcal therapy (Nishimoto, Rosch and Tuomanen 2020).
Table 4.
Natural compounds tested for their anti-PLY activity since 2010. PLY: pneumolysin.
| Natural compound | Source | Activity on PLY | References |
|---|---|---|---|
| Allicin | Active component of garlic | Inhibition of hemolytic activity in vitro. | (Arzanlou et al. 2011) |
| β-sitosterol | Inhibition of hemolytic activity and protection of human lung cells in vitro; decrease in mortality, bacterial burden and pulmonary inflammation in vivo. | (Li et al. 2015) | |
| Verbascoside | Glycoside present in plants used in Chinese medicine | Inhibition of hemolytic activity. | (Zhao et al. 2016) |
| Shikonin | Component of traditional Chinese herb | Inhibition of hemolytic activity and protection of human alveolar epithelial cells against cell death in vitro; reduction of mortality, inflammatory cell infiltration and cell damage in a murine in vivo model. | (Zhao et al. 2017) |
| Juglone | Roots, leaves, woods and fruits of Juglandaeae walnut trees | Inhibition of PLY oligomerization, needed for pore formation. | (Song et al. 2017a) |
| Epigallocathechin gallate | Major component of green tea catechins | Inhibition of PLY oligomerization, needed for pore formation; inhibition of sortase A (SrtA) leading to an in vitro decrease in biofilm formation; reduction of mortality, lung burden and overall inflammatory reactions a murine in vivo model. | (Song et al. 2017b) |
Lastly, liposomes consisting of naturally occurring cholesterol and sphingomyelin can be used to sequester PLY (Baumgartner et al. 2016). In vitro, these liposomes protect monocytes from secreted PLY. Furthermore, mice were more prone to survive a pneumococcal lung infection after treatment with liposomes. Besides a small reduction in bacterial burdens in lung and blood, the treatment was also capable of reducing tumor necrosis factor α (TNF-α) levels. This led to less signs of inflammation in the lungs. In an in vivo sepsis model, bacterial blood burden was reduced after treatment, leading to an increase in murine survival. Also in this model, TNF-α levels were decreased (Henry et al. 2015). CAL02, a mixture of liposomes, has undergone clinical trials to verify its safety and activity. While a dosage effect could not be established, the safety and tolerability of CAL02 was promising, with no adverse effects that could be linked to local tolerability events. Furthermore, all patients receiving CAL02 treatment were cured 15 to 22 days after the start of the trial (Laterre et al. 2019).
Antimicrobial peptides (AMPs)
AMPs are a large and diverse group of molecules, produced by both pro- and eukaryotes. In humans, they are important in innate immunity, while in bacteria AMPs are produced as a way to compete with and kill other bacteria (Mahlapuu et al. 2016). In a 2015 study, analogues of indolicidin and ranalexin, two natural peptides with known antibacterial activity against Gram-positive bacteria, showed in vitro bactericidal activity towards antibiotic susceptible and resistant pneumococcal strains, without cytotoxicity for eukaryotic cells (Jindal et al. 2015). In silico molecular docking pointed towards interactions with autolysin and/or PLY, two known pneumococcal virulence factors as a mechanism of action, while a later study revealed that cell membrane integrity is highly impacted by these AMPs in vitro (Jindal et al. 2015, 2017). This study also confirmed the in vivo effect of these compounds in a murine bacteremia model, leading to an increase in survival, decrease in bacterial burden and decrease in tissue damage in lungs and spleen (Jindal et al. 2017).
Quorum sensing (QS) inhibitors
Biofilm formation is known to play an important role in OM and cochlear implant infections (Cevizci et al. 2015). QS systems are known to control the maturation stage of these biofilms, as they allow communication between bacteria in a cell-density dependent manner (Brackman and Coenye 2014). Targeting this system, rather than targeting the bacteria itself, has been gaining scientific attention and S. pneumoniae uses several QS systems that have the potential for intervention. First, the ComABCDE pathway is regulated by competence-stimulating peptide (CSP). This system allows for induction of competence and controlling genetic transformation, when a biofilm has matured and pneumococcal density is high enough. Bacteriocins, which inhibit the growth of competing bacteria, are produced as a response to the BlpABCSRF pathway, which operates similarly to ComABCDE. Also, autoinducer-2 (AI-2) is known as a common QS system of Gram-positive bacteria and is also present in pneumococci. The enzyme LuxS activates AI-2, which, in turn, facilitates initial attachment of bacteria to a surface (Galante et al. 2014). Apart from LuxS, DNA adenine methyltransferase (DAM) plays an important role in the biosynthesis of AI-2, as it is part of the activated methyl cycle. It catalyzes a methyl transfer from S-adenosyl-L-methionine (SAM) to adenine, a feature unique in bacteria (Yadav et al. 2015). Lastly, in 2015, Dimarchi et al. discovered a novel QS system, TprA/PhrA, that controls the expression of bacteriocins called lantibiotics (Hoover et al. 2015). This system has been shown to be crucial for pneumococcal virulence in pneumonia, meningitis and OM models (Motib et al. 2017).
Several biofilm disruption strategies have been studied. Cevizci et al. tested the possibility of analogues of N-acyl homoserine lactone (AHL), a signaling molecule known to play a role in QS systems, to prevent pneumococcal biofilm formation in an in vivo cochlear implant model. While these results are preliminary, prolonged treatment with the AHL analogue benzoxazolone after cochlear implant surgery prevented biofilm formation on these implants (Cevizci et al. 2015). However, it should be noted that AHL is a molecule solely attributed to Gram-negative bacteria, leaving the exact mechanism of these inhibitors against pneumococci unknown. More recently, a linear molecularly imprinted polymer (LMIP) targeting the TprA receptor and its signaling peptide PhrA was evaluated. Addition of LMIP-PhrA to in vitro cultures reduced growth rate and neuraminidase activity, which is important in pneumococcal colonization and invasiveness. In a pneumonia mouse model, dissemination from lungs to blood was prevented by LMIP-PhrA, resulting in longer survival of animals (Motib et al. 2017, 2019). Lastly, Yadav et al. studied the effect of interfering with DAM or SAM. In 2012, the effect of 5-azacytidine (5-aza) on in vitro planktonic and biofilm growth and the effect on gene expression was assessed. 5-aza is a hypomethylating drug used in leukemia treatment. Only a minor effect on planktonic growth was observed, while biofilm formation was adequately and dose-dependently inhibited in vitro. Using scanning electron microscopy (SEM), 5-aza-treated biofilms were visualized and observed to be thinner, more scattered in clumps and disorganized compared to non-treated biofilms. Also genes involved in AI-2 synthesis were downregulated after 5-aza treatment (Yadav, Chae and Song 2012). In a very similar study, sinefungin, a natural nucleoside and analogue of SAM known for its inhibitory effects on transmethylation reactions and overall antifungal, antiviral and antiprotozoal activities, was evaluated. Also in this study, in vitro biofilm formation was inhibited, and AI-2 synthesis genes were downregulated. Furthermore, in vivo biofilm formation in an OM rat model showed a decrease in CFU/bulla after sinefungin treatment (Yadav et al. 2014). Inhibition of DAM by the small molecule pyrimidinedione proved equally successful in inhibiting in vitro biofilm formation and downregulating virulence-related genes, such as ply and lytB, as well as the competence-related gene comC(Yadav et al. 2015).
Development of novel antibiotics
Lastly, a variety of novel antibiotics is being investigated, which in most cases work against a wider spectrum of pathogens. However, as for in-use antibiotics, mutations leading to resistance are of concern. An overview of the antibiotics tested against pneumococci is shown in Fig. 3. Detailed information regarding the described in vivo experiments is listed in Table 6.
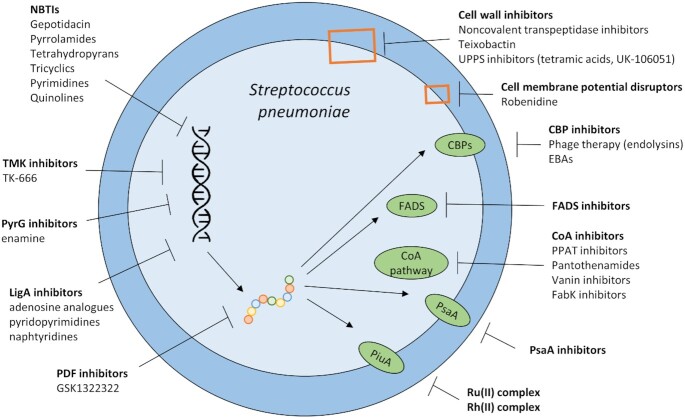
Figure 3.
Novel therapies interfering with pneumococcal survival. These therapies often focus on the inhibition of transcription, translation and enzyme elongation. Other strategies include inhibition of cell wall, CBP, FADS, the CoA pathway, PsaA and PiuA. NBTIs: novel bacterial topoisomerase II inhibitors, TMK: thymidylate kinase, LigA: NAD+-dependent DNA ligase, PDF: peptide deformylase, UPPS: undecaprenyl pyrophosphate synthetase, CBP: choline binding protein, EBAs: esters of bicyclic amines, FADS: flavin adenine dinucleotide synthetases, CoA: coenzyme A, PPAT: phosphopantetheine adenylyltransferase, PsaA: pneumococcal surface antigen A, Ru: ruthenium, Rh: rhodium.
Table 6:
In vivo results of novel antibiotics. p.i.: post-infection, CSF: cerebrospinal fluid.
| Compound | In vivo model | Treatment schedule | Endpoint | References |
|---|---|---|---|---|
| Pyrrolamides | Murine pneumonia model | 320 mg/kg orally, starting 18 h p.i., repeated twice per day | 4-log reduction in lung burden 42 h p.i. | (Eakin et al. 2012) |
| Quinolines | Murine intranasal infection model | 50 mg/kg subcutaneously, starting 1 day p.i., repeated twice per day | 4-log reduction in lung burden 48 h p.i. | (Odagiri et al. 2013) |
| Quinolines | Murine intranasal infection model | 40 mg/kg subcutaneously, starting 2h p.i., repeated twice per day | 4-log reduction in lung burden 56 h p.i. | (Odagiri et al. 2018) |
| Tetrahydropyrans | Murine thigh infection model | 80 mg/kg subcutaneously, starting 2 h p.i., repeated every 3 hours for 24 h | 4-log reduction in thigh burden 26 h p.i. | (Lepak et al. 2016) |
| Tricyclics | Rat pneumonia model | 100 mg/kg orally, 1 h, 7 h, 24 h and 31 h p.i. | At least 4-log reduction in lung burden 48 h p.i. | (Miles et al. 2016) |
| Pyrimidines | Murine pneumonia model | 100 mg/kg intraperitoneally, starting 2 h p.i., repeated 4 times per day | 4-log reduction in lung burden 26 h p.i. | (Uria-Nickelsen et al. 2013) |
| Substituted adenosine analogues | Murine pneumonia model | 45 mg/kg intraperitoneally, starting 18 h p.i., repeated four times per day | 5-log reduction in lung burden 36 h p.i. | (Mills et al. 2011) |
| Teixobactin | Murine intranasal infection model | 10 mg/kg intraveneously, 24 h and 36 h p.i. | 6-log reduction in lung burden 48 h p.i. | (Ling et al. 2015) |
| Endolysins in phages | Rat meningitis model | 20 mg/kg intracisternally or 200 mg/kg intraperitoneally, 18 h p.i. | Rapid decrease in CSF burden after intracisternal (3-log reduction after 30 min) and after intraperitoneal injection (2-log reduction after 3 h) | (Grandgirard et al. 2008) |
| Endolysins in phages | Lethal murine sepsis model | 25 µg/mouse intraperitoneally, 1 h p.i. | Increase in murine survival from 0% to 70% 7 days p.i. | (Diez-Martinez et al. 2015) |
| Combination of phages | Adult zebrafish infection model | 3.25 mg/kg total enzyme intraperitoneally, 1 h p.i. | Increase in murine survival from 27.8% to 77.8% 3 days p.i. | (Vázquez and García 2019) |
| EBAs | Embryo zebrafish model | 2 µM, starting 7 h p.i., repeated once daily for 3 days | Increase in murine survival from 50% to 97.9% 5 days p.i. | (De Gracia Retamosa et al. 2015) |
| PPAT inhibitors | Murine pneumonia model | 100 mg/kg intraperitoneally, starting 2 h p.i., repeated twice or 4 times per day | Statis of bacterial burden 24 h p.i. | (De Jonge et al. 2013) |
| NCL195 | Murine sepsis model | 50 mg/kg intraperitoneally, starting 8 h p.i., repeated after 4 h | 1-log reduction in burden 18 h p.i., prolonged 60% survival from approx. 26 h p.i. to 36 h p.i. | (Pi et al. 2020) |
Bacterial topoisomerase II inhibitors
Bacterial topoisomerases (DNA gyrase and topoisomerase IV) are targets of the well-known quinolones. However, a new class of topoisomerase II inhibitors, called novel bacterial topoisomerase II inhibitors (NBTIs), has been put forward as a new way of treating quinolone-resistant infections. These inhibitors also bind to DNA gyrase and topoisomerase IV, yet do so on a slightly different binding site, thereby evading existing resistance mechanisms. Gepotidacin represent this new drug class. It shows in vitro activity against a variety of Gram-positive bacteria, including quinolone-resistant pneumococci (Bax et al. 2010; Biedenbach et al. 2016; Flamm et al. 2017). Gepotidacin is currently recruiting for phase III trials, evaluating efficacy, safety and applicability of the drug (Kolarič, Anderluh and Minovski 2020; Koulenti et al. 2020). Other classes of bacterial topoisomerase II inhibitors are the pyrrolamides, tetrahydropyrans, tricyclics, pyrimidines and quinolines. For some of these lead compounds, only in vitro efficacy has been tested, while others also show promising effects in vivo. Regardless, none of these classes have progressed into clinical trials for pneumococcal infections (Zhang et al. 2011b; Eakin et al. 2012; Mitton-Fry et al. 2013; Odagiri et al. 2013, 2018; Uria-Nickelsen et al. 2013; Surivet et al. 2015; Lepak et al. 2016; Miles et al. 2016).
Thymidylate kinase (TMK) inhibitors
TMK is an essential enzyme in the DNA synthesis pathway. It transfers phosphate from adenosine triphosphate (ATP) to thymidine monophosphate (dTMP), which leads to the formation of thymidine diphosphate (dTDP), an essential component of the thymidine triphosphate (dTTP) pathway (Keating et al. 2012). As such, TMK is essential for bacterial growth and has been put forward as an interesting drug target (Petit and Koretke 2002). Structure-based drug design led to the development of several compounds, of which TK-666 has proven to be active in vitro against Gram-positive bacteria, while not being harmful to eukaryotic cells. Furthermore, it was shown to be equally active against antibiotic resistant and susceptible pneumococci (Keating et al. 2012; Martínez-Botella et al. 2012). Currently, TMK inhibitors are mainly evaluated for their use against Mycobacterium tuberculosis infections (Jian et al. 2019, 2020; Venugopala et al. 2020).
PyrG inhibitors
PyrG, a bacterial cytidine triphosphate (CTP) synthase, produces CTP from uridine triphosphate (UTP) and glutamine and is essential in the pyrimidine de novo biosynthetic pathway (Endrizzi et al. 2004). Furthermore, it is required for growth of bacteria such as Haemophilus influenzae. PyrG inhibitors were first reported in the literature over 40 years ago as antitumor drugs, however, thus far no PyrG inhibitors have reached the market. After an enzyme-based HTS of PyrG inhibitors, enamine proved the most interesting. However, while the IC50 value in the enzymatic assay was low (0.091 µM), the MIC was more than 128 µg/mL for pneumococci. In contrast, MICs ranging from 16 to 64 µg/mL were observed for other pathogens such as H. influenzae, E. coli and S. aureus. While this demonstrates the necessity of whole-cell based assays in drug discovery, it is also a first step towards the development of pneumococcal PyrG inhibitors (Yoshida et al. 2012). Similarly to TMK inhibitors, PyrG inhibitors are now primarily studied as a way to combat mycobacterial infections (Chiarelli et al. 2018).
NAD+-dependent DNA ligase (LigA) inhibitors
LigA is an essential enzyme in viability for both Gram-positive and Gram-negative bacteria. It is completely unrelated to eukaryotic DNA ligases, making it an interesting drug target (Pascal 2008; Mills et al. 2011). Selective inhibitors of LigA, substituted adenosine analogues, were reported to show a broad spectrum of bacterial inhibition, ranging from E. coli, Mycoplasma pneumoniae to S. pneumoniae. No binding affinity to eukaryotic DNA ligases or cytotoxicity against human red blood cells or alveolar epithelial cells A549 was observed. Furthermore, an in vivo murine lung infection model showed a dose-dependent reduction of pneumococci after treatment with one of these adenosine analogues (Mills et al. 2011). Optimization of compound classes pyridopyrimidines and naphthyridines has also been studied in vitro, leading to LigA inhibiting molecules for a wide range of bacteria, including pneumococci (Murphy-Benenato et al. 2015). More recently, the mechanism of action of the long known broad-spectrum antibiotic cordycepin was attributed to its binding to LigA (Zhou et al. 2016). However, no follow-up research on any of the aforementioned compounds has been performed.
Peptide deformylase (PDF) inhibitors
PDF is a metalloenzyme used in bacterial peptide elongation. It cleaves a formyl group from the terminal N-methionine of a newly synthesized polypeptide following ribosomal translation and elongation. Removal of this formyl group is vital for bacterial viability. Importantly, human PDF is structurally different from its prokaryotic counterpart, therefore bacterial PDF is considered an interesting novel drug target (Sangshetti, Khan and Shinde 2014). In 2011, GSK1322322 was introduced as a potential novel antibiotic against multidrug resistant S. aureus and CABP (Ross et al. 2011). It showed good in vitro activity against pneumococci and was able to reduce pneumococcal lung burdens in a murine model (Sutcliffe 2011). This molecule was undergoing clinical trials, however trials were ended due to the identification of potentially harmful metabolites.(United States National Institute of Health (NIH) 2019) IDP-73 152, another PDF inhibitor, went through a phase I clinical study in 2013, showing no adverse effects after oral administration in healthy volunteers. The results were published in 2019, but no follow-up studies have been announced (Shin et al. 2019).
Inhibition of cell wall synthesis
Beta-lactams, the most widely used antibiotics, interfere with cell wall synthesis by targeting the transpeptidase activity of penicillin-binding proteins (PBPs). They block transpeptidation by covalently binding to these enzymes, thereby killing the bacteria. However, resistance is increasing for this antibiotic class.(Macheboeuf et al. 2006) Therefore, attempts have been made to discover novel inhibitors. One of these strategies led to the screening of noncovalent inhibitors, as they are postulated to be less susceptible to resistance-inducing mutations of PBPs and production of beta-lactamases (Turk et al. 2011). In 2009, the first virtual screening of noncovalent pneumococcal inhibitors was performed. However, no biological assays were performed to confirm these results (Miguet et al. 2009). Another article from 2011 reported on 2 molecules showing promising in vitro activity against antibiotic-resistant pneumococci (Turk et al. 2011). Since then, no results on noncovalent PBP inhibitors have been published, suggesting this approach was unsuccessful.
Using a completely different mechanism, Lewis et al. published the finding of the first novel natural bacterium-derived antibiotic in decades (Durand, Raoult and Dubourg 2019). Teixobactin inhibits peptidoglycan biosynthesis by binding to lipid II and lipid III, precursors of peptidoglycan and teichoic acid, respectively. In vitro, it is active against a variety of Gram-positive bacteria, but not against Gram-negatives. Teixobactin showed a dose-dependent reduction in pneumococcal lung burden in a murine model (Ling et al. 2015). Since its discovery, numerous analogues have been synthesized, however still no clinical trials involving teixobactin or one of its analogues have been setup (McCarthy 2019; Koulenti et al. 2020).
Lastly, undecaprenyl pyrophosphate synthetase (UPPS) synthesizes undecaprenyl pyrophosphate (UPP) from isopentenyl pyrophosphate (IPP). UPP is essential in the bacterial cell wall synthesis, as it is needed for cross membrane transport of carbohydrates, and as such, UPPS has been shown to be essential for pneumococcal growth (Apfel et al. 1999). However, only few attempts have been made to develop UPPS inhibitors against pneumococci. In 2010, tetramic acids were evaluated as pneumococcal UPPS inhibitors. While these compounds showed interesting in vitro activity, no whole-cell based assays were performed (Lee et al. 2010). Later, Danley et al. reported in vitro UPPs inhibition by UK-106 051, a carboxamide analogue, leading to inhibition of pneumococcal growth, yet no in vivo confirmation has been attempted (Danley et al. 2015). Since then, few papers on the use of UPPS inhibitors against a variety of bacteria were published, all reporting early stage preclinical data (Concha et al. 2016; Inokoshi et al. 2016; Wang et al. 2016; Cherian et al. 2017; Jukic et al. 2019). While these data show potential for this target, currently there are no UPPS inhibitors in development.
Choline binding protein (CBP) interference
CBPs are a large group of proteins located on the pneumococcal cell surface, involved in pneumococcal autolysis (LytA, LytC, CbpD, CbpF), cell separation after division (LytB), interference with complement activation (PspA), adherence to host cells (PspC, PcpA, CbpG) and modulating the amount of choline (Pce). There are two main strategies to use CBP as a novel anti-pneumococcal approach, (i) through direct inhibition of CBPs and (ii) through the administration of endolysins (Maestro and Sanz 2016).
Maestro et al. published a detailed review in 2016 on the use of direct CPB inhibitors (Maestro and Sanz 2016). All inhibitors were based upon choline, such as esters of bicyclic amines (EBAs) which can be used as monomers or nanoparticle dendrimers, consisting of several ligands. As CBPs contain several binding sites, the binding affinity increases dramatically (Mammen, Choi and Whitesides 1998). De Gracia Retamosa et al. showed EBAs were capable of lysing in vitro planktonic cultures in a dose-dependent manner and increased survival in a zebrafish model (De Gracia Retamosa et al. 2015). In a 2019 study, EBAs were confirmed to in vitro lyse planktonic cultures using a LytA-dependent mechanism. Furthermore, also in vitro formation of biofilms was blocked (Roig-Molina et al. 2019). Lastly, phagocytosis of bacteria by microglial cells increased in in vitro dendrimer-treated pneumococcal cultures (Ribes et al. 2013).
Phage therapy has repeatedly been suggested in the battle against multidrug resistant bacteria. Bacteriophages have been observed to kill antibiotic resistant bacteria. In addition, some of their products, such as endolysins, show promising results (Cisek et al. 2017). Interestingly, phage resistance mostly comprises a loss of bacterial virulence (Caflisch, Suh and Patel 2019). Endolysins are produced by phage-infected bacteria to enable the release of novel phages, thereby destroying the bacterial cell wall.(Stoffels et al. 2017) In pneumococci—and other Gram-positive bacteria—these lysins belong to the CBP family. Therefore, they recognize choline residues in the teichoic acids of pneumococci (Vázquez and García 2019). Administration of purified endolysins to in vitro pneumococcal cultures led to a rapid decrease in cell density (Diez-Martinez et al. 2015). Furthermore, mice and rats showed a greater likelihood of survival after endolysin therapy following a pneumococcal challenge (Grandgirard et al. 2008; Diez-Martinez et al. 2015). Synergy between different phages has been observed both in vitro on planktonic and biofilm cultures and in a zebrafish model, leading to an increase in zebrafish survival (Vázquez and García 2019). Several clinical trials involving phage therapy have already been done, or are ongoing or planned (Vázquez, García and García 2018; Caflisch, Suh and Patel 2019). While none of these trials have focused on pneumococci, this does indicate phages or lysins are promising antibacterial drug candidates.
PsaA inhibitors
Apart from enhancing the immune system through passive immunization (see earlier), PsaA has also been proposed as a direct drug target. Inhibitors have been studied for their anti-pneumococcal properties. In 2015, Bajaj et al. virtually screened a library of small molecules for their binding properties to PsaA (Couñago et al. 2014). Hits were further optimized and tested in an in vitro model. Two molecules showed promising inhibitory properties against pneumococci, however the authors stated that more optimization of these compounds was needed (Bajaj et al. 2015). This experimental setup was later repeated by another research group, confirming PsaA as an alternative drug target. However, this research group also failed to identify a sufficiently inhibiting molecule, as its most active compounds possessed a flexible tail, which is considered a poor development prospect (Obaidullah et al. 2018).
Flavin adenine dinucleotide synthetase (FADS) inhibitors
FADSs synthesize flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). As cofactors of flavoproteins, FMN and FAD are present in all living organisms and insufficiency of either leads to cell death. In bacteria, FADS synthesizes FMN and FAD from riboflavin (RF) in two steps: production of FMN from RF (RFK module) and subsequent production of FAD from FMN (FMNAT module). While there is some similarity between pro- and eukaryotes in the RFK module, this is not the case for the FMNAT module, making it an interesting novel drug target (Serrano et al. 2013; Sebastián et al. 2017). Using an enzyme-based HTS approach based on the crystal structure of Corynebacterium ammoniagenes, several lead compounds were identified. However, biological activity against pneumococci remained low, suggesting they do not reach inhibitory concentrations at the intracellular level (Sebastián et al. 2018). Lastly, a recent study using virtual screening identified four FADS inhibitors inhibiting pneumococcal growth in vitro (Lans et al. 2020).
Coenzyme A (CoA) pathway inhibitors
CoA is an essential cofactor in all living organisms in the metabolism of fatty acids. It is synthesized from pantothenate (vitamin B5), cysteine and ATP. First, pantothenate is phosphorylated to 4’-phosphopantothenate by pantothenate kinase (CoaA). This molecule is then condensated with cysteine and subsequently decarboxylated to obtain 4’-phosphopantetheine. Using the enzyme phosphopantetheine adenylyltransferase (PPAT) and ATP, 4’-phosphopantetheine is converted to dephospho-CoA, which is subsequently phosphorylated to yield CoA (Leonardi et al. 2005).
PPAT is an essential enzyme within the CoA pathway. It has been put forward as a novel drug target numerous times, because of its structurally attractive site, high degree of conservation among bacterial species, distinct differences between bacterial and human PPAT, known kinetics and available purified enzyme (Miller et al. 2010). In 2013, de Jonge et al. identified several lead compounds capable of inhibiting PPAT through structure-based HTS. Moreover, these compounds inhibited in vitro growth of macrolide-resistant pneumococci, as well as other antibiotic-resistant Gram-positive bacteria. In a murine lung infection model, treatment with PPAT inhibitors led to a stasis of bacterial burden (De Jonge et al. 2013).
Furthermore, pantothenamides, derivates of pantothenate (vitamin B5), are known to possess antibiotic activity in vitro. They are substrates of CoaA, leading to the formation of inactive CoA analogues (Zhang, White and Rock 2006). In mammals, vanins—also called panthetheinases—hydrolyze pantetheine into vitamin B5, as a way of vitamin recycling (Jansen et al. 2013a). In 2013, studies showed that antibiotic pantothenamides were also hydrolyzed and thus inactivated by vanins, leading to the combination of pantothenamides and vanin inhibitors as a novel antibacterial strategy against Gram-positive bacteria. Vanin inhibitors were able to protect pantothenamides from degradation by host panthetheinases, making pantothenamides more stable for use (Jansen et al. 2013b). Furthermore, pantothenamides resistant towards vanin activity have been reported recently. However, activity of these modified pantothenamides towards pneumococci has not been assessed yet, as they are now mainly studied for their antiplasmodial activity (Jansen et al. 2019; Spry et al. 2020).
In turn, CoA is used in a series of reactions to produce fatty acids. In these subsequent reactions, enoyl-acyl carrier protein reductase (ENR) catalyzes the last and rate-limiting step in each round of chain elongation. Different isoforms of ENR exist, however, pneumococci only possess one of them, called FabK (Heath and Rock 1995). In silico docking revealed several potential inhibitors for FabK, however, to date, none of these have been tested further (Zhang et al. 2011a). Some ENR inhibitors have reached clinical trials, however, none of them are specific to FabK (Rana et al. 2020).
Interference with iron transport systems
Iron is an essential nutrient for bacterial growth and survival. As the concentration of free iron in the host is low, bacteria developed highly specific iron-acquisition systems on their membrane surfaces. In S. pneumoniae, PiaABC, PiuABC and PitABC are the three known iron-transport systems, respectively responsible for the acquisition of heme, ferrichrome and ferric irons (Brown et al. 2002; Cheng et al. 2013). PiaA, PiuA and PitA located on the cell surface bind these free iron-molecules. A Ru(II) complex has been tested for its inhibitory activity towards PiuA, needed for ferrichrome transport. This complex was capable of inhibiting pneumococcal growth without affecting an alveolar epithelial cell line in vitro (Yang et al. 2014a). A similar study conducted with a Rh(II) complex showed the same effects, consolidating the use of metal complexes as potential novel anti-pneumococcal drugs (Yang et al. 2019a). Since metal complexes only recently drew attention as potential antibacterial agents, development is still in a very early stage (Frei 2020).
Repurposing of existing drugs
Repurposing of existing drugs has important benefits. As these drugs are already in use, their safety, tolerability and toxicity has been extensively studied, reducing the costs for development (Cragg, Grothaus and Newman 2014).
Robenidine is an anticoccidial agent used worldwide in poultry and rabbits. Recently, it has been evaluated for its activity on bacteria such as pneumococci and S. aureus. Robenidine, together with two analogues, showed in vitro bactericidal activity against pneumococci by disrupting the cell membrane potential, leading to a thicker cell membrane and a wider periplasmic space (Ogunniyi et al. 2017). Recently, treatment of septic mice with one of these analogues, NCL195 showed a minor reduction in bacterial burden 18 h p.i. Survival was also prolonged, as all mice died 16 h p.i. in the control group. In the treatment group, 60% was still alive at this point, but eventually all mice succumbed at 46 h p.i. As such, NCL195 is not suitable for further development, but its scaffold could be used in further research (Pi et al. 2020).
Choline kinase (ChoK) is a mediator of cell growth and division of eukaryotic cells. As such, it is a drug target for tumor cells. ChoK inhibitor RSM-932A is undergoing clinical trials. However, multiple bacteria including pneumococci also express ChoK. Human ChoK inhibitors MN58b and RSM-932A have been shown to inhibit pneumococcal growth in vitro. However, as these inhibitors also inhibit human ChoK and potentially other bacterial ChoK, including those of the human microbiome, other inhibitors need to be identified (Zimmerman, Lacal and Ibrahim 2019).
Antioxidants N-acetyl-L-cysteine and cysteamine are mucolytics and have been proposed suitable in the treatment of Huntington's and Parkinson's diseases, as well as cystic fibrosis and malaria. In vitro, these two compounds have antibacterial activity against pneumococci in mixed-species biofilms with H. influenzae, killing 98% of all bacteria (Domenech and García 2017). However, it should be noted pneumococci used in this study were non-encapsulated to promote biofilm formation, which might affect results.
Lastly, auranofin, a compound used in the treatment of rheumatoid arthritis, has been tested in vitro against multiple bacteria including multidrug resistant pneumococci. Auranofin and derivative MH05 were subsequently tested in a murine sepsis model. They were shown to significantly reduce mortality and bacterial burden of infections with several pneumococcal strains (Aguinagalde et al. 2015). Currently, auranofin is in phase 2 clinical trials as therapy against M. tuberculosis(Butler and Paterson 2020).
CONCLUSION
Mostly in academia, major efforts are underway to identify potential new drug targets and to improve infection outcomes. Currently, there are three main strategies: (i) boosting host immunity by interfering with its immune responses, (ii) lowering pneumococcal virulence and (iii) developing novel antibacterials with a new mechanism of action (MOA). Each of these strategies has its own benefits and limitations. The first strategy often leads to adjuvant therapies, which can be used in concurrence with current antibiotics. While some of these therapies show highly promising results, no clinical trials have been set up to date. Importantly, interfering with host immune responses is a very delicate process. The biggest limitation to developing therapies interfering with host immunity is the risk of creating immune imbalances which can pose serious health threats (Bewersdorf et al. 2018). Still, this strategy is gaining attention, as publications increase and clinical trials are initiated. Secondly, lowering pneumococcal virulence can be done in multiple ways. The general idea is to inhibit important virulence factors to enable our own immune system to overcome the infection, without the need for bactericidal compounds. However, most of the proposed drug targets are pathogen specific. Notwithstanding their beneficial effects on disease outcome, use of these inhibitors requires certainty regarding the causative agent, which cannot always be guaranteed. Lastly, the development of novel natural antibacterials is hampered by the difficult culturing methods of soil bacteria, the primary source for natural antibiotics. Followed by the discovery of teixobactin, this issue was partially resolved by the development of the iChip technology (Ng and Chan 2016). Furthermore, while rational drug discovery (e.g. based on enzymatic screenings) shows promising results, the pharmaceutical industry is not keen on investing in novel antibiotics as investment costs are high, while the profits could be small (Arias and Murray 2015; Jackson, Czaplewski and Piddock 2018). Even when a potential hit is successfully identified, the emergence of resistance is always on the lure, lowering return on investments (Wright 2015). Therefore, the Infectious Diseases Society of America (IDSA) presented the 10 x ’20 initiative ten years ago, in 2010, to develop and approve 10 novel, efficacious and safe systemically administered antibiotics by 2020 (Gilbert et al. 2010). While the initial goal is met, in 2019 IDSA released a publication stating that even the development of 20 novel antibiotics might still not be sufficient to tackle future drug resistance problems (Talbot et al. 2019). Therapies targeting the bacteria without killing them, i.e. anti-virulence therapies, might provide an answer to this issue. However, most research involving these targets is still in an early stage, with only liposomes as PLY inhibitors currently going through clinical trials.
MATERIALS AND METHODS
Pubmed searches
‘Streptococcus pneumoniae pipeline’, ‘streptococcus pneumoniae novel drug target’, ‘streptococcus pneumoniae novel drug’, ‘streptococcus pneumoniae novel therapy’, ‘(‘Therapeutics’[Mesh] OR ‘Anti-Infective Agents’[Mesh]) AND (‘Streptococcus pneumoniae’[Mesh] OR ‘Pneumococcal Infections’[Mesh]) AND novel’. The literature reporting on anti-pneumococcal activity without explicit bacterial target (e.g. evaluation of plant extracts) was excluded. The literature reporting on novel drug targets older than 10 years (cut-off January 2010) was excluded.
Contributor Information
F Cools, Laboratory for Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium.
P Delputte, Laboratory for Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium.
P Cos, Laboratory for Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium.
Conflict of interest
None declared.
REFERENCES
- Aguinagalde L, Díez-Martínez R, Yuste Jet al. . Auranofin efficacy against MDR Streptococcus pneumoniae and Staphylococcus aureus infections. J Antimicrob Chemother. 2015;70:2608–17. [DOI] [PubMed] [Google Scholar]
- Akbari E, Negahdari B, Faraji Fet al. . Protective responses of an engineered PspA recombinant antigen against Streptococcus pneumoniae. Biotechnol Reports. 2019;24, DOI: 10.1016/j.btre.2019.e00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali YM, Hayat A, Saeed BMet al. . Low-dose recombinant properdin provides substantial protection against Streptococcus pneumoniae and Neisseria meningitidis infection. Proc Natl Acad Sci U S A. 2014;111:5301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Feldman C.. Pneumolysin as a potential therapeutic target in severe pneumococcal disease. J Infect. 2017;74:527–44. [DOI] [PubMed] [Google Scholar]
- Andre GO, Converso TR, Politano WRet al. . Role of Streptococcus pneumoniae proteins in evasion of complement-mediated immunity. Front Microbiol. 2017;8, DOI: 10.3389/fmicb.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel CM, Takács B, Fountoulakis Met al. . Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: Cloning, expression, and characterization of the essential uppS gene. J Bacteriol. 1999;181:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias CA, Murray BE.. A new antibiotic and the evolution of resistance. N Engl J Med. 2015;372:1168–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyakumaran R, Pokrovskaya V, Little DJet al. . Direct Staudinger-phosphonite reaction provides methylphosphonamidates as inhibitors of CE4 De-N-acetylases. ChemBioChem. 2015;16:1350–6. [DOI] [PubMed] [Google Scholar]
- Arzanlou M, Bohlooli S, Jannati Eet al. . Allicin from garlic neutralizes the hemolytic activity of intra- and extra-cellular pneumolysin O in vitro. Toxicon. 2011;57:540–5. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Mamidyala SK, Zuegg Jet al. . Discovery of novel pneumococcal surface antigen A (PsaA) inhibitors using a fragment-based drug design approach. ACS Chem Biol. 2015;10:1511–20. [DOI] [PubMed] [Google Scholar]
- Bangert M, Wright AK, Rylance Jet al. . Immunoactivating peptide P4 augments alveolar macrophage phagocytosis in two diverse human populations. Antimicrob Agents Chemother. 2013;57:4566–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman TK, Kumar M, Chaira Tet al. . Potential of the fluoroketolide RBx 14255 against Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae in an experimental murine meningitis model. J Antimicrob Chemother. 2019;74:1962–70. [DOI] [PubMed] [Google Scholar]
- Baumgartner D, Aebi S, Grandgirard Det al. . Clinical Streptococcus pneumoniae isolates induce differing CXCL8 responses from human nasopharyngeal epithelial cells which are reduced by liposomes. BMC Microbiol. 2016;16, DOI: 10.1186/s12866-016-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax BD, Chan PF, Eggleston DSet al. . Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935–40. [DOI] [PubMed] [Google Scholar]
- Bergenfelz C, Hakansson AP.. Streptococcus pneumoniae otitis media pathogenesis and how it informs our understanding of vaccine strategies. Curr Otorhinolaryngol Rep. 2017;5:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewersdorf JP, Grandgirard D, Koedel Uet al. . Novel and preclinical treatment strategies in pneumococcal meningitis. Curr Opin Infect Dis. 2018;31:85–92. [DOI] [PubMed] [Google Scholar]
- Biedenbach DJ, Bouchillon SK, Hackel Met al. . In vitro activity of gepotidacin, a novel triazaacenaphthylene bacterial topoisomerase inhibitor, against a broad spectrum of bacterial pathogens. Antimicrob Agents Chemother. 2016;60:1918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman G, Coenye T.. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2014;21:5–11. [DOI] [PubMed] [Google Scholar]
- Bradley JS, Byington CL, Shah SSet al. . The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite AT, Marriott HM, Lawrie A.. Divergent roles for TRAIL in lung diseases. Front Med. 2018;5, DOI: 10.3389/fmed.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles DE, Paton JC, Mukerji Ret al. . Pneumococcal vaccines. Microbiol Spectr. 2019;7, DOI: 10.1128/microbiolspec.GPP3-0028-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks LRK, Mias GI.. Streptococcus pneumoniae’s virulence and host immunity: Aging, diagnostics, and prevention. Front Immunol. 2018;9, DOI: 10.3389/fimmu.2018.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS, Gilliland SM, Ruiz-Albert Jet al. . Characterization of pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect Immun. 2002;70:4389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui NK, Turk S, Buckenmaier Set al. . Development of screening assays and discovery of initial inhibitors of pneumococcal peptidoglycan deacetylase PgdA. Biochem Pharmacol. 2011;82:43–52. [DOI] [PubMed] [Google Scholar]
- Bukvić Krajačić M, Dumić M, Novak Pet al. . Discovery of novel ureas and thioureas of 3-decladinosyl-3-hydroxy 15-membered azalides active against efflux-mediated resistant Streptococcus pneumoniae. Bioorganic Med Chem Lett. 2011;21:853–6. [DOI] [PubMed] [Google Scholar]
- Butler MS, Paterson DL.. Antibiotics in the clinical pipeline in October 2019. J Antibiot (Tokyo). 2020;73:329–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caflisch KM, Suh GA, Patel R.. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev Anti Infect Ther. 2019;17:1011–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir GJ, Lefèvre N, Corazza Fet al. . Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol Sex Differ. 2013;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Disease Control (CDC) . Antibiotic resistance threats in the United States, 2013. 2013. [Google Scholar]
- Cevizci R, Düzlü M, Dündar Yet al. . Preliminary results of a novel quorum sensing inhibitor against pneumococcal infection and biofilm formation with special interest to otitis media and cochlear implantation. Eur Arch Oto-Rhino-Laryngology. 2015;272:1389–93. [DOI] [PubMed] [Google Scholar]
- Chang J, Zhang SJ, Jiang YWet al. . Design, synthesis, and antibacterial activity of demethylvancomycin analogues against drug-resistant bacteria. ChemMedChem. 2013;8:976–84. [DOI] [PubMed] [Google Scholar]
- Cheng W, Li Q, Jiang YLet al. . Structures of Streptococcus pneumoniae PiaA and its complex with ferrichrome reveal insights into the substrate binding and release of high affinity iron transporters. PLoS One. 2013;8, DOI: 10.1371/journal.pone.0071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu P, Xu Yet al. . Synthesis and antibacterial activity of novel modified 5-O-desosamine ketolides. Bioorganic Med Chem Lett. 2012;22:7402–5. [DOI] [PubMed] [Google Scholar]
- Cherazard R, Epstein M, Doan T-Let al. . Antimicrobial resistant Streptococcus pneumoniae. Am J Ther. 2017;24:e361–9. [DOI] [PubMed] [Google Scholar]
- Cherian PT, Deshpande A, Cheramie MNet al. . Design, synthesis and microbiological evaluation of ampicillin-tetramic acid hybrid antibiotics. J Antibiot (Tokyo). 2017;70:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli LR, Mori G, Orena BSet al. . A multitarget approach to drug discovery inhibiting Mycobacterium tuberculosis PyrG and PanK. Sci Rep. 2018;8, DOI: 10.1038/s41598-018-21614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek AA, Dąbrowska I, Gregorczyk KPet al. . Phage therapy in bacterial infections treatment: One hundred years after the discovery of bacteriophages. Curr Microbiol. 2017;74:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha N, Huang J, Bai Xet al. . Discovery and characterization of a class of pyrazole inhibitors of bacterial undecaprenyl pyrophosphate synthase. J Med Chem. 2016;59:7299–304. [DOI] [PubMed] [Google Scholar]
- Cong C, Wang H, Hu Yet al. . Synthesis and antibacterial activity of novel 4″-O-benzimidazolyl clarithromycin derivatives, Eur J Med Chem. 2011;46:3105–11. [DOI] [PubMed] [Google Scholar]
- Cools F, Torfs E, Vanhoutte Bet al. . Streptococcus pneumoniae galU gene mutation has a direct effect on biofilm growth, adherence and phagocytosis in vitro and pathogenicity in vivo. Pathog Dis. 2018;76, DOI: 10.1093/femspd/fty069. [DOI] [PubMed] [Google Scholar]
- Cools F, Triki D, Geerts Net al. . In vitro and in vivo evaluation of in silico predicted pneumococcal UDPG:PP inhibitors. Front Microbiol. 2020;11:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couñago RM, Ween MP, Begg SLet al. . Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10:35–41. [DOI] [PubMed] [Google Scholar]
- Covington PS, Davenport JM, Andrae DAet al. . A Phase 2 study of the novel fluoroquinolone JNJ-Q2 in community-acquired bacterial pneumonia. J Antimicrob Chemother. 2013;68:2691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ.. New horizons for old drugs and drug leads. J Nat Prod. 2014;77:703–23. [DOI] [PubMed] [Google Scholar]
- Čipčić Paljetak H, Verbanac D, Padovan Jet al. . Macrolones are a novel class of macrolide antibiotics active against key resistant respiratory pathogens in vitro and in vivo. Antimicrob Agents Chemother. 2016;60:5337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danley DE, Baima ET, Mansour Met al. . Discovery and structural characterization of an allosteric inhibitor of bacterial cis-prenyltransferase. Protein Sci. 2015;24:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, LaRose MI, Hergott CBet al. . Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J Immunol. 2014;193:764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gracia Retamosa M, Díez-Martínez R, Maestro Bet al. . Aromatic esters of bicyclic amines as antimicrobials against Streptococcus pneumoniae. Angew Chemie - Int Ed. 2015;54:13673–7. [DOI] [PubMed] [Google Scholar]
- De Jonge BLM, Walkup GK, Lahiri SDet al. . Discovery of inhibitors of 4’-phosphopantetheine adenylyltransferase (PPAT) to validate PPAT as a target for antibacterial therapy. Antimicrob Agents Chemother. 2013;57:6005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Church D, Vanderkooi OGet al. . Streptococcus pneumoniae infection: a Canadian perspective. Expert Rev Anti Infect Ther. 2013;11:781–91. [DOI] [PubMed] [Google Scholar]
- Diez-Martinez R, De Paz HD, Garcia-Fernandez Eet al. . A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J Antimicrob Chemother. 2015;70:1–11. [DOI] [PubMed] [Google Scholar]
- DiFrancesco BR, Morrison ZA, Nitz M.. Monosaccharide inhibitors targeting carbohydrate esterase family 4 de-N-acetylases. Bioorganic Med Chem. 2018;26:5631–43. [DOI] [PubMed] [Google Scholar]
- Dockrell DH, Marriott HM, Prince LRet al. . Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J Immunol. 2003;171:5380–8. [DOI] [PubMed] [Google Scholar]
- Dockrell DH, Whyte MKB, Mitchell TJ.. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest. 2012;142:482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech M, García E. N-Acetyl-L-cysteine and cysteamine as new strategies against mixed biofilms of nonencapsulated Streptococcus pneumoniae and nontypeable Haemophilus influenzae. Antimicrob Agents Chemother. 2017;61, DOI: 10.1128/AAC.01992-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech M, Ramos-Sevillano E, Garcia Eet al. . Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae. Infect Immun. 2013;81:2606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper MP, Weir S, Macone Aet al. . Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother. 2014;58:1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugourd D, Yang H, Elliott Met al. . Antimicrobial properties of MX-2401, an expanded-spectrum lipopeptide active in the presence of lung surfactant. Antimicrob Agents Chemother. 2011;55:3720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GA, Raoult D, Dubourg G.. Antibiotic discovery: History, methods and perspectives. Int J Antimicrob Agents. 2019;53:371–82. [DOI] [PubMed] [Google Scholar]
- Eakin AE, Green O, Hales Net al. . Pyrrolamide DNA gyrase inhibitors: Fragment-based nuclear magnetic resonance screening to identify antibacterial agents. Antimicrob Agents Chemother. 2012;56:1240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrizzi JA, Kim H, Anderson PMet al. . Crystal structure of Escherichia coli cytidine triphosphate synthetase, a nucleotide-regulated glutamine amidotransferase/ATP-dependent amidoligase fusion protein and homologue of anticancer and antiparasitic drug targets. Biochemistry. 2004;43:6447–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) . Surveillance of Antimicrobial Resistance in Europe 2018., 2019. [Google Scholar]
- Fajdetić A, Vinter A, Paljetak HČet al. . Synthesis, activity and pharmacokinetics of novel antibacterial 15-membered ring macrolones. Eur J Med Chem. 2011;46:3388–97. [DOI] [PubMed] [Google Scholar]
- Fang F.Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat Rev Microbiol. 2004;2, DOI: 10.1038/NRMICRO1004. [DOI] [PubMed] [Google Scholar]
- Farrell DJ, Mendes RE, Jones RN. Antimicrobial activity of solithromycin against serotyped macrolide-resistant Streptococcus pneumoniae isolates collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2015;59:2432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Hilliard JJ, Morrow BJet al. . Efficacy of a new fluoroquinolone, JNJ-Q2, in murine models of Staphylococcus aureus and Streptococcus pneumoniae skin, respiratory, and systemic infections. Antimicrob Agents Chemother. 2011;55:5522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Coimbra J, Sarda C, Rello J.. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther. 2020, DOI: 10.1007/s12325-020-01248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RK, Farrell DJ, Rhomberg PRet al. . Gepotidacin (GSK2140944) in vitro activity against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2017;61, DOI: 10.1128/AAC.00468-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RK, Rhomberg RR, Sader HS. In vitro activity of the novel lactone ketolide nafithromycin (WCK 4873) against contemporary clinical bacteria from a global surveillance program. Antimicrob Agents Chemother. 2017;61, 10.1128/AAC.01230-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei A.Metal complexes, an untapped source of antibiotic potential?. Antibiotics. 2020;9, DOI: 10.3390/antibiotics9020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante J, Ho A, Tingey Set al. . Quorum sensing and biofilms in the pathogen, Streptococcus pneumoniae. Curr Pharm Des. 2014;21:25–30. [DOI] [PubMed] [Google Scholar]
- Geno KA, Gilbert GL, Song JYet al. . Pneumococcal capsules and their types: Past, present, and future. Clin Microbiol Rev. 2015;28:871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DN, Guidos RJ, Boucher HWet al. . The 10 × ‘20 initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–3. [DOI] [PubMed] [Google Scholar]
- Gilley RP, Orihuela CJ.. Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization. Front Cell Infect Microbiol. 2014;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgirard D, Loeffler JM, Fischetti VAet al. . Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–22. [DOI] [PubMed] [Google Scholar]
- Grossman TH, Fyfe C, O’Brien Wet al. . Fluorocycline TP-271 is potent against complicated community-acquired bacterial pneumonia pathogens. mSphere. 2017;2, 10.1128/msphere.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman TH, Murphy TM, Slee AMet al. . Eravacycline (TP-434) is efficacious in animal models of infection. Antimicrob Agents Chemother. 2015;59:2567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman TH, Starosta AL, Fyfe Cet al. . Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother. 2012;56:2559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–42. [DOI] [PubMed] [Google Scholar]
- Hegde SS, Okusanya OO, Skinner Ret al. . Pharmacodynamics of TD-1792, a novel glycopeptide-cephalosporin heterodimer antibiotic used against Gram-positive bacteria, in a neutropenic murine thigh model. Antimicrob Agents Chemother. 2012;56:1578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BD, Neill DR, Becker KAet al. . Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat Biotechnol. 2015;33:81–8. [DOI] [PubMed] [Google Scholar]
- Hernansanz-Agustín P, Izquierdo-Álvarez A, García-Ortiz Aet al. . Nitrosothiols in the immune system: signaling and protection. Antioxid Redox Signal. 2013;18:288–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O, Priller J, Prozorovski Tet al. . TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest. 2007;117:2004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SE, Perez AJ, Tsui HCTet al. . A new quorum-sensing system (TprA/PhrA) for Streptococcus pneumoniae D39 that regulates a lantibiotic biosynthesis gene cluster. Mol Microbiol. 2015;97:229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley D, Griffin C, Young Met al. . Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of qge. Clin Infect Dis. 2020, DOI: 10.1093/cid/ciaa1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokoshi J, Nakamura Y, Komada Set al. . Inhibition of bacterial undecaprenyl pyrophosphate synthase by small fungal molecules. J Antibiot (Tokyo). 2016;69:798–805. [DOI] [PubMed] [Google Scholar]
- Iovino F, Engelen-Lee JY, Brouwer Met al. . pIgR and PEC AM-1 bind to pneumococcal adhesins RrgA and PspC mediating bacterial brain invasion. J Exp Med. 2017;214:1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino F, Thorsdottir S, Henriques-Normark B.. Receptor blockade: A novel approach to protect the brain from pneumococcal invasion. J Infect Dis. 2018;218:476–84. [DOI] [PubMed] [Google Scholar]
- Jackson N, Czaplewski L, Piddock LJ V. Discovery and development of new antibacterial drugs: learning from experience?. J Antimicrob Chemother. 2018;73:1452–9. [DOI] [PubMed] [Google Scholar]
- Jansen PAM, Hermkens PHH, Zeeuwen Pet al. . Combination of pantothenamides with vanin inhibitors as a novel antibiotic strategy against Gram-positive bacteria. Antimicrob Agents Chemother. 2013b;57:4794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PAM, van der Krieken DA, Botman PNMet al. . Stable pantothenamide bioisosteres: novel antibiotics for Gram-positive bacteria. J Antibiot (Tokyo). 2019;72:682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PAM, Van Diepen JA, Ritzen Bet al. . Discovery of small molecule vanin inhibitors: New tools to study metabolism and disease. ACS Chem Biol. 2013a;8:530–4. [DOI] [PubMed] [Google Scholar]
- Jia L, Wang Y, Wang Yet al. . Synthesis and antibacterial evaluation of novel 11-O-aralkylcarbamoyl-3-O-descladinosylclarithromycin derivatives. Bioorganic Med Chem Lett. 2018;28:2471–6. [DOI] [PubMed] [Google Scholar]
- Jia L, Yan M, Shen Yet al. . Synthesis and antibacterial evaluation of novel 11-O-carbamoyl clarithromycin ketolides. Bioorganic Med Chem Lett. 2017;27:3693–7. [DOI] [PubMed] [Google Scholar]
- Jian Y, Hulpia F, Risseeuw MDPet al. . 1-(1-Arylethylpiperidin-4-yl)thymine analogs as antimycobacterial TMPK inhibitors. Molecules. 2020;25, DOI: 10.3390/molecules25122805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Y, Risseeuw MDP, Froeyen Met al. . 1-(Piperidin-3-yl)thymine amides as inhibitors of M. tuberculosis thymidylate kinase. J Enzyme Inhib Med Chem. 2019;34:1730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal HM, Le CF, Mohd Yusof MYet al. . Antimicrobial activity of novel synthetic peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PLoS One. 2015;10:e0128532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal HM, Zandi K, Ong KCet al. . Mechanisms of action and in vivo antibacterial efficacy assessment of five novel hybrid peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PeerJ. 2017;2017, DOI: 10.7717/peerj.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic M, Rožman K, Sova Met al. . Anthranilic acid inhibitors of Undecaprenyl Pyrophosphate Synthase (UppS), an essential enzyme for bacterial cell wall biosynthesis. Front Microbiol. 2019;10, DOI: 10.3389/fmicb.2018.03322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Weiser JN, Paton JCet al. . The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. [DOI] [PubMed] [Google Scholar]
- Kasanmoentalib ES, Valls Seron M, Ferwerda Bet al. . Mannose-binding lectin-associated serine protease 2 (MASP-2) contributes to poor disease outcome in humans and mice with pneumococcal meningitis. J Neuroinflammation. 2017;14, DOI: 10.1186/s12974-016-0770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanmoentalib ES, Valls Seron M, Morgan BPet al. . Adjuvant treatment with dexamethasone plus anti-C5 antibodies improves outcome of experimental pneumococcal meningitis: A randomized controlled trial. J Neuroinflammation. 2015;12, DOI: 10.1186/s12974-015-0372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanmoentalib ES, Valls Serón M, Engelen-Lee JYet al. . Complement factor H contributes to mortality in humans and mice with bacterial meningitis. J Neuroinflammation. 2019;16, DOI: 10.1186/s12974-019-1675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Yamagishi Y, Hagihara Met al. . Antimicrobial activity of solithromycin and levofloxacin against a murine pneumonia mixed-infection model caused by Streptococcus pneumoniae and anaerobic bacteria. J Infect Chemother. 2019;25:311–3. [DOI] [PubMed] [Google Scholar]
- Keating TA, Newman J V., Olivier NBet al. . In vivo validation of thymidylate kinase (TMK) with a rationally designed, selective antibacterial compound. ACS Chem Biol. 2012;7:1866–72. [DOI] [PubMed] [Google Scholar]
- Kim G-L, Seon S-H, Rhee D-K.. Pneumonia and Streptococcus pneumoniae vaccine. Arch Pharm Res. 2017;40:885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, McGee L, Tomczyk Set al. . Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: A United States perspective. Clin Microbiol Rev. 2016;29:525–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishii R, Yamaguchi Y, Takei M. Vitro activities and spectrum of the novel fluoroquinolone lascufloxacin (KRP-AM1977). Antimicrob Agents Chemother. 2017;61, 10.1128/AAC.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Scheld WM, Pfister HW.. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2:721–36. [DOI] [PubMed] [Google Scholar]
- Koelman DLH, Brouwer MC, Van De Beek D.. Targeting the complement system in bacterial meningitis. Brain. 2019;142:3325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarič A, Anderluh M, Minovski N.. Two decades of successful SAR-grounded stories of the Novel Bacterial Topoisomerase Inhibitors (NBTIs). J Med Chem. 2020;63:5664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulenti D, Xu E, Song Aet al. . Emerging treatment options for infections by multidrug-resistant Gram-positive microorganisms. Microorganisms. 2020;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian SA, Ota T, Bubeck SSet al. . Generation and improvement of effector function of a novel broadly reactive and protective monoclonal antibody against pneumococcal surface protein A of Streptococcus pneumoniae. PLoS One. 2016;11, DOI: 10.1371/journal.pone.0154616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Rathy S, Hajare AKet al. . Synthesis and antibacterial activity of a novel series of acylides active against community acquired respiratory pathogens. Bioorganic Med Chem Lett. 2012;22:476–81. [DOI] [PubMed] [Google Scholar]
- Kumura K, Wakiyama Y, Ueda Ket al. . Synthesis and antibacterial activity of novel lincomycin derivatives. I. Enhancement of antibacterial activities by introduction of substituted azetidines. J Antibiot (Tokyo). 2016;69:440–5. [DOI] [PubMed] [Google Scholar]
- Kwambana-Adams BA, Mulholland EK, Satzke Cet al. . State-of-the-art in the pneumococcal field: Proceedings of the 11th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-11). Pneumonia. 2020;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land WG.Use of DAMPs and SAMPs as therapeutic targets or therapeutics: A note of caution. Mol Diagnosis Ther. 2020;24:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans I, Anoz-Carbonell E, Palacio-Rodríguez Ket al. . In silico discovery and biological validation of ligands of FAD synthase, a promising new antimicrobial target. PLoS Comput Biol. 2020;16, DOI: 10.1371/JOURNAL.PCBI.1007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterre PF, Colin G, Dequin PFet al. . CAL02, a novel antitoxin liposomal agent, in severe pneumococcal pneumonia: a first-in-human, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2019;19:620–30. [DOI] [PubMed] [Google Scholar]
- Lee C, Choi SK, Kim RKet al. . Development of a new 15-valent pneumococcal conjugate vaccine (PCV15) and evaluation of its immunogenicity. Biologicals. 2019;61:32–7. [DOI] [PubMed] [Google Scholar]
- Lee L V., Granda B, Dean Ket al. . Biophysical investigation of the mode of inhibition of tetramic acids, the allosteric inhibitors of undecaprenyl pyrophosphate synthase. Biochemistry. 2010;49:5366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Zhang YM, Rock COet al. . Coenzyme A: Back in action. Prog Lipid Res. 2005;44:125–53. [DOI] [PubMed] [Google Scholar]
- Lepak AJ, Seiler P, Surivet JPet al. . In vivo pharmacodynamic target investigation of two bacterial topoisomerase inhibitors, ACT-387042 and ACT-292706, in the neutropenic murine thigh model against Streptococcus pneumoniae and Staphylococcus aureus. Antimicrob Agents Chemother. 2016;60:3626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepak AJ, Zhao M, Liu Qet al. . Pharmacokinetic/pharmacodynamic evaluation of a novel aminomethylcycline antibiotic, KBP-7072, in the neutropenic murine pneumonia model against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2019;63, 10.1128/AAC.02404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Li XL, Wang Het al. . Structure-activity relationships of novel alkylides: 3-O-Arylalkyl clarithromycin derivatives with improved antibacterial activities. Eur J Med Chem. 2012;49:289–303. [DOI] [PubMed] [Google Scholar]
- Li CR, Zhai QQ, Wang XKet al. . In vivo antibacterial activity of MRX-I, a new oxazolidinone. Antimicrob Agents Chemother. 2014;58:2418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhao X, Wang Jet al. . β-sitosterol interacts with pneumolysin to prevent Streptococcus pneumoniae infection. Sci Rep. 2015;5:17668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling LL, Schneider T, Peoples AJet al. . A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ma S, Yan Met al. . Synthesis and antibacterial evaluation of novel 11,4″-disubstituted azithromycin analogs with greatly improved activity against erythromycin- resistant bacteria. Eur J Med Chem. 2013;59:209–17. [DOI] [PubMed] [Google Scholar]
- Lundbo LF, Benfield T.. Risk factors for community-acquired bacterial meningitis. Infect Dis (Auckl). 2017;49:433–44. [DOI] [PubMed] [Google Scholar]
- Macheboeuf P, Contreras-Martel C, Job Vet al. . Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–91. [DOI] [PubMed] [Google Scholar]
- Macone AB, Caruso BK, Leahy RGet al. . In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother. 2014;58:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CX, Lv W, Li YXet al. . Design, synthesis and structure-activity relationships of novel macrolones: Hybrids of 2-fluoro 9-oxime ketolides and carbamoyl quinolones with highly improved activity against resistant pathogens. Eur J Med Chem. 2019;169:1–20. [DOI] [PubMed] [Google Scholar]
- Maestro B, Sanz JM.. Choline binding proteins from Streptococcus pneumoniae: A dual role as enzybiotics and targets for the design of new antimicrobials. Antibiotics. 2016;5, DOI: 10.3390/antibiotics5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Håkansson J, Ringstad Let al. . Antimicrobial peptides: An emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6, DOI: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen M, Choi S, Whitesides G.. Polyvalent interactions in bioldgical systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed Engl. 1998;37, DOI: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Martínez-Botella G, Breen JN, Duffy JESet al. . Discovery of selective and potent inhibitors of Gram-positive bacterial thymidylate kinase (TMK). J Med Chem. 2012;55:10010–21. [DOI] [PubMed] [Google Scholar]
- Ma S, Jiao B, Ju Yet al. . Synthesis and antibacterial evaluation of novel clarithromycin derivatives with C-4″ elongated arylalkyl groups against macrolide-resistant strains. Eur J Med Chem. 2011a;46:556–66. [DOI] [PubMed] [Google Scholar]
- Masouris I, Klein M, Dyckhoff Set al. . Inhibition of DAMP signaling as an effective adjunctive treatment strategy in pneumococcal meningitis. J Neuroinflammation. 2017;14, DOI: 10.1186/s12974-017-0989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang L, Wang Ret al. . Novel C-4″ modified azithromycin analogs with remarkably enhanced activity against erythromycin-resistant Streptococcus pneumoniae: The synthesis and antimicrobial evaluation. Eur J Med Chem. 2011b;46:5196–205. [DOI] [PubMed] [Google Scholar]
- McCarthy MW.Teixobactin: a novel anti-infective agent. Expert Rev Anti Infect Ther. 2019;17:1–3. [DOI] [PubMed] [Google Scholar]
- McGhee P, Clark C, Kosowska-Shick KMet al. . In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob Agents Chemother. 2010;54:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes RE, Farrell DJ, Flamm RKet al. . In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. Antimicrob Agents Chemother. 2016;60:4407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay JP, Waterer GW, Long ACet al. . Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguet L, Zervosen A, Gerards Tet al. . Discovery of new inhibitors of resistant Streptococcus pneumoniae Penicillin Binding Protein (PBP) 2x by structure-based virtual screening. J Med Chem. 2009;52:5926–36. [DOI] [PubMed] [Google Scholar]
- Miles TJ, Hennessy AJ, Bax Bet al. . Novel tricyclics (e.g., GSK945237) as potent inhibitors of bacterial type IIA topoisomerases. Bioorganic Med Chem Lett. 2016;26:2464–9. [DOI] [PubMed] [Google Scholar]
- Miller JR, Thanabal V, Melnick MMet al. . The use of biochemical and biophysical tools for triage of high-throughput screening hits - aa case study with Escherichia coli phosphopantetheine adenylyltransferase. Chem Biol Drug Des. 2010;75:444–54. [DOI] [PubMed] [Google Scholar]
- Mills SD, Eakin AE, Buurman ETet al. . Novel bacterial NAD+-dependent DNA ligase inhibitors with broad-spectrum activity and antibacterial efficacy in vivo. Antimicrob Agents Chemother. 2011;55:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton-Fry MJ, Brickner SJ, Hamel JCet al. . Novel quinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV. Bioorganic Med Chem Lett. 2013;23:2955–61. [DOI] [PubMed] [Google Scholar]
- Mollerach M, López R, García E.. Characterization of the galU gene of Streptococcus pneumoniae encoding a uridine diphosphoglucose pyrophosphorylase: a gene essential for capsular polysaccharide biosynthesis. J Exp Med. 1998;188:2047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasta L, Ronfani L, Marchetti Fet al. . Burden of disease caused by otitis media: Systematic review and global estimates. Moormann AM (ed.). PLoS One. 2012;7:e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro Pedroso M, Selleck C, Bilyj Jet al. . Reaction mechanism of the metallohydrolase CpsB from: Streptococcus pneumoniae, a promising target for novel antimicrobial agents. Dalt Trans. 2017;46:13194–201. [DOI] [PubMed] [Google Scholar]
- Mook-Kanamori BB, Geldhoff M, van der Poll Tet al. . Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24:557–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona JK, Miller DC, Morona Ret al. . The effect that mutations in the conserved capsular polysaccharide biosynthesis genes cpsA, cpsB, and cpsD have on virulence of Streptococcus pneumoniae. J Infect Dis. 2004;189:1905–13. [DOI] [PubMed] [Google Scholar]
- Morrow BJ, He W, Amsler KMet al. . In vitro antibacterial activities of JNJ-Q2, a new broad-spectrum fluoroquinolone. Antimicrob Agents Chemother. 2010;54:1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton B, Mitsi E, Pennington SHet al. . Augmented passive immunotherapy with p4 peptide improves phagocyte activity in severe sepsis. Shock. 2016;46:635–41. [DOI] [PubMed] [Google Scholar]
- Moscoso M, Garcia E, Lopez R.. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol. 2006;188:7785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motib A, Guerreiro A, Al-Bayati Fet al. . Modulation of quorum sensing in a Gram-positive pathogen by linear molecularly imprinted polymers with anti-infective properties. Angew Chemie - Int Ed. 2017;56:16555–8. [DOI] [PubMed] [Google Scholar]
- Motib AS, Al-Bayati FAY, Manzoor Iet al. . TprA/PhrA quorum sensing system has a major effect on pneumococcal survival in respiratory tract and blood, and its activity is controlled by CcpA and GlnR. Front Cell Infect Microbiol. 2019;9, DOI: 10.3389/fcimb.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Benenato KE, Gingipalli L, Boriack-Sjodin PAet al. . Negishi cross-coupling enabled synthesis of novel NAD+-dependent DNA ligase inhibitors and SAR development. Bioorganic Med Chem Lett. 2015;25:5172–7. [DOI] [PubMed] [Google Scholar]
- Nayak S, Pradhan D, Singh Het al. . Computational screening of potential drug targets for pathogens causing bacterial pneumonia. Microb Pathog. 2019;130:271–82. [DOI] [PubMed] [Google Scholar]
- Ng V, Chan WC.. New found hope for antibiotic discovery: Lipid II inhibitors. Chem - A Eur J. 2016;22:12606–16. [DOI] [PubMed] [Google Scholar]
- Nielsen AG, Nielsen RB, Riis AHet al. . The impact of statin use on pneumonia risk and outcome: A combined population-based case-control and cohort study. Crit Care. 2012;16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto AT, Rosch JW, Tuomanen EI.. Pneumolysin: Pathogenesis and therapeutic target. Front Microbiol. 2020;11, DOI: 10.3389/fmicb.2020.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaidullah AJ, Ahmed MH, Kitten Tet al. . Inhibiting Pneumococcal surface antigen A (PsaA) with small molecules discovered through virtual screening: Steps toward validating a potential target forStreptococcus pneumoniae. Chem Biodivers. 2018;15, DOI: 10.1002/cbdv.201800234. [DOI] [PubMed] [Google Scholar]
- Odagiri T, Inagaki H, Nagamochi Met al. . Design, synthesis, and biological evaluation of novel 7-[(3 aS,7 aS)-3 a-aminohexahydropyrano[3,4- c]pyrrol-2(3 H)-yl]-8-methoxyquinolines with potent antibacterial activity against respiratory pathogens. J Med Chem. 2018;61:7234–44. [DOI] [PubMed] [Google Scholar]
- Odagiri T, Inagaki H, Sugimoto Yet al. . Design, synthesis, and biological evaluations of novel 7-[7-amino-7-methyl- 5-azaspiro[2.4]heptan-5-yl]-8-methoxyquinolines with potent antibacterial activity against respiratory pathogens. J Med Chem. 2013;56:1974–83. [DOI] [PubMed] [Google Scholar]
- Ogunniyi AD, Khazandi M, Stevens AJet al. . Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent. PLoS One. 2017;12:e0183457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal JM.DNA and RNA ligases: structural variations and shared mechanisms. Curr Opin Struct Biol. 2008;18:96–105. [DOI] [PubMed] [Google Scholar]
- Paton JC, Trappetti C.. Streptococcus pneumoniae capsular polysaccharide. Microbiol Spectr. 2019;7, DOI: 10.1128/microbiolspec.gpp3-0019-2018. [DOI] [PubMed] [Google Scholar]
- Paukner S, Riedl R. Pleuromutilins: Potent drugs for resistant bugs-mode of action and resistance. Cold Spring Harb Perspect Med. 2017;7, 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner S, Sader HS, Ivezic-Schoenfeld Zet al. . Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57:4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlović D, Mutak S. Synthesis and antibacterial evaluation of novel 4′-glycyl linked quinolyl-azithromycins with potent activity against macrolide-resistant pathogens. Bioorganic Med Chem. 2016;24:1255–67. [DOI] [PubMed] [Google Scholar]
- Pereira D, Fernandes P. Synthesis and antibacterial activity of novel 4-aryl-[1,2,3]-triazole containing macrolides. Bioorganic Med Chem Lett. 2011;21:510–3. [DOI] [PubMed] [Google Scholar]
- Petit CM, Koretke KK.. Characterization of Streptococcus pneumoniae thymidylate kinase: Steady-state kinetics of the forward reaction and isothermal titration calorimetry. Biochem J. 2002;363:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettini E, Fiorino F, Cuppone AMet al. . Interferon-γ from brain leukocytes enhances meningitis by type 4 Streptococcus pneumoniae. Front Microbiol. 2015;6, DOI: 10.3389/fmicb.2015.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrani P, Mandell L, Torres Aet al. . The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13:139–52. [DOI] [PubMed] [Google Scholar]
- Pi H, Nguyen HT, Venter Het al. . In vitro activity of robenidine analog NCL195 in combination with outer membrane permeabilizers against gram-negative bacterial pathogens and impact on systemic gram-positive bacterial infection in mice. Front Microbiol. 2020;11, DOI: 10.3389/fmicb.2020.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JA, Dockrell DH.. Virulence factors in pneumococcal respiratory pathogenesis. Future Microbiol. 2008;3:205–21. [DOI] [PubMed] [Google Scholar]
- Qin Y, Qiang S, Ji Set al. . Synthesis and antibacterial activity of novel 3-O-arylalkylcarbamoyl-3-O-descladinosyl-9-O-(2-chlorobenzyl)oxime clarithromycin derivatives. Bioorganic Med Chem Lett. 2018;28:3324–8. [DOI] [PubMed] [Google Scholar]
- Qi Y, Jiao B, Ma X et al. Synthesis and antibacterial activity of novel 4″-O-carbamoyl erythromycin-A derivatives. Arch Pharm (Weinheim) 2010;343:458–64. doi: 10.1002/ardp.200900288. [DOI] [PubMed] [Google Scholar]
- Rajagopal M, Walker S.. Envelope structures of Gram-positive bacteria. Current Topics in Microbiology and Immunology. Vol 404. Springer Verlag, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam G, Anderton JM, Carlone GMet al. . Pneumococcal surface adhesin A (PsaA): A review. Crit Rev Microbiol. 2008a;34:131–42. [DOI] [PubMed] [Google Scholar]
- Rajam G, Bangert M, Hammons GMet al. . P4 peptide therapy rescues aged mice from fatal pneumococcal sepsis. Clin Vaccine Immunol. 2010;17:1823–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam G, Phillips DJ, White Eet al. . A functional epitope of the pneumococcal surface adhesin A activates nasopharyngeal cells and increases bacterial internalization. Microb Pathog. 2008b;44:186–96. [DOI] [PubMed] [Google Scholar]
- Rajam G, Skinner J, Melnick Net al. . A 28-aa Pneumococcal Surface Adhesin A–derived peptide, P4, augments passive immunotherapy and rescues mice from fatal pneumococcal infection. J Infect Dis. 2009;199:1233–8. [DOI] [PubMed] [Google Scholar]
- Raj VS, Barman TK, Kalia Vet al. . A novel ketolide, RBx 14255, with activity against multidrug-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 2014;58:4283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana P, Ghouse SM, Akunuri Ret al. . FabI (enoyl acyl carrier protein reductase) - A potential broad spectrum therapeutic target and its inhibitors. Eur J Med Chem. 2020;208, DOI: 10.1016/j.ejmech.2020.112757. [DOI] [PubMed] [Google Scholar]
- Rasko DA, Sperandio V.. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–28. [DOI] [PubMed] [Google Scholar]
- Ribes S, Riegelmann J, Redlich Set al. . Multivalent choline dendrimers increase phagocytosis of Streptococcus pneumoniae R6 by microglial cells. Chemotherapy. 2013;59:138–42. [DOI] [PubMed] [Google Scholar]
- Rodgers W, Frazier AD, Champney WS. Solithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Antimicrob Agents Chemother. 2013;57:1632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, Ding X, Chanson ALet al. . Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression. Eur J Immunol. 2007;37:3509–21. [DOI] [PubMed] [Google Scholar]
- Roig-Molina E, Domenech M, Retamosa M de Get al. . Widening the antimicrobial spectrum of esters of bicyclic amines: In vitro effect on Gram-positive Streptococcus pneumoniae and Gram-negative non-typeable Haemophilus influenzae biofilms. Biochim Biophys Acta - Gen Subj. 2019;1863:96–104. [DOI] [PubMed] [Google Scholar]
- Ross JE, Sader HS, Ivezic-Schoenfeld Zet al. . Disk diffusion and MIC quality control ranges for BC-3205 and BC-3781, two novel pleuromutilin antibiotics. J Clin Microbiol. 2012;50:3361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JE, Scangarella-Oman NE, Miller LAet al. . Determination of disk diffusion and MIC quality control ranges for GSK1322322, a novel peptide deformylase inhibitor. J Clin Microbiol. 2011;49:3928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinchik E, Schneider T, Elliott Met al. . Mechanism of action and limited cross-resistance of new lipopeptide MX-2401. Antimicrob Agents Chemother. 2011;55:2743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sag D, Ayyildiz ZO, Gunalp Set al. . The role of TRAIL/DRs in the modulation of immune cells and responses. Cancers (Basel). 2019;11, DOI: 10.3390/cancers11101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangshetti J, Khan FA, Shinde D.. Peptide deformylase: A new target in antibacterial, antimalarial and anticancer drug discovery. Curr Med Chem. 2014;22:214–36. [DOI] [PubMed] [Google Scholar]
- Savva A, Brouwer MC, Roger Tet al. . Functional polymorphisms of macrophage migration inhibitory factor as predictors of morbidity and mortality of pneumococcal meningitis. Proc Natl Acad Sci U S A. 2016;113:3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer DO, Chouake JS, Nosanchuk JDet al. . The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián M, Anoz-Carbonell E, Gracia Bet al. . Discovery of antimicrobial compounds targeting bacterial type FAD synthetases. J Enzyme Inhib Med Chem. 2018;33:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián M, Lira-Navarrete E, Serrano Aet al. . The FAD synthetase from the human pathogen Streptococcus pneumoniae: A bifunctional enzyme exhibiting activity-dependent redox requirements. Sci Rep. 2017;7, DOI: 10.1038/s41598-017-07716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self WH, Wunderink RG, Dinubile MJet al. . Low admission plasma gelsolin concentrations identify community-acquired pneumonia patients at high risk for severe outcomes. Clin Infect Dis. 2019;69:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Ferreira P, Martinez-Julvez Met al. . The prokaryotic FAD synthetase family: A potential drug target. Curr Pharm Des. 2013;19:2637–48. [DOI] [PubMed] [Google Scholar]
- Shao C, Zhou W, Zhang Set al. . Synthesis and antibacterial activity of N 4-mono alkyl derivatives of novel glycopeptide LYV07ww01. Bioorganic Med Chem Lett. 2011;21:6732–8. [DOI] [PubMed] [Google Scholar]
- Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs. 1999;8:1195–202. [DOI] [PubMed] [Google Scholar]
- Shin D, Park SI, Lee HSet al. . Pharmacokinetics and tolerability of IDP-73152 mesylate after a single oral administration under fasted and fed conditions in healthy volunteers. Drug Des Devel Ther. 2019;13:2483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Lu G, Li Met al. . Juglone Alleviates Pneumolysin-Induced Human Alveolar Epithelial Cell Injury via Inhibiting the Hemolytic Activity of Pneumolysin. Antonie Van Leeuwenhoek. 2017a;110, 10.1007/S10482-017-0880-0. [DOI] [PubMed] [Google Scholar]
- Song M, Teng Z, Li Met al. . Epigallocatechin gallate inhibits Streptococcus pneumoniae virulence by simultaneously targeting pneumolysin and sortase A. J Cell Mol Med. 2017b;21:2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C, Barnard L, Kok Met al. . Toward a stable and potent coenzyme A-targeting antiplasmodial agent: Structure-activity relationship studies of N-phenethyl-α-methyl-pantothenamide. ACS Infect Dis. 2020;6:1844–54. [DOI] [PubMed] [Google Scholar]
- Standish AJ, Salim AA, Zhang Het al. . Chemical inhibition of bacterial protein tyrosine phosphatase suppresses capsule production. PLoS One. 2012;7:e36312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwede K, Henken S, Bohling Jet al. . TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209:1937–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwede K, Tempelhof O, Bolte Ket al. . Local delivery of GM-CSF protects mice from lethal pneumococcal pneumonia. J Immunol. 2011;187:5346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffels L, Taunt HN, Charalambous Bet al. . Synthesis of bacteriophage lytic proteins against Streptococcus pneumoniae in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J. 2017;15:1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Tanikawa T, Suzuki Ket al. . Synthesis and structure-activity relationship of a novel class of 15-membered macrolide antibiotics known as “11a-azalides.”. Bioorganic Med Chem. 2012;20:5787–801. [DOI] [PubMed] [Google Scholar]
- Surivet JP, Zumbrunn C, Rueedi Get al. . Novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-Gram positive activity and improved safety profile. J Med Chem. 2015;58:927–42. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JA.Antibiotics in development targeting protein synthesis. Ann N Y Acad Sci. 2011;1241:122–52. [DOI] [PubMed] [Google Scholar]
- Tai SS.Streptococcus pneumoniae protein vaccine candidates: Properties, activities and animal studies. Crit Rev Microbiol. 2006;32:139–53. [DOI] [PubMed] [Google Scholar]
- Talbot GH, Jezek A, Murray BEet al. . The Infectious Diseases Society of America's 10 × ’20 initiative (10 new systemic antibacterial agents US Food and Drug Administration approved by 2020): Is 20 × ’20 a possibility?. Clin Infect Dis. 2019;69:1–11. [DOI] [PubMed] [Google Scholar]
- Tannous A, Levinson SL, Bolognese Jet al. . Safety and pharmacokinetics of recombinant human plasma gelsolin in patients hospitalized for nonsevere community-acquired pneumonia. Antimicrob Agents Chemother. 2020;64, DOI: 10.1128/AAC.00579-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Too LK, Ball HJ, McGregor ISet al. . The pro-inflammatory cytokine interferon-gamma is an important driver of neuropathology and behavioural sequelae in experimental pneumococcal meningitis. Brain Behav Immun. 2014;40:252–68. [DOI] [PubMed] [Google Scholar]
- Turk S, Verlaine O, Gerards Tet al. . New noncovalent inhibitors of penicillin-binding proteins from penicillin-resistant bacteria. PLoS One. 2011;6, DOI: 10.1371/journal.pone.0019418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura E, Wakiyama Y, Kumura Ket al. . Synthesis of novel lincomycin derivatives and their in vitro antibacterial activities. J Antibiot (Tokyo). 2013;66:195–8. [DOI] [PubMed] [Google Scholar]
- United States National Institute of Health (NIH) . A three-part study to determine the safety, tolerability and pharmacokinetics of GSK1322322 in healthy volunteers and healthy male Japanese subjects. 2019. [Google Scholar]
- Uria-Nickelsen M, Neckermann G, Sriram Set al. . Novel topoisomerase inhibitors: Microbiological characterisation and in vivo efficacy of pyrimidines. Int J Antimicrob Agents. 2013;41:363–71. [DOI] [PubMed] [Google Scholar]
- van de Beek D, Brouwer M, Hasbun Ret al. . Community-acquired bacterial meningitis. Nat Rev Dis Prim. 2016;2:16074. [DOI] [PubMed] [Google Scholar]
- Van Der Maten E, Van Selm S, Langereis JDet al. . Alternative pathway inhibition by exogenous factor H fails to attenuate inflammation and vascular leakage in experimental pneumococcal sepsis in mice. PLoS One. 2016;11, DOI: 10.1371/journal.pone.0149307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopala KN, Tratrat C, Pillay Met al. . In silico design and synthesis of tetrahydropyrimidinones and tetrahydropyrimidinethiones as potential thymidylate kinase inhibitors exerting anti-tb activity against Mycobacterium tuberculosis. Drug Des Devel Ther. 2020;14:1027–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W, Blanot D, De Pedro MA.. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–67. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Massidda O, Tomasz A.. The cell wall of Streptococcus pneumoniae. Microbiol Spectr. 2019;7, DOI: 10.1128/microbiolspec.gpp3-0018-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W, Tomasz A.. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect Immun. 2002;70:7176–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez R, García E, García P.. Phage lysins for fighting bacterial respiratory infections: A new generation of antimicrobials. Front Immunol. 2018;9, DOI: 10.3389/fimmu.2018.02252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez R, García P.. Synergy between two chimeric lysins to kill Streptococcus pneumoniae. Front Microbiol. 2019;10:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wache C, Klein M, Ostergaard Cet al. . Myeloid-related protein 14 promotes inflammation and injury in meningitis. J Infect Dis. 2015;212:247–57. [DOI] [PubMed] [Google Scholar]
- Wadood A, Jamal A, Riaz Met al. . Subtractive genome analysis for in silico identification and characterization of novel drug targets in Streptococcus pneumoniae strain JJA. Microb Pathog. 2018;115:194–8. [DOI] [PubMed] [Google Scholar]
- Wagner-Muñiz DA, Haughney SL, Kelly SMet al. . Room temperature stable PspA-based nanovaccine induces protective immunity. Front Immunol. 2018;9, DOI: 10.3389/fimmu.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Zhao FL, Zhang Let al. . Synthesis and antibacterial evaluation of a series of 11,12-cyclic carbonate azithromycin-3-O-descladinosyl-3-O-carbamoyl glycosyl derivatives. Molecules. 2017a;22, 10.3390/molecules22122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cong C, Chern Chai Wet al. . Synthesis and antibacterial activity of novel 4″-O-(1-aralkyl-1,2,3-triazol-4-methyl-carbamoyl) azithromycin analogs. Bioorganic Med Chem Lett. 2017b;27:3872–7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Desai J, Zhang Yet al. . Bacterial cell growth inhibitors targeting undecaprenyl diphosphate synthase and undecaprenyl diphosphate phosphatase. ChemMedChem. 2016;11:2311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JN, Roche AM, Hergott CBet al. . Macrophage migration inhibitory factor is detrimental in pneumococcal pneumonia and a target for therapeutic immunomodulation. J Infect Dis. 2015;212:1677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerink MAJ, Schroeder HW, Nahm MH.. Immune responses to pneumococcal vaccines in children and adults: Rationale for age-specific vaccination. Aging Dis. 2012;3:51–67. [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WJ, Bonten MJ, Boersma WGet al. . Management of community-acquired pneumonia in adults: 2016 guideline update from the dutch working party on antibiotic policy (SWAB) and dutch association of chest physicians (NVALT). Neth J Med. 2018;76:4–13. [PubMed] [Google Scholar]
- Woehrl B, Brouwer MC, Murr Cet al. . Complement component 5 contributes to poor disease outcome in humans and mice with pneumococcal meningitis. J Clin Invest. 2011;121:3943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead M, Blasi F, Ewig Set al. . Guidelines for the management of adult lower respiratory tract infections - Full version. Clin Microbiol Infect. 2011;17:E1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO) . Pneumonia. 2019. [Google Scholar]
- Wright G.Antibiotics: An irresistible newcomer. Nature. 2015;517:442–4. [DOI] [PubMed] [Google Scholar]
- Yadav M, Park S, Chae Set al. . Sinefungin, a natural nucleoside analogue of S-adenosylmethionine, inhibits Streptococcus pneumoniae biofilm growth. Biomed Res Int. 2014;2014, DOI: 10.1155/2014/156987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav MK, Chae SW, Song JJ.. Effect of 5-azacytidine on in vitro biofilm formation of Streptococcus pneumoniae. Microb Pathog. 2012;53:219–26. [DOI] [PubMed] [Google Scholar]
- Yadav MK, Go YY, Chae SWet al. . The small molecule DAM inhibitor, pyrimidinedione, disrupts Streptococcus pneumoniae biofilm growth in vitro. PLoS One. 2015;10, DOI: 10.1371/journal.pone.0139238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Sun B, Zhang Let al. . Chemical interference with iron transport systems to suppress bacterial growth of Streptococcus pneumoniae. PLoS One. 2014a;9, DOI: 10.1371/journal.pone.0105953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Xu JY, Meng Met al. . Dirhodium (II) complex interferes with iron-transport system to exert antibacterial action against Streptococcus pneumoniae. J Proteomics. 2019a;194:160–7. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bedugnis A, Levinson Set al. . Delayed administration of recombinant plasma gelsolin improves survival in a murine model of penicillin-susceptible and penicillin-resistant pneumococcal pneumonia. J Infect Dis. 2019b;220:1498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chiou TTY, Stossel TPet al. . Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol - Lung Cell Mol Physiol. 2015;309:L11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Huang YCT, Koziel Het al. . Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. Elife. 2014b;3, DOI: 10.7554/eLife.03711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau B, Too LK, Ball HJet al. . TIGR4 strain causes more severe disease than WU2 strain in a mouse model of Streptococcus pneumoniae meningitis: a common pathogenic role for interferon-γ. Microbes Infect. 2017;19:413–21. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nasu H, Namba Eet al. . Discovery of a compound that acts as a bacterial PyrG (CTP synthase) inhibitor. J Med Microbiol. 2012;61:1280–5. [DOI] [PubMed] [Google Scholar]
- Zavala A, Kovacec V, Levín Get al. . Screening assay for inhibitors of a recombinant Streptococcus pneumoniae UDP-glucose pyrophosphorylase. J Enzyme Inhib Med Chem. 2017;32:203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanel GG, Hartel E, Adam Het al. . Solithromycin: A novel fluoroketolide for the treatment of community-acquired bacterial pneumonia. Drugs. 2016;76:1737–57. [DOI] [PubMed] [Google Scholar]
- Zhanel GG, Walters M, Noreddin Aet al. . The ketolides: A critical review. Drugs. 2002;62:1771–804. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu C, Min Jet al. . Rational questing for potential novel inhibitors of FabK from Streptococcus pneumoniae by combining FMO calculation, CoMFA 3D-QSAR modeling and virtual screening. J Mol Model. 2011a;17:1483–92. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li G, Liu Met al. . Synthesis and in vitro antibacterial activity of 7-(3-alkoxyimino-5-amino/ methylaminopiperidin-1-yl)fluoroquinolone derivatives. Bioorganic Med Chem Lett. 2011b;21:928–31. [DOI] [PubMed] [Google Scholar]
- Zhang YM, White SW, Rock CO. Inhibiting bacterial fatty acid synthesis. J Biol Chem. 2006;281:17541–4. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li H, Wang Jet al. . Verbascoside alleviates pneumococcal pneumonia by reducing pneumolysin oligomers. Mol Pharmacol. 2016;89:376–87. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhou Y, Wang Let al. . Shikonin alleviates the biotoxicity produced by pneumococcal pneumolysin. Life Sci. 2017;177:1–7. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Du D, Cao Let al. . Synthesis and antibacterial activity of novel 11-[3-[(arylcarbamoyl)oxy]propylamino]-11-deoxy-6-O-methyl-3-oxoerythromycin A 11-N,12-O-cyclic carbamate derivatives. J Antibiot (Tokyo). 2016;69:811–7. [DOI] [PubMed] [Google Scholar]
- Zhou X, Cai G, He Yet al. . Separation of cordycepin from Cordyceps militaris fermentation supernatant using preparative HPLC and evaluation of its antibacterial activity as an NAD+-dependent DNA ligase inhibitor. Exp Ther Med. 2016;12:1812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T, Lacal JC, Ibrahim SA.. Choline kinase emerges as a promising drug target in gram-positive bacteria. Front Microbiol. 2019;6, DOI: 10.3389/fmicb.2019.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]